Abstract
We have previously shown that GABA and glycine modulate respiratory activity in the in vitro brainstem preparations of the lamprey and that blockade of GABAA and glycine receptors restores the respiratory rhythm during apnoea caused by blockade of ionotropic glutamate receptors. However, the neural substrates involved in these effects are unknown. To address this issue, the role of GABAA, GABAB and glycine receptors within the paratrigeminal respiratory group (pTRG), the proposed respiratory central pattern generator, and the vagal motoneuron region was investigated both during apnoea induced by blockade of glutamatergic transmission and under basal conditions through microinjections of specific antagonists. The removal of GABAergic, but not glycinergic transmission within the pTRG, causes the resumption of rhythmic respiratory activity during apnoea, and reveals the presence of a modulatory control of the pTRG under basal conditions. A blockade of GABAA and glycine receptors within the vagal region strongly increases the respiratory frequency through disinhibition of neurons projecting to the pTRG from the vagal region. These neurons were retrogradely labelled (neurobiotin) from the pTRG. Intense GABA immunoreactivity is observed both within the pTRG and the vagal area, which corroborates present findings. The results confirm the pTRG as a primary site of respiratory rhythm generation, and suggest that inhibition modulates the activity of rhythm-generating neurons, without any direct role in burst formation and termination mechanisms.
Introduction
The isolated brainstem of the adult lamprey spontaneously generates respiratory neuronal activity in vitro. The vast majority of respiratory motoneurons are located in the facial, glossopharyngeal and, especially, in the vagal nuclei (Rovainen, 1974, 1977, 1979; Guimond et al. 2003), while the paratrigeminal respiratory group (pTRG) that is responsible for the respiratory rhythm generation, i.e. the respiratory central pattern generator (CPG), is located in a region rostral to the trigeminal motor nucleus (Mutolo et al. 2007, 2010, 2011; Cinelli et al. 2013).
Endogenously released excitatory amino acids play a crucial role in the respiratory rhythmogenesis through an action within the pTRG (Bongianni et al. 1999; Martel et al. 2007; Cinelli et al. 2013), and the pTRG itself contains glutamatergic neurons projecting to the vagal respiratory motor nucleus (Cinelli et al. 2013). The respiratory motor output is abolished by bath application of ionotropic glutamate receptor antagonists, but is restored by removing inhibitory input through subsequent bath application of bicuculline and strychnine. In addition, the resumed respiratory rhythm can be suppressed by a blockade of α7 nicotinic acetylcholine receptors of pTRG neurons (Mutolo et al. 2011). Under basal conditions, blockade of GABAA and/or glycine receptors by bath application of the appropriate antagonists causes potent excitatory effects on respiration, while a blockade of GABAB receptors induces slight decreases in respiratory frequency (Rovainen, 1983; Bongianni et al. 2006). However, the sites responsible for all the above reported respiratory effects induced by GABAA, GABAB or glycine receptor blockade have not yet been identified.
The present study was carried out on in vitro brainstem preparations of the adult lamprey to investigate the role of GABA and glycine receptors within the pTRG and the vagal motoneuron region during blockade of glutamatergic transmission and under basal conditions. For this purpose, microinjections of specific antagonists acting on GABAA, GABAB and glycine receptors were performed into the pTRG and the vagal motoneuron area.
A blockade of GABA, but not glycine receptors within the pTRG proved to affect the respiratory activity. Thus, the presence of GABA immunoreactivity within the respiratory network was investigated by double-labelling experiments in which retrogradely labelled pTRG neurons were identified by injections of neurobiotin into the vagal motoneuron region. Evidence was also provided that GABAergic and glycinergic inputs are present at the level of the vagal region under basal conditions by providing a strong inhibitory influence on neurons sending excitatory projections to the pTRG. In support of this finding, retrogradely labelled neurons were identified within the vagal motoneuron region by injections of neurobiotin into the pTRG.
Methods
Ethical approval
Experiments were performed on a total of 43 young adult (12–15 cm) lampreys (Petromyzon marinus). All animal care and experimental procedures were conducted in accordance with the Italian legislation and the official regulations of the European Communities Council on the use of laboratory animals (directive 86/609/EEC). The study was approved by the Animal Care and Use Committee of the University of Florence as well as by the Swedish local ethical committee (Stockholm's Norra Djurförsöksetiska Nämnd). During the investigation, all efforts were made to minimize animal suffering and to reduce the number of animals used.
Anatomical experiments
Experiments were carried out on seven lampreys. The dissection, injections, fixation and sectioning of the lamprey brains were performed as described previously (Stephenson-Jones et al. 2011, 2012a; Cinelli et al. 2013). The animals were anaesthetized with tricaine methansulfonate (100 mg l−1; MS-222; Sigma-Aldrich, St Louis, MO, USA) and transected caudally at the seventh gill. The dorsal skin, muscles and cartilage were removed to expose the brain. During the dissection and the injections, the head was pinned down and submerged in ice-cooled physiological solution (in mm): 91 NaCl, 2.1 KCl, 2.6 CaCl2, 1.8 MgCl2, 4 glucose, 23 NaHCO3. The solution was continuously bubbled with 95% O2–5% CO2 to oxygenate and maintain the pH at 7.4.
Retrograde tracing
All injections were made with glass micropipettes (borosilicate, 1.5 mm outer diameter, 1.17 mm inner diameter) with a tip diameter of 10–20 μm. The micropipettes were fixed in a holder, which was attached to an air supply and a micromanipulator. Neurobiotin (20% in distilled water; Vector Laboratories, Burlingame, CA, USA) was pressure injected (∼50 nl) bilaterally into the region of vagal nuclei, where the vast majority of respiratory motoneurons are located (n = 5). Injections were made at two different adjacent sites along the vagal nucleus to affect as much as possible the entire population of respiratory motoneurons. Fast Green dye (0.2%; Sigma-Aldrich) was added to the solution to aid visualization of the injected tracer and to verify that the injection was reasonably confined to the investigated region. Similarly, neurobiotin injections were performed into the pTRG (n = 2). The preparation was the same to that employed in the electrophysiological experiments (see below for further details) with the brain exposed dorsally, the roof of the isthmic region cut along the midline and the alar plates spread laterally and pinned down.
Dissection and histology
After injections, the preparations were kept submerged in the physiological solution in the dark at 4°C for 24 h to allow retrograde transport of the tracer. The brains were then dissected out of the surrounding tissue and fixed by immersion in 4% formalin, 2% glutaraldehyde and 14% of a saturated solution of picric acid in 0.1 m phosphate buffer (PB) pH 7.4 for 12–24 h, after which they were cryoprotected in 20% sucrose in PB for 3–12 h. Transverse 20 μm-thick sections were made using a cryostat, collected on gelatin-coated slides and stored at −20°C until additional processing.
Immunohistochemistry
For the immunohistochemical detection of GABA (double-labelling experiments), the primary and secondary antibodies were diluted in 1% BSA, 0.3% Triton-X 100 in 0.1 m PB. Sections were incubated overnight with a mouse monoclonal anti-GABA antibody (1:5000, mAb, 3A12, kindly donated by Dr Peter Streit, Zürich, Switzerland; see e.g. Matute & Streit, 1986; Robertson et al. 2007) raised against GABA conjugated to BSA with gluteraldehyde. Sections were subsequently incubated with a mixture of Cy3-conjugated donkey anti-mouse IgG (GABA; 1:500; Jackson ImmunoResearch, West Grove, PA, USA) and Alexa fluor-488 conjugated streptavidin (1:1000; Jackson ImmunoResearch) for 2 h. In the experiments in which neurobiotin injections were performed into the pTRG, sections were incubated with Alexa fluor-488 conjugated streptavidin (1:1000) for 2 h. In all instances, sections were coverslipped with glycerol containing 2.5% diazabicyclooctane (Sigma-Aldrich).
Analysis
Photomicrographs of key results were taken with an Olympus XM10 (Olympus Sverige AB, Stockholm, Sweden) digital camera or a Nikon DS-Fi1 (Nikon, Japan) digital camera. Illustrations were prepared in Adobe Photoshop CS2 and 4 (Adobe Systems Incorporated, San Jose, CA, USA). Images were only adjusted for brightness and contrast.
