Abstract
BACKGROUND
Lonafarnib is an oral selective farnesyltransferase inhibitor, a class of drugs which have shown activity in preclinical glioma models. Temozolomide (TMZ) is an alkylating agent that is the first-line chemotherapy for glioblastoma.
METHODS
The current study combined the cytotoxic agent TMZ with the cytostatic agent lonafarnib for patients with recurrent glioblastoma to establish a maximum tolerated dose (MTD) of the combination and its preliminary efficacy. Three dose cohorts of lonafarnib were studied in the phase 1 component of the trial (100 mg twice daily [bid], 150 mg bid, and 200 bid) with dose-dense schedule of TMZ (150 mg/m2 daily) administered in an alternating weekly schedule. After establishing the MTD of lonafarnib, a subsequent expansion phase 1b was undertaken to evaluate efficacy, primarily measured by 6-month progression-free survival (PFS-6).
RESULTS
Fifteen patients were enrolled into the phase 1 component and 20 patients into the phase 1b component. The MTD of lonafarnib in combination with TMZ was 200 mg bid. Among the patients enrolled into the study, 34 were eligible for 6-month progression evaluation and 35 patients were evaluable for time-to-progression analysis. The PFS-6 rate was 38% (95% confidence interval [CI] = 22%, 56%) and the median PFS was 3.9 months (95% CI = 2.5, 8.4). The median disease-specific survival was 13.7 months (95% CI = 8.9, 22.1). Hematologic toxicities, particularly lymphopenia, were the most common grade 3 and 4 adverse events. There were no treatment-related deaths.
CONCLUSIONS
These results demonstrate that TMZ can be safely combined with a farnesyltransferase inhibitor and that this regimen is active, although the current study cannot determine the relative contributions of the 2 agents or the contribution of the novel administration schedule.
Keywords: glioblastoma, recurrent, lonafarnib, temozolomide, farnesyltransferase inhibitor
INTRODUCTION
Glioblastomas are rapidly growing primary brain tumors, associated with a high degree of morbidity and mortality. Current management for patients with newly diagnosed glioblastoma includes surgery followed by external beam radiation and concurrent and adjuvant temozolomide (TMZ) therapy.1 Although an international randomized trial confirmed the efficacy of this regimen compared with radiation alone, recurrent tumor growth is the rule. At present, there is no consensus on the optimal treatment approach for patients with recurrent glioblastoma multiforme, especially when recurrence occurs after exposure to TMZ (which is used as first-line treatment), with most patients have been previously treated with 6 or more cycles of adjuvant therapy or stopped early because of tumor progression.2,3 Prior to the establishment of the current standard of concurrent chemoradiation for newly diagnosed glioblastoma, several trials evaluated the efficacy of TMZ in patients with recurrent glioblastoma who were TMZ-naive. In a randomized phase 2 study that compared TMZ with oral procarbazine, patients receiving TMZ had a 6-month progression-free survival (PFS-6) of 21%, which was significantly better than those treated with procarbazine (PFS-6 = 8%).4 More recently, 2 studies in which patients treated with conventional first-line treatment with TMZ using novel dosing strategies were challenged at recurrence with TMZ at a daily dose of 50 mg/m2 showed clinical benefit with PFS-6 of 17% and 23.9%.5,6 In addition, a study that treated patients with recurrent glioblastoma using the “week on/week off” dosing schedule of TMZ at a dose of 150 mg/m2/day demonstrated clinical benefit with a PFS-6 of 43.8%.7
There have been many studies of signal transduction modulating agents for treatment of recurrent glioblastoma. Single-agent studies have demonstrated only modest results, leading to speculation that combination regimens with either 2 signal transduction modulators or with conventional cytotoxic agents may be more effective. Lonafarnib is a farnesyltransferase inhibitor (FTI), and its potential activity as an anticancer agent is related to potential inhibition of the farnesylation of a variety of intracellular proteins, such as RhoB, RAS, prelamin A, prelamin B, and CCAX phosphatase, which are involved in cell proliferation and homeostasis.8 Although FTIs were developed initially as inhibitors of the Ras pathway, subsequent studies demonstrated that alternative pathways such as geranyl geranylation are not affected by FTIs, thereby diminishing the impact of FTIs on Ras. However, with the broad impact of FTIs, these agents may affect an upstream component of the proliferation cascade, leading to a cytostasis.9 In vitro studies using several established glioblastoma cell lines confirm that treatment with an FTI significantly inhibits proliferation, causes cell cycle arrest, and can stimulate apoptosis.10,11
This study combined the cytotoxic agent TMZ with the cytostatic agent lonafarnib. The rationale for this drug combination relies on the ability of the FTI to cause cell cycle arrest.8 Subsequent cessation of the drug should then lead to an increase in farnesyltransferase activity, resulting in a rapid increase in tumor cell proliferative activity and a high percentage of cells in S phase, potentially maximizing the efficacy of the alkylating effect of TMZ.
