Phosphatidylinositol 4,5-bisphosphate has a direct role in regulating receptor-operated TRPC channel activation and inactivation.
Abstract
Transient receptor potential classical (or canonical) (TRPC)3, TRPC6, and TRPC7 are a subfamily of TRPC channels activated by diacylglycerol (DAG) produced through the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) by phospholipase C (PLC). PI(4,5)P2 depletion by a heterologously expressed phosphatase inhibits TRPC3, TRPC6, and TRPC7 activity independently of DAG; however, the physiological role of PI(4,5)P2 reduction on channel activity remains unclear. We used Förster resonance energy transfer (FRET) to measure PI(4,5)P2 or DAG dynamics concurrently with TRPC6 or TRPC7 currents after agonist stimulation of receptors that couple to Gq and thereby activate PLC. Measurements made at different levels of receptor activation revealed a correlation between the kinetics of PI(4,5)P2 reduction and those of receptor-operated TRPC6 and TRPC7 current activation and inactivation. In contrast, DAG production correlated with channel activation but not inactivation; moreover, the time course of channel inactivation was unchanged in protein kinase C–insensitive mutants. These results suggest that inactivation of receptor-operated TRPC currents is primarily mediated by the dissociation of PI(4,5)P2. We determined the functional dissociation constant of PI(4,5)P2 to TRPC channels using FRET of the PLCδ Pleckstrin homology domain (PHd), which binds PI(4,5)P2, and used this constant to fit our experimental data to a model in which channel gating is controlled by PI(4,5)P2 and DAG. This model predicted similar FRET dynamics of the PHd to measured FRET in either human embryonic kidney cells or smooth muscle cells, whereas a model lacking PI(4,5)P2 regulation failed to reproduce the experimental data, confirming the inhibitory role of PI(4,5)P2 depletion on TRPC currents. Our model also explains various PLC-dependent characteristics of channel activity, including limitation of maximum open probability, shortening of the peak time, and the bell-shaped response of total current. In conclusion, our studies demonstrate a fundamental role for PI(4,5)P2 in regulating TRPC6 and TRPC7 activity triggered by PLC-coupled receptor stimulation.
INTRODUCTION
Transient receptor potential classical/canonical (TRPC) 3, C6, and C7 channels are the closest mammalian homologues of the Drosophila melanogaster TRP channel and are expressed in various cell types, including smooth muscle and neurons (Hardie, 2003; Inoue et al., 2006; Venkatachalam and Montell, 2007). These channels conduct cations (Na+, Ca2+) in response to stimulation of receptors coupled to phospholipase C (PLC), namely Gq protein–coupled receptors and certain tyrosine kinase receptors (Ramsey et al., 2006). For that reason, the currents mediated by these channels are often called receptor-operated cation currents (Inoue and Kuriyama, 1993; Firth et al., 2007). TRPC3/6/7 channels are also activated by synthetic membrane-permeable diacylglycerol (DAG) analogues and are thus considered to be DAG-sensitive or activated channels (Hofmann et al., 1999; Okada et al., 1999). In a physiological context, DAG is produced by the hydrolytic activity of PLC, which is located downstream of the receptors for neurotransmitters and hormones. Therefore, the receptor stimulation activates TRPC3/6/7 channels through the production of DAG to generate the receptor-operated TRPC currents (Beech et al., 2004; Panda et al., 2005; Hartmann et al., 2008).
Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2, or PIP2) is the major substrate of PLC, and its hydrolysis produces DAG. PI(4,5)P2 is known to regulate numerous ion channels, modulating electrical signal outputs from metabotropic receptors in diverse physiological contexts (Gamper and Shapiro, 2007; Hilgemann, 2007; Logothetis et al., 2007). However, knowledge concerning the effect of PI(4,5)P2 on TRPC channels is still accumulating (Jardín et al., 2008; Lemonnier et al., 2008; Monet et al., 2012). We have recently demonstrated using Danio rerio voltage-sensing phosphatase (DrVSP) that reduction or depletion of PI(4,5)P2 inhibits the activity of DAG-sensitive TRPC3/6/7 channels, both in an exogenous expression system and in smooth muscle–derived cells (A7r5) (Imai et al., 2012; Itsuki et al., 2012a). The DrVSP-mediated inhibition of TRPC3/6/7 currents was detected even in currents evoked by a membrane-permeable DAG analogue (OAG), which suggests that reduction in PI(4,5)P2 can inhibit TRPC3/6/7 channel opening regardless of the presence of DAG. Under Gq protein–coupled receptor stimulation, PI(4,5)P2 hydrolysis (breakdown) by the receptor-activated PLC largely contributes to the production of DAG. Therefore, such a complex relationship of PI(4,5)P2 and DAG suggests that TRPC3/6/7 channel activity may be regulated in a self-limiting manner.
The effects of PLC-coupled receptor-driven channel regulation via enzymatic hydrolysis of PI(4,5)P2 are not known. To improve our understanding, we simultaneously measured PI(4,5)P2 or DAG dynamics and receptor-operated TRPC currents evoked by carbachol (CCh; a muscarinic receptor agonist) or vasopressin (a vasoconstrictor). To this end, we measured the levels of PI(4,5)P2 and DAG using a quantitative Förster resonance energy transfer (FRET)-based sensor alongside detection of TRPC6 and TRPC7 currents, which are more sensitive than TRPC3 currents to reduction in PI(4,5)P2 in human embryonic kidney (HEK)293 cells and smooth muscle–derived cells. We found that the temporal FRET dynamics of PI(4,5)P2 reduction closely correlate with the time course of activation and inactivation of receptor-operated TRPC6/7 channel currents. We also constructed a kinetic model of receptor-driven PI(4,5)P2–DAG signaling. This model, calibrated with DrVSP-derived functional dissociation constants for PI(4,5)P2 binding to TRPC3/6/7 channels, closely resembled our experimental results. Our study, combining experimental and computer simulation data, revealed the crucial role of receptor-stimulated PI(4,5)P2 hydrolysis in TRPC6/7 currents. Part of the work presented here has appeared in abstract form (Itsuki et al., 2012b).
MATERIALS AND METHODS
Plasmids and cells
The pcDNA3 expression vector encoding human TRPC6 (GenBank accession no. NM_004621) was provided by T. Hofmann (Institut für Pharmakologie und Toxikologie, Zürich, Switzerland); pCI-neo expression vectors encoding mouse TRPC3 (GenBank accession no. NM_019510) and TRPC7 (GenBank accession no. NM_012035) were provided by Y. Mori (Kyoto University, Kyoto, Japan). Single amino acid mutation in TRPC6 and TRPC7 was generated using the QuikChange Site-Directed Mutagenesis kit (Agilent Technologies) according to the manufacturer’s instructions. To generate bright FRET pairs, super-enhanced YFP or CFP isolated from a RhoA FRET sensor (provided by M. Matsuda, Kyoto University, Kyoto, Japan) were modified to give A207K mutants as a monomeric form (CFPmse or YFPmse) (Zacharias et al., 2002). These modified fluorophores were fused to the N-terminal side of the PLCδ Pleckstrin homology domain (PHd; provided by K. Jalink, The Netherlands Cancer Institute, Amsterdam, Netherlands) to construct PI(4,5)P2 sensor molecules consisting of CFPmse-PHd or YFPmse-PHd. For DAG detection, CFPmse was fused to the C-terminal side of PKCε (provided by M. Schaefer, Leipzig University, Leipzig, Germany), yielding PKCε-CFPmse. To be an energy acceptor of membrane-bound PKCE-CFPmse, YFPmse was attached to the C-terminal side of the GAP-43 myristoyl domain (Invitrogen) through an octaglycine (G8) linker (Myr-YFPmse). PI(4,5)P2 and DAG sensor cDNA were each incorporated into an IRES-reporter region–excluded pIRES2 expression vector (Invitrogen). A pEF-BOS expression vector encoding human muscarinic type 1 receptor (M1R) was provided by T. Haga (Gakushuin University, Tokyo, Japan). Human phosphatidylinositol-4-phosphate-5-kinase (PIP5K; β isoform) in pcDNA3.1 vector (Invitrogen) was provided by S. Kita and T. Iwamoto (Fukuoka University, Fukuoka, Japan). All PCR products were sequenced entirely.
HEK293 cells (obtained from the ATCC) were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% FBS (Gibco) and antibiotics (penicillin and streptomycin; Gibco) at 37°C (5% CO2). For transfection, cells were seeded on poly-l-lysine–coated glass coverslips (Matsunami) in 35-mm culture dishes and transfected with a mixture of plasmid vector–incorporated DNAs using the SuperFect transfection reagent (QIAGEN). For FRET-based PI(4,5)P2 detection, HEK293 cells were cotransfected with 1 µg each of plasmids encoding TRPC3, 6, or 7 together with M1R (or without it, in endogenous muscarinic receptor stimulation) and 0.3 µg each of plasmids encoding CFPmse-PHd and YFPmse-PHd. For DAG detection, 0.3 µg each of plasmids encoding Myr-YFPmse and PKCε-CFPmse were cotransfected instead of the PI(4,5)P2 sensor plasmids. For detection of local PI(4,5)P2 around the TRPC7 channel, the sequence encoding the donor protein (CFPmse) was inserted before the stop codon of TRPC7. In this case, equal amounts (1 µg) of donor, acceptor (YFPmse-PHd), and M1R plasmids were used for transfection. Measurements on transfected cells were made within 24–72 h after transfection.