Electrophysiological experiments
Animal preparation
Experiments were performed on 36 lampreys. Animal preparation and experimental procedures were similar to those described in previous reports (Bongianni et al. 1999, 2002, 2006; Mutolo et al. 2007, 2010, 2011; Cinelli et al. 2013). Anaesthetized animals were transected below the gills. Muscles and connective tissues were removed and the isolated brain-spinal cord was mounted dorsal-side up in a Sylgard-lined recording chamber continuously perfused with a cold physiological solution with the same characteristics described above using a peristaltic pump. The chamber volume was 3.0 ml and the perfusion rate was set at 2.5 ml min−1. Bath temperature was maintained at 9–10 °C. The brain was exposed and the choroid plexus removed; the brain tissue rostral to the optic tectum was cut and removed. Caudally, a transection was made caudal to the obex. The isolated brainstem of the adult lamprey spontaneously generates respiratory neuronal activity in vitro (fictive respiration); this activity closely resembles that underlying the respiratory behaviour of intact animals (Rovainen, 1977, 1983; Thompson, 1985; Russell, 1986; Bongianni et al. 1999, 2002, 2006; Martel et al. 2007; Mutolo et al. 2007, 2010, 2011; Cinelli et al. 2013). Recordings of vagal motoneurons as well as of respiration-related neurons within the pTRG and microinjections into these regions or neighboring sites were performed under microscope control (Stemi 2000, Zeiss, Göttingen, Germany). At the level of pTRG region, these manoeuvres were facilitated by cutting the roof of the isthmic region along the midline, spreading the alar plates laterally and pinning them down. In agreement with previous reports (Mutolo et al. 2007, 2010, 2011; Cinelli et al. 2013), these procedures had no significant effect on respiratory activity.
Recording procedures
Efferent respiratory activity was recorded bilaterally from the vagal nerves by means of suction electrodes. The signals were amplified, full-wave rectified and integrated (low-pass filter, time constant 10 ms). In control trials, brainstem preparations spontaneously produced a stable and regular fictive respiratory rhythm for at least 12 h (Thompson, 1985; Bongianni et al. 1999, 2002, 2006; Mutolo et al. 2007, 2010, 2011; Cinelli et al. 2013). Extracellular activity was recorded with fine (0.1 mm shaft diameter) tungsten microelectrodes (5 MΩ impedance at 1 kHz) from small groups of pTRG neurons (pauci-unit recordings) and processed in the same way as the vagal activity. Intracellular neuronal recordings from vagal motoneurons were performed using glass microelectrodes filled with 3 m potassium acetate (resistance 70–80 MΩ). The signals were amplified by an Axoclamp-2A amplifier (Molecular Devices, Sunnyvale, CA, USA) in bridge mode. The obex was used as a standard point of anatomical reference to evaluate coordinates of recording and microinjection sites. Neuronal activity was recorded from vagal motoneurons (0.4–0.6 mm rostral to the obex, 0.3–0.4 mm lateral to the midline and 0.15–0.25 mm below the dorsal surface of the rhombencephalon) as well as from respiration-related neurons of the pTRG (1.8–2.0 mm rostral to the obex, 0.8–1.0 mm lateral to the midline and 0.25–0.3 mm below the dorsal surface that, given the arrangement of our preparations in the electrophysiological experiments, corresponded to the tilted lateral wall of the IV ventricle). All the raw and integrated signals were acquired and analysed by a personal computer equipped with an analog-to-digital interface (50 kHz sampling rate; Digidata 1440, Molecular Devices), and appropriate software (Axoscope, Molecular Devices) was used. Offline analysis was performed using Clampfit software (Molecular Devices).
Drug application
Drugs were either applied through the perfusing solution or microinjected into the pTRG as well as into the region of vagal motoneurons on the basis of both extracellular recordings and coordinates. In each experiment, the preparation was perfused with the control solution for at least 60 min before control recordings. The following drugs were used: 100 μm D-(–)-2-amino-5-phosphonopentanoic acid (D-AP5, a NMDA receptor antagonist, Tocris Bioscience, Bristol, UK), 20 μm or 1 mm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, a non-NMDA receptor antagonist, Tocris Bioscience), 10 μm or 1 mm bicuculline methiodide (a GABAA receptor antagonist, Sigma-Aldrich), 0.2 mm gabazine (a GABAA receptor antagonists, Tocris Bioscience), 10 μm or 1 mm strychnine hydrochloride (a glycine receptor antagonist, Tocris Bioscience), and 25 mm CGP 35348 (a selective GABAB receptor antagonist, Tocris Bioscience). CNQX and D-AP5 were applied first to abolish the respiratory rhythm (Bongianni et al. 1999; Mutolo et al. 2011); then, bicuculline or strychnine were either microinjected into the pTRG or added to the bath. After completion of each drug challenge, the preparation was allowed to recover by perfusing it with the control solution. In addition, under basal conditions bilateral or unilateral microinjections of 1 mm bicuculline methiodide, 0.2 mm gabazine, 25 mm CGP 35348, 1 mm strychnine and 1 mm CNQX were performed. Drug concentrations and application times were similar to those employed in previous studies in lampreys (Bongianni et al. 1999, 2006; Ménard et al. 2007; Mutolo et al. 2011; Cinelli et al. 2013; see also Mutolo et al. 2007). All employed drugs were dissolved in distilled water made up to a stock solution and stored as small aliquots in a freezer until use. Stock solutions were diluted in the perfusing solution to the final desired concentration immediately prior to bath application or to microinjections.
Bilateral or unilateral microinjections (0.5–1 nl) of different drugs were performed by means of glass micropipettes (tip diameter 10–20 μm) and by applying pressure pulses of 50–100 ms with a Picospritzer (General Valve Corporation, Fairfield, NJ, USA) connected to the injection pipette. The injected volume was estimated by measuring the diameter of a droplet ejected from the tip of the pipette and by using the equation of a sphere. The diameters ranged from 0.10 to 0.12 mm. Bilateral microinjections were performed using a single micropipette that was withdrawn after the first microinjection and then introduced contralaterally for the second injection. The interval between the two microinjections was ≤20 s. The inactive dye Fast Green (0.2%, Sigma-Aldrich) was added to the drug solution to visually assess the spread and the approximate localization of the injection. The microinjections were associated with the appearance of dye spots (∼0.2 mm diameter) confined to the investigated regions (Mutolo et al. 2007, 2011; Cinelli et al. 2013). The diameters of the dye spots were slightly greater than those of the ejected droplets due to the diffusion of the injected solution according to several complex factors involving the presence of cells of different shape and the extracellular space. The localization of the pTRG was judged by the position of the dye spot with respect to the sulcus limitans of His and the isthmic Müller cell I1 (Cinelli et al. 2013). The depth of the injection (∼0.3 mm below the dorsal surface) was inferred from that of rhythmic extracellular neuronal activity previously recorded in each preparation. Control microinjections of the effective drugs were performed at sites 0.4 mm away from the pTRG, i.e. into the trigeminal motor nucleus or its lateral border, as well as at more rostral sites into the mesencephalic region. Localization of the vagal motoneuron pool was judged on the basis of the dye spot position relative to landmarks of the vagal nucleus easily visible under microscope control. The depth of the injection (∼0.2 mm below the dorsal surface) was inferred from that of rhythmic extra- or intracellular neuronal activity previously recorded in each preparation. On some occasions (n = 3), green fluorescent latex microspheres (LumaFluor, New City, NY, USA) were added (dilution 1:3) to the injectate (1 mm bicuculline) for post hoc confirmation of injection sites within the pTRG and the vagal motor nucleus. Control microinjections of equal volumes of the vehicle solution with 0.2% Fast Green dye were also made. In additional experiments, unilateral cuts (hemisections) were made with fine scissors across the caudal part of the trigeminal nucleus to separate the pTRG region from the vagal motoneurons. Then, unilateral microinjections of 1 mm bicuculline or 1 mm strychnine were performed into the vagal motoneurons of the hemisected side. In another series of experiments, unilateral microinjections of 1 mm bicuculline or 1 mm strychnine into the vagal motoneurons were performed during apnoea caused by unilateral microinjections of 1 mm CNQX into the ipsilateral pTRG.
