Abstract
It is becoming increasingly clear that steroid hormones are involved in the biology of many organs outside the reproductive system. Evidence has been accumulating since the mid-1990’s that the lung contains receptors for both estrogen and progesterone and that these hormones have some role in lung development, pulmonary inflammation, and lung cancer. The estrogen receptor β (ERβ) is the major ER expressed in lung tissues, while inflammatory cells capable of infiltrating the lung are reported to express both ERα and ERβ. Although there is evidence in animals of preferential effects of ERβ in the lungs of females, human lung tumors from males also contain ERβ positive cells and express aromatase, the enzyme that converts testosterone to estrogens. This review will discuss current literature findings on the role of the ERs and the progesterone receptor (PR) as well CYP19 (aromatase), the rate-limiting enzyme in the synthesis of estrogen, in lung cancer.
ESTROGEN RECEPTORS IN THE DEVELOPING LUNG AND IN LUNG CANCER
Estrogen receptors (ERs) are members of the nuclear steroid receptor superfamily, and mediate cellular responses to the hormone estrogen. ERs function either as estrogen-dependent transcription factors, or as phosphorylation-dependent transcription factors that are activated by kinase pathways not requiring ligand binding1. Two different genes encode the ER proteins ERα and ERβ, which are expressed with different tissue distributions2. Both ER subtypes bind β-estradiol, the most active form of estrogen, with high affinity. Multiple isoforms of ERα and ERβ exist including at least three ERα isoforms3 and five ERβ isoforms4,5. ERβ is thought to be the major functional form of ER in the lung based on two lines of evidence. First, differential expression of ERβ mRNA compared to ERα mRNA was found in human lung tissue during fetal development6 and in the adult mouse lung7. Second, female ERβ knockout (−/−) mice display a lung abnormality: at three months of age, they display a decreased number of alveoli and reduction in expression of key regulators of surfactant homeostasis7. By age five months, both female and male mice show alveolar collapse and alterations in extracellular matrix8, suggesting that estrogen does have some role in lung homeostasis in males as well as females. In the ERβ −/− mouse, female but not male offspring were protected against development of lung tumors after in utero exposure to the polycyclic hydrocarbon dibenzochrysene9. Confirmation that the lung is an estrogen responsive tissue was observed in the transgenic ERE-luciferase reporter mouse, where a 15-fold induction of reporter gene expression occurred in the lungs of both males10 and females11 after estrogen treatment.
Antibodies that distinguish between ERα and ERβ proteins are now well established, and it is apparent that full-length ERβ protein is expressed in most human NSCLC cell lines, and is frequently present in primary specimens of human NSCLCs from men as well as women12-16. ERβ protein is detected in both the nucleus and the cytoplasm and is comprised of mainly full-length protein in addition to some smaller variants12. However, the frequency and function of the different ERβ isoforms in lung cancer is not well understood because most studies have not undertaken a comparison of the five known ERβ isoforms which are the result of alternative splicing of the last coding exon 17. ERβ-1 is the full-length ERβ protein and the only fully functional isoform which can bind ligand. ERβ-2 has been reported to function as a dominant negative of ERα18 whereas isoforms -3,-4 and -5 do not have innate activities but can heterodimerize with ERβ-14. Whether there is lung tumor expression of full-length ERα is controversial. ERα staining of lung tumor tissues and cell lines was found primarily in the cytoplasm and on the cell membrane, with rare expression in the nucleus12-16, and both mRNA and protein analysis showed ERα messages to be comprised of alternatively spliced variants12. These variant isoforms lack the amino-terminus because the proteins are differentially detected by antibodies that recognize the ERα amino- and carboxy-terminal12. Immunoblotting failed to detect the expected 66kD ERα protein, while smaller variants of 42kD and 54kD were found19,20. Estrogen-mediated RNA transcription, non-genomic signaling that activates tyrosine kinases, and proliferation in lung tumor cell lines can be blocked by the ER inhibitor fulvestrant, providing evidence that ERs found in lung cancer are functional12,19,20. Comparisons of ERα and ERβ selective agonists show that biological effects are predominantly mediated by ERβ20. Although ERα protein may be found in some lung tumors, such as those with EGFR mutation21, ERβ appears to be the major ER expressed in lung cancer.
ERs and LUNG CANCER SURVIVAL
There are now many published reports examining ER status in relation to NSCLC patient survival. Recently, high cytoplasmic ERβ-1 staining was identified as a negative prognostic factor for lung cancer, independent of other prognostic factors22. Nuclear ERβ positivity was observed in the majority of lung cancer cases13-16,22 and found to be a favourable prognostic indicator in some studies. In some reports, the prognostic significance was only observed in male patients or was limited to a subset of patients with a particular mutation14-16. However, most studies utilized antibodies to total ERβ that could not distinguish different ERβ isoforms. The negative effect of ERβ-1 on survival was observed in male and female patients and showed no interaction with sex. Prognostic significance of cytoplasmic ER protein may be related to the importance of non-genomic signaling for ER action in the lung (discussed below). Isoform specificity was also reported in a study demonstrating that ERβ-1 but, not ERβ-2, was related to worse prognosis in female stage I lung cancer patients23. Nuclear ERβ-1 also correlated with poor survival in metastatic lung cancer, but not early stage lung cancer patients24. In contrast, the ERβ-2 and −5 isoforms have been linked to better lung cancer outcome25. ERβ survival studies are summarized in Table I.
Table 1.
