Abstract
The aim was to investigate the metabolic and anti-inflammatory effects of resveratrol alone and when combined with exercise training in skeletal muscle of aged human subjects. Healthy, physically inactive men (60–72 years old) were randomized to either 8 weeks of daily intake of 250 mg resveratrol or placebo or to 8 weeks of high-intensity exercise training with 250 mg resveratrol or placebo. Before and after the interventions, resting blood samples and muscle biopsies were obtained and a one-legged knee-extensor endurance exercise test was performed. Exercise training increased skeletal muscle peroxisome proliferator-activated receptor-γ co-activator-1α mRNA ∼1.5-fold, cytochrome c protein ∼1.3-fold, cytochrome c oxidase I protein ∼1.5-fold, citrate synthase activity ∼1.3-fold, 3-hydroxyacyl-CoA dehydrogenase activity ∼1.3-fold, inhibitor of κB-α and inhibitor of κB-β protein content ∼1.3-fold and time to exhaustion in the one-legged knee-extensor endurance exercise test by ∼1.2-fold, with no significant additive or adverse effects of resveratrol on these parameters. Despite an overall ∼25% reduction in total acetylation level in skeletal muscle with resveratrol, no exclusive resveratrol-mediated metabolic effects were observed on the investigated parameters. Notably, however, resveratrol blunted an exercise training-induced decrease (∼20%) in protein carbonylation and decrease (∼40%) in tumour necrosis factor α mRNA content in skeletal muscle. In conclusion, resveratrol did not elicit metabolic improvements in healthy aged subjects; in fact, resveratrol even impaired the observed exercise training-induced improvements in markers of oxidative stress and inflammation in skeletal muscle. Collectively, this highlights the metabolic efficacy of exercise training in aged subjects and does not support the contention that resveratrol is a potential exercise mimetic in healthy aged subjects.
Introduction
Ageing is directly linked to lifestyle-related metabolic diseases (Masoro, 2001; Woods et al. 2012). Although many tissues and organs are affected by ageing, loss of muscle mass in concert with decreased oxidative and antioxidant capacity of skeletal muscle (Conley et al. 2000; Hollmann et al. 2007; Chabi et al. 2008) are hallmarks in the ageing process and may have a negative impact on whole-body metabolism. It is well established that exercise training elicits numerous health-beneficial effects (Pedersen & Saltin, 2006). Specifically, in aged subjects, exercise training has been reported to counteract loss of muscle mass and strength (Frontera et al. 1988; Hollmann et al. 2007) and to increase the oxidative capacity of skeletal muscle (Suominen et al. 1977; Iversen et al. 2011), thereby postponing age-related muscle deteriorations. In addition, exercise training is believed to exert anti-inflammatory effects (Handschin & Spiegelman, 2008; Gleeson et al. 2011; Woods et al. 2012), which may contribute to the beneficial effects of exercise. However, not all individuals have the ability or desire to perform regular physical activity.
The natural polyphenol resveratrol, present in dark grapes and nuts, has in rodents been reported to induce metabolic effects similar to those observed with exercise training (Baur et al. 2006; Lagouge et al. 2006). Specifically, resveratrol has been reported to extent lifespan of C. elegans and Drosophila (Howitz et al. 2003; Wood et al. 2004), to have anti-inflammatory effects (Pearson et al. 2008; Olholm et al. 2010) and to increase exercise endurance and skeletal muscle oxidative capacity in mice (Lagouge et al. 2006). However, only few resveratrol studies have been conducted in humans, and these reports have been inconsistent (Brasnyó et al. 2011; Timmers et al. 2011; Crandall et al. 2012; Poulsen et al. 2012; Yoshino et al. 2012) and modest compared with numerous rodent studies (Baur et al. 2006; Lagouge et al. 2006; Um et al. 2010; Dolinsky et al. 2012; Park et al. 2012). Moreover, only one study has, until now, examined the metabolic effects of resveratrol in healthy aged individuals (Yoshino et al. 2012), and no previous studies have examined the combined effects of resveratrol and exercise training. The previous studies primarily have focused on individuals with pre-existing metabolic disorders, such as type 2 diabetes, insulin resistance and obesity (Brasnyó et al. 2011; Timmers et al. 2011; Crandall et al. 2012; Poulsen et al. 2012).
Although the exact molecular mechanism is debated (Baur et al. 2006; Um et al. 2010; Park et al. 2012; Price et al. 2012), resveratrol is mainly believed to exert its metabolic effects via an AMP-activated protein kinase (AMPK) and/or sirtuin 1 (SIRT1)-mediated activation of the transcriptional co-activator peroxisome proliferator activated receptor-γ co-activator-1α (PGC-1α; Baur et al. 2006; Lagouge et al. 2006). In addition, based on its antioxidant properties (Stojanović et al. 2001; Olas & Wachowicz, 2005), resveratrol may not only mediate adaptations in various organs through an AMPK–SIRT1–PGC-1α axis, but may additionally scavenge excessive reactive oxygen species, which may be particularly relevant during exercise in aged individuals.
The aim of the present study was to investigate the effects of 8 weeks of daily resveratrol intake either alone or in combination with high-intensity exercise training in healthy aged men. The study tested the hypotheses that daily intake of resveratrol elicits metabolic adaptations and anti-inflammatory effects in skeletal muscle of healthy aged subjects similar to exercise training, and that resveratrol intake combined with exercise training potentiates such metabolic and anti-inflammatory effects.
