Abstract
Oxygen uptake kinetics (τ ) are slowed when exercise is initiated from a raised metabolic rate. Whether this reflects the recruitment of muscle fibres differing in oxidative capacity, or slowed blood flow (
) are slowed when exercise is initiated from a raised metabolic rate. Whether this reflects the recruitment of muscle fibres differing in oxidative capacity, or slowed blood flow ( ) kinetics is unclear. This study determined τ
) kinetics is unclear. This study determined τ in canine muscle in situ, with experimental control over muscle activation and
in canine muscle in situ, with experimental control over muscle activation and  during contractions initiated from rest and a raised metabolic rate. The gastrocnemius complex of nine anaesthetised, ventilated dogs was isolated and attached to a force transducer. Isometric tetanic contractions (50 Hz; 200 ms duration) via supramaximal sciatic nerve stimulation were used to manipulate metabolic rate: 3 min stimulation at 0.33 Hz (S1), followed by 3 min at 0.67 Hz (S2). Circulation was initially intact (SPON), and subsequently isolated for pump-perfusion (PUMP) above the greatest value in SPON. Muscle
during contractions initiated from rest and a raised metabolic rate. The gastrocnemius complex of nine anaesthetised, ventilated dogs was isolated and attached to a force transducer. Isometric tetanic contractions (50 Hz; 200 ms duration) via supramaximal sciatic nerve stimulation were used to manipulate metabolic rate: 3 min stimulation at 0.33 Hz (S1), followed by 3 min at 0.67 Hz (S2). Circulation was initially intact (SPON), and subsequently isolated for pump-perfusion (PUMP) above the greatest value in SPON. Muscle  was determined contraction-by-contraction using an ultrasonic flowmeter and venous oximeter, and normalised to tension-time integral (TTI). τ
was determined contraction-by-contraction using an ultrasonic flowmeter and venous oximeter, and normalised to tension-time integral (TTI). τ /TTI and τ
/TTI and τ were less in S1SPON (mean ± s.d.: 13 ± 3 s and 12 ± 4 s, respectively) than in S2SPON (29 ± 19 s and 31 ± 13 s, respectively; P < 0.05). τ
were less in S1SPON (mean ± s.d.: 13 ± 3 s and 12 ± 4 s, respectively) than in S2SPON (29 ± 19 s and 31 ± 13 s, respectively; P < 0.05). τ /TTI was unchanged by pump-perfusion (S1PUMP, 12 ± 4 s; S2PUMP, 24 ± 6 s; P < 0.001) despite increased O2 delivery; at S2 onset, venous O2 saturation was 21 ± 4% and 65 ± 5% in SPON and PUMP, respectively.
/TTI was unchanged by pump-perfusion (S1PUMP, 12 ± 4 s; S2PUMP, 24 ± 6 s; P < 0.001) despite increased O2 delivery; at S2 onset, venous O2 saturation was 21 ± 4% and 65 ± 5% in SPON and PUMP, respectively.  kinetics remained slowed when contractions were initiated from a raised metabolic rate despite uniform muscle stimulation and increased O2 delivery. The intracellular mechanism may relate to a falling energy state, approaching saturating ADP concentration, and/or slowed mitochondrial activation; but further study is required. These data add to the evidence that muscle
kinetics remained slowed when contractions were initiated from a raised metabolic rate despite uniform muscle stimulation and increased O2 delivery. The intracellular mechanism may relate to a falling energy state, approaching saturating ADP concentration, and/or slowed mitochondrial activation; but further study is required. These data add to the evidence that muscle  control is more complex than previously suggested.
control is more complex than previously suggested.
Introduction
Increasing the rate of skeletal muscle power production requires an increase in the rate of intramuscular oxidative phosphorylation in order to meet the energy demands of the physical task. A rapid adjustment in oxidative ATP provision is a hallmark of an effectively integrated, and healthy, physiological system (Rossiter, 2011; Poole & Jones, 2012), as it requires a coordinated adjustment of O2 transport through the respiratory and cardiovascular systems, and O2 utilisation ( ) within the activated skeletal muscles. The dynamic control of skeletal muscle
) within the activated skeletal muscles. The dynamic control of skeletal muscle  is mediated through interactions amongst mitochondrial phosphate feedback, O2 and substrate delivery, and mitochondrial enzyme activation, each of which may be subject to control and/or limitation (Meyer, 1989; Glancy et al. 2008; Schmitz et al. 2012; Wüst et al. 2013). However, the exact mechanisms contributing to
is mediated through interactions amongst mitochondrial phosphate feedback, O2 and substrate delivery, and mitochondrial enzyme activation, each of which may be subject to control and/or limitation (Meyer, 1989; Glancy et al. 2008; Schmitz et al. 2012; Wüst et al. 2013). However, the exact mechanisms contributing to  kinetics remain incompletely understood.
kinetics remain incompletely understood.
At the onset of moderate-intensity exercise in young, healthy individuals, the time constant (τ) of  inferred from breath-by-breath pulmonary gas exchange measurement (Grassi et al. 1996) is greater (i.e.
inferred from breath-by-breath pulmonary gas exchange measurement (Grassi et al. 1996) is greater (i.e.  kinetics are slower) when exercise is initiated from an active baseline causing a raised metabolic rate (Brittain et al. 2001; MacPhee et al. 2005; Bowen et al. 2011; Williams et al. 2013). This observation is also evident during high-intensity exercise (DiMenna et al. 2008, 2009, 2010a, b2010b, c2010c). The mechanism of a slowed
kinetics are slower) when exercise is initiated from an active baseline causing a raised metabolic rate (Brittain et al. 2001; MacPhee et al. 2005; Bowen et al. 2011; Williams et al. 2013). This observation is also evident during high-intensity exercise (DiMenna et al. 2008, 2009, 2010a, b2010b, c2010c). The mechanism of a slowed  kinetic adjustment from a raised metabolic rate is typically attributed to a slower adjustment of O2 delivery (Hughson & Morrissey, 1982; MacPhee et al. 2005; DiMenna et al. 2010c) and/or an orderly fibre recruitment strategy (Brittain et al. 2001) in which highly oxidative fibres with inherently faster
kinetic adjustment from a raised metabolic rate is typically attributed to a slower adjustment of O2 delivery (Hughson & Morrissey, 1982; MacPhee et al. 2005; DiMenna et al. 2010c) and/or an orderly fibre recruitment strategy (Brittain et al. 2001) in which highly oxidative fibres with inherently faster  kinetics (Wüst et al. 2013) are recruited first. The finding that femoral artery blood flow (
kinetics (Wüst et al. 2013) are recruited first. The finding that femoral artery blood flow ( ) dynamics are also slowed during transitions from a raised metabolic rate (MacPhee et al. 2005) is consistent with the former suggestion. However, differentiating amongst these putative mechanisms in human studies is a complex challenge.
) dynamics are also slowed during transitions from a raised metabolic rate (MacPhee et al. 2005) is consistent with the former suggestion. However, differentiating amongst these putative mechanisms in human studies is a complex challenge.
Attempts to achieve this during very heavy-intensity cycle exercise transitions, using manipulations in posture (DiMenna et al. 2010c), cadence (DiMenna et al. 2009) and priming exercise (DiMenna et al. 2008, 2010b, c2010c) largely support either mechanism (Hughson & Morrissey, 1982; Brittain et al. 2001; Wilkerson & Jones, 2006; Jones et al. 2008b). More recently, attempts in humans to uncouple the influence of muscle recruitment from muscle metabolism and  (using two identical exercise bouts imposed on different background metabolic conditions) led to conflicting reports, one of which reported findings consistent with the orderly recruitment of muscle fibres with lower mitochondrial content in slowing
(using two identical exercise bouts imposed on different background metabolic conditions) led to conflicting reports, one of which reported findings consistent with the orderly recruitment of muscle fibres with lower mitochondrial content in slowing  kinetics (DiMenna et al. 2010a), whereas the other implicated an intracellular mechanism related to the raised metabolic rate itself in slowing
kinetics (DiMenna et al. 2010a), whereas the other implicated an intracellular mechanism related to the raised metabolic rate itself in slowing  kinetics (Bowen et al. 2011) (perhaps via a less negative Gibbs free energy of ATP splitting in previously active fibres; ΔGATP).
kinetics (Bowen et al. 2011) (perhaps via a less negative Gibbs free energy of ATP splitting in previously active fibres; ΔGATP).
The aim of the present study, therefore, was to determine muscle  kinetics in the isolated canine muscle in situ by direct application of Fick's principle (Hernandez et al. 2010), which allows for independent experimental control over muscle fibre recruitment, muscle
kinetics in the isolated canine muscle in situ by direct application of Fick's principle (Hernandez et al. 2010), which allows for independent experimental control over muscle fibre recruitment, muscle  , and baseline metabolic rate.
, and baseline metabolic rate.
To address the hypothesis that the orderly recruitment of muscle fibres with different mitochondrial density or capillarity is responsible for the slowed  kinetics during exercise transitions from an active baseline, muscle
kinetics during exercise transitions from an active baseline, muscle  ,
,  and microvascular oxygenation were measured with spontaneous blood flow (SPON) during increments in metabolic rate from rest to low-frequency stimulation (S1), and low-frequency to high-frequency stimulation (S2) of the sciatic nerve. The influence of muscle fibre recruitment would therefore be eliminated between S1 and S2 because all muscle fibres are maximally activated by direct nerve stimulation during both transitions.
and microvascular oxygenation were measured with spontaneous blood flow (SPON) during increments in metabolic rate from rest to low-frequency stimulation (S1), and low-frequency to high-frequency stimulation (S2) of the sciatic nerve. The influence of muscle fibre recruitment would therefore be eliminated between S1 and S2 because all muscle fibres are maximally activated by direct nerve stimulation during both transitions.
To address the hypothesis that slowed  dynamics are responsible for slowing
dynamics are responsible for slowing  kinetics on transition from an active baseline, the hindlimb muscle was pump-perfused (PUMP) at a constant high rate during both S1 and S2 transitions (Grassi et al. 1998). Were any differences to persist in muscle
kinetics on transition from an active baseline, the hindlimb muscle was pump-perfused (PUMP) at a constant high rate during both S1 and S2 transitions (Grassi et al. 1998). Were any differences to persist in muscle  kinetics between S1 and S2 during PUMP (in which both fibre recruitment and blood flow were constant), the results would indicate that neither factor is prerequisite for the slowed muscle
kinetics between S1 and S2 during PUMP (in which both fibre recruitment and blood flow were constant), the results would indicate that neither factor is prerequisite for the slowed muscle  kinetics during exercise initiated from a raised metabolic rate.
kinetics during exercise initiated from a raised metabolic rate.
Methods
Animals and ethical approval
This study was conducted with the approval of the Institutional Animal Care and Use Committee of Auburn University (Auburn, AL, USA), where the experiments were performed. Nine adult dogs of either sex were anaesthetised with pentobarbital sodium (30 mg kg−1) via an injection into the cephalic vein. After establishing a state of deep surgical anaesthesia, a jugular vein was isolated, and all subsequent doses (65–100 mg) were administered to maintain a deep surgical plane of anaesthesia. All dogs were treated with an initial bolus of heparin (1500 U kg−1) with two additional doses given at 2 h intervals to a total of 3000 U kg−1. Dogs were intubated with an endotracheal tube and mechanically ventilated (model 613; Harvard Apparatus, Holliston, MA, USA). Rectal temperature was maintained at 37°C with a heating pad. Ventilation was adjusted to maintain normal arterial 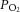 ,
, 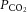 and pH.
and pH.
Surgical preparation
The surgical preparation was similar to the methods previously described in detail (Stainsby & Otis, 1964; Hernandez et al. 2010). In short, the left gastrocnemius plus superficial digital flexor (GS) was surgically isolated from surrounding muscles. A portion of the calcaneus, with the two tendons from the GS attached, was cut away at the heel and clamped around a metal rod for connection to an isometric myograph via a load cell (SM-250; Interface Inc., Scottsdale, AZ, USA) to measure tension development. The proximal end of the muscle remained attached to its origin. The hindlimb was fixed at the knee and ankle by nails placed into the femur and tibial bones and attached to a fixed platform. A turnbuckle strut was inserted between the tibial bone nail and the arm of the myograph to further minimize limb movement during contractions. The muscle was covered with saline-soaked gauze and a thin plastic sheet to reduce drying and cooling. The left sciatic nerve, which innervates the GS, was doubly ligated, cut between the ties, and the distal end secured in an epoxy-resin loop containing stimulating electrodes.