Summary of ERβ survival analysis of lung tumor tissue by IHC
| Study Name | ERβ Ab | Sample Size | Stage Distribution | Scoring System | Result |
|---|---|---|---|---|---|
| Wu 2005 (ref. 13) | Biogenix (ERβ-1 isoform specific) | Male 174 | Stage I 38% | nuclear staining only scored | Positive ERβ=lower grade tumor in men and women combined |
| Female 127 | Stage II 22% | positive = moderate/strong nuclear staining of >50% of tumor cells | ERβ overexpression= better survival | ||
| Total 301 | stage III 40% | negative = not defined | |||
| cut-off= positive versus negative | |||||
| Schw artz 2005 (ref. 14) | MCA 1974 (ERβ-1 isoform specific) | Male 64 | Local 21.9% | nuclear and cytoplasmic staining scored together | Positive ERβ= better survival in men (significant) |
| Female 214 | Regional/Distant 64.7% | % positive cells (focal <10%, moderate 11-50%, diffuse 41-100%) + staining intensity (1-3) | Positive ERβ= worse survival in women (trend) | ||
| Total 278 | Unknown 13.3% | positive = at least +1 (weak) intensity in ≥ 10%of tumor cells | |||
| negative = no nuclear staining, cytoplasmic staining or +1 intensity nuclear staining | |||||
| cut-off= positive versus negative | |||||
| Kaw aii 2005 (ref. 15) | H-150 (epitope-aa 1-150 of ERβ) | Male 76 | Stage I 50% | not specified if nuclear or cytoplasmic ERβ was examined | Positive ERβ= better survival in men and women combined |
| Female 56 | Stage II 18% | % positive score (0-5) + staining intensity (0-3)= total score (0-8) | High ERβ= better survival in men and women combined (Stage I only) | ||
| Total 132 | Stage III 27% | positive = not defined | |||
| Stage IV 5% | negative= not defined | ||||
| cut-off= positive versus negative and low (0-4) versus high (5-8) | |||||
| Skov 2005 (ref. 16) | PPG5/10 (ERβ-1 isoform specific) | Male 71 | Stage I 63% | nuclear staining only scored | Negative ERβ= better survival in women |
| Female 33 | Stage II 13% | positive= at least weak nuclear staining in >10% of tumor cells | Positive ERβ= better survival in men | ||
| Total 104 | Stage III 26% | negative= not defined | no clinical signficance with cytoplasmic ERβ in separate analysis | ||
| cut-off= positive versus negative | |||||
| Nose 2009 (ref. 60) | H-150 (epitope-aa 1-150 of ERβ) | Male 260 | Stage I 64% | nuclear staining only scored | Strong nuclear ERβ=better DFS in all patients |
| Female 187 | Stage II 9% | % positive score (0-5) + staining intensity (0-3)= total score (0-8) | Strong nuclear ERβ= better DFS in patients with EGFR mutant tumors, but not EGFR wild-type tumors | ||
| Total 447 | Stage III 23% | negative= 0 | |||
| Stage IV 4% | weak= 2-4 | ||||
| strong= 5-8 | |||||
| cut-off= negative/weak versus strong | |||||
| Raso 2009 (ref. 67) | H-150 (epitope-aa 1-150 of ERβ) | Male 150 | Stage I 63% | nuclear and cytoplasmic staining scored separately | Low nuclear ERβ= better RFS |
| Female 167 | Stage II 20% | % positive score (0-100%) X staining intensity (0-3)= total score (0-300) | no relationship with OS | ||
| Total 317 | Stage III 15% | positive= >0 | |||
| Stage IV 2% | negative=0 | ||||
| cut-off= low (0) versus high (>0) | |||||
| Stabile 2011 (ref. 20) | PPG5/10 (ERβ-1 isoform specific) | Male 91 | Stage I 39% | nuclear and cytoplasmic staining scored separately | High cytoplasmic ERβ=worse OS and shorter TTP |
| Female 92 | Stage II 20% | % positive score (0-5) + staining intensity (0-3)= total score (0-8) | no relationship with nuclear ERβ | ||
| Total 183 | Stage III 28% | low= 0-7 | ERβ associated with poor survival was in the strongest staining group only (>7) | ||
| Stage IV 11% | high=>7 | Survival effect may be for ERβ-1 overexpressing tumors only | |||
| Unknown 1% | cut-off= low versus high | ||||
| Navaratnam 2012 (ref. 22) | GC17/385P (ERβ-1 isoform specific) | Male 70 | Stage I-II 64% | nuclear staining only scored | High ERβ-1= better OS in earlier stage |
| 14C8 (total ERβ) | Female 67 | Stage III-IV 36% | nuclear staining intensity (1-3) X % tumor cells stained | High ERβ-1=worse OS in later stage | |
| 57/3 (ERβ-2 isoform specific) | Total 137 | cut-off= median IHC score | no relationship with ERβ-2 | ||
| Liu 2013 (ref. 23) | PPG5/10 (ERβ-1 isoform specific) | Male 58 | Stage I-II 36% | nuclear and cytoplasmic staining scored separately | cytoplasmic ERβ-2 and ERβ-5=longer DFS and OS |
| 57/3 (ERβ-2 isoform specific) | Female 54 | Stage III-IV 64% | % positive score (0-5) + staining intensity (0-3)= total score (0-8) | ||
| Total 112 | cut-off = Allred score>3 | ||||
| DFS, disease-free survival; RFS, recurrance-free survival; PFS, progression-free survival; OS, overall survival; TTP, time to progression | |||||
There is no consensus on effect on survival of expression of ERα protein, which as noted above is predominantly found as smaller variant proteins. It is variously reported that ERα has no effect on survival, or to correlate with poor prognosis15,16,22. Nuclear and cytoplasmic ERs may have distinct functions and each component should be assessed both separately and together in lung cancer patient tissue specimens. A growing literature also shows that ERs localize to mitochondria and that estrogen can induce expression of the mitochondrial genome as well as increase vulnerability to oxidative stressors such as hydrogen peroxide. There are recent reports of mitochondrial action of ERβ in lung cancer cells where it appears to protect against apoptosis26 and to show reduced activity during allergic airway inflammation in a mouse model of asthma27. Analysis of the different ERβ isoforms as well as their cellular localization will be necessary to completely understand the role of ERβ in lung cancer. If standardized approaches can be developed, these hormone receptor markers may become useful biomarkers, potentially able to predict the aggressiveness of lung cancers and to identify patients who might respond to hormonal therapy.
