Abstract
Numerous epidemiological studies, supported by clinical and experimental findings, have suggested beneficial effects of dietary fish or fish oil supplementation on cardiovascular health. One such experimental study showed a profound (100%) increase in myocardial efficiency (i.e. the ratio of work output to metabolic energy input) of the isolated whole heart, achieved by a corresponding decrease in the rate of myocardial oxygen consumption. However, a number of other investigations have returned null results on the latter energetic index. Such conflicting findings have motivated us to undertake a re-examination. To that effect, we investigated the effects of dietary fatty acid supplementation on myocardial mechano–energetics, with our primary focus on cardiac efficiency. We used both isolated hearts and isolated left ventricular trabeculae of rats fed with one of three distinct diets: reference (REF), fish oil-supplemented (FO) or saturated fat-supplemented (SFA). For all three groups, and at both spatial levels, we supplied 10 mm glucose as the exogenous metabolic substrate. In the working heart experiments, we found no difference in the average mechanical efficiency among the three dietary groups: 14.8 ± 1.1% (REF), 13.9 ± 0.6% (FO) and 13.6 ± 0.7% (SFA). Likewise, we observed no difference in peak mechanical efficiency of left ventricular trabeculae among the REF, FO and SFA groups: 13.3 ± 1.4, 11.2 ± 2.2 and 12.5 ± 1.5%, respectively. We conclude that there is no effect of a period of pre-exposure to a diet supplemented with either fish oil or saturated fatty acids on the efficiency of the myocardium at either spatial level: tissue or whole heart.
Introduction
Since the putative benefits of fish oil (FO) were revealed nearly 40 years ago (Bang et al. 1976), considerable evidence and counter-evidence has been published in the epidemiological, clinical and physiological literature. A particular example in the physiological field is provocative. Using isolated working rat hearts, Pepe and McLennan (2002) found no difference in work output, but a pronounced difference in the total myocardial O2 consumption (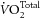 ), among rats fed with normal, FO-supplemented or saturated fat (SFA)-supplemented diets. This resulted in a dramatic (nearly two-fold) increase in myocardial efficiency (calculated from the ratio of work output to oxygen consumption) of the FO–fed rats and an equal change, but in the opposite direction, of the SFA–fed rats. This result is counter to that of several other studies. No difference in
), among rats fed with normal, FO-supplemented or saturated fat (SFA)-supplemented diets. This resulted in a dramatic (nearly two-fold) increase in myocardial efficiency (calculated from the ratio of work output to oxygen consumption) of the FO–fed rats and an equal change, but in the opposite direction, of the SFA–fed rats. This result is counter to that of several other studies. No difference in 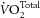 was measured in pig hearts in situ (Hartog et al. 1987) or in isolated rat hearts in vitro (de Deckere & ten Hoor, 1979, 1980; Demaison et al. 2000). The cellular mechanism underlying the proposed energetic benefits of dietary FO is also equivocal. Whereas both Yamaoka et al. (1988) and Pepe et al. (1999) reported decreased rates of O2 consumption in rat heart mitochondria in association with increased FO fatty acid incorporation into the mitochondrial membrane phospholipids, other studies have shown no changes in mitochondrial respiratory rate in FO fatty acid-rich (McMillin et al. 1992; Demaison et al. 1994; Lemieux et al. 2008) or in n-6 polyunsaturated fatty acid (PUFA)- or SFA-rich preparations (De Deckere & Ten Hoor, 1980; Royce & Holmes, 1984).
was measured in pig hearts in situ (Hartog et al. 1987) or in isolated rat hearts in vitro (de Deckere & ten Hoor, 1979, 1980; Demaison et al. 2000). The cellular mechanism underlying the proposed energetic benefits of dietary FO is also equivocal. Whereas both Yamaoka et al. (1988) and Pepe et al. (1999) reported decreased rates of O2 consumption in rat heart mitochondria in association with increased FO fatty acid incorporation into the mitochondrial membrane phospholipids, other studies have shown no changes in mitochondrial respiratory rate in FO fatty acid-rich (McMillin et al. 1992; Demaison et al. 1994; Lemieux et al. 2008) or in n-6 polyunsaturated fatty acid (PUFA)- or SFA-rich preparations (De Deckere & Ten Hoor, 1980; Royce & Holmes, 1984).
Such sharp disagreements beg clarification. To that end, we undertook to investigate the effect of dietary FO supplementation on cardiac mechano-energetics, employing two different measurements of metabolic input (oxygen consumption and heat production) at two different spatial scales (the isolated whole heart and isolated left ventricular trabeculae), respectively, and at a range of preloads and afterloads.
Methods
Ethical approval
Experiments were conducted in accordance with protocols approved by The University of Auckland Animal Ethics Committee (Approval R787).
Animal preparation
Male Wistar rats (6–7 weeks old, 250–350 g) were randomly divided into three different ‘Diet’ groups: reference (REF), fish oil-rich (FO) and saturated fatty acid-rich (SFA). Diets were prepared such that each had a unique fatty acid composition. Standard rodent pellets (2018 Teklad Global 18% Protein Rodent Diet, Harlan, Indianapolis, IN, USA) were used as the dietary base. The REF group was given these base pellets (which contained 6.2% fat by weight), whereas the FO and SFA groups were fed chows that comprised base pellets supplemented with fish oil (liquid RxOmega-3 Factors, Natural Factors, Coquitlam, BC, Canada) and beef fat (100% pure beef dripping, Farmland, New Zealand), respectively, the added fat comprising 10% by weight. The rats (n = 15 per group) were fed their respective diets for 6–8 weeks. Food and water consumption, as well as the body mass, of each rat were monitored weekly throughout the feeding period. Whereas all rats survived the dietary period, not all whole heart experiments were successful and not all hearts yielded a suitable trabecula. Hence, the number of observations per group varies somewhat among interventions. However, regardless of the success of the whole heart experiments, the wet and dry weights of all the hearts were measured for averaging purposes.
Heart preparation
At the end of the feeding period, a rat was presented to the experimenter under single-blind conditions. It was deeply anaesthetised with isoflurane (5% in O2) and killed by cervical dislocation. The heart was quickly excised and placed in ice-cold saline to induce cardiac arrest. The aorta was immediately cannulated for Langendorff perfusion with oxygenated Tyrode solution (at a perfusion pressure of 70 mmHg) at room temperature. The Tyrode solution contained (in mm): 130 NaCl, 6 KCl, 1 MgCl2, 0.5 NaH2PO4, 1.5 CaCl2, 10 Hepes and 10 glucose, and its pH was adjusted to 7.4 using Tris. Note that the exogenous metabolic substrate was 10 mm glucose for all three groups. In two separate experiments, the vasodilatory agent adenosine (5 μm) was added to investigate the extent of coronary reserve. In all experiments, the perfusate was vigorously bubbled with 100% O2 continuously.
With the heart submerged under Tyrode solution, one of the four pulmonary veins and the pulmonary artery were separately cannulated and all other vessels ligated. A unipolar stimulus electrode (Coaxial Stimulation Electrode, Harvard Apparatus, Holliston, MA, USA) was placed on the right atrium and pacing commenced at 4 Hz. Once fully instrumented, the heart was enclosed by a water-jacketed chamber to maintain constant temperature (32°C) while preventing tissue desiccation.