Methods
Ethical statement and subjects
The study was approved by the ethics Committee of Copenhagen and Frederiksberg communities (H-2-2011-079) and was conducted in accordance with the guidelines of the Declaration of Helsinki. All subjects provided written informed consent before the initiation of the study.
The present study was part of a larger study, and data covering cardiovascular adaptations to exercise training with or without resveratrol supplementation have already been published (Gliemann et al. 2013).
Forty-three 60- to 72-year-old, physically inactive but otherwise healthy, male subjects participated (Supporting information Table S1 and Gliemann et al. 2013). All subjects were non-smokers and underwent a medical examination. None had been diagnosed with cardiovascular disease, hypertension, renal dysfunction, insulin resistance or type 2 diabetes, and all subjects had normal ECG. Two subjects (one in the training + resveratrol group and one in the training + placebo group) were diagnosed with hypercholesterolaemia regulated by their own physician (medication was maintained during the experimental period). The other participants had normal cholesterol levels.
Experimental set-up
Study design
The study was divided into two parts, which were both 8 week randomized, double-blinded, placebo-controlled trials. Subjects from the first part were assigned to either a combination of exercise training with placebo (n = 13) or exercise training with 250 mg resveratrol day−1 (Fluxome Inc., Stenlose, Denmark; n = 14). Subjects from the second part were assigned to either placebo (n = 7) or 250 mg resveratrol day−1 (n = 9). The allocation was based on body mass index, fasting blood glucose, cholesterol and maximal oxygen consumption (Table S1 and Gliemann et al. 2013). All participants were instructed to take one tablet each morning. For each tablet, subjects noted the time of consumption and any discomfort that might appear throughout the intervention period. Furthermore, the subjects were instructed to continue their habitual lifestyle throughout the intervention period.
Exercise training protocol
The exercise training intervention consisted of supervised high-intensity interval spinning training (cycle ergometer) twice per week and full-body circuit training once per week. In addition, the subjects were instructed to walk 5 km once per week. The intensity of the exercise was controlled by TEAM2 WearLink+ heart rate monitors (Polar, Kempele, Finland).
Endurance test
On the first experimental day, a dual-energy X-ray absorptiometry scan was performed in addition to an incremental time-to-exhaustion dynamic one-legged knee-extensor exercise test. After acclimating and a short warm-up, the test started at 6 W and gradually increased by 6 W every 5 min until exhaustion. The total energy output (in kilojoules) was calculated based on duration (in seconds) and workload (in watts).
Muscle biopsies and blood samples
On the second experimental day (∼48 h after the first experimental day), the subjects arrived after an overnight fast. Resting blood samples were taken from an arm vein, and a vastus lateralis muscle biopsy was obtained under local anaesthesia (lidocaine; AstraZeneca, Södertälje, Sweden) using the percutaneous needle biopsy technique (Bergstrom, 1975) with suction. Muscle biopsies were quick-frozen in liquid nitrogen and stored (−80°C) until analysis. After the intervention period, the two experimental days were repeated in the same order, with blood samples and muscle biopsies obtained ∼1 h after consumption of either the placebo or the resveratrol tablet.
Analyses
Plasma cytokines
Plasma cytokines were analysed using an ultrasensitive MSD multi-spot 96-well assay system precoated with antibodies (MesoScaleDiscovery, Gaithersburg, MD, USA) according to the manufacturer's protocol. The MSD plates were measured on a MSD Sector Imager 2400 plate reader. Raw data were measured as electrochemiluminescence signal (light) detected by photodetectors and analysed using the Discovery Workbench 3.0 software (MSD). A standard curve was generated for each analyte and used to determine the concentration of analytes in each sample.
Isolation of RNA, reverse transcription and real-time PCR
Total RNA was isolated from 15–20 mg muscle tissue by a modified guanidinium thiocyanate–phenol–chloroform extraction method (Chomczynski & Sacchi, 1987) as described previously (Pilegaard et al. 2000), except that the tissue was homogenized for 2 min at 30 revolutions s−1 in a TissueLyserII (Qiagen, Hilden, Germany).
The final pellets were resuspended in Diethylpyrocarbonate-treated H2O containing 0.1 mm EDTA. The RNA was quantified based on the absorbance at 260 nm (Nanodrop 1000; Thermo Scientific, Rockford, IL, USA). Purity of the RNA samples was evaluated from the 260 nm/280 nm ratio, and all samples were above 1.8.
The Superscript II RNase H− system and Oligo dT (Invitrogen, Carlsbad, CA, USA) were used to reverse transcribe the mRNA to cDNA as described previously (Pilegaard et al. 2000). The amount of single-stranded DNA (ssDNA) was determined in each cDNA sample by use of OliGreen reagent (Molecular Probes, Leiden, The Netherlands) as described previously (Lundby et al. 2005).
Real-time PCR was performed using an ABI 7900 sequence-detection system (Applied Biosystems, Foster City, CA, USA). Primers and TaqMan probes for amplifying gene-specific mRNA fragments were designed using the human-specific database from ensemble (http://www.ensembl.org/Homo_sapiens/Info/Index) and Primer Express (Applied Biosystems). All TaqMan probes were 5′-FAM and 3′-TAMRA labelled, and primers and Taqman probes were obtained from TAG Copenhagen (Copenhagen, Denmark; Table 1). Real-time PCR was performed in triplicate in a total reaction volume of 10 μl using Universal Mastermix (Applied Biosystems). Cycle threshold (Ct) was converted to a relative amount by use of a standard curve constructed from a serial dilution of a pooled RT sample analysed together with the samples. For each sample, target gene mRNA content was normalized to ssDNA content.
