Abstract
Importance
The study provides novel data to inform the mechanisms by which poverty negatively impacts childhood brain development.
Objective
To investigate whether income to needs ratio experienced in early childhood impacts brain development at school age and to explore the mediators of this effect.
Design
Data from a prospective longitudinal study of emotion development in preschool children who participated in neuroimaging at school age were used to investigate the effects of poverty on brain development. Children were assessed annually for 3-6 years prior to the time of a MRI scan during which they were evaluated on psychosocial, behavioral and other developmental dimensions.
Setting
An academic research unit at the Washington University School of Medicine.
Participants
Preschoolers 3- 6 years of age were ascertained from primary care and day care sites in the St. Louis metropolitan area and annually assessed behaviorally for 5-10 years. Healthy preschoolers and those with clinical symptoms of depression participated in neuroimaging at school age/early adolescence.
Main Outcome Measure(s)
The main outcomes of interest were brain volumes of children's white matter and cortical gray matter as well as hippocampus and amygdala obtained using MRI. Mediators of interest were caregiver support/hostility measured observationally during the preschool period and stressful life events measured prospectively.
Results
Poverty was associated with smaller white and cortical gray matter and hippocampal and amygdala volumes. The effects of poverty on hippocampal volume were mediated by caregiving support/hostility on the left and right as well as stressful life events on the left.
Conclusions and Relevance
The findings that exposure to poverty in early childhood materially impacts brain development at school age further underscores the importance of attention to the well established deleterious effects of poverty on child development. Findings that these effects on the hippocampus are mediated by caregiving and stressful life events suggest that attempts to enhance early caregiving should be a focused public health target for prevention and early intervention. Findings substantiate the behavioral literature on the negative effects of poverty on child development and provide new data confirming that effects extend to brain development. Mechanisms for these effects on the hippocampus are suggested to inform intervention.
INTRODUCTION
The deleterious effects of poverty on child development have been well established in psychosocial research, with poverty identified as among the most powerful risk factors for poor developmental outcomes.1,2 Children exposed to poverty have poorer cognitive outcomes and school performance as well as higher risk for antisocial behaviors and mental disorders.3 Notably, developmental deficits associated with poverty have been detected as early as infancy.4,5 Despite these established and alarming poor developmental outcomes, to date there has been little neurobiological data in humans to inform the mechanism(s) of these relationships. This represents a critical gap in the literature and an urgent national and global public health problem based on statistics that over one in five children are now living below the poverty line in the United States alone.6
The tangible effect of early environmental exposures on brain development has been well established in laboratory animals. Animals exposed to enriched environments high in stimulation have been shown to display increased hippocampal cell proliferation and neurogenesis compared to those reared in relative deprivation.7 Poverty represents a form of human deprivation that may parallel this animal model, raising the question of whether low levels of stimulation and relative psychosocial neglect associated with poverty have a similar negative effect on human brain development. A few studies have directly investigated the relationship between poverty and childhood brain development. Consistent with animal data, Noble and colleagues detected smaller hippocampus and amygdala in 5-17 year old children living in poverty.8 In a large community sample, Hanson et al., reported smaller hippocampal gray matter volumes among children from lower income backgrounds.9 Lower socioeconomic status was associated with smaller hippocampal gray matter volumes bilaterally in a small sample of healthy 10 year olds.10
These findings suggest that exposure to poverty has deleterious effects on human amygdala and hippocampal development. These brain regions, involved in stress regulation and emotion processing, are known to be sensitive to environmental stimuli. However, what remains unclear, and critical to addressing this public health problem, are the specific factors that mediate this association in humans. Poverty is strongly associated with a number of risk factors implicated in poor developmental outcomes in behavioral studies, such as unsupportive parenting, poor nutrition and education, lack of caregiver education, and high levels of traumatic and stressful life events, making the income to needs ratio a good proxy for cumulative developmental stress.11 These as well as other associated factors could serve as mechanisms mediating the negative impact of poverty on brain development. Whether such mediators of risk are also operative at the neurobiological level in humans remain unclear.