Histology
Injection sites were localized in brainstems fixed (4% formalin in 0.1 m phosphate buffer, pH 7.4, overnight), cryoprotected with 30% sucrose, frozen, and cut at 20 μm thickness on a cryostat. Coronal sections stained with Cresyl Violet were used for the histological control. Sections were examined in a light and/or epifluorescence microscopy (Eclipse E400 Nikon) equipped with the Nikon Intensilight C-HGFI mercury-fibre illuminator. Photomicrographs of key results were taken with a Nikon DS-Fi1 digital camera. Illustrations were prepared in Adobe Photoshop CS2 and 4 (Adobe Systems Incorporated). Images were only adjusted for brightness and contrast.
Data analysis
Respiratory frequency (cycles min−1), vagal burst duration (ms, measured on raw activity), and peak amplitude of integrated vagal activity (taken as an index of the intensity of vagal bursts, arbitrary units) were measured and averaged for 20 s in the period immediately preceding each trial (control values) and, unless otherwise stated, at 2 min intervals during drug application and after recovery. Average values of respiratory variables observed in control conditions, at the time when the maximum response occurred and after recovery were considered for statistical analysis (Sigma Stat; SPSS, Chicago, IL, USA). Student's paired t tests were employed to evaluate changes in respiratory variables induced by bicuculline either microinjected into the pTRG or added to the bath during apnoea caused by CNQX–d-AP5. In microinjection trials, respiratory variables were measured and averaged for 20 s immediately before the microinjections, at the time when the maximum response occurred and after recovery. One-way repeated-measures ANOVA followed by Student–Newman–Keuls tests was used to assess the effects of drug microinjections. To analyse changes induced by drug microinjections into neighbouring sites 0.4 mm away from the pTRG region, comparisons between mean values of respiratory variables before and ∼5 min after microinjections in the same preparation were performed by means of unpaired t tests. Paired t tests were used to evaluate the effects caused by vehicle microinjections. Changes in respiratory variables were also expressed as percentage variations of control values. The number of preparations is indicated by n. The same preparations could be used for different types of trials. All values are presented as means ± SEM; P < 0.05 was considered as significant.
Results
Rhythmogenic role of GABAA receptors during ionotropic glutamate receptor blockade
The respiratory role of GABAA and glycine receptors during ionotropic glutamate receptor blockade was studied in six preparations. In agreement with previous findings (Bongianni et al. 1999; Mutolo et al. 2011), bath application of 20 μm CNQX and 100 μm d-AP5 caused the cessation of the respiratory rhythm within ∼20 min. Figure 1A illustrates respiratory activity under control conditions and the apnoea caused by ionotropic glutamate receptor blockade (AMPA and NMDA). Following bilateral microinjections of 1 mm bicuculline (0.5–1 pmol; n = 3) into the pTRG, respiratory activity resumed after a few seconds and reached a maximum after 3–4 min. The effects were similar to those previously observed following bath application of bicuculline and strychnine during apnoea induced by bath application of CNQX and d-AP5 or following bath application of a cocktail solution containing antagonists of both fast synaptic excitatory and inhibitory transmission (see Fig. 4 in Mutolo et al. 2011). As compared with control levels, the resumed respiratory frequency was lower (32.3 ± 3.6 vs. 61.3 ± 2.4 cycles min−1; P < 0.05), whereas the duration of vagal bursts was longer (53.1 ± 3.2 vs. 29.2 ± 1.2 ms; P < 0.05), in the absence of concomitant changes in peak amplitude (Fig. 1A, right). The activity disappeared progressively within 8–10 min of the injections. In the same preparations, bilateral microinjections of 1 mm bicuculline into the vagal motoneuron region (n = 3) did not restore respiratory rhythm, but caused the appearance of a low level of tonic vagal activity (Fig. 1A).
Figure 1. Role of GABAA receptors in the resumption of respiratory activity during blockade of ionotropic glutamate receptors.
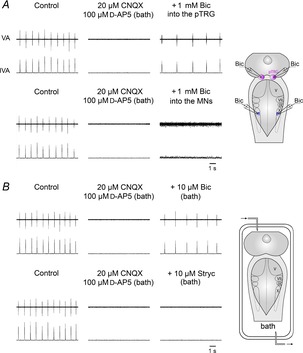
A, in one preparation, during apnoea caused by bath application of 20 μm CNQX and 100 μm d-AP5, bilateral microinjections of 1 mm bicuculline (Bic) into the pTRG restored the respiratory rhythm within 4 min after the completion of the injections, while similar microinjections into the region of vagal motoneurons (MNs) caused the appearance of a low level of tonic vagal activity. The sites (pink area, pTRG; blue area, vagal MN region) where Bic was microinjected are shown on a schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon. B, in another preparation, bath application of 20 μm CNQX and 100 μm d-AP5 abolished the respiratory rhythm that was restored by 10 μm Bic, but not by 10 μm strychnine (Stryc). A schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon in the perfused recording chamber is shown. pTRG, paratrigeminal respiratory group; V, trigeminal motor nucleus; VII, facial motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus; VA, raw vagal nerve activity; IVA, integrated vagal nerve activity.
Figure 1B illustrates that the resumption of respiratory activity during blockade of ionotropic glutamate receptors was also obtained by bath application of 10 μm bicuculline (n = 3). Respiratory activity resumed at a reduced frequency and an increased vagal burst duration. This activity started after ∼1 min and after ∼10 min displayed characteristics similar to those described above following bicuculline microinjections into the pTRG. Thereafter, the rhythmic activity gradually disappeared within 20 min of washout. In contrast, bath application of 10 μm strychnine (n = 3) did not restore the respiratory rhythm during apnoea caused by CNQX–d-AP5 (Fig. 1B). Similarly, respiratory activity did not resume (not shown) following bilateral microinjections of 1 mm strychnine (0.5–1 pmol) performed into the pTRG (n = 2) or the vagal motoneuron region (n = 2).
The localization of bicuculline injections within the pTRG and the vagal motor nucleus is illustrated in Fig. 2. It closely corresponds to that illustrated in our previous reports (Mutolo et al. 2007, 2011). The injected area has a diameter of ∼0.2 mm in agreement with the measurement of the dye spots generated by the microinjections. Control microinjections of equivalent volumes of the vehicle solution containing 0.2% Fast Green at the responsive site (n = 3) did not cause any obvious respiratory response. Bilateral microinjections of bicuculline into neighbouring sites 0.4 mm away from the pTRG region (n = 3) did not restore respiratory rhythm.
Figure 2. Localization of injection sites.
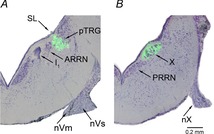
Photomicrographs of transverse sections of the rhombencephalon showing the location of the fluorescent beads (green) added to 1 mm bicuculline microinjected into the pTRG (A) and the vagal motor nucleus (B). Sections are counterstained with Cresyl Violet. Light-field and fluorescence photomicrographs have been superimposed. ARRN, anterior rhombencephalic reticular nucleus; I1, isthmic Müller cell; nVm, motor root of the trigeminal nerve; nVs, sensory root of the trigeminal nerve; nX, vagal nerve; PRRN, posterior rhombencephalic reticular nucleus; pTRG, paratrigeminal respiratory group; SL, sulcus limitans of His; X, vagal motor nucleus.
