Abstract
In this double-blind, randomised, controlled trial, we investigated the effects of vitamin C and E supplementation on endurance training adaptations in humans. Fifty-four young men and women were randomly allocated to receive either 1000 mg of vitamin C and 235 mg of vitamin E or a placebo daily for 11 weeks. During supplementation, the participants completed an endurance training programme consisting of three to four sessions per week (primarily of running), divided into high-intensity interval sessions [4–6 × 4–6 min; >90% of maximal heart rate (HRmax)] and steady state continuous sessions (30–60 min; 70–90% of HRmax). Maximal oxygen uptake ( ), submaximal running and a 20 m shuttle run test were assessed and blood samples and muscle biopsies were collected, before and after the intervention. Participants in the vitamin C and E group increased their
), submaximal running and a 20 m shuttle run test were assessed and blood samples and muscle biopsies were collected, before and after the intervention. Participants in the vitamin C and E group increased their  (mean ± s.d.: 8 ± 5%) and performance in the 20 m shuttle test (10 ± 11%) to the same degree as those in the placebo group (mean ± s.d.: 8 ± 5% and 14 ± 17%, respectively). However, the mitochondrial marker cytochrome c oxidase subunit IV (COX4) and cytosolic peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1α) increased in the m. vastus lateralis in the placebo group by 59 ± 97% and 19 ± 51%, respectively, but not in the vitamin C and E group (COX4: −13 ± 54%; PGC-1α: −13 ± 29%; P ≤ 0.03, between groups). Furthermore, mRNA levels of CDC42 and mitogen-activated protein kinase 1 (MAPK1) in the trained muscle were lower in the vitamin C and E group than in the placebo group (P ≤ 0.05). Daily vitamin C and E supplementation attenuated increases in markers of mitochondrial biogenesis following endurance training. However, no clear interactions were detected for improvements in
(mean ± s.d.: 8 ± 5%) and performance in the 20 m shuttle test (10 ± 11%) to the same degree as those in the placebo group (mean ± s.d.: 8 ± 5% and 14 ± 17%, respectively). However, the mitochondrial marker cytochrome c oxidase subunit IV (COX4) and cytosolic peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1α) increased in the m. vastus lateralis in the placebo group by 59 ± 97% and 19 ± 51%, respectively, but not in the vitamin C and E group (COX4: −13 ± 54%; PGC-1α: −13 ± 29%; P ≤ 0.03, between groups). Furthermore, mRNA levels of CDC42 and mitogen-activated protein kinase 1 (MAPK1) in the trained muscle were lower in the vitamin C and E group than in the placebo group (P ≤ 0.05). Daily vitamin C and E supplementation attenuated increases in markers of mitochondrial biogenesis following endurance training. However, no clear interactions were detected for improvements in  and running performance. Consequently, vitamin C and E supplementation hampered cellular adaptations in the exercised muscles, and although this did not translate to the performance tests applied in this study, we advocate caution when considering antioxidant supplementation combined with endurance exercise.
and running performance. Consequently, vitamin C and E supplementation hampered cellular adaptations in the exercised muscles, and although this did not translate to the performance tests applied in this study, we advocate caution when considering antioxidant supplementation combined with endurance exercise.
Introduction
Aerobic endurance exercise is highly recommended by health authorities for its rewarding effects on health (Garber et al. 2011) and, in many sports, high muscular aerobic energy capacity and  are prerequisites for elite performance (Saltin & Astrand, 1967). Strategies for obtaining optimal endurance training effects include not only certain training methods, such as interval training (Gibala, 2007), but also nutritional measures (Hawley et al. 2011). Supplements containing antioxidants and vitamins are widely used for the purpose of improving health and athletic achievement (Petroczi et al. 2007; Kennedy et al. 2013). Isolated vitamin C and E supplements are among the most commonly used, despite tentative evidence for the purported effects of these vitamins on health, sport performance and recovery from muscle damage (Padayatty et al. 2003; Nikolaidis et al. 2012).
are prerequisites for elite performance (Saltin & Astrand, 1967). Strategies for obtaining optimal endurance training effects include not only certain training methods, such as interval training (Gibala, 2007), but also nutritional measures (Hawley et al. 2011). Supplements containing antioxidants and vitamins are widely used for the purpose of improving health and athletic achievement (Petroczi et al. 2007; Kennedy et al. 2013). Isolated vitamin C and E supplements are among the most commonly used, despite tentative evidence for the purported effects of these vitamins on health, sport performance and recovery from muscle damage (Padayatty et al. 2003; Nikolaidis et al. 2012).
Contrary to common beliefs, studies have recently demonstrated that antioxidant supplementation may interfere with exercise-induced cell signalling in skeletal muscle fibres (Ristow & Zarse, 2010; Hawley et al. 2011). In turn, such changes in cell signalling may potentially blunt or block adaptations to training (Peternelj & Coombes, 2011; Gliemann et al. 2013; Morales-Alamo & Calbet, 2014). For example, Gomez-Cabrera et al. (2008) investigated whether high dosages of vitamin C affected adaptation to endurance exercise training in both an animal and a human model (1000 mg day−1 in the human study; male participants). Interestingly, endurance performance increased to a greater extent in animals treated with the placebo compared with animals treated with vitamin C. Furthermore, markers for mitochondrial biogenesis [i.e. peroxisome proliferator-activated receptor-γ co-activator 1-α (PGC-1α)] increased only in animals treated with the placebo. In the human experiment, changes in  did not differ significantly between the supplement and placebo groups. Unfortunately, these authors did not test endurance capacity or collect muscle biopsies from participants to verify the results of the animal study. In another study with untrained and trained male participants, Ristow et al. (2009) demonstrated that 4 weeks of vitamin C (1000 mg day−1) and E (400 IU day−1) supplementation blunted training-induced increases in the mRNA expression of genes associated with mitochondrial biogenesis and endogenous antioxidant systems in skeletal muscle (e.g. PGC-1α and glutathione peroxidase). Furthermore, Braakhuis et al. (2014) observed that supplementation with 1000 mg day−1 of vitamin C for 3 weeks slowed female runners during training, although no differences were found in a 5 km time trial or in an incremental treadmill test after the intervention period.
did not differ significantly between the supplement and placebo groups. Unfortunately, these authors did not test endurance capacity or collect muscle biopsies from participants to verify the results of the animal study. In another study with untrained and trained male participants, Ristow et al. (2009) demonstrated that 4 weeks of vitamin C (1000 mg day−1) and E (400 IU day−1) supplementation blunted training-induced increases in the mRNA expression of genes associated with mitochondrial biogenesis and endogenous antioxidant systems in skeletal muscle (e.g. PGC-1α and glutathione peroxidase). Furthermore, Braakhuis et al. (2014) observed that supplementation with 1000 mg day−1 of vitamin C for 3 weeks slowed female runners during training, although no differences were found in a 5 km time trial or in an incremental treadmill test after the intervention period.
Contrary to these studies, Yfanti et al. (2010, 2011, 2012) found no negative effects of vitamin C (500 mg day−1) and E (400 IU day−1) supplementation in male participants who trained five times per week for 12 weeks on a cycle ergometer. The antioxidant supplementation did not influence changes in  and maximal power output (cycling), or activity of the enzymes citrate synthase (CS) and β-hydroxyacyl-CoA dehydrogenase (β-HAD) in skeletal muscle. Similarly, Roberts et al. (2011) reported no effects of vitamin C (1000 mg day−1) supplementation on adaptations to high-intensity running training in male participants.
and maximal power output (cycling), or activity of the enzymes citrate synthase (CS) and β-hydroxyacyl-CoA dehydrogenase (β-HAD) in skeletal muscle. Similarly, Roberts et al. (2011) reported no effects of vitamin C (1000 mg day−1) supplementation on adaptations to high-intensity running training in male participants.  and endurance performance (10 km time trial and YoYo tests) improved equally in supplemented and placebo groups. The conflicting results from these human studies are reflected in the findings of recent animal studies (Braakhuis, 2012; Gomez-Cabrera et al. 2012; Nikolaidis et al. 2012).
and endurance performance (10 km time trial and YoYo tests) improved equally in supplemented and placebo groups. The conflicting results from these human studies are reflected in the findings of recent animal studies (Braakhuis, 2012; Gomez-Cabrera et al. 2012; Nikolaidis et al. 2012).