A7r5 cells, the cell line derived from rat thoracic aortic smooth muscle (Brandt et al., 1976), were obtained from the ATCC, maintained in medium identical to that used for HEK293 cells, and passaged every 5–7 d. The transfection protocol was essentially the same as the one used with HEK293 cells. A7r5 cells transfected with CFPmse-PHd and YFPmse-PHd were reseeded on poly-l-lysine–coated glass coverslips and incubated at 37°C (5% CO2) for at least 15 min before use. Cells were always used within 2 h of reseeding.
Solutions and drugs
The standard external solution contained (mM): 140 NaCl, 5 KCl, 1 CaCl2, 1.2 MgCl2, 10 HEPES, and 10 glucose (pH 7.4, adjusted with Tris base; 300 mOsm, adjusted with glucose). The pipette solution contained (mM): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgCl2, 5 EGTA, 1.5 CaCl2, 10 HEPES, 2 ATP-Na2, 0.1 GTP, and 10 glucose (pH 7.2, adjusted with Tris base; 290–295 mOsm, adjusted with glucose). CCh (Sigma-Aldrich) was diluted in the standard external solution from its stock concentration (100 mM in H2O). To confirm monovalent cationic currents, NMDG solution (150 mM N-methyl-d-glucamine chloride, 10 mM HEPES, and 1 mM CaCl2, with pH 7.4 adjusted with HCl) was applied at the end of each stimulus. RHC80267 (EMD Millipore) was dissolved in DMSO (Wako Chemicals USA). Stock solutions of Arginine8 vasopressin (AVP; 100 µM; MP Biomedicals) and nifedipine (10 mM; EMD Millipore) were dissolved in H2O and DMSO, respectively. AVP and nifedipine were freshly prepared in the standard external solution to final concentrations of 1 and 5 µM, respectively, before applying to A7r5 cells. During experiments, HEK293 and A7r5 cells were continuously perfused with external solution and gravity-fed at a flow rate of 0.25 ml/min. The perfusion was turned on and off using electromagnetic solenoid microvalves (The Lee Co.).
Simultaneous measurements of TRPC currents and FRET
Electrophysiology.
The whole-cell patch-clamp technique was used for current detection. Patch electrodes with a resistance of 4–6 MΩ (when filled with internal solution) were made from 1.5-mm borosilicate glass capillaries (Sutter Instrument). Series resistance errors were compensated >60%. Voltage generation and current signal acquisition were accomplished using a patch-clamp amplifier (AxoPatch 200B; Axon Instruments) with an A/D D/A converter (Digidata 1200; Axon Instruments). Sampled data were low-pass filtered and digitized at 1 kHz using pClamp 9.0 (Axon Instruments) and analyzed using custom-written software (MATLAB; MathWorks). The currents were recorded at a holding potential of −50 mV. For activation of DrVSP, depolarizing step pulses (from 20 to 180 mV, 500-ms duration) were delivered every 20 s. The ratio of the currents before and after DrVSP activation was used to quantitate DrVSP-mediated inhibition, “r (I).” Before its calculation, the leak that was defined by the current in NMDG-containing solution was subtracted. All experiments were performed at room temperature (22–25°C).
FRET detection.
Fluorescence from voltage-clamped cells was detected using a microscope (60 × 0.9 N.A. objective; TE300 Eclipse; Nikon) equipped with a two-channel simultaneous beam-splitter (Dual-View2; Photometrics) and a high sensitivity EMCCD camera (Evolve512; Photometrics). Excitation light filtered at 427/10 and 504/12 nm was alternately introduced via an optical fiber from a lamp house equipped with a high speed excitation wavelength selector (75 W xenon lamp; OSP-EXA; Olympus). Epifluorescence from the cells was prefiltered using a multiband dichroic mirror (449–483 and 530–569 nm) contained in the microscope, and then further separated in the beam-splitter (at 505 nm) and filtered at 464/23 nm (detection of the donor fluorescence) or 542/27 nm (detection of the acceptor fluorescence). Optical filters were obtained from Semrock, except the splitter (Chroma Technology Corp.). The duration of camera exposure was 100 ms and occurred within 150-ms periods of illumination at each excitation wavelength. Images were captured with an EM gain of 300 and then digitized as 512 × 512 pixels by 16-bit arrays in the microscope software (Micro-manager v.1.4). The image pixel resolution was ∼0.26 µm. Averaged intensities from the whole-cell region (typically 20 × 20 to 40 × 40 square pixels) were analyzed to calculate FRET using a custom-written MATLAB program. The electrophysiology and FRET measurements were synchronized using brief triggers from the A/D D/A converter linked to the excitation light shutter. All of the data in this paper were recorded from the first application of any of the agonists.
Calculation of FRET.
Fluorescence signal output obtained from a given sample is denoted by the descriptor FX(Y), where X and Y are the fluorescence filter settings for the emission and excitation light, respectively. The emission filter for donor fluorescence (464 nm) is denoted as “464,” and that for acceptor fluorescence (542 nm) as “542.” The excitation filter for donor excitation (427 nm) is denoted as “D,” and that for acceptor excitation (504 nm) as “A.” Background intensity, captured using the corresponding filter setting with nontransfected cells, was subtracted from specimen fluorescence signals. Finally, the FRET ratio (FR) was calculated according to the “3-cube” method (Erickson et al., 2001):
| (1) |
where RD1 = F542(D)/F464(D), RD2 = F542(A)/F464(D), and RA = F542(D)/F542(A). Constants of RD1, RD2, and RA were predetermined using measurements from single cells expressing only donor- (CFPmse) and acceptor- (YFPmse) tagged molecules, respectively. From the FR values, we can compute the effective FRET efficiency (EEFF):
| (2) |
where εYFPmse and εCFPmse are the molar extinction coefficients for the FRET cube excitation filters obtained using the 427-nm excitation band-pass filter. We determined the ratio in brackets to be 0.11, based on maximal extinction coefficients for YFPmse and CFPmse (Mori et al., 2011).
Establishment of a relationship between PI(4,5)P2 concentration and FRET
Here, we calculated the relationship between FRET and PI(4,5)P2. To this end, we considered two different cases: (1) FRET between TRPC7 channel-anchored CFPmse and YFPmse-PHd, where YFPmse-PHd can transit between a soluble cytoplasmic state and a PI(4,5)P2-bound membrane state; and (2) FRET between CFPmse-PHd and YFPmse-PHd, where both probes transition between a soluble and membrane-bound state.
Case 1 is described by:
| (3) |
where F is the fraction of PHd bound to PI(4,5)P2, Kd(PHd) is the dissociation constant of PHd bound to PI(4,5)P2 (reported as 2.0 µM; Lemmon et al., 1996; Hirose et al., 1999), and FRmax is the maximum FR at an infinitely high concentration of PI(4,5)P2 that induces all the fluorophore-fused PHd probes to bind to the plasma membrane. FRmax is a purely theoretical value, because physiological PI(4,5)P2 levels (5–40 µM) are insufficient to localize all PHd proteins to the plasma membrane (Bunce et al., 1993; McLaughlin and Murray, 2005). Nevertheless, although FRmax is impossible to demonstrate, overexpression of PIP5K can greatly increase the cellular levels of PI(4,5)P2 by approximately two- to threefold (Winks et al., 2005). We observed that cells overexpressing PIP5K demonstrated ∼1.2 times higher FR than control cells with resting PI(4,5)P2 levels (Fig. 5 A). We thus calculated the FRmax value by multiplying the resting FR by this correction factor of 1.2. Eq. 3 was used to simulate FR dynamics in cells expressing TRPC7-CFPmse and YFPmse-PHd, as shown in Eq. 25 (Table 3) and Fig. 7 D. Conversion of the FRET between CFPmse-PHd and YFPmse-PHd to PI(4,5)P2 concentration (i.e., case 2) is described in the Results.
Figure 5.
Functional dissociation constants of PI(4,5)P2 binding to TRPC3/6/7 channels. (A) Comparison of FR or EEFF in the resting condition between cells expressing TRPC6 channel and CFPmse-PHd and YFPmse-PHd (control), and those overexpressing PIP5K (+PIP5K). The FR of cells overexpressing PIP5K increased on average by ∼1.2-fold compared with control cells. *, P < 0.05; unpaired t test. (B) Steady-state plots for estimating the functional Kd of PI(4,5)P2 binding to TRPC3/6/7 channels. Horizontal axis indicates the estimated PI(4,5)P2 concentration based on the conversion from FR to PI(4,5)P2, according to Eq. 5.
Table 3.
Equations for the back-calculated FR
| Comment to the equation | Equation | No. |
| Fraction of IP3 bound PHd (PHd): | 21 | |
| Concentration of membrane bound PHd: | 22 | |
| Concentration of cytosolic PHd upon PI(4,5)P2 reduction: | 23 | |
| Concentration of IP3 bound PHd: | 24 | |
| Norm.FR between TRPC7-CFPmse and YFPmse-PHd: | 25 | |
| Norm.FR between CFPmse-PHd and YFPmse-PHd: | 26 |
Figure 7.
