Abstract
Background & Aims
Adult hepatocytes undergo cell cycle progression and proliferation in response to partial hepatectomy (PH). Transient lipid accumulation within hepatocytes preceding the peak proliferative phase is a characteristic feature of regenerating livers. However, the molecular mediators and mechanisms responsible for lipid accumulation in regenerating livers are not well understood. Adipose differentiation related protein (ADRP; Plin2) regulates hepatic triglyceride storage and Plin2-deficient (Plin2−/−) mice have significantly reduced triglyceride (TG) content in the liver. We sought to determine the functional significance of PLIN2 in liver regeneration in response to PH and toxic liver injury and examined whether absence of Plin2 expression modulates hepatocyte proliferation and liver regeneration.
Methods
We subjected wild-type (WT) and Plin2−/− mice to 70% PH or acute carbon tetrachloride (CCL4) treatment and examined the hepatic lipid content, the expression profile of lipid metabolism-related genes, the rate of cellular proliferation and the dynamics of liver regeneration in the treated animals.
Results
In response to PH, Plin2−/− mice showed decreased hepatic triglyceride accumulation and delayed cell cycle progression, which was associated with impaired liver regeneration. Fatty acid (FA) synthesis and lipid transfer gene expression profile were comparable between Plin2−/− and wild-type mice, while VLDL secretion rate was higher in the Plin2−/− mice. Downregulated β-oxidation and reduced cytosolic FA level in Plin2−/− mice may have contributed to the attenuation of the liver regeneration capacity in these animals. In parallel experiments, we also observed attenuated hepatic lipid accumulation and proliferation in response to CCl4-mediated acute toxic liver injury in Plin2−/− mice.
Conclusions
We conclude that PLIN2-mediated lipid accumulation and utilization by the liver is important for efficient liver regeneration in response to PH and toxic liver injury.
Keywords: ADRP, ADFP, PLIN2, Partial hepatectomy, Lipid oxidation, Triglyceride, Fatty acids
Introduction
The liver is a central metabolic organ that is involved in the synthesis, storage, metabolism, and repartitioning of the macronutrients carbohydrate, protein, and fat. It has a unique capacity to maintain its size for proper function, a property that is quickly activated upon loss of liver mass, as it happens after partial hepatectomy (PH) [1], a maneuver that has been used to study liver regeneration in rodents [2,3]. In this model, the left and medial lobes of the liver are removed, resulting in loss of 70% of the liver mass. As an acute response to PH, most remaining hepatocytes rapidly undergo proliferation, as they also start accumulating large amounts of lipids, including triglycerides (TG), fatty acids (FA), and cholesteryl esters, which are normally stored inside intracellular lipid droplets (LDs). The proliferative response is most marked within the first 3 days, and the original liver mass is restored within a week in rodents [4]. The hepatic LDs are thought to be critical for the process, as lipids stored in these droplets may be used as the source of new cell membrane formation as well as the energy source required for hepatocyte proliferation. However, the exact role of lipid accumulation and utilization in post-PH liver regeneration is poorly understood [5].
Obese patients with fatty livers tend to have poor outcome after liver resection or transplantation [6,7]. In rodents, pre-existing steatosis in high fat diet or genetic models, as well as in other models of augmented hepatic steatosis, are associated with impairment of liver regeneration after PH [8–11]. On the other hand, models such as liver-specific glucocorticoid receptor-inactivated [12] and caveolin 1 deficient mice [13], also display reduced hepatic TG and defective liver regeneration after PH. So, both insufficient and excessive liver fat may negatively impact the normal process of liver regeneration.
Adipose differentiation related protein (ADRP; Plin2) is a major LD protein readily detected in fatty liver. Interestingly, Plin2−/− mice display markedly reduced hepatic TG content [14,15]. In addition, they also exhibit evidence of abnormal lipid homeostasis in other tissues, e.g., reduced cholesteryl ester storage and increased cholesterol efflux in macrophages [16], modestly lowered milk fat [17], and compromised retinyl ester transport and storage in the retina [18]. The reduction of hepatic TG content in Plin2−/− mice can be accounted for, wholly or partly, by an increased output of very low density lipoprotein (VLDL) from the liver. Furthermore, in comparison with wildtype mice, the liver of Plin2−/− mice displays altered TG compartmentalization; the TG is markedly depleted in most subcellular compartments except the microsomes where the TG concentration is actually increased. To understand the role of Plin2 and hepatic TG handling in regenerating livers, we studied the lipid dynamics and the regenerative response to 70% partial hepatectomy and carbon tetrachloride (CCl4)-mediated toxic liver injury in Plin2+/+ and Plin2−/− mice.
Materials and methods
Animals
We generated and maintained Plin2−/− mice in C57BL/6J background as described previously [14]; C57BL/6J mice purchased from Jackson Lab (Bar Harbor, Maine) served as wild-type (WT) controls. All experimental procedures were carried out under a protocol approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. Mice were kept on 12 h dark-light cycle and maintained on standard chow and water before and after the surgery. We performed PH on 8–12 week old male mice by separately ligating and resecting the right upper, left upper (equivalent to median lobe), and left lower lobes (also known as left lateral lobe), which together make up about 70% of the liver [3]. The mortality rate is less than 5% for both the wild-type and Plin2−/− mice.
In order to evaluate liver regeneration in response to a different acute liver injury, we treated 8–12 weeks old Plin2−/− and wild-type male mice with a single intraperitoneal injection of carbon tetrachloride (CCl4; 750 µl/kg BW in corn oil) and harvested the liver tissue after 48 and 72 h. BrdU (50 mg/kg BW) was administered intraperitoneally 2 h prior to tissue harvest, tissues were snap frozen in liquid nitrogen and stored in −80 °C for further analysis. For histological analysis, we fixed tissues in 10% formaldehyde and embedded in paraffin or used frozen tissues for further analysis.
Serum chemistry assays
We measured serum triglycerides (Infinity assay by Thermo Electron, Melbourne, Australia), non-esterified free-fatty acids (Wako Chemicals USA, Hercules, CA), Glycerol (Sigma, St. Louis, MO), β-hydroxybutyrate (Cayman Chemical, Ann Arbor, MI) by colorimetric assay. Serum IL-1β, IL-6, and TNF-α (eBioscience, San Diego, CA) were measured using an enzyme immunoassay kit according to the manufacturer’s instructions.