Accordingly, it seems clear that antioxidant supplementation potentially inhibits favourable cellular responses to endurance training. By contrast, the discrepancy between studies invites further investigation. Therefore, we studied the influence of vitamin C and E supplementation on adaptations to aerobic endurance training, hypothesising that high dosages of vitamin C and E, ingested shortly before and after exercise, would blunt physiological adaptations to 11 weeks of endurance training. The hypothesis was tested in a study using a double-blind, randomised, controlled trial design, in which both training and nutrition were tightly controlled. We combined performance tests with physiological measurements ( ) and biochemical/molecular analyses of blood and muscle.
) and biochemical/molecular analyses of blood and muscle.
Methods
Participants
Fifty-four young, healthy men and women participated in the experiment (Table 1 and Fig. 1). Forty of the volunteers were defined as recreationally endurance-trained individuals because they had been endurance training between one and four times per week for 6 months prior to the study. The endurance training consisted mainly of running and cycling. Fourteen volunteers were defined as untrained because they had not trained regularly (maximum of one session per week) during the previous 6 months. Sixty-eight volunteers were recruited to the study, but 14 participants (seven from each group) dropped out of the study during the training intervention. Five participants were injured during training (ankle sprains, Achilles pains) and nine dropped out for reasons unrelated to the study.
Table 1.
Characteristics of participants in the vitamin C and E supplementation group and the placebo group
| Vitamin C and E group (n = 27: 14 women, 13 men) | Placebo group (n = 27: 14 women, 13 men) | |
|---|---|---|
| Age (years) | 25 ± 5 | 24 ± 6 |
| Height (m) | 1.74 ± 0.10 | 1.76 ± 0.10 |
| Body mass (kg) | 74 ± 14 | 70 ± 12 |
 (ml·min−1·kg−1) (ml·min−1·kg−1) |
53 ± 9 | 53 ± 8 |
Data are mean ± s.d.
Figure 1. Numbers of endurance-trained and untrained participants in each group.
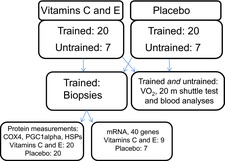
Numbers of participants in tests and analyses applied. PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1 α; HSPs, heat shock proteins.
The volunteers were instructed not to take any form of supplements or medication (except contraceptives). Individuals who did use multi-vitamin supplements were asked to stop taking them at least 2 weeks before the beginning of the study.
The study was approved by the Regional Ethics Committee for Medical and Health Research of South-East Norway and performed in accordance with the Helsinki Declaration. All participants signed a written consent form.
Experimental design
After pre-tests and assessments (e.g.  and muscle biopsies), participants were randomly allocated to a vitamin C and E supplement group or a placebo group. The randomisation was stratified by gender and
and muscle biopsies), participants were randomly allocated to a vitamin C and E supplement group or a placebo group. The randomisation was stratified by gender and  . All participants started to take supplements or placebo as they started on the endurance training programme. All tests were replicated after 11 weeks of training. The experiment was a double-blind, randomised, controlled trial.
. All participants started to take supplements or placebo as they started on the endurance training programme. All tests were replicated after 11 weeks of training. The experiment was a double-blind, randomised, controlled trial.
Blood samples and muscle biopsies collected before the intervention period were preceded with 3 days of rest and were scheduled to occur again 3 days after the last exercise session. However, for practical reasons, a few participants provided samples 2 or 4 days after the last exercise session. There was no group bias in the sampling time-points.
Supplementation and nutrition
The C and E vitamin and placebo pills were produced under Good Manufacturing Practice (GMP) requirements at Petefa AB (Västra Frölunda, Sweden). Each vitamin pill contained 250 mg of ascorbic acid and 58.5 mg DL-α-tocopherol acetate. The placebo pills had the same shape and appearance as the vitamin pills.
The pills were analysed by a commercial company (Vitas AS, Oslo, Norway) 2 years after production; no sign of degradation of the vitamins was found (per pill: mean ± s.d. vitamin C: 255 ± 7 mg; mean ± s.d. vitamin E: 62 ± 2 mg). The experiments were conducted within this time period. No traces of the vitamins were found in the placebo pills.
Participants consumed two pills (500 mg vitamin C and 117 mg vitamin E) 1–3 h before every training session and two pills within 1 h after training. On non-training days, participants ingested two pills in the morning and two pills in the evening. Thus, the daily dosage was 1000 mg of vitamin C and 235 mg of vitamin E. Supplement intake was confirmed in a training diary.
Participants were asked to drink no more than two glasses of juice and four cups of coffee or tea per day. Juices especially rich in antioxidants, such as grape juice, were to be avoided.
We aimed to keep the participants in energy balance and encouraged them to continue their normal diets. Participants completed a weighed food registration dietary assessment over 4 days (Black et al. 1991) at the start and end of the intervention period. Participants used a digital food scale with precision to 1 g (Vera 67002; Soehnle-Waagen GmbH & Co., Murrhardt, Germany). The dietary registrations were analysed with a nutrient analysis programme (Mat på data 4.1; Norwegian Nutrition Society, Oslo, Norway).
Body composition
A bioimpedance apparatus (Inbody 720; Biospace Co., Ltd, Seoul, South Korea) was used to assess body composition before and after the training intervention. The apparatus has been validated (compared with Dual-energy X-ray absorptiometry, DXA) for estimating fat mass and lean mass in men and women (Anderson et al. 2012).
Endurance training
The training programme was divided into three periods (Table 2). In period 1, participants exercised three times per week in two continuous sessions (30 min and 60 min) and one interval session (4 × 4 min). In period 2, one extra interval session was added to give a total of four sessions per week. In periods 2 and 3, the number of runs per interval session was increased, although exercise intensity was similar throughout the training period. The exception was that less experienced runners (untrained participants) used three to six sessions to gradually increase the intensity. Intensity was high in every session, except during the 60 min run (moderate intensity). Running was the main form of exercise, but one running session per week could be substituted by cycling, cross-country skiing or a similar whole-body activity.
Table 2.
Outline of the endurance training programme
| Weeks | Period | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|---|
| 1–3 | 1 | Continuous: 30 min: 82–87% of HRmax; Borg: 15–17 | Interval: 4 × 4 min: >90% of HRmax; Borg: 16–18 | Continuous: 45–60 min: 72–82% of HRmax; Borg: 13–16 | |
| 4–8 | 2 | Continuous: 30 min: 82–87% of HRmax; Borg: 15–17(18) | Interval: 5 × 4 min: >90% of HRmax; Borg: 16–18 | Continuous: 60 min: 72–82% of HRmax; Borg: 13–16 | Interval: 4 × 6 min: >90% of HRmax; Borg: 16–18 |
| 9–11 | 3 | Continuous: 30 min: 82–87% of HRmax; Borg: 15–17(18) | Interval: 6 × 4 min: >90% of HRmax; Borg: 16–18 | Continuous: 60 min: 72–82% of HRmax; Borg: 13–16 | Interval: 5 × 6 min: >90% of HRmax; Borg: 16–18 |
HRmax, maximal heart rate; Borg, Borg scale of perceived exertion (6–20).
Training intensity was controlled using the Borgs scale (rating of perceived exertion) and heart rate monitors (RS400/RS800CX; Polar Electro Oy, Kempele, Finland). Heart rate monitors were worn in every session and training data were collected and controlled by the investigators. Moreover, each participant was instructed to complete a training diary in which he or she logged mean heart rate, running distance and perceived effort (not reported).
 and submaximal workloads
and submaximal workloads
All participants took part in a familiarisation session for  measurements using a mixing chamber (Oxycon Pro; Erich Jaeger GmbH, Hoechberg, Germany) on a treadmill (ELG 90/200 Sport; Woodway GmbH, Weil am Rhein, Germany). The pre-test for
measurements using a mixing chamber (Oxycon Pro; Erich Jaeger GmbH, Hoechberg, Germany) on a treadmill (ELG 90/200 Sport; Woodway GmbH, Weil am Rhein, Germany). The pre-test for  started with 7 min at two submaximal running speeds (5.3% inclination), corresponding to 60% and 85% of the
started with 7 min at two submaximal running speeds (5.3% inclination), corresponding to 60% and 85% of the 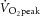 reached during the familiarisation session.
reached during the familiarisation session. 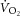 , respiratory exchange ratio (RER), heart rate (measured using the RS400; Polar Electro Oy) and ratings of perceived exertion (Borgs scale) were measured during the last 2 min at each speed. Capillary blood from a finger-stick was sampled within 1 min after each workload and blood lactate concentration was measured (YSI 1500 Sport Lactate Analyzer; YSI, Inc., Yellow Springs, OH, USA). The same submaximal running velocities were used in both the pre- and post-tests.
