Abstract
Background
Ulcerative colitis (UC) is a chronic inflammatory condition of the colon characterized by episodes of disease activity and symptom-free remission. There is paucity of evidence regarding the efficacy and safety of complementary or alternative medicines for the management of UC. Curcumin, an anti-inflammatory agent, has been used in many chronic inflammatory conditions such as rheumatoid arthritis, esophagitis and post-surgical inflammation. The efficacy of this agent for maintenance of remission in patients with UC has not been systematically evaluated.
Objectives
The primary objective was to systematically review the efficacy and safety of curcumin for maintenance of remission in UC.
Search methods
A computer-assisted literature search of MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Inflammatory Bowel Disease Specialized Trial Register was performed on July 11, 2012 to identify relevant publications. Proceedings from major gastroenterology meetings and references from published articles were also searched to identify additional studies.
Selection criteria
Randomized placebo-controlled trials (RCT) of curcumin for maintenance of remission in UC were included. Studies included patients (of any age) who were in remission at the time of recruitment. Co-interventions were allowed.
Data collection and analysis
Two authors independently extracted data and assessed the methodological quality of the included studies using the Cochrane risk of bias tool. Data were analyzed using Review Manager (RevMan 5.1). We calculated the relative risk (RR) and 95% confidence interval (95% CI) for each dichotomous outcome. For continuous outcomes we calculated the mean difference (MD) and 95% CI.
Main results
Only one trial (89 patients) fulfilled the inclusion criteria. This trial randomized 45 patients to curcumin and 44 patients to placebo. All patients received treatment with sulfasalazine or mesalamine. The study was rated as low risk of bias. Curcumin was administered orally in a dose of 2 g/day for six months. Fewer patients relapsed in the curcumin group than the placebo group at six months. Four per cent of patients in the curcumin group relapsed at six months compared to 18% of patients in the placebo group (RR 0.24, 95%CI 0.05 to 1.09; P = 0.06). There was no statistically significant difference in relapse rates at 12 months. Twenty-two per cent of curcumin patients relapsed at 12 months compared to 32% of placebo patients (RR 0.70, 95% CI 0.35 to 1.40; P = 0.31). A total of nine adverse events were reported in seven patients. These adverse events included sensation of abdominal bulging, nausea, transient hypertension, and transient increase in the number of stools. The authors did not report which treatment group the patients who experienced adverse events belonged to. The clinical activity index (CAI) at six months was significantly lower in the curcumin group compared to the placebo group (1.0 ± 2.0 versus 2.2 ± 2.3;MD −1.20, 95%CI −2.14 to −0.26). The endoscopic index (EI) at six months was significantly lower in the curcumin group than in the placebo group (0.8 ± 0.6 versus 1.6 ± 1.6; MD −0.80, 95% CI −1.33 to −0.27).
Authors’ conclusions
Curcumin may be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine. However, further research in the form of a large scale methodologically rigorous randomized controlled trial is needed to confirm any possible benefit of curcumin in quiescent UC.
Medical Subject Headings (MeSH): Anti-Inflammatory Agents, Non-Steroidal [*therapeutic use]; Colitis, Ulcerative [*drug therapy]; Curcumin [*therapeutic use]; Drug Therapy, Combination [methods]; Maintenance Chemotherapy [*methods]; Mesalamine [therapeutic use]; Randomized Controlled Trials as Topic; Recurrence; Sulfasalazine [therapeutic use]
MeSH check words: Humans
PLAIN LANGUAGE SUMMARY
Curcumin for maintenance of remission in ulcerative colitis
Curcumin is a natural anti-inflammatory agent that is often used in many chronic inflammatory conditions including rheumatoid arthritis, esophagitis and post-surgical inflammation. The purpose of this systematic review was to examine the effectiveness and safety of curcumin therapy for the maintenance of remission in patients with ulcerative colitis (UC), a chronic inflammatory condition of the colon. Currently available agents for the management of this condition have been reported to result side effects, particularly when used for prolonged periods. This review includes one randomized trial with a total of 89 participants. All patients received treatment with sulfasalazine or mesalamine (drugs containing 5-aminosalicylic acid). Fewer patients in the curcumin group relapsed at six months compared to patients who received placebo (e.g. fake drug).However, this result was not statistically significant. Patients in the curcumin group had significantly lower disease activity index and endoscopic index scores at six months than patients in the placebo group. No serious side effects were reported. A total of nine mild side effects were reported in seven patients. These side effects included a sensation of abdominal bulging, nausea, a brief increase in blood pressure, and a brief increase in the number of stools. The results of this systematic review suggest that curcumin may be a safe and effective therapy for maintenance of remission in ulcerative colitis when given as additional therapy with mesalamine or sulfasalazine. Further research is needed to confirm any possible benefit of curcumin for maintenance therapy in ulcerative colitis.
BACKGROUND
Curcumin is a natural phenol found in the large-leafed herb curcuma longa L. (common names Turmeric, Indian Saffron). The rhizome (underground stem) of the turmeric plant contains up to 5% curcumin, in combination with essential oils and other compounds. Turmeric has been used for various medicinal uses in both Indian (Ayurvedic) and Chinese medicine systems for thousands of years (Joshi 1983). Only recently has modern medicine begun to critically evaluate turmeric and its extracts and antioxidant, anti-inflammatory, antiplatelet, cholesterol lowering, antibacterial and antifungal effects have been identified. Curcumin may also induce apoptosis and inhibit carcinogenesis (Basile 2009; Lee 2009; O’Sullivan-Coyne 2009). Curcumin scavenges reactive oxygen species including superoxide and hydroxyl radical (Sreejayana 1997) reduces inducible nitric oxide (NO) synthetase expression (Ukil 2003), decreases the expression of c-jun, cfos and c-myc proto-oncogenes (Kakar 1994), suppresses activation of NF-κB (Singh 1995), induces glutathione transferase and NADPH quinone reductase involved in detoxification via xenobiotic metabolism (Arbiser 1998).
Description of the condition
Ulcerative colitis (UC) is a chronic inflammatory condition of the colon characterized by diffuse, superficial inflammation of colonic mucosa variably extending from the rectum to the cecum (Podolosky 1991). The extent of colonic involvement is used for sub-classification of UC into right sided, left sided or pancolitis. The incidence and prevalence of UC varies by geographical region, ethnicity and race. Currently available epidemiological data suggest that it is more frequent in industrialized countries where incidence ranges from 6.5 to 16.0 per 100,000 person-years and prevalence ranges from26 to 214 patients per 100,000 persons. Inflammatory bowel disease (IBD) is less frequent in less industrialized nations with incidence and prevalence rates ranging from 0.08 to 5.0 and from 3.6 to 70.0 patients per 100,000 persons per year, respectively (Victoria 2009). The burden of UC on the healthcare system is profound, accounting for nearly 500,000 physician visits and more than 47,000 hospitalizations per year in the United States alone (Hanauer 2004).