MATERIALS AND METHODS
Patients
The study enrolled adult patients (≥18 years), with a Karnofsky performance status (KPS) score of ≥60, who had histologically confirmed supratentorial glioblastoma or gliosarcoma. Patients were required to have unequivocal radiographic evidence of tumor progression or recurrence, as determined by magnetic resonance imaging or computed tomography scan, after radiation therapy. Additional eligibility criteria included adequate bone marrow, renal, and liver function. Patients might have had as many as 2 prior chemotherapy regimens for recurrent or progressive tumor but could not have had prior treatment with an FTI (lonafarnib or tipifarnib). Patients in phase 1b expansion were required to have a treatment history of a minimum of 2 cycles of adjuvant TMZ. An imaging study, to serve as baseline, had to be performed within 14 days prior to registration and on a steroid dose that has been stable or decreasing for at least 5 days. Patients must have recovered from the toxic effects of prior therapy. Patients who had undergone recent resection of recurrent or progressive tumor were eligible even if they did not have radiologically measurable disease, provided they had completely recovered from surgery. Patients were not permitted to use enzyme-inducing anti-epileptic drugs (EIAEDs) because of well-established drug-drug interactions that lead to alterations in the pharmacokinetics of lonafarnib. Patients changing from EIAEDs to non-EIAEDs had to be off EIAEDs for at least 72 hours prior to the initiation of treatment. Patients with a history of any other cancer (except nonmelanoma skin cancer or carcinoma in situ of the cervix) were ineligible unless in complete remission and off of all therapy for that disease for a minimum of 3 years. Subjects deemed to have progressed immediately after completing concurrent TMZ and radiation therapy were not considered for entry in order to reduce possibility of “pseudo-progression.” All patients were required to provide informed consent, and the institutional review board approved the study.
Treatment Plan and Endpoints
TMZ at a dose of 150 mg/m2/day was administered orally, after fasting for 1 hour, once a day for 7 consecutive days (days 1 through 7 and days 15 through 21) of each 28-day course of therapy. Courses were repeated every 28 days. Patients might be premedicated with prochlorperazine (10 mg) orally 30 to 60 minutes before temozolamide dosing and repeated every 4 to 6 hours as necessary to prevent nausea and vomiting. Lonafarnib was given orally, with water, in the morning and in the evening for 7 consecutive days (days 8 through 14 and days 22 through 28) 1 hour before or after morning and evening meals. Courses were repeated every 28 days.
Phase 1 Treatment Plan
The primary endpoint of the phase 1 study was to define the maximum tolerated dose (MTD) of lonafarnib, keeping the dose and schedule of TMZ unchanged. The dose of lonafarnib was escalated according to a standard 3 + 3 design, starting at dose level 0 with 100 mg bid. Further prespecified dose escalations were 150 mg bid and 200 mg bid for 7 consecutive days. The MTD was defined as the dose at which no more than 0 of 3 or 1 of 6 patients experienced a dose-limiting toxicity (DLT) with the next higher dose having at least 2 of 3 or 2 of 6 patients encountering DLT. The definitions of DLTs were based on the Common Terminology Criteria for Adverse Events (version 3) and included the following events: grade 4 thrombocytopenia, grade 4 anemia and/or grade 4 neutropenia or any nonhematologic grade 3 toxicity, excluding alopecia but including grade 3 vomiting or diarrhea uncontrolled by supportive therapy.