Distribution of GABA immunoreactivity within the pTRG and the vagal motor nucleus
The presence of GABA immunoreactivity within the pTRG and the vagal motor nucleus was investigated by double-labelling experiments (n = 5). Examples of retrogradely labelled neurons in the pTRG region identified by bilateral injections of neurobiotin into the vagal motoneuron pool are illustrated in Fig. 3A and C (merged, neurobiotin (green) + GABA immunoreactivity (red) signals). In agreement with our recent results (Cinelli et al. 2013), retrogradely labelled neurons were confined within a small area of the rostral rhombencephalon/isthmic region in the dorsal aspect of the anterior rhombencephalic reticular nucleus (ARRN) at the level of the isthmic Müller cell I1, close to the sulcus limitans of His (green signal, Fig. 3A). The location corresponds closely to that already reported in our previous studies for the pTRG (Mutolo et al. 2007, 2011). As illustrated in Fig. 3A, retrogradely labelled neurons were lacking GABA but they were peppered with GABA-immunoreactive structures (red signal). Figure 3B shows at higher magnification retrogradely labelled pTRG neurons (green signal) and GABA immunoreactivity (red signal) in the same section. No GABA-immunoreactive cells were intermingled with the population of pTRG neurons. GABA-immunopositive fibres also surrounded unlabelled cells located within the pTRG area. Intense GABA immunoreactivity was also found within the vagal motoneuron region (Fig. 3C). Figure 3D and E illustrates at higher magnification the cell bodies of vagal motoneurons (green signal) and GABA-immunoreactive structures (red signal) in the same section. The merged image (Fig. 3F) shows that vagal motoneurons were surrounded by GABAergic fibres.
Figure 3. Distribution of GABA immunoreactivity in the pTRG region and the vagal motor nucleus.
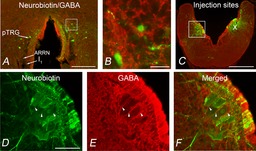
A, photomicrograph of a transverse section from the isthmic region at the level of the pTRG showing retrogradely labelled neurons (green) after bilateral injections of neurobiotin into the vagal motoneuron pools. GABA immunoreactivity is shown in red. B, photomicrograph at a higher magnification of the portion of the transverse section (white box in A) showing retrogradely labelled neurons in the pTRG surrounded by GABA-immunoreactive structures. C, photomicrograph of a transverse section in the caudal rhombencephalon showing the site of bilateral neurobiotin injections into the vagal motor nuclei. The distribution of GABA immunoreactivity is shown in red. D–F, photomicrographs at a higher magnification of the portion of the transverse section indicated by the white box in C. Arrowheads point to GABAergic fibres surrounding the cell bodies of vagal motoneurons. D, vagal motoneurons stained by neurobiotin injection. E, GABA-immunoreactive structures. F, merged image. Scale bars, 200 μm in A, 25 μm in B, 500 μm in C, 100 μm in D–F. ARRN, anterior rhombencephalic reticular nucleus; I1, isthmic Müller cell; pTRG, paratrigeminal respiratory group; X, vagal motor nucleus.
Role of GABAA receptors within the pTRG
The role of GABAA receptors within the pTRG in the absence of ionotropic glutamate receptor blockade was investigated in nine preparations. Bilateral microinjections of 1 mm bicuculline (0.25–0.5 pmol; n = 5) or 0.2 mm gabazine (0.1–0.2 pmol; n = 4) into the pTRG caused the appearance within 20–30 s of patterns of breathing characterized by irregular, prolonged vagal bursts (seizure-like activity) associated with tonic activity. Thereafter, a pattern of breathing characterized by rhythmic activity assembled in multiple bursts progressively developed and stabilized in a double-burst pattern (interburst interval 80.5 ± 7.1 ms) within ∼5 min (Fig. 4A and B). The specific GABAA receptor antagonist gabazine was used since it has been reported that bicuculline, in addition to its effects on GABAA receptors, affects calcium-dependent K+ channels (Debarbieux et al. 1998; Schmitt et al. 2004). Figure 4B shows in more detail the effects observed on the respiratory motor output as well as on the intracellular activity of a vagal motoneuron 5 min following the injections of 0.2 mm gabazine into the pTRG. The interval between vagal duplets was 1.0 ± 0.05 s, leading to a frequency of 60.5 ± 2.6 cycles min−1, a value slightly, but not significantly higher than that of respiratory frequency under control conditions (59.2 ± 2.7 cycles min−1). The duration of the first and second vagal bursts of the duplets increased without concomitant changes in peak amplitude. The duration of the first and second bursts changed from 29.3 ± 0.7 ms (control value) to 36.1 ±1.4 ms (23.0 ± 3.6%; P < 0.05) and to 41.7 ± 2.8 ms (43.2 ± 10.8%; P < 0.01), respectively. The respiratory activity recovered within 45 min of the injections (burst duration 28.6 ± 1.2 ms). In the same preparations, bilateral microinjections of bicuculline (n = 3) or gabazine (n = 4) into neighbouring sites 0.4 mm away from the pTRG region did not cause the above described pattern of breathing nor significant changes in the control respiratory activity.
Figure 4. Respiratory role of GABAA receptors within the pTRG in one brainstem preparation.
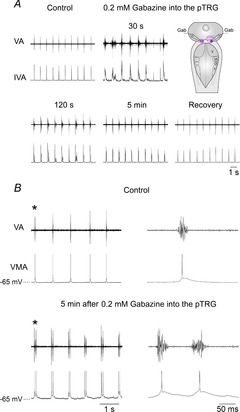
A, respiratory responses induced by bilateral microinjections of 0.2 mm gabazine into the pTRG at different time points after the completion of the injections. Traces are raw vagal nerve activity (VA) and integrated vagal nerve activity (IVA). The microinjections caused within 20–30 s the appearance of irregular, prolonged vagal bursts (seizure-like activity) associated with tonic activity. Thereafter, a respiratory activity characterized by rhythmic bursts assembled in multiple bursts developed progressively and stabilized in a double-burst pattern. The sites where gabazine (Gab) was microinjected into the pTRG (pink area) are shown on a schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon. B, bilateral microinjections of 0.2 mm gabazine into the pTRG caused the appearance of vagal bursts grouped in duplets within 5 min of the completion of the injections. Traces are raw vagal nerve activity (VA) and vagal motoneuron activity (VMA). The first single or double vagal and neuronal bursts (marked with an asterisk) are shown with an expanded time scale on the right of each trace. Note delayed repolarization in motoneuron membrane potential after gabazine. pTRG, paratrigeminal respiratory group; V, trigeminal motor nucleus; VII, facial motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus.
Role of GABAA receptors within the vagal motoneuron region
To verify whether the vagal motoneuron regions have a role in the mediation of excitatory effects on respiration caused by bath application of bicuculline (Bongianni et al. 2006), we performed bilateral or unilateral microinjections of bicuculline into these regions in a total of ten preparations (Fig. 5A). Bilateral microinjections of 1 mm bicuculline (n = 5) increased respiratory frequency from 66.3 ± 3.3 to 93.9 ± 2.7 cycles min−1 (43.9 ± 8.6%; P < 0.01) without significant changes in peak amplitude and duration of vagal bursts. The respiratory responses reached a maximum within ∼4 min. Respiratory frequency recovered within 30 min of the injections (66.9 ± 3.1 cycles min−1). Unilateral bicuculline microinjections (n = 5) caused similar, but less pronounced bilateral effects on respiratory frequency (from 63.0 ± 5.1 to 80.3 ± 5.8 cycles min−1; 27.8 ± 2.2%; P < 0.05). The respiratory responses reached a maximum within ∼4 min and the recovery was achieved in 20 min (63.7 ± 5.5 cycles min−1). Pauci-unit recordings (n = 3) from pTRG neurons displayed rhythmic activity synchronous with vagal bursts (Fig. 5A). Respiratory effects induced by unilateral GABAA receptor blockade within the vagal motoneuron region were prevented by a hemisection between the pTRG and the vagal motoneuron pool. It is worth noting that the rhythmic respiratory activity ceased on the hemisected side, but it was still present, although slightly depressed, on the contralateral side (n = 3; Fig. 5B). Unilateral bicuculline microinjections into the region of vagal motoneurons of the hemisected side caused a low level of tonic vagal activity only on the ipsilateral side. Similarly, increases in respiratory frequency caused by bicuculline microinjections into the vagal motoneurons were prevented by 1 mm CNQX (0.5–1 pmol; n = 3) microinjected into the pTRG. In agreement with our previous results (Cinelli et al. 2013), unilateral CNQX microinjections caused the suppression of rhythmic activity on both sides within ∼1 min for 6–10 min. During these apnoeic effects, unilateral bicuculline microinjections into the vagal motoneuron region on the CNQX injection side provoked the appearance of low levels of tonic vagal activity only in the ipsilateral vagal nerve (Fig. 5C).