In human and various animal models of IBD, including the trinitrobenzene sulfonic acid (TNBS)-induced colitis model, increases in mucosal pro-inflammatory cytokines such as interleukin (IL)-1b, IL-6 tumour necrosis factor (TNF)-α, IL-12, and interferon (IFN)-y have been demonstrated (Ammon 1991). The expression of the corresponding genes is mainly regulated by the transcription factor NF-κB (Singh 1995). Previous studies have shown that NF-κB activation is elevated in UC.
Description of the intervention
Chemical constituents
Chemical analysis of turmeric has yielded essential oil, fatty oil, and moisture (Ammon 1991). The pale yellow to orange oil is composed of a number of monoterpenes and sesquiterpenes, including zingiberene, curcumene, α and β turmerone, and curlone amongst others. The yellow pigments are curcuminoids consisting of curcumin (di-feruloyl-methane), p-hydroxycinnamoyl (feruloyl)methane and p, p’ - dihydroxy-dicinnamoyl-methane in three to five per cent concentration (Ammon 1991).
Pharmacokinetic data
Studies in rats suggest that around 60% of a dose of curcumin suspended in water is absorbed and furthermore that suspending it in oil increases the absorbed fraction. About a third of the amount administered orally remains in the colon 24 hours after oral dosing (Ravindranath 1980). However, unchanged curcumin is not detected in the urine or blood and does not accumulate in the tissues or fat. Hence it is likely that curcumin undergoes rapid metabolism following absorption in the enterocytes or the liver, although other metabolic pathways have not been completely elucidated. Use of 14C labeled curcumin has shown that major biliary metabolites are glucuronides of tetrohydrocurcumin and hexahydrocurcumin. Minor biliary metabolites are dehydroferulic acid and ferulic acid. Only traces of intact curcumin and six per cent of total radioactivity are excreted in the urine (Wahlstrom 1978). Thus curcumin if absorbed at all, is mainly excreted in bile. The effects of all of its metabolites are unknown. Luminal curcumin or its derivatives possibly have topical activity on the colonic epithelial cells independent of systemic absorption. While oral intake of curcumin results in therapeutic concentration in the intestinal mucosa in vivo (Sugimoto 2002), detailed metabolic studies have not been carried out in humans.
Safety data
Studies in rats, guinea-pigs, dogs and monkeys have reported no adverse effects. Curcumin has been demonstrated to be safe in six human trials (Chainani-Wu 2003). However, the herbal medicine literature cautions against usage of high dosages in pregnancy given risk of premature uterine contractions (Blumenthal 1998).
How the intervention might work
Curcumin inhibits the activation of NF-kB inducing kinase and IKB kinase in intestinal epithelial cells (Jobin 1999). Sugimoto 2002 evaluated the effects of different concentrations of curcumin (0.5%, 2.0% or 5.0% in diet) in tri-nitrobenzene sulfonic acid (TNBS) induced colitis in mice. Curcumin prevented colonic inflammation and furthermore, CD4+ T-Cell infiltration, NF-κB activation and expression of pro-inflammatory cytokine messenger RNA in the colonic mucosa were inhibited. Ukil 2003 reported similar results with curcumin in TNBS-induced colitis in mice; a decrease in neutrophil infiltration, lipid peroxidation, levels ofNO and oxy-radicals. Consistent with these observations, NF-κB activation in the colonic mucosa is suppressed by curcumin. Salh 2003 also reported that curcumin attenuates inflammation in a murine colitis model. The anti-inflammatory effects of curcumin include a reduction of myeloperoxidase activity, number of infiltrating neutrophils and IL-1β production. The DNA binding activity of NF-κB as well as activation of P3B-MAPK was inhibited. Other data demonstrate a switch fromTh1 to Th2 immune response following treatment with curcumin (Ukil 2003). Ung 2009 demonstrated that in IL-10 gene-deficient mice oral curcumin can have modifying effects on colitis by reducing levels of inflammatory cytokines and MPO. However, curcumin was not able to induce histological improvement as had been demonstrated in chemically induced mouse colitis models. Arafa 2009 reported that curcumin may have a protective role in ulcerative colitis through regulation of oxidant/anti-oxidant balance and modulation of the release of some inflammatory endocoids including TNF-a and NO.
It has been demonstrated that curcumin inhibits colon carcinogenesis by down regulating tumor necrosis factor alpha, transcription factor NF-kappa B and modulation of arachidonic acid metabolism via cyclooxygenase and lipoxygenase pathways during initiation and promotion stages of carcinogenesis (Chan 1995); Singh 1995; Hong 2004) and significantly inhibits tumor development by inducing apoptosis via suppression of NF-κB and AP-1 during promotion and progression stages of carcinogenesis (Huang 1991; Kawamori 1999).
Why it is important to do this review
While sulfasalazine, mesalamine and immunosuppressives such as azathioprine and 6-mercaptopurine are clinically efficacious for treatment of UC, they are associated with adverse events. Sulfasalazine has been linked to hematological abnormalities, hypersensitivity reactions, liver and renal abnormalities. Azathioprine has been associated with lymphoma and pancreatitis. Corticosteroids have been associated with impaired glucose tolerance, adrenal suppression, susceptibility to infection, and osteoporosis. Mesalamine is generally considered to be a safe drug. Serious adverse events such as pancreatitis (Pitchumoni 2010) or interstitial nephritis are rare (Arend 2004). The identification of safer and effective medications continues to be an unmet need for patients with this condition. Recent studies have documented that a significant proportion of patients with UC use complementary or alternative medicines to treat their condition. There is little evidence-based-medicine to guide physicians and patients in this field (Ausfeld-Hafter 2005). It is therefore important to identify well-tolerated agents that can help induce and maintain remission as an alternative to agents such as sulfasalazine. Curcumin is widely available, inexpensive, safe and possibly an effective alternative treatment for UC.
OBJECTIVES
The primary objective was to assess the efficacy and safety of curcumin for maintenance of remission in ulcerative colitis.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing curcumin preparations to placebo or other agents were considered for inclusion.