Phase 1b Treatment Plan
The primary endpoint of the phase 1b component was to further elucidate the tolerability of an alternating 7-day on, 7-day off combination of TMZ and lonafarnib in patients who had failed prior TMZ treatment, defined as having had tumor progression/recurrence while under or having recently completed treatment with adjuvant TMZ.
Secondary endpoints included treatment response as measured by overall PFS, the 6-month PFS rate, objective response, and overall survival from study entry. Patients enrolled on the phase 1b component of this trial were treated at the established MTD from the phase 1 study. Therefore, the dose of TMZ was 150 mg/m2 orally administered on a 7-day on, 7-day off schedule and alternating with lonafarnib orally administered at 200 mg twice a day using a 7-day on, 7-day off schedule that did not overlap with TMZ administration. Significant (grade 3 or 4) hematologic and nonhematologic treatment-related toxicities resulted in either treatment cessation or treatment toxicity-specific dose reductions.
Patient Evaluation During the Study
Baseline evaluation included a physical examination with a neurological evaluation, complete blood counts, blood chemistries including renal and hepatic function, a serum pregnancy test (where appropriate) and tumor imaging with either magnetic resonance (preferred) or computed tomography. Patients had blood counts and blood chemistries measured on day 22 (±3 days) of each course (cycle duration of 28 days) and prior to each new course; a repeat tumor imaging study was performed prior to the initiation of every odd course or as clinically indicated. Neurologic examinations were performed within 14 days prior to the initiation of every odd course, or at any time that was clinically indicated. Patients were evaluated for adverse events weekly during their first cycle of therapy for the determination of the MTD. Adverse events for subsequent cycles were used for dose modifications but did not affect the determination of the MTD. Clinical response was assessed using neurological examination and KPS. Radiological response was assessed using McDonald criteria.12 Toxicities were recorded and graded according to the CTCAE, version 3.0.
Statistical Analyses
Phase 1
The MTD of the combination of lonafarnib and TMZ was defined as the dose at which 0 of 3 or 1 of 6 patients experience DLT with the next higher dose having at least 2 of 3 or 2 of 6 patients encountering DLT. Three additional patients were tested at the putative MTD to confirm the safety of this dose level.
Phase 1b
Efficacy analyses combined outcomes from patients enrolled on both the phase 1 and phase 1b components of the clinical trial. For overall efficacy combining the phase 1 with phase 1b components, a well-established historical data set was used to define efficacy where the proportion of patients remaining alive and free from progression at 6 months was 15% (95% confidence interval [CI] ranged from 10% to 19%).11 We therefore set p0 = 15% and p1 = 30% using a doubling of the historical 6-month PFS rate. Based on these design parameters, a 2-stage design would require that at least 4 of the initial 15 patients are without progression at 6 months. If the study continued accrual to a total of 34 patients, with 26 treated at the MTD, the combination regimen in phase 2 testing will be considered as effective if more than 7 of 34 patients have not progressed at 6 months. This provides a 1-tailed alpha = 0.11 with a power of 79% for the 30% alternative. The Kaplan-Meier survival function was used to estimate PFS and survival.
RESULTS
Baseline characteristics for the patients enrolled on both the phase 1 and phase 1b components of the study are provided in Table 1.
TABLE 1.