The partial pressures of O2 in the solution upstream (arterial 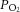 , measured just superior to the coronary ostia) and downstream (venous
, measured just superior to the coronary ostia) and downstream (venous 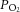 , measured in the pulmonary artery cannula) of the coronary vasculature was continuously measured by a pair of fibre optic oxygen sensors (FOXY-R-8CM, Ocean Optics Inc., Dunedin, FL, USA). The rates of aortic and coronary outflow were measured using perivascular flow probes (T206 and T106, Transonic system, Ithaca, NY, USA) modified for in-line measurent. Mean arterial pressure was obtained using a pressure transducer (SP 844 Transducer, MEMSCAP, Crolles, France) located in the aortic outflow catheter. Data acquisition and recording were achieved using PowerLab LabChart Pro software (ADInstruments, Dunedin, New Zealand).
, measured in the pulmonary artery cannula) of the coronary vasculature was continuously measured by a pair of fibre optic oxygen sensors (FOXY-R-8CM, Ocean Optics Inc., Dunedin, FL, USA). The rates of aortic and coronary outflow were measured using perivascular flow probes (T206 and T106, Transonic system, Ithaca, NY, USA) modified for in-line measurent. Mean arterial pressure was obtained using a pressure transducer (SP 844 Transducer, MEMSCAP, Crolles, France) located in the aortic outflow catheter. Data acquisition and recording were achieved using PowerLab LabChart Pro software (ADInstruments, Dunedin, New Zealand).
Isolated working heart experiments
Upon attainment of steady state (typically about 60 min post-death), perfusion was switched to working heart mode (preload 10 mmHg, afterload 50 mmHg). Preload and afterload could be varied independently by changing their respective pressure heads. When the heart reached a new steady state under the specified condition, a different preload–afterload combination was imposed. The first intervention comprised fixed preload but variable afterload (from 40 mmHg to near-isovolumic pressure, corresponding to near-zero aortic outflow) and was completed in 15–20 min. The second intervention comprised fixed afterload (75 mmHg) but variable preload (from 5 to 20 mmHg in steps of 5 mmHg) and was completed in 60–90 min.
Myocardial 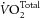 was recorded continuously and retrospectively corrected for the epicardial exchange of O2 between the heart and its surroundings inside the heart chamber, as previously described (Loiselle, 1989; Goo et al. 2013). Upon completion of both work protocols, the heart was Langendorff-perfused with high-K+ (26 mm) cardioplegic Tyrode solution to measure its resting metabolic rate.
was recorded continuously and retrospectively corrected for the epicardial exchange of O2 between the heart and its surroundings inside the heart chamber, as previously described (Loiselle, 1989; Goo et al. 2013). Upon completion of both work protocols, the heart was Langendorff-perfused with high-K+ (26 mm) cardioplegic Tyrode solution to measure its resting metabolic rate.
The rate of work (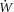 ) was calculated as the product of mean arterial pressure, rate of arterial flow and heart rate. The rate of oxygen consumption (
) was calculated as the product of mean arterial pressure, rate of arterial flow and heart rate. The rate of oxygen consumption (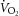 ) was calculated as the product of coronary flow rate, the difference between the upstream and downstream
) was calculated as the product of coronary flow rate, the difference between the upstream and downstream 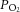 , and the solubility of oxygen in saline at 32°C (0.0249 mLO2 atm−1 mL−1). Total efficiency (εTotal) and mechanical efficiency (εMech) were calculated as follows:
, and the solubility of oxygen in saline at 32°C (0.0249 mLO2 atm−1 mL−1). Total efficiency (εTotal) and mechanical efficiency (εMech) were calculated as follows:
| (1) |
| (2) |
The rates of change of total (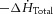 ) and basal (
) and basal (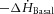 ) enthalpy were calculated from their appropriate measurements of
) enthalpy were calculated from their appropriate measurements of 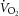 , using the energetic equivalent of O2 (20 kJ l−1). In total, whole heart results were obtained from 9 rats from the REF, 14 from FO and 14 from SFA groups.
, using the energetic equivalent of O2 (20 kJ l−1). In total, whole heart results were obtained from 9 rats from the REF, 14 from FO and 14 from SFA groups.
Trabecula experiments
At the completion of each working heart experiment, the heart was perfused with Tyrode solution containing 20 mm butanedione monoxime (BDM) and low Ca2+ (0.25 mm) in preparation for isolation of left ventricular trabeculae. The arrested heart was detached from the working heart cannulae and, while submerged under the BDM/low Ca2+ solution, a geometrically uniform, free-running ventricular trabecula was excised. It was then mounted in the measurement chamber of a work-loop microcalorimeter (Taberner et al. 2011; Han et al. 2013) maintained at 32°C. The microcalorimeter allows simultaneous measurement of force, change of length and heat production. The trabecula, electrically stimulated at 3 Hz, was gradually stretched to its optimal length (Lo, where developed force was maximal). The total time between removing the heart from the working heart apparatus and achieving steady-state twitch force production at Lo was typically 60 min. Completion of both protocols was typically achieved during a further 60 min.
Trabeculae were subjected to two different modes of contraction. The first mode required the trabecula to undergo force-length work-loops at six to seven different afterloads. Work (W), heat, change of enthalpy (−ΔH; work plus heat) and mechanical efficiency (W/−ΔH) were calculated. In the second intervention, the trabecula was required to contract isometrically at progressively diminishing lengths below Lo. Economy of contraction and activation heat were calculated from the resulting heat–stress relationship. Results were obtained from 12, 9 and 12 trabeculae from the REF, FO and SFA groups, respectively.
Normalisation of quantitative data
At the end of each trabecula experiment, the ventricular tissues were blot-dried and the wet mass of the ventricles was measured. A small sample of ventricular tissue was left in an oven at 60°C for 24 h and reweighed. The wet/dry mass ratio of each heart was determined from the sample. All the measurements made in the working heart experiments were normalised to the dry mass of the ventricles, whereas those made in the trabecula experiments were normalised to the volume of wet tissue (m3), calculated from measurements of the diameter and length under the assumption that trabeculae were cylindrical.
Myocardial content of fatty acids
The remaining ventricular tissue was frozen in liquid N2 and stored at −80°C for subsequent analysis of myocardial fatty acid content. Samples from three hearts from each of the three diet groups were analysed commercially (AsureQuality, Auckland, New Zealand).
Statistical analyses
Maximum values of ‘contractile power’ and ‘efficiency’ were derived from third-order polynomial functions fitted to the observed afterload-dependent data using MATLAB software (Mathworks, Natick, MA, USA). All values of interest are expressed as means ± standard error of the mean (SEM). The data for each variable were subjected to analysis of variance (ANOVA), using the Generalised Linear Model of SAS (SAS Institute Inc., Cary, NC, USA). Statistical significance was declared at P < 0.05. Post hoc tests of differences among means were applied, where appropriate, using mutually orthogonal contrast vectors.
Results
Fatty acid contents of diets and hearts
The fatty acid contents of the diets and hearts of the current study (n = 3) are summarised in Table 1. Also shown for comparison are the equivalent fatty acid contents used by Pepe and McLennan (2002), based on values used in their previous (1996) study. In both cases, the FO group showed greater dietary and myocardial contents of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the FO fatty acids of principal concern, compared to the other two groups.
Table 1.