Table 1.
Primer and TaqMan probe sequences
| Forward primer | Reverse primer | Probe | |
|---|---|---|---|
| PGC-1α | 5′-CAAGCCAAACCAACAACTTTATCTCT-3′ | 5′-CACACTTAAGGTGCGTTCAATAGTC-3′ | 5′-AGTCACCAAATGACCCCAAGGGTTCC-3′ |
| TNFα | 5′-TCTGGCCCAGGCAGTCAGAT-3′ | 5′-AGCTGCCCCTCAGCTTGA-3′ | 5′-CAAGCCTGTAGCCCATGTTGTAGCAAACC-3′ |
| iNOS | 5′-AGCGGGATGACTTTCCAAGA-3′ | 5′-TAATGGACCCCAGGCAAGATT-3′ | 5′-CCTGCAAGTTAAAATCCCTTTGGCCTTATG-3′ |
Peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α), tumour necrosis factor α (TNFα) and inducible nitric oxide synthase (iNOS) primer and TaqMan probe sequences used for real-time PCR.
Lysate generation and protein determination
Freeze-dried muscle biopsies were dissected free of visible fat, blood and connective tissue under a stereo microscope in a temperature- (∼18°C) and humidity (<30%)-controlled room. Muscle lysate was produced from ∼5–10 mg dry weight as previously described (Birk & Wojtaszewski, 2006), except that the tissue was homogenized for 3 min at 30 revolutions s−1 in a TissueLyser (TissueLyser II; Qiagen). Homogenates were centrifuged for 20 min, at 16,000g, 4°C and lysates (supernatant) collected. The protein content in lysates was measured by the bicinchoninic acid method (Thermo Scientific).
SDS-PAGE and Western blotting
Protein content and phosphorylation levels were measured in muscle lysates by SDS-PAGE and Western blotting. Equal amounts of total protein were loaded for each sample. Band intensity was quantified using Carestream IS 4000 MM (Fisher Scientific, Thermo Fisher Scientific, Waltham, MA, USA) and Carestream Health Molecular Imaging software. Commercially available antibodies were used to detect AMPKThr172 (#2535), SIRT1 (#2493), acetylated lysine residues (#9441), tumour necrosis factor α (TNFα; #3707), inducible nitric oxide synthase (iNOS; #2977), inhibitor of κB-α (IκB-α; #9242), inhibitor of κB-β (IκB-β; #9248), transcription factor RelA (p65; #4764), p65ser536 (#3033), inhibitor of κB kinase (IKK; #2678), IKKser176,180 (#2697), c-Jun N-terminal kinases (JNK; #9252), JNKThr183,Tyr185 (#9251), p38 mitogen-activated protein kinases (p38; #9212), p38Thr180,Tyr182 (#4511) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; #2118), all from Cell Signaling (Danvers, MA, USA); cytochrome c (cyt c; #556433; BD Pharmigen, Franklin Lakes, NJ, USA), cytochrome c oxidase I (COXI; #459600; Invitrogen), acetyl-CoA carboxylase ACCser79 (#07-303, Millipore, Billirica, MA, USA) and AMPKα2 (a kind gift from Professor Grahame Hardie, University of Dundee, Dundee, UK). Protein content as well as phosphorylation levels were expressed as arbitrary units relative to control samples loaded on each site of each gel and normalized to GAPDH protein.
Enzyme activities
A portion of the freeze-dried muscle biopsies, free of connective tissue, blood and visible fat was homogenized (1:400) in 0.3 m phosphate buffer (pH 7.7) containing 0.05% bovine serum albumin by use of a TissueLyser (3 min, 30 revolutions s−1). Maximal citrate synthase (CS) activity was determined according to the manufacturer's protocol (Sigma-Aldrich, St Louis, MO, USA), with absorbance kinetically measured at 405 nm (Multiscan; Thermo Scientific) at baseline and after addition of oxaloacetate (Sigma-Aldrich). 3-Hydroxyacyl-CoA dehydrogenase (HAD) activity was kinetically determined at 355 nm/460 nm (excitation/emission; Fluoroscan; Thermo Scientific) as previously described (Lowry et al. 1978). After addition of acetoacetyl-CoA (Sigma-Aldrich), the change in emission was converted to activity. The CS and HAD activities were normalized to protein content measured in the respective samples by the bicinchoninic acid method.
Protein carbonylation
Protein carbonylation was determined in homogenates made in a 0.3 m phosphate buffer (described in the ‘Enzyme activities’ subsection above) using an OxiSelectTM ELISA-kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer's protocol, with absorbance measured at 450 nm (Multiscan; Thermo Scientific). Based on a serially diluted oxidized/reduced bovine serum albumin standard, the absorbance was converted to protein carbonyl concentration and normalized to protein content in the samples as determined by the bicinchoninic acid method.
Statistical analysis
Two-way repeated-measures ANOVA was applied to test the effect of resveratrol vs. placebo and the combined resveratrol + exercise training vs. combined placebo + exercise training. If normality or variance of the data set was skewed, the data were logarithmically transformed before applying the ANOVA. If a main effect was observed, pairwise differences were located by Student–Newman–Keul's multiple comparison post hoc test. In addition, within-group comparisons were analysed by Student's paired t test. A value of P < 0.05 was considered significant, and all values are presented as means ± SEM.
Results
Compliance
Based on self-reports, all subjects took their provided daily tablet and none of them reported any significant side-effects throughout the intervention.