SPD model fitting to experimentally observed TRPC6/7 currents and AVP-evoked TRPC6/7-like currents in A7r5 cells. (A) Fitting of the receptor-operated TRPC6 current simultaneously measured with PI(4,5)P2 by FRET with the SPD model (top; light blue trace). The experimental data were obtained from the low strength of receptor stimulation carried by CCh application (100 µM) to endogenous muscarinic receptor in HEK293 cells. Back-calculated FR of PI(4,5)P2 concentrations at the respective points (bottom; solid) was normalized against the time zero FR (Norm.FR; middle; light blue), and that was overlaid on the experimental Norm.FR (middle; circles). The bottom panel displays the fitting resultant changes in PI(4,5)P2 (solid), DAG (dashed), and PA (thin dashed). The similarity of Norm.FR was assessed by SD as described in Materials and methods. (B) Fitting of experimental TRPC6 current data from M1R-overexpressing cells. The initial parameters are identical to those in A. (C) Fitting of experimental TRPC6/7-like currents recorded from A7r5 aorta–derived smooth muscle cells. The currents were evoked by 1 µM AVP. The inset in the top panel shows A7r5 cells expressing the PI(4,5)P2 sensor. (D) Fitting of TRPC7 currents. Local PI(4,5)P2 dynamics were detected using a FRET donor linked to the TRPC7 channel at the end of its C-terminal domain (top; inset). Transient increments in FRET were detected during the plateau or biphasic response (middle; arrow).
Estimation of expressed fluorophore-tagged PHd proteins
We determined that the average concentration of fluorophore-tagged PHd in single cells was 1.6 µM. This value was calculated based on the intensity from fluorescein (Sigma-Aldrich) as follows. Fluorescein was dissolved in 50 mM borate buffer (pH 9.1). The fluorescence intensities in small droplets of this fluorescein solution were measured, under the same conditions as the cells overexpressing YFPmse-PHd. The respective intensities were standardized by the quantum yields for fluorescein (0.92) and YFPmse (0.57), and then the average [YFPmse-PHd] in living cell was determined to be 0.8 µM. Equal amounts of CFPmse-PHd and YFPmse-PHd plasmids were transfected, so total amounts of CFPmse-PHd and YFPmse-PHd proteins could be extrapolated from this value.
Modeling and fitting statistics
Kinetic models of PI(4,5)P2 hydrolysis pathway by PLC were formulated as ordinary differential equations, and the concentrations of PI(4,5)P2 and DAG derived from these were incorporated into the channel-operation models. Simulations were performed in Excel (Microsoft) using the forward Euler method with a time step of 0.05 s. Individual steps were translated into differential equations based on the proposed kinetic scheme. Fitting the models to the experimental data of TRPC6/7 currents was performed by a Generalized Reduced Gradient algorithm of the Solver function in Excel. Details of model formulations and model fitting to the experimental data are described in the Results.
The errors between the experimental and the back-calculated FR by model fitting to the currents (Figs. 6 and 7) were evaluated by the standard deviations of residual as follows:
where Norm.FR and Norm.FRbk denote the normalized experimental and back-calculated FR at the i points. n indicates the total number of time points (20/s).
Figure 6.
PI(4,5)P2 reaction model and channel gating models (DG and SPD) used for simulations. (A) Minimal PI(4,5)P2–DAG reaction scheme (top). Hydrolysis of PI(4,5)P2 (local) is the first step in this model (ki). The produced DAG can serve as a substrate for DAG kinase, DAG lipase, and DAG acetyltransferase. For simplicity, we refer only to DAG kinase (kii). PA, phosphatidic acid. Further catalytic steps for the producing of CDP-DAG and PI were bound to directly generate PI(4)P (kiii). The PI(4,5)P2 recovery from PI(4)P by PIP5K is referred to as “kiv”. PI(4,5)P2 and DAG concentrations are linked to the three-state DG (bottom left) and the four-state SPD (bottom right) models. (B) Comparison of TRPC6 current data processed through the DG and SPD simulation models with parameters of the accelerated PLC kinetics. The top panel shows that a rapid decay of TRPC6 current was seen with the SPD model (red trace), but not with the DG model (orange trace). The current amplitudes were normalized to their peak currents (Norm.current). The bottom panel shows the simulated dynamics of PI(4,5)P2 (solid line) and DAG (dashed line) concentrations.
Online supplemental material
Plotting the currents versus FRmin/FRresting relationship (Fig. S1). DrVSP-mediated inhibition at the single-channel level (Fig. S2). Diffused PI(4,5)P2 was incorporated in the self-limiting regulation by PI(4,5)P2–DAG signaling (SPD) model-based simulation (Fig. S3). Incomplete matching of the DG model to the experimental FR (Fig. S4). The quick recovery of FR under the AVP stimulation (Fig. S5). Fitting the SPD model to TRPC7 current demonstrated less matching to FR dynamics and vice versa (Fig. S6). Effect of an inactive ATP analogue in the patch pipette on the receptor-operated TRPC7 currents (Fig. S7). The online supplemental material is available at http://www.jgp.org/cgi/content/full/jgp.201311033/DC1.
RESULTS
Simultaneous measurement of PI(4,5)P2 and TRPC currents
The PHd of PLCδ binds both PI(4,5)P2 and inositol 1,4,5-trisphosphate (IP3) (Hirose et al., 1999). In the resting state, however, most of the fluorophore-tagged PHds are located at the plasma membrane, making it possible to measure PI(4,5)P2 at the plasma membrane (van der Wal et al., 2001; Jensen et al., 2009; Yudin et al., 2011) (Fig. 1 A). Using this approach, we first demonstrated the simultaneous measurements of PI(4,5)P2 and receptor-operated TRPC6 current. The FRET pairs, which consisted of donor (CFPmse) or acceptor (YFPmse) fused to the PHd, were coexpressed with TRPC6 channel and M1R in mammalian HEK293 cells (Fig. 1 B). Fig. 1 C shows a typical example of the simultaneous measurement of a TRPC6 current and PI(4,5)P2 levels in a HEK293 cell after stimulation with 10 µM CCh. Soon after CCh application, an inward TRPC6 current (Fig. 1 C, top) and FRET reduction (middle) were observed concurrently.
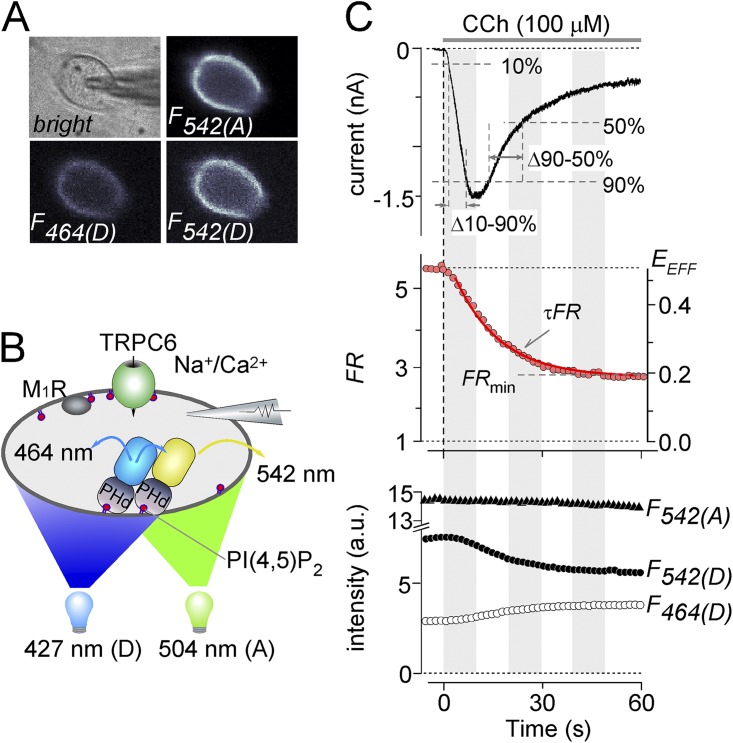
Figure 1.
Simultaneous measurement of receptor-operated TRPC6 currents and PI(4,5)P2 detected by 3-cube FRET. (A) Images of PI(4,5)P2 sensor expressed in HEK293 cells using a 3-cube filter. Phase-contrast image (top left), YFP channel (F542(A); top right), CFP channel (F464(D); bottom left), and FRET channel (F542(D); bottom right) are shown. (B) Diagram of molecules transfected into HEK293 cells. TRPC6, PI(4,5)P2 sensor (CFPmse-PHd [blue box] and YFPmse-PHd [yellow box]), and M1R were expressed. The excitation wavelengths, 427 and 504 nm, were alternately illuminated. (C) Typical example of CCh-induced TRPC6 currents (top) and the corresponding FRET changes (middle). The FR (middle; circles) calculated by 3-cube methods can yield near absolute FRET efficiency (EEFF; right axis). Measured parameters were the duration of Δ10–90% and Δ90–50% of the peak current, the kinetics of FRET decay (τFR), and minimum FR (FRmin). The decline of FRET (FR) was fitted with a single-exponential decay (red solid curve): The bottom panel shows the changes in the fluorescence intensities (a.u.) that passed through the respective filter setting.