Liver histology and immunofluorescence
We assessed liver morphology based on 5 µm hematoxylin and eosin-stained paraffin sections. The frequency of nuclear PCNA staining (anti-PCNA antibody; Cell Signaling Technology, Danvers, MA) was determined by examination of at least three random 200× fields and at least 300 cells and nuclei in each tissue section. We stained liver sections for BrdU positive nuclei with the BrdU labeling and detection kit (Roche, Indianapolis, IN) according to manufacturer’s instructions. Sections of liver frozen in OCT compound (Sakura Finetek, Torrance, CA) were stained with Oil Red O (Sigma, St. Louis, MO) for analysis of hepatic fat accumulation.
Liver lipid analysis
We homogenized 200 mg liver tissues in 2 ml of PBS, extracted lipids from these homogenates according to Bligh and Dyer [19] and fractioned different lipid species by one dimensional thin layer chromatography (silica Gel-60, Analtech, Newark, DE), using petroleum ether/ether/acetic-acid (85:25:1). Lipids were visualized by incubating the TLC plate in a saturated iodine chamber and quantified by comparing the optical density of samples with lipid standards using Image J software [14,15].
Determination of rate of very low density lipoprotein (VLDL) secretion in vivo
At 24 h after partial hepatectomy, we quantified the rate of VLDL secretion in vivo by injecting intraperitoneally Pluronic F-127 (2 mg/g BW in PBS, BASF Corporation, Florham Park, NJ), a lipoprotein lipase inhibitor, and monitored the serum triglyceride before, and 1, 2, 3, and 4 h after injection [14].
Quantitative reverse transcription PCR
We extracted total RNA from the liver by using the Absolutely RNA™ Miniprep Kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions. RNA was treated with DNase I, reverse transcribed using the Superscript III First Strand Synthesis System and used for quantitative PCR (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using the iQ™ SYBR Green Supermix (BIO-RAD, Hercules, CA) and an Mx3000P quantitative PCR machine (Stratagene, La Jolla, CA). Gapdh, Hmbs, and Eef1g were used as the housekeeping control genes.
Western blotting
We analyzed total liver protein extracts by 10% SDS-PAGE, and transferred the protein to nitrocellulose membrane (Bio-Rad) at 250 mA for 90 min. Membranes were blocked with 5% non-fat dry milk dissolved in TBST (Tris-buffered saline Tween) for 1 h at RT prior to incubation with antibodies specific for PCNA, cyclins D1 and A (Santa Cruz, CA), PLINs and MTTP (the latter two antibodies were developed in our laboratory [14,15]). To ensure equal loading, we stripped the membranes and reprobed them with anti-β-actin or anti-GAPDH antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
We expressed all values as means ± S.D. with the exception of quantitative PCR analyses, which were expressed as means ± S.E. Difference between means was analyzed by using Student’s t-test. A difference was considered significant when p <0.05.
Results
PH induces PLIN2 expression
Partial hepatectomy (PH) is a well-established procedure for studying liver regeneration, and a large body of studies have established the chronological changes of hepatic lipids after PH [4,20,21]. However, in order to identify critical time points for comparative studies with the Plin2−/− mice, we first determined the chronology of biochemical and molecular parameters at various time points after PH in wild-type mice. Our initial characterization of plasma and hepatic lipid profiles and gene expression patterns after PH in the wild-type mice recapitulated previous studies (Supplementary Figs. 1 and 2).
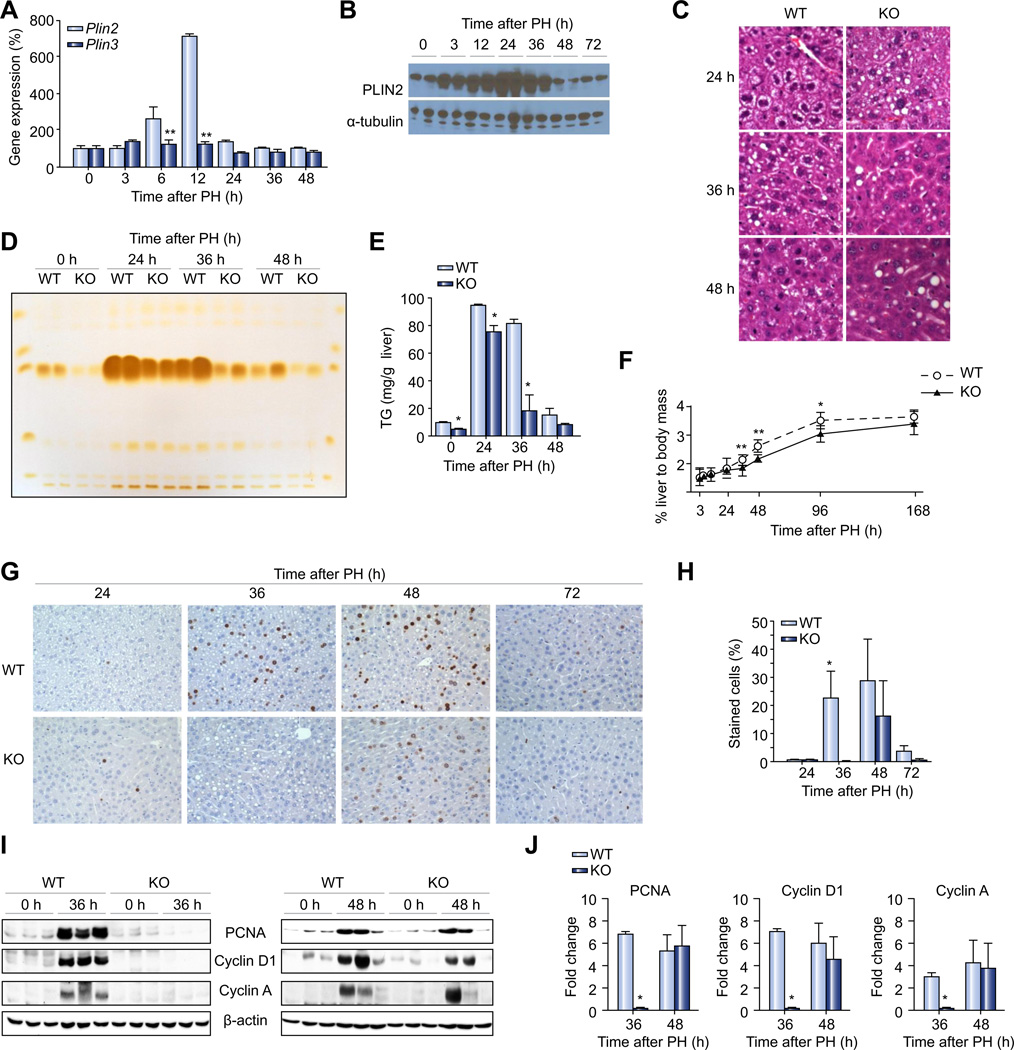
Plin2 (Adrp) and Plin3 (Tip47) are major LD proteins that are highly expressed in the liver. We examined the expression pattern of these LD proteins in response to PH and found that Tip47 mRNA expression is not influenced by PH, whereas Plin2 mRNA level doubled at 6 h and went up ~7-fold 12 h after PH, returning to baseline by 24 h post-PH (Fig. 1A). The PLIN2 protein expression increased rapidly starting at 3 h and peaked 24 h after PH (Fig. 1B). It remained high at 36 h, but returned to baseline 48 h after PH (Fig. 1B).