Venous outflow from the GS was isolated by ligating all veins draining into the popliteal vein except the GS veins. The popliteal vein was cannulated, and this venous blood flow was returned to the animal via a reservoir attached to a cannula in the left jugular vein. An indwelling oximeter probe (Oximetrix 3; Abbott Laboratories, North Chicago, IL, USA) and flowmeter (T206; Transonic Systems, Ithaca, NY, USA) were inserted into the cannulated vein to continuously monitor the fraction of saturated haemoglobin (Hb) and blood flow, respectively. For the SPON condition the arterial circulation was left intact. For the PUMP condition the arterial circulation to the GS was isolated by the ligation of all vessels from the popliteal artery that do not enter the GS. The contralateral femoral artery was isolated, cannulated, and the cannula was passed through a peristaltic pump (Minipuls 3 MP2/HF; Gilson, Inc., Middleton, WI, USA) into the popliteal artery supplying the contracting muscle. A T-connector in the tubing was connected to a pressure transducer (model RP-1500; Narco Bio-Systems, Inc., Austin, TX, USA) for the measurement of perfusion pressure. During the SPON condition, perfusion pressure was measured via a cannula in the right, contralateral femoral artery.
After all experiments, the muscle was removed from the animal, cleared of surface connective tissue, and weighed. The wet weight was used to normalise physiological variables to muscle mass (e.g.  and
and  ). All dogs were killed at the end of the experimental series with an overdose of pentobarbital sodium and saturated potassium chloride.
). All dogs were killed at the end of the experimental series with an overdose of pentobarbital sodium and saturated potassium chloride.
Protocol
At the start of each experiment, the muscle was set at an optimal length (Lo) by progressive lengthening until a peak was obtained in developed tension during stimulation at 0.2 Hz. Once Lo was determined, at least 5 min of rest was given before experiments began. Before the start of each experimental condition, the resting muscle tension was checked and the muscle reset to Lo as necessary. Isometric tetanic contractions (0.2 ms pulses at 50 Hz; 200 ms duration) were elicited via maximal nerve stimulation, with 3 min of stimulation at 0.33 Hz (S1) immediately followed by 3 min at 0.67 Hz (S2) (Fig. 1A). Both stimulation rates are expected to elicit submaximal rates of  : ∼40% and 60% of maximum
: ∼40% and 60% of maximum  , respectively (Kelley et al. 1996; Ameredes et al. 1998). In the first instance, muscle
, respectively (Kelley et al. 1996; Ameredes et al. 1998). In the first instance, muscle  was spontaneous (SPON) with the circulation intact. Subsequently, the arterial circulation was isolated and muscle
was spontaneous (SPON) with the circulation intact. Subsequently, the arterial circulation was isolated and muscle  was maintained at a constant high rate by peristaltic pump (PUMP); the rate was maintained above the steady-state rate measured in each individual dog at 0.67 Hz contractions during SPON in order to ensure that
was maintained at a constant high rate by peristaltic pump (PUMP); the rate was maintained above the steady-state rate measured in each individual dog at 0.67 Hz contractions during SPON in order to ensure that  was not limiting. This was achieved over ∼5 min prior to the contractile bout by a combination of adenosine infusion into the muscle arterial circulation and a series of small step increases in the pump rate to maintain perfusion pressure within the physiological range (<160 mmHg, with one exception). Stimulated contractions under the two perfusion conditions were separated by at least 1.5 h.
was not limiting. This was achieved over ∼5 min prior to the contractile bout by a combination of adenosine infusion into the muscle arterial circulation and a series of small step increases in the pump rate to maintain perfusion pressure within the physiological range (<160 mmHg, with one exception). Stimulated contractions under the two perfusion conditions were separated by at least 1.5 h.
Figure 1. Contractile protocol and group mean contraction-by-contraction responses of muscle tension development, blood flow ( ) and oxygen uptake (
) and oxygen uptake ( ).
).
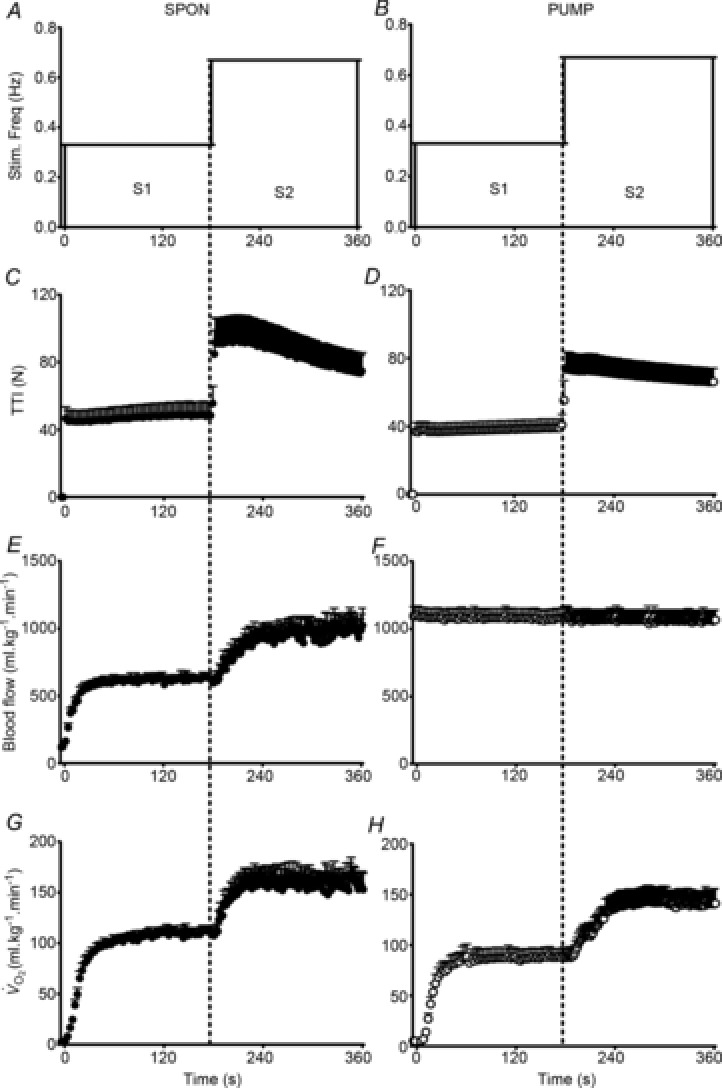
Spontaneous perfusion (SPON) is displayed in the left columns and pump-perfusion (PUMP) in the right columns. The two-step contractile protocol (S1 and S2) is visualised in A and B as a function of stimulation frequency. The measured tension-time-integral normalised per second (TTI) is displayed in C and D, indicating the increase in tension development at the higher stimulation frequency. The dynamic adjustment of blood flow was faster in S1SPON than in S2SPON (E), but was maintained at a constant high rate during S1PUMP and S2PUMP (F).  was slowed when stimulations were initiated from a raised metabolic baseline (S2) in both SPON (G) and PUMP (H). Group mean kinetic parameter values are presented in Table 1. Values are mean ± s.e.m.
was slowed when stimulations were initiated from a raised metabolic baseline (S2) in both SPON (G) and PUMP (H). Group mean kinetic parameter values are presented in Table 1. Values are mean ± s.e.m.
Measurements
Maximal tension (Tmax) was determined as the highest value during the contraction (load cell 90% response time was <1 ms). The tension-time integral (TTI; N·s) was calculated for each contraction during both S1 and S2 stimulations, as an indication for ATP turnover, and expressed as the mean value per second (N·s·s−1 = N).
The flowmeter was set to its highest pulsatile cut-off frequency of 100 Hz and manually calibrated with a graduated cylinder before, during and after each series of contractions.
The inline oximeter used to measure venous O2 saturation (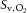 ) had a 90% response time of 5 s. To account for the oximeter response characteristics, the
) had a 90% response time of 5 s. To account for the oximeter response characteristics, the 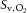 signal was filtered using a Butterworth filter and deconvoluted by a first-order transfer function, as previously described (Hernandez et al. 2010). The oximeter was calibrated for each individual contractile period using venous blood samples collected at rest and during steady state. These samples, as well as the arterial samples collected before and after each series of contractions, were analysed immediately at 37°C for
signal was filtered using a Butterworth filter and deconvoluted by a first-order transfer function, as previously described (Hernandez et al. 2010). The oximeter was calibrated for each individual contractile period using venous blood samples collected at rest and during steady state. These samples, as well as the arterial samples collected before and after each series of contractions, were analysed immediately at 37°C for 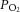 ,
, 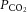 , pH, and lactate concentration ([lactate−]) by a blood gas, pH, metabolite analyser (GEM Premier 3000; Instrumentation Laboratory, Inc., Lexington, MA, USA) and for haemoglobin concentration ([Hb]), and percent saturation of Hb with O2 (
, pH, and lactate concentration ([lactate−]) by a blood gas, pH, metabolite analyser (GEM Premier 3000; Instrumentation Laboratory, Inc., Lexington, MA, USA) and for haemoglobin concentration ([Hb]), and percent saturation of Hb with O2 (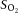 ) by a CO-Oximeter (IL 682; Instrumentation Laboratory, Inc.) set for dog blood.
) by a CO-Oximeter (IL 682; Instrumentation Laboratory, Inc.) set for dog blood.
Muscle oxygenation was determined using continuous-wave near-infrared spectroscopy (NIRS) (Oxymon MkIII; Artinis Medical Systems, Zetten, the Netherlands). Briefly, two fibre optic bundles communicate between the data-acquisition system and the muscle. At the end of one cable, NIR light is emitted from an optode in two wavelengths (860 nm and 784 nm); at the end of the other cable a second optode received NIR light for transmission back to the data acquisition unit to determine the relative concentrations of deoxygenated and oxygenated heme groups. This method does not distinguish between the contributions of Hb and myoglobin (Mb) to the NIRS signal. An optode holder was placed on the GS with a Velcro strap and opaque black plastic was placed over the optodes to block contamination from external light. NIR signals were biased to zero ∼30 s before the onset of stimulation. The relative change in oxygenated Hb plus Mb (Δ[HbMbO2]) and deoxygenated Hb plus Mb (Δ[HHbMb]), and their total sum (Δ[THbMb]) are presented in arbitrary units. Measurements of NIRS, muscle tension, venous  ,
, 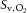 and muscle oxygenation were sampled at 125 Hz.
and muscle oxygenation were sampled at 125 Hz.
Calculations and kinetic analysis
 across the muscle was calculated using the Fick principle as
across the muscle was calculated using the Fick principle as 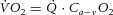 (the product of blood flow and the arteriovenous O2 concentration difference) and expressed as ml O2 per kg muscle mass per minute.
(the product of blood flow and the arteriovenous O2 concentration difference) and expressed as ml O2 per kg muscle mass per minute.  and
and 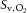 were averaged over each contraction cycle, allowing the calculation of
were averaged over each contraction cycle, allowing the calculation of  on a contraction-by-contraction basis as previously described (Hernandez et al. 2010). NIRS signals for muscle oxygenation changes were also averaged over each contraction cycle.
on a contraction-by-contraction basis as previously described (Hernandez et al. 2010). NIRS signals for muscle oxygenation changes were also averaged over each contraction cycle.
The kinetics of  (both the absolute value, and normalised to TTI;
(both the absolute value, and normalised to TTI;  /TTI),
/TTI),  and Δ[HHbMb] were fitted using OriginLab 8 (OriginLab Corp., Northampton, MA, USA) from the onset of contractions using the general equation:
and Δ[HHbMb] were fitted using OriginLab 8 (OriginLab Corp., Northampton, MA, USA) from the onset of contractions using the general equation:
| (1) |
where the variable value (y) any time t after the onset of contractions was characterised by the steady state increment (Δ[y]ss) above the baseline ([y]b), and TD and τ represent the delay and time constant of the exponential response, respectively. To determine the best fit, several criteria were used (Rossiter et al. 2001; Whipp & Rossiter, 2005). The [y]b value for S1 was determined from the average of ∼10 s before the onset of stimulations, and [y]b for S2 was determined from the average of the last 30 s of the S1 steady-state and then fixed for non-linear least squares fitting. The best fit fundamental kinetic response characteristics (Δ[y]ss, TD and τ) were isolated using the iterative method described previously (Rossiter et al. 2001; Whipp & Rossiter, 2005), by moving the exponential fitting window to minimize the 95% confidence interval (CI) for τ (C95), maximize the flatness of the residual, and minimize the reduced χ2 of the fit (in that order). The presence and magnitude of any  ‘slow component’ (
‘slow component’ ( sc) in the kinetic responses was identified by comparison of the fitted exponential steady-state and the measured end-exercise value of each variable averaged from the final 30 s of the S1 and S2 stimulation periods.
sc) in the kinetic responses was identified by comparison of the fitted exponential steady-state and the measured end-exercise value of each variable averaged from the final 30 s of the S1 and S2 stimulation periods.