The subjects enrolled in the exercise training protocol had a similar high compliance and completed 1.9 ± 0.1 (spinning) and 1.0 ± 0.09 (CrossFit) sessions per week. The intensity during the supervised training was equal between the two groups and was on average above 70% of maximal heart rate during 67% of each training session and above 90% of maximal heart rate during 14% of each training session (Gliemann et al. 2013).
Subject characteristics
Baseline characteristics as well as results obtained after the exercise training intervention with or without resveratrol supplementation have already been published (Gliemann et al. 2013). Detailed baseline characteristics as well as the results after the intervention of the subjects enrolled in the placebo and resveratrol intervention without exercise training are given in the Supporting information (Table S1). Briefly, for the groups without exercise training, general blood profile (fasting glucose, total cholesterol, high-density lipoprotein, low-density lipoprotein, high-density lipoprotein/low-density lipoprotein ratio and triglycerides), body mass index, body fat percentage and mean arterial blood pressure were similar in the placebo and the resveratrol groups before the intervention (Table S1) and did not change with either placebo or resveratrol supplementation (Table S1).
Endurance
Eight weeks of resveratrol or placebo intake did not affect endurance in a one-legged knee-extensor exercise test (presented as the percentage change in energy output in kilojoules). However, endurance increased (P < 0.05) ∼1.2-fold (from 25.8 ± 1.7 to 31.6 ± 2.1 kJ) with exercise training and ∼1.5-fold (from 22.9 ± 2.4 to 32.2 ± 2.6 kJ) with exercise training combined with resveratrol supplementation, with no significant additive effects of resveratrol supplementation (Fig. 1A). The individual changes in endurance are given in Fig. 1B.
Figure 1. Percentage change in endurance (A) and individual changes in endurance (B) from before (Pre) to after (Post) 8 weeks of intervention in placebo (n = 7), resveratrol (RSV; n = 9; 250 mg day-1), exercise-trained and placebo (n = 13) and exercise-trained and RSV-supplemented subjects (n = 14; 250 mg day-1).

Abbreviations: T, trained; and UT, untrained. Values in A are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
AMPK and ACC phosphorylation, SIRT1 protein and total acetylation in skeletal muscle
Two suggested mediators of the metabolic effects of resveratrol in skeletal muscle, AMPK and SIRT1, were examined. While resveratrol alone did not affect AMPK (Fig. 2A) and ACC phosphorylation (data not shown) or the SIRT1 protein content (Supporting information Fig. S1) in skeletal muscle, the overall acetylation level decreased (P < 0.05) on average by 27% with resveratrol in skeletal muscle (Fig. 2B). Sirtuin 1 protein data from the exercise training study have already been published (Gliemann et al. 2013).
Figure 2. AMP-activated protein kinase (AMPK)Thr182 phosphorylation (A) and total acetylation of lysine residues (B) in muscle lysates from placebo (n = 7), RSV (n = 9), exercise-trained (T) and placebo (n = 13) and exercise-trained and RSV-supplemented subjects (n = 14) before (Pre) and after (Post) 8 weeks of intervention.
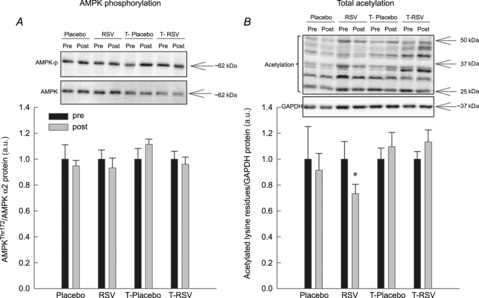
AMPK phosphorylation is normalized to AMPKα2 and acetylation levels are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein content. Protein content and acetylation are given in arbitrary units (a.u.). Representative blots are shown in each panel, with samples loaded in the same order as depicted in the graph. Values are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
Messenger RNA content of PGC-1α in skeletal muscle
The mRNA level of PGC-1α, the downstream target of AMPK and SIRT1, was determined in skeletal muscle. While resveratrol alone did not affect PGC-1α mRNA content in skeletal muscle, exercise training increased (P < 0.05) the PGC-1α mRNA content ∼1.5-fold, with no additional effect of resveratrol (Fig. 3).
Figure 3. Peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α) mRNA normalized to single-stranded DNA (ssDNA) content in skeletal muscle from placebo (n = 7), RSV (n = 9), exercise-trained (T) and placebo (n = 13) and exercise-trained and RSV-supplemented subjects (n = 14) before (Pre) and after (Post) 8 weeks of intervention.
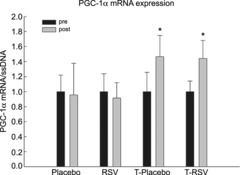
Values are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
Enzyme activities and oxidative protein content in skeletal muscle
Resveratrol supplementation alone had no effect on the CS and HAD activity or the cyt c and COXI protein content in skeletal muscle. In contrast, exercise training increased (P < 0.05) CS and HAD activity ∼1.3-fold, cyt c protein ∼1.2-fold and COXI protein content ∼1.5-fold, with no significant additional effects of resveratrol supplementation when combined with exercise training (Fig. 4).
Figure 4. Citrate synthase (CS) activity (A), 3-hydroxyacyl-CoA dehydrogenase (HAD) activity (B), cytochrome c (cyt c) protein (C) and cytochrome c oxidase I (COXI) protein (D) in skeletal muscle from placebo (n=7), RSV (n=9), exercise-trained (T) and placebo (n=13) and exercise-trained and RSV-supplemented subjects (n = 14) before (Pre) and after (Post) 8 weeks of intervention.
