Abstract
When exposed to a hypoxic environment the body's first response is a reflex increase in ventilation, termed the hypoxic ventilatory response (HVR). With chronic sustained hypoxia (CSH), such as during acclimatization to high altitude, an additional time-dependent increase in ventilation occurs, which increases the HVR. This secondary increase persists after exposure to CSH and involves plasticity within the circuits in the central nervous system that control breathing. Currently these mechanisms of HVR plasticity are unknown and we hypothesized that they involve glutamatergic synapses in the nucleus tractus solitarius (NTS), where afferent endings from arterial chemoreceptors terminate. To test this, we treated rats held in normoxia (CON) or 10% O2 (CSH) for 7 days and measured ventilation in conscious, unrestrained animals before and after microinjecting glutamate receptor agonists and antagonists into the NTS. In normoxia, AMPA increased ventilation 25% and 50% in CON and CSH, respectively, while NMDA doubled ventilation in both groups (P < 0.05). Specific AMPA and NMDA receptor antagonists (NBQX and MK801, respectively) abolished these effects. MK801 significantly decreased the HVR in CON rats, and completely blocked the acute HVR in CSH rats but had no effect on ventilation in normoxia. NBQX decreased ventilation whenever it was increased relative to normoxic controls; i.e. acute hypoxia in CON and CSH, and normoxia in CSH. These results support our hypothesis that glutamate receptors in the NTS contribute to plasticity in the HVR with CSH. The mechanism underlying this synaptic plasticity is probably glutamate receptor modification, as in CSH rats the expression of phosphorylated NR1 and GluR1 proteins in the NTS increased 35% and 70%, respectively, relative to that in CON rats.
Introduction
The hypoxic ventilatory response (HVR) is a reflex increase in ventilation that occurs in response to decreased arterial oxygen tension (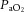 ). With chronic sustained hypoxia (CSH) of days to months (e.g. during acclimatization to altitude), additional time-dependent increases in ventilation occur that further improve
). With chronic sustained hypoxia (CSH) of days to months (e.g. during acclimatization to altitude), additional time-dependent increases in ventilation occur that further improve 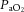 (Powell et al. 1998). This secondary increase is termed ventilatory acclimatization to hypoxia (VAH) and persists after the removal of hypoxic stimulation, indicating plasticity within the ventilatory control circuits (Aaron & Powell, 1993; Hupperets et al. 2004). Two mechanisms have been identified that contribute to VAH: (1) sensitivity of carotid body arterial chemoreceptors to O2 increases, and (2) CNS responsiveness to afferent inputs from carotid bodies increases (Bisgard & Neubauer, 1995; Dwinell & Powell, 1999; Wilkinson et al. 2010a; Kumar & Prabhakar, 2012). Considerable progress has been made towards elucidating mechanisms of enhanced carotid body sensitivity to O2 during CSH but mechanisms that mediate increased CNS gain of the HVR are not well understood.
(Powell et al. 1998). This secondary increase is termed ventilatory acclimatization to hypoxia (VAH) and persists after the removal of hypoxic stimulation, indicating plasticity within the ventilatory control circuits (Aaron & Powell, 1993; Hupperets et al. 2004). Two mechanisms have been identified that contribute to VAH: (1) sensitivity of carotid body arterial chemoreceptors to O2 increases, and (2) CNS responsiveness to afferent inputs from carotid bodies increases (Bisgard & Neubauer, 1995; Dwinell & Powell, 1999; Wilkinson et al. 2010a; Kumar & Prabhakar, 2012). Considerable progress has been made towards elucidating mechanisms of enhanced carotid body sensitivity to O2 during CSH but mechanisms that mediate increased CNS gain of the HVR are not well understood.
In the CNS, carotid body afferent neurons project to the nucleus tractus solitarius (NTS) (Lipski et al. 1977; Housley & Sinclair, 1988), and there is considerable evidence implicating glutamate as the main excitatory neurotransmitter at this synapse. For example, during hypoxia, glutamate is released into the NTS coincident with the HVR, glutamate injection into the NTS mimics the HVR and both the HVR and glutamate release are abrogated by carotid body denervation (Mizusawa et al. 1994; Richter et al. 1999). Furthermore, NTS microinjection or systemic injection of NMDA receptor (NMDAR) antagonists attenuate the HVR in a variety of species (Connelly et al. 1992; Mizusawa et al. 1994; Soto-Arape et al. 1995; Ohtake et al. 1998), and specifically attenuate VAH in mice and rats (Reid & Powell, 2005; El Hasnaoui-Saadani et al. 2007). In addition, there is evidence that glutamatergic AMPA receptors (AMPARs) play a role in short-term responses to hypoxia. For example, >85% of the neurons expressing cFOS (an indicator of neuronal firing) in the NTS during carotid body chemoreceptor stimulation also express AMPARs (GlurR2/3; Whitney et al. 2000), NTS microinjection of a broad-spectrum excitatory amino acid antagonist further attenuates responses to short-term hypoxia beyond the effect of NMDAR blockade alone (Mizusawa et al. 1994), while simultaneous antagonism of NMDARs and AMPARs within the NTS reduces ventilatory and cardiac responses to chemical or CO2-mediated carotid body stimulation in anaesthetized and awake rats (Vardhan et al. 1993; Zhang & Mifflin, 1993; Haibara et al. 1999; Braga et al. 2007). Together, these results show that during hypoxia, glutamate released from carotid sinus nerve afferents at the NTS acts on both AMPARs and NMDARs to cause the HVR.
Considering the role of glutamate neurotransmission in other models of neural plasticity (e.g. long-term potentiation, see Discussion), we hypothesized that plasticity in glutamatergic synaptic transmission in the NTS during CSH contributes to VAH. However, previous studies of the role of glutamate in the HVR have not investigated chronic hypoxia. In addition, most researchers have employed systemic approaches to manipulate NMDARs and AMPARs, which makes the results difficult to interpret. For example, within the pons and medulla, MK801 has been shown to bind to the ventrolateral and dorsolateral pons, parapyramidal nucleus, NTS and reticular formation, all of which also affect breathing (Coles et al. 1998). The goal of the present paper was to determine if synaptic plasticity mediated by glutamate receptors in the NTS contribute to the CNS component of VAH in awake rats.
Methods
Experimental animals and surgery
All surgical procedures and protocols were performed in accordance with the relevant guidelines of The University of California San Diego Institutional Animal Care and Use Committee. Male Sprague–Dawley Rats (Charles River) weighing 250–300 g were housed under a 12:12 h light–dark cycle and fed a standard diet ad libitum.
Animals were divided into two groups treated with either (1) the NMDAR antagonist dizocilpine (MK801; n = 31), or (2) the AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoaxaline-2,3-dione (NBQX; n = 26). Within these two groups, animals were further divided into two experimental groups: (1) normoxic sea level controls (CON; n = 13 for each), and (2) chronically hypoxic rats (CSH; n = 8 and 11, respectively). Additional groups of animals in each category were utilized for acute AMPA and NMDA injection experiments and sham artificial cerebral spinal fluid (ACSF) experiments. The CSH rats were acclimatized to a simulated altitude of 5500 m in a hypobaric chamber at 380 Torr (PIO2 = 70 Torr, equivalent to ≈10% O2 at sea-level barometric pressure) for 7 days. The chamber was returned to sea level for 15 min every 3–4 days for general cage maintenance or when it was necessary to remove animals for experimentation. This level of hypoxia was chosen for the present study because it is the most severe level of sustained hypoxia tolerated by rats that does not induce a stress response (Powell, unpublished observation). Furthermore, 10% O2 is a commonly utilized level of experimental hypoxia in the literature studying the effects of chronic hypoxia in lab rodents and other models (e.g. Fidone et al. 1982; Bee & Pallot, 1995; Gozal et al. 1996). Finally, this level of hypoxia is consistent with previous experiments in our laboratory in both rats and mice (Aaron & Powell, 1993; Huey et al. 2000; Reid et al. 2002; Wilkinson et al. 2010b), and therefore simplifies comparison between the literature and our present findings. Some studies have shown that VAH is manifest in some species within a few days of exposure to CSH (Forster et al. 1976); however, we have chosen a 7-day exposure to ensure that VAH is fully manifest in these animals and because a 7-day exposure (or longer) is a common experimental set-up in studies of lab rodents (e.g. Aaron & Powell, 1993; El Hasnaoui-Saadani et al. 2007; Pichon et al. 2009). Furthermore, it is likely that different mechanisms underlie VAH during short-term (24–48 h) or long-term (days–months) CSH, and we are interested particularly in the long-term mechanisms that appear to be more dependent on increased CNS gain of the respiratory control circuits (see Discussion).
At least 2 days before acclimatization in chronic sustained normoxia or hypoxia all animals underwent surgery for implantation of guide cannulae, arterial catheters and body temperature telemetry probes. All surgeries were performed under isoflurane anaesthesia (initially 5% isoflurane in 100% O2 and maintained at 2–3% isoflurane). Stereotaxic surgery (Kopf Instruments, Tujunga, CA, USA) was used to implant a stainless steel guide cannula (Plastics One, Roanoke, VA, USA) bilaterally into the NTS (co-ordinates: anteroposterior –0.3 mm (calamus scriptorius), mediolateral –0.7 mm, dorsoventral –0.5 mm) to deliver pharmacological agents. Two holes were drilled into the cranium into which screws would fit firmly and the guide cannula was secured to the skull using acrylic resin that fixed the guide cannula to these screws. The microinjection needle was 1 mm longer than the guide cannula and projected into the NTS.