Fatty acid analyses of rat food (‘Dietary’) and hearts (‘Myocardial’); comparison of current study with those of Pepe and McLennan (1996, 2002)
| Dietary (mol/100 mol) |
Myocardial (mol/100 mol) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current study |
Pepe and McLennan (1996) |
Current study |
Pepe and McLennan (1996) |
|||||||||
| Fatty acid* | REF | FO | SFA | REF | FO | SFA | REF | FO | SFA | REF | FO | SFA |
| 18:2 (n-6), LNA | 53.3 | 26.2 | 26.3 | 33.9 | 5.6 | 6.7 | 18.4 | 9.5 | 15.3 | 21.6 | 10.2 | 13.9 |
| 18:3 (n-3), ALNA | 5.8 | 3.0 | 3.1 | 3.3 | 1.2 | 1.5 | 0.4 | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 |
| 20:4 (n-6), AA | <0.1 | 1.2 | <0.1 | 0.6 | 1.0 | 0.1 | 16.3 | 13.9 | 16.7 | 17.7 | 14.1 | 20.3 |
| 20:5 (n-3), EPA | <0.1 | 21.1 | 0.1 | 2.2 | 24.3 | 0.4 | 0.1 | 2.3 | 0.1 | 0.3 | 3.1 | 0.3 |
| 22:5 (n-3), DPA | <0.1 | 2.8 | <0.1 | — | 1.5 | 0.1 | 2.1 | 2.4 | 2.8 | 1.4 | 1.8 | 1.8 |
| 22:6 (n-3), DHA | <0.1 | 10.6 | <0.1 | 6.2 | 11.8 | 0.8 | 8.3 | 20.4 | 9.2 | 15.4 | 27.2 | 19.5 |
| Σ(n-6) PUFA | 54.0 | 29.3 | 26.8 | 34.5 | 6.6 | 7.0 | 36.4 | 24.5 | 33.4 | 39.8 | 24.7 | 34.6 |
| Σ(n-3) PUFA | 6.8 | 39.5 | 3.7 | 11.8 | 38.9 | 2.8 | 11.2 | 25.7 | 12.8 | 17.2 | 32.3 | 21.4 |
| (n-3):(n-6 PUFA) | 0.1 | 1.3 | 0.1 | 0.3 | 5.9 | 0.4 | 0.3 | 1.1 | 0.4 | 0.4 | 1.3 | 0.6 |
| ΣSFA | 19.5 | 12.4 | 38.0 | 25.3 | 25.3 | 54.9 | 34.9 | 34.5 | 35.3 | 33.9 | 34.9 | 34.8 |
| ΣMUFA | 23.1 | 18.8 | 30.9 | — | — | — | 10.2 | 8.1 | 10.3 | — | — | — |
| ΣPUFA | 61.0 | 68.5 | 30.7 | 46.2 | 45.5 | 9.6 | 47.8 | 50.4 | 46.4 | 57.0 | 57.0 | 56.0 |
| PUFA:SFA | 3.1 | 5.5 | 0.8 | 1.6 | 1.8 | 0.2 | 1.4 | 1.5 | 1.3 | 1.7 | 1.6 | 1.7 |
| PUFA:MUFA | 2.6 | 3.7 | 1.0 | — | — | — | 4.8 | 6.2 | 4.6 | — | — | — |
| Total fat content (% by weight) | 7.7 | 14.6 | 13.4 | 7.6 | 15.3 | 15.3 | 2.2 | 2.43 | 2.13 | — | — | — |
LNA, linoleic acid; ALNA, α-linoleic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid.
Morphometric characteristics of the rats, hearts and trabeculae
The physical characteristics of the animals and their whole heart and trabecula preparations are summarised in Table 2. The FO–fed rats had marginally greater average heart wet mass than the other two diet groups, but there were no differences in the wet/dry mass ratios. Likewise, their trabeculae were of similar dimensions (diameter and length): 220 ± 17 μm and 2.3 ± 0.2 mm for REF (n = 12), 185 ± 10 μm and 2.2 ± 0.3 mm for FO (n = 9) and 221 ± 13 μm and 2.4 ± 0.2 mm for SFA (n = 12).
Table 2.
Physical characteristics of rats, hearts and trabeculae
| REF (n = 15) | FO (n = 15) | SFA (n = 15) | |
|---|---|---|---|
| Body mass (g) | 482.4 ± 7.9 | 465.5 ± 8.6 | 498.2 ± 7.9 |
| Heart/body (%) | 0.30 ± 0.01 | 0.32 ± 0.01 | 0.29 ± 0.01 |
| Heart wet mass (g) | 1.44 ± 0.03 | 1.50 ± 0.04* | 1.44 ± 0.03 |
| Heart dry mass (g) | 0.27 ± 0.02 | 0.28 ± 0.01 | 0.27 ± 0.01 |
| Heart wet/dry mass | 5.41 ± 0.23 | 5.40 ± 0.10 | 5.40 ± 0.10 |
Values are means ± SEM for n observations.
P < 0.05.
Isolated working heart experiments
Aortic and coronary flow rates as functions of afterload and preload
ANOVA revealed no effect of diet on aortic or coronary flow rates (Fig. 1A). However, diet did have an effect on total ventricular outflow (aortic + coronary; Fig. 1B), which was higher in the REF group than either the SFA group, or the average of the SFA and FO groups. Over the entire range of afterloads, the downstream 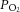 (Fig. 1C) remained well above the value of venous
(Fig. 1C) remained well above the value of venous 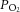 commonly observed in vivo. Figure 1D reveals the effect of adding 5 μm adenosine to the perfusate; the coronary flow rate increased significantly over the same range of afterloads during the administration period, an effect that was completely abolished by wash-out.
commonly observed in vivo. Figure 1D reveals the effect of adding 5 μm adenosine to the perfusate; the coronary flow rate increased significantly over the same range of afterloads during the administration period, an effect that was completely abolished by wash-out.
Figure 1. Aortic and coronary flow as functions of afterload.
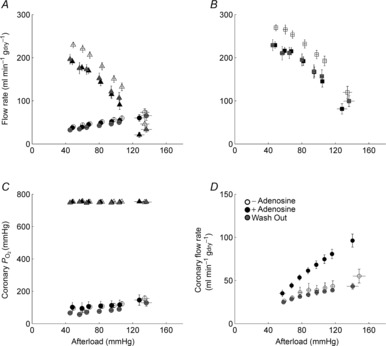
A, aortic (triangles) and coronary (circles) flow rates, and B, total ventricular outflow (the sum of aortic and coronary flow rates, significantly higher in the REF group) as functions of afterload. C, upstream (coronary arterial) 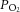 (triangles) and downstream (coronary venous)
(triangles) and downstream (coronary venous) 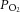 (circles) as functions of afterload at a preload of 10 mmHg. Symbols represent mean ± SEM of n = 9 REF (open symbols), n = 14 FO (black symbols) and n = 14 SFA (grey symbols) diet groups . D, coronary flow rate before (‘– Adenosine’), during (‘+ Adenosine’) and after (‘Wash Out’) 5 μm adenosine (mean ± SEM, n = 2).
(circles) as functions of afterload at a preload of 10 mmHg. Symbols represent mean ± SEM of n = 9 REF (open symbols), n = 14 FO (black symbols) and n = 14 SFA (grey symbols) diet groups . D, coronary flow rate before (‘– Adenosine’), during (‘+ Adenosine’) and after (‘Wash Out’) 5 μm adenosine (mean ± SEM, n = 2).