Experimental studies of the neurobiological impact of poverty cannot be conducted in humans for obvious ethical reasons. However, the negative effect of early unsupportive parenting in the form of maternal deprivation and stress on hippocampal and amygdala development has been well established in rodents. Stress paradigms in rodent models have been associated with elevated anxiety and contrasting alterations in neuronal morphology in the hippocampus and amygdala, with dendritic atrophy observed in the hippocampus and increased dendritic arborization in amygdala.12,13 Developing rodents deprived of maternal nurturance show decreased hippocampal volume and altered stress reactivity.14 An epigenetic mechanism for this effect has been elaborated.15 Importantly, controlled trials that have randomized institutionalized toddlers to early therapeutic foster care versus institutionalization have documented the deleterious effects of early relative deprivation on cognitive outcomes.16
A few studies have investigated the effects of early caregiving on amygdala and hippocampal volumes in children. Consistent with animal data, Tottenham et al., showed an association between early institutional rearing and larger amygdala volumes.17 While animal data would suggest that institutional rearing would lead to reduced hippocampal volume, some investigators have suggested that such effects may not become evident in humans until later in life.18 Consistent with this, decreased hippocampal volumes have been found in numerous studies of adults who experienced high levels of childhood stress/trauma.19,20 In spite of this hypothesized delayed hippocampal effect, a positive impact of early supportive parenting on hippocampal development has been detected as early as school age.21
To investigate the effects of poverty on childhood brain development and to begin to inform the mediating mechanisms of these negative effects, we investigated associations between poverty and total white and total cortical gray matter volume, as well as hippocampus and amygdala volumes in a sample of children ages 6 to 12 years followed longitudinally since the preschool period. Based on the behavioral data in humans and the neurobiological data in animals, we hypothesized that an effect of poverty on these brain volume outcomes would be found. We also hypothesized that key variables associated with poverty and known to negatively impact child development outcomes, including caregiving support, caregiver education and stressful life events, would mediate the association between poverty and brain volumes.
METHODS
Participants
A total of n=145 right-handed children were obtained from a larger sample enrolled in the 10-year longitudinal Preschool Depression Study (PDS, N=305 at baseline). The larger sample was recruited from metropolitan St. Louis daycares and preschools using a screening checklist to include healthy children and to oversample preschoolers with depressive symptoms. Subjects and their caregivers participated in 3-6 comprehensive annual diagnostic and developmental assessments prior to the first neuroimaging session (see 22 for full description). Subjects were screened for standard imaging contraindications. There were no significant differences on demographic variables between the imaging sub-sample and the original sample. Table 1 shows the characteristics of the study sample. All study procedures were reviewed and approved by the IRB.
Table 1.
Demographics for current sample
| Mean (SD; range) or N (%) | |
|---|---|
| Average parent education (years) | |
| < High school diploma | 10 (7%) |
| High school diploma | 11 (8%) |
| Some college | 57 (38%) |
| College degree | 27 (19%) |
| Some graduate school or graduate/professional degree | 40 (28%) |
| Income-to-needs ratioa | 2.14 (1.27; 0.00 – 4.74) |
| Family size | 4.27 (1.21; 2 – 8) |
| Race | |
| African-American | 47 (56%) |
| Caucasian | 81 (32%) |
| Other | 17 (12%) |
| Supportive-to-nonsupportive caregiving ratio | 0.67 (0.45; −0.44 – 1.75) |
| Children's Age (years) | 9.78 (1.29; 6 - 12) |
| Child Gender | |
| Female | 73 (51%) |
Footnote:
Total family income divided by the federal poverty level for a family of that size closest to the year data were collected.
Measures
Income to Needs
Income-to-needs ratio was operationalized as the total family income divided by the federal poverty level based on family size in the year most proximal to data collection.23 The value was calculated through baseline PDS data of caregiver reported total family income and total number of people living in the household.
Psychiatric Diagnostic Status/Stressful Life Events/Caregivers’ Education
Subjects were assessed annually using Preschool Age Psychiatric Assessment (parent interview, age 3-8) and Child and Adolescent Psychiatric Assessment (parent/child interview, age ≥ 9).24 Both measures also reliably capture experiences of stressful and traumatic life events.25,26 Life events between baseline and time of scan were used for the current analysis.