Fig. 1. Hepatic lipids, lipid droplet protein and regeneration after PH.
(A) Quantitative RT-PCR analysis of mRNA expression of Plin2 and Plin3 lipid droplet protein genes (n = 4 of each group) in wild-type mice before (0 h) or various time points after PH. (B) PLIN2 protein expression at 0, 3, 12, 24, 36, 48, and 72 h after PH. Two representative samples from each time point are shown and α-tubulin was used as an internal control for equal loading. (C) Microscopic examination of H&E stained liver sections from wild-type mice and Plin2−/− mice 24, 36, 48 h after PH. (D) Thin-layer chromatography analysis of lipids isolated from livers of wild-type and Plin2−/− mice at basal (0 h) and 24, 36, 48 h after PH. (E) Quantitative densitometry of hepatic TG contents in wild-type, and Plin2−/− mice (based on TLC results; n = 4). (F) Liver regeneration index in wild-type and Plin2−/− mice (n = 5–16 of each group) determined by dividing the weight of liver at indicated time points with the body weight before PH. (G) Immunohistochemical analysis of BrdU incorporation at 24, 36, 48, and 72 h post-PH of wild-type and Plin2−/− mice. (H) Percent of BrdU positive cells in wild-type and Plin2−/− mice at indicated time points after PH (n = 3). (I) Western blotting of total liver homogenates of resected lobes (0 h) and remnant livers (36 h and 48 h post-PH) of wild-type and Plin2−/− mice. (J) Densitometric analysis of band intensities of Western blotting for PCNA, Cyclin D1 and Cyclin A. In all panels, data are expressed as means ± S.D. (n = 3). *p <0.05, *p <0.01 vs. wild-type group. WT, wild-type; KO, Plin2−/−. (This figure appears in colour on the web.)
Plin2−/− mice have attenuated lipid droplet formation and delayed liver regeneration in response to PH
We have previously shown that Plin2−/− mice have marked TG deficiency in the liver under basal conditions [18]. Because Plin2 was highly upregulated both at transcriptional and posttranscriptional levels after PH (Fig. 1A and B), we decided to use Plin2−/− mice as a model to dissect the role of Plin2 in hepatic TG dynamics during the liver regeneration in response to PH.
Light microscopic examination of remnant liver sections revealed that LD sizes in Plin2−/− mice appeared more heterogeneous 24 h after PH (Fig. 1C) with reduced LD formation 36 h and 48 h after PH (Fig. 1C), as compared to wild-type mice. Oil-red-O (ORO) staining of liver sections revealed similar morphological features (Supplementary Fig. 3), though the morphology was not as well defined compared to H & E staining in distinguishing smaller droplets due to solvent (isopropanol) effects [22] (Fig. 1C). We previously noted a similar change in hepatic LD size in Plin2−/− hepatocytes in conditions other than PH that are normally associated with fatty liver [14,15]. As compared to wild-type, Plin2−/− remnant livers had reduced TG content as analyzed by thin layer chromatography of livers harvested at 24, 36, and 48 h after PH (Fig. 1D and E). Sham operation did not alter the hepatic TG in the wild-type and Plin2−/− mice 36 h post-operation (Supplementary Fig. 4A and B).
We next studied the PH-induced liver regeneration in wild-type and Plin2−/− mice. The liver regeneration index (the weight of remnant liver divided by initial body weight) rose sharply between 36 to 48 h, and plateaued after at 96 h after PH. In contrast, the liver regeneration in Plin2−/− mice was attenuated as compared to wild-type mice (Fig. 1F). In a separate experiment, we calculated the liver regeneration index based on the remnant liver weight and body weight at 48 h post-PH, and confirmed that liver regeneration is significantly impaired in Plin2−/− mice (Supplementary Fig. 5A). Notably, body weights of mice recovering from PH were comparable between the wild-type and Plin2−/− mice (Supplementary Fig. 5B).
Hepatocyte cell-cycle entry and proliferation in response to PH is delayed in Plin2−/− mice
Adult hepatocytes are highly differentiated quiescent cells which rarely undergo cell division. However, in response to PH almost all hepatocytes underwent proliferation with concerted cell cycle entry and progression. In order to evaluate hepatocyte proliferation in response to PH, we analyzed liver sections by BrdU incorporation. In the wild-type mice, BrdU-positive nuclei were detectable at 24 h and peaked between 36 to 48 h and near-basal levels were reached by 72 h post-PH. Interestingly, BrdU incorporation was significantly attenuated at 36 h and peaked at 48 h post-PH, suggestive of delayed cell-cycle progression, in Plin2−/− mice (Fig. 1G and H). Correspondingly, induction of cyclins D1 and A, key mediators of cell cycle progression at G1 and G1/S phase of cell cycle, as well as PCNA, a marker of proliferating hepatocytes, were attenuated at 36 h post PH in the remnant livers of Plin2−/−, as compared to wild-type (Fig. 1I and J). The expression levels of these markers in the remnant livers of Plin2−/− mice, however, were comparable to wild-type mice at 48 h post-PH (Fig. 1I and J).