Women with advanced NSCLC live longer than men28, although this observation is not specific to lung cancer’ but is found in many tumor types. How much of this survival differences might be attributed to hormonal differences is not clear. A study of lung cancer presentation in pre- versus post-menopausal women showed more advanced disease including poorly differentiated tumors with less favourable histologies in pre-menopausal women29. Despite this, a significant survival difference between pre-and post-menopausal women was not seen. In a more recent study, women over the age of 60 had a significant survival advantage over both men and younger women, a difference potentially attributable to hormonal status since men did not show survival differences by age30.
HORMONE REPLACEMENT AND LUNG CANCER SURVIVAL
Exposure to hormone replacement therapy (HRT) has negative effects on lung cancer survival. Ganti et al.31 reported that a significant association between both a lower median age at lung cancer diagnosis and a shorter median survival time in women who used HRT around the time of diagnosis versus those who did not. This effect was more apparent in women who smoked, suggesting an interaction between estrogens and tobacco carcinogens. The Women’s Health Initiative, a randomized, placebo-controlled trial in which more than 16,000 post-menopausal women received placebo or daily HRT for 5 years, also reported a strong negative effect on survival after a lung cancer diagnosis in women on the HRT arm32. The HRT group had a significantly greater likelihood of dying from lung cancer with a trend toward more lung cancer diagnoses compared to the placebo group. An increase in lung cancer incidence associated with HRT was also observed in the Vitamins and Lifestyle Study, and this effect on lung cancer risk was duration dependent33. However, other reports suggest that HRT use prior to diagnosis could protect women from developing lung cancer, especially if they smoked34. An inverse relationship was also observed between HRT use and NSCLC risk in postmenopausal women with ER-positive, but not ER-negative lung tumors35. There are several possible explanations for these differing observations. There could be different effects on the balance between induction of cell differentiation and cell proliferation by estrogen in normal lung epithelium compared to malignant epithelium. ERβ is over-expressed in lung tumors compared to matched normal lung tissues22, which could lead to abnormal responses to estrogen. The immune system is also regulated by estrogen, and the ability of the immune system to reject malignant lung tissues early in the cancer process could be enhanced by HRT. Lung tumors are also known to produce aromatase (see below);thus it is possible that exogenous hormone use reduces local estrogen production by inhibiting pulmonary aromatase expression. Exact HRT used, duration of use and timing of use may modulate the effects of HRT on lung cancer risk prior to diagnosis and survival of lung cancer patients after diagnosis. Since it is now recommended that HRT use be of limited duration in post-menopausal women, due to hazards of long-term use, HRT effects on lung cancer risk or outcome may be less pronounced in the future.
A role for estrogen in lung cancer presentation is supported by several retrospective population studies demonstrating that anti-estrogen use improves survival of female lung cancer patients. An observational study which included more than 6500 breast cancer survivors found that women who received any anti-estrogen treatment had significantly lower subsequent lung cancer mortality36. The Manitoba Cancer Registry also evaluated 2320 women with or without exposure to anti-estrogens37. Anti-estrogen used both before and after lung cancer diagnosis was significantly associated with decreased mortality. Published studies on HRT and anti-estrogen use support the idea of estrogen acting as a promoter of lung cancer aggressiveness that may play a key role not only in the biology but also the outcome of lung cancer.
A body of preclinical evidence now demonstrates that estrogen is a driver of lung cancer. Estrogens induce cell proliferation of NSCLC cells in cell culture12,38, human tumor xenografts12, and in animal models of lung cancer39. Estrogen can also modulate expression of genes in NSCLC cell lines that are important for inducing cell proliferation such as c-myc and cyclin D119. Estrogen signaling through ERE and AP-1 promoter elements was shown to occur primarily through ERβ in NSCLC cells20,40. In addition, fulvestrant, a pure ER antagonist, inhibits proliferation in NSCLC cell lines and in lung tumor xenograft models in immunocompromised mice12. This preclinical evidence combined with population data showing that HRT reduces lung cancer survival strongly support targeting the estrogen pathway therapeutically.
AROMATASE IN LUNG CANCER
CYP19, otherwise known as aromatase, a member of the cytochrome P450 family, catalyzes the conversion of androstenedione and testosterone to estrone and β-estradiol, respectively, and both CYP19 mRNA and protein have been detected in the lung41,42. High β-estradiol levels were detected by mass spectroscopy in intra-tumoral extracts of primary NSCLC43. Several studies have now shown the ability of lung cancer cells to synthesize their own estrogen. Aromatase protein was expressed in NSCLC cell lines and primary lung tumor tissues and aromatase positive NSCLC cell lines were shown to produce β-estradiol44. In patient specimens of lung cancer, aromatase positivity detected by immunohistochemistry found aromatase protein localized in both the epithelial cell component of lung tumors as well as in infiltrating macrophages (Figure 1), suggesting that release of estrogen might occur locally in the tumor microenvironemnt. A large decrease in growth of human NSCLC tumor xenografts treated with the aromatase inhibitor anastrozole has been observed44.
Figure 1.
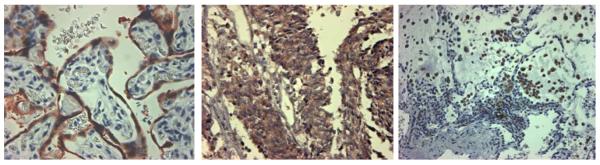
Detection of aromatase (CYP19) by immunohistochemistry in control human placental tissue (left), in a primary human lung tumor with staining in both the epithelial component and in infiltrating inflammatory cells (center), and in a primary lung tumor with staining only in the inflammatory cell component (right).
Anastrozole was also demonstrated to prevent lung carcinogenesis in female mice exposed to a nitrosamine carcinogen found in tobacco, and this prevention effect was increased when combined with fulvestrant45. Interestingly, in this animal model of lung cancer prevention, aromatase expression was confined almost exclusively to inflammatory cells that had infiltrated preneoplastic and neoplastic areas of the lungs, whereas the abnormal epithelial cells were mostly negative45. Thus, an important source of estrogen synthesis may be inflammatory cells that infiltrate the lungs in response to carcinogens, beginning early in the carcinogenesis process. This local production of estrogen may be part of the chronic inflammatory reaction occurring in lung tumors. Use of an aromatase inhibitor to treat lung cancer is further supported by Coombes and colleagues which reported a lower incidence of primary lung cancer in breast cancer patients treated with exemestane after 2 to 3 years of tamoxifen therapy (4 cases) compared with continued tamoxifen treatment (12 cases)46.