Tanner Staging Questionnaire
The Tanner was used to measure children's pubertal status at the time of scan.27,28
Parental Supportive/Hostile Caregiving
At the second assessment wave (ages 4 -7 years), parent-child dyads were observed interacting during the “waiting task,” a structured task designed to elicit mild dyadic stress29. This laboratory task requires the child to wait for 8 min before opening a brightly wrapped gift within arm's reach. Children are told that they can open the gift once their caregiver completes questionnaires. Blind raters, trained to reliability coded the interaction for caregivers’ use of both supportive (e.g., praising the child for waiting) and hostile (e.g., threats about negative consequences) strategies. This task has acceptable psychometric properties and is a well-validated and widely used parenting measure.29-32 Hostility scores were subtracted from support scores to provide a difference score.
MRI Acquisition
Two 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) scans were acquired on a Siemens 3.0-T Tim Trio scanner without sedation (sagittal acquisition, repetition time 2,300ms, echo time 3.16ms, inversion time 1,200ms, flip angle 8°, 160 slices, 256 × 256 matrix, field of view 256mm, 1.0-mm3 voxels, total time 12:36min).
Image Analyses: Whole Brain
Total gray and white matter volumes were obtained using FreeSurfer (v5.1). The white and pial FreeSurfer surfaces were visually inspected and were regenerated with manual intervention when necessary. Cortical gray matter volume was defined as the volume between the pial and white matter surfaces. White matter volume was calculated by subtracting the subcortical and ventricular volumes from the volume bounded by the white matter surface.
Image Analyses: Amygdala and Hippocampus
The hippocampus was segmented by an automated high-dimensional template-based transformation. The manual template, delineated on one subject with typical anatomy, was reviewed by neuroanatomical gold standard experts following boundary definitions.33,34 The gold template surface, generated from the manual template, included grey and white matter. Subject images, landmarked by an experienced rater blind to subject characteristics, were aligned to the template through an affine transformation followed by a non-linear large deformation transformation to increase alignment precision. After matching subject-template voxel intensities, a high-dimensional subject-template transformation was generated through large deformation diffeomorphic metric mapping 35. Results were blindly reviewed (C.B.) for surface quality. The reliability of this process is well established.34 The amygdala segmentation paralleled the methodology of the hippocampus.
Statistical Analyses
Potential Covariates
T-tests and Pearson Correlations were conducted to explore variation in brain volumes related to children's gender, age, pubertal status, history of psychiatric disorders (Y/N), and children's history of psychotropic medication (Y/N) use. Covariates were included in the final analyses if significant for that particular region.
Associations between income-to-needs ratio and brain volume
Hierarchical multiple linear regression analyses were conducted to test whether income-to-needs ratio predicted brain volumes. For all models, covariates were entered at step 1 and income-to-needs ratio was entered at step 2.
Mediators of the hypothesized associations between income-to-needs & brain volumes
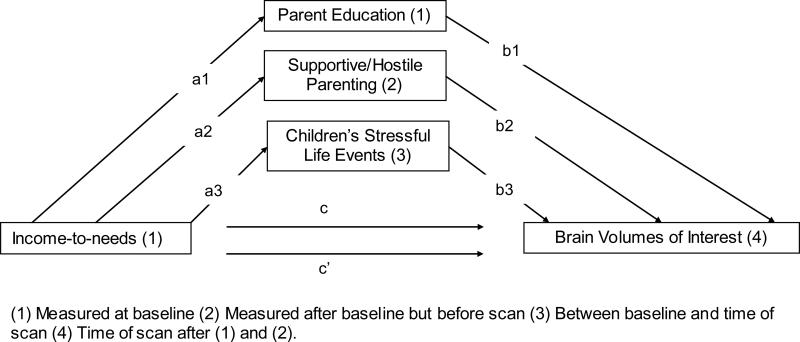
Three variables were tested as possible mediators of the relations between baseline income-to-needs ratios and children's brain volumes (see Figure 1). Mediators were tested by calculating bias-corrected 95% confidence intervals using bootstrapping with n = 10,000 resamples via the PROCESS procedure for SPSS.36,37 Given our data cannot establish temporal precedence between caregivers’ income-to-needs ratio and highest level of education, we chose to use baseline data for both variables.
Figure 1.
Conceptual Model Testing Multiple Mediators of the Hypothesized Association between Income-to-needs and Variation in Brain Volume
RESULTS
eTable 1 shows the results of analyses testing potential covariates. Based on these results, gender was included as a covariate in all analyses except those examining right hippocampal volume. For analyses of white matter volume, children's age and pubertal status were also included as covariates. None of the brain volumes differed significantly in relation to children's history of DSM-IV Axis I disorder or psychotropic medication exposure. For all analyses examining hippocampus or amygdala volumes, children's total cortical brain volume (total white + total cortical grey) was included as a covariate to assess specificity.