Arterial catheters were inserted through the femoral artery and reached the abdominal aorta of the rat. Polyethylene tubing (PE-50) was heated and stretched to fit the diameter of the artery, and sutured in place. The stretch allowed for a single tube with no joints that was more resistant to the formation of blood clots. The catheter was tunnelled beneath the dorsal skin and exited at the back of the neck through a stainless steel headbutton that was sutured in place for easy access. A stainless steel ring was screwed to the headbutton to protect the catheter.
A telemetry thermometer probe (Emitter, Respironics, Bend, OR, USA) was implanted to monitor body temperature. The body temperature is required for an accurate calculation of the tidal volume (VT, see below). The emitter was implanted into the abdominal cavity and sutured in place to the interior wall of the abdomen.
Plethysmography
Ventilation (VI) was measured using the barometric pressure method of plethysmography modified for continuous flow (Jacky, 1978). On the day of experimentation, individual animals were sealed into a 7 litre Plexiglas chamber. An electronic gas mixer (MFC-4; Sable Systems, Las Vegas, NV, USA) was used to supply the animal with an inflowing gas mixture (3 l min−1) of controlled O2 and CO2 (balance N2). Inflowing gas entered the chamber through a tube (7 cm long and 1 cm in diameter) that was filled with smaller PE-50 tubing of similar length. This double-jacketed design created a high impedance input to reduce the loss of pressure signals. Continuous flow and proper chamber pressure were achieved by a vacuum pump (Dayton Electric, Chicago, IL, USA) that sucked gas from the chamber through a valve. Pressure inside the box was referenced to atmospheric pressure using a water manometer. Atmospheric pressure corrected for standard gravity, room temperature and elevation was recorded on each experimental day as a reference. To ensure a controlled gas mixture in the chamber, the pressure inside the chamber was positive (<0.5 cmH2O). Chamber gas concentrations were measured using a mass spectrometer (Perkin-Elmer 1100 Medical Gas Analyser, Pomona, CA, USA) that was calibrated for O2 and CO2 on each experimental day. A chamber temperature probe (Thermalert TH-5; Physitemp, Clifton, NJ, USA) was sealed inside the box, and a humidity probe was placed into the box through a hole that was cut and sealed for this purpose. Inspiration produces humidity related changes in pressure that can be monitored with a differential pressure transducer (DP45; Validyne, Northridge, CA, USA) referenced to atmosphere. Output from the transducer was recorded on a digital data acquisition system (Labdat, see below). Respiratory frequency (fR) was calculated directly from the ventilation-induced pressure swings. Tidal volume (VT) was calculated from the ventilation-induced pressure changes using an equation from Drorbaugh and Fenn (1955) and modified for flow-through plethysmography by Jacky (Drorbaugh & Fenn, 1955; Jacky, 1978):
where Vcal = volume of the calibration pulse (ml); Pm = peak height (A–D units); Pcal = peak height of calibration pulse (A–D units); PB = barometric pressure (mmHg); 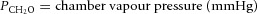 ;
; 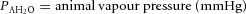 ; TA = body temperature of the animal (K); TC = chamber temperature (K).
; TA = body temperature of the animal (K); TC = chamber temperature (K).
Before each experiment, calibration pulses (0.2, 0.5 and 1.0 ml) were injected into the chamber at a rate similar to the rats’ inspiratory time. Ventilation was determined under poikilocapnic conditions. The animals were given at least 40 min to habituate to the plethysmograph at their chronic inspired O2 levels before study. Two inspired O2 levels (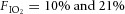 ) were used in the study. VI was measured 10–15 min after the rats were exposed to an acute inspired gas concentration unless otherwise noted (Fig. 1).
) were used in the study. VI was measured 10–15 min after the rats were exposed to an acute inspired gas concentration unless otherwise noted (Fig. 1).
Figure 1. Experimental protocol.
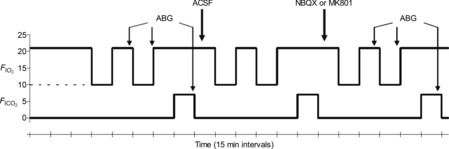
Schematic indicates changes in inspired gas concentrations during experiments. Animals were placed in the plethysmograph for 25–45 min at their chronic inspired O2 level before the start of an experiment to allow them time to acclimate to the environment. Continuous line indicates normoxia (21% O2); dashed line indicates chronic sustained hypoxia (10% O2). ABG samples were collected at the indicated time points. Animals received injections of ACSF (sham), NBQX, or MK801 at the times indicated. ABG, arterial blood gas; ACSF, artificial cerebral spinal fluid.
Arterial blood gas measurements
Single arterial batch samples (0.2 ml) were taken while the rats were breathing 10% or 21% O2 (n = 3–5 for each CON and CSH group), as well as 7% CO2 in 21% O2 (n = 3–5 for each CON group and CSH group). Samples were obtained after control and drug microinjections (Fig. 1). These samples were corrected for body temperature and analysed (Instrumentation Laboratory Gem Premier 5000, Lexington, MA, USA) for 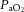 , arterial CO2 pressure (
, arterial CO2 pressure (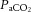 ) and arterial pH.
) and arterial pH.
Microinjections
All of the drugs that were used were prepared in ACSF (in mm: 115 NaCl, 2.0 KCl, 2.2 KH2PO4, 25 NaHCO3, 10 d-glucose, 1.2 MgSO4 and 2.5 CaCl2, adjusted to pH 7.4 with HCl) (Youssef et al. 2001). The ACSF was microinjected as a sham in a subset of all experimental groups studied. All drugs were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise indicated.
A microinjection needle made to fit the guide cannula was connected to a 500 nl Hamilton microsyringe through a polyethylene tube. The polyethylene tube ran through the lid of the plethysmograph and was sealed. After control ventilatory measurements were made at 10% and 21% O2, 50 nl of NBQX (0.2 nmol) or MK801 (0.2 nmol) was injected into the NTS. The pharmacological doses used in the present study are sufficient to inhibit physiological responses to NTS-mediated stimulation throughout the time frame of our experiments in awake or anaesthetized rats, in accordance with studies conducted in similar preparations from other laboratories (Ohta et al. 1993; Mizusawa et al. 1994; Machado et al. 2000). Following drug microinjection, ventilatory measurements were again collected in each gas mixture. Ventilatory effects of the drugs were determined by comparing the data obtained following the control measurements with those collected following the drug microinjection. Animals were permitted at least 1 h to rest in their home cages between the completion of sampling following the first injection and beginning the second experimental trial. Animals received ACSF injections before drug injections and experimental protocols were not randomized because the effects of these drugs take hours to days to wash off. In some experiments, animals received a second injection of ACSF in place of cocktail microinjection to assess the impact of the experimental time course. No differences were observed between the effects of ACSF in multiple trials in the same animal (data not shown).
To confirm that glutamatergic AMPAR and NMDAR activity was blocked by NBQX or MK801, respectively, AMPA or NMDA were microinjected into the NTS in the presence and absence of NBQX or MK801. For these experiments, the rat was placed in the plethysmograph and allowed to stabilize for 40 min. After baseline ventilatory measurements were made, 50 nl of AMPA or NMDA (12.5 pmol) were injected bilaterally into the NTS and ventilatory data were collected by analysing 1 min intervals at 0–1, 1–2, 3–4, 4–5, 5–6, 10–11, 15–16 and 30–31 min postinjection. For ventilatory measurements in the presence of the blocker, the rat was microinjected with 50 nl of NBQX (0.2 nmol) or MK801 (0.2 nmol) before being placed in the plethysmograph. At least 10 consecutive representative breaths were selected for analysis from the final 5 min of each experimental treatment epoch and at least 10 sets of breaths were analysed for each period. Breaths were chosen from periods where the animal was not physically active and clear peaks could be determined relative to a flat baseline.
Localization of microinjections
Colloidal gold or Evan's blue microinjections were used to localize the microinjection sites (Fig. 2). At the end of the experiment, colloidal gold (50 nl) or Evan's blue (50 nl) was microinjected through the guide cannulae into the NTS at the same site as the drug delivery. The animals were anaesthetized with an overdose of sodium pentobarbital and transcardially perfused with ice-cold ACSF followed by 4% paraformaldehyde. The brainstem was removed and postfixed in 2% paraformaldehyde for 1 day, and then stored in sucrose (30%). The brainstems were frozen in isopentane (–140°C) and sectioned (slice thickness = 30–50 μm) on a cryostat (Cryocut 1800; Leica Biosystems, Wetzlar, Germany). For animals microinjected with the colloidal gold, stains were enhanced using silver intensification solution (Ted Pella Inc., Redding, CA, USA), which marked the microinjection sites with black staining. Aqueous Eosin Y was used as a cytoplasmic counter-stain. Glutamate, AMPA or NMDA were microinjected in rats on a day before they were studied with the full protocol. No ventilatory responses were observed if the histological data showed a microinjection that was more than 500 μm away from the commissural caudal NTS target site, but similar responses were obtained if microinjections were localized within 500 μm of the target. Figure 2A shows the spread of our 50 nl microinjections is about 250 μm, although it is not known exactly how the pharmacological effects of glutamate receptor agonists and antagonists spread compared to a histological marker. For data analysis, we only used animals that had a positive response to glutamate, NMDA or AMPA microinjection or in which the microinjection site was located within 500 μm of the NTS target.