To evaluate the kinetics of the current activation and inactivation, we measured the time required for receptor-operated TRPC6 current to increase from 10 to 90% of its peak amplitude and then to decay from 90 to 50% (Fig. 1 C, top). We quantitatively examined the real-time alteration in PI(4,5)P2 level using the 3-cube FRET measurement (described in Materials and methods). FRET changes over time between CFPmse-PHd and YFPmse-PHd (Fig. 1 C, middle) were calculated from the respective fluorescence intensities (Fig. 1 C, bottom). The donor fluorescence (Fig. 1 C, open circles; F464(D)) and resonance fluorescence upon donor excitation (black circles; F542(D)) displayed inverted changes, whereas acceptor fluorescence (filled triangles; F542(A)) stayed largely constant (Fig. 1 C, bottom). These configurations ensured the FRET reduction and low level of quenching of sensor proteins during the recordings. Kinetics of FR reduction (τFR) and the minimum amount of FR (FRmin) under the receptor stimulation were obtained by fitting to the FRET data with a single-exponential decay function (Fig. 1 C, middle).
PI(4,5)P2 dynamics at different levels of receptor stimulation and TRPC6/7 currents
We then explored the effect of PI(4,5)P2 reduction on TRPC6 or TRPC7 channel currents at varying levels of receptor stimulation. Stimulation of endogenous muscarinic receptors in HEK293 cells with 100 µM CCh evoked prolonged TRPC6 current and a small amount of reduction in FR (Fig. 2 A). The time for TRPC6 current activation (Δ10–90%) and inactivation (Δ90–50%) were 32 ± 7 s and 39.2 ± 6.7 s (n = 6), respectively. In accordance with the current time course, τFR was lengthened to 55.3 ± 4.5 s, and FRmin stayed near the resting level. Reduction in FR was only 18 ± 6%. Previous studies have shown that depletion of PI(4,5)P2 can be induced by overexpression of M1R (Xie et al., 2011; Dickson et al., 2013). Consistent with these studies, overexpressing M1R and treating the cells with a high concentration of CCh (100 µM) greatly accelerated the TRPC6 current and FRET reduction (Fig. 2 B) (Δ10–90% = 1.6 ± 0.3 s; Δ90–50% = 6.2 ± 0.9 s; τFR = 4.5 ± 0.9 s), and enhanced the FR reduction with a near zero FRET efficiency (FRmin = 1.15 ± 0.1 and EEFF = 0.016 ± 0.01; n = 11). Intriguingly, the time to reach the peak current was remarkably shortened compared with endogenous muscarinic receptor stimulation at the same concentration of CCh (100 µM; +M1R = 2.8 ± 0.7 s; endo = 63 ± 8 s). Similar tendencies in the FR reduction and the peak time were observed when TRPC7 was expressed instead of TRPC6, except for the shorter activation and inactivation time for TRPC7 current (Fig. 2, C and D). In addition, TRPC7’s τFR was slightly delayed and FRmin was also slightly attenuated compared with that of TRPC6. (Data from the different strength of receptor stimulation was summarized in Fig. 2 E.)
Figure 2.
Correlation between the TRPC6/7 currents and the decay of PI(4,5)P2. (A and B) Example traces of TRPC6 currents (top) and FRET of PI(4,5)P2 sensor (CFPmse-PHd and YFPmse-PHd) (bottom) upon stimulation with 100 µM CCh of either endogenous (A) or overexpressed M1R (B). (C and D) Same as in A and B, but in cells expressing TRPC7. (E) Summary of currents and FRET changes. TRPC6/7 current increase (Δ10–90%) and decay (Δ90–50%), kinetics of FRET reduction (τFR), and degree of FRET reduction (FRmin) were accelerated in a CCh concentration and M1R expression-dependent manner. The data depicted by the stripe and the white bars show without channel expression (transfected only PI(4,5)P2 sensor) and endogenous receptor simulation, respectively. Numbers in parentheses indicate the number of cells measured, here and throughout. (F and G) Time courses of the Δ10–90% and Δ90–50% of receptor-operated currents were plotted against the simultaneously measured τFR (F, TRPC6; G, TRPC7). These data were obtained from various concentrations of CCh or level of M1R expression. The slope with a linear fit highlights a relationship between the time courses and τFR.
To elucidate the functionality of PI(4,5)P2 hydrolysis, we focused on the kinetic relationships between TRPC6/7 currents and FRET reduction. For that purpose, the log–log plots for the activation or the inactivation of TRPC6/7 currents and the values of τFR were made using data obtained at varying levels of agonist stimulation, with and without M1R overexpression (Fig. 2, F and G). These log–log plots showed a clear correlation between τFR and the current time courses (Δ10–90% [left panels] and Δ90–50% [right panels]) for both TRPC6 and TRPC7 currents. The linear relationship that appeared in a plot of the relation of Δ90–50% to τFR in TRPC7-expressing cells showed significantly steeper slopes (slope = 1.33) than that in TRPC6-expressing cells (slope = 0.76) (Fig. 2, F and G). This steepness may reflect higher TRPC7 sensitivity to reduction in PI(4,5)P2 than TRPC3 or TRPC6 (Imai et al., 2012). In addition to the FRET decay, the extent of the reduction or depletion of PI(4,5)P2 levels (FRmin) also showed a similar tendency to the time course of current activation or inactivation of each channel (Fig. S1). These plots, however, were slightly more scattered than those of τFR. Such a scattering was probably caused by cell-to-cell variability in the released IP3 in response to the hydrolysis of PI(4,5)P2 (Irvine and Schell, 2001). The simultaneous detection of PI(4,5)P2 and TRPC currents demonstrated that the activation and inactivation time courses related to both the kinetics and the extent of PI(4,5)P2 reduction.
Simultaneous detection of DAG and receptor-operated TRPC channel current
TRPC3/6/7 channels are DAG-sensitive ion channels, but the manner in which DAG dynamics correlate with these TRP channels’ activity remains largely unknown. To address this question, DAG production was concurrently monitored with receptor-operated TRPC6/7 channel currents. The detection of DAG dynamics relies on membrane translocation of DAG-activated PKC in response to increasing DAG levels at the plasma membrane (Violin et al., 2003) (Fig. 3 A). We used Ca2+-insensitive PKCε as a fluorescence donor molecule to exclude Ca2+-dependent translocation of PKC (Sinnecker and Schaefer, 2004). Upon stimulation with CCh through the endogenous muscarinic receptors, TRPC6 current and DAG production were initiated almost simultaneously (Fig. 3 B). This parallel response is consistent with TRPC6 being a DAG-sensitive channel. Furthermore, during the inactivation of the TRPC6 channel current, DAG level also declined. This synchronicity indicated that when the strength of receptor stimulation was weak, the production of DAG levels seemed to be a critical factor to the current appearances.
Figure 3.
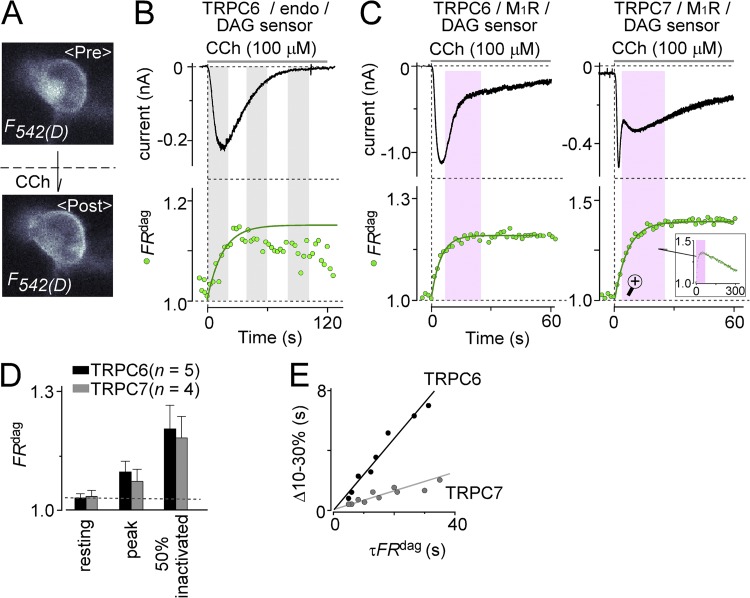
Incompatible correlation between receptor-operated TRPC6/7 currents and DAG production. (A) Principle of DAG detection by PKC probe FRET. Increments in FRET caused by the translocation of PKCε-CFPmse to the plasma membrane were detected by a coexpressed membrane-resident acceptor protein (Mry-YFPmse) in HEK293 cells. (B) Whole-cell TRPC6 currents (top) and FRET changes caused by DAG increments, “FRdag” (bottom, green circles), recorded from endogenous muscarinic receptor stimulation with 100 µM CCh. The rise of FRET was fitted to the exponential equation: (green solid curve). (C) Traces of currents and FRdag in M1R-overexpressing cells with 100 µM CCh. (Left) TRPC6 currents. (Right) TRPC7 currents. Prolonged DAG production was observed. Purple zones indicate inconsistencies between current inactivation and DAG production. The inset in the TRPC7 panel shows FRdag changes over a longer time scale (300 s). (D) Summary of FRdag levels at the respective current points observed in the robust receptor stimulation (+M1R and 100 µM). The black and gray bars denote expression of TRPC6 and TRPC7 channels, respectively. (E) Time courses of initial phase of current increase (Δ10–30%) were plotted against kinetics of DAG production (τFRdag).
Contrary to the synchronicity of the TRPC6 current and DAG dynamics in the weak receptor stimulation, when the cells overexpressed M1R, the simultaneous measurement was revealed to be inconsistent. The activation of TRPC6 channel current paralleled DAG production, whereas the inactivation of the channel did not; there was no decline in DAG production (Fig. 3 C, left, purple zone). This lack of temporal consistency between the current decay and DAG levels was even more prominent in the TRPC7 channel, which exhibited inactivation while DAG levels were still increasing (Fig. 3 C, right, and summarized in D). This inconsistency may be explained by the idea that PKC-mediated phosphorylation inhibits channel opening, which has been proposed for TRPC3 (Soboloff et al., 2007).