Income-to-Needs Predicting Total White and Cortical Gray Matter Volumes
White Matter Volume
Children's age, gender, and pubertal status were entered at step 1. Income-to-needs ratio was entered at step 2 and was a positive predictor of white matter volume, accounting for a significant increase in variance, Fchange (1,137) = 8.12, p = .005. The R2adjusted for each step of the model as well as the unstandardized regression coefficients (B), standard error (SE), and standardized regression coefficients (β) are reported in Table 2.
Table 2.
Income-to-Needs Predicting Total White Matter & Cortical Gray Matter Volume
| Region | Regression Step | R2adjusted | B | SE | β |
|---|---|---|---|---|---|
| Total White Matter Volume | Step 1 | .18c | |||
| Gender | 35825.52 | 8289.96 | .33c | ||
| Age | 527.91 | 380.08 | .16 | ||
| Pubertal Status | 16157.45 | 11320.94 | .15 | ||
| Step 2 | .22c | ||||
| Gender | 33101.50 | 8140.35 | .31c | ||
| Age | 705.30 | 373.53 | .19a | ||
| Pubertal Status | 11585.00 | 11155.72 | .11 | ||
| Income-to-Needs | 9349.11 | 3280.85 | .22b | ||
| Total Cortical Gray Matter Volume | Step 1 | .11c | |||
| Gender | 36014.24 | 8353.40 | .34c | ||
| Step 2 | .22c | ||||
| Gender | 32716.59 | 7836.99 | .31c | ||
| Income-to-Needs | 14828.42 | 3176.82 | .35c |
Footnote:
p < .05
p < .01
p < .001
Cortical Gray Matter
Gender was included at step 1 of the model. Income-to-needs ratio was entered at step 2 and was a positive predictor of gray matter volume, accounting for a significant increase in variance, Fchange(1,142) = 21.79, p < .001 (Table 2).
Income-to-needs Predicting Left and Right Hippocampus and Amygdala Volumes
Covariates, including whole brain volume, were entered in step 1. As seen in Table 3, for children's left hippocampus volume, including income-to-needs at step 2 resulted in a significant increase in the amount of variance accounted for, Fchange(1,115) = 5.76, p = .018. Income-toneeds was a positive predictor of children's left hippocampus volumes. For right hippocampus, the increase in variance accounted for after including income-to-needs at step 2 only approached significance, Fchange(1,119) = 2.94, p = .09. For children's left amygdala volume, including income-to-needs at step 2 resulted in a significant increase in the amount of variance accounted for, Fchange(1,120) = 6.28, p = .014. Income-to-needs was a positive predictor of children's left amygdala volumes. For right amygdala volumes, the increase in variance accounted for after including income-to-needs at step 2 only approached significance, Fchange(1,127) = 2.79, p = .09.
Table 3.
Hierarchical Regression: Income-to-Needs Variable Predicting Hippocampus and Amygdala Volumes
| Region | Regression Step | R2adjusted | B | SE | β |
|---|---|---|---|---|---|
| Left Hippocampus | Step 1 | .15c | |||
| Gender | 7.40 | 31.83 | .02 | ||
| Cortical Brain Volume | .001 | .000 | .41c | ||
| Step 2 | .19c | ||||
| Gender | 7.19 | 31.20 | .02 | ||
| Cortical Brain Volume | .001 | .000 | .34c | ||
| Income-to-Needs | 30.30 | 12.62 | .21 a | ||
| Right Hippocampus | Step 1 | .27b | |||
| Cortical Brain Volume | .001 | .000 | .52c | ||
| Step 2 | .28b | ||||
| Cortical Brain Volume | .001 | .000 | .49c | ||
| Income-to-Needs | 20.56 | 12.41 | .14 | ||
| Left Amygdala | Step 1 | .25c | |||
| Gender | 53.65 | 26.49 | .17a | ||
| Cortical Brain Volume | .001 | .000 | .42c | ||
| Step 2 | .28c | ||||
| Gender | 58.20 | 25.99 | .18a | ||