Figure 2. Location of NTS microinjection site.
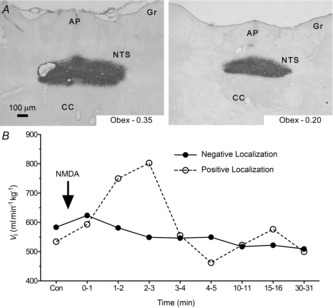
A, representative images show coronal sections of the brainstem of a rat showing the sites of successful microinjection with colloidal gold in the caudal region of the NTS. Aqueous Eosin Y is used as a cytoplasmic counterstain. Magnification to 6.3×. B, sample time course of ventilatory responses to 50 μl NMDA microinjections in two different rats. Animals were not used for further measurements or analysis if they showed no response to AMPA, NMDA or glutamate microinjection (negative localization). AP, area postrema; CC, central canal; GR, gracile nucleus; NTS, nucleus tractus solitarius.
Protein extraction and Western blots
Rats exposed to 7 days of room air or CSH (n = 6 each) were killed with a pentobarbital overdose. The skull was rapidly opened, the brainstem extracted and immediately placed in liquid nitrogen. The NTS was extracted under liquid nitrogen to prevent protein denaturation. Briefly, the obex was visually identified and a 1 mm thick coronal section was obtained 1.5 mm caudal to 1.5 mm rostral to the obex. The dorsal half of this brainstem section was carefully removed under liquid nitrogen, weighed and stored at –80°C until analysis. To extract proteins, tissues were homogenized on ice with a tissue blender in RIPA buffer (Cell Signaling, Boston, MA, USA) supplemented with complete protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA) and Halt phosphatases inhibitor cocktail (Thermo Scientific, Rockford, IL, USA). The resulting homogenate was centrifuged for 10 min at 10,000 g at 4°C to remove cell debris. Supernatants were taken as whole cell lysates and protein concentration was measured using a bicinchoninic acid kit, according to the manufacturer's instructions (Sigma-Aldrich).
For Western blot analysis, equal amounts of protein (75 μg well−1) were separated at 80 V for 3 h on 4–12% Bolt Mini Bis-Tris Plus gels (Life Technologies, Carlsbad, CA, USA) and transferred on to a PVDF membrane (Millipore, Billerica, MD, USA). Western blots were performed with antibodies against AMPA receptor GluR 1 (1:1000; Millipore), GluR 2 (1:500; Neuromab, Davis, CA, USA), and phopho-GluR 1 (1:1000; Millipore) subunits; NMDA receptor NR1 (1:250; Santa Cruz, Dallas, TX, USA) and phospho-NR1 (1:250; Millipore) subunits; and GAPDH (1:1000; Millipore). Membranes were incubated in primary antibodies overnight at 4°C, rinsed 3× 15 min in TBS + 0.1% Tween20 and then blocked in odyssey blocking buffer (LI-COR, Lincoln, NE, USA). Specific bands were visualized after 2 h incubation with the respective fluorescent secondary antibodies (1:15,000) using an Odyssey fluorescent scanner (LI-COR). Densitometry of Western blots from each experimental group were obtained using ImageJ (NIH), and absolute values were normalized to GAPDH expression on the same blot. Following analysis, blots were stripped with stripping buffer (LI-COR) and re-probed with antibodies such that all antibodies were probed on each gel to facilitate accurate comparison of the expression of related protein within the same sample. Results were analysed in arbitrary units, comparing each value with that obtained from each respective GAPDH measurement on each blot, and results are expressed as fold-change relative to normoxic controls run simultaneously. Gels were repeated three times.
Data collection and analysis
Ventilatory measurements were recorded on a PC using an analog-to-digital data acquisition system (DT-2801-A; National Instruments, Richmond, BC, USA) sampling at 200 Hz. Representative periods of 30 s were selected for analysis using a custom peak detection program developed in-house.
Statistical analysis was performed using commercial software (SPSS 15.0; SPSS Inc., Chicago, IL, USA). The null hypothesis in our model is that ventilation increases in animals breathing acute hypoxic or hypercapnic gas mixtures. Our study was designed to test for effects of drug or acclimatization to hypoxia that prevent or enhance these expected changes. For all experiments, individual n values correspond to a single animal treated with CON or CSH acclimatization protocols and then treated with ACSF alone or AMPAR, or NMDAR agonists or antagonists dissolved in ACSF as described above, before and after exposure to 21% O2 and then either acute hypoxia (10% O2) or acute hypercapnia (7% CO2). Values are presented as means ± s.e.m. P < 0.05 was considered to achieve statistical significance. All data were normally distributed (Shapiro–Wilk: P > 0.05) with equal variance (P > 0.05).
For acute AMPA and NMDA responses, a repeated measures ANOVA was used to determine whether there was a statistically significant difference in the time-dependent response to AMPA or NMDA in the presence and absence of NBQX or MK801, respectively. The between-subject factors (CON vs. CSH, and AMPA or NMDA in the presence vs. absence of NBQX or MK801, respectively) were considered against time. A Dunnett's multiple comparisons test was used to determine whether there was a statistically significant difference at each time point to the pre-AMPA or pre-NMDA control (‘Con’; t = 0) value.
For drug microinjection data, a three-way repeated measures ANOVA was used to determine if there was a statistically significant difference between the three independent factors considered: (1) acute ventilatory response (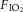 of 0.10 vs. 0.21); (2) chronic oxygen condition (CON vs. CSH); and (3) treatment [control (ACSF) vs. drug microinjection]. Sphericity could not be assumed, so the Greenhouse–Geisser test was used because it is the most conservative. Two-way comparisons between the independent variables or their changes with acute hypoxia or hypercapnia (e.g. the HVR or ΔVI between 10% and 21% O2) were performed using two-way ANOVA. Bonferonni post hoc multiple comparisons tests were run on each of the dependent variables to compare the single point means of interest. The dependent variables analysed were: total minute ventilation (VI), respiratory frequency (fr), tidal volume (VT), hypoxic and hypercapnic Δs for VI,
fR and VT, inspiratory time (TI), expiratory time (TE), the inspiratory slope (VT/TI) as an index of the ventilatory drive to breathe, and arterial
of 0.10 vs. 0.21); (2) chronic oxygen condition (CON vs. CSH); and (3) treatment [control (ACSF) vs. drug microinjection]. Sphericity could not be assumed, so the Greenhouse–Geisser test was used because it is the most conservative. Two-way comparisons between the independent variables or their changes with acute hypoxia or hypercapnia (e.g. the HVR or ΔVI between 10% and 21% O2) were performed using two-way ANOVA. Bonferonni post hoc multiple comparisons tests were run on each of the dependent variables to compare the single point means of interest. The dependent variables analysed were: total minute ventilation (VI), respiratory frequency (fr), tidal volume (VT), hypoxic and hypercapnic Δs for VI,
fR and VT, inspiratory time (TI), expiratory time (TE), the inspiratory slope (VT/TI) as an index of the ventilatory drive to breathe, and arterial 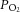 ,
, 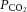 and pH.
and pH.
Unpaired two-tailed t tests were used to compare changes in the expression of AMPAR and NMDAR proteins between CON and CSH groups. These data were normalized to the expression of the housekeeping protein GAPDH to account for loading differences between samples. For ease of comparison, normalized data are the percentage of the expression of the protein in question in matching samples from CON animals (CON = 100%).
Results
Acute and chronic hypoxia, but not artificial cerebral spinal fluid microinjections increase ventilation
Ventilation increased in response acute hypoxia or hypercapnia as expected (Fig. 3, and see below). The aim of the present study was to test hypotheses related to plasticity of changes in the acute HVR (i.e. effects of drugs or CSH on the established effects of acute hypoxia on ventilation. Therefore, as the null hypothesis is an increase in ventilation with acute hypoxia (or hypercapnia), for simplicity, we assume significant effects of acute alterations in O2 or CO2 on ventilation. We emphasize results wherein changes do not occur in response to acute hypoxia or hypercapnia with double daggers (‡) in all figures.
Figure 3. Effects of hypoxia and sham ACSF injections on ventilation in CON and CSH rats.