Comparable de novo lipogenesis and lipid uptake in Plin2−/− and wild-type mice after PH
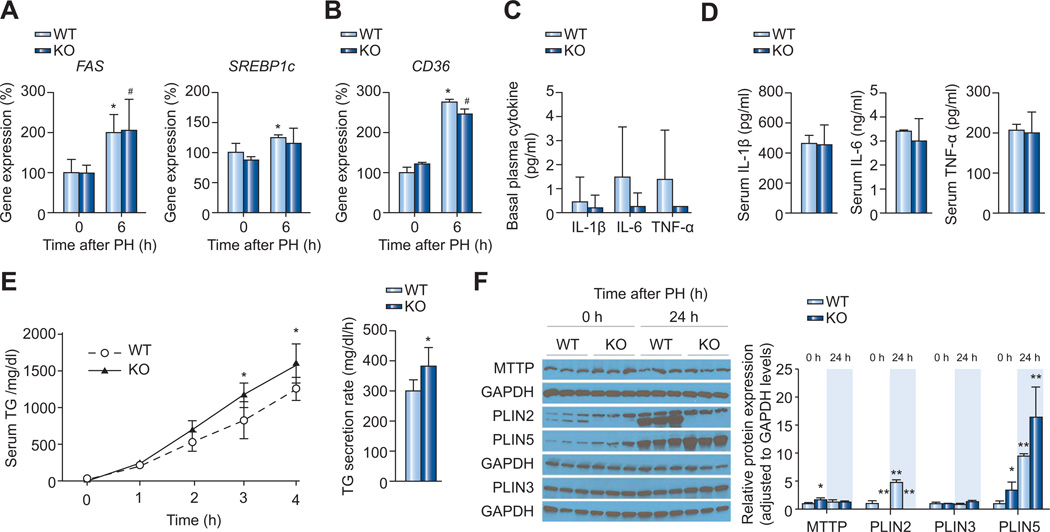
To explore the mechanism for the altered lipid homeostasis in Plin2−/− mice during liver regeneration after PH, we evaluated mRNA expression of rate-limiting and regulatory genes essential for efficient lipogenesis by quantitative real-time reverse transcription PCR. Basal mRNA expression of FAS and SREBP1c was comparable between Plin2−/− and wild-type mice (Fig. 2A), corroborating with our previous findings [18]. Induction of FAS and SREBP1c expression in response to PH was also comparable between Plin2−/− and wild-type mice at 6 h post PH, time of peak expression post-PH (Supplementary Fig. 2A). We also did not find any difference in ACC1 and SCD1 mRNA induction between Plin2−/− and wild-type mice (data not shown). Furthermore, the CD36 mRNA level in Plin2−/− mice was not different from that of wild-type mice (Fig. 2B), and no difference was detected in the level of LDL-R mRNA (data not shown). Finally, the time course of changes in serum TG, FA, and glycerol for Plin2−/− mice after PH was not detectably different from that in wild-type mice (data not shown).
Fig. 2. Mechanisms of hepatic TG reduction in the liver of Plin2−/− compared to that of the wild-type (WT) mice after PH.
Quantitative RT-PCR analysis of mRNA expression of (A) lipogenic genes, FAS and SREBP1c, and (B) the fatty acid transporter, CD36, in wild-type and mice at indicated time after PH. (n = 4 of each group). (C) Basal plasma cytokine levels in wild-type and Plin2−/− mice. (D) Serum cytokine levels measured at 6 h after PH (n = 4–5 of each group). Plasma IL-1β, IL-6, and TNF-α were measured by using ELISA kits (n = 4). (E) VLDL secretion determined at 24 h after PH (n = 5 of each group). Plasma TG levels were determined at indicated time after i.p. injection of Pluronic F-127, a lipoprotein lipase inhibitor at 2 mg/g BW (left panel). We calculated the rate of VLDL secretion (right panel) using data obtained between 1 and 4 h when the secretion was occurring at near-linear rate. (F) Western blot analyses of MTTP and LD proteins (PLIN2, 3, and 5 are shown; PLIN4 was undetectable) before (0 h) and 24 h after PH in the wild-type and Plin2−/− mice (left panel). Three independent polyacrylamide gels were analyzed and GAPDH was used as an internal control in each case. Quantitative analyses of proteins (right panel) were done using Image J software. W, wild-type; KO, Plin2−/− mice. In all panels, data are expressed as means ± S.D. *p <0.05, **p <0.01 vs. wild-type group. (This figure appears in colour on the web.)
It is known that inflammatory cytokines influence hepatocyte lipogenesis as well as liver regeneration after PH [23–26]. The basal cytokine levels were barely detectable (Fig. 2C). We found that the serum levels of IL-1β, IL-6, and TNF-α increased after PH and reached their peak at 6 h post PH (data not shown), but the levels of these cytokines were similar in Plin2−/− and wild-type mice (Fig. 2D). Furthermore, we also found no difference in the serum level of hepatic growth factor between wild-type and Plin2−/− mice under basal conditions and 12 h after PH (Supplementary Fig. 6). These findings suggest that the lower liver regeneration index detected in Plin2−/− mice was not accounted for by the serum cytokine levels that stimulate liver regeneration.
Unbridled VLDL secretion in Plin2−/− mice after PH
We next examined the rate of VLDL secretion 24 h after PH in wild-type and Plin2−/− mice. We measured plasma TG levels at different times after administration of the lipase inhibitor, Pluronic F-127, and found a significantly increased rate of VLDL secretion in Plin2−/− mice compared with wild-type mice (Fig. 2E), as we reported previously in the basal state in Plin2−/− deficient mice [18,19]. Taken together, these findings suggest that the decreased lipid accumulation in the liver of Plin2−/− mice appears not to be related to impaired de novo FA synthesis or lipid uptake from the circulation, but the unbridled VLDL secretion in Plin2−/− mice seems to offer a partial explanation.
As we have shown before, microsomal triglyceride transfer protein (MTP), the rate limiting enzyme in VLDL secretion, was modestly increased in Plin2−/− mice compared to that of the wild-type before PH (Fig. 2F). However, its level was similar at 24 h after PH between the two groups of mice indicating mechanisms other than MTP protein may also be involved.
PH caused an increase of PLIN2 protein in the wild-type mice. As PLIN2 belongs to a family of five related genes (Plin1–5) [27], we examined whether loss of PLIN2 caused a compensatory change of other members of the gene family. PLIN1 and 4 were not detectable in the liver of the control or PH mice (data not shown). The PLIN3 protein level was not different between wild-type and Plin2−/− mice; however, the protein abundance of PLIN5 was increased in both groups at 24 h after PH and the level was higher in the Plin2−/− mice compared to that of the wild-type both before and 24 h after PH (Fig. 2F).