Mah et al.47 found aromatase to be a predictive biomarker of lung cancer survival in early stage lung cancer. Women over age 65 with lower levels of aromatase in tumor tissue had a greater chance of survival compared to those with higher aromatase expression. The prognostic value of aromatase expression was greatest in Stage I and II lung cancer patients. In this post-menopausal population of patients whose circulating estrogen levels are low due to decreased production by the ovaries, local estrogen production through tumor expression of aromatase could be the determinant of estrogen levels. However, no general association between aromatase and lung cancer survival was observed in a separate cohort that included all stages of lung cancer unless combined with other markers such as ERβ, EGFR and PR expression22. No effect of sex or menopausal status was found for aromatase in this study, but the study did not focus on older post-menopausal women. Taken together, literature observations about aromatase in NSCLC strongly suggest the use of aromatase inhibitors, already in use for breast cancer treatment, to treat or possibly prevent lung cancer. Since aromatase inhibitors are contraindicated in premenopausal women and in men, this approach would be targeted to post-menopausal women whose lung tumors are aromatase positive, and may give clinicians a biomarker to predict survival of post-menopausal women at an early stage of disease where more treatment options are available. A phase I clinical trial is underway that will evaluate side effects and best dose of the irreversible steroidal aromatase inhibitor exemestane in combination with chemotherapy for late stage lung cancer therapy (NCT01664754). Other enzymes involved in intratumoral production and metabolism of estrogens are also under investigation as potential targets for lung cancer therapy48.
NON-GENOMIC ESTROGEN SIGNALING AND INTERACTIONS WITH GROWTH FACTOR RECEPTOR SIGNALING PATHWAYS
Although most breast cancer studies focus on nuclear actions of ERs, involving changes in gene transcription that take place over several hours or longer through direct binding of ERs to promoter elements of estrogen response genes, estrogen can also rapidly activate cytoplasmic kinase signaling in seconds to minutes. This rapid signaling is termed non-genomic and occurs via non-nuclear ERs located in the membrane or the cytoplasm. In breast cancer cells, an additional membrane ER was identified as a G protein-coupled receptor called GPR3049. Expression of GPR30 has recently been demonstrated in lung cancer cells but the function and regulation of GPR30 in the lung is still unknown50. In NSCLC cells, extra nuclear ERs besides GPR30 have been identified in plasma membrane fractions and cytoplasmic fractions and treatment with estrogen or ERβ-specific ligands has been shown to promote rapid stimulation of tyrosine kinase signaling pathways20,38. These effects can be inhibited by the addition of fulvestrant.
The mechanism of non-genomic ER signaling is through activation of tyrosine kinase growth factor pathways, such as the epidermal growth factor receptor (EGFR/HER-1) and the insulin-like growth factor 1 (IGF-1R). EGFR is a member of the tyrosine kinase receptor family that also includes HER-2, HER-3 and HER-451, and many lung tumors are highly dependent on these pathways for proliferation, cell motility, angiogenesis, cell survival, and differentiation52. Over-expression of EGFR correlates with poor prognosis in NSCLC patients53. An interaction between the ER and EGFR has been demonstrated in lung cancer cells40,54; estrogen can activate the EGFR in lung cancer cell lines within 5-10 minutes through release of EGFR ligands, and the combination of the anti-estrogen fulvestrant and an EGFR tyrosine kinase inhibitor such as gefitinib or erlotinib can maximally inhibit cell proliferation, induce apoptosis and reduce downstream signaling pathways both in vitro and in vivo40,55. Erlotinib, the EGFR inhibitor that is FDA-approved for NSCLC also gave the best anti-tumor effects in NSCLC tumor xenografts when combined with fulvestrant55. The multi-targeted tyrosine kinase inhibitor, vandetanib, which targets EGFR and VEGFR, also showed additive effects when combined with fulvestrant56. A synergistic effect of gefitinib combined with the reversible non-steroidal aromatase inhibitor, anastrozole, was also observed in lung cancer cell lines, further suggesting a functional interaction between EGFR and ER pathways57. Additionally, membrane ERs were co-localized with EGFR in lung tumors39. Ligand-independent signaling may also occur through activation of ERs by tyrosine kinase receptors such as EGFR. For example, EGFR can directly phosphorylate ER at specific serine residues58. These residues were found to be phosphorylated in 87.5% of ER positive lung tumors examined54.
Reciprocal control of expression was also observed between ER and EGFR in lung cancer cells. In NSCLC cells, EGFR protein expression was down-regulated in response to estrogen and up-regulated in response to fulvestrant, suggesting that the EGFR pathway is activated when estrogen is depleted40. Conversely, ERβ protein expression was down-regulated after treatment with EGF and up-regulated following treatment with gefitinib, providing a further rationale to target these pathways together40. Similar cross-talk has also been found between ER signaling and IGF-1R signaling in lung cancer, a pathway that has also been implicated in lung cancer development. Estrogen was demonstrated to up-regulate IGF-1R expression through ERβ activation in lung cancer cells and tissues59. Both aromatase and ERβ expression were positively correlated with IGF-1 and IGF-1R expression in this study59, and these pathways acted synergistically to promote the development of lung adenocarcinoma in mice60. The combined treatment with fulvestrant and an IGF-1R inhibitor showed maximum anti-tumor effects compared to single agent treatment in this a carcinogen-induced mouse adenocarcinoma model60.