| Cortical Brain Volume | .001 | .000 | .36c | ||
| Income-to-Needs | 25.63 | 10.23 | .20 b | ||
| Right Amygdala | Step 1 | .32c | |||
| Gender | 58.64 | 26.17 | .18a | ||
| Cortical Brain Volume | .001 | .000 | .49c | ||
| Step 2 | .33c | ||||
| Gender | 1.11 | 26.03 | .18b | ||
| Cortical Brain Volume | .001 | .000 | .44c | ||
| Income-to-Needs | 17.38 | 10.41 | .13 |
Footnote:
p < .05
p < .01
p < .001
Caregivers’ Education, Parenting, & Stressful Life Events as Mediators of the Associations Between Income-to-Needs & Brain Volumes
The analyses described above established a relationship between income-to-needs and later brain volumes. We hypothesized that there would also be indirect (i.e., mediated) effects through caregivers’ education, observed use of supportive/hostile parenting, as well as children's experience of stressful life events. Figure 1 provides a conceptual diagram of the meditational analyses conducted. MacKinnon (2002) and colleagues suggest that mediation analyses be conducted when there is a relation between a predictor and mediator (paths a1, a2, a3 in Figure 1) as well as a relation between a mediator and outcome (paths b1, b2, and b3 in Figure 1). To be considered a mediator, the strength of the direct relation between predictor and outcome (path c Figure 1) will be diminished when the mediator is entered into the analysis (path c’ in Figure 1). Covariates included in the meditational analyses were parallel with prior analyses and were only applied to outcome variables. Below we first establish the relationship between the predictor (income-to-needs) and the potential mediators (caregiver education, parenting and life events), and then examine the relationships of the mediators to the outcome (brain volume), and when significant, whether they reduce the direct effect of income-to-needs on brain volumes.
Income-to-Needs Predicting Potential Mediators
Regression analyses confirmed income-toneeds ratio: (path a1) was significantly associated with caregivers’ education (ranges across all regions: p-values <.001 in all models), (path a2) predicted caregiving support/hostility assessed 1-year after baseline controlling for caregivers’ education (p-values <.001), and (path a3) predicted children's experience of stressful life events between baseline and time of scan when covarying for caregivers’ education and supportive/hostile parenting (p-values <.001 in all models).
Mediators of Total White Matter and Cortical Gray Matter Volumes
Paths b1, b2, and b3 from the mediators to white matter and cortical gray matter volume were all non-significant, p's > .05. Thus, neither caregiving behaviors, education, nor life stress, mediated the relationship between income-to-needs and cortical gray or white matter volume.
Mediators of Hippocampal Volumes
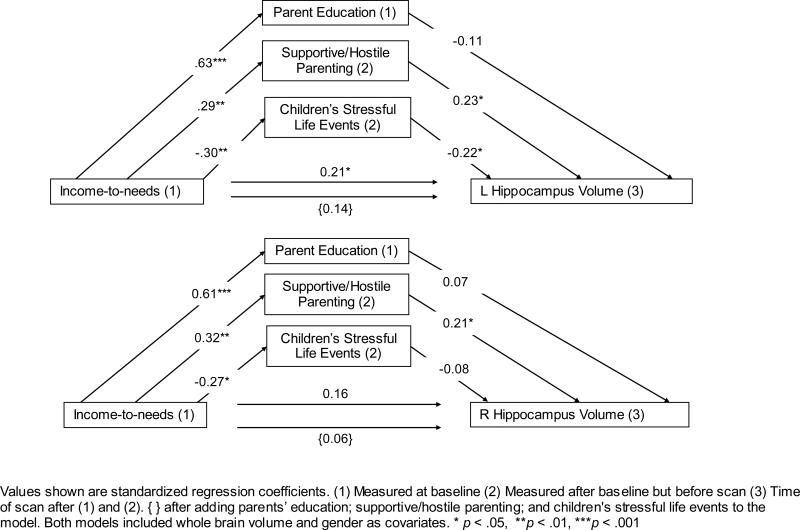
Figure 2 illustrates that two of the mediating variables, stressful life events (path b1) and caregiving behaviors (path b3), positively predicted children's left hippocampus volumes. For right hippocampus volume, caregiving behavior (path b3) was the only significant mediator. When mediators were included in the model, the direct paths (i.e., paths c’) from income-to-needs to left hippocampus, p > .51, and right hippocampus, p > .55, volumes were no longer significant, indicating full mediation (see Figure 2). eTable 2 shows the mediated effects of income-to-needs ratio on left and right hippocampus volumes.