Types of participants
Participants of any age diagnosed with ulcerative colitis as confirmed by clinical and endoscopic criteria and who were in remission at the time of enrolment (as defined by any activity index) were considered for inclusion.
Types of interventions
Studies in which patients in the treatment group received curcumin at any dose versus a comparison group receiving placebo, no treatment, or any other active intervention were considered for inclusion. Any duration of follow-up was allowed.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of patients who experienced clinical or endoscopic relapse (as defined by the included studies).
Secondary outcomes
Secondary outcomes included:
Frequency and nature of adverse events;
Changes in disease activity score (modified Mayo Score);
Changes in the endoscopy score (Mayo Score);
Time to relapse; and
Changes in laboratory measures of inflammation, haemoglobin, platelet count, erythrocyte sedimentation rate, and serum albumin.
Search methods for identification of studies
Electronic searches
The following databases were searched from inception to July 11, 2012 using the strategies described in Appendix 1:
Cochrane Central Register of Controlled Trials;
Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders (IBD/FBD) Group Specalised Trial Register;
Pubmed
MEDLINE
EMBASE
Ongoing trials were identified using the registry link http://ClinicalTrials.gov.
Searching other resources
The reference lists of studies and review articles identified by the literature search were reviewed for additional pertinent studies. The abstracts and proceedings of major gastrointestinal meetings (Digestive Diseases Week-USA, Canadian Digestive Diseases Week, American Gastroenterological Association, British Society of Gastroenterology, United European Gastroenterology Week) presented since 2006 were handsearched. Authors and experts were contacted for additional information as necessary.
Data collection and analysis
Selection of studies
Two review authors (SK, VA) independently screened studies to identify potentially relevant trials for full review based on the title, abstract, and descriptors. Full manuscripts of relevant trials identified for study inclusion were obtained. These trials were critically appraised by two independent review authors (SK, VA) and any disagreements was resolved by discussion with a third review author (AK).
Data extraction and management
Data were independently extracted by two authors (SK, VA) using a pre-designed data collection form, and saved electronically with appropriate version control. The data collection form was reviewed for clarity, relevance to the study questions and completeness. Any disagreement in the collected data was resolved by discussion with the third reviewer (AK). SK entered the data into RevMan. Additional information was sought from the authors of trials with inadequate or missing data.
Data extracted included the following:
Total patients enrolled and patients assigned to each treatment group;
Curcumin: dose, frequency, duration, and if treatment was blinded;
Concomitant medication(s), route of administration and dose: e.g. corticosteroids, 5-ASA, sulfasalazine, azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, or infliximab;
Patient characteristics including age, gender, duration of symptoms and disease severity; and
Study outcomes including number of patients completing treatment, total number of dropouts, number of dropouts due to adverse effects, number of patients with relapse. Where possible, the median number of days to remission and the mean change in disease activity indices were assessed.
If trials had multiple treatment groups, the ‘shared’ comparison group was to be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group were to be treated as independent comparisons.
Assessment of risk of bias in included studies
Three authors independently assessed the risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Factors assessed were:
Sequence generation (i.e. was the allocation sequence adequately generated?);
Allocation sequence concealment (i.e. was allocation adequately concealed?);
Blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
Incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
Selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
Other potential sources of bias (i.e. was the study apparently free of other problems that could put it at a high risk of bias?). A judgement of ’Yes’ indicates low risk of bias, ’No’ indicates high risk of bias, and ’Unclear’ indicates unclear or unknown risk of bias. Study authors was contacted when insufficient information was provided to determine risk of bias. Risk of bias was summarized for the primary outcomes within and across studies. A risk of bias graph was used to illustrate risk across studies. Any disagreements were resolved by consensus and, if necessary, by adjudication with a third author.
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes and constructed a Summary of Findings (SoF) table (Schünemann 2011).
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Curcumin versus placebo for maintenance of remission in ulcerative colitis | |||||||
| Patient or population: Maintenance of remission in ulcerative colitis | |||||||
| Settings: Tertiary care centres in a high income country | |||||||
| Intervention: Curcumin versus placebo1 | |||||||
| Outcomes |
Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) |
No of Participants (studies) |
Quality of the evidence (GRADE) |
Comments | ||
| Assumed risk10 | Corresponding risk | ||||||
| Control | Curcumin placebo | versus | |||||
|
Relapse at 6 months Clinical Disease Activty Index more than 42 Follow-up: 6 months |
182 per 1000 |
44 per 1000 (9 to 198) |
RR 0.24 (0.05 to 1.09) |
89 (1 study) |
⊕⊕○○ low3,4,5,6,7 |
||
|
Relapse at 12 months Clinical Disease Activty Index more than 42 Follow-up: 12 months |
318 per 1000 |
223 per 1000 (111 to 445) |
RR 0.7 (0.35 to 1.4) |
89 (1 study) |
⊕⊕○○ low3,4,6,7,7 |
||
|
Clinical Activity Index at 6 months Clinical Disease Activty Index 2. Scale from: 0 to 12. Follow-up: 6 months |
The mean clinical activ- ity index at 6 months in the control groups was 2. 2 points |
The mean Clinical Activity Index at 6 months in the intervention groups was 1.2 lower (2.14 to 0.26 lower) |
82 (1 study) |
⊕⊕○○ low3,4,6,7 |
|||
|
Endoscopic Index at 6 months Endoscopic Index9. Scale from: 1 to 4. Follow-up: 6 months |
The mean endoscopic in- dex at 6 months in the control groups was 1.6 points |
The mean Endoscopic In- dex at 6 months in the in- tervention groups was 0.8 lower (1.33 to 0.27 lower) |
82 (1 study) |
⊕⊕○○ low3,4,6,7 |
|||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| CI: Confidence interval; RR: Risk ratio; | |||||||
| GRADE Working Group grades of evidence | |||||||
| High quality: Further research is very unlikely to change our confidence in the estimate of effect. | |||||||
| Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||||
| Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | |||||||
| Very low quality: We are very uncertain about the estimate. | |||||||
Both arms received standard care and mesalamine or sulfasalazine
Scores range from 0 to 12 (high scores reflect more severe disease)
No study limitations: Central randomization and allocation concealed; investigators, outcome assessors and participants were blinded; analysis was by intention to treat; no overt reporting biases; no conflicts of interest
No serious indirectness: This trial included only adults (who constitute about 95% of those with ulcerative colitis). This is a validated, clinically relevant outcome measure. Not downgraded
Serious imprecision: There are relatively few events and wide confidence intervals around the estimate of effect that suggests appreciable benefit with curcumin as well as non-appreciable benefit with placebo. Downgraded by 1
Publication Bias: Unlikely as the search was comprehensive
Very serious imprecision: There are relatively few events and wide confidence intervals around the estimate of effect that suggests appreciable benefit with curcumin as well as with placebo and no statistically significance between the interventions. Downgraded by 2
Serious imprecision: There are relatively few events and wide confidence intervals (CI) around the estimate of effect with the lower limit of the CI crossing 0.5. Downgraded by 1
Scores assess healing of ulcers on sigmoidoscopy; range 1 to 4, with high scores indicating severe disease activity
The assumed risk is the risk in the control group
Measures of treatment effect
Statistical analysis was performed using the RevMan software version 5.1.0.We planned to use a fixed-effect model for meta-analysis in the absence of significant heterogeneity (I2 more than 50%) across the studies. We constructed two by two tables (e.g. relapse versus remission for curcumin versus placebo) from the raw extracted data. We calculated risk ratios (RR) with 95% confidence intervals (95% CI) for dichotomous outcomes. Where appropriate, we expressed estimated effects as NNT (number needed to treat), which corresponds mathematically to the inverse of the absolute risk difference and clinically to the number of patients to be treated to avoid one undesired event. We planned to calculate hazard ratios (HR; generic variance method) for time-to-event outcomes. We calculated the mean difference (MD) along with 95% CI for continuous variables. We intended to pool data using the MD, if continuous data from two or more studies were available for the same instrument of evaluation with the same units of measurement. Conversely, we planned to use the standardized mean difference (SMD) if the studies had expressed the same variables through different instruments or different units of measurement with the same instrument.