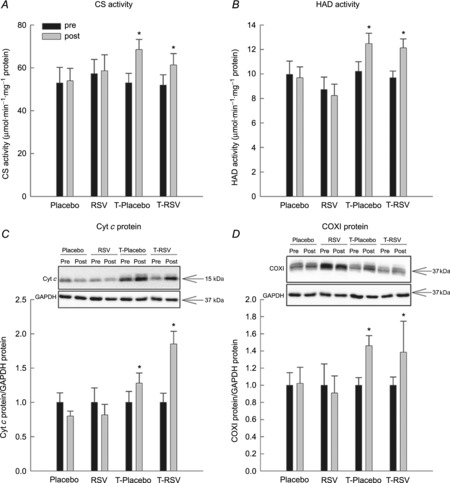
Protein content is normalized to GAPDH and given in arbitrary units (a.u.). Representative blots are shown in each panel, with samples loaded in the same order as depicted in the graph. Values are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
Protein carbonylation in skeletal muscle
The protein carbonylation level in skeletal muscle was evaluated as a marker of oxidative stress. While resveratrol intake alone did not affect protein carbonylation in skeletal muscle, exercise training decreased (P < 0.05) protein carbonyl levels by ∼20%. Resveratrol combined with exercise training blunted the exercise training-induced reduction in protein carbonylation in skeletal muscle (Fig. 5).
Figure 5. Protein carbonylation levels in skeletal muscle from placebo (n = 7), RSV (n = 9), exercise-trained (T) and placebo (n = 13) and exercise-trained and RSV-supplemented subjects (n = 14) before (Pre) and after (Post) 8 weeks of intervention.
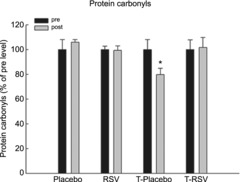
Values are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
Inflammatory signalling in skeletal muscle
Nuclear factor-κB, JNK and p38 activation were analysed in order to examine the inflammatory status of the skeletal muscle.
Resveratrol alone did not affect the abundance of the IκB-α and IκB-β (Fig. 6) or the JNK, p65, p38 and IKK phosphorylation (Supporting information Fig. S2). Exercise training increased (P < 0.05) the protein abundance of IκB-α and IκB-β 1.2- to 1.3-fold, with no additive effects when combined with resveratrol supplementation, whereas p65, JNK, p38 and IKK phosphorylation was unaffected by exercise training (Fig. S2).
Figure 6. Inhibitor of κB-α (IκB-α) protein (A) and inhibitor of κB-β (IκB-β) protein (B) in muscle lysates from placebo (n = 7), RSV (n = 9), exercise-trained (T) and placebo (n = 13) and exercise-trained and RSV-supplemented subjects (n = 14) before (Pre) and after (Post) 8 weeks of intervention.
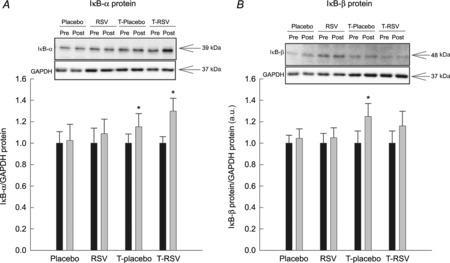
Protein content is normalized to GAPDH and given in arbitrary units (a.u.). Representative blots are shown in each panel, with samples loaded in the same order as depicted in the graph. Values are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
Inflammatory markers in skeletal muscle
Resveratrol did not affect TNFα mRNA (Fig. 7) and TNFα protein or iNOS mRNA and iNOS protein content in skeletal muscle (Supporting information Fig. S3). However, exercise training decreased (P < 0.05) TNFα mRNA content by ∼40%, and resveratrol combined with exercise training blunted this effect (Fig. 7). Muscle TNFα protein and iNOS protein were not affected by exercise training, with or without resveratrol (Fig. S3).
Figure 7. Tumour necrosis factor α (TNFα) mRNA content in skeletal muscle from placebo (n = 7), RSV (n = 9), exercise-trained (T) and placebo (n = 13) and exercise-trained and RSV-supplemented subjects (n = 14) before (Pre) and after (Post) 8 weeks of intervention.
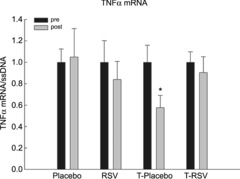
Tumour necrosis factor α mRNA is normalized to single-stranded DNA (ssDNA). Values are presented as means ± SEM. *Significantly different from Pre within treatment, P < 0.05.
Plasma levels of inflammatory markers
No differences were observed in systemic levels of TNFα, interleukin-6 or C-reactive protein in any of the interventions (Supporting information Table S2).
Discussion
The main findings of the present study are that while exercise training markedly increased muscle endurance and the content and activity of oxidative proteins as well as decreased the TNFα mRNA content and protein carbonylation level in skeletal muscle, resveratrol did not affect these parameters. In fact, resveratrol even impaired the exercise training-induced reductions in TNFα mRNA content and the protein carbonylation level in skeletal muscle. These latter findings challenge our hypotheses and previous resveratrol studies in rodents.