Figure 5. Respiratory role of GABAA receptors within the vagal motor nucleus.
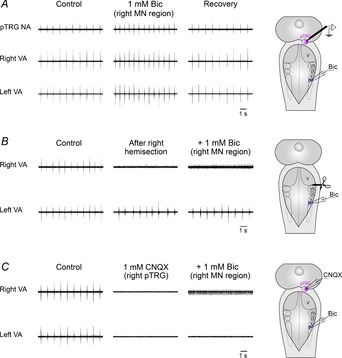
A, marked increases in respiratory frequency ∼4 min after a unilateral microinjection of 1 mm bicuculline (Bic) into the region of vagal motoneurons (MN region) in one preparation. Note the excitatory effects on pTRG neuronal activity (pauci-unit recordings; NA) as well as on the right and left raw vagal nerve activity (VA). Sites where extracellular recordings of pTRG neurons (pink area) and Bic microinjections (blue area) were performed are shown on a schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon. B, in another preparation, hemisection of the brainstem between the pTRG and the vagal motoneuron region abolished the ipsilateral vagal output and slightly reduced the contralateral vagal bursts. Unilateral microinjection of 1 mm Bic into the region of vagal motoneurons on the hemisected side caused slight tonic activity in the ipsilateral vagal output, but did not change the pattern of rhythmic activity on the contralateral side. A schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon showing the hemisection (scissors) and the Bic microinjection site (blue area) is reported. C, during apnoea caused by 1 mm CNQX unilateral microinjection into the pTRG, unilateral microinjection of 1 mm Bic into the region of vagal motoneurons of the same side caused the appearance of a low level of tonic vagal activity on the ipsilateral side of another preparation. Injection sites (pink area, pTRG; blue area, vagal MN region) are schematically depicted on a dorsal view of the lamprey mesencephalon–rhombencephalon. pTRG, paratrigeminal respiratory group; V, trigeminal motor nucleus; VII, facial motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus.
Projections from the vagal motoneuron region to the pTRG
To further support the results observed following bicuculline microinjections into the vagal motoneuron region, a tracer (neurobiotin) was injected into the pTRG (n = 2) to explore if there was direct projection from the vagal area to the pTRG. Figure 6 shows examples of retrogradely labelled neurons within the vagal motor region (green signal) and the injection site into pTRG (green signal). Retrogradely labelled neurons were found mainly in the rostral and central portions of the ipsilateral vagal motoneuron region either intermingled with vagal motoneurons or in close vicinity to them. In these experiments, our attention was only focused on the region of the vagal motor nucleus. Labelled neurons were round or bipolar and the largest diameters of their soma varied from 9 to 20 μm. In contrast, vagal motoneurons were much larger and most of them had a piriform or elongated shape (see also Rovainen, 1974; Guimond et al. 2003).
Figure 6. Retrogradely labelled neurons in the vagal motoneuron region.
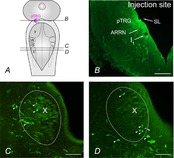
A, schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon showing the levels of the coronal sections illustrated in B–D (continuous lines) and the location of the pTRG (pink area). B, photomicrograph of a transverse section of the isthmic region showing the site of neurobiotin injection into the pTRG (green signal). C and D, photomicrographs of transverse sections, 100 μm apart, of the vagal motoneuron region (dotted line) showing retrogradely labelled neurons (green signal) after an injection of neurobiotin into the pTRG. Arrowheads point to some retrogradely labelled neurons. ARRN, anterior rhombencephalic reticular nucleus; I1, isthmic Müller cell; pTRG, paratrigeminal respiratory group; SL, sulcus limitans of His; V, trigeminal motor nucleus; VII, facial motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus. Scale bars, 200 μm in B, 100 μm in C and D.
Role of GABAB receptors
In five preparations, bilateral microinjections of the GABAB receptor antagonist CGP 35348 were performed into either the pTRG or the vagal motoneuron pool to ascertain whether these neural structures have a role in the GABAB receptor modulation of respiratory activity (Bongianni et al. 2006). Bilateral microinjections of 25 mm CGP 35348 (12.5–25 pmol; n = 5) into the pTRG significantly decreased respiratory frequency from 63.4 ± 2.5 to 51.8 ± 1.3 cycles min−1 (18.0 ± 4.8%; P < 0.05) without significant changes in the duration and amplitude of vagal bursts (Fig. 7A). The respiratory effect started within 4–5 min and reached a maximum within ∼15 min; a complete recovery was achieved after 30 min (63.8 ± 2.1 cycles min−1). Bilateral microinjections of CGP 35348 into neighbouring sites 0.4 mm away from the pTRG region (n = 3) did not cause any obvious or consistent effects. As shown in Fig. 7B, bilateral microinjections of 25 mm CGP 35348 (12.5–25 pmol; n = 4) into the region of vagal motoneurons did not cause any obvious effect.
Figure 7. Respiratory role of GABAB receptors.
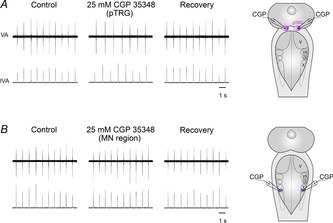
A, decreases in respiratory frequency ∼15 min after bilateral microinjections of 25 mm CGP 35348 into the pTRG in one preparation. B, absence of appreciable respiratory effects ∼15 min after bilateral microinjections of 25 mm CGP 35348 into the region of vagal motoneurons (MN region) in a different preparation. The sites (pink area, pTRG; blue area, vagal MN region) where CGP 35348 (CGP) was microinjected are shown on a schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon. pTRG, paratrigeminal respiratory group; V, trigeminal motor nucleus; VII, facial motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus; VA, raw vagal nerve activity; IVA, integrated vagal nerve activity.
Role of glycine receptors
In six preparations, microinjections of 1 mm strychnine were performed into the pTRG and the vagal motoneuron pool to verify whether these regions are sites of action of strychnine applied to the bath under basal conditions (Bongianni et al. 2006). Bilateral microinjections of 1 mm strychnine (0.5–1 pmol) performed into the pTRG (n = 3) did not provoke any effect (Fig. 8A). Conversely, bilateral or unilateral microinjections of 1 mm strychnine into the region of vagal motoneurons caused marked increases in respiratory frequency without significant changes in peak amplitude and duration of vagal bursts (Fig. 8B). Bilateral microinjections of 1 mm strychnine (n = 4) increased respiratory frequency from 62.8 ± 2.1 to 87.3 ± 4.4 cycles min−1 (38.8 ± 4.9%; P < 0.005), and the respiratory responses reached a maximum within 3 min. The recovery was observed within 30 min of the injections (63.0 ± 1.8 cycles min−1). Unilateral strychnine microinjections (n = 4) caused slightly less pronounced bilateral effects; the respiratory frequency increased from 63.5 ± 1.3 to 81.6 ± 3.9 cycles min−1 (28.6 ± 6.2%; P < 0.01). The respiratory responses reached a maximum within ∼3 min and the recovery was achieved within 20 min (63.2 ± 1.8 cycles min−1). As reported above for GABAA receptor blockade, respiratory effects induced by unilateral glycine receptor blockade within the vagal motoneuron region were also prevented by a hemisection between the pTRG and the vagal motoneuron region (n = 2) or by 1 mm CNQX microinjections (n = 2) into the pTRG (not shown). Also these outcomes are strongly corroborated by the finding of retrogradely labelled neurons identified within the vagal region by injections of neurobiotin into the pTRG (see Fig. 6).
Figure 8. Respiratory role of glycine receptors.