Downregulated β-oxidation in Plin2−/− mice after PH
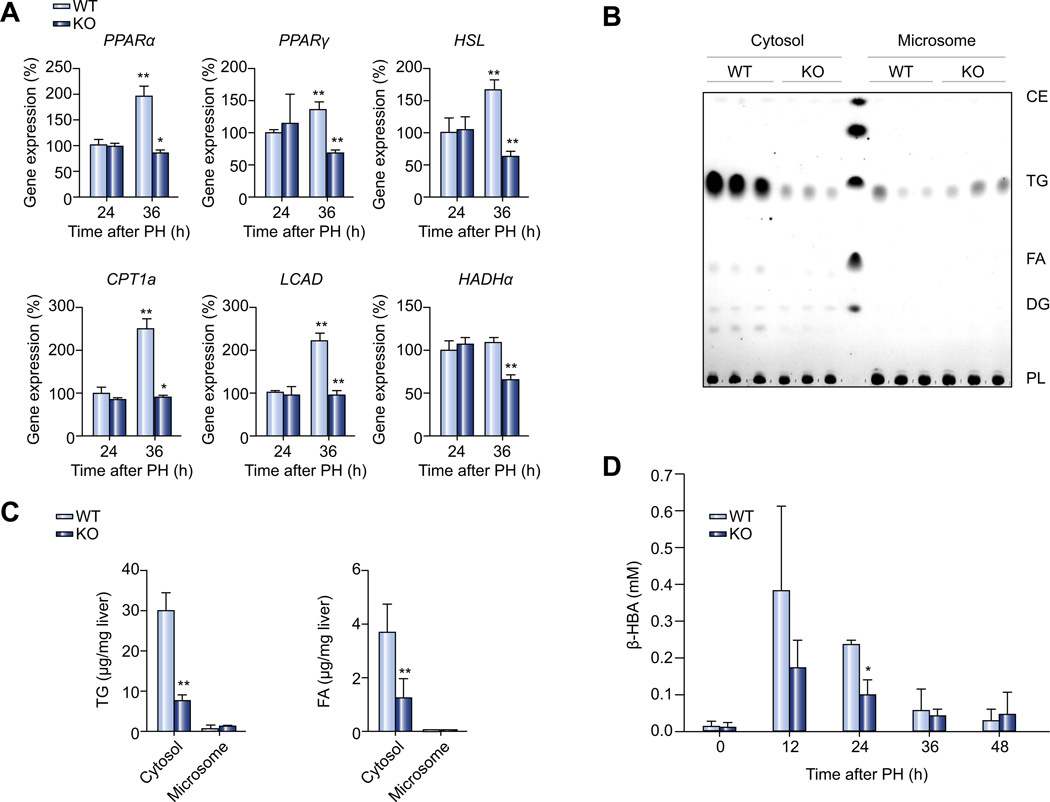
Finally, we examined the expression of genes involved in lipolysis and β-oxidation in the liver of Plin2−/− mice, at 36 h after PH, when lipolysis and β-oxidation were at their peak, as observed from the PH of wild-type animals (Supplementary Fig. 2B and C). Interestingly, all of the transcripts that we examined, including those for PPARα and PPARγ (critical regulators of hepatic lipid oxidation), HSL (lipolytic enzyme), CPT1a (a rate limiting enzyme involved in the transport of long chain FA into mitochondrial matrix for β-oxidation), as well as LCAD and HADHα (enzymes for β-oxidation), were downregulated in the liver of Plin2−/− mice compared to those of wild-type animals (Fig. 3A).
Fig. 3. β-oxidation and lipid partitioning in the liver of wild-type and Plin2−/− mice after PH.
(A) Quantitative RT-PCR analysis of mRNA expression of genes related to lipid metabolism at 24 h (24) and 36 h (36) after PH (n = 4). (B) Thin-layer chromatography analysis of lipids in cytosolic and microsomal compartments isolated from wild-type and Plin2−/− livers 36 h after PH. (C) Quantification of cytosolic and microsomal triglyceride and fatty acids content in livers of wild-type, and Plin2−/− mice 36 h after PH (n = 5). (D) Serum β-HBA from wild-type and Plin2−/− mice (n = 5) at basal and different time points after PH. In all panels, data are expressed as means ± S.D. *p <0.05 and **p <0.01 vs. wild-type group. WT, wild-type; KO, Plin2−/−; TG, triglyceride; FA, fatty acids.
We next analyzed the subcellular distribution of lipids in different cellular compartments by fractionation. We separated liver homogenates isolated from Plin2−/− and wild-type mice 36 h after PH into cytosolic and microsomal fractions by differential centrifugation and extracted the lipids from these fractions for TLC analysis. As shown in Fig. 3B and C, TG, and FA content was significantly reduced in the cytosolic fraction of the Plin2−/− mice compared to that of the wild-type mice. On the other hand, TG content in the microsomal fraction tended to be higher in the Plin2−/− mice as compared with the wild-type, though the difference did not reach statistical significance, unlike what we found previously, in which the microsomal TG was elevated in the Plin2−/− mice compared to the wild-type under basal conditions, despite an overall reduction of hepatic TG in Plin2−/− mice [14].
To determine whether β-oxidation was altered in Plin2−/− mice, we measured serum β-HBA, a ketone body produced after β-oxidation in the liver. The serum β-HBA level of Plin2−/− mice at 24 h after PH was significantly lower than that of the wild-type mice (Fig. 3D); a similar trend was found at 12 h after PH, which corroborates with the down-regulation of β-oxidation genes associated with Plin2 deficiency suggesting a lowering of β-oxidation in the liver after PH in Plin2−/− mice.
Liver regeneration after carbon tetrachloride treatment is impaired in Plin2−/− mice
To determine if the delayed liver regeneration is unique to PH in the Plin2−/− mice or if PLIN2 also plays a role in liver regeneration in response to toxic liver injury, we treated the wild-type and Plin2−/− mice with carbon tetrachloride to induce acute liver injury.