Statistical analysis
All data are presented as the mean ± s.d., unless otherwise stated. Data were analysed using one-way repeated-measures ANOVA or, where appropriate, a non-paired t test. Two dogs were omitted from SPON because of low perfusion and excessive fatigue. Therefore, comparisons between S1SPON and S2SPON were based on n = 7, as were comparisons among the SPON and PUMP conditions. Comparisons between S1PUMP and S2PUMP were based on n = 9. The correlation between 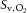 and Δ[HHbMb] was analysed using a related-samples Wilcoxon signed rank test. When a significant difference was found in ANOVA, least significant differences (LSD) post hoc testing was used to determine the location of significant differences. The level of significance was set at P ≤ 0.05 and all statistical analyses were performed in IBM spss Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA).
and Δ[HHbMb] was analysed using a related-samples Wilcoxon signed rank test. When a significant difference was found in ANOVA, least significant differences (LSD) post hoc testing was used to determine the location of significant differences. The level of significance was set at P ≤ 0.05 and all statistical analyses were performed in IBM spss Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
With the exception of one muscle, the average weight (n = 8) of the gastrocnemius complex was 72 ± 15 g and the average percentage of water was 77.6 ± 1.4%; these data are very similar to previous findings with this model. The one exception was slightly oedematous because PUMP pressure was >160 mmHg; wet weight for this muscle was corrected to the average water percentage of the other eight muscles.
Mechanical output
Spontaneous flow
Tmax in S1SPON tended to be lower at the last compared to the first contraction (96 ± 6% of initial force; P = 0.086). Tmax fell to 79 ± 8% during S2SPON (P < 0.001 versus S1SPON). As expected, the TTI approximately doubled between S1SPON (56 ± 25 N) and S2SPON (117 ± 50 N; P < 0.001) (Fig. 1C). The TTI at the end of stimulations was 105 ± 10% of the initial value in S1SPON, whereas TTI fell during S2SPON to 83 ± 8% of the initial value (P = 0.001).
Pump perfusion
The absolute force and TTI were significantly greater in SPON compared to PUMP (P = 0.002), probably as the result of a time effect as the spontaneous flow condition necessarily always preceded the pump-perfusion condition. Tmax was unchanged throughout S1PUMP (100 ± 4%; P = 0.772), but fell during S2PUMP (84 ± 5%; P < 0.001). Similar to SPON, the TTI was approximately doubled between S1PUMP (48 ± 22 N) and S2PUMP (94 ± 42 N; P < 0.001) (Fig. 1D). The TTI at the end of stimulations was 104 ± 4% of the initial value in S1PUMP and 91 ± 6% in S2PUMP (P = 0.001). TTI tended to be better maintained in S2PUMP than S2SPON (P = 0.065).
Muscle blood flow ( ) and oxygen uptake (
) and oxygen uptake ( ) kinetics
) kinetics
Spontaneous flow
Mean responses of  and
and  in SPON are shown in Fig. 1E and G, respectively. In SPON
in SPON are shown in Fig. 1E and G, respectively. In SPON  kinetics were more rapid in S1SPON (TD = 0 ± 1 s, τ = 12 ± 4 s) than S2SPON (TD = 4 ± 3 s, τ = 31 ± 13 s; P < 0.001) (Table 1). Similarly, τ
kinetics were more rapid in S1SPON (TD = 0 ± 1 s, τ = 12 ± 4 s) than S2SPON (TD = 4 ± 3 s, τ = 31 ± 13 s; P < 0.001) (Table 1). Similarly, τ was less in S1SPON (11 ± 3 s) than S2SPON (17 ± 7 s; P < 0.003), but the TD for
was less in S1SPON (11 ± 3 s) than S2SPON (17 ± 7 s; P < 0.003), but the TD for  was slightly greater in S1SPON (9 ± 2 s versus 6 ± 3 s; P = 0.016). Adjustment of bulk blood flow (τ
was slightly greater in S1SPON (9 ± 2 s versus 6 ± 3 s; P = 0.016). Adjustment of bulk blood flow (τ ) was similar to τ
) was similar to τ in S1SPON, but significantly slower in S2SPON (P = 0.023). In addition, the association (r2) between τ
in S1SPON, but significantly slower in S2SPON (P = 0.023). In addition, the association (r2) between τ and τ
and τ was stronger for S1SPON (0.78) than for S2SPON (0.39). Between-condition differences in τ were greater than the 95% CI for all τ parameter estimates.
was stronger for S1SPON (0.78) than for S2SPON (0.39). Between-condition differences in τ were greater than the 95% CI for all τ parameter estimates.
Table 1.
Muscle oxygen uptake ( ) and blood flow (
) and blood flow ( ) kinetic parameters for step transitions in two different stimulation protocols (S1 and S2) under spontaneous flow and pump-perfusion
) kinetic parameters for step transitions in two different stimulation protocols (S1 and S2) under spontaneous flow and pump-perfusion
| SPON |
PUMP |
||||
|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | ||
 bl bl
|
ml·kg−1·min−1 | 3.0 ± 1.0 | 107 ± 12† | 4.6 ± 5.0 | 84 ± 21†* |
Δ ss ss
|
ml·kg−1·min−1 | 98 ± 11 | 50 ± 21† | 74 ± 17* | 50 ± 22† |
 sc sc
|
ml·kg−1·min−1 | 7 ± 5 | 0 ± 0† | 4 ± 3* | 2 ± 2* |
 sc/TTI sc/TTI |
ml·kg−1 min−1 N−1 | 2 ± 5 | 5 ± 8 | 1 ± 4 | 2 ± 6 |
TD  /TTI /TTI |
s | 7 ± 3 | 4 ± 2† | 6 ± 2 | 3 ± 4† |
τ /TTI /TTI |
s | 13 ± 3 | 29 ± 19† | 12 ± 4 | 24 ± 6† |
MRT  /TTI /TTI |
s | 20 ± 3 | 32 ± 20‡ | 18 ± 6 | 27 ± 7† |
 bl bl
|
ml kg−1 min−1 | 120 ± 38 | 623 ± 79† | 1042 ± 198* | 1042 ± 198* |
Δ ss ss
|
ml kg−1 min−1 | 488 ± 76 | 370 ± 166† | — | — |
TD 
|
s | 0 ± 1 | 4 ± 3† | — | — |
τ
|
s | 12 ± 4 | 31 ± 13† | — | — |
MRT 
|
s | 12 ± 4 | 35 ± 13† | — | — |
| Δ[HHbMb]ss | a.u. | 21 ± 2 | 2 ± 1† | 17 ± 2 | 15 ± 2* |
| Δ[HHbMb] nadir time | s | 6 ± 2 | 0 ± 1 | 4 ± 1 | 3 ± 3 |
| τΔ[HHbMb] | s | 8 ± 2 | § | 11 ± 4 | 20 ± 5† |
| MRT Δ[HHbMb] | s | 16 ± 4 | § | 14 ± 4 | 23 ± 7† |
Values are mean ± s.d. for SPON (n = 7) and PUMP (n = 9). Abbreviations: bl, baseline; MRT, mean response time (equal to TD+τ); ss, steady-state asymptote; sc, slow-component; TD, time delay; τ, time constant; TTI, tension time integral; SPON, spontaneous perfusion; PUMP, pump perfusion. Δ[HHbMb] is the deoxygenation signal for haemoglobin (Hb) and myoglobin (Mb) measured by NIRS. Significant difference (P < 0.05) compared to SPON (*) and S1 (†).
P = 0.065 compared to S1SPON.
Note the very small amplitude in Δ[HHbMb] during S2SPON precluded confident kinetic fits in all cases (Fig. 2A).
The Δ ss (P < 0.001) and Δ
ss (P < 0.001) and Δ ss (P = 0.038) were each significantly greater in S1SPON than in S2SPON (Table 1). These differences were partly explained by a significant decrease in TTI that was apparent in S2SPON but not in S1SPON (Fig. 1C). Δ
ss (P = 0.038) were each significantly greater in S1SPON than in S2SPON (Table 1). These differences were partly explained by a significant decrease in TTI that was apparent in S2SPON but not in S1SPON (Fig. 1C). Δ ss/TTI was 0.88 ± 0.42 ml min−1 kg−1 N−1 in S1SPON and 1.51 ± 0.69 ml min−1 kg−1 N−1 in S2SPON (P = 0.004), which reflected a 1.7-fold change on doubling the contractile frequency. Accounting for these differences, however, did not alter the kinetic slowing between S1SPON and S2SPON: τ
ss/TTI was 0.88 ± 0.42 ml min−1 kg−1 N−1 in S1SPON and 1.51 ± 0.69 ml min−1 kg−1 N−1 in S2SPON (P = 0.004), which reflected a 1.7-fold change on doubling the contractile frequency. Accounting for these differences, however, did not alter the kinetic slowing between S1SPON and S2SPON: τ /TTI was 13 ± 3 s and 29 ± 19 s in S1SPON and S2SPON, respectively (P = 0.036) (Table 1). The tendency for a small
/TTI was 13 ± 3 s and 29 ± 19 s in S1SPON and S2SPON, respectively (P = 0.036) (Table 1). The tendency for a small  sc (7 ± 5 ml kg−1 min−1 and 0 ± 0 ml kg−1 min−1 in S1SPON and S2SPON, respectively) was removed by TTI normalisation and did not differ from zero (P > 0.14) (Table 1).
sc (7 ± 5 ml kg−1 min−1 and 0 ± 0 ml kg−1 min−1 in S1SPON and S2SPON, respectively) was removed by TTI normalisation and did not differ from zero (P > 0.14) (Table 1).
Pump perfusion
Mean responses of  and
and  in PUMP are shown in Fig. 1F and H, respectively. In the PUMP condition
in PUMP are shown in Fig. 1F and H, respectively. In the PUMP condition  was maintained at 1.04 ± 0.20 l kg−1 min−1 throughout stimulations (Fig. 1F), which was above the greatest value in SPON (0.99 ± 0.23 l kg−1 min−1). Despite this, τ
was maintained at 1.04 ± 0.20 l kg−1 min−1 throughout stimulations (Fig. 1F), which was above the greatest value in SPON (0.99 ± 0.23 l kg−1 min−1). Despite this, τ in S1PUMP (12 ± 4 s) was significantly less than in S2PUMP (17 ± 5 s; P = 0.003) (Fig. 1H). The TD for
in S1PUMP (12 ± 4 s) was significantly less than in S2PUMP (17 ± 5 s; P = 0.003) (Fig. 1H). The TD for  was longer in S1PUMP than S2PUMP (7 ± 2 s versus 4 ± 2 s; P = 0.016). Of interest, despite a significant TD in both SPON and PUMP,
was longer in S1PUMP than S2PUMP (7 ± 2 s versus 4 ± 2 s; P = 0.016). Of interest, despite a significant TD in both SPON and PUMP,  was not constant but increased gradually in a manner inconsistent with a ‘pure’ delayed mono-exponential
was not constant but increased gradually in a manner inconsistent with a ‘pure’ delayed mono-exponential  response. The differences in Δ
response. The differences in Δ ss between S1PUMP and S2PUMP (P = 0.001) (Table 1) were largely explained by a decrease in TTI during S2PUMP (Fig. 1D). As in SPON, the kinetic differences between conditions remained when the Δ
ss between S1PUMP and S2PUMP (P = 0.001) (Table 1) were largely explained by a decrease in TTI during S2PUMP (Fig. 1D). As in SPON, the kinetic differences between conditions remained when the Δ ss response was normalised for changes in TTI: τ
ss response was normalised for changes in TTI: τ /TTI was 12 ± 4 s and 24 ± 6 s in S1PUMP and S2PUMP, respectively (P < 0.001) (Table 1). There was no evidence of a
/TTI was 12 ± 4 s and 24 ± 6 s in S1PUMP and S2PUMP, respectively (P < 0.001) (Table 1). There was no evidence of a  sc during the PUMP condition, either in the absolute response (4 ± 3 ml kg−1 min−1 and 2 ± 2 ml kg−1 min−1 for S1SPON and S2SPON, respectively) or when normalised to TTI (P > 0.33 compared to zero) (Table 1).
sc during the PUMP condition, either in the absolute response (4 ± 3 ml kg−1 min−1 and 2 ± 2 ml kg−1 min−1 for S1SPON and S2SPON, respectively) or when normalised to TTI (P > 0.33 compared to zero) (Table 1).