However, for TRPC6 channel, there was no significant difference in the current inactivation (Δ90–50%) between cells overexpressing PKCε (TRPC6, M1R, and DAG sensor–expressing, 7.5 ± 2.5 s; n = 5) and control (TRPC6 and M1R, 6.2 ± 0.9 s; n = 11). In addition, a previous report has shown that PKCδ exerts a negative feedback effect via phosphorylation of Ser448 of TRPC6 (Bousquet et al., 2010). Using the phosphorylation-insensitive mutant TRPC6S448A and its corresponding mutant TRPC7S394A, we tested whether the PKC-mediated phosphorylation of these residues is involved in the current decay. Under the robust receptor stimulation, unexpectedly, we did not detect any clear difference in the Δ90–50% time (100 µM CCh: TRPC6wt, 11.4 ± 2.4 s; TRPC6S448A, 12.7 ± 2.7 s; TRPC7wt, 1.8 ± 1.1 s; TRPC7S394A, 1.9 ± 0.9 s; n = 8). Furthermore, the basal phosphorylation site of TRPC6 has been identified at Ser814 (Bousquet et al., 2011), which may play a role in the channel function. We also tested the mutant, TRPC6S814A, but it failed to show any significant differences, including Δ90–50% (10.1 ± 3.5 s; n = 7), which is consistent with the previous report (Bousquet et al., 2011). Our data do not exclude the possibility that PKC-mediated phosphorylation inhibits channel opening, because of the variety of cell and measurement conditions. Nevertheless, these results suggest that the DAG production level and PKC phosphorylation–mediated channel modulation may contribute less to the inactivation of TRPC6/7 channels at the robust stimulation.
A simulation model was built for further understanding of dual regulation by PI(4,5)P2 and DAG, as described in the latter section. For that purpose, we presented the relation between the current increase and the production of DAG. Plotting the early phase of the current increase (Δ10–30%) versus DAG production kinetics (τFRdag) exhibited a clear correlation, with a smaller slope for the TRPC7 compared with the TRPC6-expressing cells (Fig. 3 E). This result suggests that the TRPC7 channel is highly sensitive to the increment of DAG. We thus set the DAG sensitiveness in the initial parameter as TRPC7 > TRPC6 in the model simulation (Table 4, rows 21 and 22).
Table 4.
Parameters and initial conditions for the fitting
| Row | Parameters (unit) | Setting | Initial value of TRPC6 endo/C6 + M1R/A7r5/C7 + M1R | Source or comments |
| 1 | PI(4,5)P2 (µM) at resting | Free | 20/20/20/20 | Bunce et al., 1993; McLaughlin and Murray, 2005 |
| 2 | ki_PLC (s−1) | Free | 0.04/1/1/1 | Rational to PI(4,5)P2 reduction with our data |
| 3 | kii_DAG kinase (s−1) | Free | 0.03/0.03/0.08/0.03 | Rational to DAG changes with our data |
| 4 | kiii_PA to PIP reactions (s−1) | Free | 0.01/0.01/0.01/0.01 | Appropriate for PI(4,5)P2 synthesis |
| 5 | kiv_PIP5K (s−1) | Free | 0.1/0.1/0.25/0.1 | Appropriate for PI(4,5)P2 resynthesis |
| 6 | kv_IP3 phosphatase (s−1) | Free | 0.5/0.5/0.5/0.5 | Appropriate for IP3 hydrolysis |
| 7 | τrd (s) | Free | 5/2/1/4 | Appropriate for Receptor desensitization |
| 8 | τsd (s) | Free | 50/10/5/10 | Same as above |
| 9 | Rd_f (no unit) | Free | 0.5/0.5/0.5/0.5 | Same as above |
| 10 | Sd_f (no unit) | Free | 0.5/0.5/0.5/0.5 | Same as above |
| 11 | spot (distance global to local; µm) | Free | 4/4/3/4 | 5 times more than the diffusion coefficient of PI(4,5)P2 |
| 12 | dcoef of PI(4,5)P2 (µm2/s) | Free | 0.8/0.8/0.8/0.8 | Golebiewska et al., 2008 |
| 13 | Ratio of local ki / global ki | Free | 1/4/2/5 | Approximate from the uneven FRET reduction (Fig. S3) |
| 14 | PI4P (µM) | Fixed | 10/10/10/10 | Brown et al., 2008 |
| 15 | Activation delay (no unit) | Free | 0.01/0.001/0.001/0.001 | Appropriate for receptor activation |
| 16 | Activation power (no unit) | Free | 0.5/0.3/0.7/0.3 | Same as above |
| 17 | Vrev (mV) | Fixed | 0/0/0/0 | |
| 18 | Vhold (mV) | Fixed | −50/−50/−50/−50 | |
| 19 | No. of channels | Free | 100–7,000 | Appropriate for the current density |
| 20 | Channel conductance (pS) | Fixed | 35/35/35/70 | Hofmann et al., 1999; Lemonnier et al., 2008 |
| 21 | K1 (Kd for DAG1; µM) | Free | 60/60/35/10 | Effective OAG concentrations are 10 to 100 µM in Hofmann et al., 1999; Okada et al., 1999; Imai et al., 2012 |
| 22 | K2 (Kd for DAG2; µM) | Free | 30/30/10/10 | Same as above |
| 23 | K3 (Kd for PI(4,5)P2; µM) | Free | 2/2/5/5 | This paper, Fig. 4 D |
| 24 | Expressed PHd (µM) | Fixed | 1.6/1.6/1.6/1.6 | This paper, Materials and methods |
| 25 | Kd PI(4,5)P2 of PHd (µM) | Fixed | 2.0/2.0/2.0/2.0 | Hirose et al., 1999 |
| 26 | Kd IP3 of PHd (µM) | Fixed | 0.1/0.1/0.1/0.1 | Hirose et al., 1999 |
Functional dissociation constants of PI(4,5)P2 binding to TRPC3/6/7 channels
These results have shown the substantial importance of the dissociation of PI(4,5)P2 to the inactivation of TRPC6/7 channel currents, but the affinity of PI(4,5)P2 to these channels is not yet known. To obtain this parameter, DrVSP, which functions as a membrane-resident voltage-controllable phosphoinositides phosphatase, was used to reduce intrinsic PI(4,5)P2 (Okamura et al., 2009). Our previous report demonstrated that reduction of PI(4,5)P2 by activation of DrVSP led to concomitant inhibition of TRPC3/6/7 currents (Imai et al., 2012). We simultaneously measured the voltage-dependent stepwise controls of FRET between CFPmse-PHd and YFPmse-PHd, and the DrVSP-mediated inhibition. To evoke the currents, the DAG lipase inhibitor, RHC80267, was used. This compound is suitable to produce stabilized inward currents, mainly caused by elevating the resting level of DAG (Albert et al., 2005). The step-pulse protocol, from 20 to 180 mV with a duration of 500 ms, enables the current inhibition and FRET reduction to be observed simultaneously (Fig. 4 A).
Figure 4.
TRPC current inhibition and PI(4,5)P2 reduction in response to the protocol for measuring the voltage dependence of DrVSP activation. (A) TRPC6, CFPmse-PHd, YFPmse-PHd (PI(4,5)P2 sensor), and voltage-sensing phosphatase (DrVSP) were coexpressed in HEK293 cells. Gradual current inhibition and reduction in PI(4,5)P2 caused by the step-pulse protocol (left; from 20 to 180 mV; duration of 500 ms; repeated every 25 s). A DAG lipase inhibitor (RHC80267; 100 µM) was applied to induce the currents (gray horizontal bar). The ratio of current inhibition, r(I), and FRET reduction, r(FR), upon the depolarization pulses was used to quantify the channel activity and PI(4,5)P2 changes after DrVSP activation (right). (B) The voltage dependence of current inhibition (left axis; circles) and FR reduction (right axis; triangles) after DrVSP activation in cells expressing TRPC3 (left), TRPC6 (middle), and TRPC7 (right) channels.
By plotting the current inhibition “r(I)” and FRET reduction “r(FR)” against the depolarizing pulses that activate DrVSP, the various sensitivities of the TRPC3, TRPC6, and TRPC7 channels were quantified (Fig. 4 B). The inhibition of the TRPC3 current (Fig. 4 B, left, circles) was relatively insensitive, compared with the reduction in FRET (triangles). In contrast, the inhibition of TRPC7 was highly sensitive to the reduction in FRET (Fig. 4 B, right). TRPC6 exhibited a similar level of gradual changes in r(I) and r(FR) (Fig. 4 B, middle). This result confirms our previous observations showing differential sensitivities of TRPC3/6/7 channels to PI(4,5)P2 reduction, with an order of TRPC7 > C6 > C3 (Imai et al., 2012).