Patient Characteristics
| Characteristic | Phase 1 | Phase 1b | Total |
|---|---|---|---|
| Sex | |||
| Male | 10 | 13 | 23 |
| Female | 3 | 8 | 11 |
| Age, y, median (range) | 47 (29–68) | 51 (31–69) | 50 (29–69) |
| Karnofsky performance status | |||
| 90–100 | 9 | 14 | 23 |
| 60–80 | 4 | 7 | 11 |
| TMZ historya | |||
| A | 2 | 0 | 2 |
| B | 7 | 15 | 22 |
| C | 3 | 5 | 8 |
| D | 1 | 1 | 2 |
| Total | 13 | 21 | 34 |
Temozolomide (TMZ) history: A = No history of treatment with TMZ. B = Tumor progression while receiving adjuvant TMZ before completion of 6 cycles. Only 8 patients in this study had imaging worsening within the 3 months following completion of chemoradiation (the “pseudoprogression window”). Five of these had pathologically proven progression from either a biopsy or tumor resection. C = Tumor progression while receiving extended adjuvant TMZ beyond the standard 6 cycles but before completion of adjuvant treatment. D = Tumor progression after completion of adjuvant treatment and a treatment-free interval of greater than 2 months
Phase 1
A total of 15 patients were accrued to the phase 1 component between December 22, 2004, and July 5, 2005 (6 patients to the first dose level 0, 3 patients to dose level 1, 6 patients to dose level 2). There were no treatment-related dose-limiting toxicities. However, 1 of the first 3 patients at dose level 1 died on day 29 of an unrelated cardiac event. An additional 3 patients were accrued to this dose level to confirm safety of the dose. Further dose escalation ensued without additional cardiac events. As planned, 3 additional patients were enrolled onto the third dose level (dose 2) to confirm treatment tolerability.
Phase 1b
Twenty-one patients were accrued and treated on the phase 1b expansion component between July 27, 2005, and February 9, 2007. All patients had received prior TMZ and were considered to have failed TMZ treatment. Importantly, only 8 patients on our study had imaging worsening within the 3 months following completion of chemoradiation (the “pseudoprogression window”). Five of these had pathologically proven progression from either a biopsy or tumor resection;
All patients were treated with alternating 7-day on, 7-day off combination of TMZ and lonafarnib (200 mg bid). Combining the phase 1 and phase 1b components to evaluate overall treatment efficacy yields 34 evaluable patients for evaluation progression at 6 months, because 2 patients were excluded (1 with cardiac death at day 29, 1 withdrew consent). In this efficacy cohort, 2 patients had no prior TMZ exposure, 22 patients had tumor progression while receiving adjuvant TMZ before completion of 6 cycles of adjuvant TMZ, 8 patients had tumor progression while receiving extended adjuvant TMZ beyond the standard 6 cycles but before completion of adjuvant treatment, and 2 patients had progression after completion of adjuvant treatment and a treatment-free interval of greater than 2 months.
Treatment Results
The average number of treatment cycles on the study was 7.8 (median = 3, range = 1–69). For all patients, the Kaplan-Meier estimated median disease-specific survival from study entry was 13.7 months (95% CI = 8.9, 22.1) (Fig. 1) and median progression-free survival was 3.9 months (95% CI = 2.5, 8.4) (Fig. 2). The 6-month PFS rate was approximately 38%. One patient remains progression-free at 3.5 years, this patient underwent partial tumor resection prior to enrollment that confirmed recurrent tumor. The residual enhancing tumor was 2.3 cm2 and resolved with treatment to achieve a radiographic complete response.
Figure 1.
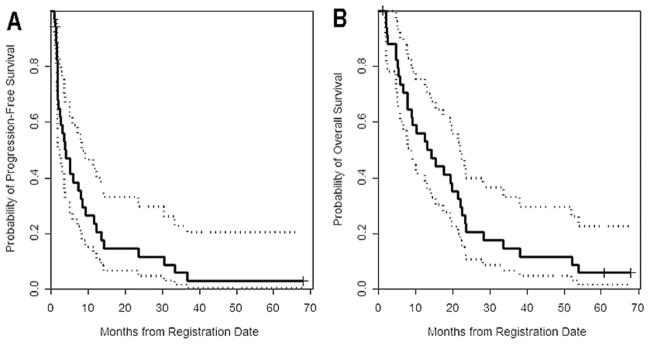
(A) Kaplan-Meier progression-free survival curve is shown for all patients included in the study. (B) Kaplan-Meier overall survival curve is shown for all patients included in the study.
Figure 2.