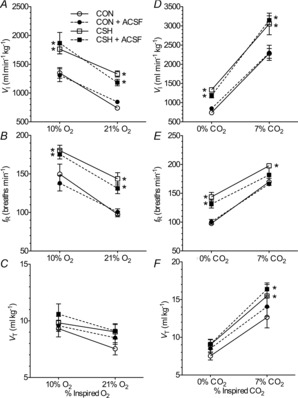
A, effect of hypoxia and ACSF microinjections on VI. B, effect of hypoxia and ACSF on fR. C, effect of hypoxia and ACSF on VT. D–F, effect of ACSF microinjections on hypoxic ventilatory response and hypercapnic ventilatory response of VI (D), fR (E) and VT (F). Data are the time response following bilateral microinjections of NMDA in the presence (continuous lines) or absence (dotted lines) of ACSF. Data are means ± s.e.m. from n = 7 animals per group. *Significant differences between CON and CSH values (P < 0.05). ACSF, artificial cerebral spinal fluid; CON, controls; CSH, chronic sustained hypoxia; fR, breathing frequency; VI, total minute ventilation; VT, tidal volume.
Acute and chronic exposure to hypoxia had the same effects on ventilation in all treatment groups before drug microinjections (Fig. 3), and were similar to those reported previously (Aaron & Powell, 1993; Reid & Powell, 2005). Acute hypoxia (10% O2) increased VI 80% in CON rats and 55% in CSH rats (Fig. 3A–C; P = 0.0001 for CON, 0.026 for CSH). These increases were predominately due to increased fR (Fig. 3B; P = 0.0001 for CON, 0.007 for CSH) and VT was not significantly altered in either group (Fig. 3C). Comparing baseline ventilation, relative to CON animals VI was elevated by 80% in CSH animals breathing 21% O2 and by 30% in CSH rats breathing 10% O2 (Fig. 3A–C; P = 0.049 and 0.0001 for 10 and 21% O2, respectively). A plot of VI
versus
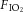 shifted upwards with CSH and was roughly parallel to CON data, as previously reported for the poikilocapnic HVR in CSH rats (Aaron & Powell, 1993). The increase in ventilation was due to increases in fR compared to the controls (P = 0.156 and 0.0058 for 10 and 21% O2, respectively), and VT was not significantly altered by hypoxia (Fig. 3A–C).
shifted upwards with CSH and was roughly parallel to CON data, as previously reported for the poikilocapnic HVR in CSH rats (Aaron & Powell, 1993). The increase in ventilation was due to increases in fR compared to the controls (P = 0.156 and 0.0058 for 10 and 21% O2, respectively), and VT was not significantly altered by hypoxia (Fig. 3A–C).
Animals were also exposed to hypercapnia (7% CO2) as a control experiment to determine whether glutamate receptor antagonism impinged upon the response to all ventilatory chemostimuli, or if the role for these receptors is specific to the hypoxic chemoreflex pathway. In ACSF-treated animals breathing 7% CO2 VI increased ∼3-fold in all groups and this increase was markedly greater than that induced by hypoxia (Fig. 3D–F; P = 0.0001 and 0.0007 for CON and CSH, respectively). Elevated VI during hypercapnia was the product of significant increases in both fR and VT (Fig. 3E and F; P = 0.0027 and 0.0123 for fR in CON and CSH, respectively, and 0.0008 and 0.0049 for VT in CON and CSH, respectively). Bilateral microinjections of ACSF (50 nl side−1) into the NTS had no effect on ventilation in 0% CO2 or 7% CO2 in either group relative to control measurements made before microinjection treatment (Fig. 3).
Acute NMDA or AMPA injections increased ventilation in control and chronic sustained hypoxia rats
Before examining the effect of glutamate receptor antagonists on the HVR and VAH we first tested the efficacy of specific NMDAR and AMPAR antagonists (MK801 and NBQX, respectively) at blocking agonist-induced channel activation. Bilateral microinjection of NMDA increased VI ∼2-fold within seconds of drug application in both CON and CSH rats (Fig. 4A; P = 0.025 for CON, 0.001 for CSH). This increase was due to a combination of 50% increases of both fR and VT (Fig. 4B and C; P = 0.025 and 0.017 for fR in CON and CSH, respectively, 0.0022 and 0.001 for VT in CON and CSH, respectively). Breathing activity remained significantly elevated for 10–15 min following microinjections before returning to baseline levels. Microinjecting MK801 into the NTS before treatment with NMDA abolished responses to NMDA in both CON and CSH groups (Fig. 4, continuous lines).
Figure 4. Effects of NMDA and AMPA microinjections into the NTS in the presence and absence of MK801 or CNQX on ventilation in CON and CSH rats.
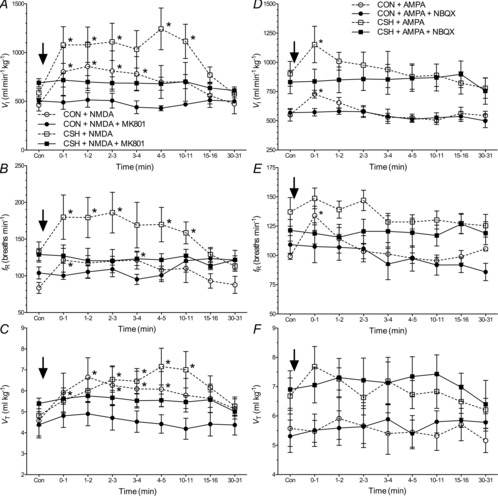
Effects of NMDA on (A) VI, (B) fR and (C) VT in the presence (continuous lines) or absence (dotted lines) of the NMDAR antagonist MK801. Effects of AMPA on (D) VI, (E) fR and (F) VT in the presence (continuous lines) or absence (dotted lines) of the AMPAR antagonist NBQX. Arrow indicates injection of NMDA or AMPA into the NTS. All measurements were made in 21% O2. Data are mean ± s.e.m. n = 6 CON and eight CSH rats for NMDA experiments and four CON and six CSH for AMPA experiments. *Significance from control value before injection (Con) (P < 0.05). CON, controls; CSH, chronic sustained hypoxia; fR, breathing frequency; VI, total minute ventilation; VT, tidal volume.
Bilateral microinjection of AMPA had smaller but similar effects on ventilation. AMPA increased VI at the first minute of microinjection in both the CON and CSH groups (Fig. 4D; P = 0.0003 for CON, 0.0001 for CSH). In normoxic rats, VI was increased 50%, and in CSH rats VI was increased 20% by AMPA microinjections. The response to AMPA in the normoxic rats was mainly the result of a 35% increase in fR at 1 min following microinjection (Fig. 4E; P = 0.0011). Tidal volume was not significantly changed in either group in all time periods (Fig. 4F), although non-significant increases in VT and fR at 1 min following AMPA injections in CSH rats combined to underlie the significant increase in VI. Microinjecting NBQX into the NTS before treatment with AMPA abolished responses to AMPA in both CON and CSH groups (Fig. 4, continuous lines).
NMDA receptor antagonism depressed the hypoxic ventilatory response in chronic sustained hypoxia but not control animals
In CON rats, MK801 microinjections in the NTS had no significant effect on VI during normoxia or acute hypoxia but MK801 did decrease the slope of the HVR in CON rats (Fig. 5A and B; P = 0.001), mediated by decreases in the HVR of both the fR and VT components (Fig. 5D and F; P = 0.001 for fR and 0.004 for VT). Although VI was unchanged in CON rats, MK801 decreased fR in acute hypoxia (Fig. 5C; P = 0.026) increased TE and tended to increase TI (Fig. 6A and B; P = 0.049). VT (Fig. 5E) and ventilatory drive (VT/TI; Fig. 6C) did not change with MK-801.
Figure 5. Effect of MK801 on the HVR in CON and CSH rats.
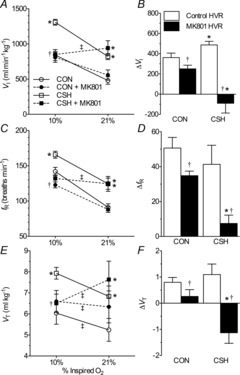
Effects of chronic hypoxia and an NMDAR antagonist (MK801) on (A) VI, (C) fR and (E) VT in CON and CSH rats before and after microinjection of MK801. Effect of chronic hypoxia and MK801 on the magnitude of the HVR of (B) VI, (D) fR and (F) VT. Data are means ± s.e.m. from n = 13 CON and 8 CSH rats. *Significant difference between CON and CSH values. †Significant difference between artificial cerebral spinal fluid and drug injections. ‡No significant difference between 10% and 21% O2 (P < 0.05). CON, controls; CSH, chronic sustained hypoxia; fR, breathing frequency; HVR, hypoxic ventilatory response; VI, total minute ventilation; VT, tidal volume.
Figure 6. Effect of chronic hypoxia and/or MK801 on ventilation in CON and CSH rats.
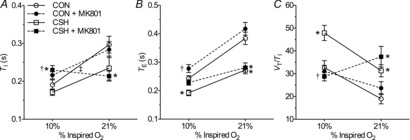
Effects of chronic hypoxia and an NMDA receptor antagonist (MK801) on (A) TI, (B) TE and (C) VT/TI for CON and CSH rats before and after microinjection of MK801. Data are means ± s.e.m. from n = 13 CON and 8 CSH rats. *Significant difference between CON and CSH values. †Significant difference between artificial cerebral spinal fluid and drug injections. ‡No significant difference between 10% and 21% O2 (P < 0.05). CON, controls; CSH, chronic sustained hypoxia; TE, expiratory time; TI, inspiratory time; VT/TI, ventilatory drive to breathe.