Blood gases and muscle oxygenation kinetics
Arterial blood gas and CO-Oximetry variables did not differ between the resting baseline of SPON and PUMP (Table 2). At the end of S1SPON, 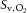 and
and 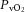 were 21 ± 4% and 16 ± 2 mmHg respectively, and were similar at the end of S2SPON (Fig. 2E, Table 2). In PUMP,
were 21 ± 4% and 16 ± 2 mmHg respectively, and were similar at the end of S2SPON (Fig. 2E, Table 2). In PUMP, 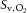 and
and 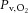 were greater throughout the rest and stimulation protocol in comparison with SPON (P < 0.001) (Table 2, Fig. 2E and F). In addition, and in contrast to SPON,
were greater throughout the rest and stimulation protocol in comparison with SPON (P < 0.001) (Table 2, Fig. 2E and F). In addition, and in contrast to SPON, 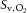 and
and 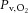 were lower in S2PUMP than in S1PUMP. Similarly,
were lower in S2PUMP than in S1PUMP. Similarly, 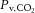 was less and venous pH greater throughout the PUMP condition compared to SPON (Table 2). Venous [lactate−] increased slightly between rest and S2SPON (from 1.4 ± 0.4 mM to 2.1 ± 0.2 mM; P < 0.01), whereas venous [lactate−] did not differ between rest and S2PUMP (1.6 ± 0.5 mM and 2.0 ± 0.4 mM, respectively; P > 0.05).
was less and venous pH greater throughout the PUMP condition compared to SPON (Table 2). Venous [lactate−] increased slightly between rest and S2SPON (from 1.4 ± 0.4 mM to 2.1 ± 0.2 mM; P < 0.01), whereas venous [lactate−] did not differ between rest and S2PUMP (1.6 ± 0.5 mM and 2.0 ± 0.4 mM, respectively; P > 0.05).
Table 2.
Blood gas, pH and blood metabolites at rest and steady-state in two different stimulation protocols (S1 and S2) under spontaneous flow and pump-perfusion
| SPON |
PUMP |
|||||
|---|---|---|---|---|---|---|
| Rest | S1 | S2 | Rest | S1 | S2 | |
| Arterial | ||||||
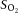 (%) (%) |
97 ± 1 | — | — | 97 ± 1 | — | — |
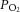 (mmHg) (mmHg) |
107 ± 10 | — | — | 108 ± 12 | — | — |
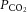 (mmHg) (mmHg) |
31 ± 1 | — | — | 31 ± 2 | — | — |
| pH | 7.41 ± 0.02 | — | — | 7.37±0.03* | ||
| [Lactate−] (mM) | 1.2 ± 0.4 | — | — | 1.7 ±0.5 | — | — |
| Venous | ||||||
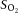 (%) (%) |
89 ± 3 | 21 ± 4† | 23 ± 8† | 96 ± 1* | 65 ± 5*† | 44 ± 7*†‡ |
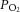 (mmHg) (mmHg) |
60 ± 5 | 16 ± 2† | 18 ± 4† | 88 ± 6* | 37 ± 5*† | 27 ± 5*†‡ |
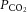 (mmHg) (mmHg) |
33 ± 1 | 49 ± 3† | 58 ± 3†‡ | 30 ± 1* | 38 ± 2*† | 48 ± 4*†‡ |
| pH | 7.40 ± 0.02 | 7.34 ± 0.01† | 7.26 ± 0.02†‡ | 7.38 ± 0.02* | 7.34 ± 0.02† | 7.28 ± 0.02*†‡ |
| [Lactate−] (mM) | 1.4 ± 0.4 | 1.6 ± 0.2 | 2.1 ± 0.2†‡ | 1.6 ± 0.5 | 1.6 ± 0.4 | 2.0 ± 0.4 |
Values are mean ± s.d. Abbreviations: 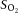 , oxygen saturation; P, partial pressure; SPON, spontaneous perfusion; PUMP, pump-perfusion. Significant difference (P < 0.05) compared with SPON (*), rest (†) or S1 (‡).
, oxygen saturation; P, partial pressure; SPON, spontaneous perfusion; PUMP, pump-perfusion. Significant difference (P < 0.05) compared with SPON (*), rest (†) or S1 (‡).
Figure 2. Microvascular deoxygenation and whole-muscle venous oxygen saturation dynamics during a two-step contractile protocol.
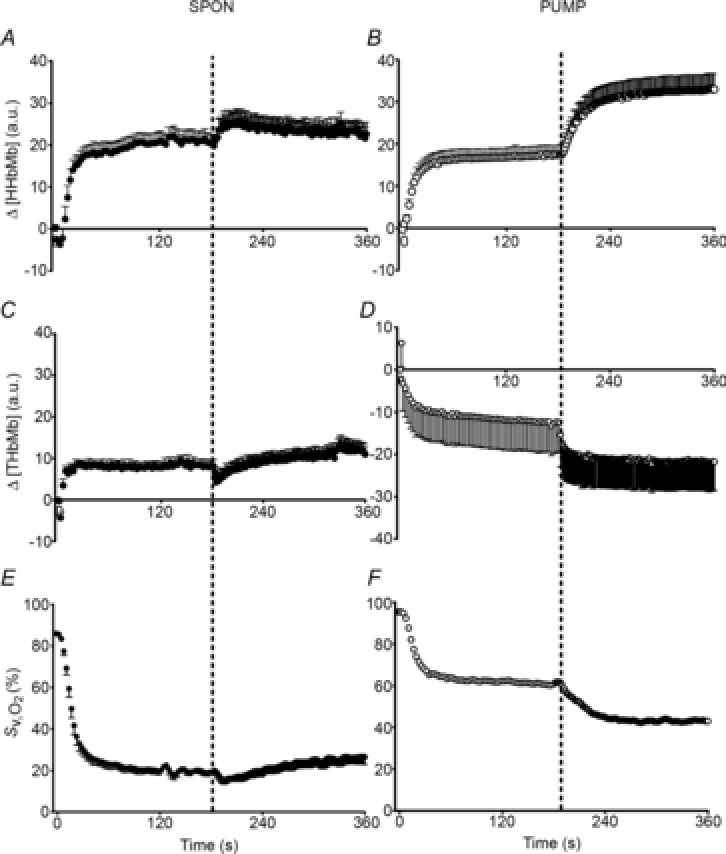
Spontaneous perfusion (SPON) is displayed in the left columns and pump-perfusion (PUMP) in the right columns. Muscle microvascular relative concentrations of deoxygenated Hb plus deoxygenated Mb (Δ[HHbMb]) by near-infrared spectroscopy (NIRS) are presented in A and B. Total Hb and Mb (Δ[THbMb]) by NIRS are presented in C and D. Venous O2 saturation (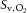 ) from inline oximetry during contractions is displayed in E and F. Note the rapid and large increase in microvascular deoxygenation and fall in
) from inline oximetry during contractions is displayed in E and F. Note the rapid and large increase in microvascular deoxygenation and fall in 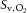 in SPON that are attenuated in PUMP. Values are mean ± s.e.m.
in SPON that are attenuated in PUMP. Values are mean ± s.e.m.
The kinetic profiles of Δ[HHbMb] and Δ[THbMb] are displayed in Fig. 2A–D, and kinetic parameter estimates are presented in Table 1. The deoxygenation amplitude (Δ[HHbMb]ss) was significantly greater in S1SPON than in S2SPON (P < 0.001) (Table 1). In contrast, Δ[HHbMb]ss did not differ between S1PUMP and S2PUMP (P = 0.797). The kinetics of tissue deoxygenation were well represented by an exponential during S1SPON (with a τ of 8 ± 2 s), but not during S2SPON. Here, the very small amplitude of the Δ[HHbMb] and non-exponential response profile precluded confident kinetic characterisation using eqn (1) (Fig. 2A). There was a good association between Δ[HHbMb] and 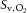 in SPON (r2 = 0.69 ± 0.12; P < 0.001 between conditions), particularly after the initial 20 s of contractions (Fig. 3A). Muscle deoxygenation responded exponentially in both S1PUMP and S2PUMP, and τ Δ[HHbMb] was significantly less in S1PUMP (11 ± 4 s) than in S2PUMP (20 ± 5 s; P < 0.001) (Table 1, Fig. 2B). As a result there was a very good inverse linear relationship between Δ[HHbMb] and
in SPON (r2 = 0.69 ± 0.12; P < 0.001 between conditions), particularly after the initial 20 s of contractions (Fig. 3A). Muscle deoxygenation responded exponentially in both S1PUMP and S2PUMP, and τ Δ[HHbMb] was significantly less in S1PUMP (11 ± 4 s) than in S2PUMP (20 ± 5 s; P < 0.001) (Table 1, Fig. 2B). As a result there was a very good inverse linear relationship between Δ[HHbMb] and 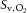 in PUMP (r2 = 0.93 ± 0.06) (Fig. 3B) throughout the entire transition. In PUMP, the kinetics of local microvascular deoxygenation (τ Δ[HHbMb]; 11 ± 4 s and 20 ± 5 s, in S1PUMP and S2PUMP, respectively) did not differ from τ
in PUMP (r2 = 0.93 ± 0.06) (Fig. 3B) throughout the entire transition. In PUMP, the kinetics of local microvascular deoxygenation (τ Δ[HHbMb]; 11 ± 4 s and 20 ± 5 s, in S1PUMP and S2PUMP, respectively) did not differ from τ measured across the whole muscle (12 ± 4 s and 17 ± 5 s; P = 0.32).
measured across the whole muscle (12 ± 4 s and 17 ± 5 s; P = 0.32).
Figure 3. Kinetic association between microvascular deoxygenation and whole-muscle venous oxygen saturation during a two-step contractile protocol.
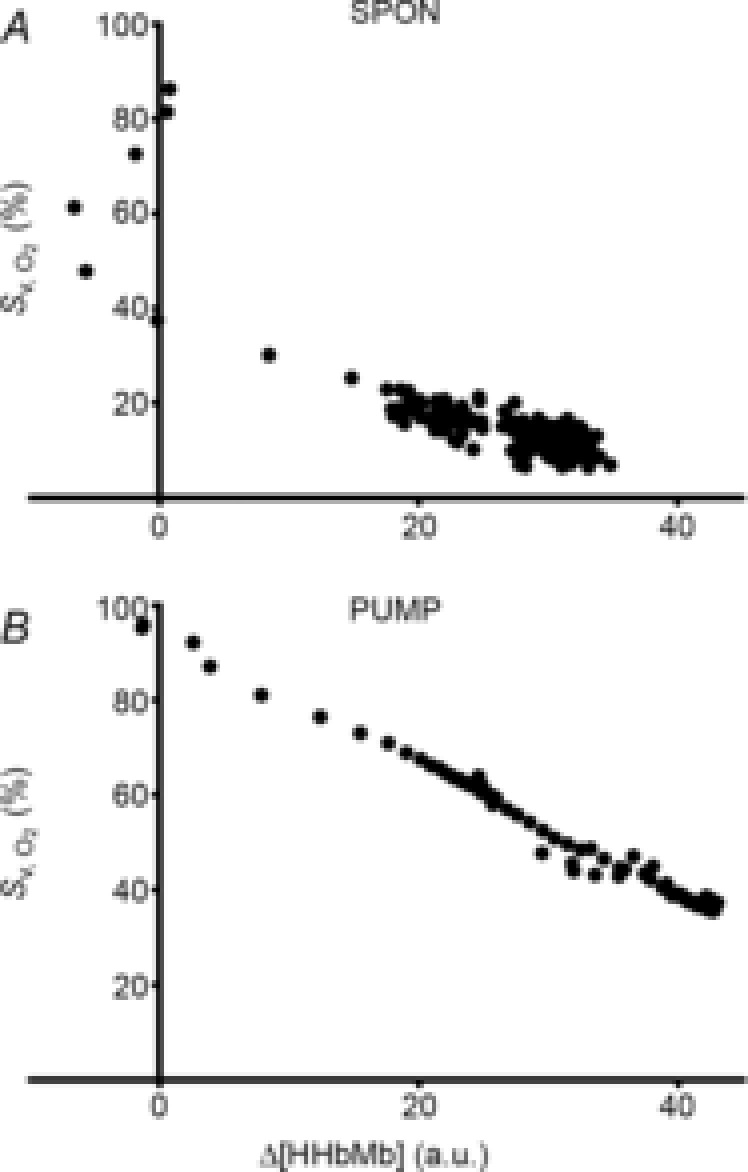
Representative examples from an individual animal of the relationship between Δ[HHbMb] and 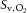 in SPON (A) and PUMP (B). Group mean r2 values were significantly lower in SPON (0.69 ± 0.12) than in PUMP (0.93 ± 0.06; P < 0.001).
in SPON (A) and PUMP (B). Group mean r2 values were significantly lower in SPON (0.69 ± 0.12) than in PUMP (0.93 ± 0.06; P < 0.001).