Dealing with missing data
At the outcome level, data that were measured but not reported were sought from study authors. If there was a discrepancy in the number randomized and the number analyzed in each treatment group, the percentage lost to follow up was calculated in each group and reported. No assumptions about loss to follow up for continuous data was made and analyses were based on those completing the trial. For dichotomous data, if drop-outs had exceeded 10% for any trial, we were to assign the worse outcomes to those who were lost to follow-up and assess the impact on study results using sensitivity analyses.
Assessment of heterogeneity
We planned to assess heterogeneity between the included trials using the Chi2 test in conjunction with the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (Higgins 2011b). We considered a I2 value of 50% or greater to denote significant heterogeneity and 75% or greater to denote substantial and major heterogeneity. A P value of ≤ 0.10 would have been considered statistically significant for the Chi2 test.
Assessment of reporting biases
If there had been more than 10 studies, apart from assessing the risk of selective outcome reporting considered under “Assessment of risk of bias in included studies”, potential publication bias would have been assessed using funnel plots (Egger 1997). Publication bias occurs when the publication of research results depends on their nature and direction (Dickersin 1990). In order to quantify this asymmetry in meta-analyses with binary outcomes, we planned to apply the arcsine test as proposed by Rücker 2008.
Data synthesis
If a sufficient number of clinically similar studies had been available, we planned to pool results in meta-analyses. However, if substantial heterogeneity (I2 > 75%)was identified, we would not pool studies for meta-analyses. For dichotomous outcomes the pooled RR and 95%CI would have been calculated. For continuous outcomes, the pooled MD or SMD and 95% CI would have been calculated. For time-to-event data, we intended to pool Hazard ratios (HR) using the generic inverse variance method. We planned to display the results using forest plots, with a summary statistic presented if no substantial heterogeneity was detected. Had the included trials compared different interventions verus curcumin, we would have attempted indirect comparisons, using the methods of Bucher 1997 to compare competing interventions that had not been compared directly with each other.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses included:
Disease severity (mild, moderate, severe);
Disease extent (proctitis, proctosigmoiditis left-sided colitis, pancolitis);
Age at onset of symptoms (pediatric versus adult);
Curcumin formulation;
Dose of curcumin;
Duration of treatment;
Duration of disease;
Concurrent medications (e.g. 5-ASA drugs, corticosteroids and immunosuppressants such as azathioprine, 6-mercaptopurine, methotrexate, cyclosporine and mycophenolate mofetil); and
Length of follow up.
Sensitivity analysis
Where possible, sensitivity analyses were to be performed to explore the effects of various aspects of study methodology such as loss to follow-up and allocation concealment in the included studies. If sufficient data were available, sensitivity analyses would have been conducted to determine the impact of exclusion of studies with lower methodological quality, for example:
Excluding trials at high or unclear risk of bias;
Excluding unpublished studies, since these may not have been subjected to the peer review process and may have intrinsic bias issues;
Excluding industry sponsored studies; and
Excluding trials that had not assessed compliance.