, respiratory exchange ratio (RER), heart rate (measured using the RS400; Polar Electro Oy) and ratings of perceived exertion (Borgs scale) were measured during the last 2 min at each speed. Capillary blood from a finger-stick was sampled within 1 min after each workload and blood lactate concentration was measured (YSI 1500 Sport Lactate Analyzer; YSI, Inc., Yellow Springs, OH, USA). The same submaximal running velocities were used in both the pre- and post-tests.
After a 10 min rest, the participants performed the  test. Running speed (5.3% inclination) was increased by 1 km h−1 in three 1 min stages, before 0.5 km h−1 increases per minute until exhaustion (total duration: 4–8 min). Lactate was measured as detailed above.
test. Running speed (5.3% inclination) was increased by 1 km h−1 in three 1 min stages, before 0.5 km h−1 increases per minute until exhaustion (total duration: 4–8 min). Lactate was measured as detailed above.
20 m shuttle run test (Beep test)
The 20 m shuttle run test is a multi-stage shuttle run test that measures aerobic fitness and has demonstrated good reliability (Leger et al. 1988). Participants ran a distance of 20 m between two lines and placed one foot on the line each time a beep sounded (from a CD player); the interval between beeps decreased over time. The test had 21 levels. It started at a speed of 8 km h−1 and increased by 0.5 km h−1 per minute. Participants ran until exhaustion, which was defined as failure to complete the distance within the time limit after one warning. Untrained participants completed a familiarisation session before this test.
Muscle tissue sampling and pre-analytic handling
Muscle biopsies from the mid-portion of the right m. vastus lateralis were collected before and after the training intervention. The post-training insertion was located proximally to the site of the pre-training biopsy (approximately 3 cm). The procedure was conducted under local anaesthesia (xylocain adrenalin, 10 mg ml−1 + 5 μg ml−1; AstraZeneca PLC, London, UK). Approximately 200 mg (2–3 × 50–150 mg) of muscle tissue was obtained using a modified Bergström technique. Tissue intended for homogenisation and protein measurements was quickly washed in physiological saline, and fat, connective tissue and blood were removed before the sample was weighed and quickly frozen in isopentane cooled on dry ice. Tissue intended for mRNA analyses was placed in RNAlater (Ambion; Life Technologies, Inc., Carlsbad, CA, USA). Samples for immunohistochemistry were mounted in Tissue-Tek (cat. no. 4583; Sakura Finetek USA, Inc., Torrance, CA, USA) and quickly frozen in isopentane cooled on liquid nitrogen. All muscle samples were stored at −80°C for later analyses.
Protein immunoblot
About 50 mg of muscle tissue was homogenised and fractionated into cytosol, membrane, nuclear and cytoskeletal fractions, using a commercial fractionation kit according to the manufacturer's procedures (ProteoExtract Subcellular Proteo Extraction Kit, no. 539790, Calbiochem; EMD Biosciences GmbH, Schwalbach, Germany). Protein concentrations were assessed with a commercial kit (BioRad DC protein micro plate assay, nos 0113, 0114, 0115; Bio-Rad Laboratories, Inc., Hercules, CA, USA), a filter photometer (Expert 96; ASYS Hitech Cambridge, UK), and the software provided (Kim Version 5.45.0.1; Daniel Kittrich).
Cytosol, membrane and nuclear fractions were analysed by the western blotting technique. Equal amounts of protein were loaded per well (9–30 μg) and separated on 4–12% SDS-PAGE gels under denatured conditions for 35–45 min at 200 volts in cold MES running buffer (NuPAGE MES SDS running buffer; Invitrogen, Inc., Carlsbad, CA, USA). Proteins were thereafter transferred onto a PDVF-membrane (Immuno-blot, cat. no. 162-0177; Bio-Rad Laboratories, Inc.), at 30 volts for 90 min in cold transfer buffer (NuPAGE transfer buffer, cat. no. NP0006-1; Life Technologies, Inc.). Membranes were blocked at room temperature for 2 h in a 5% fat-free skimmed milk and 0.05% TBS-T solution [TBS, cat. no. 170–6435 (Bio-Rad Laboratories, Inc.); Tween 20, cat. no. 437082Q (VWR International, Radnor, PA, USA); skimmed milk, cat. no. 1.15363 (Merck KGaA, Darmstadt, Germany)]. Blocked membranes were incubated with antibodies against heat shock protein 60 (HSP60) (mouse anti-HSP60, cat. no. ADI-SPA-807, diluted 1 : 4000; Enzo Life Sciences, Inc., Farmingdale, NY, USA), HSP70 (mouse anti-HSP70, cat. no. ADI-SPA-810, diluted 1 : 4000; Enzo Life Sciences Inc.), and COX 4 (mouse anti-COX4, cat. no. Ab14744, diluted 1 : 1000; Abcam Plc, Cambridge, UK) overnight at 4°C, followed by incubation with secondary antibody (goat anti-mouse, cat. no. 31430, diluted 1 : 30000; Thermo Fisher Scientific, Inc., Hanover Park, IL, USA) at room temperature for 1 h. All antibodies were diluted in a 1% fat-free skimmed milk and 0.05% TBS-T solution. Membranes with the PGC-1α molecular weight were blocked at room temperature for 2 h in a 1% BSA solution (BSA 10% in PBS; deionized H2O; cat. no. 37525; Thermo Fisher Scientific, Inc.). Blocked membranes were incubated with primary antibodies against PGC-1α [rabbit-anti-PGC-1α, C-Terminal (777–7979), cat. no. 516557, diluted 1 : 2000; Calbiochem, EMD Millipore Corp., Billerica, MA, USA) overnight at 4°C, followed by incubation with secondary antibody (goat anti-rabbit IgG, cat. no. 7074, diluted 1 : 1000; Cell Signaling Technology, Inc., Beverly, MA, USA) at room temperature for 1 h. Both primary and secondary antibodies were diluted in 1% BSA and deionized H2O solution. Between stages, membranes were washed in 0.05% TBS-T solution. Bands were visualised using an HRP-detection system (Super Signal West Dura Extended Duration Substrate, cat. no. 34076; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Chemiluminescence was measured using a CCD image sensor (Image Station 2000R or Image Station 4000R; Eastman Kodak, Inc., Rochester, NY, USA), and band intensities were calculated with Carestream molecular imaging software (Carestream Health, Inc., Rochester, NY, USA). All samples were run as duplicates and mean values were used for statistical analyses.
Immunohistochemistry
Cross-sections 8 μm thick were cut using a microtome at −20°C (CM3050; Leica Microsystems GmbH, Wetzlar, Germany) and mounted on microscope slides (Superfrost Plus; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The sections were then air-dried and stored at −80°C. The muscle sections were blocked for 30 min with 1% BSA (cat. no. A4503; Sigma-Aldrich Corp., St Louis, MO, USA) and 0.05% PBS-T solution (cat. no. 524650; Calbiochem, EMD Biosciences, Inc., San Diego, CA, USA). They were then incubated with antibodies against myosin heavy chain type 2 (1 : 1000; SC71; gift from Professor S. Schiaffino), CD31 (capillaries; 1 : 200; clone JC70A, M0823; Dako A/S, Glostrup, Denmark) and dystrophin (1 : 1000; cat. no. ab15277; Abcam Plc) overnight at 4°C followed by incubation with appropriate secondary antibodies (Alexa Fluor, cat. no. A11005 or A11001; Invitrogen, Inc.). Between stages, sections were washed for 3 × 5 min in 0.05% PBS-T solution. Muscle sections were finally covered with a coverslip and glued with ProLong Gold Antifade Reagent with DAPI (cat. no. P36935; Invitrogen Molecular Probes, Eugene, OR, USA) and left to dry overnight at room temperature. Muscle sections were visualised using a high-resolution camera (DP72; Olympus Corp., Tokyo, Japan) mounted on a microscope (BX61; Olympus Corp.) with a fluorescence light source (X-Cite 120PCQ; EXFO Photonic Solutions Inc., Mississauga, Ontario, Canada). Fibre type distribution, fibre cross-sectional area and capillaries were identified using TEMA software (CheckVision, Hadsund, Denmark). All staining counts were manually approved or corrected independently by two investigators. Capillarisation was expressed as capillaries around each fibre (CAF) and CAF related to fibre area (CAFA), for type 1 and type 2 (2A and 2X) fibres.