Discussion
The phenomenon of a slowed  kinetic response when exercise is initiated from a raised metabolic rate has been extensively investigated during moderate (Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Bowen et al. 2011; Williams et al. 2013) and high-intensity (Wilkerson & Jones, 2006; DiMenna et al. 2008, 2009, 2010a, b2010b, c2010c; Jones et al. 2008b; Breese et al. 2012) exercise in humans. These investigations implicated mechanisms mediated through the orderly recruitment of muscle fibres (beginning with highly oxidative and progressing to less oxidative) and/or slowed muscle
kinetic response when exercise is initiated from a raised metabolic rate has been extensively investigated during moderate (Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Bowen et al. 2011; Williams et al. 2013) and high-intensity (Wilkerson & Jones, 2006; DiMenna et al. 2008, 2009, 2010a, b2010b, c2010c; Jones et al. 2008b; Breese et al. 2012) exercise in humans. These investigations implicated mechanisms mediated through the orderly recruitment of muscle fibres (beginning with highly oxidative and progressing to less oxidative) and/or slowed muscle  kinetics limiting O2 delivery during the transition from a raised metabolic rate. To investigate these hypotheses, the present study used an isolated canine stimulated muscle preparation in situ, which for the first time provided independent experimental control over muscle fibre activation and muscle
kinetics limiting O2 delivery during the transition from a raised metabolic rate. To investigate these hypotheses, the present study used an isolated canine stimulated muscle preparation in situ, which for the first time provided independent experimental control over muscle fibre activation and muscle  on contractions from rest (S1) and a raised metabolic rate (S2). To our surprise, we found that
on contractions from rest (S1) and a raised metabolic rate (S2). To our surprise, we found that  kinetics remained slowed in S2 (τ
kinetics remained slowed in S2 (τ in S2 was double that in S1) even when all muscle fibres were activated simultaneously and
in S2 was double that in S1) even when all muscle fibres were activated simultaneously and  was maintained in excess of the spontaneous rate by pump perfusion. These findings indicate that: (i)
was maintained in excess of the spontaneous rate by pump perfusion. These findings indicate that: (i)  kinetics are slower when initiated from a raised metabolic rate in this stimulated canine muscle model (a model that employs essentially only highly oxidative muscle fibres), just as they are in intact humans, and (ii) neither orderly fibre recruitment nor slow
kinetics are slower when initiated from a raised metabolic rate in this stimulated canine muscle model (a model that employs essentially only highly oxidative muscle fibres), just as they are in intact humans, and (ii) neither orderly fibre recruitment nor slow  kinetics are prerequisites for the slowed
kinetics are prerequisites for the slowed  kinetics initiated from a raised metabolic rate. Therefore, although these results do not rule out recruitment- or blood flow-related mechanisms in slowing S2
kinetics initiated from a raised metabolic rate. Therefore, although these results do not rule out recruitment- or blood flow-related mechanisms in slowing S2  kinetics in humans, they suggest that these mechanisms are not exclusive mediators.
kinetics in humans, they suggest that these mechanisms are not exclusive mediators.
Traditional theories of the control of oxidative metabolism suggest that, in the absence of a mitochondrial O2 or NADH delivery limitation,  kinetics conform to a first-order rate reaction via ADP-feedback, in which τ
kinetics conform to a first-order rate reaction via ADP-feedback, in which τ is dependent on mitochondrial volume or activity in the recruited muscles (e.g. Chance & Williams, 1955). The finding in 1970 that
is dependent on mitochondrial volume or activity in the recruited muscles (e.g. Chance & Williams, 1955). The finding in 1970 that  kinetics were not constant within the moderate intensity domain in which it was presumed that uniform highly oxidative muscle fibres were recruited (di Prampero et al. 1970) was seemingly counter to this fundamental observation and led to the development of alternative hypotheses that O2 delivery and/or the recruited mitochondrial volume varied between S1 and S2 (Hughson & Morrissey 1982; Brittain et al. 2001). The combination of these hypotheses therefore satisfied the requirement for a first-order system and the experimental observation of slowed S2
kinetics were not constant within the moderate intensity domain in which it was presumed that uniform highly oxidative muscle fibres were recruited (di Prampero et al. 1970) was seemingly counter to this fundamental observation and led to the development of alternative hypotheses that O2 delivery and/or the recruited mitochondrial volume varied between S1 and S2 (Hughson & Morrissey 1982; Brittain et al. 2001). The combination of these hypotheses therefore satisfied the requirement for a first-order system and the experimental observation of slowed S2  kinetics. The present findings, however, directly challenge this understanding of the kinetic control of muscle
kinetics. The present findings, however, directly challenge this understanding of the kinetic control of muscle  in vivo that has prevailed for more than 50 years and demand consideration of alternative mediators for the
in vivo that has prevailed for more than 50 years and demand consideration of alternative mediators for the  kinetic responses to contractions in skeletal muscle.
kinetic responses to contractions in skeletal muscle.
Influence of muscle recruitment
By using an isolated muscle preparation in situ with stimulation via the sciatic nerve, we were able to uniformly activate the entire muscle to either a low metabolic rate (S1; one contraction every 3 s) or a higher, but submaximal, metabolic rate (S2; two contractions per 3 s). This allowed us to overcome the complexity inherent in human studies in which the oxidative capacity of motor units recruited in S1 may be greater than those recruited in S2. During muscle stimulation with spontaneous perfusion, we reasoned that if slowed  kinetics were observed at the higher stimulation frequency, they could not be attributable to the additional recruitment of motor units differing in oxidative capacity. Nevertheless, τ
kinetics were observed at the higher stimulation frequency, they could not be attributable to the additional recruitment of motor units differing in oxidative capacity. Nevertheless, τ /TTI (and τ
/TTI (and τ ) remained markedly faster in S1 (13 ± 3 s) than in S2 (29 ± 19 s). This ∼2-fold difference is consistent with observations during human moderate-intensity exercise, in which an ∼2-fold increase in τ
) remained markedly faster in S1 (13 ± 3 s) than in S2 (29 ± 19 s). This ∼2-fold difference is consistent with observations during human moderate-intensity exercise, in which an ∼2-fold increase in τ is generally observed between S1 and S2 conditions (e.g. Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Bowen et al. 2011; Williams et al. 2013).
is generally observed between S1 and S2 conditions (e.g. Hughson & Morrissey, 1982; Brittain et al. 2001; MacPhee et al. 2005; Bowen et al. 2011; Williams et al. 2013).
Since the original observations of a slowed  kinetics response on transition from a raised metabolic rate (Hughson & Morrissey, 1982; Brittain et al. 2001), muscle recruitment has been suggested to underlie the phenomenon. The increased motor demand in S2 was presumed to activate fibres with lower oxidative capacity and, therefore, inherently slower
kinetics response on transition from a raised metabolic rate (Hughson & Morrissey, 1982; Brittain et al. 2001), muscle recruitment has been suggested to underlie the phenomenon. The increased motor demand in S2 was presumed to activate fibres with lower oxidative capacity and, therefore, inherently slower  kinetics (Kushmerick et al. 1992; Wüst et al. 2013). Consistent with this, muscle EMG activity increases to a greater extent in S2 than S1 during cycling exercise in humans, particularly when transitioning from steady-state exercise in the moderate domain into the very heavy-intensity domain, which is likely to involve the recruitment of less oxidative type II fibres (Breese et al. 2012). In addition, manipulation of fibre activation patterns using extreme pedal rates (DiMenna et al. 2009) showed that a higher cadence exacerbated the slowed
kinetics (Kushmerick et al. 1992; Wüst et al. 2013). Consistent with this, muscle EMG activity increases to a greater extent in S2 than S1 during cycling exercise in humans, particularly when transitioning from steady-state exercise in the moderate domain into the very heavy-intensity domain, which is likely to involve the recruitment of less oxidative type II fibres (Breese et al. 2012). In addition, manipulation of fibre activation patterns using extreme pedal rates (DiMenna et al. 2009) showed that a higher cadence exacerbated the slowed  kinetics phenomenon: S2
kinetics phenomenon: S2  kinetics were slowed (versus S1) to a greater extent at extreme high cadence (versus low cadence), which was presumed to reflect a greater reliance on low-oxidative type II fibres. Additionally, Williams et al. (2013) found that
kinetics were slowed (versus S1) to a greater extent at extreme high cadence (versus low cadence), which was presumed to reflect a greater reliance on low-oxidative type II fibres. Additionally, Williams et al. (2013) found that  kinetics during S2 may be speeded by high-intensity exercise training, which is known to promote mitochondrial biogenesis (amongst other effects) in recruited muscle fibres. Each of these findings is consistent with a role for recruitment patterns in the slowed S2
kinetics during S2 may be speeded by high-intensity exercise training, which is known to promote mitochondrial biogenesis (amongst other effects) in recruited muscle fibres. Each of these findings is consistent with a role for recruitment patterns in the slowed S2  kinetics phenomenon.
kinetics phenomenon.
Canine muscle is comprised almost entirely of highly oxidative muscle fibres (Maxwell et al. 1977), nevertheless the hierarchical recruitment of fibres varying in oxidative capacity may play an important role in determining  kinetics during voluntary activity in the vastus lateralis in humans (Hodson-Tole & Wakeling, 2009). However, the present data do not support the recruitment of less oxidative muscle fibres as a requirement for the slowing in
kinetics during voluntary activity in the vastus lateralis in humans (Hodson-Tole & Wakeling, 2009). However, the present data do not support the recruitment of less oxidative muscle fibres as a requirement for the slowing in  kinetics during exercise initiated from a raised metabolic rate. Congruently, the premise of intrinsically slower on-transient
kinetics during exercise initiated from a raised metabolic rate. Congruently, the premise of intrinsically slower on-transient  kinetics in poorly oxidative muscle fibres has recently been challenged (Wüst et al. 2013). In isolated single frog muscle fibres, τ
kinetics in poorly oxidative muscle fibres has recently been challenged (Wüst et al. 2013). In isolated single frog muscle fibres, τ at contractions onset is poorly related to oxidative capacity, implicating an allosteric (Wüst et al. 2011; Schmitz et al. 2012) or parallel activation process (Korzeniewski, 2007), perhaps mediated via mitochondrial [Ca2+] (Glancy & Balaban, 2012), that dissociates the kinetic relationship between cellular maximal
at contractions onset is poorly related to oxidative capacity, implicating an allosteric (Wüst et al. 2011; Schmitz et al. 2012) or parallel activation process (Korzeniewski, 2007), perhaps mediated via mitochondrial [Ca2+] (Glancy & Balaban, 2012), that dissociates the kinetic relationship between cellular maximal  and τ
and τ predicted in a first-order rate reaction. It is possible then, that slowed
predicted in a first-order rate reaction. It is possible then, that slowed  kinetics in S2 are related to a blunting of this activation process at higher metabolic rates compared with lower rates independently of fibre recruitment.
kinetics in S2 are related to a blunting of this activation process at higher metabolic rates compared with lower rates independently of fibre recruitment.
Influence of muscle  kinetics
kinetics
The other mechanism proposed to mediate slowed  kinetics is slowed muscle
kinetics is slowed muscle  kinetics in S2 (MacPhee et al. 2005; Hernandez et al. 2010; Breese et al. 2012; Goodwin et al. 2012). Indeed, in a human study using knee-extension exercise, MacPhee et al. (2005) demonstrated that femoral artery τ
kinetics in S2 (MacPhee et al. 2005; Hernandez et al. 2010; Breese et al. 2012; Goodwin et al. 2012). Indeed, in a human study using knee-extension exercise, MacPhee et al. (2005) demonstrated that femoral artery τ was slowed from 21 s to 39 s between S1 and S2. We, therefore, addressed this hypothesis through pump perfusion (PUMP) in the stimulated canine hindlimb. Despite the experimental increase in
was slowed from 21 s to 39 s between S1 and S2. We, therefore, addressed this hypothesis through pump perfusion (PUMP) in the stimulated canine hindlimb. Despite the experimental increase in  to exceed the rate measured in S2SPON, once again we found τ
to exceed the rate measured in S2SPON, once again we found τ /TTI (and τ
/TTI (and τ ) remained 2-fold less in S1 than S2.
) remained 2-fold less in S1 than S2.