However, the inhibition of TRPC3 and TRPC6 currents was not complete, and the FRET reduction by DrVSP activation was also insufficient to reach a zero FRET level (FR = 1.46 ± 0.07; n = 14 at 120 mV). We thus speculated that this partial current inhibition may simply be because of the incomplete depletion of PI(4,5)P2. This idea raises a challenging question: how is TRPC3/6/7 inhibition by DrVSP activation observed at the single-channel level? To answer this question, DrVSP was activated during receptor stimulation in the cell-attached patch mode. Robust depolarization led to a brief, but almost complete, inhibition of the TRPC6 channel during the bursting activity (Fig. S2). This observation enabled us to use a standard ligand-binding isotherm for PI(4,5)P2–TRPC3/6/7 channel binding. The calculation of PI(4,5)P2 concentration from the reduction in FR was done by a boundary function (described in Materials and methods). Because the FRET between CFPmse-PHd and YFPmse-PHd proteins is cancelled by detachment of either the donor or acceptor fluorophore-fused PHd proteins from the membrane, FR reduction could be approximated as a cooperative square law of the membrane-bound fraction of PHd (Eq. 3) as follows:
| (4) |
where Kd(PHd) is the dissociation constant of PI(4,5)P2 binding to the PHd. The left side of Eq. 4 is always positive, and solving PI(4,5)P2 yields:
| (5) |
where FRmax is the maximum FR value at an infinite concentration of PI(4,5)P2. According to the resting FR of PIP5K-overexpressing cells, that value was estimated as 1.2-fold higher than the resting FR of the control cells (Fig. 5 A). After solving Eq. 5, r(I) versus the estimated PI(4,5)P2 concentration (Est, PI(4,5)P2) plots were fitted using the Hill equation (Hill, 1910) (Fig. 5 B). Assuming a dissociation constant of PI(4,5)P2 − PHd = 2 µM (Hirose et al., 1999), the functional dissociation constants of PI(4,5)P2 binding to TRPC3, TRPC6, and TRPC7 channels were estimated at 1, 2, and 5 µM, respectively. These factors were incorporated into K3 as the initial parameter for simulation in channel regulation by PI(4,5)P2 (see below).
Modeling TRPC channel activity coupled to PI(4,5)P2–DAG signaling
Having determined the functional constants of PI(4,5)P2 binding to TRPC channels, we attempted to simulate channel activity and compare the results with the experimental data. Our model consists of three components. Part 1 covers the minimal PI(4,5)P2-to-DAG reaction, including the process of PI(4,5)P2 recovery (Table 1, Eqs. 6–17). Part 2 is the calculation of the open probability (Po) and the resultant current based on the dynamics of DAG and PI(4,5)P2 concentrations (Table 2, Eqs. 18–20). Part 3 is a back-calculation of normalized FR of PI(4,5)P2 sensor according to the concentrations of PI(4,5)P2 and IP3 (Table 3; Eqs. 21–26). Details of the respective components are described in full below.
Table 1.
Equations for minimal PI(4,5)P2–DAG reaction scheme
| Equation | No. |
| 6 | |
| 7 | |
| 8 | |
| 9 | |
| 10 | |
| 11 | |
| 12 | |
| 13 | |
| 14 | |
| 15 | |
| 16 | |
| 17 |
Table 2.
Equations for channel gating
| Comment to the equation | Equation | No. |
| Open probability in DG model: | 18 | |
| Open probability in SPD model: | 19 | |
| TRPC6 / C7 currents: | 20 |
(Part 1) Scheme of the minimum essential PI(4,5)P2–DAG reaction.
The goals of this simulation were to reproduce the experimental data and elucidate the functional role of the reduction in PI(4,5)P2 caused by agonist-induced receptor-operated currents. Thus, we focused on the activation of PLC by Gq protein–coupled receptors as follows. The initial step is hydrolysis of PI(4,5)P2 by PLC, which generates DAG and IP3 (Fig. 6 A, ki, and Table 1, Eqs. 6–8). Activation of PLC starts at the first time step (0.05 s) after time zero. In the factor of PLC activity, we also included the adjusted factors of receptor desensitization (Table 1, Eq. 9) and the time-dependent acceleration of PLC activity by receptor stimulation (Eq. 10). The latter factor has been demonstrated as Ca2+-dependent positive feedback in PLC activity (Horowitz et al., 2005). The next step is DAG phosphorylation by DAG kinase to generate phosphatidic acid (PA) (Fig. 6 A, kii, and Table 1, Eq. 11). The third step is production of PI(4)P, a precursor of PI(4,5)P2, from PA, skipping the intermediate products of CDP-DAG and phosphatidylinositol (Fig. 6 A, kiii, and Table 1, Eq. 12). For recovery of PI(4,5)P2, we considered two pathways: (1) resynthesis of PI(4,5)P2 from PI(4)P by PIP5K (Fig. 6 A, kiv, and Table 1, Eq. 13) and (2) diffusion of PI(4,5)P2 from global to local channel area (Eqs. 14 and 15). The idea and necessity of the diffusion pathway are described in the Discussion and the legend of Fig. S4. The amount of scavenged PI(4,5)P2 arising from the binding of PHd proteins was solved by Eq. 16 (Table 1). The total available amount of PI(4,5)P2 to transfer to Part 2 (channel gating) was calculated by Eq. 17 (Table 1).
(Part 2) Channel gating.
Two channel gating models were constructed. The first, a simple channel gating model, only consists of DAG binding and unbinding for channel opening and closing, and it lacks the inhibitory regulation by PI(4,5)P2 reduction (termed the “DG model” in Fig. 6 A, bottom left). The second is a realistic model that takes into account the inhibitory effect caused by a reduction of PI(4,5)P2 that directly induces transition of the channel to an inactive state from any closed or open state. This model is referred to as the “SPD model” (Fig. 6 A, bottom right). Open probabilities (Po) expressed as a function of agonist concentration, calculated according to the DG and SPD models, are described in Eqs. 18 and 19, respectively (Table 2). Finally, whole-cell currents were calculated using Eq. 20.
(Part 3) Back-calculation of FR from the simulated PI(4,5)P2 concentration.
As described in the previous section, the observed FR changes were converted to PI(4,5)P2 concentrations, according to Eq. 5. Here, we attempted to do the opposite, using these equations to explore FRET dynamics. Receptor stimulation produces DAG and IP3 after PLC-mediated hydrolysis of PI(4,5)P2. IP3 is highly diffusible in the cytoplasm and binds to PHd proteins with higher affinity (0.1 µM) than PI(4,5)P2 (Hirose et al., 1999). The factor of FRET reduction caused by the binding of IP3 to PI(4,5)P2 sensor was incorporated into the back-calculation of FR dynamics (Table 3, Eqs. 21–24). Although it was difficult to calculate the absolute efficiency of FRET, because of various expression levels of fluorophore-fused PHd, we could estimate the normalized FR (Norm.FR) changes by the resting level of FR. The alternations in Norm.FR, caused by CFPmse-fused TRPC7 channel versus YFPmse-PHd and CFPmse-PHd versus YFPmse-PHd, were solved by Eqs. 25 and 26 (Table 3), respectively.
Insufficient matching of the DG model
First, we examined whether a model without PI(4,5)P2 regulation (DG model) was able to reproduce the experimental data of the simultaneous measurements. Fitting to the TRPC6 currents was accomplished by minimizing the sum of the squared errors with 19 free parameters, the initial values of which are listed in Table 4. The fidelity of the model was assessed by a similarity of FR between the experimentally measured FR and simulated FR, which was obtained from the back-calculation of the resultant PI(4,5)P2 concentrations by fitting to the currents, according to the equations described in the section on model Part 3 and Table 3.
When the currents were induced by the weak receptor stimulation through endogenous muscarinic receptors, the experimental FR was substantially compatible with the calculated FR from the simulated PI(4,5)P2 changes (Fig. S4 A; SD = 0.12). In contrast, fitting the currents in M1R-overexpressing cells to the DG model highly deviated from the experimental FR changes (Fig. S4 B; SD = 0.63). Furthermore, the DAG production was quite more transient than that observed in the PKC-based FRET dynamics (Fig. S4 B, bottom panel, dashed line). Therefore our fitting examination indicates that the DG model may be useful for mimicking the delayed receptor-operated currents, but it is not totally suitable for the rapid case. As we demonstrated, when the parameter for PLC activity (ki) was set to an accelerated kinetics (ki = 0.7), a marked TRPC6 current inactivation emerged only in the SPD model but not in the DG model (Fig. 6 B, top).
Fitting the SPD model to the experimental data
The SPD model was then tested. The same basic fitting strategy as in the DG model was used. After fitting the SPD model to the experimental receptor-operated current data, the computed PI(4,5)P2 data were compared with the experimental FR dynamics. In this case, in addition to the 19 parameters, the dissociation constant of PI(4,5)P2 binding was incorporated at K3 (Table 4, row 23). The fitting of the SPD model to the whole-cell TRPC6 currents did indeed show overlapping of the dynamics of the experimental Norm.FR and the back-calculated Norm.FR, with a smaller SD value than in the DG model (SD = 0.04; n = 4). Furthermore, similar overlapping was clearly evident in TRPC6 currents in M1R-overexpressing cells (SD = 0.05; n = 4; Fig. 7 B, middle). The fitting expressed a prolonged existence of DAG, persisting well beyond the current decay (Fig. 7 B, bottom, solid dashed line). Such prolonged existence of DAG was seen experimentally after robust receptor stimulation (Fig. 3 B) and in recent work by Falkenburger et al. (2013). The correlation between the experimental and modeled data supports the description of TRPC6 currents and PI(4,5)P2 dynamics in the SPD model (fitted parameters are summarized in Table 5).
Table 5.