Gene expression analyses
Total RNA was isolated using an RNeasy Fibrous Tissue Mini Kit (cat. no. 74704; Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions. RNA quantity and quality were determined using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and Agilent Bioanalyser combined with an Agilent RNA 6000 Nano Kit (Agilent Technologies, Inc., Palo Alto, CA, USA). A High-Capacity cDNA Reverse Transcription Kit (cat. no. 4368814; Applied Biosystems, Inc., Foster City, CA, USA) was used for cDNA synthesis. Quantitative RT–PCR was performed in a 7900HT Fast Real-Time PCR System (Applied Biosystems, Inc.) using 140 ng cDNA in a custom-made Taq-Man Low Density Array (Applied Biosystems, Inc.). Primers for the following genes were included in the array (abbreviated name; Applied Biosystems assay ID): CRYAB (Hs00157107_m1); CAT (Hs00156308_m1); CDC42 (Hs00741586_mH); CS (Hs00830726_sH); COL4A1 (Hs01007469_m1); COX4I1 (Hs00971639_m1); CYCS (Hs01588973_m1); ESRRA (Hs00607062_gH); FOXO1 (Hs01054576_m1); SLC2A4 (Hs00168966_m1); GPX1 (Hs00829989_gH); HIF1A (Hs00936368_m1); HMOX1 (Hs00157965_m1); HSPB2 (Hs00155436_m1); HSPD1 (Hs01036747_m1); HSPA1A:HSPA1B (Hs00359147_s1); HSF1 (Hs00232134_m1); IGF2 (Hs00171254_m1); IL6 (Hs99999032_m1); LAMA4 (Hs00158588_m1); MAPK1 (Hs01046830_m1); MAPK3 (Hs00385075_m1); NFKB1 (Hs00231653_m1); NFKB2 (Hs00174517_m1); NID2 (Hs00201233_m1); NOX1 (Hs00246589_m1); CYBB (Hs00166163_m1); NOX3 (Hs00210462_m1); NOX4 (Hs01558199_m1); NOX5 (Hs00225846_m1); NQO1 (Hs00168547_m1); NFE2L1 (Hs00231457_m1); NFE2L2 (Hs00232352_m1); NRF1 (Hs00602161_m1); PPARGC1B (Hs00991676_m1); PPARGC1A (Hs01016724_m1); PPARA (Hs00947539_m1); PPARG (Hs01115512_m1); RELA (Hs00153294_m1); SOD1 (Hs00916176_m1); SOD2 (Hs00167309_m1); TXN (Hs00828652_m1), and VEGFA (Hs00900055_m1). Endogenous controls included in the assay were: 18S, GAPDH (Hs99999905_m1); GUSB (Hs99999908_m1); HPRT1 (Hs99999909_m1), and TBP (Hs99999910_m1). RQ Manager Version 1.2 (Applied Biosystems, Inc.) and Microsoft Excel 2010 were used for data analysis. Expression levels were quantified using the cycle threshold (Ct) normalised against the average of the endogenous controls GUSB and HPRT1. ΔCt represents the Ct value of the target gene minus (average) Ct value of the endogenous control and is used to calculate 2–ΔCt. A target gene was determined as ‘not expressed’ when the average Ct was ≥35.
Blood sampling and handling
Venous blood was collected in the morning after 12 h of fasting. Heparin- and EDTA-coated tubes were immediately centrifuged at 1500 g for 10 min at 4°C. Care was taken to keep the collected plasma cooled (on ice) between steps, and to freeze the treated samples rapidly in dry ice. Heparin plasma destined for vitamin C analysis was immediately mixed in equal volumes with metaphosphoric acid before freezing; the further procedure is described by Karlsen et al. (2005). Vitamin E was analysed in EDTA plasma, as described by Bastani et al. (2012). Plasma (heparin) 8-iso PGF-2α analyses have previously been described by Bastani et al. (2009). All samples were stored at −80°C until analysis.
Statistics
The numbers of participants included in the different tests and analyses are given in Fig. 1. All data were tested for Gaussian distribution with the D'Agostino–Pearson omnibus normality test. A two-way ANOVA was used to evaluate the effect of training (time) and vitamin C and E supplementation (absolute values, pre and post). A Holm–Sidak multiple comparisons test was applied for post hoc analyses. Between-groups differences in relative changes (%) from before to after the intervention period (pre–post changes) were assessed with an unpaired Student's t test or the Mann–Whitney test (depending on distribution). Relative changes within each group were assessed with a paired Student's t test or Wilcoxon signed rank test (depending on distribution). For mRNA data, Mann–Whitney U tests were used to compare changes between groups, and Wilcoxon signed rank tests were used for within-group analyses. Data are given as the mean ± s.d. in the text and tables. The figures display maximum and minimum values, 25th and 75th quartiles and medians (boxplots) as some of the biochemical variables were not normally distributed. Outliers were defined by Tukey's rule. Effect size was calculated as the differences between the group means divided by the combined s.d. Graphpad Prism Version 6.00 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analyses.
Results
The participants reported 97 ± 5% adherence to the supplements. A survey conducted after the training period confirmed that group affiliation had remained concealed from participants. The vitamin C and E supplementation raised plasma levels of both vitamin C [before: 81 ± 24 μm; after: 114 ± 30 μm (P < 0.001)] and vitamin E [α-tocopherol; before: 27 ± 7 μm; after: 35 ± 11 μm (P = 0.009)] (Fig. 2). No changes were found in the placebo group [vitamin C: before: 80.9 ± 17.2 μm; after: 81.1 ± 19.9 μm (P = 0.70); vitamin E: before 25.9 ± 6.6 μm; after: 26.6 ± 4.2 μm (P = 0.66)].
Figure 2. Percentage changes in plasma levels of vitamin C and E in the vitamin C and E group and the placebo group.
A, vitamin C levels. B, vitamin E levels. Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); #, difference between groups; *, within-group changes.
In contrast to the vitamin C and E supplementation group (before: 87.1 ± 49 pg ml−1; after: 85.5 ± 43 pg ml−1), 8-iso PGF-2α increased in the placebo group [before: 74 ± 33 pg ml−1; after: 88.2 ± 29 pg ml−1 (P = 0.03)]. This difference between the groups was statistically significant (P = 0.03) (Fig. 3).
Figure 3. Percentage changes in plasma 8-iso-prostane in the vitamin C and E group and the placebo group.
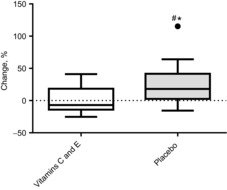
Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); #, difference between groups; *, within-group changes.
We found no significant difference in energy intake between the vitamin C and E supplementation group and placebo group (∼10500 ± 3500 kJ in both groups), nor in macro- or micro-nutrients (data not shown). In their regular diet, the vitamin C and E supplementation group consumed 104 ± 72 mg of vitamin C and 11 ± 4 mg of vitamin E per day, whereas the placebo group consumed 102 ± 50 mg and 11 ± 4 mg of vitamin C and E, respectively (P > 0.7 between groups).
The vitamin C and E supplementation group reduced body mass by 1.0 ± 2.0% (P = 0.02) as a result of a 5.3 ± 8.6% (P = 0.005) loss of fat mass, but these changes did not differ from those in the placebo group (Table 3). Estimated muscle mass was stable in both groups.
Table 3.
Body composition before and after the 11 week intervention period
| Vitamin C and E group |
Placebo group |
||||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Change, % | Pre | Post | Change, % | P-value* | |
| Body mass (kg) | 73.9 ± 14.2 | 73.1 ± 13.7† | −1.0 ± 2.0‡ | 70.2 ± 11.8 | 69.5 ± 12.5 | −1.1 ± 2.8 | 0.856 |
| Fat mass (kg) | 15.5 ± 7.1 | 14.6 ± 6.8† | −5.3 ± 8.9‡ | 12.6 ± 5.8 | 12.2 ± 5.9 | −3.3 ± 12.1 | 0.497 |
| Fat% | 20.8 ± 8.2 | 19.8 ± 7.9† | −4.6 ± 7.7‡ | 18.1 ± 7.1 | 17.6 ± 7.2 | −2.0 ± 11.0 | 0.324 |
| Muscle mass (kg) | 32.9 ± 7.2 | 33.0 ± 7.1 | 0.4 ± 2.2 | 32.4 ± 6.6 | 32.3 ± 6.8 | −0.4 ± 2.6 | 0.206 |
Values are mean ± s.d.