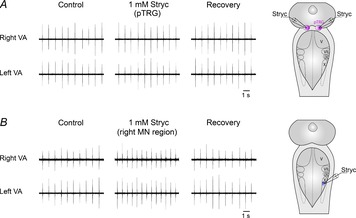
A, absence of appreciable respiratory effects ∼3 min after bilateral microinjections of 1 mm strychnine (Stryc) into the pTRG in one preparation. B, marked increases in respiratory frequency ∼3 min after a unilateral microinjection of 1 mm Stryc into the region of vagal motoneurons (MN region) in a different preparation. The sites (pink area, pTRG; blue area, vagal MN region) where Stryc was microinjected are shown on a schematic illustration of a dorsal view of the lamprey mesencephalon–rhombencephalon. pTRG, paratrigeminal respiratory group; V, trigeminal motor nucleus; VII, facial motor nucleus; IX, glossopharyngeal motor nucleus; X, vagal motor nucleus; VA, raw vagal nerve activity; IVA, integrated vagal nerve activity.
Discussion
This study demonstrates that the removal of GABAergic, but not glycinergic inhibition within the pTRG causes the resumption of rhythmic respiratory activity during apnoea caused by blockade of ionotropic glutamate receptors. Present data indicate that within the pTRG, under basal conditions, there is a modulatory control exerted by GABAA and GABAB receptors, while a glycinergic modulation is lacking. On the other hand, the present study is the first to provide evidence that GABAergic and glycinergic inputs to neurons within the vagal motoneuron region are involved in the regulation of respiratory frequency via ascending excitatory projections to the pTRG. Accordingly, retrogradely labelled neurons were identified in the vagal area by injections of neurobiotin into the pTRG. The results on the respiratory role of GABA are corroborated by the presence of intense GABA immunoreactivity within the pTRG and the vagal motoneuron region. These findings are represented in a schematic drawing together with earlier results (Fig. 9).
Figure 9. Schematic drawing representing present and previous findings on the connectivity within the respiratory network and relevant neurotransmitter influences.
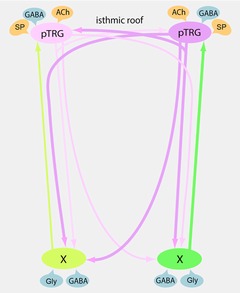
The pTRG region is shown with its projections (pink) to ipsilateral and contralateral vagal motoneuron regions and to the contralateral pTRG (Gariépy et al. 2012; Cinelli et al. 2013). Excitatory (yellow) and inhibitory (blue) influences on the pTRG region (present findings and Mutolo et al. 2010, 2011; Cinelli et al. 2013) and the vagal motoneuron region (present findings) are illustrated. Newly identified excitatory projections to the pTRG (green) from neurons located in the vagal area have also been reported. ACh, acetylcholine; GABA, γ-aminobutyric acid; Gly, glycine; pTRG, paratrigeminal respiratory group region; SP, substance P; X, vagal motoneuron region.
GABAergic mechanisms within the pTRG
In agreement with our previous discussion on the role of inhibition in the respiratory rhythm generation in the lamprey (Bongianni et al. 2006) and in the rabbit (Bongianni et al. 2010), the involvement of inhibitory amino acids in the respiratory regulation is controversial. In this context, it is of interest to recall that the respiratory rhythmogenesis persists after a blockade of synaptic inhibition, not only in neonatal rodent preparations (reviewed in Feldman et al. 2013), but also in the adult lamprey (Rovainen, 1983; Bongianni et al. 2006; see also present results) and in the adult turtle (Johnson et al. 2002, 2007). In addition, a blockade of synaptic inhibition in the pre-metamorphic tadpole abolishes fictive gill ventilation, but not lung ventilation (Galante et al. 1996; Broch et al. 2002). These findings are consistent, at least to some extent, with recent results in adult mammals (e.g. Bongianni et al. 2010; Feldman et al. 2013; Janczewski et al. 2013; see Kam et al. 2013 for further references).
Here, we show, using microinjection techniques, that the pTRG is under a modulatory control exerted only by GABA acting on GABAA and GABAB receptors to fine tune the respiratory CPG. Endogenous activation of GABAA receptors seems to act on the mechanisms generating respiratory bursts with little influence on the interburst period and may conceivably represent a mechanism to control mainly the excitability of pTRG neurons and to generate a more regular respiratory rhythm. Consistent with our interpretation, a pattern of breathing characterized by the presence of double bursts has recently been reported by increasing neuronal excitability of the preBötzinger complex (preBötC), the proposed mammalian respiratory CPG, through blockade of inhibitory synaptic transmission both in in vitro and in vivo preparations (Kam et al. 2013). The observed decrease in respiratory frequency due to blockade of GABAB receptors is mediated at the level of the pTRG. This effect could tentatively be explained by the presence of presynaptic GABAB receptors exerting an inhibitory control on GABA release (Bettler et al. 2004). Noticeably, the GABAergic system is also a strong modulator of the lamprey locomotor activity, but is not required for burst generation (Tegner et al. 1993; Schmitt et al. 2004; Grillner & Jessell, 2009).
The prominent role of GABAergic mechanisms within the pTRG is especially emphasized by the fact that the resumption of rhythmic activity occurs only following the application of bicuculline during apnoea caused by blockade of ionotropic glutamate receptors. As recently discussed (Mutolo et al. 2011), this event is obviously caused by disinhibition phenomena at the level of the pTRG containing both glutamatergic and cholinergic neurons. The present findings are also consistent with previous results on the resumption of respiratory rhythm after substance P or nicotine microinjections into the pTRG that increase network excitability during CNQX–d-AP5-induced apnoea (Cinelli et al. 2013). The results on the respiratory role of GABA within the pTRG are also corroborated by the presence of intense GABA immunoreactivity in this region.
GABAergic and glycinergic control of an ascending excitatory pathway to the pTRG
This study provides evidence that GABAergic and glycinergic systems are active at the level of the vagal motoneuron region, providing a marked depression of local neurons sending ascending excitatory projections to the pTRG. In fact, the increases in respiratory frequency caused by a blockade of GABAA and glycine receptors within the vagal area were prevented by the hemisection between the pTRG and the vagal motoneuron region or by CNQX microinjections into the pTRG. These findings are confirmed by the presence of retrogradely labelled neurons within the vagal area after neurobiotin injections into the pTRG.
Increases in respiratory frequency caused by bath application of bicuculline or strychnine (Rovainen, 1983; Bongianni et al. 2006) were mimicked by microinjections of these drugs within the vagal motoneuron region. The effects are conceivably not due to a direct action on vagal motoneurons since they were not retrogradely labelled from the pTRG. Our finding that GABA immunoreactivity is present within the vagal motoneuron region is consistent with previous observations by Robertson et al. (2007) and strongly corroborates the present neuropharmacological results. Indeed, we have found that vagal motoneurons were surrounded by GABAergic fibres, thus suggesting the presence of GABAergic synaptic inputs impinging also on them, but the functional role of these hypothetical inputs is not clear. This GABAergic inhibitory control could, at least in part, be revealed by the appearance of a low level of tonic vagal activity following microinjections of bicuculline into the vagal motoneuron region during the observed apnoeic effects. However, bicuculline applied to the vagal area never restored the respiratory rhythm, thus suggesting that this area does not possess the rhythmogenic properties hypothesized by previous studies (see e.g. Kawasaki, 1979, 1984; Thompson, 1985, 1990; Martel et al. 2007).
Functional implications
Inhibitory inputs to the respiratory network may arise from several regions of the lamprey brain where GABAergic and glycinergic neurons have been identified (Robertson et al. 2007; Villar-Cerviño et al. 2008). Interestingly, these neurons have been found within or in close vicinity to the facial, glossopharyngeal and vagal nuclei, and the nucleus tractus solitarii (NTS). In particular, since the NTS receives fibres from the afferent divisions of facial, glossopharyngeal and vagal nerves (Nieuwenhuys, 1972), we may tentatively propose that a potent inhibitory GABAergic and glycinergic control could be exerted at the NTS level by local interneurons and possibly by other unidentified sources onto second-order sensory neurons which may provide different influences on respiratory activity including those excitatory on the pTRG. These findings are consistent with the functional organization of NTS neurons in mammals (e.g. Miyazaki et al. 1999; Ezure & Tanaka, 2004; Bonham et al. 2006; Kubin et al. 2006; Bailey et al. 2008; Kang et al. 2012).