Mechano-energetic variables as functions of afterload and preload
The three mechano-energetic indices (power, rate of change of enthalpy and total efficiency) are presented as functions of afterload and preload in Fig. 2. Figure 2A, C and E show average data of each diet group for variable afterloads, at preload of 10 mmHg, while Fig. 2B, D and F show results for variable preloads at a fixed afterload of 75 mmHg. For the variable-afterload protocol, power (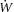 ) was greater in the REF group compared to the average of the FO and SFA groups (P = 0.037; Fig. 2A). However, the rate of change of enthalpy (
) was greater in the REF group compared to the average of the FO and SFA groups (P = 0.037; Fig. 2A). However, the rate of change of enthalpy ( ) did not differ among the diet groups (Fig. 2C). Total efficiency, which is the ratio of these two indices (eqn 1 and Fig. 2E), was not different among the diet groups. For the variable-preload protocol, there was no difference in any of these indices among the diet groups.
) did not differ among the diet groups (Fig. 2C). Total efficiency, which is the ratio of these two indices (eqn 1 and Fig. 2E), was not different among the diet groups. For the variable-preload protocol, there was no difference in any of these indices among the diet groups.
Figure 2. Power, rate of change of enthalpy and total efficiency as functions of afterload and preload for the three diet groups.
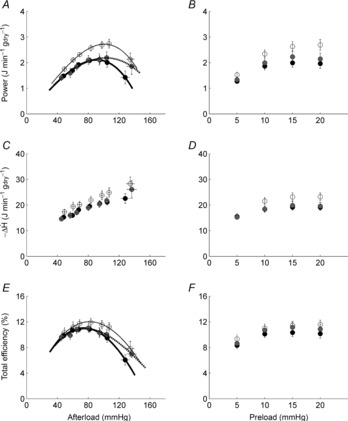
Values are means ± SEM of n = 9 REF (open circles), n = 14 FO (filled circles) and n = 14 SFA (grey circles) working hearts. Power and efficiency data were fitted with third-order polynomial functions.
Peak power and peak total efficiency
For each heart, the power–afterload and total efficiency–afterload data were fitted using third-order polynomials constrained to pass through the origin (Fig. 2A and E). From these relationships, the peak values (Fig. 3A and B), and the afterloads at which they occurred (Fig. 3C and D), were calculated. ANOVA revealed no difference in the average values of either index among the diet groups. Average peak total efficiencies were 12.3 ± 0.6, 11.5 ± 0.5 and 11.5 ± 0.6% for REF, FO and SFA, respectively (Fig. 3B).
Figure 3. Peak power and peak total efficiency of the whole heart, and the afterloads at which they occur.
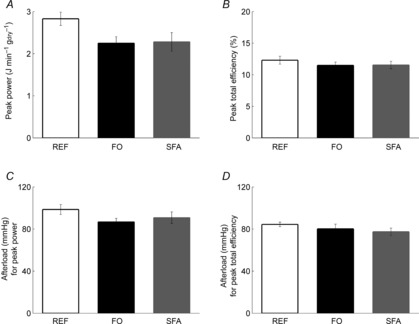
Peak power (A) and peak total efficiency (B), and the afterloads yielding the respective peak power (C) and peak total efficiency (D); values are mean ± SEM from n = 9 REF, n = 14 FO and n = 14 SFA hearts.
Mechanical efficiency (eqn 2) was also calculated for each diet group, by subtracting the basal rate of oxygen consumption. Basal metabolism, measured at an afterload of 75 mmHg, did not differ among the diet groups: 3.9 ± 0.7, 3.4 ± 0.5 and 2.6 ± 0.5 J s−1 kgdry−1 for REF, FO and SFA, respectively. In consequence, there was no difference in the mechanical efficiency across the diet groups: 14.8 ± 1.1% for REF, 13.9 ± 0.6% for FO and 13.6 ± 0.7% for SFA.
Trabecula experiments
Figure 4A and B shows representative records of stress (force per cross-sectional area) and heat rate. Work-loop contractions with variable afterloads (labels b–h) were bracketed by isometric contractions (labelled ‘a’); note the constancy of their profiles throughout the duration of the experiment. By contrast, for the afterloaded isotonic contractions, the rate of heat production decreased with diminishing afterload. The accompanying steady-state twitches and length-change traces are shown in Fig. 4C and D. The area within a stress–length loop (Fig. 4E) quantifies the external mechanical work performed by the trabecula. Note that for isometric contraction (L/Lo = 1), work is zero.
Figure 4. Typical experimental records arising from the work-loop protocol.
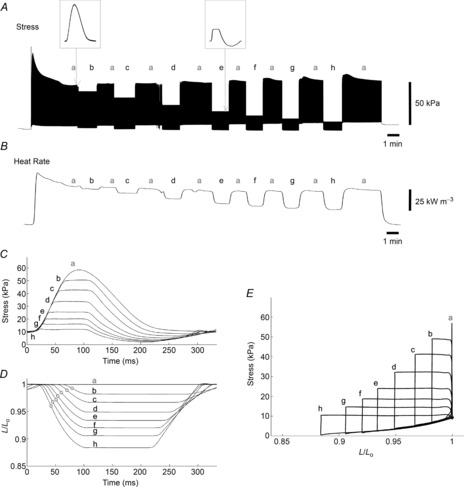
Twitch stress (A) and rate of heat output (B) of a trabecula undergoing a decreasing order of selected afterloads (b–h), bracketed by isometric contractions (a). The left-hand inset shows a single isometric contraction (a) and the right-hand inset depicts a work-loop contraction at a selected afterload (e). C, overlaid steady-state twitches at various afterloads: isometric (a) and work-loops (b–h). D, instantaneous muscle length (relative to optimal length, L/Lo) as a function of time during the contractions shown in C. For the isometric contraction (a) of this particular trabecula, the peak systolic stress was 60 kPa and passive stress 10 kPa. The point of maximum shortening velocity for each isotonic contraction is shown as a circle. E, parametric plot of the data shown in C and D; overlaid isometric contraction (a) and work-loop contractions (b–h).
Change of enthalpy, work and mechanical efficiency as functions of relative afterload
Change of enthalpy (−H) was calculated as the sum of external mechanical work and the associated heat production (in accordance with the 1st Law of Thermodynamics). The relationship between −ΔH and the relative total afterload (S/So) of a trabecula, and the average relationships for the three diet groups are presented in Fig. 5A and B. External mechanical work, calculated as the area enclosed by the stress–length work-loop (Fig. 4E), and its relationship with normalised stress (S/So) is presented in Fig. 5C and D. There were no differences among the diet groups in either the enthalpy–load relationships (Fig. 5B), the values of peak work (Fig. 5D) or the relative afterloads at which peak work occurred (Fig. 5D).
Figure 5. Change of enthalpy (-ΔH) andwork (W) as functions of relative total afterload (S/So).
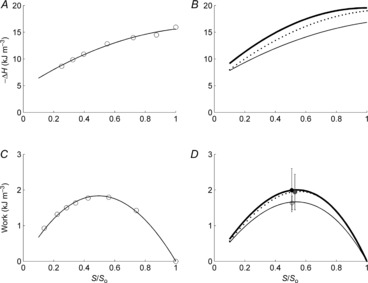
Data from a representative trabecula (A and C). Data for −ΔH were fitted using second-order polynomials and the work versus S/So data with third-order polynomials (constrained to intersect the abscissa at both [0, 0] and [1, 0]). B and D, average relationships of the trabeculae for each diet group: n = 12 REF (thin lines), n = 9 FO (thick lines) and n = 12 SFA (broken lines). Means ± SEM of peak work are superimposed in D for each diet group: REF (open circle), FO (black filled circle) and SFA (grey filled circle).