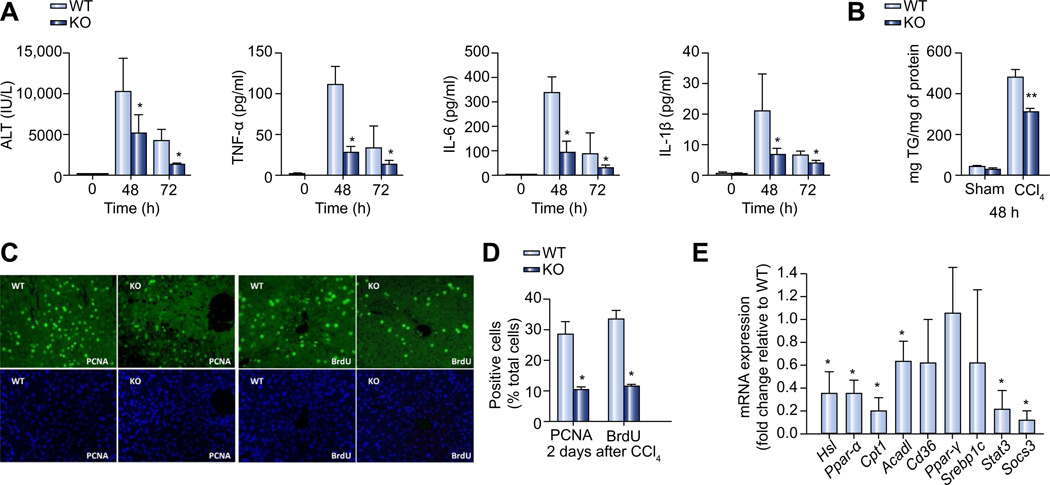
As anticipated, the serum markers of liver injury and inflammation, ALT, IL1β, IL-6, and TNF-α were all elevated at 48 h after CCl4 treatment (Fig. 4A) in the wild-type mice. The levels of these inflammatory markers subsided at 72 h after treatment (Fig. 4A). Plin2−/− mice that received CCl4 treatment had a similar temporal profile of inflammatory response, but with an attenuated induction of serum markers of inflammation, as compared to wild-type mice. This is quite different from the inflammatory responses after PH (Fig. 2D) in which these two groups of mice had comparable serum cytokine expression.
Fig. 4. Impaired liver regeneration after CCl4 treatment in Plin2−/− mice.
(A) Plasma liver enzyme (ALT) and inflammatory cytokine (TNF-α, IL-6, and IL-1β) levels before (0 h) and 48 and 72 h after CCl4 treatment in wild-type and Plin2−/− mice (n = 6). (B) Hepatic TG content in wild-type and Plin2−/− mice 48 h after CCl4 or sham treatments. (C) PCNA and BrdU staining (upper panel) of liver sections from 48 h after CCl4 treatment in wild-type and Plin2−/− mice. DAPI (lower panel) staining was used to identify nuclei and for cell counting. (D) Percent of PCNA and BrdU positive cells 2 days (48 h) after CCl4 treatment in the wild-type and Plin2−/− mice. (E) Quantitative analysis of mRNA expression 72 h after CCl4 treatment. Relative mRNA expression in Plin2−/− mice was shown by using house-keeping genes as controls and expressed as ratios to the levels of the wild-type. *p <0.05 and **p <0.01 between wild-type and Plin2−/− mice (n = 6). WT, wild-type; KO, Plin2−/−. (This figure appears in colour on the web.)
The hepatic TG levels increased dramatically 48 h after CCl4 treatment in the wild-type mice with attenuated induction in Plin2−/− mice (60% of the wild-type) (Fig. 4B).
Next, we evaluated hepatocyte proliferation by BrdU incorporation at 48 h after CCl4 treatment. Similar to our findings in response to PH, hepatocyte proliferation in response to CCl4-mediated injury was impaired in Plin2−/− mice. About 30% of the hepatocytes underwent cell proliferation (which were BrdU-positive; Fig. 4C) in the wild-type mice at 48 h post-PH, while only 12% of cells in Plin2−/− mice were BrdU-positive (Fig. 4D). Immunostaining for PCNA, a well-established marker for proliferating cells, validated our findings that hepatocyte proliferation in response to CCl4-mediated injury is impaired in Plin2−/− mice (Fig. 4C and D).
In order to understand the molecular changes associated with the differential response of wild-type and Plin2−/− mice to CCl4 hepatotoxicity, we harvested liver tissues at 72 h post-CCl4 treatment and analyzed these by quantitative RT-PCR. Mirroring our observations in response to PH, mRNA expression of several genes associated with lipolysis (Hsl) and β-oxidation (Ppar-α, Cpt1α, and Acadl) were downregulated in the liver of Plin2−/− mice 72 h after CCl4 treatment (Fig. 4E). The expression of lipogenic genes Ppar-γ and Srebp1c, and fatty acid transporter, Cd36 were comparable between wild-type and Plin2−/− mice. In addition, the expression of Stat3 and Socs3 were downregulated in the Plin2−/− mice compared to those of the wild-type at 72 h after CCl4 treatment (Fig. 4E).
Discussion
It is well known that transient hepatosteatosis occurs at 12 to 48 h after PH in rodents [4,5,26]. Early studies suggested a role for uptake of free fatty acids and subsequent storage as triglycerides in the lipid accumulation in regenerating livers [20,28,29]. Delahunty et al. contend that lipid accumulation, early on during liver regeneration, is a consequence of excessive esterification of systemically derived free fatty acids into triglycerides at rates in excess of the liver’s ability to secrete as β-lipoproteins and at later times the rate of lipid secretion matches the free fatty acid flux, thus preventing further accumulation [30].
In this study, we first investigated the time course of changes in lipid metabolism in response to PH in wild-type mice. During early liver regeneration, the expression of genes for de novo FA synthesis was upregulated at 3 to 6 h, and mRNA for CD36 was increased from 12 to 24 h after PH. Furthermore, serum FA, and glycerol were increased after PH, and drastically decreased at 24 h after PH. These findings suggest that de novo lipogenesis in the liver provides the initial supply of fatty acids for TG synthesis at the early phase (0–6 h) and subsequently uptake of lipolytic products from peripheral tissues serve as a source of hepatic lipids.
The transcript for the major hepatic LD gene Plin2 is also upregulated at 12 h after PH. Our studies corroborate previous reports that lipid droplet formation and triglyceride accumulation peak at 24 h post-PH (Fig. 1C). We noted that the PLIN2 protein level started to increase at 3 h after PH when the transcription of Plin2 was unchanged in the wild-type mice (Fig. 1A and B). It appears that both transcriptional as well as post-transcriptional mechanisms influence PLIN2 protein levels in regenerating livers, as PLIN2 protein is known to be regulated by the proteasome degradation pathway, which may be modulated by the availability of intracellular lipids [31,32].
Absence of Plin2 was previously shown to lead to marked reduction in hepatic TG storage. We therefore took advantage of the Plin2−/− mouse model and compared the effect of presence or absence of Plin2 expression on liver regeneration after PH. Plin2−/− mouse liver has a significantly lower TG content than wild-type after PH [14,15]. The Plin2−/− hepatocytes recovering from PH maintain the ability to form LDs, however, the size of post-PH hepatic LDs is more heterogeneous in Plin2−/− mice, and the number of LDs is substantially lower than that in wild-type mice, an observation similar to what we noted previously in Plin2−/− mice [14,15].