The observation that resveratrol supplementation did not affect oxidative proteins, inflammatory markers and protein carbonylation in skeletal muscle or blood lipid profile, body fat percentage and mean arterial blood pressure (Table S1) is in contrast to previous reports in rodents (Baur et al. 2006; Lagouge et al. 2006; Um et al. 2010; Dolinsky et al. 2012; Park et al. 2012) and a study in obese human subjects (Timmers et al. 2011). However, recent studies in humans (Poulsen et al. 2012; Yoshino et al. 2012) and rodents (Higashida et al. 2013; Olesen et al. 2013) have, in agreement with the present study, not observed any metabolic effects of resveratrol. More strikingly, resveratrol has even been reported to impair AMPK-mediated metabolic signalling in human primary myotubes (Skrobuk et al. 2012). The lack of resveratrol-induced effects on AMPK signalling and SIRT1 protein content in skeletal muscle in the present study is in accordance with two previous studies (Poulsen et al. 2012; Yoshino et al. 2012) and may underlie the absence of effects on PGC-1α and its downstream targets, cyt c and COXI. However, resveratrol did seem to affect the overall acetylation level in skeletal muscle, potentially reflecting increased SIRT1 activity (Baur et al. 2006; Lagouge et al. 2006), although seemingly insufficient to induce any downstream metabolic effects in the present settings.
Although the bioavailability of resveratrol has been reported as relatively low in humans (Walle, 2011), the discrepancies between the various human studies do not seem to be dose related. Hence, the study by Timmers et al. (2011) reporting metabolic improvements used an intermediate resveratrol dose of 150 mg day−1 for 30 days, reaching a plasma resveratrol concentration of 0.8 μm, whereas the present study (250 mg day−1 for 8 weeks) and the studies by Youshino et al. (2012) and Poulsen et al. (2012), using 75 mg day−1 for 12 weeks and 1500 mg day−1 for 4 weeks, respectively, did not find similar metabolic effects. It may be argued that the pharmacological window of resveratrol-mediated metabolic effects is narrow, and it is likely that an optimal dose exists, at least in vitro (Higashida et al. 2013). However, evidence from rodents does not support this, because resveratrol doses ranging from ∼20 mg (kg body weight)−1 day−1 to ∼1 g (kg body weight)−1 day−1 all have been reported to elicit metabolic effects (Baur et al. 2006; Lagouge et al. 2006; Dolinsky et al. 2012; Price et al. 2012). In addition, a pilot study performed by Poulsen et al. (2012), using the same supplier of resveratrol as in the present study, reported that the plasma levels after an acute intake of 500 mg of resveratrol resulted in a plasma resveratrol concentration of ∼1.5 μm. Hence, it may be expected that the present dose (250 mg) has resulted in a plasma resveratrol concentration of ∼0.75 μm, which is similar to the plasma resveratrol concentration observed in the study by Timmers et al. (2011). In contrast, it may be speculated that the subjects in the present study as well as in the previous study with aged, non-obese women (Yoshino et al. 2012) were too ‘metabolically healthy’ to experience any improvements with resveratrol. Accordingly, resveratrol does not improve plasma lipid profile, glucose tolerance, insulin sensitivity or lifespan in ‘metabolically healthy’ rodents (Turrens et al. 1997; Juan et al. 2002; Miller et al. 2011; Higashida et al. 2013; Strong et al. 2013). Collectively, these observations may indicate that a certain degree of metabolic dysfunction is a prerequisite to obtain favourable effects of resveratrol. However, future human studies are needed to verify this and to elucidate the apparent species-related discrepancies.
The present observations that exercise training led to a co-ordinated increase in muscle endurance, mitochondrial oxidative proteins and CS and HAD enzyme activity in skeletal muscle are in accordance with previous studies in young (Holloszy, 1967; Gollnick et al. 1973; Henriksson & Reitman, 1977) and aged subjects (Suominen et al. 1977; Iversen et al. 2011) and emphasize the high plasticity of skeletal muscle even with increasing age (Short et al. 2003; Ghosh et al. 2011). In addition, these data collectively support that exercise training improves muscle function in aged individuals through several intracellular metabolic adaptations.
The observed increase in PGC-1α mRNA with exercise training is in accordance with some previous studies (Russell et al. 2003; Short et al. 2003), but in contrast to others (Pilegaard et al. 2003; Nordsborg et al. 2010). Previous reports have shown that PGC-1α mRNA content increases transiently during recovery from a single exercise bout (Baar et al. 2002; Pilegaard et al. 2003), returning to baseline within 24 h of recovery. The present increase in PGC-1α ∼48 h after the last exercise training session is therefore surprising, but may be due to a combination of the age of the subjects and the intense exercise training protocol, which may have led to an accumulation of PGC-1α mRNA in these aged subjects. Hence, PGC-1α mRNA has previously been reported to be lower in elderly subjects (Ling et al. 2004), and the exercise training may therefore have elicited a more marked increase in basal PGC-1α mRNA level in the aged subjects than in young subjects. Although PGC-1α has been shown not to be mandatory for exercise training-induced adaptations in oxidative proteins in skeletal muscle of young mice (Leick et al. 2008; Geng et al. 2010), other studies have emphasized a role of PGC-1α in basal mitochondrial function (Lin et al. 2002, 2004; Handschin et al. 2007; Wende et al. 2007) and exercise training-induced mitochondrial adaptations (Geng et al. 2010; Leick et al. 2010). Taken together, these data may suggest that an increased PGC-1α expression underlies the co-ordinated exercise training-induced metabolic adaptations in skeletal muscle in the present study.