Targeting the EGFR using TKIs as a single therapy is of limited use in the absence of an EGFR mutation, which occurs in about 20% of adenocarcinoma patients. The patients who respond well to EGFR TKIs are mainly females and never smokers which may relate to cross-talk in signaling between the EGFR and ER in lung cancer61. As noted above, some studies have reported a correlation between EGFR mutation and ER expression21,62. These observations were translated in a phase I clinical trial using drugs that target EGFR and ER, that assess the toxicity of combined treatment of gefitinib with fulvestrant63. Targeting both pathways was found to be safe and to have anti-tumor activity in female patients with advanced, pre-treated NSCLC. Additionally, high ERβ expression was correlated with better patient survival. A phase II trial examining the combination of erlotinib (the now-preferred EGFR TKI) with fulvestrant compared to erlotinib alone has recently been completed, in which 100 patients were treated64. Combination treatment was well tolerated. Progression-free survival and response rate were similar between the two treatment arms in unselected patients. However, among patients with EGFR wild-type tumors, the clinical benefit rate (which included partial responders and those with stable disease) was significantly higher among patients treated with the combination compared to erlotinib alone, with trends towards improved survival. It is yet to be determined what ER-related biomarkers will be informative in defining the patients most likely to benefit. These clinical trials suggest that targeting the ER pathway in conjunction with the EGFR pathway, or other aberrantly expressed tyrosine kinase receptors, will have beneficial antitumor effects in NSCLC as has been observed in breast cancer cells65, particularly in patients whose tumors do not contain an EGFR mutation. The combination of anti-estrogen therapy with an IGF-1R inhibitor also warrants clinical investigation.
PROGESTERONE RECEPTORS IN LUNG CANCER
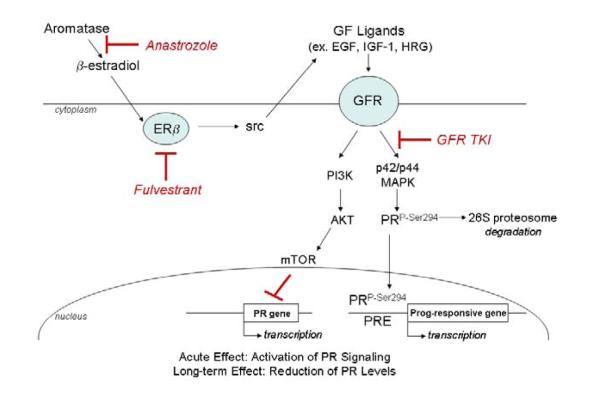
There are two major isoforms of PR, PR-A and PR-B, which play different roles in modulating cellular responses to progesterone. PR is an estrogen response gene, and PR-positive breast cancers are usually more differentiated tumors that respond to anti-estrogen therapy. The ratio of PR-A:PR-B is thought to affect clinical outcome for breast cancer, with high levels of PR-A associating with more differentiation and better survival. There are several reports of expression of total PR by primary NSCLC tissues, although there is a great deal of variability in the reported frequency of expression22,66,67,68,69. Several reports found little or no PR in NSCLC16,70,71, while in another study, lower PR was observed in lung tumors compared to matched normal lung tissue22. Two reports have shown that PR is a strong protective factor for lung cancer22,70. The antibodies used in these lung cancer survival studies do not distinguish between the PR-A and PR-B isoforms, which could exert different functions. Enzymes capable of synthesizing progesterone were also detected in many NSCLC tumors. Apositive correlation was observed between intratumoral levels of progesterone and the presence of three enzymes that participate in progesterone synthesis: steroidogenic acute regulatory protein, P450 side chain cleavage and 3β-hydroxysteroid dehydrogenase70. Progesterone treatment led to growth inhibition of tumor xenografts and along with induction of apoptosis, in agreement with clinical data suggesting presence of PR was correlated with longer overall survival in NSCLC patients70. Progesterone has also been shown to inhibit migration and invasion of lung cancer cell lines72. In breast cancer, PR is known to signal though ligand-independent mechanisms due to phosphorylation by kinases, leading to degradation of the phosphorylated form by the proteasome73. One mechanism for low tumor PR expression in breast tumors is through increased growth factor signaling which leads to a more aggressive tumor biology with faster progression, while also causing PR phosphorylation and down-modulation74. Whether or not this same mechanism of kinase-directed PR phosphorylation occurs in lung tumors is unknown and is currently being investigated (Figure 2).
Figure 2. Proposed Model of Ligand-Independent PR Signaling in Lung Cancer.
Growth Factor (GF) ligands will induce Growth Factor Receptor (GFR) signaling, resulting in phosphorylation of PR at ser294 by the MAPK pathway, leading to both ligand-independent receptor activation and induction of proteosome-mediated PR degradation, and/or may lead to direct suppression of PR transcription by the Akt/mTor pathway (with a resultant decrease in PR protein expression). Such a pathway might explain why low PR in lung tumors was associated with worse survival, because it is indicative of high levels of GFR siginaling.
Progesterone derivatives have been useful in the treatment of both endometrial cancer and breast cancer75,76. Agents such as medroxyprogesterone acetate, which can be given orally, have potential for treatment of lung cancer, perhaps in combination with agents that suppress either the ER pathway or act on growth factor pathways such as EGFR, c-Met, or other TKIs. Whether progesterone could be used for prevention is open to debate since it can also have angiogenic properties.
ESTROGEN RECEPTORS IN OTHER NON-REPRODUCTIVE MALIGNANCIES
Expression of ER has been observed in tumors derived from other non-endocrine target tissues, such as head and neck squamous cell carcinoma (HNSCC). HNSCC tumors express ERs, although with variable results reported in the literature. Exogenous estrogen has been shown to stimulate HNSCC proliferation and invasion in vitro77 and to increase tumor growth in mice78. Similar to lung cancer, estrogen has been shown to induce both genomic (transcriptional responses) as well as non-genomic (rapid P-MAPK signaling) in HNSCC77. The main difference between these tumor types is that both ERα and ERβ are important in HNSCC whereas ERβ is the predominant receptor in lung tumors. Nuclear ERα was increased in HNSCC tumors compared to adjacent normal tissue and high nuclear ERα tumor levels have been linked to poor survival in HNSCC77. In contrast, estrogen appears to have protective effects in colorectal cancer, which appear to be mediated through ERβ expression79. Population studies have shown exposure to HRT protects women against colorectal cancer, the opposite of what has been observed for lung cancer. Estrone is produced in the colon by conversion from β-estradiol by 17-β hydroxysteroid dehydrogenases, and estrone has anti-proliferative effects in the colon. Non-genomic signaling that results in suppression of genes such as c-Myc and cyclins may be involved in these anti-proliferative effects. Rather than being over-expressed (as observed in lung cancer), ERβ expression appears to be lost during the carcinogenic process in the colon79. These findings demonstrate that the pro- or anti-cancer signaling initiated by ERs have remarkable tissue specificity, which may depend upon interactions with other signaling molecules and receptor co-activators, as well as the extent to which different growth factors produce phosphorylation of ERs Differences in the effects of ERs in non-endocrine tissues is a fruitful subject for further investigation.