P-values for between-group differences in percentage change. Within-group changes:
P < 0.05;
P < 0.01.
All participants performed 38–45 exercise sessions during the 11 week intervention. The training diaries and heart rate data showed no differences in training intensity or perceived exertion between the groups (data not shown).
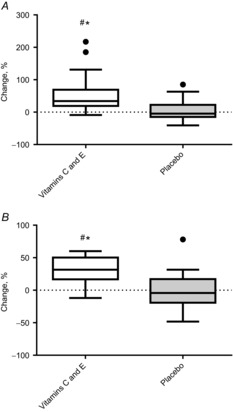
 improved to the same degree in both groups (vitamin C and E supplementation group: from 52.9 ± 7.6 ml min−1 kg−1 to 57.2 ± 9.6 ml min−1 kg−1; placebo group: from 52.9 ± 8.6 ml min−1 kg−1 to 57.1 ± 7.4 ml min−1 kg−1), as did performance in the 20 m shuttle run test (vitamin C and E supplementation group: from 1660 ± 570 m to 1800 ± 540 m, placebo group: from 1670 ± 550 m to 1870 ± 550 m) (Fig. 4).
improved to the same degree in both groups (vitamin C and E supplementation group: from 52.9 ± 7.6 ml min−1 kg−1 to 57.2 ± 9.6 ml min−1 kg−1; placebo group: from 52.9 ± 8.6 ml min−1 kg−1 to 57.1 ± 7.4 ml min−1 kg−1), as did performance in the 20 m shuttle run test (vitamin C and E supplementation group: from 1660 ± 570 m to 1800 ± 540 m, placebo group: from 1670 ± 550 m to 1870 ± 550 m) (Fig. 4).
Figure 4. Percentage changes in  and the 20 m shuttle run test in the vitamin C and E group and the placebo group.
and the 20 m shuttle run test in the vitamin C and E group and the placebo group.
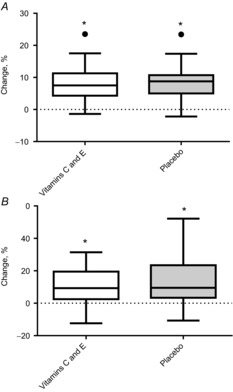
A, changes in  . B, changes in 20 m shuttle run. Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); #, difference between groups; *, within-group changes.
. B, changes in 20 m shuttle run. Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); #, difference between groups; *, within-group changes.
The subgroup of previously untrained participants increased their  more than the trained participants (12.6 ± 6.2%; P < 0.001, untrained versus trained), but there were no differences between the untrained participants in the vitamin C and E supplementation group and those in the placebo group (P = 0.98).
more than the trained participants (12.6 ± 6.2%; P < 0.001, untrained versus trained), but there were no differences between the untrained participants in the vitamin C and E supplementation group and those in the placebo group (P = 0.98).
During submaximal running, corresponding to 58 ± 7% and 80 ± 7% of pre-intervention  , putative training effects were slightly larger in the placebo than in the vitamin C and E supplementation group, specifically for heart rate and RER values (Table 4). However, the group differences only tended towards statistical significance (P = 0.08–0.09; effect size = 0.5 for both variables).
, putative training effects were slightly larger in the placebo than in the vitamin C and E supplementation group, specifically for heart rate and RER values (Table 4). However, the group differences only tended towards statistical significance (P = 0.08–0.09; effect size = 0.5 for both variables).
Table 4.
Changes in oxygen uptake (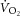 ), heart rate (HR), respiratory exchange rate (RER) and lactate during submaximal workloads at approximately 60% and 80% of
), heart rate (HR), respiratory exchange rate (RER) and lactate during submaximal workloads at approximately 60% and 80% of  at baseline
at baseline
| Vitamin C and E group |
Placebo group |
||||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Change, % | Pre | Post | Change, % | P-value* | |
60% of pre 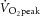
| |||||||
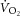 (ml·min−1·kg−1) (ml·min−1·kg−1) |
30.9 ± 5.9 | 30.4 ± 6.3 | −1.4 ± 8.7 | 30.3 ± 4.5 | 29.1 ± 5.2 | −3.6 ± 11.3 | 0.430 |
| HR (beats· min−1) | 140.8 ± 13.2 | 136.2 ± 12.7 | −3.0 ± 6.7 | 140.9 ± 17.3 | 131.7 ± 15.9 | −6.3 ± 7.2‡ | 0.095 |
RER (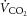 : : 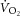 ) ) |
0.89 ± 0.05 | 0.89 ± 0.05 | 0.3 ± 5.4 | 0.91 ± 0.04 | 0.89 ± 0.04 | −1.7 ± 5.1 | 0.168 |
| Lactate (mmol·l) | 1.6 ± 0.9 | 1.3 ± 0.5 | −3.5 ± 33.4 | 1.5 ± 0.9 | 1.3 ± 0.7 | −6.1 ± 32.5 | 0.776 |
80% of pre 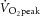
| |||||||
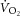 (ml·min−1·kg−1) (ml·min−1·kg−1) |
42.4 ± 7.9 | 42.5 ± 8.9 | −0.1 ± 6.7 | 41.7 ± 5.2 | 41.4 ± 6.0 | −0.4 ± 9.0 | 0.919 |
| HR (beats· min−1) | 170.1 ± 11.1 | 165.0 ± 13.3 | −2.9 ± 5.9 | 169.8 ± 15.5 | 161.6 ± 14.6 | −4.7 ± 4.4‡ | 0.214 |
RER (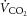 : : 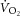 ) ) |
0.93 ± 0.04 | 0.92 ± 0.05 | −1.5 ± 5.3 | 0.95 ± 0.04 | 0.91 ± 0.03 | −3.9 ± 4.5‡ | 0.083 |
| Lactate (mmol·l) | 3.8 ± 2.2 | 2.5 ± 1.3 | −27.4 ± 25.1‡ | 3.3 ± 2.2 | 2.4 ± 1.3 | −18 ± 26.0‡ | 0.270 |
Values are mean ± s.d.
P-values for between-group differences in percentage change. Within-group changes:
P < 0.05;
P < 0.01.
The COX4 protein content in membrane fractions (including the mitochondrial components) of samples from the m. vastus lateralis increased with training only in the placebo group (P = 0.01). A similar trend was seen in COX4 mRNA levels from muscle biopsies (Fig. 5).
Figure 5. Percentage changes in COX4 mRNA, COX4 (protein), HSP60 mRNA and HSP60 (protein) in the vitamin C and E group and the placebo group.
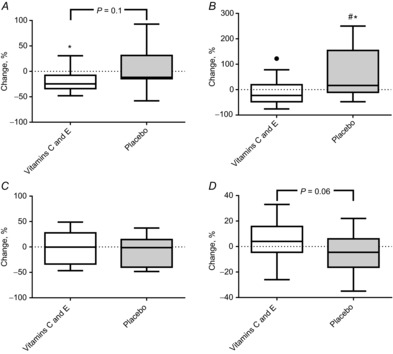
A, COX4 mRNA. B, COX4 (protein). C, HSP60 mRNA. D, HSP60 (protein). Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); *, within-group changes. Exact P-values denote tendencies for group differences.
The PGC-1α mRNA levels increased during training only in the vitamin C and E supplementation group (Fig. 6), but no significant changes were found for PGC-1α protein content in either the cytosol or nuclear fractions in either group. However, a small but significant group difference was found for the change in PGC-1α protein levels in the cytosolic fraction (P = 0.03).
Figure 6. Percentage changes in PGC-1α mRNA and PGC-1α in cytosol and nuclear fractions in the vitamin C and E group and the placebo group.
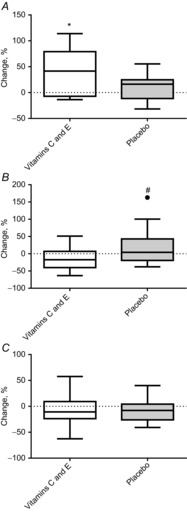
A, PGC-1α mRNA. B, PGC-1α in cytosol. C, PGC-1α in nuclei. Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); #, difference between groups; *, within-group changes.