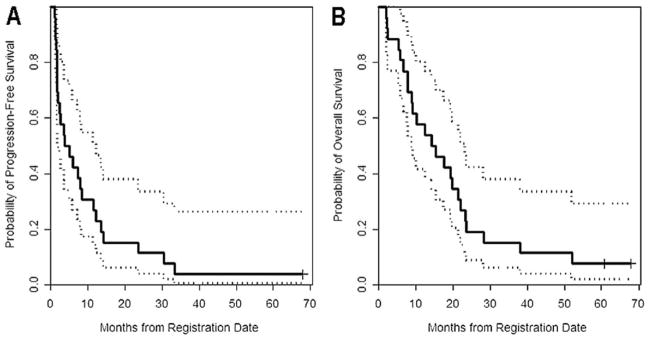
(A) Kaplan-Meier progression-free survival curve is shown for the 26 patients who were treated with the maximal tolerated dose. (B) Kaplan-Meier overall survival curve is shown for the 26 patients who were treated with the maximal tolerated dose.
For the 26 patients who were treated at the MTD, median disease-specific survival was 14.9 months (95% CI = 8.9, 23.3) and 6-month PFS was 42.3% (95% CI = 27%, 66%) (Fig. 2).
Six patients (17.6%) demonstrated a partial tumor response as defined by a reduction in the size of the enhancing lesion by >50% using cross-sectional area, while on stable or decreasing corticosteroid dose (McDonald criteria) and 2 patients (5.9%) achieved a radiographic complete response. In addition, 16 (47.1%) patients had stable disease. The remaining 10 (29.4%) patients demonstrated progressive disease.
Factors that were associated with a better prognosis included patient age (<60) for overall survival (P = .03). There was a trend toward longer survival in the group of patients who had early (< 6 cycles of adjuvant TMZ) progression compared with the other groups; however, this difference was not statistically significant. High KPS had a nonsignificant trend toward improved outcome. Other factors such as sex and tumor resection before initiating treatment did not impact prognosis.
Safety and Toxicity
Grade 3 and 4 lymphopenia were the most common toxicity occurring in 25 patients (78% of patients, 48% of cycles), with only one case of opportunistic infection (Table 2). Serious neutropenia and thrombocytopenia occurred in 8 (25%) and 7 (22%) patients and 6.7% and 4.4% of all cycles, respectively. Six patients stopped treatment and 5 patients required dose reduction, secondary to hematologic toxicity. The most common serious nonhematologic toxicity was fatigue (grade 3 or 4 occurring in 2.4% of all cycles). There were no treatment-related deaths.
TABLE 2.
Grade 3 or 4 Adverse Events Possibly, Probably, or Definitely Related to Study Drugsa
| Toxicity | Grade
|
||
|---|---|---|---|
| 3 | 4 | Total | |
| ALT/SGPT | 1 | 0 | 1 |
| Diarrhea | 1 | 0 | 1 |
| Dyspnea | 1 | 0 | 1 |
| Electrolyte disturbance | 7 | 0 | 7 |
| Esophagitis | 2 | 0 | 2 |
| Fatigue | 5 | 0 | 5 |
| Hemoglobin | 2 | 0 | 2 |
| Leukocytes | 12 | 3 | 15 |
| Lymphopenia | 81 | 29 | 110 |
| Neutrophils (ANC/AGC) | 14 | 2 | 16 |
| Opportunistic infection | 1 | 0 | 1 |
| Pain | 2 | 0 | 2 |
| Platelets | 7 | 4 | 11 |
| Pneumonitis | 1 | 1 | 2 |
| VZV herpetic dermatitis | 1 | 0 | 1 |
| Total | 138 | 39 | 177 |
Abbreviations: ALT, alanine aminotransferase; ANC/AGC, absolute neutrophil count/absolute granulocyte count; SGPT, serum glutamic pyruvic transaminase; VZV, varicella zoster virus.
A total of 251 treatment cycles.
DISCUSSION
Single-agent administration of signal transduction modulators has not proven to be effective in recurrent glioblastoma, even with agents targeting pathways that are thought to be important in glioma tumor biology.13 Single-agent use of FTIs in malignant gliomas has not demonstrated significant efficacy. A phase 2 trial of tipifarnib, an oral FTI, revealed a 6-month PFS rate of 12%.14 However, combination regimens with cytotoxic chemotherapy may demonstrate improved efficacy due to additive or synergistic effects. The current study began as a dose-finding phase 1 trial, but was expanded after tumor responses were seen in patients who had previously failed initial treatment with temozolomide.