SUMMARY
Research on steroid hormones in lung cancer is likely to benefit both men and women. Lung cancer in both male and female patients is ER and PR positive, as well as often being aromatase positive. Cell lines derived from both sexes are responsive to estrogens, and show responses to therapeutic agents targeting the estrogen pathway. Endocrine-based therapeutic treatments may therefore be beneficial for both men and women. Endocrine therapies also have potential for lung cancer prevention. Possible strategies to target the estrogen signalling pathway for lung cancer are summarized in Figure 3. Published data show protection from tobacco carcinogens in female mice, while similar unpublished preclinical evidence suggests that these hormonal therapies are also effective in male mice (unpublished observations). Local production of estrogens in the lung, either by lung cells or by infiltrating macrophages and other inflammatory cells, may be a significant source of estrogen that could drive the tumor process, independent of reproductive tissues. Estrogen produced as a result of pulmonary inflammation may be an important driver of the pro-tumor consequences of chronic inflammation in the lung. Several clinical trials are underway to test endocrine therapies in combination with either targeted therapy or chemotherapy in advanced lung cancer patients. Positive results would suggest these treatments should also be examined in earlier stages of lung cancer. Greater understanding of the role of endocrine pathways in lung cancer will provide a rationale for future hormone-based therapies earlier in the course of disease and possibly for lung cancer prevention. Additional understanding of the role of non-nuclear versus nuclear ERs as well as PR function in lung cancer will be crucial for exploiting these pathways clinically. Biomarker identification that predicts which lung cancer patients are the best candidates for hormonal therapy will also be needed. Because endocrine therapies are relatively safe and are amenable for long-term treatment, the potential to bring them to clinical use for lung cancer is great.
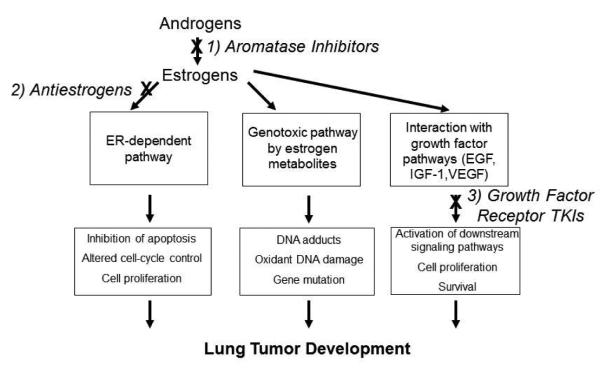
Figure 3.
Available strategies to target the estrogen signaling pathway for lung cancer treatment or prevention. Strategies include: 1) inhibition of estrogen synthesis with aromatase inhibitors; 2) down-regulation of ERs using antiestrogens; 3) targeting growth factor pathways that are activated by estrogens, such as the EGF, IGF-1 and VEGF pathways using growth factor receptor TKIs. These strategies can be used as single agents or in combination.
ACKNOWLEDGEMENTS
This work was supported in part by the following grants: SPORE in Lung Cancer P50 090440 from the National Cancer Institute to JMS, Career Development Award from the SPORE in Lung Cancer P50 090440 to LPS, an award from the V Foundation for Cancer Research to JMS, and an award from the Lung Cancer Research Foundation to LPS. We thank Ms. Carmella Campbell for assistance in preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors have no disclosures relevant to this report.
REFERENCES
- 1.Blaustein JD. Steroid hormone receptors: long- and short-term integrators of the internal milieu and the external environment. Horm. Metab. Res. 2012;44:563–8. doi: 10.1055/s-0032-1311605. [DOI] [PubMed] [Google Scholar]
- 2.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 3.Lin AHY, Li RWS, Ho EYW, et al. Differential ligand binding affinities of human estrogen receptor-α isoforms. PLOS One. 2013;8:e63199. doi: 10.1371/journal.pone.0063199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung YK, Mak P, et al. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc Natl Acad Sci USA. 2006;103:13162–7. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaaban AM, Green AR, Karthik S, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–35. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 6.Brandenberger AW, Tee MK, Lee JY, et al. Tissue distribution of estrogen receptors alpha (ER-α) and beta (ER-β) mRNA in the midgestational human fetus. J Clin Endocrin Metab. 1997;82:3509–12. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 7.Patrone C, Caael TN, Pettersson K, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor β. Mol Cell Biol. 2003;23:8542–52. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morani A, Barros RP, Imamov O, et al. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta−/−) mice. Proc. Natl Acad Sci USA. 2006;103:7165–9. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benninghoff AD, Williams DE. The role of estrogen receptor β in transplacental cancer prevention by indole-3-carbinol. Cancer Prev Res. 2013;6:339–48. doi: 10.1158/1940-6207.CAPR-12-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemmen JG, Arends RJ, van Boxtel AL, et al. Tissue-and time-dependent estrogen receptor activation in estrogen reporter mice. J Mol Endocrinol. 2004;32:689–701. doi: 10.1677/jme.0.0320689. [DOI] [PubMed] [Google Scholar]
- 11.Ciana P, DiLuccio G, Belcredito S. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Molec Endocrinol. 2001;15:1104–13. doi: 10.1210/mend.15.7.0658. [DOI] [PubMed] [Google Scholar]
- 12.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- 13.Wu CT, Chang YL, et al. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130:979–86. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AG, Prysak GM, Murphy V, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11:7280–7. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 15.Kawai H, Ishii A, Washiya K, et al. Combined overexpression of EGFR and estrogen receptor alpha correlates with a poor outcome in lung cancer. Anticancer Res. 2005;25:4693–98. [PubMed] [Google Scholar]
- 16.Skov BG, Fischer BM, Pappot H. Oestrogen receptor b over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Moore JT, McKee DD, Slent-Kesler K, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998 Jun 9;247(1):75–8. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Inous S, Watanabe T, et al. Molecular cloning and characterization of human estrogen receptor betacs: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998 Aug 1;26(15):3505–12. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberger PA, Vasquez AC, Kanterewicz B, et al. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598–605. doi: 10.1158/0008-5472.CAN-04-2694. [DOI] [PubMed] [Google Scholar]