HSP60 and HSP70 did not change significantly during training at either the mRNA level (some data given in Fig. 5) or protein level in the cytosolic and nuclear fractions (Fig. 7).
Figure 7. Percentage changes in HSP60 and HSP70 levels in cytosol and nuclear fractions in the vitamin C and E group and the placebo group.
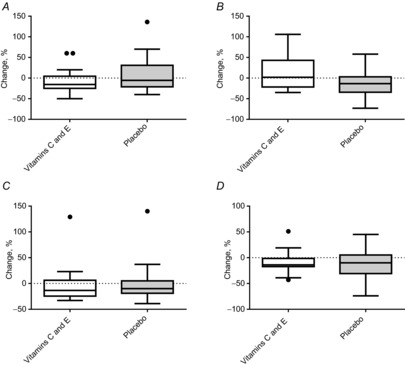
A, HSP60 in cytosol. B, HSP60 in nuclei. C, HSP70 in cytosol. D, HSP70 in nuclei. Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule).
The mRNA levels of CDC42 and MAPK1 decreased in the vitamin C and E supplementation group; these changes differed significantly from those in the placebo group (P ≤ 0.05) (Fig. 8).
Figure 8. Percentage changes in CDC42 mRNA and MAPK1 mRNA in the vitamin C and E group and the placebo group.
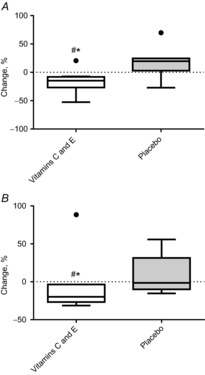
A, CDC42 mRNA. B, MAPK1 mRNA. Boxplots show maximum–minimum values, 25–75th quartiles, and medians. ●, outliers (Tukey's rule); #, difference between groups; *, within-group changes.
With no group differences in mRNA levels, vascular endothelial growth factor (VEGF) mRNA (P = 0.018) and CRYAB mRNA (αB-crystallin) (P = 0.018) decreased in the placebo group. (See online supporting information for this article for results for all analysed genes.)
No changes or group differences were found for fibre cross-sectional area or capillarisation (Table 5). When data for both groups were combined, a trend towards an increased proportion of type 2 fibres emerged (P = 0.08).
Table 5.
Fibre type distribution, fibre area, and capillarisation
| Vitamin C and E group |
Placebo group |
||||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Change, % | Pre | Post | Change, % | P-value | |
| Fibre type 1 (%) | 54 ± 12 | 51 ± 12 | −3.9 ± 22.9 | 49 ± 13 | 44 ± 11 | −7.4 ± 28.5 | 0.124 |
| CSA (μm2) fibre type 1 | 5070 ± 1614 | 5202 ± 1409 | 5.6 ± 22.1 | 5021 ± 1702 | 4893 ± 1206 | 3.7 ± 35.2 | 0.455 |
| CAF fibre type 1 | 4.4 ± 0.9 | 4.4 ± 0.9 | −0.6 ± 13.1 | 4.1 ± 0.8 | 4.2 ± 0.7 | 1.3 ± 13.6 | 0.774 |
| CAFA fibre type 1 | 0.9 ± 0.2 | 0.9 ± 0.2 | −1.6 ± 26.0 | 0.9 ± 0.2 | 0.9 ± 0.3 | 7.0 ± 35.9 | 0.746 |
| CSA (μm2) fibre type 2 | 4831 ± 1646 | 5245 ± 2048 | 11.3 ± 34.3 | 5845 ± 2207 | 6019 ± 2368 | 4.5 ± 34.2 | 0.234 |
| CAF fibre type 2 | 3.8 ± 1.0 | 3.8 ± 1.0 | 3.1 ± 17.1 | 4.0 ± 0.7 | 4.0 ± 0.9 | 0.7 ± 14.4 | 0.730 |
| CAFA fibre type 2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.0 ± 30.3 | 0.7 ± 0.2 | 0.8 ± 0.5 | 10.4 ± 57.0 | 0.579 |
Values are mean ± s.d. CAF, capillaries around each fibre; CAFA, CAF/fibre area. *P-value for between-group difference in percentage change.
Discussion
In the present study, we investigated the effects of vitamin C and E supplementation on adaptations to endurance exercise during an 11 week double-blind, randomised, controlled trial (n = 54). The main findings were that the supplementation blunted the training-induced upregulation of cytosolic PGC-1α and the mitochondrial COX4 protein in m. vastus lateralis, without altering training-induced improvements in  or running performance. The supplementation decreased gene expression of the signalling proteins CDC42 and MAPK1, but did not alter stress proteins or capillarisation.
or running performance. The supplementation decreased gene expression of the signalling proteins CDC42 and MAPK1, but did not alter stress proteins or capillarisation.
Cellular effects
Although conflicting results exist, animal models have demonstrated that high dosages of antioxidant supplements can shut down specific (redox-sensitive) cell signalling pathways and thereby decrease the synthesis of new muscle mitochondria and endogenous antioxidant production (Kang et al. 2009; Hawley et al. 2011; Strobel et al. 2011; Villanueva & Kross, 2012; Feng et al. 2013). Importantly, both the health benefits and improved athletic performance that occur in response to endurance training seem to depend on such cellular adaptations (Coffey & Hawley, 2007; Ristow & Zarse, 2010). Using human participants, we herein provide novel evidence that high dosages of vitamin C and E reduce the endurance training-induced increase of COX4 (in the m. vastus lateralis), which suggests a blunted mitochondrial biogenesis. It was not possible to decipher the exact mechanism behind this effect. However, as suggested by Ristow et al. (2009, 2010), we assume that the antioxidants attenuated the generation of reactive oxygen and/or nitrogen species (RONS), and thereby inhibited redox-sensitive signalling and blunted the induction of genes such as PGC-1α (as discussed further below).
Our observations conflict with findings in a recent human study by Yfanti et al. (2010), who reported that supplementation with vitamin C and E did not alter training adaptations, as assessed by changes in CS and β-HAD activity in m. vastus lateralis. A plausible explanation for this discrepancy could be that Yfanti et al. (2010) used a vitamin C supplement of 500 mg day−1, rather than the 1000 mg day−1 used in the present study. Furthermore, our participants were instructed to take the supplements in two doses (half dosage: 500 mg vitamin C and 117.5 mg vitamin E) 1–3 h before and within 1 h after each exercise session. By contrast, participants in the study by Yfanti et al. (2010) consumed their vitamin supplement only at breakfast. Given the pharmacokinetics of vitamin C in plasma [which decrease within a few hours; (Padayatty et al. 2004)], this might have caused a different cellular response to the supplementation.
We and others (Morton et al. 2009a; Feng et al. 2013) have used COX4 as a marker of mitochondrial content, and COX4 and total mitochondrial contents are found to correlate significantly (Larsen et al. 2012). Nevertheless, as a surrogate marker for mitochondrial content, COX4 content is not directly comparable with changes in enzyme activity, such as in CS as measured by Yfanti et al. (2010).
Mitochondrial biogenesis seems to be primarily regulated by PGC-1α, which controls the expression of both nuclear and mitochondrial gene transcription through proteins such as NFR1/2 and TFAM (Lanza & Sreekumaran, 2010). The upstream activators of PGC-1α comprise MAPK (p38 and ERK1/2) and AMPK (Lanza & Sreekumaran, 2010; Hawley et al. 2011). In the present study, we observed that vitamin C and E supplementation blunted any rise in muscle cytosolic PGC-1α levels and lowered gene expression of CDC42 and MAPK1 (ERK2). These responses are consistent with the changes observed for COX4. By contrast, PGC-1α mRNA was increased only in the vitamin C and E supplementation group, and nuclear PGC-1α protein levels were unchanged in both groups. Further complicating the issue, others have recently reported that PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in mice (Rowe et al. 2012).
Notably, our biopsies were collected 2–4 days after the last training session and thus they do not reflect any immediate activation, subcellular movement of proteins (e.g. nuclear translocation of PGC-1α) or gene expression during exercise.