In the PUMP condition, 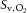 was greater throughout the entire S1–S2 protocol in comparison with SPON (Fig. 1E and F) and venous [lactate−] was lower (Table 2). At the end of S2PUMP,
was greater throughout the entire S1–S2 protocol in comparison with SPON (Fig. 1E and F) and venous [lactate−] was lower (Table 2). At the end of S2PUMP, 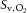 and
and 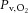 were 44 ± 7% and 27 ± 5 mmHg, respectively, whereas
were 44 ± 7% and 27 ± 5 mmHg, respectively, whereas 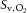 and
and 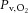 at the end of the S1SPON transition were 21 ± 4% and 16 ± 2 mmHg, respectively. Clearly, therefore, the PUMP intervention was successful in increasing O2 delivery, thereby increasing the potential for the O2 extraction required to support muscle O2 utilisation. Whether this was effective in relieving a potential limitation of capillary-to-mitochondrial O2 diffusion is unknown. However, we made simple model calculations of the critical
at the end of the S1SPON transition were 21 ± 4% and 16 ± 2 mmHg, respectively. Clearly, therefore, the PUMP intervention was successful in increasing O2 delivery, thereby increasing the potential for the O2 extraction required to support muscle O2 utilisation. Whether this was effective in relieving a potential limitation of capillary-to-mitochondrial O2 diffusion is unknown. However, we made simple model calculations of the critical 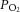 required to avoid an anoxic myocellular core ([Mb] = 0.311 mm) (Schuder et al. 1979), (P50 for Mb = 3.8 mmHg; fibre cross-sectional area = 3650 μm2) (Maxwell et al. 1977); for more details, see Wüst et al. (2009). This calculation suggests that the estimated critical
required to avoid an anoxic myocellular core ([Mb] = 0.311 mm) (Schuder et al. 1979), (P50 for Mb = 3.8 mmHg; fibre cross-sectional area = 3650 μm2) (Maxwell et al. 1977); for more details, see Wüst et al. (2009). This calculation suggests that the estimated critical 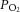 is lower in S1 than in S2; thus, as [MbO2] decreases and
is lower in S1 than in S2; thus, as [MbO2] decreases and  increases between S1 and S2, a greater extracellular
increases between S1 and S2, a greater extracellular 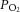 is required to maintain diffusive O2 flux. Therefore, as
is required to maintain diffusive O2 flux. Therefore, as  increases in the transition to S2SPON, O2 diffusion may become limiting despite an unchanged capillary
increases in the transition to S2SPON, O2 diffusion may become limiting despite an unchanged capillary 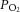 (inferred from the relatively flat
(inferred from the relatively flat 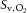 and Δ[HHbMb] responses). However, the venous
and Δ[HHbMb] responses). However, the venous 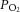 measured immediately prior to S2PUMP (65 ± 5 mmHg) greatly exceeds the critical
measured immediately prior to S2PUMP (65 ± 5 mmHg) greatly exceeds the critical 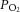 estimated by this model (<12 mmHg), reducing the likelihood of a regional capillary
estimated by this model (<12 mmHg), reducing the likelihood of a regional capillary 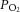 limitation to O2 flux. Therefore, we propose that the finding that τ
limitation to O2 flux. Therefore, we propose that the finding that τ did not differ between S2SPON and S2PUMP despite greatly increased oxygenation, argues that O2 delivery is not a requisite cause of the increased τ
did not differ between S2SPON and S2PUMP despite greatly increased oxygenation, argues that O2 delivery is not a requisite cause of the increased τ in S2 in the present in situ model.
in S2 in the present in situ model.
Although moderate-intensity  kinetics appear to be sensitive to slowed convective O2 delivery in this canine model (Goodwin et al. 2012), increasing the rate of O2 provision through raised muscle
kinetics appear to be sensitive to slowed convective O2 delivery in this canine model (Goodwin et al. 2012), increasing the rate of O2 provision through raised muscle  above the spontaneous condition does not appear to speed the
above the spontaneous condition does not appear to speed the  response (Grassi et al. 1998), at least at the contraction frequencies used in the present study. In humans, studies that have attempted to increase muscle
response (Grassi et al. 1998), at least at the contraction frequencies used in the present study. In humans, studies that have attempted to increase muscle  prior to the S2 transition [e.g. by priming or supine exercise (DiMenna et al. 2008, 2010c; Jones et al. 2008a)] found that the slowed S2
prior to the S2 transition [e.g. by priming or supine exercise (DiMenna et al. 2008, 2010c; Jones et al. 2008a)] found that the slowed S2  kinetics remained. Indeed, other evidence suggests that bulk blood flow dynamics are not limiting to
kinetics remained. Indeed, other evidence suggests that bulk blood flow dynamics are not limiting to  kinetics during submaximal exercise in healthy normoxic humans (Poole & Jones, 2012) or in canine muscle (Grassi et al. 1998; Grassi, 2000; Goodwin et al. 2012). Taken together, these data do not support a major role for O2 delivery in the slowed
kinetics during submaximal exercise in healthy normoxic humans (Poole & Jones, 2012) or in canine muscle (Grassi et al. 1998; Grassi, 2000; Goodwin et al. 2012). Taken together, these data do not support a major role for O2 delivery in the slowed  kinetics observed when exercise is initiated from a raised metabolic rate.
kinetics observed when exercise is initiated from a raised metabolic rate.
Another way in which the kinetics of the circulation may contribute to slowing S2  kinetics is via circulatory modulation between muscle and lung (Benson et al. 2013). An increase in the baseline
kinetics is via circulatory modulation between muscle and lung (Benson et al. 2013). An increase in the baseline  prior to S2 alters the vascular transit and mixing characteristics of the venous blood draining the muscles and other organs. However, it appears that this effect is unlikely to account for more than ∼2 s of the observed slowing of pulmonary
prior to S2 alters the vascular transit and mixing characteristics of the venous blood draining the muscles and other organs. However, it appears that this effect is unlikely to account for more than ∼2 s of the observed slowing of pulmonary  dynamics in S2 in humans (Bowen et al. 2011). The present data essentially remove much of the uncertainty surrounding this suggestion, by using direct Fick measurement of
dynamics in S2 in humans (Bowen et al. 2011). The present data essentially remove much of the uncertainty surrounding this suggestion, by using direct Fick measurement of  kinetics proximal to the muscle (rather than at the lung) and maintaining a constant high rate of
kinetics proximal to the muscle (rather than at the lung) and maintaining a constant high rate of  . The greater intramuscular phosphocreatine (PCr) breakdown observed in S2 in humans is also consistent with the suggestion that slowed
. The greater intramuscular phosphocreatine (PCr) breakdown observed in S2 in humans is also consistent with the suggestion that slowed  kinetics reflect an ‘intramuscular’ effect rather than a ‘transit’-related distortion in gas exchange kinetics (Jones et al. 2008b).
kinetics reflect an ‘intramuscular’ effect rather than a ‘transit’-related distortion in gas exchange kinetics (Jones et al. 2008b).
Therefore, although the present results do not rule out muscle fibre recruitment profiles or  dynamics as active mechanisms that contribute to slowing S2
dynamics as active mechanisms that contribute to slowing S2  kinetics during voluntary exercise in humans, they do suggest that these putative mediators are not exclusive mechanisms causing the slowed
kinetics during voluntary exercise in humans, they do suggest that these putative mediators are not exclusive mechanisms causing the slowed  kinetics observed during transitions from a raised metabolic rate.
kinetics observed during transitions from a raised metabolic rate.
What slows  kinetics when exercise is initiated from a raised metabolic rate?
kinetics when exercise is initiated from a raised metabolic rate?
The experimental evidence from the present study points towards additional intracellular factors in mediating these slowed  kinetics.
kinetics.
The kinetic control of  is typically proposed to be a simple linear feedback loop mediated via ADP, which is, in turn, largely dependent upon [PCr] (Meyer, 1988). The steady-state relationship between [PCr] and
is typically proposed to be a simple linear feedback loop mediated via ADP, which is, in turn, largely dependent upon [PCr] (Meyer, 1988). The steady-state relationship between [PCr] and  is thought to be essentially linear over the aerobic range, implying that
is thought to be essentially linear over the aerobic range, implying that  kinetics should be identical in both the S1 and S2 steps of the current protocol. Using the same dog hindlimb model, we recently observed sigmoidal [ADP]–
kinetics should be identical in both the S1 and S2 steps of the current protocol. Using the same dog hindlimb model, we recently observed sigmoidal [ADP]– kinetic response curves, consistent with the notion of a mitochondrial activation process at the onset of contractions (Wüst et al. 2011). These data suggest that a simple ADP feedback mechanism is not sufficient to explain
kinetic response curves, consistent with the notion of a mitochondrial activation process at the onset of contractions (Wüst et al. 2011). These data suggest that a simple ADP feedback mechanism is not sufficient to explain  kinetics in skeletal muscle, and an allosteric or parallel activation process might contribute to shaping the response (Jeneson et al. 1996, 2009; Gurd et al. 2006; Korzeniewski, 2007; Wüst et al. 2011, 2013; Glancy & Balaban, 2012; Schmitz et al. 2012). Allosteric activation would seem to contradict the observed slowing in
kinetics in skeletal muscle, and an allosteric or parallel activation process might contribute to shaping the response (Jeneson et al. 1996, 2009; Gurd et al. 2006; Korzeniewski, 2007; Wüst et al. 2011, 2013; Glancy & Balaban, 2012; Schmitz et al. 2012). Allosteric activation would seem to contradict the observed slowing in  kinetics in S2. That is, ‘mitochondrial priming’ by prior contractions predicts a slower
kinetics in S2. That is, ‘mitochondrial priming’ by prior contractions predicts a slower  adjustment during S1 when [ADP] sensitivity is low (but increasing), and a faster
adjustment during S1 when [ADP] sensitivity is low (but increasing), and a faster  adjustment during S2 when [ADP] sensitivity is high from the outset.
adjustment during S2 when [ADP] sensitivity is high from the outset.
The hyperbolic or sigmoidal relationship between [ADP] and  (Jeneson et al. 1996; Glancy et al. 2008; Wüst et al. 2011; Schmitz et al. 2012) means that
(Jeneson et al. 1996; Glancy et al. 2008; Wüst et al. 2011; Schmitz et al. 2012) means that  kinetics would slow as [ADP] approaches ‘saturating’ concentrations: thus,
kinetics would slow as [ADP] approaches ‘saturating’ concentrations: thus,  increments that are low in the metabolic range reside on the steeper, linear portion of the [ADP]–
increments that are low in the metabolic range reside on the steeper, linear portion of the [ADP]– response curve, whereas the same increment higher in the metabolic range may encroach on the flatter portion of the curve and be kinetically slower. However, in such an event,
response curve, whereas the same increment higher in the metabolic range may encroach on the flatter portion of the curve and be kinetically slower. However, in such an event,  would approach
would approach 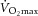 . The rate of
. The rate of  during S2 in the present study averaged ∼150 ml kg−1 min−1, which is well below the maximal values for this model (Kelley et al. 1996; Ameredes et al. 1998). In addition, observations in human studies show slowed
during S2 in the present study averaged ∼150 ml kg−1 min−1, which is well below the maximal values for this model (Kelley et al. 1996; Ameredes et al. 1998). In addition, observations in human studies show slowed  kinetics at <50%
kinetics at <50% 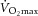 , suggesting that encroaching peak myocellular
, suggesting that encroaching peak myocellular  is unlikely to be responsible for the phenomenon.
is unlikely to be responsible for the phenomenon.