Figure 6 shows the average relationships of mechanical efficiency (the ratio of work to −ΔH, whose values are quantified in Fig. 5) versus relative total afterload, S/So, and compares the values of peak mechanical efficiency and the relative afterload corresponding to the peaks (Fig. 6B) among the diet groups. Peak mechanical efficiency values were 13.3 ± 1.4, 11.2 ± 2.2 and 12.5 ± 1.5% for the REF, FO and SFA groups, respectively, with no significant differences. Neither was there any difference in the relative afterloads at which these peaks occurred.
Figure 6. Mechanical efficiency of isolated trabeculae as a function of relative total afterload.
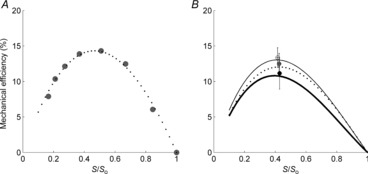
Mechanical efficiency as functions of relative total afterload (S/So) for a representative trabecula, fitted with a third-order polynomial with constraints at points (0,0) and (1,0) (A), and the average relationships for the diet groups (B); there were no significant differences in the peak values of mechanical efficiency or the afterload achieving peak efficiency among the groups. Means ± SEM of peak mechanical efficiency are superimposed in B for each diet group: REF (open circle), FO (black filled circle) and SFA (grey filled circle).
Activation heat and the economy of contraction
From the isometric length-change protocol, linear heat–active stress and heat–stress time integral (STI) relationships were obtained (Fig. 7). The STI of a single twitch is defined as the area enclosed under the twitch stress–time profile, and is calculated by integrating twitch stress with respect to time. The slope and y-intercept of each of the average relationships for each diet group represent ‘economy of contraction’ and ‘activation heat’, respectively. There were no differences in either the slopes or the y-intercepts among the diet groups. The slope and intercept values of each diet group for the heat–active stress relationship (Fig. 7C) were 0.21 ± 0.02 and 5.1 ± 1.1 kJ m−3 for REF, 0.23 ± 0.03 and 3.8 ± 1.5 kJ m−3 for FO, and 0.22 ± 0.03 and 3.8 ± 1.5 kJ m−3 for SFA, respectively. The slopes of the heat–STI relationships (Fig. 7D) were 1.8 ± 0.2 in all three diet groups, with their intercepts not different from those found in the heat–stress relationships.
Figure 7. Typical experimental records arising from the isometric length-change protocol.
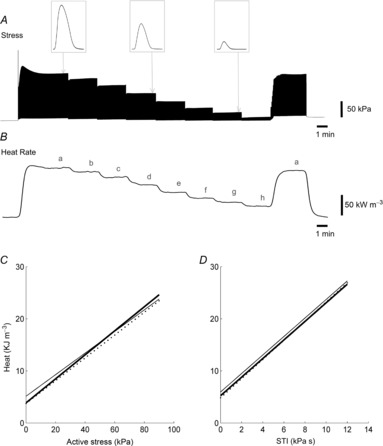
Twitch stress (A) and rate of heat output (B) of a trabecula at various lengths (b–h) below its optimal length (a). Insets in A represent individual twitch profiles at lengths a, e and g. Linear regression of the average heat–stress relationships (C) and the average heat–STI relationships (D) for the three diet groups: REF (thin line), FO (thick line) and SFA (broken line); no differences among diet groups.
Other mechanical indices of interest
Isometric total stress, passive stress, twitch duration (at both the 50% and the 5% peak stress levels), rate of rise and fall of twitch stress, maximum velocity of shortening, maximum power of shortening and STI did not differ among the diet groups (data not shown).
Discussion
The literature contains a number of contradictory experimental findings regarding the mechano-energetics of hearts enriched with either FO fatty acids or SFAs. To clarify the presence and/or extent of the putative effects of different fatty acids, we employed two distinct measurement techniques at two physiological scales: isolated whole heart and ventricular trabeculae. To our knowledge, this is the first comprehensive study to look at the effect of different dietary fatty acids on mechano-energetic indices, including cardiac efficiency (the appropriately scaled ratio of work output to oxygen consumption), over a range of afterloads in isolated working heart preparations. It is also the first to examine the effects on the energetics of isolated cardiac muscle. Results from both experimental moieties are unequivocal: no effect of dietary FO fatty acid or SFA on either ‘total’ or ‘mechanical’ cardiac efficiency.
In order not to misattribute our observed null effect of diet on cardiac mechano-energetic outcomes, it is first necessary to show that the diets achieved the desired concentrations of components in the myocardium. The diet of the REF group was 6.2% fat by weight, while the other two groups received 15% fat, but with reciprocal amounts of either high fish oil (FO group) or high saturated fatty acids (SFA group) for 6–8 weeks. As a result of the feeding regimen, the intended differences in fatty acid contents of the hearts from the different diet groups were achieved. In particular, the n-3 to n-6 PUFA ratio of the myocardia, which reflects the extent of incorporation of FO, was increased in the FO group compared to the REF and SFA groups (Table 1). For example, the ratio was increased 4.5-fold in the FO group compared to the SFA group, and by 1.6-fold with respect to the REF group. Such changes in the cellular composition of fatty acids following differential supplementation have been consistently shown in the literature (Gudbjarnason et al. 1978; Tahin et al. 1981; Hartog et al. 1987; Pepe & McLennan, 1996; Katan et al. 1997), and support our claim of absence of effect of high fat diets on the contractile efficiency of the heart (Fig. 2E).
These findings are in accordance with those from several other studies. No effect of dietary fatty acids on cardiac mechano-energetics was found in porcine hearts in situ (Hartog et al. 1987) or in isolated working rat hearts (Demaison et al. 2000). However, de Deckre and ten Hoor (1979, 1980) did see beneficial effects on left ventricular work capacity and coronary flow rate of dietary n-6 PUFAs with respect to SFAs in isolated rat hearts, but with no effect on total myocardial oxygen consumption (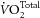 ). Whole body results from human subjects are equivocal. Thus, whereas Peoples et al. (2008) and Peoples & McLennan (2010) showed that dietary supplementation with fish oils effected a reduction of
). Whole body results from human subjects are equivocal. Thus, whereas Peoples et al. (2008) and Peoples & McLennan (2010) showed that dietary supplementation with fish oils effected a reduction of 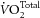 during exercise, the equally comprehensive study by Bortolotti et al. (2007) concluded that fish oil supplementation does not alter energy efficiency in healthy males.
during exercise, the equally comprehensive study by Bortolotti et al. (2007) concluded that fish oil supplementation does not alter energy efficiency in healthy males.
It can be seen that, in each of the studies referenced above, only a single component of the efficiency ratio was reported, i.e. either the numerator (work) or the denominator (oxygen consumption). The sole exception, of which we are aware, is the study by Pepe and McLennan (2002). These investigators showed dramatic alterations in 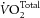 , with no change of work output, between FO- and SFA-rich diet groups. The consequences were a near doubling of myocardial efficiency in rat hearts enriched with fish oils, and a near halving of efficiency in the group fed saturated fatty acids compared to the control group. As our results, from both whole heart and trabecula experiments, differ radically from those of Pepe and McLennan (2002), we critically examine discrepancies between our respective results that might have led to our diametrically opposed conclusions.