Figure 2.
Caregivers education, Supportive/Hostile Parenting, Children's Experiences of Stressful Life Events as Mediators of the Relation between Income-to-Needs and Hippocampus Volumes
Mediators of Amygdala Volumes
Paths b1, b2, and b3 from the mediators to left and right amygdala volumes were all non-significant, p's > .14.
DISCUSSION
Study findings demonstrate that exposure to poverty during early childhood is associated with smaller white matter, cortical gray matter and hippocampal and amygdala volumes measured at school age/early adolescence. These findings extend the substantial body of behavioral data demonstrating the deleterious effects of poverty on child developmental outcomes into the neurodevelopmental domain and are consistent with prior results.8,9 Further, these study findings extend the available structural neuroimaging data in children exposed to poverty by informing the mechanism of the effects of poverty on hippocampal volumes. Findings indicated that the effects of poverty on hippocampal volumes were mediated by caregiving support/hostility on both left and right hippocampus. On the left, stressful life events also emerged as a significant mediator. Caregiver education was not a significant mediator. As exposure to poverty is well known to be strongly associated with a variety of negative life experiences, the role that these risk factors appeared to play in the relationship between poverty and alterations in brain development elucidates more specific targets for prevention.
Notably, alterations in brain volume associated with poverty were detected more globally in cortical gray and white matter volume, although mediation in these regions was not identified. The finding that mediation associated with parenting and life stress was selective to the hippocampus suggests regional specificity to these mechanistic relationships. The key role of caregiver nurturance in hippocampal development and its relationship to adaptive stress responses has well been established in animal studies. Consistent findings have been provided from an earlier sub-group of this study sample suggesting that supportive parenting also plays a key role in child hippocampal development independent of income.21 Thus, the current findings add to and extend the literature underscoring the critical role of nurturance for childhood well being.38 The finding that experiences of stressful life events also mediated the relationship between poverty and left hippocampal volume is consistent with the extensive body of animal data that have elucidated the negative effects of early stress on HPA function and hippocampal volume.39 Understanding these mechanisms is key to the design of more targeted interventions, providing a feasible alternative to changing psychosocial status itself, a much more challenging goal that vulnerable rapidly developing young children do not have time to await.
Limitations of the current data are that the original study sample was oversampled for preschoolers with symptoms of depression, limiting generalizability. Further, the relationships in the mediation model may be bi-directional. A sample with multiple waves of imaging data starting earlier in development would be necessary to adequately test directionality. Future studies with such designs and more detailed assessments of the correlates of poverty such as nutrition, parental psychopathology and genetic factors are needed to further elucidate the mechanisms of risk.
We believe these findings may be useful to inform preventive interventions for this high-risk population facing a multitude of psychosocial stressors and suggest that caregiving should be a specific target. The importance of early interventions that target caregiving is underscored by studies demonstrating high cost effectiveness through greatly enhanced long-term outcomes.40 Further, children who receive more nurturing caregiving may also be protected from exposure to stressful life events suggesting this central target may have positive ramifications on brain development.41 Considering these issues, study findings are relevant to the public policy debate on the importance of early preschool programs for young children living in poverty. The finding that the effects of poverty on hippocampal development are mediated through caregiving and stressful life events further underscore the importance of high quality early childhood caregiving, a task that can be achieved through parenting education and support as well as through preschool programs that provide high quality supplementary caregiving and safe haven to vulnerable young children.
Supplementary Material
Acknowledgments
The authors thank Michael I. Miller Ph.D. and Tilak Ratnanather Ph.D. of the Center for Imaging Science, The Johns Hopkins University, for their technical assistance and insights with the implementation of the large deformation diffeomorphic metric mapping to quantify the hippocampus and amygdala volumes.
Funding/Support: This study was supported by NIH Grants 2R01 MH064769-06A1 (Luby), and R01MH090786 (Luby, Barch, Botteron). Dr. Belden's work on this manuscript was supported by NIH Grant 5K01MH090515-04.
Role of the Sponsor: The funding source had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Luby had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Luby, Barch, Botteron
Acquisition of data: Luby, Barch, Botteron, Babb, Nishino
Analysis and interpretation of data: Belden, Luby, Barch, Harms, Marrus, Babb
Drafting of the manuscript: Luby, Belden, Barch
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Belden, Barch
Obtained funding: Luby, Barch, Botteron
Administrative, technical, or material support: Babb, Nishino, Marrus
Study supervision: Luby, Barch, Botteron
Additional Contributions: We wish to acknowledge our child participants and their parents whose participation and cooperation made this research possible.