Fatty acid synthesis and lipid transfer gene expression profiles were similar between the Plin2−/− and wild-type mice prior to and at various times after PH. The serum cytokine levels were also comparable between Plin2−/− and wild-type. On the other hand, rate of VLDL secretion in Plin2−/− mice was significantly higher than that of wild-type mice. We have reported similar results for these mice at normal state [14], suggesting that Plin2−/− mice had unbridled VLDL secretion both before and after PH compared to wild-type mice. Although at the basal condition before PH, the rate limiting enzyme in hepatic VLDL production, MTTP, is upregulated in Plin2−/− mice, its level at 24 h after PH is not different from that of the wild-type (Fig. 2F). This indicates that other factors may be involved in the elevated VLDL secretion under PH condition in the Plin2−/− mice. Therefore, even at a time when the cell needs TG as a source of energy for tissue repair and cellular proliferation, Plin2−/− mice show a persistent defect in their ability to retain sufficient amounts of fuel as a source of energy for the tissue growth and repair process.
β-oxidation was not altered in Plin2−/− mice under basal conditions [14]. However, the hepatic mRNA levels of lipolytic enzyme (HSL), β-oxidation (CPT1a, LCAD, HADHα), and key transcriptional regulators of lipid metabolism (PPARα, and PPARγ) were uniformly downregulated in Plin2−/− mice compared to wild-type mice 36 h after PH (Fig. 3A). The difference in gene expression was accompanied by a distinct delay in liver regeneration and impaired hepatocyte proliferation response in Plin2−/− mice after PH (Fig. 1F). It is likely that the elevated lipolysis and β-oxidation induced by PH, 36–48 h post PH, are necessary for optimal liver regeneration in wild-type mice. Reduced hepatic lipids after PH in Plin2−/− mice may hamstring the hepatocytes from regulating the genes involved in lipolysis and β-oxidation that are required for full recovery.
Intracellular partitioning of lipids was different in Plin2−/− mouse livers as compared with those of wild-type mice. Not only was TG affected, but FA content in the cytosolic fraction of Plin2−/− mice was also reduced compared with wild-type mouse livers after PH. It is possible that reduced cytosolic FA in the hepatocytes of Plin2−/− mice could adversely affect the optimal activation of PPARα, which controls the expression of genes involved in β-oxidation.
In response to partial hepatectomy, caveolins, key components of caveolae, exhibit a dramatic redistribution from the cell surface to the newly formed lipid droplets within hepatocytes. Cav-1−/− mice fail to accumulate hepatic TG and show impaired liver regeneration and decreased survival after PH [13]. The distinct defects in hepatic triglyceride accumulation and hepatocyte proliferation in response to PH in Cav-1−/− mice primarily stems from its genetic defect in lipid droplet formation [13,33]. It is interesting to note the similarities between Cav-1−/− and Plin2−/− mice in their response to PH, i.e., inherent defects in efficient lipid droplet formation early on in response to PH have a profound impact on the subsequent hepatocyte proliferative response. Our finding of delayed hepatocyte proliferation in response to PH in Plin2−/− mice further underscores the importance of optimal lipid droplet formation and triglyceride accumulation in regenerating livers.
Impaired β-oxidation was also found to hamstring liver regeneration after PH. Adiponectin-deficient mice that show downregulation of genes necessary for efficient β-oxidation also have impaired liver regeneration [33]. PPARα-null mice in the sv129 genetic background have reduced β-oxidation, increased hepatic TG content, and suppressed liver regeneration capacity [10]. Interestingly, however, Newberry et al. [34] found that PPARα-null mice in the C57BL/6 genetic background have increased hepatic TG content but normal post-PH liver regeneration. The same authors used other mouse models with altered hepatic TG content, and showed that hepatic TG content does not appear to affect liver regeneration and hepatocyte proliferation after PH in the models tested. In view of the different findings in different laboratories, it is reasonable to conclude that absolute levels of hepatic TG content alone may not control the capacity of liver regeneration following PH. It is likely a combination of alterations of hepatic TG content and the underlying genetic and metabolic changes that may impact the capacity of the liver to regenerate optimally after PH. Liver regeneration is a multi-factorial process under the influence of numerous humoral and hemodynamic factors which activate multiple redundant cell signaling cascades and pathways essential for survival and proliferation in order to ensure efficient liver regeneration. Therefore, it is not surprising that despite delays in hepatocyte proliferation in Plin2−/−, as evidenced by attenuated induction of markers of proliferation at 36 h post-PH, restoration of liver mass is comparable to wild-type mice at 168 h post-PH.
Finally, we examined the liver regeneration in these mice in a model based on CCl4 liver toxicity. Again, Plin2−/− mice showed a delayed liver regenerative response after CCl4 treatment compared to wild-type mice (Fig. 4C). However, the mechanism may not be exactly the same as that of PH. Although the gene expression profiles suggested a defect in β-oxidation in Plin2−/− mice (Fig. 4E), we noted that the inflammatory cytokine levels such as Tnf-α, IL-1β, and IL-6 were also attenuated in Plin2−/− mice (Fig. 4A), whereas these factors were not different between Plin2−/− and the wild-type mice in the PH model. We speculate that the CCl4 may have enticed a more global inflammatory response in the animals resulting in a difference in plasma cytokine profile compared to PH. As these cytokines are known to stimulate liver regeneration [35], it is likely that the delayed liver regeneration in Plin2−/− mice responding to CCl4 toxicity was also due to the attenuated levels of these cytokines – an observation that should be investigated in the future.
We also noticed that the STAT3 mRNA level was decreased 72 h after CCl4 treatment in the Plin2−/− compared to the wild-type mice (Fig. 4E). Although STAT3 exerts its function through protein phosphorylation, a previous study has shown that liver specific Stat3 knockout mice had decreased DNA synthesis in response to CCl4 treatment compared to the wild-type mice [36].