CDC42 is a member of the Rho family of small GTPases (Jaffe & Hall, 2005). Among various functions, CDC42 exerts certain effects via MAPKs (Maillet et al. 2009) and has been shown to be ROS-sensitive (Li et al. 2009). Nielsen et al. (2010) reported no changes in protein levels of CDC42 in response to 12 weeks of endurance training, but a decrease with cessation of training. Cessation of training is certainly strongly associated with a decrease in muscular fitness, including mitochondrial capacity (Henriksson, 1992). Accordingly, the lower CDC42 gene expression may reflect an adverse effect of the vitamin C and E supplementation, further supporting the negative effect observed on COX4 levels, and shedding light on possible mechanisms for antioxidant interactions.
There were no significant changes in HSP60 and HSP70 levels (mRNA or cytosolic and nucleic protein). This suggests the absence of any accumulation of cellular stress during endurance training, with or without C and E vitamin supplementation (Morton et al. 2009b). Stable HSP levels contrast with the observations of previous studies (Liu et al. 2006; Morton et al. 2009b). This difference may reflect the fact that our participants (from whom we collected muscle biopsies) were recreational endurance athletes as they entered the study (Morton et al. 2009b). Similarly, the training status of the participants was probably the reason for the stability in capillary density.
 and performance
and performance
The various cellular effects of the vitamin C and E supplementation are interesting, but performance outcomes are more important for athletes. Thus, in contrast to the cellular observations, the increases in  (∼8%) and improvements in running performance (20 m shuttle run test; ∼10–14%) were similar in both groups. This is in line with recent human studies in which an increase in
(∼8%) and improvements in running performance (20 m shuttle run test; ∼10–14%) were similar in both groups. This is in line with recent human studies in which an increase in  attributable to endurance training was unaffected by vitamin C and E supplementation (Aguilo et al. 2007; Yfanti et al. 2010; Roberts et al. 2011). Interestingly, Gomez-Cabrera et al. (2008) reported that rats supplemented with vitamin C showed the same increases in
attributable to endurance training was unaffected by vitamin C and E supplementation (Aguilo et al. 2007; Yfanti et al. 2010; Roberts et al. 2011). Interestingly, Gomez-Cabrera et al. (2008) reported that rats supplemented with vitamin C showed the same increases in  as placebo-treated animals. However, the vitamin C supplementation strongly suppressed improvements in endurance performance (running to exhaustion). No group differences were detected in the present study, yet it is intriguing to note that the four participants with the largest improvements in running performance were all in the placebo group (effect size = 0.3 in favour of the placebo group). Although speculative, this may suggest that there are considerable inter-individual differences in the effects of vitamin C and E supplementation. However, subgroup analyses showed no effect of initial training status or gender on gains in
as placebo-treated animals. However, the vitamin C supplementation strongly suppressed improvements in endurance performance (running to exhaustion). No group differences were detected in the present study, yet it is intriguing to note that the four participants with the largest improvements in running performance were all in the placebo group (effect size = 0.3 in favour of the placebo group). Although speculative, this may suggest that there are considerable inter-individual differences in the effects of vitamin C and E supplementation. However, subgroup analyses showed no effect of initial training status or gender on gains in  or running performance during the training period (data not shown).
or running performance during the training period (data not shown).
In further support of the (mild) negative effects of the vitamin C and E supplementation, we observed improved fat oxidation (indicated by reduced RER values) and reduced heart rates at submaximal workloads in the placebo group, but no significant changes were detected in the vitamin C and E group. The group differences were of moderate effect size and did not reach statistical significance (P = 0.08–0.09). Theoretically, improved fat oxidation at steady state submaximal workloads may reflect both a selective upregulation of enzymes, such as β-HAD, or a gross increase in mitochondrial mass, or both (Spina et al. 1996). Unfortunately, we did not measure cellular markers for fat oxidation; however, our observation of a group difference in COX4 levels, indicating increased levels of mitochondrial proteins, may be related to the RER findings.
Although we recruited a high number of participants, compared with similar studies (Nikolaidis et al. 2012), this study may have been insufficiently powered to detect small but potentially true biological effects (e.g. changes in RER values and running performance). For these variables, we had only 30–45% power to detect statistical group differences of the 3–4% observed.
Vitamin C and E in plasma and changes in 8-iso PGF-2α
Plasma measurements supported the efficiency of the vitamin C and E supplementation, albeit that vitamin C and E levels among our young, healthy participants were in the upper range of reference values at baseline (Karlsen et al. 2005; Gomez-Cabrera et al. 2008; Yfanti et al. 2010; Braakhuis et al. 2014).
It is interesting that vitamin C and E supplementation inhibited an elevation of 8-iso PGF-2α, which is an established oxidative stress marker (Basu & Helmersson, 2005), that occurred in the placebo group. Vitamin C and E supplements (alone) have been found to reduce 8-iso PGF-2α levels (Basu & Helmersson, 2005), although, intriguingly, vitamin E has been shown to act as a pro-oxidant in certain experiments (Bowry et al. 1992; Abudu et al. 2004). Endurance training has been found to lower 8-iso PGF-2α plasma concentration, especially in individuals with initially high levels (Roberts et al. 2002; Campbell et al. 2010; Arikawa et al. 2013). Contrary to these training studies, we observed an increase in the placebo group. This increase may be explained by the intensive, high-frequency running programme for participants with normal baseline 8-iso PGF-2α levels.
Supplement considerations
Our participants were supplemented with DL-α-tocopherol acetate, the synthetic form of vitamin E. The bioavailability and biological action of natural D-α-tocopherol/RRR-α-tocopherol may differ (Traber et al. 1994; Burton et al. 1998). Thus, we must be careful when comparing our results with those of studies that have administered the natural form of vitamin E. Concerning vitamin C, there seem to be no differences in blood and tissue bioavailability of synthetic and natural or flavonoid-rich vitamin C (Carr et al. 2013).
Conclusion
Vitamin C and E supplementation did not affect the endurance training-induced increases in  and running performance (20 m shuttle test). However, at the muscle cellular level, the supplementation blunted the training-induced increase in mitochondrial COX4 protein content. Group differences in PGC-1α (cytosolic protein level), and CDC42 and MAPK1 mRNA levels provide further evidence that antioxidant supplementation may interfere with exercise-induced cell signalling in skeletal muscle. Moreover, the cellular results appeared to some degree to be reflected in physiological adaptations, as measured under submaximal workloads (heart rate and RER). Thus, supplementation with high dosages of vitamin C and E appears to diminish some of the endurance training-induced adaptations in human skeletal muscles. We suggest that high dosages of isolated antioxidants should be used with caution in individuals who are simultaneously engaged in endurance training.
and running performance (20 m shuttle test). However, at the muscle cellular level, the supplementation blunted the training-induced increase in mitochondrial COX4 protein content. Group differences in PGC-1α (cytosolic protein level), and CDC42 and MAPK1 mRNA levels provide further evidence that antioxidant supplementation may interfere with exercise-induced cell signalling in skeletal muscle. Moreover, the cellular results appeared to some degree to be reflected in physiological adaptations, as measured under submaximal workloads (heart rate and RER). Thus, supplementation with high dosages of vitamin C and E appears to diminish some of the endurance training-induced adaptations in human skeletal muscles. We suggest that high dosages of isolated antioxidants should be used with caution in individuals who are simultaneously engaged in endurance training.
Key points
Recent studies have indicated that antioxidant supplementation may blunt adaptations to exercise, such as mitochondrial biogenesis induced by endurance training. However, studies in humans are sparse and results are conflicting.
Isolated vitamin C and E supplements are widely used, and unravelling the interference of these vitamins in cellular and physiological adaptations to exercise is of interest to those who exercise for health purposes and to athletes.
Our results show that vitamin C and E supplements blunted the endurance training-induced increase of mitochondrial proteins (COX4), which is important for improving muscular endurance.
Training-induced increases in
 and running performance were not detectably affected by the supplementation.
and running performance were not detectably affected by the supplementation.The present study contributes to understanding of how antioxidants may interfere with adaptations to exercise in humans, and the results indicate that high dosages of vitamins C and E should be used with caution.
Acknowledgments
The authors thank Jonathan M. Peake for valuable comments and language corrections in the manuscript.
Additional information
Competing interests
None declared.
Author contributions
G.P., T.R., J.H., H.B.B., B.R.R., O.S., A.S. and R.B. contributed to the study conception and design. G.P, T.R., J.H., O.S., A.S., K.T.C., G.H., I.P., N.E.B., H.N.Ø., C.B., M.M., F.F., H.W., E.T.U. and I.G. contributed to data analysis and interpretation. All authors contributed to the drafting and critical revision of the paper and approved the final manuscript for publication.