Another suggestion is that the raised pre-transition metabolic rate itself exerts an influence on subsequent  kinetics. Bowen et al. (2011) proposed that a reduction in the cellular energetic state in the active fibres in S1 [i.e. reduced [PCr] and increased [ADP] and [Pi], resulting in a less negative ΔGATP (Jones et al. 2008a)] would slow ‘mitochondrial power’ delivery to ATP consuming processes [where ‘mitochondrial ‘power’ is the product of ATP production rate and ΔGATP, linked to
kinetics. Bowen et al. (2011) proposed that a reduction in the cellular energetic state in the active fibres in S1 [i.e. reduced [PCr] and increased [ADP] and [Pi], resulting in a less negative ΔGATP (Jones et al. 2008a)] would slow ‘mitochondrial power’ delivery to ATP consuming processes [where ‘mitochondrial ‘power’ is the product of ATP production rate and ΔGATP, linked to  by P/O ratio (Glancy et al. 2008)]. A raised
by P/O ratio (Glancy et al. 2008)]. A raised  prior to S2 (and therefore less negative ΔGATP) would necessitate a greater ATP production rate by muscle mitochondria to sustain a constant mitochondrial power delivery. However, this suggestion is controversial because human data show conflicting results: a reduced intracellular energetic state per se does (Bowen et al. 2011) or does not (DiMenna et al. 2010a) coincide with slowed
prior to S2 (and therefore less negative ΔGATP) would necessitate a greater ATP production rate by muscle mitochondria to sustain a constant mitochondrial power delivery. However, this suggestion is controversial because human data show conflicting results: a reduced intracellular energetic state per se does (Bowen et al. 2011) or does not (DiMenna et al. 2010a) coincide with slowed  kinetics in S2. Unfortunately, we were unable to address this proposal directly in the present study using serial muscle ΔGATP measurements by biopsy, because it was more important to maintain the integrity of the preparation over the entire SPON and PUMP protocol, and also as a result of the placement of NIRS optodes over the muscle. Nevertheless, although a mechanism related to less negative ΔGATP fits the limited evidence available, it seems unlikely. This is because the probability of cross-bridge detachment on ATP splitting would need to be greater in S1 than S2 (Meyer & Foley, 1996), which seems improbable because the energy release by ATP hydrolysis (∼−50 kJ mol−1 in physiologic conditions) is thought to exceed that required to detach the cross bridge, even when ΔGATP is less favourable as in S2. Therefore, other factors must be responsible.
kinetics in S2. Unfortunately, we were unable to address this proposal directly in the present study using serial muscle ΔGATP measurements by biopsy, because it was more important to maintain the integrity of the preparation over the entire SPON and PUMP protocol, and also as a result of the placement of NIRS optodes over the muscle. Nevertheless, although a mechanism related to less negative ΔGATP fits the limited evidence available, it seems unlikely. This is because the probability of cross-bridge detachment on ATP splitting would need to be greater in S1 than S2 (Meyer & Foley, 1996), which seems improbable because the energy release by ATP hydrolysis (∼−50 kJ mol−1 in physiologic conditions) is thought to exceed that required to detach the cross bridge, even when ΔGATP is less favourable as in S2. Therefore, other factors must be responsible.
The combination of alterations in mitochondrial enzyme activity and ADP feedback (Hogan et al. 1992) might explain the slowed S2  kinetic response if allosteric activation were slower than ADP accumulation, or absent, in S2. In this way [ADP] could encroach upon a flat portion of the [ADP]–
kinetic response if allosteric activation were slower than ADP accumulation, or absent, in S2. In this way [ADP] could encroach upon a flat portion of the [ADP]– response curve prior to maximal mitochondrial activation and S2
response curve prior to maximal mitochondrial activation and S2  kinetics would be slowed. Evidence to support this suggestion is sparse. Maximal mitochondrial activation occurs very rapidly (<10 s) following the onset of tetanic contractions at
kinetics would be slowed. Evidence to support this suggestion is sparse. Maximal mitochondrial activation occurs very rapidly (<10 s) following the onset of tetanic contractions at 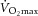 (Wüst et al. 2011; Chess et al. 2013), but little is known about mitochondrial activation kinetics at submaximal rates of
(Wüst et al. 2011; Chess et al. 2013), but little is known about mitochondrial activation kinetics at submaximal rates of  . In addition, this mechanism suggests that slow S2
. In addition, this mechanism suggests that slow S2  kinetics would be sensitive to high-intensity ‘priming’ exercise, which appears not to be the case in humans (DiMenna et al. 2008, 2010b). Nevertheless, the kinetics of mitochondrial activation in comparison to [ADP] feedback have not been investigated in conditions similar to the S1–S2 protocol used in the present study, and hence we are unable to confirm or rule out this mechanism as a mediator of the current findings. Considerable investigation will be required to understand the contribution of these control factors to skeletal muscle mitochondrial respiration during various experimental conditions in vivo. Nevertheless, it seems that mediators other than muscle recruitment and O2 delivery are necessary to explain the observed slowed
kinetics would be sensitive to high-intensity ‘priming’ exercise, which appears not to be the case in humans (DiMenna et al. 2008, 2010b). Nevertheless, the kinetics of mitochondrial activation in comparison to [ADP] feedback have not been investigated in conditions similar to the S1–S2 protocol used in the present study, and hence we are unable to confirm or rule out this mechanism as a mediator of the current findings. Considerable investigation will be required to understand the contribution of these control factors to skeletal muscle mitochondrial respiration during various experimental conditions in vivo. Nevertheless, it seems that mediators other than muscle recruitment and O2 delivery are necessary to explain the observed slowed  kinetics when exercise is initiated from a raised metabolic rate.
kinetics when exercise is initiated from a raised metabolic rate.
Comparison of whole-muscle extraction and microvascular oxygenation dynamics
Continuous-wave NIRS provides access to the relative oxygenation (Δ[HbMbO2]) and deoxygenation (Δ[HHbMb]) dynamics of the microvasculature within contracting skeletal muscles during stimulations or exercise. Its non-invasive nature makes it an attractive technique for human studies and Δ[HHbMb] is commonly used both in vivo and in situ to make inferences of microvascular O2 delivery-to-utilisation ‘matching’ or O2 extraction (e.g. DeLorey et al. 2003; Grassi et al. 2003; Koga et al. 2007; Breese et al. 2012; Goodwin et al. 2012). However, the interpretation of Δ[HHbMb] is complex because it is sensitive both to changes in  /
/ and the total volume of heme chromophores within the optical field (amongst other variables). Indeed, despite changes in total heme chromophore, Δ[HHbMb] provided a good index of
and the total volume of heme chromophores within the optical field (amongst other variables). Indeed, despite changes in total heme chromophore, Δ[HHbMb] provided a good index of 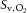 dynamics in SPON (r2 = 0.69 ± 0.12) (Fig. 3A) particularly after the initial ∼ 20 s of the transition when Δ[THbMb] changes were most rapid. This highlights the likelihood that the kinetics of Δ[THbMb] change are most relevant in maintaining a strong association between NIRS-estimated and actual O2 extraction: when the kinetics of Δ[THbMb] and Δ[HHbMb] are similar, Δ[HHbMb] may provide a very good index of microvascular O2 extraction.
dynamics in SPON (r2 = 0.69 ± 0.12) (Fig. 3A) particularly after the initial ∼ 20 s of the transition when Δ[THbMb] changes were most rapid. This highlights the likelihood that the kinetics of Δ[THbMb] change are most relevant in maintaining a strong association between NIRS-estimated and actual O2 extraction: when the kinetics of Δ[THbMb] and Δ[HHbMb] are similar, Δ[HHbMb] may provide a very good index of microvascular O2 extraction.
In the PUMP condition in the present study, we used adenosine infusion to vasodilate the muscle prior to contractions, and pump perfusion to maintain  at a constant high rate during the S1–S2 transition. Under these conditions the relationship between microvascular Δ[HHbMb] and whole-muscle O2 extraction (as reflected by
at a constant high rate during the S1–S2 transition. Under these conditions the relationship between microvascular Δ[HHbMb] and whole-muscle O2 extraction (as reflected by 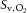 ) were remarkably similar across the entire S1–S2 contraction protocol (Fig. 2B and F, Fig. 3B). τΔ[HHbMb] (11 ± 4 s and 20 ± 5 s, in S1PUMP and S2PUMP, respectively) did not differ from τ
) were remarkably similar across the entire S1–S2 contraction protocol (Fig. 2B and F, Fig. 3B). τΔ[HHbMb] (11 ± 4 s and 20 ± 5 s, in S1PUMP and S2PUMP, respectively) did not differ from τ (12 ± 4 s and 17 ± 5 s in S1PUMP and S2PUMP, respectively), despite a dynamic reduction in Δ[THbMb] (Fig. 2D). Note here that the fall in Δ[THbMb] during PUMP is kinetically similar to that in Δ[HHbMb] (Figs 2 and 3), despite (or perhaps because of) being imposed on a pre-exercise vasodilation and raised
(12 ± 4 s and 17 ± 5 s in S1PUMP and S2PUMP, respectively), despite a dynamic reduction in Δ[THbMb] (Fig. 2D). Note here that the fall in Δ[THbMb] during PUMP is kinetically similar to that in Δ[HHbMb] (Figs 2 and 3), despite (or perhaps because of) being imposed on a pre-exercise vasodilation and raised  induced by adenosine infusion and pump perfusion, unlike in SPON, in which a kinetically dissociated increase in Δ[THbMb] is more typical. Nevertheless, the similarity between τΔ[HHbMb] and τ
induced by adenosine infusion and pump perfusion, unlike in SPON, in which a kinetically dissociated increase in Δ[THbMb] is more typical. Nevertheless, the similarity between τΔ[HHbMb] and τ during constant
during constant  suggests, in this canine model, in which myocyte mitochondrial density, [Mb] and capillarity are relatively uniform, and the NIRS optodes are placed directly on the muscle surface, that whole-muscle
suggests, in this canine model, in which myocyte mitochondrial density, [Mb] and capillarity are relatively uniform, and the NIRS optodes are placed directly on the muscle surface, that whole-muscle  kinetics provide a close proxy of cellular
kinetics provide a close proxy of cellular  kinetics.
kinetics.
Conclusion
The present study addressed the mechanism of slowed  kinetics when exercise is initiated from a raised metabolic rate. Using electrical stimulation of the sciatic nerve and pump-controlled perfusion, we were able to investigate the role of muscle fibre activation and muscle O2 delivery in mediating this phenomenon. The results are consistent with pulmonary
kinetics when exercise is initiated from a raised metabolic rate. Using electrical stimulation of the sciatic nerve and pump-controlled perfusion, we were able to investigate the role of muscle fibre activation and muscle O2 delivery in mediating this phenomenon. The results are consistent with pulmonary  measurements in that slowed
measurements in that slowed  kinetics during contractions initiated from a raised metabolic rate were observed when measured across the contracting muscle. To our surprise, however, we found that slowed
kinetics during contractions initiated from a raised metabolic rate were observed when measured across the contracting muscle. To our surprise, however, we found that slowed  kinetics were not alleviated when all muscle fibres were simultaneously recruited or when muscle O2 delivery was experimentally increased to a rate exceeding spontaneous perfusion conditions. Thus these data do not support the notion that the hierarchical recruitment of fibres of progressively lower oxidative capacity or an O2 delivery limitation represent the requisite mechanisms responsible for slowing of
kinetics were not alleviated when all muscle fibres were simultaneously recruited or when muscle O2 delivery was experimentally increased to a rate exceeding spontaneous perfusion conditions. Thus these data do not support the notion that the hierarchical recruitment of fibres of progressively lower oxidative capacity or an O2 delivery limitation represent the requisite mechanisms responsible for slowing of  kinetics during exercise initiated from a raised metabolic rate. The intracellular mechanism mediating this phenomenon may relate to a falling energy state, approaching saturating ADP concentration, and/or some other mechanism of slowed mitochondrial activation. Further study will be required to elucidate the responsible mechanisms. Certainly, the current data add to the growing body of evidence that control of muscle
kinetics during exercise initiated from a raised metabolic rate. The intracellular mechanism mediating this phenomenon may relate to a falling energy state, approaching saturating ADP concentration, and/or some other mechanism of slowed mitochondrial activation. Further study will be required to elucidate the responsible mechanisms. Certainly, the current data add to the growing body of evidence that control of muscle  during contractions may be more complex than previously thought.
during contractions may be more complex than previously thought.
Key points
A slow adjustment of skeletal muscle oxygen uptake (
 ) to produce energy during exercise predisposes to early fatigue.
) to produce energy during exercise predisposes to early fatigue.In human studies,
 kinetics are slow when exercise is initiated from an elevated baseline; this is proposed to reflect slow blood flow regulation and/or recruitment of muscle fibres containing few mitochondria.
kinetics are slow when exercise is initiated from an elevated baseline; this is proposed to reflect slow blood flow regulation and/or recruitment of muscle fibres containing few mitochondria.To investigate this, we measured
 kinetics in canine muscle, with experimental control over muscle activation and blood flow.
kinetics in canine muscle, with experimental control over muscle activation and blood flow.We found that
 kinetics remained slow when contractions were initiated from an elevated baseline despite experimentally increased blood flow and uniform fibre activation.
kinetics remained slow when contractions were initiated from an elevated baseline despite experimentally increased blood flow and uniform fibre activation.These data challenge our current understanding of the control of muscle
 and demand consideration of new alternative mediators for
and demand consideration of new alternative mediators for  control.
control.
Acknowledgments
None
Glossary
- GS
gastrocnemius plus superficial digital flexor
- Hb
haemoglobin
- Mb
myoglobin
- P
partial pressure
- PCr
phosphocreatine
- PUMP
pump-controlled blood flow

blood flow
- S1
stimulation at 0.33 Hz
- S2
stimulation at 0.67 Hz
- SPON
spontaneous blood flow
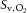
venous oxygen saturation
- TD
time delay
- Tmax
maximal tension
- TTI
tension-time integral

oxygen uptake
- τ
time constant
- Δ[HbMbO2]
oxygenated Hb plus Mb
- Δ[HHbMb]
deoxygenated Hb plus Mb
- Δ[THbMb]
total sum Hb plus Mb
Additional information
Competing interests
None declared.