Resultant parameters by fitting to TRPC currents with SPD model
| Cell | HEK293 | HEK293 | A7r5 | HEK293 | |
| Transfected plasmids | TRPC6/CFPmse-PHd/YFPmse-PHd | TRPC6/M1R/CFPmse-PHd/YFPmse-PHd | CFPmse-PHd/YFPmse-PHd | TRPC7-CFPmse/M1R/YFPmse-PHd | |
| Receptor agonist (conc. µM) | CCh (100) | CCh (100) | Arg-Vasopressin (1) | CCh (100) | |
| The number of fitted cells | 4 | 4 | 4 | 4 | |
| Row | Parameters (unit) | ||||
| 1 | PI(4,5)P2 (µM) at resting | 22.5 ± 0.4 | 23.0 ± 0.4 | 22.2 ± 0. 2 | 14.6 ± 0.1 |
| 2 | ki_PLC (s−1) | 0.05 ± 0.02 | 1.2 ± 0.20 | 1.3 ± 0.42 | 0.93 ± 0.09 |
| 3 | kii_DAG kinase (s−1) | 0.04 ± 0.01 | 0.03 ± 0.02 | 0.07 ± 0.03 | 0.02 ± 0.01 |
| 4 | kiii_PA to PIP reactions, (s−1) | 0.008 ± 0.002 | 0.006 ± 0.002 | 0.004 ± 0.001 | 0.006 ± 0.0001 |
| 5 | kiv_PIP5K (s−1) | 0.050 ± 0.004 | 0.07 ± 0.04 | 0.17 ± 0.083 | 0.04 ± 0.03 |
| 6 | kv_IP3 phosphatase (s−1) | 0.7 ± 0.4 | 0.2 ± 0.1 | 0.88 ± 0.093 | 0.84 ± 0.14 |
| 7 | τrd (s) | 12.0 ± 3.8 | 1.4 ± 0.6 | 1.4 ± 0.2 | 4.4 ± 0.4 |
| 8 | τsd (s) | 24 ± 11 | 17 ± 5.2 | 6 ± 2.4 | 38 ± 15 |
| 9 | Rd_f (no unit) | 0.65 ± 0.08 | 0.55 ± 0.03 | 0.60 ± 0.12 | 0.46 ± 0.05 |
| 10 | Sd_f (no unit) | 0.35 ± 0.05 | 0.44 ± 0.02 | 0.43 ± 0.124 | 0.53 ± 0.06 |
| 11 | spot (distance global to local; µm) | 0.64 ± 0.09 | 2.78 ± 0.79 | 2.83 ± 0.98 | 1.90 ± 0.29 |
| 12 | dcoef of PI(4,5)P2 (µm2/s) | 0.36 ± 0.08 | 0.8 ± 0.47 | 0.5 ± 0.11 | 0.29 ± 0.06 |
| 13 | Ratio of local ki / global ki | 1.1 ± 0.28 | 4.0 ± 1.6 | 2.8 ± 0.98 | 6.0 ± 1.3 |
| 14 | Activation delay factor | 0.03 ± 0.024 | 0.01 ± 0.002 | 0.003 ± 0.002 | 0.03 ± 0.025 |
| 15 | Activation power factor | 0.6 ± 0.33 | 0.32 ± 0.07 | 0.7 ± 0.17 | 0.3 ± 0.12 |
| 16 | The number of channels | 1,644 ± 277 | 5,808 ± 959 | 317 ± 121 | 2,384 ± 1,896 |
| 17 | K1 (kd for DAG1; µM) | 39 ± 6.3 | 26 ± 2.4 | 23 ± 7.6 | 7.0 ± 0.3 |
| 18 | K2 (kd for DAG2; µM) | 29 ± 5.1 | 22 ± 2.0 | 8 ± 3.4 | 6.3 ± 0.25 |
| 19 | K3 (kd for PI(4,5)P2; µM) | 1.9 ± 0.6 | 3.0 ± 0.66 | 9 ± 1.5 | 9.7 ± 0.14 |
| SD | 0.04 | 0.05 | 0.12 | 0.14 | |
| Sr0.01a | 82.7% | 80.9% | 70.1% | 56.2% | |
Parameters are presented as mean ± SEM. (Typical results are displayed in Fig. 7.)
Percentage of the squared residuals lower than the value of 0.01.
The SPD model was also examined for TRPC6/7-like currents in aortic smooth muscle–derived A7r5 cells (Brueggemann et al., 2006; Maruyama et al., 2006). The PI(4,5)P2 sensor proteins (CFPmse-PHd and YFPmse-PHd) were coexpressed in A7r5 cells without exogenous expression of channels or receptors (Fig. 7 C, inset). By applying AVP, TRPC6/7-like currents and FRET reduction were observed similarly to the observations after stimulation of HEK293 cells with CCh (Fig. 7 C, top and middle). A noticeable difference was that PI(4,5)P2 recovered quickly (5–20 s after the application of AVP). Such rapid FRET recovery is expected with the rapid desensitization of vasopressin receptors, and was reproduced by coexpressing vasopressin type 1A receptors with TRPC6 or TRPC7 channel in HEK293 cells (Fig. S5). When fitting these AVP-evoked TRPC6/7-like currents, the simulation parameters for rapid desensitization (Table 4, rows 7 and 8) also overlapped the Norm.FR dynamics (Fig. 7 C, middle, blue line; SD = 0.12). These results indicate that the SPD model is useful even in the context of physiological cells.
Contrary to these positive results, the back-calculated FRET demonstrated less similarity to the experimental FRET achieved by fitting to TRPC7 currents observed in HEK293 cells and vice versa (Fig. S6; SD = 0.18; n = 4). In addition, TRPC7 currents often demonstrated a plateau or biphasic response when M1R was overexpressed, but the FRET did not clearly show such irregular dynamics. We reasoned that the FRET detection by fluorophore-fused PHd was too slow to respond to the PI(4,5)P2 dynamics compared with the time course of TRPC7 current, recorded by the electrophysiological method. To improve this issue, we redesigned the FRET pairs to detect PI(4,5)P2 changes in the local vicinity of the TRPC7 channels. The donor fluorophore (CFPmse) directly fused to the channel was coexpressed with YFPmse-PHd (Fig. 7 D, inset). Intriguingly, this FRET pair showed a transient increment of FRET during the plateau or biphasic current (Fig. 7 D, red arrow). By fitting the TRPC7 currents with the corresponding “down-up-down” PI(4,5)P2 dynamics (Fig. 7 D, bottom), we achieved an improved matching of Norm.FR (SD = 0.14) as well. This transient up-regulation of PI(4,5)P2 reflects a quick replenishment by diffusible PI(4,5)P2 in the SPD model (Fig. S3). These results show that corresponding Norm.FR responses in various cell and channel settings strongly support the fidelity of the SPD model for reproducing simultaneous events of receptor-operated TRPC currents and FR changes.
DISCUSSION
Because PI(4,5)P2 hydrolysis by PLC is the critical event in receptor-operated TRPC3/6/7 currents, the parallel observation of PI(4,5)P2 or DAG with channel activity is fundamental to advancing our understanding of channel regulation. To address this point, we performed concerted quantitative FRET measurements of sensitized fluorescence emission (3-cube method) and electrophysiological measurements on patch-clamped cells. We accomplished this by using CFPmse- or YFPmse-fused PHd proteins as a FRET sensor of PI(4,5)P2, or CFPmse-fused PKCε and YFPmse-fused myristoyl membrane–attached peptides to monitor DAG. CCh or AVP was used to induce TRPC6/7 currents, which were measured to monitor the synchronicity of receptor-operated currents and changes in either PI(4,5)P2 or DAG levels. The experimental results demonstrated a correlation between the kinetics of PI(4,5)P2 reduction and the activation or the inactivation of receptor-operated TRPC6/7 currents. Fitting the experimental data to the DG or SPD model revealed that the idea of self-limiting regulation by PI(4,5)P2 and DAG is critical for reproducing receptor-operated TRPC6/7 currents.
Reproducing the currents and FRET reduction in the SPD model
Our results show that reduction in PI(4,5)P2 by PLC-mediated hydrolysis appears to be fundamental to the inactivation of TRPC6/7 channels. This contribution is even more apparent after robust receptor stimulation, either by high concentrations of receptor agonist or by overexpression of functional receptors. Here, we simulate in a virtual setting the inhibitory role played by a reduction in PI(4,5)P2 levels in the SPD model in the context of different levels of PLC activity. In the averaged fitted data for TRPC6 currents, PLC activity (ki) was gradually decreased or increased, as shown in Fig. 8 A. The simulated TRPC6 currents and FRET by the PHd clearly demonstrated PLC-dependent dynamics in regard to the amplitudes or kinetics in the currents (Fig. 8 A, top) and the kinetics or the extent of FRET reduction (bottom). We then depicted log–log plots of the time course of current growth (Δ10–90%) or decay (Δ90–50%) versus τFR (Fig. 8 B, red circles). For comparison, we also simulated this plot with the DG model (Fig. 8 B, black circles) as well as TRPC7 channels. The log–log plots of Δ10–90% to τFR showed a linear relationship in both SPD and DG models of TRPC6/7 currents. These plots closely resemble the experimental data, which are shown as gray lines in Fig. 8 B (solid and dashed line for TRPC6 and TRPC7 channels, respectively). In contrast, the linear relationship between current decay (Δ90–50%) and τFR was reproduced only when modeled with the SPD model (Fig. 8 B, right, red symbols), but not with the DG model.