- 20.Hershberger PA, Stabile LP, Kanterewicz B, et al. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol. 2009;116:102–9. doi: 10.1016/j.jsbmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazieres J, Rouquette I, Lepage B, et al. Specificities of lung adenocarcinoma in women who have never smoked. J Thorac Oncol. 2013;8:923–9. doi: 10.1097/JTO.0b013e3182904dfb. [DOI] [PubMed] [Google Scholar]
- 22.Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor b-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;17:154–64. doi: 10.1158/1078-0432.CCR-10-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi S, Coti M, Lonardo F. Expression of estrogen receptor beta 1 but not estrogen receptor beta 2 or alpha is linked to worse prognosis in stage I adenocarcinoma, in women, in a large epidemiological cohort but not in a smaller, single hospital based series. United States and Canadian Academy of Pathology. 2010 Abstract 1843. [Google Scholar]
- 24.Navaratnam S, Skliris G, Qing G, et al. Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm Cancer. 2012;3:93–100. doi: 10.1007/s12672-012-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Liao Y, Tang H, et al. The expression of estrogen receptors b2,5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine. 2013 doi: 10.1007/s12020-013-9916-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Zhang G, Yanamala N, Lathrop KL, et al. Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells. Mol Endocrinol. 2010;24:1737–47. doi: 10.1210/me.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simoes DC, Psarra AM, Mauad T, et al. Glucocorticoid and estrogen receptors are reduced in mitochondria of lung epithelial cells in asthma. PLoS One. 2012;7:e39183. doi: 10.1371/journal.pone.0039183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albain KS, Crowley JJ, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 29.Moore KA, Mery CM, Jaklitsch MT, et al. Menopausal effects on presentation, treatment, and survival of women with non-small cell lung cancer. Ann Thorac Surg. 2003;766:1789–95. doi: 10.1016/s0003-4975(03)01024-5. [DOI] [PubMed] [Google Scholar]
- 30.Wakelee HA, Dahlberg SE, Schiller JA, et al. Menopausal status of women may affect survival in advanced NSCLC: Analysis of recent Eastern Cooperate Oncology Group (ECOG) studies using age of 60 years or older as a surrogate marker: P1-052. J Thoracic Oncology. 2007b;2:S570. [Google Scholar]
- 31.Ganti AK, Sahmoun AE, Panwalkar AW, et al. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 32.Chelbowski, Schwartz AG, Wakelee H, et al. Estrogen plus progestin and lung cancer in postmenopausal women. Lancet. 2009;375:1243–51. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol. 2010;28:1540–6. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schabath MB, Wu X, Vassilopoulou-Sellin R, et al. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10:113–23. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz AG, Wenzlaff AS, Prysak GM, et al. Reproductive factors, hormone use, estrogen receptor expression and risk of non-small cell lung cancer in women. Journal of Clinical Oncology. 2007;25:5785–92. doi: 10.1200/JCO.2007.13.3975. [DOI] [PubMed] [Google Scholar]
- 36.Bouchardy C, Benhamou S, Schaffar R, et al. Lung cancer mortality risk among breast cancer patients treated with anti-estrogens. Cancer. 2011;117:1288–95. doi: 10.1002/cncr.25638. [DOI] [PubMed] [Google Scholar]
- 37.Lother SA, Harding GA, Musto G, et al. Antiestrogen use and survival of women with non-small cell lung cancer in Manitobe, Canada. Horm Cancer. 2013 doi: 10.1007/s12672-013-0149-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–76. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 39.Hammoud Z, Tan B, et al. Estrogen promotes tumor progerssion in a genetically defined mouse model of lung adenocaricoam. Endocr Relat Cancer. 2088;15:475–83. doi: 10.1677/ERC-08-0002. [DOI] [PubMed] [Google Scholar]
- 40.Stabile LP, Lyker JS, Gubish CT, et al. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–70. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 41.Martel C, Melner MH, et al. Widespread tissue distribution of steroid sulfatase, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD), 17 beta-HSD 5 alpha-reductase and aromatase activities in the rhesus monkey. Mol Cell Endocrinol. 1994;104:103–11. doi: 10.1016/0303-7207(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 42.Price T, Aitken J, Simpson ER. Relative expression of aromatase cytochrome P450 in human fetal tissues as determined by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab. 1992;74:879–83. doi: 10.1210/jcem.74.4.1548354. [DOI] [PubMed] [Google Scholar]
- 43.Niikawa H, Suzuki T, Miki Y, et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417–26. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg OK, Marquez-Garban DC, Fishbein MC, et al. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–91. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 45.Stabile LP, Rothstein ME, Cunningham DE, et al. Prevention of tobacco carcinogen-induced lung cancer in female mice using antiestrogens. Carcinogenesis. 2012;33:2181–9. doi: 10.1093/carcin/bgs260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;35011:1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 47.Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Research. 2007;67:10484–90. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verma MK, Miki Y, Abe K, et al. Intratumoral localizastion and activity of 17beta-hydroxysteroid dehydrogenase type 1 in non-small cell lung cancer: a potent prognostic factor. J Transl Med. 2013:11–167. doi: 10.1186/1479-5876-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas P, Pang Y, et al. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 50.Jala VR, Radde BBN, Haribabu B, et al. Enhanced expression of G-protein coupled estrogen receptor (GPER/GPR30) in lung cancer. BMC Cancer. 2012;12:624. doi: 10.1186/1471-2407-12-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajkumar T, Gullick WJ. The type I growth factor receptors in human breast cancer Breast Cancer Res Treat. 1994;29:3–9. doi: 10.1007/BF00666177. [DOI] [PubMed] [Google Scholar]