, with no change of work output, between FO- and SFA-rich diet groups. The consequences were a near doubling of myocardial efficiency in rat hearts enriched with fish oils, and a near halving of efficiency in the group fed saturated fatty acids compared to the control group. As our results, from both whole heart and trabecula experiments, differ radically from those of Pepe and McLennan (2002), we critically examine discrepancies between our respective results that might have led to our diametrically opposed conclusions.
The two principal differences between our study and that of Pepe and McLennan (2002) are that the latter supplemented their bicarbonate-buffered experimental solution with porcine erythrocytes (haematocrit of 40%) and they conducted their experiments at 37°C. Hence, it might be argued that the current study starved the hearts of O2, as the amount of O2 contained per volume of perfusate was much lower. However, we consider that the delivery of O2 to the myocardium in our study was adequate given that: (1) the downstream 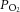 was well above the normal physiological venous
was well above the normal physiological venous 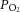 at all afterloads (Fig. 1C), (2) an increase in downstream
at all afterloads (Fig. 1C), (2) an increase in downstream 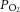 was observed at high afterloads, thereby ruling out oxygen starvation at all submaximal workloads, and (3) there was clearly coronary vascular reserve present, as shown by administration of 5 μm adenosine (Mawson et al. 1994; Krams et al. 2004) (Fig. 1D). This evidence notwithstanding, to be safe we lowered the experimental temperature to 32°C and paced the hearts at 4 Hz, thereby simultaneously increasing the oxygen capacitance of the Tyrode perfusate, while lowering the energy demand on the heart. We considered a fixed pacing frequency to be prudent since omega-3 PUFAs are known to reduce heart rate (Peoples et al. 2008; Kang, 2012) while increasing its variability (Christensen, 2011). All hearts followed the pacing frequency. Our concerns regarding the adequacy of oxygenation may not have been necessary, however, because Duvelleroy et al. (1976) found the efficiency of the rat heart to be 12%, whether hearts were perfused in the absence of red blood cells or with 40% haematocrit – the identical condition adopted by Pepe and McLennan (2002). Note that this value of efficiency falls well within the accepted range (8–20%) reported in other isolated rat working heart studies (Neely et al. 1967; Starnes & Rumsey, 1988; Starnes et al. 1992). We therefore conclude that the difference in the efficiency values of the current study (12.3%, REF at 32°C) and those of Pepe and McLennan (2002) and Starnes et al. (1992) (6%, REF at 37°C) cannot be reconciled by the difference in oxygen supply.
was observed at high afterloads, thereby ruling out oxygen starvation at all submaximal workloads, and (3) there was clearly coronary vascular reserve present, as shown by administration of 5 μm adenosine (Mawson et al. 1994; Krams et al. 2004) (Fig. 1D). This evidence notwithstanding, to be safe we lowered the experimental temperature to 32°C and paced the hearts at 4 Hz, thereby simultaneously increasing the oxygen capacitance of the Tyrode perfusate, while lowering the energy demand on the heart. We considered a fixed pacing frequency to be prudent since omega-3 PUFAs are known to reduce heart rate (Peoples et al. 2008; Kang, 2012) while increasing its variability (Christensen, 2011). All hearts followed the pacing frequency. Our concerns regarding the adequacy of oxygenation may not have been necessary, however, because Duvelleroy et al. (1976) found the efficiency of the rat heart to be 12%, whether hearts were perfused in the absence of red blood cells or with 40% haematocrit – the identical condition adopted by Pepe and McLennan (2002). Note that this value of efficiency falls well within the accepted range (8–20%) reported in other isolated rat working heart studies (Neely et al. 1967; Starnes & Rumsey, 1988; Starnes et al. 1992). We therefore conclude that the difference in the efficiency values of the current study (12.3%, REF at 32°C) and those of Pepe and McLennan (2002) and Starnes et al. (1992) (6%, REF at 37°C) cannot be reconciled by the difference in oxygen supply.
Other possible causes of differences between the current study and that of Pepe and McLennan (2002) are the total period of feeding and the age of the rats. In the latter case, rats were fed for 10 weeks longer than in the current study. In consequence, the rats were 8 months old at the time of experimentation, compared to 3 months in the current study. The duration of feeding itself, however, is not likely to be the cause because the kinetics of incorporation of FO fatty acids into the membranes are rapid, saturation of FO fatty acids in the myocardium happening as early as the third week from commencement of feeding (Tahin et al. 1981). As shown in Table 1, DHA and EPA content of the hearts in the FO-enriched diet groups of both studies were quantitatively and qualitatively similar with respect to the other two diet groups (Pepe & McLennan, 1996). The age of the rats at the time of experimentation is not likely to have affected the efficiency, either; a decline of myocardial efficiency, from approximately 10% to 8%, has been observed only in much older rats (18–24 months) and under a very high afterload (150 mmHg) (Starnes & Rumsey, 1988).
On the other hand, the profile of efficiency as a function of afterload could have been altered by the diets. One possible scenario is that the different diets could have differentially affected ventricular wall compliance, rendering those of the FO group more compliant, for instance, as FO fatty acids have been found to reduce arterial stiffness (Pase et al. 2011). If the older age of the rats and/or the longer feeding period in the study by Pepe and McLennan (2002) had altered tissue properties, then the efficiency–afterload profiles of the diet groups could have been differentially affected. Examples of such possible changes are illustrated in Fig. 8B and C. Given that Pepe and McLennan (2002) reported the efficiency values at only a single afterload (75 mmHg), such changes (Fig. 8B and C) could have incorrectly resulted in the conclusion of different values of efficiency among the diet groups when there had been, in fact, no actual difference in the peak values. Whereas this putative phenomenon could be invoked to explain the qualitative difference of results between the two studies, it is quantitatively improbable that it could account for a doubling of efficiency. We emphasise the necessity of examining mechano-energetic variables over a wide range of loading conditions (particularly afterload) when performing isolated working heart experiments.
Figure 8. Schematic diagrams showing various possibilities that might have produced the differences among the diet groups in Pepe and McLennan (2002).
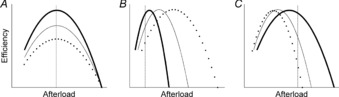
REF (thin continuous line), FO (thick continuous line) and SFA (dotted line). A, example of genuine differences in peak efficiencies, despite being observed at only the selected afterload (vertical broken line). B and C, further examples of no differences in peak efficiency among the groups nevertheless leading to faulty conclusions because of different efficiency profiles.
Mechano-energetics assayed using left ventricular trabeculae
Trabeculae may be considered to be the one-dimensional equivalent of the three-dimensional ventricle. Following completion of a whole heart experiment, a trabecula of suitable dimensions was dissected from the left ventricle and mounted in our work-loop calorimeter (Taberner et al. 2011). Each trabecula performed work-loops and underwent an isometric length-change protocol. There were no differences among the trabeculae from the diet groups in either active or passive stress, rate of stress development or relaxation, stress–time integral or twitch duration (results not shown). Published reports on the effect of FOs on such mechanical properties of isolated cardiac tissue are not all in agreement. McLennan et al. (1987) found a depressive effect of dietary PUFAs on maximum developed tension and maximum rate of tension development, while others found no effect (Awumey & Pehowich, 1995; Chemla et al. 1995; Skuladottir & Johannsson, 1997; Billman et al. 2010). Our results are in accordance with those of the latter studies; we found no effect of dietary FO or SFA on the mechanics of left ventricular trabeculae.