Conversely, MK801 abolished the sensitivity of VI to acute hypoxia in CSH rats and blocked VAH (Fig. 5A and B). The effect of MK-801 on fR and VT was similar, and statistically significant changes in VAH of all three parameters was prevented by MK801 in CSH rats (Fig. 5A–F). The prevention of hypoxia-mediated changes in fR was due to increases in both TI and TE with acute hypoxia and MK801 treatment (Fig. 6A and B; P = 0.039 and 0.039 for TI and TE, respectively), and MK-801 abolished the increase in ventilatory drive associated with acute hypoxia in CSH rats (Fig. 6C).
Hence, NMDA receptor blockade in the NTS primarily affected ventilation in hypoxia after acclimatization to CSH by eliminating the normal response to increased fR with acute hypoxia in CSH rats. NMDAR blockade also had a similar effect on the slope of the HVR in CON rats due to a significant decrease in breathing frequency response to acute hypoxia.
AMPA receptor antagonism depressed effects of acute and chronic hypoxia
In CON rats, NBQX microinjections in the NTS had no effect on VI during normoxia but reduced VI by ∼20% during acute hypoxia (Fig. 7A; P = 0.0022). Consequently, the slope of the poikilocapnic HVR was depressed by NBQX (Fig. 7A and B; P = 0.042). The decrease in VI of CON rats was due to a decrease in fR (Fig. 7C and D; P = 0.0001) while VT also increased (Fig. 7E and F; P = 0.031). Both TI and TE increased with NBQX in all conditions (Fig. 8A and B; P = 0.0001 for both TI and TE). Ventilatory drive (VT/TI) changed in parallel with VI: decreasing in acute hypoxia but not changing in normoxia with NBQX (Fig. 8C; P = 0.012 for 10% O2).
Figure 7. Effect of NBQX on the HVR in CON and CSH rats.
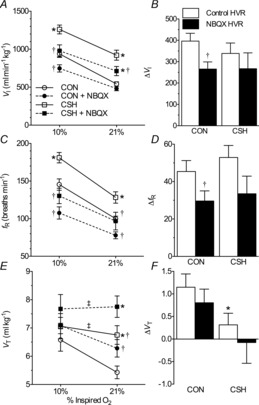
Effects of chronic hypoxia and an AMPA receptor antagonist (NBQX) on (A) VI, (C) fR and (E) VT for CON and CSH rats before and after microinjection of NBQX. Effect of chronic hypoxia and NBQX on the magnitude of the HVR of (B) VI, (D) fR and (F) VT. Data are means ± s.e.m. from n = 13 CON and 8 CSH rats. *Significant difference between CON and CSH values. †Significant difference between artificial cerebral spinal fluid and drug injections. ‡No significant difference between 10% and 21% O2 (P < 0.05). CON, controls; CSH, chronic sustained hypoxia; fR, breathing frequency; HVR, hypoxic ventilatory response; VI, total minute ventilation; VT, tidal volume.
Figure 8. Effect of chronic hypoxia and/or NBQX on ventilation in CON and CSH rats.
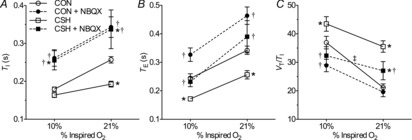
Effects of chronic hypoxia and an AMPA receptor antagonist (NBQX) on (A) TI, (B) TE and (C) VT/TI for CON and CSH rats before and after microinjection of NBQX. Data are means ± s.e.m. from n = 13 CON and 11 CSH rats. *Significant difference between CON and CSH values. †Significant difference between artificial cerebral spinal fluid and drug injections. ‡No significant difference between 10% and 21% O2 (P < 0.05). CON, controls; CSH, chronic sustained hypoxia; TE, expiratory time; TI, inspiratory time; VT/TI, ventilatory drive to breathe.
In CSH animals NBQX decreased VI during both normoxia and acute hypoxia by ∼20%, similar in magnitude to the decrease observed in CON animals exposed to acute hypoxia (Fig. 7A; P = 0.0006 and 0.01 for 10% and 21% O2, respectively). As the magnitude of this effect was similar between treatment conditions, NBQX had no significant effect on the slope of the HVR (Fig. 7B). These changes in VI were due to decreases in fR of ∼25% in both 10% and 21% O2 (Fig. 7C and D; P = 0.0001 and 0.001 for 10% and 21% O2, respectively), whereas VT trended slightly up to compensate (Fig. 7E and F). As in CON animals, TI and TE were both increased with NBQX in both 10% (P = 0.0002 and 0.003 for TI and TE, respectively) and 21% O2 (P = 0.0002 and 0.01 for TI and TE, respectively) in CSH animals (Fig. 8A and B). Similarly, the drive to breathe (VT/TI) mirrored VI such that NBQX significantly reduced VT/TI in CSH animals in both normoxia and hypoxia (Fig. 8C; P = 0.001 and 0.021 for 10% and 21% O2, respectively).
Taken together these data show that AMPAR blockade in the NTS tended to decrease ventilation and ventilatory drive under all conditions where these variables were elevated relative to normoxic controls (i.e. during acute hypoxia in CON and CSH animals and during normoxia in CSH animals), primarily through effects on breathing frequency.
NBQX but not MK801 affected the hypercapnic ventilatory response in control or chronic sustained hypoxia rats
The ventilatory response to hypercapnia (7% CO2) was not altered in any ventilatory parameter by MK801 microinjection in any treatment group or experimental condition (Fig. 9A–C), as indicated by three-way ANOVA analysis. Conversely, NBQX microinjection tended to decrease ventilation but this only achieved significance in the fR of CSH animals during hypercapnia, i.e. the condition in which breathing was stimulated to the highest level (Fig. 9D–F; P = 0.005). Despite this change, a two-way ANOVA indicated that the magnitude of the slope of the hypercapnic ventilatory response (HCVR) was not different between CON and CSH rats in any parameter. Hence, the effects of different gluamate receptor blockers on the HCVR were different from their effects on the HVR.
Figure 9. Effect of MK801 and CNQX on the hypercapnic ventilatory response of CON and CSH rats.
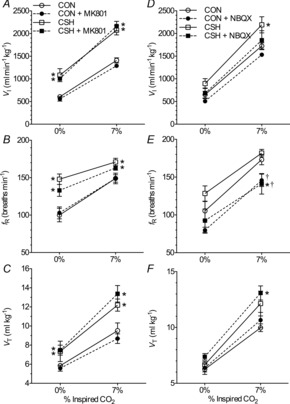
Effects of chronic hypoxia and an NMDAR antagonist (MK801) on (A) VI, (B) fR and (C) VT for CON and CSH rats before and after microinjection of MK801. Effects of chronic hypoxia and an AMPA receptor antagonist (NBQX) on (D) VI, (E) fR and (F) VT for CON and CSH rats before and after microinjection of NBQX. Data are means ± s.e.m. n = 13 CON and 8 CSH rats for MK801, 13 CON and 11 CSH for CNQX. *Significant difference between CON and CSH values (P < 0.05). CON, controls; CSH, chronic sustained hypoxia; fR, breathing frequency; VI, total minute ventilation; VT, tidal volume.
NMDA and AMPA receptor antagonists do not affect arterial blood gases
Arterial blood gases were examined in a subset of animals from each treatment group (Fig. 10). 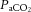 was depressed and
was depressed and 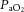 was increased in CSH rats relative to CON animals, as expected for ventilatory acclimatization to hypoxia (Olson & Dempsey, 1978; Aaron & Powell, 1993). Arterial pH in animals breathing 10% O2 was similar in CON and CSH groups (7.56 ± 0.05 and 7.52 ± 0.02, respectively) despite the lower
was increased in CSH rats relative to CON animals, as expected for ventilatory acclimatization to hypoxia (Olson & Dempsey, 1978; Aaron & Powell, 1993). Arterial pH in animals breathing 10% O2 was similar in CON and CSH groups (7.56 ± 0.05 and 7.52 ± 0.02, respectively) despite the lower 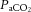 with CSH, as expected for metabolic compensation of the respiratory alkalosis (Olson & Dempsey, 1978; Aaron & Powell, 1993). Microinjection of MK801 or NBQX had no significant effects on arterial blood gases or pH values in either group as indicated by three-way ANOVA (Fig. 10A and B). Therefore, effects of glutamate receptor antagonists on breathing cannot be explained by changes in arterial blood gases and changes were consistent with the effects predicted from changes in ventilation and are in good agreement with previous studies (Smith et al. 2001). For example,
with CSH, as expected for metabolic compensation of the respiratory alkalosis (Olson & Dempsey, 1978; Aaron & Powell, 1993). Microinjection of MK801 or NBQX had no significant effects on arterial blood gases or pH values in either group as indicated by three-way ANOVA (Fig. 10A and B). Therefore, effects of glutamate receptor antagonists on breathing cannot be explained by changes in arterial blood gases and changes were consistent with the effects predicted from changes in ventilation and are in good agreement with previous studies (Smith et al. 2001). For example, 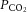 tended to increase when tidal volume decreased with drugs, particularly in normoxia. Such increases in arterial
tended to increase when tidal volume decreased with drugs, particularly in normoxia. Such increases in arterial 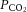 should increase ventilatory drive and tidal volume if the drugs were not having direct effects on the ventilatory chemoreflexes.
should increase ventilatory drive and tidal volume if the drugs were not having direct effects on the ventilatory chemoreflexes.