Funding
The study was partly funded by Smartfish AS (Oslo, Norway), Sana Pharma AS (Oslo, Norway) and the Norwegian Olympic Federation.
References
- Abudu N, Miller JJ, Attaelmannan M, Levinson SS. Vitamins in human arteriosclerosis with emphasis on vitamin C and vitamin E. Clin Chim Acta. 2004;339:11–25. doi: 10.1016/j.cccn.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Aguilo A, Tauler P, Sureda A, Cases N, Tur J, Pons A. Antioxidant diet supplementation enhances aerobic performance in amateur sportsmen. J Sports Sci. 2007;25:1203–1210. doi: 10.1080/02640410600951597. [DOI] [PubMed] [Google Scholar]
- Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res. 2012;32:479–485. doi: 10.1016/j.nutres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Arikawa AY, Thomas W, Gross M, Smith A, Phipps WR, Kurzer MS, Schmitz KH. Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp Clin Trials. 2013;34:212–217. doi: 10.1016/j.cct.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastani NE, Gundersen TE, Blomhoff R. Determination of 8-epi PGF2α concentrations as a biomarker of oxidative stress using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:2885–2890. doi: 10.1002/rcm.4197. [DOI] [PubMed] [Google Scholar]
- Bastani NE, Kostovski E, Sakhi AK, Karlsen A, Carlsen MH, Hjeltnes N, Blomhoff R, Iversen PO. Reduced antioxidant defense and increased oxidative stress in spinal cord injured patients. Arch Phys Med Rehabil. 2012;93:2223–2228. doi: 10.1016/j.apmr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7:221–235. doi: 10.1089/ars.2005.7.221. [DOI] [PubMed] [Google Scholar]
- Black AE, Goldberg GR, Jebb SA, Livingstone MB, Cole TJ, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Eur J Clin Nutr. 1991;45:583–599. [PubMed] [Google Scholar]
- Bowry VW, Ingold KU, Stocker R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem J. 1992;288:341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakhuis AJ. Effect of vitamin C supplements on physical performance. Curr Sports Med Rep. 2012;11:180–184. doi: 10.1249/JSR.0b013e31825e19cd. [DOI] [PubMed] [Google Scholar]
- Braakhuis AJ, Hopkins WG, Lowe TE. Effects of dietary antioxidants on training and performance in female runners. Eur J Sport Sci. 2014 doi: 10.1080/17461391.2013.785597. [in press; doi: 10.1080/17461391.2013.785597] [DOI] [PubMed] [Google Scholar]
- Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue α-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010;42:1448–1453. doi: 10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AC, Bozonet SM, Pullar JM, Simcock JW, Vissers MC. A randomized steady-state bioavailability study of synthetic versus natural (kiwifruit-derived) vitamin C. Nutrients. 2013;5:3684–3695. doi: 10.3390/nu5093684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37:737–763. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- Feng H, Kang C, Dickman JR, Koenig R, Awoyinka I, Zhang Y, Ji LL. Training-induced mitochondrial adaptation: role of peroxisome proliferator-activated receptor γ coactivator-1α, nuclear factor-κB and β-blockade. Exp Physiol. 2013;98:784–795. doi: 10.1113/expphysiol.2012.069286. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gibala MJ. High-intensity interval training: a time-efficient strategy for health promotion? Curr Sports Med Rep. 2007;6:211–213. [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Ristow M, Vina J. Antioxidant supplements in exercise: worse than useless? Am J Physiol Endocrinol Metab. 2012;302:E476–E477. doi: 10.1152/ajpendo.00567.2011. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Burke LM, Phillips SM, Spriet LL. Nutritional modulation of training-induced skeletal muscle adaptations. J Appl Physiol. 2011;110:834–845. doi: 10.1152/japplphysiol.00949.2010. [DOI] [PubMed] [Google Scholar]
- Henriksson J. Effects of physical training on the metabolism of skeletal muscle. Diabetes Care. 1992;15:1701–1711. doi: 10.2337/diacare.15.11.1701. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-γ coactivator-1α signalling is redox sensitive. Free Radic Biol Med. 2009;47:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Karlsen A, Blomhoff R, Gundersen TE. High-throughput analysis of vitamin C in human plasma with the use of HPLC with monolithic column and UV-detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;824:132–138. doi: 10.1016/j.jchromb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Kennedy ET, Luo H, Houser RF. Dietary supplement use pattern of US adult population in the 2007–2008 National Health and Nutrition Examination Survey (NHANES) Ecol Food Nutr. 2013;52:76–84. doi: 10.1080/03670244.2012.706000. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Sreekumaran NK. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol (Oxf) 2010;199:529–547. doi: 10.1111/j.1748-1716.2010.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6:93–101. doi: 10.1080/02640418808729800. [DOI] [PubMed] [Google Scholar]
- Li QF, Spinelli AM, Tang DD. Cdc42GAP, reactive oxygen species, and the vimentin network. Am J Physiol Cell Physiol. 2009;297:C299–C309. doi: 10.1152/ajpcell.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gampert L, Nething K, Steinacker JM. Response and function of skeletal muscle heat shock protein 70. Front Biosci. 2006;11:2802–2827. doi: 10.2741/2011. [DOI] [PubMed] [Google Scholar]
- Maillet M, Lynch JM, Sanna B, York AJ, Zheng Y, Molkentin JD. Cdc42 is an antihypertrophic molecular switch in the mouse heart. J Clin Invest. 2009;119:3079–3088. doi: 10.1172/JCI37694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Alamo D, Calbet JA. Free radicals and sprint exercise in humans. Free Radic Res. 2014;48:30–42. doi: 10.3109/10715762.2013.825043. [DOI] [PubMed] [Google Scholar]
- Morton JP, Croft L, Bartlett JD, MacLaren DP, Reilly T, Evans L, McArdle A, Drust B. Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J Appl Physiol. 2009a;106:1513–1521. doi: 10.1152/japplphysiol.00003.2009. [DOI] [PubMed] [Google Scholar]
- Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009b;39:643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK, Laye MJ. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588:4029–4037. doi: 10.1113/jphysiol.2010.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis MG, Kerksick CM, Lamprecht M, McAnulty SR. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid Med Cell Longev. 2012;2012:707941. doi: 10.1155/2012/707941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- Peternelj TT, Coombes JS. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 2011;41:1043–1069. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Petroczi A, Naughton DP, Mazanov J, Holloway A, Bingham J. Performance enhancement with supplements: incongruence between rationale and practice. J Int Soc Sports Nutr. 2007;4:19. doi: 10.1186/1550-2783-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106:2530–2532. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Beattie K, Close GL, Morton JP. Vitamin C consumption does not impair training-induced improvements in exercise performance. Int J Sports Physiol Perform. 2011;6:58–69. doi: 10.1123/ijspp.6.1.58. [DOI] [PubMed] [Google Scholar]
- Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One. 2012;7:e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Astrand PO. Maximal oxygen uptake in athletes. J Appl Physiol. 1967;23:353–358. doi: 10.1152/jappl.1967.23.3.353. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol (1985) 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2011;43:1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- Traber MG, Ramakrishnan R, Kayden HJ. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-α-tocopherol. Proc Natl Acad Sci U S A. 1994;91:10005–10008. doi: 10.1073/pnas.91.21.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva C, Kross RD. Antioxidant-induced stress. Int J Mol Sci. 2012;13:2091–2109. doi: 10.3390/ijms13022091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc. 2010;42:1388–1395. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- Yfanti C, Fischer CP, Nielsen S, Akerstrom T, Nielsen AR, Veskoukis AS, Kouretas D, Lykkesfeldt J, Pilegaard H, Pedersen BK. Role of vitamin C and E supplementation on IL-6 in response to training. J Appl Physiol. 2012;112:990–1000. doi: 10.1152/japplphysiol.01027.2010. [DOI] [PubMed] [Google Scholar]
- Yfanti C, Nielsen AR, Akerstrom T, Nielsen S, Rose AJ, Richter EA, Lykkesfeldt J, Fischer CP, Pedersen BK. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am J Physiol Endocrinol Metab. 2011;300:E761–E770. doi: 10.1152/ajpendo.00207.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