In terms of the energetics of isolated left ventricular trabeculae, we found no effect of diet on the enthalpy–afterload relationships (Fig. 5B). The linear heat–stress relationships (Fig. 7C), which are consistent with previous reports using isolated cardiac trabeculae (Han et al. 2010) and isolated papillary muscles (Gibbs & Gibson, 1970; Loiselle & Gibbs, 1979; Gibbs et al. 1988; Kiriazis & Gibbs, 2001), were unaffected by the differential cellular content of FO fatty acids or SFAs. Likewise, there was no difference among the diet groups in the relationship between heat and STI (Fig. 7D).
The extrapolated intercepts on the ordinates of the active heat–stress and heat–STI relationships quantify ‘activation heat’. Activation heat is attributed to the energy required for activation of the contractile unit. Several cellular processes contribute to the energy expenditure: Ca2+ cycling (sarcoplasmic reticulum Ca2+ATPase, SERCA) and Na+ extrusion (Na+–K+-ATPase) (Chapman et al. 1970; Gibbs et al. 1988). Since we found no differences in the heat intercepts of the heat–stress or heat–STI relationships among the diet groups, we infer that the dietary fatty acids do not alter the energetics of the activation mechanisms. The slope of the heat–stress relationship is considered to be an index of the ‘economy of contraction’, which describes the amount of energy that has to be expended per unit of active stress. The absence of a difference in this index among the diet groups implies that the dietary supplementation of FO does not render the contractile apparatus any more economical. Finally, given the preceding constellation of null effects, we found no differences among the diet groups in the peak mechanical efficiency of isolated left ventricular trabeculae. These results emphasise that the dietary regimen does not affect the mechano-energetics of cardiac muscle.
Conclusion
Our results, obtained from two independent measures of mechano-energetics at two different spatial scales of the heart, are unequivocal. We find no effect of a period of pre-exposure to dietary fatty acids (whether saturated or unsaturated) on either the economy or the efficiency of the whole heart or its left ventricular trabeculae, using 10 mm glucose as exogenous metabolic substrate. These findings are in stark contrast to those of Pepe and McLennan (2002) who, under comparable conditions of metabolic substrate, reported dramatic differences of efficiency in isolated rat hearts enriched with either FO fatty acids or SFAs.
Key points
Dietary fish oil has been found to have protective effects against cardiovascular disease, particularly in its role as an anti-arrhythmic agent.
An additional mechanism proposed for the putative cardio-protective effect is enhanced efficiency of metabolic energy usage by the heart.
We tested whether dietary supplementation of fish oil enhances cardiac efficiency or otherwise improves mechano-energetic performance. Experiments were performed at two distinct physiological levels (whole organ and isolated tissues) employing two independent metabolic indices (oxygen consumption and heat production), respectively.
Feeding rats with either a fish oil-enriched or a saturated fatty acid-enriched diet did not alter the efficiency of either the isolated whole heart or its left ventricular trabeculae.
Glossary
- BDM
butanedione monoxime
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FO
fish oil
- PUFA
polyunsaturated fatty acid
- SFA
saturated fat
- STI
stress time integral
Additional information
Competing interests
None.
Author contributions
Conception and design of the experiments: S.G., J.-C.H., I.J.L, A.J.T and D.S.L. Collection, analysis and interpretation of data: S.G., J.-C.H., L.A.N., A.J.T and D.S.L. Drafting and critically revising the manuscript: S.G., J.-C.H., I.J.L., A.J.T. and D.S.L. All authors approved the final version of the manuscript. The experiments were performed in the Department of Physiology and at the Auckland Bioengineering Institute, The University of Auckland.
Funding
This study was supported by grants from the National Heart Foundation of New Zealand (Small Project Grants No. 1428 and No. 1529, and Limited Budget Grant No. 1524), the Maurice and Phyllis Paykel Trust (University of Auckland Project No. 3701355), the University of Auckland Faculty Research Development Fund (Project Nos. 3627115 and 3627220), and the Royal Society of New Zealand Marsden Fund 11-UOA-199. S.G. was the recipient of a University of Auckland Doctoral Scholarship.
References
- Awumey EMK, Pehowich DJ. n-3 and n-6 fatty acids modulate the inotropic response to calcium in hypothyroid rat papillary muscle. J Cardiovas Pharmacol. 1995;25:473–480. doi: 10.1097/00005344-199503000-00019. [DOI] [PubMed] [Google Scholar]
- Bang HO, Dyerberg J, Hjørne N. The composition of food consumed by Greenland Eskimos. Acta Medica Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- Billman GE, Nishijima Y, Belevych AE, Terentyev D, Xu Y, Haizlip KM, Monasky MM, Hiranandani N, Harris WS, Gyorke S, Carnes CA, Janssen PML. Effects of dietary omega-3 fatty acids on ventricular function in dogs with healed myocardial infarctions: in vivo and in vitro studies. Am J Physiol Heart Circ Physiol. 2010;298:H1219–1228. doi: 10.1152/ajpheart.01065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotti M, Tappy L, Schneiter P. Fish oil supplementation does not alter energy efficiency in healthy males. Clin Nutr. 2007;26:225–230. doi: 10.1016/j.clnu.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Chapman JB, Gibbs CL, Gibson WR. Effects of calcium and sodium on cardiac contractility and heat production in rabbit papillary muscle. Circ Res. 1970;XXVII:601–610. doi: 10.1161/01.res.27.4.601. [DOI] [PubMed] [Google Scholar]
- Chemla D, Javouhey-Donzel A, Suard I, Maupoil V, Lecarpentier Y, Poumy JC, Rocquelin G, Rochette L. Influence of dietary polyunsaturated fatty acids on contractility, lusitropy and compliance of isolated rat myocardium. J Mol Cell Cardiol. 1995;27:1745–1755. doi: 10.1016/s0022-2828(95)90920-6. [DOI] [PubMed] [Google Scholar]
- Christensen JH. Omega-3 polyunsaturated fatty acids and heart rate variability. Front Physiol. 2011;2 doi: 10.3389/fphys.2011.00084. doi: 10.3389/fphys.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deckere EAM, ten Hoor F. Effects of dietary fats on the coronary flow rate and the left ventricular function of the isolated rat heart. Nutr Metab. 1979;23:88–97. doi: 10.1159/000176245. [DOI] [PubMed] [Google Scholar]
- De Deckere EAM, Ten Hoor F. Influences of dietary fats on coronary flow rate and left ventricular work of the isolated rat heart: sunflower seed oil versus lard. Nutr Metab. 1980;24:396–408. doi: 10.1159/000176357. [DOI] [PubMed] [Google Scholar]
- Demaison L, Blet J, Sergiel J-P, Gregoire S, Argaud D. Effect of dietary polyunsaturated fatty acids on contractile function of hearts isolated from sedentary and trained rats. Reprod Nutr Dev. 2000;40:113–125. doi: 10.1051/rnd:2000124. [DOI] [PubMed] [Google Scholar]