RESULTS
Description of studies
See Characteristics of included studies
Characteristics of included studies [ordered by study ID]
|
Hanai 2006 | ||
|---|---|---|
| Methods | Multicenter, randomised, parallel group, placebo-controlled clinical trial (N = 89) | |
| Participants | Age: 13 to 65 years | |
| Gender: both | ||
Inclusion Criteria
| ||
Exclusion Criteria:
| ||
| Setting: | ||
| Eight hospitals in Japan; outpatients | ||
| Interventions |
|
|
| Duration: six months | ||
| Outcomes |
|
|
| Notes | Study dates: Between April 2004 and July 2005 (16 months) | |
| Source of funding: Broad Medical Research Program (IBD-0069) from The Eli and Edythe L. Broad Foundation; intervention and placebo supplied by API co. Ltd, Japan | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
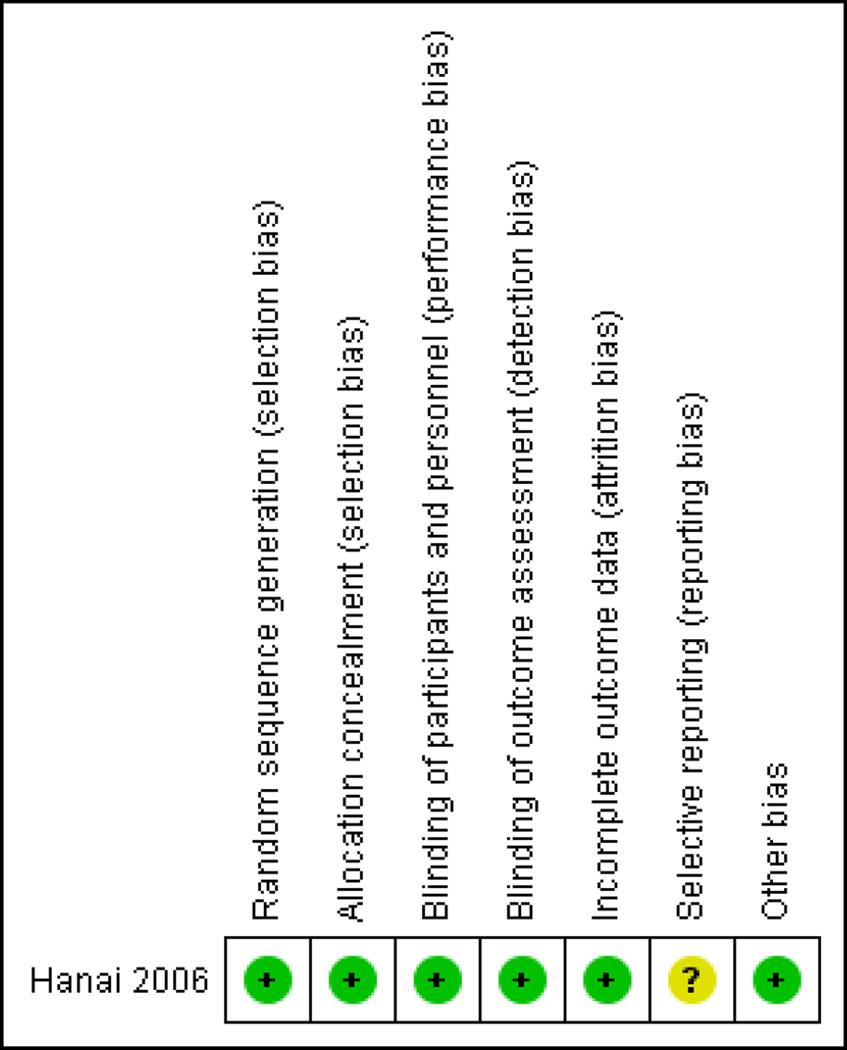
| Random sequence generation (selection bias) | Low risk | Quote from report: “Assignment to curcumin or placebo was according to a computer-generated randomization scheme done by the clinical pharmacist” |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation concealment is not explicitly mentioned in the report, but since randomization was centrally done and implemented by the clinical pharmacist, the review authors feel that the risk of bias was low |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from report: “All study personnel and participants were blinded to treatment assignment for the duration of the study.” Quote from report: “ Curcumin and placebo were made to have identical appearance (yellow)...” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from report: “Only the study statisticians and the data monitoring committee could see unblinded data, but none had any contact with the study patients” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were more dropouts in the placebo group (n = 5) than in the curcumin group (n = 2). Three patients withdrew from the study due to adverse events (1 from the placebo group and 2 from curcumin group). Attrition bias does not appear to be a serious problem in the study as ITT analyses were used |
| Selective reporting (reporting bias) | Unclear risk | Comment: We could not assess this as the study was not registered in any of the international trial registries |
| Other bias | Low risk | Comment: None identified |
Results of the search
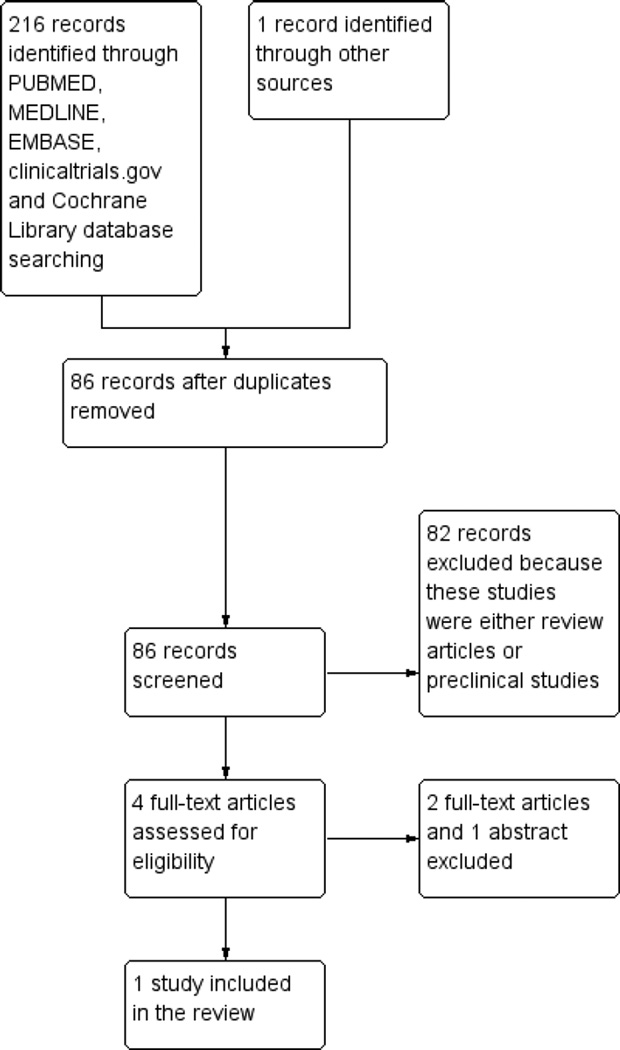
A literature search conducted on July 11, 2012 identified 216 records. One additional study was identified through searching of conference abstracts. After duplicates were removed, a total of 86 trials remained for review of titles and abstracts. Two authors independently reviewed the titles and abstracts of these trials and 4 studies were selected for full text review (see Figure 1). Three of these studies were excluded (See: Characteristics of excluded studies). One trial met the pre-defined inclusion criteria and was included in the review.
Figure 1.
Study flow diagram.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahuja 2011 | This randomized, double-blind, trial studied the efficacy and safety of curcumin enema plus oral 5-ASA, compared with placebo enema plus oral 5-ASA in 43 ulcerative colitis patients with active mild to moderate proctitis |
| Atkinson 2003 | The study, published as an abstract, enrolled patients with IBD and randomised them to either curcumin therapy or placebo. Since the data for patients with ulcerative colitis were not provided separately by the authors, we excluded this study. We attempted to contact the first author of this study but did not get any reply |
| Holt 2005 | This study was a case-series in which all patients were administered curcumin therapy |
Following the above mentioned search strategy, we also searched various international trial registries and identified two ongoing studies. The recruitment was complete but the results were yet to be reported. Of these, only one study was identified that met the inclusion criteria for this review and is detailed in the “Characteristics of ongoing studies” table.