Author contributions
All experiments were performed at Auburn University (Auburn, AL, USA). R.C.I.W., J.R.M., J.M.K., L.B.G. and H.B.R. contributed to the conception and design of the experiments. All authors contributed to the collection, analysis and interpretation of data. R.C.I.W. and H.B.R. wrote the first draft of the article. All authors contributed to the critical revision of the paper and approved the final manuscript for submission.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC; BB/F019521/1) and the National Sciences and Engineering Research Council of Canada (NSERC).
References
- Ameredes BT, Brechue WF, Stainsby WN. Mechanical and metabolic determination of VO2 and fatigue during repetitive isometric contractions in situ. J Appl Physiol (1985) 1998;84:1909–1916. doi: 10.1152/jappl.1998.84.6.1909. [DOI] [PubMed] [Google Scholar]
- Benson AP, Grassi B, Rossiter HB. A validated model of oxygen uptake and circulatory dynamic interactions at exercise onset in humans. J Appl Physiol (1985) 2013;115:743–755. doi: 10.1152/japplphysiol.00184.2013. [DOI] [PubMed] [Google Scholar]
- Bowen TS, Murgatroyd SR, Cannon DT, Cuff TJ, Lainey AF, Marjerrison AD, Spencer MD, Benson AP, Paterson DH, Kowalchuk JM, Rossiter HB. A raised metabolic rate slows pulmonary O2 uptake kinetics on transition to moderate-intensity exercise in humans independently of work rate. Exp Physiol. 2011;96:1049–1061. doi: 10.1113/expphysiol.2011.058321. [DOI] [PubMed] [Google Scholar]
- Breese BC, Barker AR, Armstrong N, Jones AM, Williams CA. The effect of baseline metabolic rate on pulmonary O2 uptake kinetics during very heavy intensity exercise in boys and men. Respir Physiol Neurobiol. 2012;180:223–229. doi: 10.1016/j.resp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Brittain CJ, Rossiter HB, Kowalchuk JM, Whipp BJ. Effect of prior metabolic rate on the kinetics of oxygen uptake during moderate-intensity exercise. Eur J Appl Physiol. 2001;86:125–134. doi: 10.1007/s004210100514. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Chess DJ, Billings E, Covian R, Glancy B, French S, Taylor J, de Bari H, Murphy E, Balaban RS. Optical spectroscopy in turbid media using an integrating sphere: mitochondrial chromophore analysis during metabolic transitions. Anal Biochem. 2013;439:161–172. doi: 10.1016/j.ab.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol (1985) 2003;95:113–120. doi: 10.1152/japplphysiol.00956.2002. [DOI] [PubMed] [Google Scholar]
- di Prampero PE, Davies CT, Cerretelli P, Margaria R. An analysis of O2 debt contracted in submaximal exercise. J Appl Physiol. 1970;29:547–551. doi: 10.1152/jappl.1970.29.5.547. [DOI] [PubMed] [Google Scholar]
- DiMenna FJ, Bailey SJ, Vanhatalo A, Chidnok W, Jones AM. Elevated baseline VO2per se does not slow O2 uptake kinetics during work-to-work exercise transitions. J Appl Physiol. 2010a;109:1148–1154. doi: 10.1152/japplphysiol.00550.2010. [DOI] [PubMed] [Google Scholar]
- DiMenna FJ, Fulford J, Bailey SJ, Vanhatalo A, Wilkerson DP, Jones AM. Influence of priming exercise on muscle [PCr] and pulmonary O2 uptake dynamics during ‘work-to-work’ knee-extension exercise. Respir Physiol Neurobiol. 2010b;172:15–23. doi: 10.1016/j.resp.2010.04.017. [DOI] [PubMed] [Google Scholar]
- DiMenna FJ, Wilkerson DP, Burnley M, Bailey SJ, Jones AM. Influence of extreme pedal rates on pulmonary O2 uptake kinetics during transitions to high-intensity exercise from an elevated baseline. Respir Physiol Neurobiol. 2009;169:16–23. doi: 10.1016/j.resp.2009.08.001. [DOI] [PubMed] [Google Scholar]
- DiMenna FJ, Wilkerson DP, Burnley M, Bailey SJ, Jones AM. Priming exercise speeds pulmonary O2 uptake kinetics during supine ‘work-to-work’ high-intensity cycle exercise. J Appl Physiol. 2010c;108:283–292. doi: 10.1152/japplphysiol.01047.2009. [DOI] [PubMed] [Google Scholar]
- DiMenna FJ, Wilkerson DP, Burnley M, Jones AM. Influence of priming exercise on pulmonary O2 uptake kinetics during transitions to high-intensity exercise from an elevated baseline. J Appl Physiol. 2008;105:538–546. doi: 10.1152/japplphysiol.90357.2008. [DOI] [PubMed] [Google Scholar]
- Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy B, Barstow T, Willis WT. Linear relation between time constant of oxygen uptake kinetics, total creatine, and mitochondrial content in vitro. Am J Physiol Cell Physiol. 2008;294:C79–C87. doi: 10.1152/ajpcell.00138.2007. [DOI] [PubMed] [Google Scholar]
- Goodwin ML, Hernandez A, Lai N, Cabrera ME, Gladden LB. VO2 on-kinetics in isolated canine muscle in situ during slowed convective O2 delivery. J Appl Physiol. 2012;112:9–19. doi: 10.1152/japplphysiol.01480.2010. [DOI] [PubMed] [Google Scholar]
- Grassi B. Skeletal muscle VO2 on-kinetics: set by O2 delivery or by O2 utilization? New insights into an old issue. Med Sci Sports Exerc. 2000;32:108–116. doi: 10.1097/00005768-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol (1985) 2003;95:149–158. doi: 10.1152/japplphysiol.00695.2002. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH, Kowalchuk JM. Prior heavy exercise elevates pyruvate dehydrogenase activity and speeds O2 uptake kinetics during subsequent moderate-intensity exercise in healthy young adults. J Physiol. 2006;577:985–996. doi: 10.1113/jphysiol.2006.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Goodwin ML, Lai N, Cabrera ME, McDonald JR, Gladden LB. Contraction-by-contraction VO2 and computer-controlled pump perfusion as novel techniques to study skeletal muscle metabolism in situ. J Appl Physiol. 2010;108:705–712. doi: 10.1152/japplphysiol.00963.2009. [DOI] [PubMed] [Google Scholar]
- Hodson-Tole EF, Wakeling JM. Motor unit recruitment for dynamic tasks: current understanding and future directions. J Comp Physiol. 2009;179:57–66. doi: 10.1007/s00360-008-0289-1. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD. Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol. 1992;73:728–736. doi: 10.1152/jappl.1992.73.2.728. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Morrissey M. Delayed kinetics of respiratory gas exchange in the transition from prior exercise. J Appl Physiol. 1982;52:921–929. doi: 10.1152/jappl.1982.52.4.921. [DOI] [PubMed] [Google Scholar]
- Jeneson JA, Schmitz JP, van den Broek NM, van Riel NA, Hilbers PA, Nicolay K, Prompers JJ. Magnitude and control of mitochondrial sensitivity to ADP. Am J Physiol Endocrinol Metab. 2009;297:E774–E784. doi: 10.1152/ajpendo.00370.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem. 1996;271:27995–27998. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- Jones AM, Fulford J, Wilkerson DP. Influence of prior exercise on muscle [phosphorylcreatine] and deoxygenation kinetics during high-intensity exercise in men. Exp Physiol. 2008a;93:468–478. doi: 10.1113/expphysiol.2007.041897. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Fulford J. Muscle [phosphocreatine] dynamics following the onset of exercise in humans: the influence of baseline work-rate. J Physiol. 2008b;586:889–898. doi: 10.1113/jphysiol.2007.142026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley K, Hamann J, Aschenbach W, Gladden L. Canine gastrocnemius muscle in situ: VO2max. Med Sci Sports Exerc. 1996;28:367. [Google Scholar]
- Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol (1985) 2007;103:2049–2056. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- Korzeniewski B. Regulation of oxidative phosphorylation through parallel activation. Biophys Chem. 2007;129:93–110. doi: 10.1016/j.bpc.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast- and slow-twitch muscle. Am J Physiol Cell Physiol. 1992;263:C598–C606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- MacPhee SL, Shoemaker JK, Paterson DH, Kowalchuk JM. Kinetics of O2 uptake, leg blood flow, and muscle deoxygenation are slowed in the upper compared with lower region of the moderate-intensity exercise domain. J Appl Physiol. 2005;99:1822–1834. doi: 10.1152/japplphysiol.01183.2004. [DOI] [PubMed] [Google Scholar]
- Maxwell LC, Barclay JK, Mohrman DE, Faulkner JA. Physiological characteristics of skeletal muscles of dogs and cats. Am J Physiol Cell Physiol. 1977;233:C14–C18. doi: 10.1152/ajpcell.1977.233.1.C14. [DOI] [PubMed] [Google Scholar]
- Meyer R, Foley J. Cellular processes integrating the metabolic response to exercise. Handbook of Physiology – Exercise: Regulation and Integration of Multiple Systems. 1996;12:841–869. [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer RA. Linear dependence of muscle phosphocreatine kinetics on total creatine content. Am J Physiol Cell Physiol. 1989;257:C1149–C1157. doi: 10.1152/ajpcell.1989.257.6.C1149. [DOI] [PubMed] [Google Scholar]
- Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol. 2012;2:933–996. doi: 10.1002/cphy.c100072. [DOI] [PubMed] [Google Scholar]
- Rossiter H. Exercise: Kinetic considerations for gas exchange. Compr Physiol. 2011;1:203–244. doi: 10.1002/cphy.c090010. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JP, Jeneson JA, van Oorschot JW, Prompers JJ, Nicolay K, Hilbers PA, van Riel NA. Prediction of muscle energy states at low metabolic rates requires feedback control of mitochondrial respiratory chain activity by inorganic phosphate. PLoS ONE. 2012;7:e34118. doi: 10.1371/journal.pone.0034118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuder S, Wittenberg JB, Haseltine B, Wittenberg BA. Spectrophotometric determination of myoglobin in cardiac and skeletal muscle: separation from hemoglobin by subunit-exchange chromatography. Anal Biochem. 1979;92:473–481. doi: 10.1016/0003-2697(79)90687-0. [DOI] [PubMed] [Google Scholar]
- Stainsby WN, Otis AB. Blood flow, blood oxygen tension, oxygen uptake, and oxygen transport in skeletal muscle. Am J Physiol. 1964;206:858–866. doi: 10.1152/ajplegacy.1964.206.4.858. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Rossiter HB. The kinetics of oxygen uptake. Physiological inferences from the parameters. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. Oxon: Routledge; 2005. pp. 62–94. [Google Scholar]
- Wilkerson DP, Jones AM. Influence of initial metabolic rate on pulmonary O2 uptake on-kinetics during severe intensity exercise. Respir Physiol Neurobiol. 2006;152:204–219. doi: 10.1016/j.resp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Williams AM, Paterson DH, Kowalchuk JM. High-intensity interval training speeds the adjustment of pulmonary O2 uptake, but not muscle deoxygenation, during moderate-intensity exercise transitions initiated from low and elevated baseline metabolic rates. J Appl Physiol (1985) 2013;114:1550–1562. doi: 10.1152/japplphysiol.00575.2012. [DOI] [PubMed] [Google Scholar]
- Wüst RCI, Grassi B, Hogan MC, Howlett RA, Gladden LB, Rossiter HB. Kinetic control of oxygen consumption during contractions in self-perfused skeletal muscle. J Physiol. 2011;589:3995–4009. doi: 10.1113/jphysiol.2010.203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst RCI, Jaspers RT, van Heijst AF, Hopman MT, Hoofd LJ, van der Laarse WJ, Degens H. Region-specific adaptations in determinants of rat skeletal muscle oxygenation to chronic hypoxia. Am J Physiol Heart Circ Physiol. 2009;297:H364–H374. doi: 10.1152/ajpheart.00272.2009. [DOI] [PubMed] [Google Scholar]
- Wüst RCI, van der Laarse WJ, Rossiter HB. On-off asymmetries in oxygen consumption kinetics of single Xenopus laevis skeletal muscle fibres suggest higher-order control. J Physiol. 2013;591:731–744. doi: 10.1113/jphysiol.2012.241992. [DOI] [PMC free article] [PubMed] [Google Scholar]