Figure 10. Arterial blood gases are not changed by NMDA or AMPA receptor antagonism.
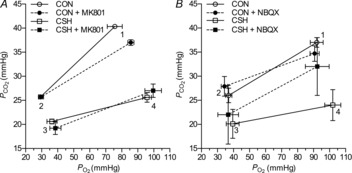
A, average changes in 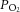 and
and 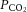 in response to microinjection of the NMDA receptor antagonist MK801 into the nucleus tractus solitarius of CON and CSH rats breathing acute levels of 10% and 21% O2. B, average changes in
in response to microinjection of the NMDA receptor antagonist MK801 into the nucleus tractus solitarius of CON and CSH rats breathing acute levels of 10% and 21% O2. B, average changes in 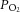 and
and 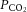 in response to microinjection of the AMPA receptor antagonist NBQX into the nucleus tractus solitarius of CON and CSH rats breathing acute levels of 10% and 21% O2. All blood gases are corrected for body temperature. Numbers refer to the following: (1) CON rats breathing 21% O2; (2) CON rats breathing 10% O2; (3) CSH rats breathing 10% O2; (4) CSH rats breathing 21% O2. Data are means ± s.e.m. from n = 3 rats per group. Significance was assessed as P < 0.05. CON, controls; CSH, chronic sustained hypoxia.
in response to microinjection of the AMPA receptor antagonist NBQX into the nucleus tractus solitarius of CON and CSH rats breathing acute levels of 10% and 21% O2. All blood gases are corrected for body temperature. Numbers refer to the following: (1) CON rats breathing 21% O2; (2) CON rats breathing 10% O2; (3) CSH rats breathing 10% O2; (4) CSH rats breathing 21% O2. Data are means ± s.e.m. from n = 3 rats per group. Significance was assessed as P < 0.05. CON, controls; CSH, chronic sustained hypoxia.
Expression of phosphorylated NMDA and AMPA receptor protein subunits increases in the nucleus tractus solitarius of chronic sustained hypoxia rats
Changes in NMDAR and AMPAR protein subunit expression in the NTS were analysed in a subset of rats treated with CON or CSH (n = 3 rats per treatment) and normalized to a housekeeping gene to correct for differences in the amount of protein loaded between gels, and then CSH values were expressed relative to the average CON value (set to 100%). Expression of the NR1 and GluR2 subunits did not change with CSH treatment, indicating that the number of receptors expressed was unchanged by CSH (Fig. 11A and B). Conversely, expression of the phosphorylated form of the NR1 (phospho-NR1) and GluR1 (phospho-GluR1) subunits increased 35% and 70%, respectively, in CSH relative to CON rats (Fig. 11A and B; P = 0.0081 for phospho-NR1 and 0.0065 for phospho-GluR1). The expression of unphosphorylated GluR1 subunit protein did not change significantly, but tended to decrease in CSH, and as a result, the relative expression of phospho-GluR1 relative to total GluR1 increased 100% in CSH rats relative to control.
Figure 11. Effect of hypoxia on nucleus tractus solitarius glutamate receptor protein expression.
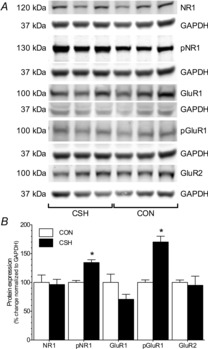
A, sample Western blots of NMDAR and AMPAR subunit protein expression and phosphorylation state from nucleus tractus solitarius samples isolated from CON rats or rats treated with CSH for 7 days. Each glutamate receptor subunit protein blot is paired with a GAPDH protein blot (below the respective glutamate protein) from the same gel. B, summary of fold-change in protein expressions from (A) normalized to GAPDH protein expression in the same samples. Data are means ± s.e.m. from three separate experiments for each antibody. The expression of all proteins was examined on each blot by stripping bound antibodies with stripping buffer and re-probing. *Significant difference between CSH and CON samples (P < 0.05).
Discussion
Our results confirm that glutamate receptors in the NTS contribute to ventilatory chemoreflexes and demonstrate a specific role for NMDARs in VAH. NMDARs are necessary for a normal HVR in CON rats and their role is significantly increased in chronically hypoxic rats, as MK801 completely blocks the ventilatory response to acute hypoxia after chronic hypoxia. In addition, we show that AMPARs in the NTS contribute to, but are not necessary for, increased ventilatory drive stimulated by acute or chronic hypoxia and hypercapnia. Our results also demonstrate increased phosphorylation of NR1 and GluR1 receptors with chronic hypoxia, without changes in receptor protein levels, which may contribute to these effects. This is an advance over previous studies of glutamate receptors and ventilatory chemoreflex plasticity, which have been limited to acute responses in normoxic control animals, have utilized anaesthetized preparations, and/or have not targeted specific brain regions or synaptic pathways but have instead utilized systemic glutamate receptor antagonists (Vardhan et al. 1993; Mizusawa et al. 1994; Ohtake et al. 1998; Reid & Powell, 2005; El Hasnaoui-Saadani et al. 2007).
Microinjection of NMDA into the NTS leads to marked and sustained increases in ventilation in CON and CSH animals mediated by increased VI, fR and VT, as previously shown for glutamate microinjections in the NTS of CON rats (Vardhan et al. 1993; Mizusawa et al. 1994). These increases are abolished by pretreatment with the specific NMDAR antagonist MK801, confirming they are NMDAR-mediated. Antagonism of NMDARs in the NTS moderately reduces the acute HVR in CON rats, primarily through a small decrease in fR. Consistent with previous experiments in which MK801 was injected systemically, the HVR is significantly reduced in our study by MK801 in CON animals (Ohtake et al. 1998; Reid & Powell, 2005). This finding is also consistent with a study in anaesthetized dogs, in which an NMDAR antagonist abolished the excitatory response of bulbospinal neurons to CO2 bolus stimulation of carotid bodies (Dogas et al. 1995). The effects on fR and VT are different with NMDAR blockade targeted to the NTS versus systemic blockade, indicating the involvement of multiple brain regions in ventilatory control circuits. In CSH animals, VAH is completely abolished in all ventilatory parameters by MK801 and there is no difference between untreated CON and MK801-treated CSH animals breathing hypoxic gas mixtures (i.e. in VT, fR, VI and VT/TI, although there are small offsetting changes in TE and TI between these populations). Hence, NMDARs in the NTS are necessary for the hypoxic increase in ventilation with CSH in conscious rats. However, it is also possible that plasticity occurs in ventilatory chemoreflex sites distal to those involving NMDA in the caudal NTS, for example in bulbospinal or phrenic motor neurons.
In addition to a critical role for NMDARs in VAH, we also demonstrate a central role for AMPARs in ventilatory responses to both acute hypoxia and VAH. However in contrast to the effects of NMDARs in the NTS, which are specific to VAH, AMPARs appear to mediate increased ventilation due to multiple stimuli in normoxia (with hypercapnia), hypoxia and after chronic hypoxia. Similar to NMDA injection, acute injection of AMPA into the NTS increases breathing in both groups. This response is more transient than following NMDA injection, and ventilatory parameters return to baseline 1–2 min after AMPA microinjection. Blocking AMPARs in the NTS significantly attenuates ventilatory responses in all experimental conditions in which the drive to breathe is elevated, i.e. in both groups during hypoxia and hypercapnia, and in CSH rats during normoxia. In CON animals breathing hypoxic gas mixtures, the depression in VI with NBQX is primarily mediated by a decrease in fR, while there is no change in VT. NBQX also decreases fR in normoxia but this leads to a compensatory increase in VT, and VI does not change. Similar changes in fR and VT with carotid body chemoreceptor stimulation were found in anaesthetized rats using another AMPAR antagonist (DNQX) microinjected in the NTS, although the net effect on VI was not significant (Vardhan et al. 1993). In addition, our results are consistent with reported effects of systemic NBQX in developing (5–15-day-old) rats, which decreases fR and increases VT without changing VI in hypoxia (Whitney et al. 2000).
Results testing the ventilatory response to CO2 also show different effects with different glutamate receptor blockers in the NTS and support a unique effect of NMDARs in the HVR and VAH. MK801 had no effects on the HCVRs but NBQX tended to decrease ventilation in 7% CO2, and significantly decreased fR in CSH rats. This is consistent with a central role for AMPARs in mediating increased ventilation whenever ventilatory drive increases, while NMDARs appear to have unique effects on the O2-sensitive ventilatory chemoreflexes.