Characteristics of ongoing studies [ordered by study ID]
| NCT00889161 | |
|---|---|
| Trial name or title | Curcumin in Pediatric Inflammatory Bowel Disease |
| Methods | Double blinded placebo controlled study |
| Participants | Age: 8 to 18 years |
| Gender: Both | |
| Criteria | |
Inclusion Criteria:
| |
Exclusion Criteria:
| |
| Interventions | Curcumin at an initial dosage of 500 mg twice a day for 3 weeks. Using the forced dose titration design, dose will be titrated up to 1g twice a day at Week 3 for a total of three weeks and then titrated again to 2 g twice a day at Week 6 for three weeks |
| Outcomes | Tolerability and safety data in pediatric patients with IBD |
| Starting date | May 2009 |
| Contact information | David Suskind MD, Seattle Children’s Hosptial, Seattle, Washington, United States, 98105 |
| Notes | |
Included studies
The single included trial (Hanai 2006) randomised 89 patients (49 male) with quiescent ulcerative colitis, defined by typical clinical, radiographic, endoscopic, and pathological criteria. The enrolled patients were randomised to either curcumin 2 g/day (n = 45) or placebo (n = 44). All patients were receiving maintenance therapy with sulfasalazine ormesalamine at entry and continued to receive these medications throughout the study. Other medications were stopped four weeks before starting the study. The interventions were administered for six months with an additional six months of follow-up. The primary study outcome was the proportion of patients relapsing at 6 and 12 months defined as an ulcerative Clinical Activity Index (CAI) score of more than four. Secondary outcomes included CAI and Endoscopic Index (EI) scores before and after treatment and adverse events. Further details including inclusion and exclusion criteria are provided in the Characteristics of included studies table.
Excluded studies
We identified one small case-series (Holt 2005) in which five patients with ulcerative colitis and five patients with Crohn’s disease were administered a pure curcumin preparation. As this was not a randomised controlled trial, the study was excluded. Atkinson 2003 was available as only an abstract. The study enrolled 27 patients with IBD and randomised them to receive curcumin or placebo for a period of 16 weeks. Since the results for patients with ulcerative colitis were not provided separately by the study authors, we could not include this study in the review. We attempted to contact the first author of this study but could not get any additional information. Ahuja 2011 studied the efficacy and safety of curcumin enema plus oral 5-ASA, compared with placebo enema plus oral 5-ASA in 43 ulcerative colitis patients with active disease.
Risk of bias in included studies
The risk of bias analysis is summarized in Figure 2. The Hanai 2006 study was assessed as having a low risk of bias. Hanai 2006 used adequate methods of randomization, allocation concealment, and blinding. A central computer-generated randomization scheme was used and implemented by a clinical pharmacist ensuring that allocation to treatment groups was concealed. All study participants and personnel were blinded to treatment for the duration of the study. The study authors did not report the measures followed to ensure compliance in either of the groups. There were more dropouts in the placebo group (n = 5) than in the curcumin group (n = 2). Three patients withdrew from the study due to adverse events (1 from the placebo group and 2 from the curcumin group). Attrition bias, however, does not appear to be a serious limitation as the total dropouts were only 7% and intention-to-treat analyses were used. This study was funded by non-industry sources.
Figure 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Curcumin versus placebo for maintenance of remission in ulcerative colitis
Relapse at 6 months
There was a non-significant trend towards benefit in the curcumin group at six months. Two patients relapsed at six months in the curcumin arm (4%) compared to eight patients in the placebo arm (18%) (RR 0.24, 95% CI 0.05 to 1.09; P = 0.06).
Relapse at 12 months
There was no statistically significant difference in the proportion of patients relapsing at 12 months follow-up. Ten patients in the curcumin group (22%) relapsed compared to 14 patients (32%) in the placebo group (RR 0.70, 95% CI 0.35 to 1.40; P = 0.31).
Frequency and nature of adverse events
A total of nine adverse events were reported in seven patients. Adverse events included sensation of abdominal bulging, nausea, transient hypertension, and transient increase in the number of stools. However, the authors did not provide the break-down of these events in the two randomised groups.
Clinical activity Index (CAI) at 6 months
The CAI at six months was significantly lower in the curcumin group compared to the placebo group (1.0 ± 2.0 versus 2.2 ± 2.3; MD −1.20, 95% CI −2.14 to −0.26).
Endoscopic Index (EI) at 6 months
The EI at six months was significantly lower in the curcumin group than in the placebo group (0.8 ± 0.6 versus 1.6 ± 1.6; MD −0.80, 95% CI −1.33 to − 0.27).
Time to relapse
Hanai 2006 did not report the time to clinical relapse in the enrolled patients.
Change in laboratory measures of inflammation
These outcomes were not reported in the Hanai 2006 study.
DISCUSSION
Summary of main results
One randomised controlled trial of 89 patients (Hanai 2006) demonstrated that curcumin at a dose of 2 g/day, when added to sulfasalazine or mesalamine, significantly reduced clinical and endoscopic activity indices at six months in patients with UC in remission. There was also a non-significant trend towards reduction in the proportion of patients with relapse in the first six months after initiation of therapy. However, there was no statistically significant difference in the proportion of patients who experienced relapse by 12 months after initiation of treatment. This could possibly be explained by the small sample size and the consequent limited power of the study. No serious adverse events were noted with curcumin therapy. Mild adverse events included sensation of abdominal bulging, nausea, transient hypertension, and transient increase in the number of stools.
Overall completeness and applicability of evidence
This review identified only one trial that was underpowered to detect potentially important differences between curcumin and placebo. Thus, the results of this study should be interpreted with caution. The dose of curcumin used in this trial was 2 g per day. A Phase I human trial with 25 subjects using up to 8 g of curcumin per day for 3 months did not find any toxicity (Cheng 2001). It is unclear if higher doses or a longer duration of treatment with curcumin would have had additional benefits. We did not find trials that compared curcumin with other active interventions. Curcumin is a relatively inexpensive intervention that is not associated with serious adverse events in healthy volunteers as well as in this trial when administered over a six month period. The results of the included trial can be extrapolated to settings in low and middle income countries. However, since the trial included only adults, this review is unable to address the efficacy of curcumin in maintaining remission in pediatric populations with ulcerative colitis. Further research is required to confirm any possible benefit of curcumin therapy in quiescent UC.