The current study, which combined lonafarnib with TMZ, demonstrated a 6-month PFS rate of 38% in all treatment doses and 6-month PFS of 42% in patients treated with the MTD. All but 2 patients had prior treatment with TMZ. These promising results do suggest that there is either synergy between the agents in this combination or that the novel schedule of treatment administration contributed to the efficacy. The theoretical basis of the alternating-week treatment is based on laboratory studies suggesting that cytostatic agents including the FTIs act to halt cell cycle activity, thereby leading to a marked increase in the mitotic cascade when the drug is stopped.15 This would potentially increase the efficacy of TMZ as the tumor cells re-enter the cell cycle after cessation of administration of the cytostatic agent. Conversely, concurrent or overlapping administration of lonafarnib with TMZ may lessen treatment efficacy. Therefore, it is not surprising that a recently published phase 1 study which evaluated combination of lonafarnib with TMZ administered on the standard 5-day dosing schedule showed minimal efficacy.16 Moreover, the aim of that phase 1 study was dose finding, and therefore, most patients did not receive treatment at the MTD.
A published preclinical study supports our findings by reporting that with in vitro brain tumor models, lonafarnib alone had limited tumor cell cytotoxicity, although it did demonstrate significant inhibition in tumor cell proliferation. In orthotropic in vivo glioblastoma murine models, combining radiation, TMZ, and lonafarnib demonstrated significant antitumor activity, with the majority of animals demonstrating a decrease in tumor volume.17
The issue of pseudoprogression is germane in all recurrent trials in glioblastoma. However, as described above, only 8 patients on our study had imaging worsening within the 3 months following completion of chemo-radiation (the “pseudoprogression window”). Five of these had pathologically proven progression from either a biopsy or tumor resection; therefore, only 3 patients who progressed within 3 months from radiation were diagnosed with progression according to imaging studies only. Therefore, we do not think the efficacy seen with this regimen is due to unrecognized “pseudoprogression.”18
Similar to the recent report from the RESCUE study that used continuous daily dosing of TMZ (50 mg/m2/day) in patients with recurrent malignant glioma, the highest response was in the patient groups who had received fewer than 6 cycles of prior TMZ and were stopped because of concerns for tumor progression.7 In our study, this subgroup had a 6-month PFS of 48%, comparing favorably to the RESCUE study, where the 6-month PFS in this subgroup was 27%. However, even in the other patients on our study who were less likely to have pseudoprogression, the 6-month PFS was 27%, strongly supporting that the treatment combination had efficacy even in truly TMZ-refractory disease.
In the RESCUE study, the group of patients who experienced progression after a treatment-free interval appeared to derive the most benefit from the therapy.7 In our study, the group of patients who were rechallenged after treatment-free interval of greater than 2 months included only 2 patients, and therefore no conclusions could be drawn regarding this group of patients.
Overall, the treatment was well tolerated, and most of the grade 3 and 4 hematologic toxicities were from lymphopenia. This lymphopenia was not associated with opportunistic infections, but this should mandate monitoring blood counts carefully in patients treated with this combination.
There are limitations in interpreting the efficacy results of this study, because it was designed as a phase 1 dose-finding study, and although included as a secondary endpoint, it was the unanticipated responses in the phase 1 component that led to the phase 1b expansion. Furthermore, the study did not compare the combination regimen with dose-dense temozolomide alone, limiting the ability to attribute efficacy to the combination. Although these results look promising, the limited scope of efficacy of lonafarnib in other cancers such as single-agent treatment in colon cancer and head and neck cancer, and in combination with carboplatin and paclitaxel for ovarian cancer, precluded additional investigation.19–21 However, a randomized study comparing the cytotoxic agent alone with the combination regimen would help elucidate the role of the combination regimen or the potential impact of enrollment of patients with unsuspected pseudoprogression.