- 52.Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 53.Selvaggi G, Novello S, Torri V, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;151:28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 54.Marquez-Garban DC, Chen H-W, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–43. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garon EB, Pietras RJ, Finn RS, et al. Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small cell lung cancer. J Thorac Oncol. 2013;8:270–8. doi: 10.1097/JTO.0b013e31827d525c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegfried JM, Gubish CT, Rothstein ME, et al. Combining the multitargeted kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2012;7:485–95. doi: 10.1097/JTO.0b013e31824177ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen L, Li Z, Shen S, et al. The synergistic effect of EGFR tyrosine kinase inhibitor gefitinib in combination with aromtase inhibitor anastrozole in non-small cell lung cancer cell lines. Lung Cancer. 2012;78:193–200. doi: 10.1016/j.lungcan.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 59.Tang H, Liao Y, Chen G, et al. Estrogen upregulates the IGF-1 signaling pathway in lung cancer through estrogen receptor-B. Med Oncol. 2012;29:2640–8. doi: 10.1007/s12032-012-0198-8. [DOI] [PubMed] [Google Scholar]
- 60.Tang H, Liao Y, Xu L, et al. Estrogen and insulin-like growth factor 1 synergistically promote the development of lung adenocarcinoma in mine. Int J Cancer. 2013 doi: 10.1002/ijc.28262. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Yang SH, Mechanic LE, Yang P, et al. Mutations in the tyrosine kinase domain of the epidermal growth factor receptor in non-small cell lung cancer. Clin Cancer Res. 2005;11:2106–10. doi: 10.1158/1078-0432.CCR-04-1853. [DOI] [PubMed] [Google Scholar]
- 62.Nose N, Sugio K, Oyama T, et al. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27:411–7. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 63.Traynor AM, Schiller JH, Stabile LP, et al. Pilot study of gefitinib and fulvestrant in the treatment of post-menopausal women with advanced non-small cell lung cancer. Lung Cancer. 2009;64:51–9. doi: 10.1016/j.lungcan.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garon EB, Siegfried JM, Dubinett SM, et al. Results of TORI-L-03, a randomized, multicenter phase II clinical trial of erlotinib (E) or E + fulvestrant (F) in previously treated advanced non-small cell lung cancer (NSCLC). Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; Washington DC. Philadelphia (PA). AACR; 2013. 2013 Apr 6-10. Abstract nr 4664. [Google Scholar]
- 65.Okubo S, Kurebayashi J, Otsuki T, et al. Additive antitumour effect of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (Iressa, ZD1839) and the antioestrogen fulvestrant (Faslodex, ICI 182,780) in breast cancer cells. Br J Cancer. 2004;901:236–44. doi: 10.1038/sj.bjc.6601504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su JM, Hsu HK, Chang H, et al. Expression of estrogen and progesterone receptors in non-small-cell lung cancer: immunohistochemical study. Anticancer Res. 1996;16:3803–6. [PubMed] [Google Scholar]
- 67.Kaiser U, Hofmann J, Schilli M, et al. Steroid-hormone receptors in cell lines and tumor biopsies of human lung cancer. Int J Cancer. 1996;67:357–64. doi: 10.1002/(SICI)1097-0215(19960729)67:3<357::AID-IJC9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 68.Di Nunno L, Larsson LG, et al. Estrogen and progesterone receptors in non-small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med. 2000;124:1467–70. doi: 10.5858/2000-124-1467-EAPRIN. [DOI] [PubMed] [Google Scholar]
- 69.Raso MG, Behrens C, Herynk MH, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Can Res. 2009;15:5359–68. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishibashi H, Suzuki T, Suzuki S, et al. Progesterone receptor in non-small cell lung cancer-a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65:6450–8. doi: 10.1158/0008-5472.CAN-04-3087. [DOI] [PubMed] [Google Scholar]
- 71.Abe K, Miki Y, Ono K, et al. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol. 2010;41:190–8. doi: 10.1016/j.humpath.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Xie M, You S, Chen Q, et al. Progesterone inhibits the migration and invasion of A549 lung cancer cells through membrane progesterone receptor a-mediated mechanisms. Oncol Rep. 2013;29:1873–80. doi: 10.3892/or.2013.2336. [DOI] [PubMed] [Google Scholar]
- 73.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 my mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97:1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cui X, Lazard Z, Zhang P, et al. Progesterone crosstalks with insulin-like growth factor signaling in breast cancer cells via induction of insulin receptor substrate-2. Oncogene. 2003;22:6937–41. doi: 10.1038/sj.onc.1206803. [DOI] [PubMed] [Google Scholar]
- 75.Sutton G. Hormonal aspects of endometrial cancer. Curr Opin Obstet Gynecol. 1990;2:69–73. [PubMed] [Google Scholar]
- 76.Kelley RM, Baker WH. Progestational agents in the treatment of carcinoma of the endometrium. N Engl J Med. 1961;264:216–22. doi: 10.1056/NEJM196102022640503. [DOI] [PubMed] [Google Scholar]
- 77.Egloff AM, Rothstein ME, Seethala R, et al. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin Cancer Res. 2009 Nov 1;15(21):6529–40. doi: 10.1158/1078-0432.CCR-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Somers KD, Koening M, Schechter GL. Growth of head and neck squamous cell carcinoma in nude micd: potentialtion of laryngeal carcinoma by 17β-estrodiol. J Natl Cancer Inst. 1988 Jul 6;80(9):688–91. doi: 10.1093/jnci/80.9.688. [DOI] [PubMed] [Google Scholar]
- 79.Barzi A, Lenz A, LaBonte M, et al. Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-13-0325. Published online on Aug 21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]