AMPARs increase ventilation in all conditions with elevated ventilatory drive, including with VAH. This is consistent with the additive effect of glutamate on AMPARs of respiratory premotor neurons (McCrimmon et al. 1997). Hence, the overall increase in ventilatory drive with CSH, which is observed in acute hypoxia, normoxia or hypercapnia, could result from increased AMPAR activity with tonic increases in afferent activity from carotid bodies that occurs with CSH (Powell, 2007). Indeed, AMPARs activate and desensitize rapidly, thereby mediating fast excitatory synaptic transmission, whereas NMDARs generally control motorneuron excitability by modulating repetitive firing behaviour and contributing to non-linear behaviours and use-dependent synaptic plasticity (Rekling et al. 2000).
The evidence supporting a central role for glutamatergic neurotransmission in the NTS in the carotid body–ventilatory chemoreflex is clear (see Introduction). However, the potential role of various glutamate receptors in the plasticity of the HVR as it changes during acclimatization to CSH and in conscious animals has been unknown but it is notable that VAH shows some similarities to long-term potentiation (LTP), which is a cellular model for learning and memory that also involves NMDARs (Lynch, 2004). VAH is an increase in ventilatory output that persists for hours to days after removal of the hypoxic stimulus and the induction of VAH appears specific to a threshold level of carotid body chemoreceptor stimulation. In the mammalian hippocampal model of LTP, glutamatergic neurotransmission is enhanced by increased presynaptic glutamate release (induced by retrograde nitric oxide signalling from the post-synaptic neuron) and/or increased post-synaptic sensitivity to glutamate (e.g. due to increased AMPAR or NMDAR expression and/or sensitivity, primarily due to receptor phosphorylation) (Malenka & Nicoll, 1999; Malenka & Bear, 2004). There is little available information regarding the synaptic plasticity mechanisms of VAH. During acute hypoxia, phrenic nerve activity increases and this increase correlates with elevated glutamate levels in the NTS (Mizusawa et al. 1994; Richter et al. 1999); however, measurements of glutamate release have not been made during periods of hypoxia >30 min, and it is not known whether this increase is sustained during CSH. Presently it is not known whether new protein synthesis or receptor modification is required to sustain or enhance the HVR during longer-term hypoxia (i.e. VAH), which occurs during CSH or in chronic lung disorders.
Our observation that phospho-NR1 and -GluR1 protein expression increases significantly with CSH, whereas expression of NR1 and GluR2 proteins is unchanged, suggests that the total number of receptors expressed at the synapse is unchanged by CSH and that receptor modification via protein phosphorylation is the primary molecular component of the synaptic plasticity that underlies the glutamatergic component of increased CNS gain of VAH (Greger et al. 2007; Paoletti et al. 2013). The underlying molecular pathway is likely mediated by protein kinase C (PKC): phosphorylation of NR1 by PKC prevents the inhibition of NMDARs by calmodulin, thereby reducing Ca2+-mediated disinhibition of the channel and contributing to LTP (Hisatsune et al. 1997; Lee, 2006), and GluR1 is also phosphorylated by PKC, which causes an increase in AMPAR open probabilities (Derkach et al. 1999; Lee, 2006). Our measurements of NMDAR subunit expression agree with previous observations of no change in NMDAR NR1, NR2A and NR2B subunit expression in rat brainstem during up to a month of chronic hypoxia exposure (Reeves et al. 2003). However, others have reported that NR1 expression increases ∼33% in the whole medulla of rats exposed to 2 weeks of 8% O2 (El Hasnaoui-Saadani et al. 2007). Conversely, our measurements of AMPAR subunit expression differ partially from a previous study in rats exposed to 7–8 days of hypoxia in which NTS GluR1 expression was unchanged but GluR2 expression increased three-fold (Zhang et al. 2009). To our knowledge, ours are the first measurements of phosphorylated glutamate receptor expression changes with chronic hypoxia.
One limitation of our study is that we investigated only two levels of acute O2 exposure (21% and 10%) and did not generate a more complete oxygen-response curve to each treatment condition. We chose this approach because we were concerned about the duration of antagonist effects and potential complications from repeated dosing in the same animal. Furthermore, our protocol requires a return to baseline conditions for 15 min between ventilatory challenges (Fig. 1) and is therefore a relatively long protocol. Additional challenges would have significantly increased the time that a given rat was being experimented upon, which would increase stress and raise additional potential complications. None the less, the study of the effect of different neurochemicals on the shape of the HVR would be a fascinating addition to the literature and this question may be targeted in future experiments utilizing chronic neurotransmitter blockade. It is also important to note that our study is focused on the long-term model of CSH, which is generally stable in rats between 4–5 days and 2 months of hypoxic exposure; however, the underlying mechanism of VAH in this time frame probably differs from that of VAH in the first 24–48 h of CSH. VAH in response to short-term hypoxia is likely predominately due to carotid body plasticity and several studies have demonstrated that carotid body oxygen-sensitivity increases more rapidly than the CNS gain of the HVR (Nielsen et al. 1988; Dwinell et al. 1996; Wilkinson et al. 2009).
Our study was specifically focused on the mechanism underlying the CNS gain component of VAH during long-term CSH in rats and our results confirm previous studies showing ionotropic glutamate receptors mediate the acute HVR. The results also demonstrate for the first time a critical role for a specific type of glutamate receptor, i.e. NMDARs in central neural plasticity in VAH, which is specific to the O2-sensitive ventilatory chemoreflex (cf. no differences in the effects of NMDAR blockers on the HCVR after CSH). Changes in NR1 phosphorylation in the NTS probably mediate this plasticity, which may share a common signalling mechanism with other types of neural plasticity such as LTP (see above). Further study is necessary to determine the signals for NMDAR plasticity in VAH (e.g. hypoxia versus activity) and to understand how different glutamate receptors in the NTS interact in chronic sustained hypoxia. For example, the effects of simultaneously blocking NMDAR and non-NMDAR in the NTS on ventilatory chemoreflexes appear different in awake and anaesthetized rats (Vardhan et al. 1993; Mizusawa et al. 1994). Also, metabotropic glutamate receptors, which mediate differential effects on intrinsic and synaptic properties via distinct synaptic mechanisms (Rekling et al. 2000) and are involved in LTP (Lynch, 2004), warrant investigation. Indeed, both metabotropic and ionotropic glutamate receptors in the NTS mediate cardiovascular reflexes, which share many common properties with ventilatory reflexes (Zhang & Mifflin, 1993). Finally, the relative roles and interactions between excitatory and inhibitory neurotransmission in VAH need to be determined because the inhibitory effects of GABA in the NTS on the HVR are depressed in chronic hypoxia (Chung et al. 2006).
Key points
Ventilation increases more with chronic than acute hypoxia and does not return to control levels when normoxia is restored, indicating plasticity in the reflexes that control breathing.
Glutamate is the primary excitatory neurotransmitter between arterial chemoreceptors that sense hypoxia and neural circuits that control breathing in the brainstem.
We microinjected specific glutamate receptor antagonists into the brainstem of awake unrestrained rats and found NMDA-type glutamate receptors explain increased ventilatory sensitivity to hypoxia after chronic hypoxia. AMPA-type glutamate receptors mediate increased ventilatory drive in normoxia after chronic hypoxia, as well as increased ventilation in acute hypoxia after chronic hypoxia and in control conditions.
Phosphorylation of AMPA and NMDA receptors is increased by chronic hypoxia.
The results indicate that plasticity in different glutamate receptors have unique effects on the reflexes that control breathing in chronic hypoxia and may share cellular mechanisms with other models of neural plasticity.
Acknowledgments
We graciously thank Ms Isabelle Marfone and Mr Shashank Gupta for technical assistance.
Glossary
- ACSF
artificial cerebral spinal fluid
- AMPAR
AMPA receptor
- fR
breathing frequency
- CSH
chronic sustained hypoxia
- MK-801
dizocilpine
- HCVR
hypercapnic ventilatory response
- HVR
hypoxic ventilatory response
- NBQX
2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoaxaline-2,3-dione
- NMDAR
NMDA receptor
- NTS
nucleus of the solitary tract (nucleus tractus solitarius)
arterial oxygen content
- TE
expiratory time
- TI
inspiratory time
- VAH
ventilatory acclimatization to hypoxia
- VI
minute ventilation
- VT
tidal volume
- VT/TI
ventilatory drive
Additional information
Competing interests
None declared.
Author contributions
M.E.P., J.A.C., A.G., Z.F. and S.G.R. performed experiments. J.A.C., A.G., S.G.R. and F.L.P. contributed to the conception and design of experiments. M.E.P., J.A.C., S.G.R. and F.L.P. contributed to the analysis and interpretation of data. M.E.P. and F.L.P. wrote the manuscript and J.A.C., A.G., Z.F. and S.G.R. contributed to the revision of the manuscript. All authors approved the final version of the manuscript. Experiments were performed at the University of California San Diego.
Funding
This work was supported by NHLBI 1R01HL081823 (F.L.P.).