Acknowledgments
FUNDING SOURCES
The clinical trial was supported by a research grant from Schering-Plough/Merck.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
Dr. Gilbert has received honoraria from Merck, Genetech, Abbott Laboratories and has been on the advisory board of Merck, Genetech, and Abbott Laboratories. Dr. Groves has received research support from Merck, Celldex, Genentech, and GSK; has received honoraria from Merck, Sanofi-Aventis, and Genentech; has been on the advisory board of Sanofi-Aventis and Genentech; and has been on the speakers bureau of Enzon. Dr. Puduvalli has received clinical trial funding from Merck, Celgene, and Genentech and has received an honorarium from Novartis. Dr. Yung has been a paid consultant for Novartis, Merck, Eden, and Actelion; has received research support from Novartis and Daiichi; has received honoraria from Novartis, Merck, Eden, and Actelion; and has been on the advisory board for Merck and Novartis. All other authors made no disclosures.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolmide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Fine H, Dear K, Loeffler J, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;7181:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Hau P, Koch D, Hundsberger T, et al. Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology. 2007;68:688–690. doi: 10.1212/01.wnl.0000255937.27012.ee. [DOI] [PubMed] [Google Scholar]
- 4.Yung WKA, Albright RE, Olson J, et al. A phase II study of temozolomide vs procarbazine in patients with glioblastoma multiforme in first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry JR, Rizek P, Cashman R, Morrison M, Morrison T. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the “rescue” approach. Cancer. 2008;113:2152–2157. doi: 10.1002/cncr.23813. [DOI] [PubMed] [Google Scholar]
- 6.Perry JR, Bélanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 7.Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357–3361. doi: 10.1200/JCO.2007.10.7722. [DOI] [PubMed] [Google Scholar]
- 8.Sebti SM, Hamilton AD, editors. Farnesyltransferase Inhibitors in Cancer Therapy. Totowa, NJ: Humana Press; 2001. [Google Scholar]
- 9.Prendergast GC. Farnesyltransferase inhibitors: antineoplastic mechanism and clinical prospects. Curr Opin Cell Biol. 2000;12:166–173. doi: 10.1016/s0955-0674(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 10.Feldkamp MM, Lau N, Guha A. Growth inhibition of astrocytoma cells by farnesyltransferase inhibitors is mediated by a combination of anti-proliferative, pro-apoptotic, and anti-angiogenic effects. Oncogene. 1999;18:7514–7526. doi: 10.1038/sj.onc.1203105. [DOI] [PubMed] [Google Scholar]
- 11.Glass TL, Liu TJ, Yung WK. Inhibition of cell growth in human glioblastoma cell lines by farnesyltransferase inhibitor SCH66336. Neuro Oncol. 2000;2:151–158. doi: 10.1093/neuonc/2.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 13.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 14.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 15.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 16.Desjardins A, Reardon DA, Peters KB, et al. A phase I trial of the farnesyl transferase inhibitor, SCH 66336, with temozolomide for patients with malignant glioma. J Neurooncol. 2011;105:601–606. doi: 10.1007/s11060-011-0627-0. [DOI] [PubMed] [Google Scholar]
- 17.Chaponis D, Barnes JW, Dellagatta JL, et al. Lonafarnib (SCH66336) improves the activity of temozolomide and radiation for orthotopic malignant gliomas. J Neurooncol. 2011;104:179–189. doi: 10.1007/s11060-010-0502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Kemeny N, Kelsen DP, et al. A phase II trial of farnesyl protein transferase inhibitor SCH 66336, given by twice-daily oral administration, in patients with metastatic colorectal cancer refractory to 5-fluorouracil and irinotecan. Ann Oncol. 2002;13:1067–1071. doi: 10.1093/annonc/mdf173. [DOI] [PubMed] [Google Scholar]
- 20.Hanrahan EO, Kies MS, Glisson BS, et al. A phase II study of Lonafarnib (SCH66336) in patients with chemorefractory, advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2009;32:274–279. doi: 10.1097/COC.0b013e318187dd57. [DOI] [PubMed] [Google Scholar]
- 21.Meier W, du Bois A, Rau J, et al. Randomized phase II trial of carboplatin and paclitaxel with or without lonafarnib in first-line treatment of epithelial ovarian cancer stage IIB-IV. Gynecol Oncol. 2012;126:236–240. doi: 10.1016/j.ygyno.2012.04.050. [DOI] [PubMed] [Google Scholar]