- Demaison L, Sergeil JP, Moraeau D, Grynberg A. Influence of the phospholipid n-6/n-3 polyunsaturated fatty acid ratio on the mitochondrial oxidative metabolism before and after myocardial ischemia. Biochim Biophys Acta. 1994;1227:53–59. doi: 10.1016/0925-4439(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Duvelleroy MA, Duruble M, Martin JL, Teisseire B, Droulez J, Cain M. Blood-perfused working isolated rat heart. J Appl Physiol. 1976;41:603–607. doi: 10.1152/jappl.1976.41.4.603. [DOI] [PubMed] [Google Scholar]
- Gibbs CL, Gibson WR. Energy production in cardiac isotonic contractions. J Gen Physiol. 1970;56:732–750. doi: 10.1085/jgp.56.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CL, Loiselle DS, Wendt IR. Activation heat in rabbit cardiac muscle. J Physiol. 1988;395:115–130. doi: 10.1113/jphysiol.1988.sp016911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo S, Pham T, Han J-C, Nielsen P, Taberner A, Hickey A, Loiselle D. Multiscale measurement of cardiac energetics. Clin Exp Pharmacol Physiol. 2013;40:671–681. doi: 10.1111/1440-1681.12139. [DOI] [PubMed] [Google Scholar]
- Gudbjarnason S, Doell B, Oskarsdottir G. Docosahexaenoic acid in cardiac metabolism and function. Acta Biol Med Germ. 1978;37:774–784. [PubMed] [Google Scholar]
- Han J-C, Taberner AJ, Nielsen PMF, Kirton RS, Ward M-L, Loiselle DS. Energetics of stress production in isolated cardiac trabeculae from the rat. Am J Physiol Heart Circ Physiol. 2010;299:H1382–1394. doi: 10.1152/ajpheart.00454.2010. [DOI] [PubMed] [Google Scholar]
- Han J-C, Taberner AJ, Nielsen PMF, Loiselle DS. Interventricular comparison of the energetics of contraction of trabeculae carneae isolated from the rat heart. J Physiol. 2013;591:701–717. doi: 10.1113/jphysiol.2012.242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog JM, Verdouw PD, Klompe M, Lamers JM. Dietary mackerel oil in pigs: effect on plasma lipids, cardiac sarcolemmal phospholipids and cardiovascular parameters. J Nutr. 1987;117:1371–1378. doi: 10.1093/jn/117.8.1371. [DOI] [PubMed] [Google Scholar]
- Kang JX. Reduction of heart rate by omega-3 fatty acids and the potential underlying mechanisms. Front Physiol. 2012;3 doi: 10.3389/fphys.2012.00416. doi: 10.3389/fphys.2012.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–2022. [PubMed] [Google Scholar]
- Kiriazis H, Gibbs CL. Effects of ageing on the activation metabolism of rat papillary muscles. Clin Exp Pharmacol Physiol. 2001;28:176–183. doi: 10.1046/j.1440-1681.2001.03416.x. [DOI] [PubMed] [Google Scholar]
- Krams R, ten Cate FJ, Carlier SG, van der Steen AFW, Serruys PW. Diastolic coronary vascular reserve: a new index to detect changes in the coronary microcirculation in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:670–677. doi: 10.1016/j.jacc.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Lemieux H, Blier PU, Tardif J-C. Does membrane fatty acid composition modulate mitochondrial functions and their thermal sensitivities? Comp Biochem Physiol A Mol Int Physiol. 2008;149:20–29. doi: 10.1016/j.cbpa.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Loiselle DS. Exchange of oxygen across the epicardial surface distorts estimates of myocardial oxygen consumption. J Gen Physiol. 1989;94:567–590. doi: 10.1085/jgp.94.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiselle DS, Gibbs CL. Species differences in cardiac energetics. Am J Physiol. 1979;237:H90–98. doi: 10.1152/ajpheart.1979.237.1.H90. [DOI] [PubMed] [Google Scholar]
- Mawson DA, Hunter PJ, Kenwright DN, Loiselle DS. Oxygen exchange in the isolated, arrested guinea-pig heart: theoretical and experimental observations. Biophys J. 1994;66:789–800. doi: 10.1016/s0006-3495(94)80855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan PL, Abeywardena MY, Charnock JS. A comparison of the long-term effects of n-3 and n-6 polyunsaturated fatty acid dietary supplements and the action of indomethacin upon the mechanical performance and susceptibility of the rat heart to dysrhythmia. Prostaglandins Leukot Med. 1987;27:183–195. doi: 10.1016/0262-1746(87)90070-9. [DOI] [PubMed] [Google Scholar]
- McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–1485. doi: 10.1152/ajpheart.1992.263.5.H1479. [DOI] [PubMed] [Google Scholar]
- Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967;212:804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutrit. 2011;106:974–980. doi: 10.1017/S0007114511002819. [DOI] [PubMed] [Google Scholar]
- Peoples GE, Howe P, McLennan PL, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol. 2008;52:540–547. doi: 10.1097/FJC.0b013e3181911913. [DOI] [PubMed] [Google Scholar]
- Peoples GE, McLennan PL. Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. Br J Nutr. 2010;104:1771–1779. doi: 10.1017/S0007114510002928. [DOI] [PubMed] [Google Scholar]
- Pepe S, McLennan PL. Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. J Nutr. 1996;126:34–42. doi: 10.1093/jn/126.1.34. [DOI] [PubMed] [Google Scholar]
- Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardiacl oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- Royce SM, Holmes RP. The saturation and isomerization of dietary fatty acids and the respiratory properties of rat heart mitochondria. Biochem Biophys Acta. 1984;792:371–375. doi: 10.1016/0005-2760(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Skuladottir GV, Johannsson M. Inotropic response of rat heart papillary muscle to alpha 1- and beta-adrenoceptor stimulation in relation to dietary n-6 and n-3 polyunsaturated fatty acids (PUFA) and age. Pharmacol Toxicol. 1997;80:85–90. doi: 10.1111/j.1600-0773.1997.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Starnes JW, Rumsey WL. Cardiac energetics and performance of exercised and food-restricted rats during aging. Am J Physiol. 1988;254:H599–608. doi: 10.1152/ajpheart.1988.254.3.H599. [DOI] [PubMed] [Google Scholar]
- Starnes JW, Seiler KS, Bowles DK, Giardina B, Lazzarino G. Fructose-1,6-bisphosphate improves efficiency of work in isolated perfused rat hearts. Am J Physiol. 1992;262:H380–384. doi: 10.1152/ajpheart.1992.262.2.H380. [DOI] [PubMed] [Google Scholar]
- Taberner AJ, Han J-C, Loiselle DS, Nielsen PMF. An innovative work-loop calorimeter for in vitro measurement of the mechanics and energetics of working cardiac trabeculae. J Appl Physiol. 2011;111:1798–1803. doi: 10.1152/japplphysiol.00752.2011. [DOI] [PubMed] [Google Scholar]
- Tahin QS, Blum M, Carafoli E. The fatty acid composition of subcellular membranes of rat liver, heart, and brain: diet-induced modifications. Eur J Biochem. 1981;121:5–13. doi: 10.1111/j.1432-1033.1981.tb06421.x. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Urade R, Kito M. Mitochondrial function in rats is affected by modification of membrane phospholipids with dietary sardine oil. J Nutr. 1988;118:290–296. doi: 10.1093/jn/118.3.290. [DOI] [PubMed] [Google Scholar]