References
- Aaron EA, Powell FL. Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J Appl Physiol. 1993;74:1635–1640. doi: 10.1152/jappl.1993.74.4.1635. [DOI] [PubMed] [Google Scholar]
- Bee D, Pallot DJ. Acute hypoxic ventilation, carotid body cell division, and dopamine content during early hypoxia in rats. J Appl Physiol. 1995;79:1504–1511. doi: 10.1152/jappl.1995.79.5.1504. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer MS. Peripheral and central efffects of hypoxia. In: Dempsey JA, Pack AIJ, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 617–618. [Google Scholar]
- Braga VA, Soriano RN, Braccialli AL, de Paula PM, Bonagamba LG, Paton JF, Machado BH. Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol. 2007;581:1129–1145. doi: 10.1113/jphysiol.2007.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Ivy GO, Reid SG. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1449–R1456. doi: 10.1152/ajpregu.00645.2005. [DOI] [PubMed] [Google Scholar]
- Coles SK, Ernsberger P, Dick TE. A role for NMDA receptors in posthypoxic frequency decline in the rat. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1546–R1555. doi: 10.1152/ajpregu.1998.274.6.R1546. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Otto-Smith MR, Feldman JL. Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res. 1992;596:99–110. doi: 10.1016/0006-8993(92)91537-o. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogas Z, Stuth EA, Hopp FA, McCrimmon DR, Zuperku EJ. NMDA receptor-mediated transmission of carotid body chemoreceptor input to expiratory bulbospinal neurones in dogs. J Physiol. 1995;487(Pt 3):639–651. doi: 10.1113/jphysiol.1995.sp020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anaesthetized rats. J Appl Physiol. 1999;87:817–823. doi: 10.1152/jappl.1999.87.2.817. [DOI] [PubMed] [Google Scholar]
- Dwinell MR, Janssen PL, Pizarro J, Bisgard GE. The role of carotid body CO2 during ventilatory acclimatization to hypoxia in the goat. Adv Exp Med Biol. 1996;410:387–390. doi: 10.1007/978-1-4615-5891-0_60. [DOI] [PubMed] [Google Scholar]
- El Hasnaoui-Saadani R, Alayza RC, Launay T, Pichon A, Quidu P, Beaudry M, Leon-Velarde F, Richalet JP, Duvallet A, Favret F. Brain stem NO modulates ventilatory acclimatization to hypoxia in mice. J Appl Physiol. 2007;103:1506–1512. doi: 10.1152/japplphysiol.00486.2007. [DOI] [PubMed] [Google Scholar]
- Fidone S, Gonzalez C, Yoshizaki K. Effects of hypoxia on catecholamine synthesis in rabbit carotid body in vitro. J Physiol. 1982;333:81–91. doi: 10.1113/jphysiol.1982.sp014440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Bisgard GE, Rasmussen B, Orr JA, Buss DD, Manohar M. Ventilatory control in peripheral chemoreceptor-denervated ponies during chronic hypoxemia. J Appl Physiol. 1976;41:878–885. doi: 10.1152/jappl.1976.41.6.878. [DOI] [PubMed] [Google Scholar]
- Gozal D, Torres JE, Gozal YM, Littwin SM. Effect of nitric oxide synthase inhibition on cardiorespiratory responses in the conscious rat. J Appl Physiol (1985) 1996;81:2068–2077. doi: 10.1152/jappl.1996.81.5.2068. [DOI] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Haibara AS, Bonagamba LG, Machado BH. Sympathoexcitatory neurotransmission of the chemoreflex in the NTS of awake rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R69–R80. doi: 10.1152/ajpregu.1999.276.1.R69. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Umemori H, Inoue T, Michikawa T, Kohda K, Mikoshiba K, Yamamoto T. Phosphorylation-dependent regulation of N-methyl-D-aspartate receptors by calmodulin. J Biol Chem. 1997;272:20805–20810. doi: 10.1074/jbc.272.33.20805. [DOI] [PubMed] [Google Scholar]
- Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol. 1988;406:99–114. doi: 10.1113/jphysiol.1988.sp017371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey KA, Low MJ, Kelly MA, Juarez R, Szewczak JM, Powell FL. Ventilatory responses to acute and chronic hypoxia in mice: effects of dopamine D-2 receptors. J Appl Physiol. 2000;89:1142–1150. doi: 10.1152/jappl.2000.89.3.1142. [DOI] [PubMed] [Google Scholar]
- Hupperets MD, Hopkins SR, Pronk MG, Tiemessen IJ, Garcia N, Wagner PD, Powell FL. Increased hypoxic ventilatory response during 8 weeks at 3800 m altitude. Respir Physiol Neurobiol. 2004;142:145–152. doi: 10.1016/j.resp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol. 1978;45:644–647. doi: 10.1152/jappl.1978.45.4.644. [DOI] [PubMed] [Google Scholar]
- Kumar P, Prabhakar N. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, McAllen RM, Spyer KM. The carotid chemoreceptor input to the respiratory neurones of the nucleus of tractus solitarus. J Physiol. 1977;269:797–810. doi: 10.1113/jphysiol.1977.sp011930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Machado BH, Castania JA, Bonagamba LG, Salgado HC. Neurotransmission of autonomic components of aortic baroreceptor afferents in the NTS of awake rats. Am J Physiol Heart Circ Physiol. 2000;279:H67–H75. doi: 10.1152/ajpheart.2000.279.1.H67. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol. 1994;478(Pt 1):55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AM, Bisgard GE, Vidruk EH. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol (1985) 1988;65:1796–1802. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- Ohta H, Lewis SJ, Talman WT. Spermidine and cardiovascular control in nucleus tractus solitarii in rat. Brain Res. 1993;620:72–77. doi: 10.1016/0006-8993(93)90272-o. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Dempsey JA. Rat as a model for humanlike ventilatory adaptation to chronic hypoxia. J Appl Physiol. 1978;44:763–769. doi: 10.1152/jappl.1978.44.5.763. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Pichon A, Zhenzhong B, Favret F, Jin G, Shufeng H, Marchant D, Richalet JP, Ge RL. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): role of nNOS and dopamine. Am J Physiol Regul Integr Comp Physiol. 2009;297:R978–R987. doi: 10.1152/ajpregu.00108.2009. [DOI] [PubMed] [Google Scholar]
- Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol. 2007;157:154–161. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Reeves SR, Gozal E, Guo SZ, Sachleben LR, Jr, Brittian KR, Lipton AJ, Gozal D. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol. 2003;95:1767–1774. doi: 10.1152/japplphysiol.00759.2002. [DOI] [PubMed] [Google Scholar]
- Reid SG, Powell FL. Effects of chronic hypoxia on MK-801-induced changes in the acute hypoxic ventilatory response. J Appl Physiol. 2005;99:2108–2114. doi: 10.1152/japplphysiol.01205.2004. [DOI] [PubMed] [Google Scholar]
- Reid SG, Aguilar NM, Kim CB, Nguyen JC, Vargas E, Powell FL. The role of NMDA receptors in ventilatory acclimatization to hypoxia. FASEB J. 2002;16:A69. [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Dempsey JA, Hornbein TF. Control of breathing at high altitude. In: Hornbein TF, Schoene RB, editors. High Altitude. New York: Marcel Dekker; 2001. pp. 139–173. [Google Scholar]
- Soto-Arape I, Burton MD, Kazemi H. Central amino acid neurotransmitters and the hypoxic ventilatory response. Am J Respir Crit Care Med. 1995;151:1113–1120. doi: 10.1164/ajrccm/151.4.1113. [DOI] [PubMed] [Google Scholar]
- Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol Regul Integr Comp Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- Whitney GM, Ohtake PJ, Simakajornboon N, Xue YD, Gozal D. AMPA glutamate receptors and respiratory control in the developing rat: anatomic and pharmacological aspects. Am J Physiol Regul Integr Comp Physiol. 2000;278:R520–R528. doi: 10.1152/ajpregu.2000.278.2.R520. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Nichols NL, Putnam RW, Powell FL. Chronic hypoxia decreases response to central chemoreceptor stimulation in the nucleus tractus solitarius (NTS) FASEB J. 2009;23(Suppl):621.16. [Google Scholar]
- Wilkinson KA, Huey K, Dinger B, He L, Fidone S, Powell FL. Chronic hypoxia increases the gain of the hypoxic ventilatory response by a mechanism in the central nervous system. J Appl Physiol. 2010a;109:424–430. doi: 10.1152/japplphysiol.01311.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Huey K, Dinger B, He LA, Fidone S, Powell FL. Chronic hypoxia increases the gain of the hypoxic ventilatory response by a mechanism in the central nervous system. J Appl Physiol. 2010b;109:424–430. doi: 10.1152/japplphysiol.01311.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef F, Addae J, McRae A, Stone T. Long-term potentiation protects rat hippocampal slices from the effects of acute hypoxia. Brain Res. 2001;907:144–150. doi: 10.1016/s0006-8993(01)02594-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin SW. Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in rats. Am J Physiol Heart Circ Physiol. 1993;265:H770–H773. doi: 10.1152/ajpheart.1993.265.2.H770. [DOI] [PubMed] [Google Scholar]
- Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained hypoxia enhances both evoked EPSCs and norepinephrine inhibition of glutamatergic afferent inputs in the nucleus of the solitary tract. J Neurosci. 2009;29:3093–3102. doi: 10.1523/JNEUROSCI.2648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