Among the possible sources of GABAergic inhibition to pTRG neurons, there is a population of small quite strongly labelled GABA-immunoreactive cells in the isthmic region (see also Fig. 4 in Robertson et al. 2007), i.e. in a location very close to the pTRG. These cells could play a role in the control of excitability of pTRG neurons. However, since the removal of GABAergic inhibition within the pTRG does not affect substantially the respiratory frequency, the observed inhibitory control of respiratory frequency appears to be exerted at the level of neurons located in the vagal area sending projections to the pTRG, rather than directly on the pTRG.
The pTRG shows many similarities (Mutolo et al. 2007, 2010, 2011; Cinelli et al. 2013) with the preBötC (Smith et al. 2000; Feldman & Del Negro, 2006; Bongianni et al. 2010; Feldman et al. 2013). In agreement with Kinkead (2009), we believe that the lamprey pTRG displays a high homology not only with the mammalian preBötC, but also with the neural mechanisms generating lung ventilation in amphibians (Wilson et al. 2002; Vasilakos et al. 2005; Chen & Hedrick, 2008) and turtles (Johnson et al. 2002, 2007), rather than with those that generate the gill rhythm in tadpoles (Galante et al. 1996; Broch et al. 2002). All these different rhythm generators are located rostrally in the medulla, have as a possible underlying rhythm generating mechanism the ‘group-pacemaker’ model, opioid sensitivity and, at least in frogs and mammals, substance P sensitivity. As far as birds are concerned, they show a general organization of the neural structures subserving the respiratory control very similar to that of mammals, although the functional characteristics of the respiratory CPG have not yet been investigated (Chatonnet et al. 2006; McLean et al. 2013). Indeed, in the lamprey the active phase is expiration, thus the pTRG should more appropriately correspond to the retrotrapezoid nucleus/parafacial respiratory group, i.e. the rostral expiratory oscillator in mammals (see e.g. Thoby-Brisson et al. 2009; Onimaru et al. 2009; Guyenet & Mulkey, 2010; Feldman et al. 2013; Smith et al. 2013). However, like the preBötC, the retrotrapezoid nucleus/parafacial respiratory group consists of glutamatergic neurons that express neurokinin 1 receptors, but is not sensitive to opioids. In addition, it should be kept in mind that the pTRG is a small neuronal population generating a simple breathing pattern and could correspond only to a component of the complex circuitry underlying respiratory rhythm generation in mammals. The latter comprises reciprocal interactions in the preBötC and Bötzinger complex that may contribute to generation of the normal three-phase respiratory pattern (Smith et al. 2007, 2013).
In conclusion, the inhibitory influences exerted by GABA receptors within the pTRG and by GABA and glycine receptors within the vagal area may represent mechanisms suitable to regulate the excitability level of the respiratory network and to adjust ventilation to changes in sensory inputs as well as to different behavioural and environmental conditions. Similarly to other neurophysiological features (Ericsson et al. 2011, 2013; Stephenson-Jones et al. 2011, 2012a,b2012b), the characteristics of inhibitory mechanisms appear to be highly conserved throughout phylogenesis and may represent a general organizational principle relevant to rhythmic activities, such as locomotion and respiration (Grillner & Jessell, 2009).
Key points
In this study we investigated the role of GABA and glycine receptors within the respiratory central pattern generator, i.e. the paratrigeminal respiratory group (pTRG), and the vagal motoneuron region of the lamprey.
Only GABA-mediated inhibition modulates the pTRG both during apnoea induced by blockade of glutamatergic transmission and under basal conditions.
Both GABA- and glycine-mediated inhibition within the vagal region are involved in the regulation of respiratory frequency via ascending excitatory projections to the pTRG.
Projecting neurons are retrogradely labelled from the pTRG, and intense GABA immunoreactivity is present within the pTRG and the vagal motoneuron region.
Inhibitory mechanisms, which appear to be evolutionarily conserved, regulate network excitability and may provide an important contribution to rhythmic activities, such as respiration and locomotion.
Glossary
- ACh
acetylcholine
- ARRN
anterior rhombencephalic reticular nucleus
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CPG
central pattern generator
- d-AP5
d-(–)-2-amino-5-phosphonopentanoic acid
- I1
isthmic Müller cell
- IVA
integrated vagal nerve activity
- GABA
γ-aminobutyric acid
- Gly
glycine
- MN
motoneuron
- nVm
motor root of the trigeminal nerve
- nVs
sensory root of the trigeminal nerve
- nX
vagal nerve
- NTS
nucleus tractus solitarii
- preBötC
preBötzinger complex
- PRRN
posterior rhombencephalic reticular nucleus
- pTRG
paratrigeminal respiratory group
- SL
sulcus limitans of His
- SP
substance P
- VA
raw vagal nerve activity
- VMA
vagal motoneuron activity
- V
trigeminal motor nucleus
- VII
facial motor nucleus
- IX
glossopharyngeal motor nucleus
- X
vagal motor nucleus
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
E.C., D.M., B.R., M.C., F.B. conducted the experiments and primary data analysis and developed the experimental design together with S.G. and T.P. All authors contributed to the evaluation of the data. F.B. wrote the manuscript in consultation with all authors. All authors approved the final version.
Funding
This study was supported by grants from the Ministry of Education, University, and Research of Italy, the A. Menarini United Pharmaceutical Industries, Swedish Research Council grants VR-M K2013-62X-03026 and VR-NT 621-2010-5666, and research funds from the Karolinska Institutet. E.C. is supported by a Postdoctoral Fellowship from Regione Toscana and Menarini United Pharmaceutical Industries.