Quality of the evidence
The overall quality of the evidence as assessed by the GRADE approach was low for the primary outcome (relapse at six months). This indicates that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. The overall quality of the evidence for relapse at 12months follow-up was also judged to be low, indicating little confidence in this effect estimate. The overall quality of the continuous outcomes was also judged to be low (See Summary of findings for the main comparison).
Potential biases in the review process
We did not identify any potential biases in the review process. A comprehensive search was performed and eligible studies were identified by two independent authors. Data extraction was performed by independently by two review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
Agreements and disagreements with other studies or reviews
The results of this systematic review agree with a recent systematic review of curcumin treatment in patients with inflammatory bowel disease (Taylor 2011). A systematic review of the safety and anti-inflammatory activity of curcumin reported it to be safe in healthy volunteers as well as in patients with rheumatoid arthritis and AIDS (Chainani-Wu 2003).
AUTHORS’ CONCLUSIONS
Implications for practice
Curcumin may be an effective and safe therapy in maintenance of remission in ulcerative colitis when given with standard treatments (sulfasalazine or mesalamine) over a period of six months. However, its use for this indication should occur in the context of a well-conducted randomised clinical trial. Curcumin is not currently approved by regulatory bodies in the U.S. or Europe for clinical use for the treatment of ulcerative colitis. This review was unable to answer the question of its efficacy compared to other interventions in maintaining remission, or the optimal dose to be used, or the optimal duration of therapy for this purpose.
Implications for research
Well designed RCTs are required to determine the efficacy and safety of curcumin at varying doses in patients with mild to moderately active ulcerative colitis and in patients with quiescent disease, either as an alternative or adjunctive therapy to mesalamine. These trials should include validated clinical and endoscopic endpoints, and be appropriately sized to detect clinically meaningful effect sizes. Based on the relapse rates in the placebo group at 6 months, we estimated that at least 706 patients per group need to be enrolled to detect a 30%relative reduction in the proportion of patients with relapse following curcumin therapy (assuming alpha error of 0.05 and power of 80%). The topical effects of curcumin on the colonic mucosa should also be measured in vitro, and compared to mesalamine in down-regulating local inflammation in models of colitis.
Supplementary Material
DIFFERENCES BETWEEN PROTOCOL AND REVIEW.
Risk of bias assessments
In our protocol we stated that we intended to explore the relationship between trial outcomes and the role of industry funding or “funding bias”. Funding bias is defined as biases in the design, outcome, and reporting of industry sponsored research in order to show that a drug produces a favourable outcome (Bekelman 2003). Relationships between industry, scientific investigators and academic institutions are widespread and often result in conflicts of interest (Bekelman 2003).We plan to explore funding bias in updates of this review, should we find additional trials.
Data Analysis
For continuous data that are missing, we planned to derive standard deviations from other available data such as standard errors, or to impute them using methods suggested byHiggins 2011a. A sensitivity analysis would have been performed by calculating the treatment effect including and excluding the imputed data to see whether this would alter the outcome of the analysis. We had also stated that we would use change from baseline to endpoint scores for the reported disease activity scores and sigmoidoscopic changes. However, for the review, we used only endpoint scores, since there are many problems with using change scores, particularly when there many drop-outs from a trial (Higgins 2011c).
ACKNOWLEDGEMENTS
Funding for the IBD/FBD Review Group (September 1, 2010 – August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON - 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry ofHealth and Long TermCare (HLTC3968FL-2010-2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
This document is an output of a workshop organized by Dr Prathap Tharyan, Director, South Asian Cochrane Network and Centre at Christian Medical College, Vellore, India April 11–15, 2011. This centre is funded partly by Indian Council of Medical Research, New Delhi, and partly by the Effective Healthcare Research Consortium via a grant from the Department of International Development (DFID) through the University of Liverpool (Paul Garner). We are grateful to Richard Kirubakaran (statistician), Aneesh George (Research Fellow) from the Prof. BV Moses & ICMR Advanced Centre for Evidence-Informed Healthcare at CMC Vellore
ACM is supported by NIDDK (K23DK084338) and the Dept of Medicine, BIDMC.
SOURCES OF SUPPORT
Internal sources
Deptartment of Gastroenterology, All India Institute of Medical Science, New Delhi, India.
All India Institute of Medical Science, New Delhi, India, India.
Library, Biostatistics
Gasteroenterology and Hepatology, VA Medical Centre, Northport, Northport, New York, USA.
Clinical Epidemiology Unit, All India Institute of Medical Science, New Delhi, India.
Biostastistics
Dept of Medicine, BIDMC, USA.
Salary support (ACM)
External sources
Parthap Tharyan, India.
This document is an output of a Review completion workshop organized by the South Asian Cochrane Network and Centre under directorship of Dr Parthap Tharyan at Christian Medical College, Vellore, India in April 2011. This centre is funded by Indian Council of Medical Research, New Delhi
NIDDK (K23DK084338), USA.
Salary support (ACM)
APPENDICES
Appendix 1. Search strategies
MEDLINE and EMBASE (Ovid) search strategy:
(colitis, ulcerative or ulcerative colitis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
proctocolitis.mp. or proctocolitis/
1 or 2
curcumin.mp. or exp curcumin/
turmeric.mp.
4 or 5
3 and 6
PUBMED search strategy:
#1 ((colitis, ulcerative[mh]) OR (proctocolitis[mh])) AND ((curcumin[mh]) OR (turmeric[mh]))
Cochrane Library search strategy:
#1(colitis, ulcerative):ti,ab,kw
#2proctocolitis
#3(#1 OR #2)
#4curcumin
#5turmeric
#6(#4 OR #5)
#7(#3 AND #6)
Footnotes
CONTRIBUTIONS OF AUTHORS
Designing the review: SK, VA, AK
Coordinating the review: SK, VA
Developing search strategy: SK, VA
Data collection: SK, VA
Performing literature search: SK, VA, AK
Selecting papers for full review: SK, VA
Obtaining papers for review: SK, VA
Screening papers for inclusion in review: SK, VA
Assessing quality of papers: SK, VA
Extracting data from papers: SK, VA
Data management: SK, MJS
Entering data into RefMan software: SK
Analysis of data: SK, VA, MJS, ACM
Interpretation of data: SK, VA, MJS, ACM
Writing the review: SK, VA, MJS, AK, ACM
DECLARATIONS OF INTEREST
Dr. Vineet Ahuja was an investigator for RCTs of oral curcumin for induction of remission in ulcerative colitis and rectal curcumin enema for induction of remission in ulcerative colitis. Mr Sushil Kumar participated in these studies as part of the research team (studies unpublished as of April 2011).