In summary, our results suggest that Plin2 upregulation at 12 h after PH may be an important component of a concerted homeostatic response to liver injury and loss of liver mass leading to LD accumulation within hepatocytes. The early accumulation of lipids within hepatocytes could serve as a source of β-oxidation during the peak hepatocyte proliferative phase in regenerating livers. Plin2−/− mice display decreased hepatic lipid accumulation and impaired liver regeneration. Lipid insufficiency in the liver of Plin2−/− mice is not the result of decreased lipogenesis or lipid uptake, but the consequence of an unbridled VLDL secretion. Plin2−/− mice also exhibit decreased cytosolic FA levels and attenuated β-oxidation in regenerating livers, as evidenced by decreased expression of β-oxidation-related genes and lower serum β-HBA levels post-PH. Collectively, a combination of these defects in lipid accumulation and utilization during critical periods of liver regeneration may compromise the liver regeneration capacity in Plin2−/− mice after PH. Moreover, our findings suggest that optimal subcellular distribution of lipids maintained by Plin2 is necessary for optimal liver regeneration after PH. These findings highlight the significance of metabolic control of liver regeneration and provide additional new insights on the role of lipid droplet-associated proteins in efficient liver regeneration in response to loss of liver mass and toxic liver injury.
Supplementary Material
Acknowledgements
This research was supported in part by NIH grants HL51586 (to LC), DK84495 (to BHC), DK069558 (to ST) and by the Diabetes & Endocrinology Research Center (P30DK079638) at Baylor College of Medicine. LC was also supported by the Betty Rutherford Chair for Diabetes Research from St. Luke’s Episcopal Hospital and Baylor College of Medicine.
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2013.07.025.
References
- 1.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 3.Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 4.Glende EA, Jr, Morgan WS. Alteration in liver lipid and lipid fatty acid composition after partial hepatectomy in the rat. Exp Mol Pathol. 1968;8:190–200. doi: 10.1016/0014-4800(68)90015-4. [DOI] [PubMed] [Google Scholar]
- 5.Farrell GC. Probing Prometheus: fat fueling the fire? Hepatology. 2004;40:1252–1255. doi: 10.1002/hep.20522. [DOI] [PubMed] [Google Scholar]
- 6.Todo S, Demetris AJ, Makowka L, Teperman L, Podesta L, Shaver T, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47:903–905. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi H, Uetsuka K, Okada T, Nakayama H, Doi K. Impaired liver regeneration after partial hepatectomy in db/db mice. Exp Toxicol Pathol. 2003;54:281–286. doi: 10.1078/0940-2993-00265. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis RA, Markiewski MM, Taub R, Lambris JD. A high-fat diet impairs liver regeneration in C57BL/6 mice through overexpression of the NF-kappaB inhibitor, IkappaBalpha. Hepatology. 2005;42:1148–1157. doi: 10.1002/hep.20879. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SP, Yoon L, Richard EB, Dunn CS, Cattley RC, Corton JC. Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice. Hepatology. 2002;36:544–554. doi: 10.1053/jhep.2002.35276. [DOI] [PubMed] [Google Scholar]
- 11.Beyer TA, Xu W, Teupser D, Auf dem KU, Bugnon P, Hildt E, et al. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40:1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, et al. Caveolin-1 is essential for liver regeneration. Science. 2006;313:1628–1632. doi: 10.1126/science.1130773. [DOI] [PubMed] [Google Scholar]
- 14.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, et al. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang BH, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J Lipid Res. 2010;51:2132–2142. doi: 10.1194/jlr.M004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul A, Chang BH, Li L, Yechoor VK, Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res. 2008;102:1492–1501. doi: 10.1161/CIRCRESAHA.107.168070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell TD, Palmer CA, Orlicky DJ, Bales ES, Chang BH, Chan L, et al. Mammary glands of adipophilin-null mice produce an amino-terminally truncated form of adipophilin that mediates milk lipid droplet formation and secretion. J Lipid Res. 2008;49:206–216. doi: 10.1194/jlr.M700396-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Imanishi Y, Sun W, Maeda T, Maeda A, Palczewski K. Retinyl ester homeostasis in the adipose differentiation-related protein-deficient retina. J Biol Chem. 2008;283:25091–25102. doi: 10.1074/jbc.M802981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Fex G, Olivecrona T. Secretion of triglyceride by the liver after partial hepatectomy. Biochim Biophys Acta. 1968;164:424–426. doi: 10.1016/0005-2760(68)90167-7. [DOI] [PubMed] [Google Scholar]
- 21.Gove CD, Hems DA. Fatty acid synthesis in the regenerating liver of the rat. Biochem J. 1978;170:1–8. doi: 10.1042/bj1700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukumoto S, Fujimoto T. Deformation of lipid droplets in fixed samples. Histochem Cell Biol. 2002;118:423–428. doi: 10.1007/s00418-002-0462-7. [DOI] [PubMed] [Google Scholar]
- 23.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 24.Sudo K, Yamada Y, Saito K, Shimizu S, Ohashi H, Kato T, et al. TNF-alpha and IL-6 signals from the bone marrow derived cells are necessary for normal murine liver regeneration. Biochim Biophys Acta. 2008;1782:671–679. doi: 10.1016/j.bbadis.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–970. doi: 10.1002/hep.510280410. [DOI] [PubMed] [Google Scholar]
- 27.Kimmel AR, Brasaemle DL, Ndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camargo AC, Cornicelli J, Cardoso SS. Alteration in lipid content of the liver in the rat after partial hepatectomy. Proc Soc Exp Biol Med. 1966;122:1151–1154. doi: 10.3181/00379727-122-31349. [DOI] [PubMed] [Google Scholar]
- 29.Infante R, Alcindor G, Raisonnier A, Petit D, Polonovski J, Caroli J. Biosynthesis of plasma lipoproteins by the regenerating liver. Biochim Biophys Acta. 1969;187:335–344. [PubMed] [Google Scholar]
- 30.Delahunty TJ, Rubinstein D. Accumulation and release of triglycerides by rat liver following partial hepatectomy. J Lipid Res. 1970;11:536–543. [PubMed] [Google Scholar]
- 31.Masuda Y, Itabe H, Odaki M, Hama K, Fujimoto Y, Mori M, et al. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res. 2006;47:87–98. doi: 10.1194/jlr.M500170-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, Dorward H, et al. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem. 2005;280:42841–42847. doi: 10.1074/jbc.M506569200. [DOI] [PubMed] [Google Scholar]
- 33.Pol A, Martin S, Fernandez MA, Ferguson C, Carozzi A, Luetterforst R, et al. Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol Biol Cell. 2004;15:99–110. doi: 10.1091/mbc.E03-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, et al. Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology. 2008;48:1097–1105. doi: 10.1002/hep.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohm F, Kohler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2:294–305. doi: 10.1002/emmm.201000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.