The present findings that combined exercise training and resveratrol supplementation did not elicit additive effects on oxidative proteins in skeletal muscle are in contrast to a previous study in mice (Menzies et al. 2013), but in accordance with a mouse study from our laboratory (Ringholm et al. 2013). The discrepancies between these studies are difficult to explain, but the present similar increase in skeletal muscle oxidative capacity in the two exercise training groups may be explained by the observed similar increase in PGC-1α mRNA in the two groups.
The exercise training-induced reduction in protein carbonylation indicates that the subjects had a reduced level of oxidative stress after the training period, which may be explained by the concomitant exercise training-induced increase in superoxide dismutase 2, as previously published (Gliemann et al. 2013). The novel observation that resveratrol impaired the exercise training-induced reduction in protein carbonylation is surprising. Although some previous studies have reported no impact of antioxidant treatment on exercise training-induced effects, other studies have reported that supplementation with various antioxidants in combination with exercise training blunts exercise training-induced adaptations (Gomez-Cabrera et al. 2008; Ristow et al. 2009). Previous studies have shown that reactive oxygen species are important inducers of antioxidant enzymes (Jackson et al. 2002) and important for insulin action (Loh et al. 2009). Given the previously reported direct antioxidant properties of resveratrol (Stojanović et al. 2001; Olas & Wachowicz, 2005), it may be speculated that resveratrol has scavenged reactive oxygen species and blunted the exercise training-induced reduction in oxidative stress. However, the previously published similar protein levels of catalase, glutathione peroxidase 1 and superoxide dismutase 2 in the two exercise training groups (Gliemann et al. 2013) do not support this, underlining that additional studies are needed to elucidate the effects of resveratrol on the handling of reactive oxygen species during exercise.
The increased abundance of IκB-α and IκB-β protein content with exercise training is in accordance with previous studies (Sriwijitkamol et al. 2006; Schenk et al. 2009) and indicates that exercise training led to reduced nuclear factor-κB signalling. These findings are intriguing and may explain the reduced TNFα mRNA level and support the finding that exercise training has anti-inflammatory effects (Pedersen & Saltin, 2006; Gleeson et al. 2011; Woods et al. 2012). The novel finding that resveratrol blunted the exercise training-induced reduction in TNFα mRNA content is interesting and may, to some extent, be explained by the impaired exercise training-induced increase in IκB-β protein content. As nuclear factor-κB is a redox-sensitive transcription factor, the parallel impaired exercise training-induced reduction in oxidative stress in skeletal muscle of these subjects may indirectly underlie these observations. However, the similar p65 phosphorylation level and IκB-α protein content between the two exercise training groups does not fully support this. It should also be noted that the decreased TNFα mRNA level with exercise training was not reflected at the protein level in skeletal muscle or in plasma, which again suggests that the subjects were too ‘metabolically healthy’ for systemic anti-inflammatory effects to be detected.
In conclusion, the present findings indicate that resveratrol supplementation did not elicit metabolic improvements in healthy aged subjects. In contrast, resveratrol even impaired the observed exercise training-induced reduction in TNFα mRNA and protein carbonylation in skeletal muscle. These findings contradict our hypotheses and earlier studies in rodents (Baur et al. 2006; Kim et al. 2007; Jackson et al. 2011). The observed improvements in muscle endurance, oxidative proteins and markers of oxidative stress and inflammation in skeletal muscle after the exercise training period underline the efficacy of exercise training and highlight the remarkable plasticity of skeletal muscle even with increasing age. Taken together, the present data support the notion that exercise training may have numerous health-beneficial effects in aged individuals, potentially postponing age-related metabolic deterioration, while use of resveratrol as a daily supplement in conjunction with exercise training may be questioned in healthy aged people.
Key points
Ageing is associated with lifestyle-related metabolic diseases, and exercise training has been suggested to counteract such metabolic deteriorations.
The natural antioxidant resveratrol has been reported to exert ‘exercise-like’ health beneficial metabolic and anti-inflammatory effects in rodents, but little is known about the metabolic effects of resveratrol supplementation alone and in combination with exercise training in humans.
The present findings showed that exercise training markedly improved muscle endurance, increased content and activity of oxidative proteins in skeletal muscle and reduced markers of oxidative stress and inflammation in skeletal muscle of aged men.
Resveratrol alone did not elicit metabolic effects in healthy aged subjects, but even impaired the exercise training-induced improvements in markers of oxidative stress and inflammation in skeletal muscle.
Acknowledgments
The authors would like thank Fluxome Inc. (Stenlose, Denmark) for providing us with trans-resveratrol and placebo tablets. A special thanks to Iben Plate for establishing the contact with Fluxome, Ninna Iversen and Anne Finne Pihl for technical assistance and Line Nielsen, Marie Linander Henriksen, Sebastian Peronard and Simon Grandjean for their skilful training of the subjects.
Glossary
- ACC
Acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- COXI
cytochrome c oxidase I
- CS
citrate synthase
- cyt c
cytochrome c
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HAD
3-hydroxyacyl-CoA dehydrogenase
- IκB-α
inhibitor of κB-α
- IκB-β
inhibitor of κB-β
- IKK
inhibitor of κB kinase
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinases
- p38
p38 mitogen-activated protein kinases
- PGC-1α
peroxisome proliferator-activated receptor-γ co-activator-1α
- SIRT1
sirtuin1
- TNFα
tumour necrosis factor α
- p65
transcription factor RelA
Additional information
Competing interests
None declared.