References
- Bailey TW, Appleyard SM, Jin YH, Andresen MC. Organization and properties of GABAergic neurons in solitary tract nucleus (NTS) J Neurophysiol. 2008;99:1712–1722. doi: 10.1152/jn.00038.2008. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Deliagina TG, Grillner S. Role of glutamate receptor subtypes in the lamprey respiratory network. Brain Res. 1999;826:298–302. doi: 10.1016/s0006-8993(99)01251-2. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Carfi M, Pantaleo T. Group I and II metabotropic glutamate receptors modulate respiratory activity in the lamprey. Eur J Neurosci. 2002;16:454–460. doi: 10.1046/j.1460-9568.2002.02098.x. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Respiratory responses induced by blockades of GABA and glycine receptors within the Botzinger complex and the pre-Botzinger complex of the rabbit. Brain Res. 2010;1344:134–147. doi: 10.1016/j.brainres.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Nardone F, Pantaleo T. GABAergic and glycinergic inhibitory mechanisms in the lamprey respiratory control. Brain Res. 2006;1090:134–145. doi: 10.1016/j.brainres.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Chen CY, Sekizawa S, Joad JP. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol. 2006;101:322–327. doi: 10.1152/japplphysiol.00143.2006. [DOI] [PubMed] [Google Scholar]
- Broch L, Morales RD, Sandoval AV, Hedrick MS. Regulation of the respiratory central pattern generator by chloride-dependent inhibition during development in the bullfrog (Rana catesbeiana. J Exp Biol. 2002;205:1161–1169. doi: 10.1242/jeb.205.8.1161. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, Borday C, Wrobel L, Thoby-Brisson M, Fortin G, McLean H, Champagnat J. Ontogeny of central rhythm generation in chicks and rodents. Respir Physiol Neurobiol. 2006;154:37–46. doi: 10.1016/j.resp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Chen AK, Hedrick MS. Role of glutamate and substance P in the amphibian respiratory network during development. Respir Physiol Neurobiol. 2008;162:24–31. doi: 10.1016/j.resp.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli E, Robertson B, Mutolo D, Grillner S, Pantaleo T, Bongianni F. Neuronal mechanisms of respiratory pattern generation are evolutionary conserved. J Neurosci. 2013;33:9104–9112. doi: 10.1523/JNEUROSCI.0299-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- Ericsson J, Silberberg G, Robertson B, Wikström MA, Grillner S. Striatal cellular properties conserved from lampreys to mammals. J Physiol. 2011;589:2979–2992. doi: 10.1113/jphysiol.2011.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson J, Stephenson-Jones M, Kardamakis A, Robertson B, Silberberg G, Grillner S. Evolutionarily conserved differences in pallial and thalamic short-term synaptic plasticity in striatum. J Physiol. 2013;591:859–874. doi: 10.1113/jphysiol.2012.236869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante RJ, Kubin L, Fishman AP, Pack AI. Role of chloride-mediated inhibition in respiratory rhythmogenesis in an in vitro brainstem of tadpole, Rana catesbeiana. J Physiol. 1996;492:545–558. doi: 10.1113/jphysiol.1996.sp021328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy JF, Missaghi K, Chartre S, Robert M, Auclair F, Dubuc R. Bilateral connectivity in the brainstem respiratory networks of lampreys. J Comp Neurol. 2012;520:1442–1456. doi: 10.1002/cne.22804. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond JC, Auclair F, Lund JP, Dubuc R. Anatomical and physiological study of respiratory motor innervation in lampreys. Neuroscience. 2003;122:259–266. doi: 10.1016/s0306-4522(03)00601-8. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol. 2010;173:244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Wiegel LM, Majewski DJ. Are pacemaker properties required for respiratory rhythm generation in adult turtle brain stems in vitro. Am J Physiol Regul Integr Comp Physiol. 2007;293:R901–R910. doi: 10.1152/ajpregu.00912.2006. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JE, Wenninger MR, Henderson DR, Mitchell GS. Role of synaptic inhibition in turtle respiratory rhythm generation. J Physiol. 2002;544:253–265. doi: 10.1113/jphysiol.2002.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBotzinger complex. J Neurosci. 2013;33:9235–9245. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Sun B, Park YS, Park CS, Jin YH. GABAA and GABAB receptors have opposite effects on synaptic glutamate release on the nucleus tractus solitarii neurons. Neuroscience. 2012;209:39–46. doi: 10.1016/j.neuroscience.2012.02.025. [DOI] [PubMed] [Google Scholar]
- Kawasaki R. Breathing rhythm-generation in the adult lamprey, Entosphenus japonicus. Jpn J Physiol. 1979;29:327–338. doi: 10.2170/jjphysiol.29.327. [DOI] [PubMed] [Google Scholar]
- Kawasaki R. Breathing rhythm-generation mechanism in the adult lamprey (Lampetra japonica. Jpn J Physiol. 1984;34:319–335. doi: 10.2170/jjphysiol.34.319. [DOI] [PubMed] [Google Scholar]
- Kinkead R. Phylogenetic trends in respiratory rhythmogenesis: insights from ectothermic vertebrates. Respir Physiol Neurobiol. 2009;168:39–48. doi: 10.1016/j.resp.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J, Bricault S, Schmidt MF. Characterization of respiratory neurons in the rostral ventrolateral medulla, an area critical for vocal production in songbirds. J Neurophysiol. 2013;109:948–957. doi: 10.1152/jn.00595.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel B, Guimond JC, Gariepy JF, Gravel J, Auclair F, Kolta A, Lund JP, Dubuc R. Respiratory rhythms generated in the lamprey rhombencephalon. Neuroscience. 2007;148:279–293. doi: 10.1016/j.neuroscience.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Matute C, Streit P. Monoclonal antibodies demonstrating GABA-like immunoreactivity. Histochemistry. 1986;86:147–157. doi: 10.1007/BF00493380. [DOI] [PubMed] [Google Scholar]
- Ménard A, Auclair F, Bourcier-Lucas C, Grillner S, Dubuc R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. J Comp Neurol. 2007;501:260–273. doi: 10.1002/cne.21258. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp Brain Res. 1999;129:191–200. doi: 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Pantaleo T. Role of neurokinin receptors and ionic mechanisms within the respiratory network of the lamprey. Neuroscience. 2010;169:1136–1149. doi: 10.1016/j.neuroscience.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Einum J, Dubuc R, Pantaleo T. Opioid-induced depression in the lamprey respiratory network. Neuroscience. 2007;150:720–729. doi: 10.1016/j.neuroscience.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Cinelli E, Bongianni F, Pantaleo T. Identification of a cholinergic modulatory and rhythmogenic mechanism within the lamprey respiratory network. J Neurosci. 2011;31:13323–13332. doi: 10.1523/JNEUROSCI.2764-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R. Topological analysis of the brain stem of the lamprey Lampetra fluviatilis. J Comp Neurol. 1972;145:165–177. doi: 10.1002/cne.901450204. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Robertson B, Auclair F, Ménard A, Grillner S, Dubuc R. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503:47–63. doi: 10.1002/cne.21348. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Respiratory motoneurons in lampreys. J Comp Physiol. 1974;94:57–68. [Google Scholar]
- Rovainen CM. Neural control of ventilation in the lamprey. Fed Proc. 1977;36:2386–2389. [PubMed] [Google Scholar]
- Rovainen CM. Neurobiology of lampreys. Physiol Rev. 1979;59:1007–1077. doi: 10.1152/physrev.1979.59.4.1007. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Generation of respiratory activity by the lamprey brain exposed to picrotoxin and strychnine, and weak synaptic inhibition in motoneurons. Neuroscience. 1983;10:875–882. doi: 10.1016/0306-4522(83)90225-7. [DOI] [PubMed] [Google Scholar]
- Russell DF. Respiratory pattern generation in adult lampreys (Lampetra fluviatilis): interneurons and burst resetting. J Comp Physiol [A] 1986;158:91–102. doi: 10.1007/BF00614523. [DOI] [PubMed] [Google Scholar]
- Schmitt DE, Hill RH, Grillner S. The spinal GABAergic system is a strong modulator of burst frequency in the lamprey locomotor network. J Neurophysiol. 2004;92:2357–2367. doi: 10.1152/jn.00233.2004. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia; Dual output pathways conserved throughout vertebrate phylogeny. J Comp Neurol. 2012a;520:2957–2973. doi: 10.1002/cne.23087. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Floros O, Robertson B, Grillner S. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc Natl Acad Sci U S A. 2012b;109:E164–E173. doi: 10.1073/pnas.1119348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Tegner J, Matsushima T, El Manira A, Grillner S. The spinal GABA system modulates burst frequency and intersegmental coordination in the lamprey: differential effects of GABAA and GABAB receptors. J Neurophysiol. 1993;69:647–657. doi: 10.1152/jn.1993.69.3.647. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Thompson KJ. Organization of inputs to motoneurons during fictive respiration in the isolated lamprey brain. J Comp Physiol A. 1985;157:291–302. doi: 10.1007/BF00618119. [DOI] [PubMed] [Google Scholar]
- Thompson KJ. Control of respiratory motor pattern by sensory neurons in spinal cord of lamprey. J Comp Physiol A. 1990;166:675–684. doi: 10.1007/BF00240017. [DOI] [PubMed] [Google Scholar]
- Vasilakos K, Wilson RJ, Kimura N, Remmers JE. Ancient gill and lung oscillators may generate the respiratory rhythm of frogs and rats. J Neurobiol. 2005;62:369–385. doi: 10.1002/neu.20102. [DOI] [PubMed] [Google Scholar]
- Villar-Cerviño V, Barreiro-Iglesias A, Anadón R, Rodicio MC. Distribution of glycine immunoreactivity in the brain of adult sea lamprey (Petromyzon marinus). Comparison with gamma-aminobutyric acid. J Comp Neurol. 2008;507:1441–1463. doi: 10.1002/cne.21634. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Vasilakos K, Harris MB, Straus C, Remmers JE. Evidence that ventilatory rhythmogenesis in the frog involves two distinct neuronal oscillators. J Physiol. 2002;540:557–570. doi: 10.1113/jphysiol.2001.013512. [DOI] [PMC free article] [PubMed] [Google Scholar]