ACM has received grant support from Proctor & Gamble, Salix and Shire. He has acted on advisory boards for Abbott, UCB and Salix. He has received speaker’s honoraria from Abbott and Schering-Plough.
The other authors have no known declarations of interest.
REFERENCES
* Indicates the major publication for the study
References to studies included in this review
- Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clinical Gastroenterology and Hepatology. 2006;4(12):1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ahuja V, Singla V, Choudhury B, Verma P, Tiwari V, Kumar S, et al. Evaluation of efficacy and safety of induction therapy with curcumin enema in ulcerative colitis patients with mild to moderate proctitis. Journal of Crohn’s and Colitis. 2011;5(1):S133. [Google Scholar]
- Atkinson RJ, Hunter JO. A double blind,placebo controlled randomised trial of curcuma extract in the treatment of steroid dependent inflammatory bowel disease. Gastroenterology. 2003;124(4 Suppl 1):A205. [Google Scholar]
- Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Digestive Diseases and Sciences. 2005;50(11):2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- NCT00889161. Curcumin in pediatric inflammatory bowel disease. [accessed 11 July 2012]; http://clinicaltrials.gov/ct2/show/NCT00889161.
Additional references
- Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Medica. 1991;57(1):1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- Arafa HM, Hemeida RA, El-Bahrawy AI, Hamada FM. Prophylactic role of curcumin in dextran sulfate sodium (DSS)-induced ulcerative colitis murine model. Food and Chemical Toxicology. 2009;47(6):1311–1317. doi: 10.1016/j.fct.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fischer C, et al. Curcumin is an in vivo inhibitor of angiogenesis. Molecular Medicine. 1998;4(6):376–383. [PMC free article] [PubMed] [Google Scholar]
- Arend LJ, Springate JE. Interstitial nephritis from mesalazine: case report and literature review. Pediatric Nephrology. 2004;19(5):550–553. doi: 10.1007/s00467-004-1411-6. [DOI] [PubMed] [Google Scholar]
- Ausfeld-Hafter B, Hoffmann S, Seibold F, Quattropani C, Heer P, Straumann A. Status of alternative medicine in Crohn disease and ulcerative colitis patents: a questionnaire survey. Forschende Komplementarmedizin und klassische Naturheilkunde. 2005;12(3):134–138. doi: 10.1159/000084785. [DOI] [PubMed] [Google Scholar]
- Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochemical Pharmacology. 2009;78(10):1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003;289(4):454–465. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T, Klein S, et al., editors. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Boston, MA: The American Botanical Council; 1998. [Google Scholar]
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology. 1997;50(6):683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) Journal of Alternative and Complementary Medicine. 2003;9 (1):161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- Chan MM-Y. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochemical Pharmacology. 1995;49(11):1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Research. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990;263(10):1359–1385. [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB. Update on the etiology, pathogenesis and diagnosis of ulcerative colitis. Nature Clinical Practice. Gastroenterology and Hepatology. 2004;1(1):26–31. doi: 10.1038/ncpgasthep0031. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane–handbook.org. [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane–handbook.org. [Google Scholar]
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane–handbook.org. [Google Scholar]
- Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25(9):1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Research. 1991;51(3):813–819. [PubMed] [Google Scholar]
- Jobin C, Bradham CA, Russo MP, Juma B, Narulla AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NFkappa B activation and pro inflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. Journal of Immunology. 1999;163(6):3474–3482. [PubMed] [Google Scholar]
- Joshi U, Wadhwani AM, Johri BM. Dictionary of Economic Plants in India. 2nd Edition. New Delhi: Indian Council of Agricultural Research; 1983. p. 62. [Google Scholar]
- Kakar SS, Roy D. Curcumin inhibit TPA-induced expression of c-fos, c-jun and c-myc proto-oncogenes messenger RNAs in mouse skin. Cancer Letters. 1994;87(1):85–89. doi: 10.1016/0304-3835(94)90413-8. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Research. 1999;59(3):597–601. [PubMed] [Google Scholar]
- Lee YK, Park SY, Kim YM, Park OJ. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Annals of the New York Academy of Sciences. 2009;1171:489–494. doi: 10.1111/j.1749-6632.2009.04699.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan-Coyne G, O’Sullivan GC, O’Donovan TR, Piwocka K, McKenna SL. Curcumin induces apoptosisindependent death in oesophageal cancer cells. British Journal of Cancer. 2009;101(9):1585–1595. doi: 10.1038/sj.bjc.6605308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. Journal of Clinical Gastroenterology. 2010;44(4):246–253. doi: 10.1097/MCG.0b013e3181cadbe1. [DOI] [PubMed] [Google Scholar]
- Podolosky DK. Inflammatory Bowel Disease. New England Journal of Medicine. 1991;325(13):928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Ravindranath V, Chandrasekhar N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16(3):259–266. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine. 2008;27(5):746–763. doi: 10.1002/sim.2971. [DOI] [PubMed] [Google Scholar]
- Salh B, Assi K, Templeman V, Parthar K, Owen D, Gomenz-Munoz A, et al. Curcumin attenuates DNB-induced murine colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2003;285(1):G235–G243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. Available from www.cochrane–handbook.org. [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] Journal of Biological Chemistry. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [PUBMED: 7559628] [DOI] [PubMed] [Google Scholar]
- Sreejayana Rao MN. Nitric oxide scavenging by curcuminoids. Journal of Pharmacy and Pharmacology. 1997;49(1):105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123(6):1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Leonard MC. Curcumin for inflammatory bowel disease: a review of human studies. Alternative Medicine Review. 2011;16(2):152–156. [PubMed] [Google Scholar]
- Ukil A, Maity S, Karmakar S, Datta N, Vedasiromani JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitorobenzene sulfonic acid-induced colitis. British Journal of Pharmacology. 2003;139(2):209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung VY, Foshaug RR, Macfarlane SM, Churchill TA, Doyle JS, Sydora BC, et al. Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent antiinflammatory effect in the IL-10 gene-deficient mouse model of IBD. Digestive Diseases and Sciences. 2009 Jun 10;55(5):1272–1277. doi: 10.1007/s10620-009-0843-z. [DOI] [PubMed] [Google Scholar]
- Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arquivos de Gastroenterologia. 2009;46(1):20–25. doi: 10.1590/s0004-28032009000100009. [DOI] [PubMed] [Google Scholar]
- Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacologica et Toxicologica. 1978;43(2):86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.