Author contributions
J.O., L.G., R.B., Y.H. and H.P. designed and conceived the study. Y.H. and H.P. acquired funding for the study and the analyses. J.O., L.G., R.B., J.S. and H.P. performed the analyses. J.O. and H.P. wrote the manuscript. L.G., R.B., J.S. and Y.H. reviewed the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by The Danish Ministry of Culture for Sports Research, The Danish Council for Independent Research – Medical Sciences and by Gigtforeningen, Denmark. The Centre of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (DNRF55). The Centre for Physical Activity Research (CFAS) is supported by a grant from Trygfonden. CIM is part of the UNIK Project: Food, Fitness & Pharma for Health and Disease, supported by the Danish Ministry of Science, Technology, and Innovation. CIM is a member of DD2 – the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724). The Copenhagen Muscle Research Centre (CMRC) is supported by a grant from the Capital Region of Denmark.
Supporting Information
The following supporting information is available in the online version of this article.
Table S1. Basic characteristics.
Table S2. Plasma cytokines.
References
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couter D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Birk JB, Wojtaszewski JF. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, Mészáros LG, Sümegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JR. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590:2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fibre-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C572–C579. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP, Garduno-Garcia JdeJ, Abdul-Ghani M, Richardson A, DeFronzo RA, Mandarino L, Van Remmen H, Musi N. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060. doi: 10.2337/db11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Biensø RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW4th, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34:107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1α in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson J, Reitman JS. Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome oxidase activities and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand. 1977;99:91–97. doi: 10.1111/j.1748-1716.1977.tb10356.x. [DOI] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann W, Strüder HK, Tagarakis CV, King G. Physical activity and the elderly. Eur J Cardiovasc Prev Rehabil. 2007;14:730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Iversen N, Krustrup P, Rasmussen HN, Rasmussen UF, Saltin B, Pilegaard H. Mitochondrial biogenesis and angiogenesis in skeletal muscle of the elderly. Exp Gerontol. 2011;46:670–678. doi: 10.1016/j.exger.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ, Papa S, Bolaños J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B, Lorusso M, Lund E, Øivind Moskaug J, Moser U, Di Paola M, Polidori MC, Signorile A, Stahl W, Viña-Ribes J, Astley SB. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209–285. doi: 10.1016/s0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park KG, Yoo EK, Kim YH, Kim YN, Kim HS, Kim HT, Park JY, Lee KU, Jang WG, Kim JG, Kim BW, Lee IK. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid Redox Signal. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H. PGC-1α is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Ling C, Poulsen P, Carlsson E, Ridderstråle M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1α and PGC-1β gene expression in twins. J Clin Invest. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CV, Kimmey JS, Felder S, Chi MM, Kaiser KK, Passonneau PN, Kirk KA, Lowry OH. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978;253:8269–8277. [PubMed] [Google Scholar]
- Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD, Pilegaard H. Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol. 2005;95:351–360. doi: 10.1007/s00421-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Physiology of aging. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S218–S222. doi: 10.1123/ijsnem.11.s1.s218. [DOI] [PubMed] [Google Scholar]
- Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg NB, Lundby C, Leick L, Pilegaard H. Relative workload determines exercise-induced increases in PGC-1α mRNA. Med Sci Sports Exerc. 2010;42:1477–1484. doi: 10.1249/MSS.0b013e3181d2d21c. [DOI] [PubMed] [Google Scholar]
- Olas B, Wachowicz B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets. 2005;16:251–260. doi: 10.1080/09537100400020591. [DOI] [PubMed] [Google Scholar]
- Olesen J, Ringholm S, Nielsen MM, Brandt CT, Pedersen JT, Halling JF, Goodyear LJ, Pilegaard H. Role of PGC-1α in exercise training- and resveratrol-induced prevention of age-associated inflammation. Exp Gerontol. 2013;48:1274–1284. doi: 10.1016/j.exger.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–1553. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couter D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2012;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholm OlesenJ, Pedersen J, Brandt C, Halling J, Hellsten Y, Prats C, Pilegaard H. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1α. Exp Gerontol. 2013;48:1311–1318. doi: 10.1016/j.exger.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Dériaz O. Endurance training in humans leads to fibre type-specific increases in levels of peroxisome proliferator-activated receptor-γ coactivator-1 and peroxisome proliferator-activated receptor-α in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- Schenk S, Harber MP, Shrivastava CR, Burant CF, Horowitz JF. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J Physiol. 2009;587:4949–4961. doi: 10.1113/jphysiol.2009.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Skrobuk P, von Kraemer S, Semenova MM, Zitting A, Koistinen HA. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologia. 2012;55:3051–3060. doi: 10.1007/s00125-012-2691-1. [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N. Reduced skeletal muscle inhibitor of κBβ content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–767. doi: 10.2337/diabetes.55.03.06.db05-0677. [DOI] [PubMed] [Google Scholar]
- Stojanović S, Sprinz H, Brede O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch Biochem Biophys. 2001;391:79–89. doi: 10.1006/abbi.2001.2388. [DOI] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suominen H, Heikkinen E, Liesen H, Michel D, Hollmann W. Effects of 8 weeks’ endurance training on skeletal muscle metabolism in 56–70-year-old sedentary men. Eur J Appl Physiol Occup Physiol. 1977;37:173–180. doi: 10.1007/BF00421772. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Lariccia J, Nair MG. Resveratrol has no effect on lipoprotein profile and does not prevent peroxidation of serum lipids in normal rats. Free Radic Res. 1997;27:557–562. doi: 10.3109/10715769709097859. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1α in muscle refueling. J Biol Chem. 2007;282:36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3:130–140. [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi FF, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Basic characteristics.
Table S2. Plasma cytokines.