Conflict of Interest Disclosure: The authors declare no conflict of interest
Online-Only Material: eTables 1 and 2 are available at http://www.jama.com
REFERENCES
- 1.Carneiro PM, Heckman JJ. Human Capital Policy. In: Heckman JJ, Krueger AB, Friedman BM, editors. Inequality in America : What role for human capital policies? MIT Press; Cambridge, MA: 2003. [Google Scholar]
- 2.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7:55–71. [PubMed] [Google Scholar]
- 3.Yoshikawa H, Aber JL, Beardslee WR. The effects of poverty on the mental, emotional, and behavioral health of children and youth: implications for prevention. Am Psychol. 2012;67:272–284. doi: 10.1037/a0028015. [DOI] [PubMed] [Google Scholar]
- 4.Lipina SJ, Martelli MI, Vuelta B, Colombo JA. Performance on the A-not-B task of Argentinean infants from unsatisfied and satisfied basic needs homes. Revista Interamericana de Psicología. 2005;39:49–60. [Google Scholar]
- 5.Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Paul H Brookes Publishing; Baltimore, MD: 1995. [Google Scholar]
- 6.U.S. Census Bureau [February 2013];Child poverty in the United States 2009 and 2010: Selected race groups and hispanic origin. 2011 http://www.census.gov/prod/2011pubs/acsbr10-05.pdf.
- 7.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 8.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS One. 2011;6:e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jednoróg K, Altarelli I, Monzalvo K, et al. The influence of socioeconomic status on children's brain structure. PLoS One. 2012;7:e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council and Institute of Medicine . Family Resources. In: Shonkoff JP, Phillips D, editors. From Neurons to Neighborhoods: The Science of Early Childhood Development. National Academy Press; Washington, D. C.: 2000. [PubMed] [Google Scholar]
- 12.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky RM. Mothering style and methylation. Nat Neurosci. 2004;7:791–792. doi: 10.1038/nn0804-791. [DOI] [PubMed] [Google Scholar]
- 16.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 17.Tottenham N, Hare TA, Quinn BT, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luby JL, Barch DM, Belden A, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Nat Acad Sci USA. 2012;109:2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luby J, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: Impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 24.Egger H, Ascher B, Angold A. Preschool Age Psychiatric Assessment (PAPA): Version 1.1. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; Durham, NC: 1999. [Google Scholar]
- 25.Angold A, Costello E. A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C). Psychol Med. 1995;25:755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- 26.Angold A, Costello E. The Child and Adolescent Psychiatric Assessment (CAPA). J Am Acad Child Adolesc Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 28.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 29.Carmichael-Olson H, Greenberg M, Slough N. Manual for the Waiting Task. University of Washington; 1985. [Google Scholar]
- 30.Cole PM, Teti LO, Zahn-Waxler C. Mutual emotion regulation and the stability of conduct problems between preschool and early school age. Dev Psychopathol. 2003;15:1–18. [PubMed] [Google Scholar]
- 31.Cole PM, Dennis TA, Smith-Simon KE, Cohen LH. Preschoolers’ emotion regulation strategy understanding: Relations with emotion socialization and child self-regulation. Social Development. 2009;18:324–352. [Google Scholar]
- 32.Mirabile S, Scaramella L, Sohr-Preston S, Robison S. Mothers’ socialization of emotion regulation: The moderating role of children's negative emotional reactivity. Child Youth Care Forum. 2009;38:19–37. doi: 10.1007/s10566-008-9063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 34.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 35.Beg MF, Miller MI, Trouvé A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vision. 2005;61:139–157. [Google Scholar]
- 36.Hayes AF. Introduction to mediation, moderation, and conditional process analysis : A regression-based approach. The Guilford Press; New York, NY: 2013. [Google Scholar]
- 37.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 38.Biglan A, Flay BR, Embry DD, Sandler IN. The critical role of nurturing environments for promoting human well-being. Am Psychol. 2012;67:257–271. doi: 10.1037/a0026796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 40.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- 41.Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Dev Psychol. 1994;30:467–483. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.