Figure 8.
Proposed contribution of PI(4,5)P2 reduction in receptor-operated TRPC6/7 currents. (A) Representative SPD model simulations for receptor-operated TRPC6 currents (top) and FRET by PI(4,5)P2 sensor (bottom) from various PLC activities (ki). ki varied from 0.03 to 1.0 (s−1). The same color curves displayed in each panel were calculated from the same ki value. (B) The various kinetics of PI(4,5)P2 reduction (τFR) according to the changes in PLC activity (ki) impact activation (left; Δ10–90%) and inactivation (right; Δ90–50%) of TRPC6/7 channel currents. Shown are the prediction made by the DG model (black) and the SPD model (red) for TRPC6 (circles) and TRPC7 channels (triangles). The star in the panels indicates the result from the ki setting of 0.7. The solid and dashed lines indicate the current–τFR relationships experimentally observed in TRPC6 and TRPC7, respectively. The direction of the arrows and its size indicate the gap from the DG to SPD model. (C) Simulation results of PLC-dependent characteristics of TRPC6/7 channels by DG (black) and SPD (red) models. (Left) The relationship between maximum open probability (Pomax) and ki. (Middle) The time required to peak currents (s) and ki. (Right) The total ionic influx and ki. t = 30 s.
The simulation result indicates that when the strength of receptor stimulation PI(4,5)P2 is low or the reduction of PI(4,5)P2 is delayed (τFR, >50 s), the DG model may be useful to reproduce such delayed receptor-operated TRPC currents. However, the situation alters as receptor stimulation is strengthened or the reduction of PI(4,5)P2 is accelerated, and the DG model deviates from the experimental data. Furthermore, the SPD model–based relationship in Δ90–50% TRPC7 currents to τFR resulted in a slight increase in the steepness to TRPC6 (Fig. 8 B). This result is similar to the TRPC7 current to τFR relationship observed experimentally (Fig. 8 B, dotted line). In contrast, the SPD model–based TRPC6 relationship resulted in an even steeper slope compared with the experimentally observed relationship (Fig. 8 B, solid line). The difference implies further unknown characteristics that underlie the regulation by PI(4,5)P2 and DAG in receptor-operated TRPC6/7 currents. Recently, several reports have addressed an alternation of PI(4,5)P2 regulation by intracellular Ca2+ in KCNQ2/3 (Kosenko et al., 2012; Falkenburger et al., 2013) and TRPV6 channels (Cao et al., 2013). According to Kosenko et al. (2012), calmodulin binding to KCNQ2 channels stabilized PI(4,5)P2 channel binding. The involvement of Ca2+ regulation to PI(4,5)P2 or DAG regulations requires further investigation. Nevertheless, because of the consistency of the SPD model simulation with the experimental results, we concluded that the inhibitory effect of reductions in PI(4,5)P2 would accelerate the channel inactivation with the increased receptor stimulation.
Proposed function of the self-limiting regulation in receptor-operated TRPC6/7 currents
Based on the model simulation, we explored several PLC-dependent features of the self-limiting regulation by PI(4,5)P2 and DAG. The first characteristic is the maximum Po (Pomax). As shown in the left plot in Fig. 8 C, in both models, Pomax values gradually increased as PLC activity increased in the range where the ki values were small (ki of <0.1). However, Pomax in the SPD model was sustained or slightly attenuated when the ki values were >0.1. Kwon et al. (2007) showed that disruption of the potential PI(4,5)P2-sensing residue of the TRPC6 channel nearly doubles the maximum current amplitude. This is consistent with our simulation, where the Pomax at ki = 0.7 s−1 eventually increased by two- to threefold in the DG model compared with that in the SPD model. The consistency of our simulation and the previous report strongly supports the functionality of the self-limiting regulation in TRPC6/7 channels for protecting the excess cation influx.
The second characteristic we measured was the shift in peak appearance. When we applied robust receptor stimulation, the time to peak currents were drastically shortened compared with the endogenous receptor stimulation (see PI(4,5)P2 dynamics at different levels… in Results). Essentially, the similar tendency was reproduced in both models (Fig. 8 C, middle). However, the time to peak shortening was even more striking in the SPD model (Fig. 8 C, red symbols) than in the DG model (black symbols). Petersson et al. (2011) computationally demonstrated that TRPC channel activity in the nervous system contributes to the temporal integration of the generation of a lower rate-firing pattern at distal dendrites. Their simulation result suggests that the rate of firing may be controlled by the peak time of TRPC currents. Therefore, TRPC6/7 channels may play a critical role in determining the neural firing rate, depending on the strength of receptor stimulation.
The third characteristic is the total ionic influx relation (Fig. 8 C, right). The total ionic influx, which was calculated by integration of the flow during the receptor stimulation (t = 30 s), was predicted as the bell-shape response to the PLC activity in the SPD model. Therefore, the optimum PLC activity appeared neither weak nor robust, but was in the middle (0.1 < ki < 0.3; Fig. 8 C, right, red symbols). TRPC3/6/7 channels contribute to vascular tone, inflammation, cell remodeling, and intestinal contraction through increased Na+ or Ca2+ levels (Tsvilovskyy et al., 2009; Smedlund et al., 2010; Tauseef et al., 2012; Weissmann et al., 2012; Koenig et al., 2013). Hence, this bell-shaped relationship may be linked to these multiple pathophysiological responses.
Collectively, channel inhibition by PI(4,5)P2 reduction in DAG-sensitive TRPC3/6/7 channels not only accelerates the rate of current decay but also contributes to suppression of the current amplitude, shortening of the peak time, and the bell shape of the ionic influx, leading to the multiple functionality of these channels.
PI(4,5)P2 replenishment mechanism by diffusion
It has been suggested that phosphatidylinositol 4-phosphate is critical for replenishing PI(4,5)P2 via its phosphorylation by PIP5K (Suh and Hille, 2002; Loew, 2007). Furthermore, PIP5K substantially contributes to the quick recovery of PI(4,5)P2 after its depletion by DrVSP activation (Falkenburger et al., 2010; Imai et al., 2012). However, in case of receptor-operated TRPC6/7 currents, we observed that a high concentration of ATP or its inactive analogue AMP-PNP in the patch-pipette solution had little effect on TRPC6/7 currents (Fig. S7), even in the plateau or biphasic phase. We speculate that an additional PI(4,5)P2 replenishment pathway, such as the detachment of PI(4,5)P2 from proteins, dispersion from its clustered complex (van den Bogaart et al., 2011), translocation of phosphoinositides from the PIS organelle (Kim et al., 2011), or an unknown mechanism (Hammond et al., 2012), may be involved.
However, here we focused on the lateral diffusion of PI(4,5)P2. When we analyzed FRET in regions of small compartments, a nonuniform reduction in FR by PI(4,5)P2 sensor upon receptor stimulation was observed (Fig. S3). This variability of a region-specific PI(4,5)P2 reduction was consistent with an electron microscopic study showing that PI(4,5)P2 located in a membrane microdomain (known as caveolae) decreased slower than in an undifferentiated membrane upon receptor stimulation (Fujita et al., 2009). We have speculated that such nonuniformity of PI(4,5)P2 reduction creates the opportunity for PI(4,5)P2 diffusion to occur. For that reason, we incorporated the pathway of PI(4,5)P2 replenishment by lateral diffusion into our model (Part 1). By incorporation of this pathway, the plateau or biphasic currents after the peak current can be enhanced as the gap of PI(4,5)P2 dynamics between the local and global domain increases (Fig. S3). In the case of TRP channels in flies, the plateau phase of the TRP current in response to light adaptation has been demonstrated to have a key role in phototransduction (Lo and Pak, 1981; Minke, 2010). Therefore, incorporation of replenishment of PI(4,5)P2 in our model may provide an important contribution to understanding the physiological consequences.
Molecular insight into PI(4,5)P2 and DAG binding to TRPC channels
The fitting result of the SPD model predicted the dissociation constant of DAG binding to TRPC6 channel to be 20 to ∼40 µM and to TRPC7 to be <10 µM (Table 5, rows 17 and 18). This parameter has not been well characterized, despite being critical for DAG-sensitive TRPC channel activation. In the experiment, receptor-operated TRPC7 currents demonstrated a higher sensitivity to DAG production (Fig. 3 E) than TRPC6 currents, which was consistent with the result predicted by the SPD model, thus providing a fundamental parameter into the TRPC6/7 channel activation. However, it is still unclear exactly where these lipids bind to the TRPC3/6/7 channels.
Among the TRP superfamily, heat- and capsaicin-activated TRPV channels are relatively well characterized and have at least two PI(4,5)P2-binding sites in the C-terminal domains, proximal and distal regions to the S6 domain (Prescott and Julius, 2003; Doerner et al., 2011; Ufret-Vincenty, et al., 2011). In the case of the TRPC family, earlier studies have also shown that the distal region of the TRPC6 C-terminal domain contributes to the current decay (Kwon et al., 2007), whereas PI(4,5)P2 binding to the PH-like domain in the N-terminal region reduces TRPC3 current amplitudes (van Rossum et al., 2005). These results indicate that PI(4,5)P2 binding may be supported by multiple channel domains/regions in a complex manner. Furthermore, another complex point regarding the PI(4,5)P2 regulation is that various heteromeric TRPC3/6/7 channels can be formed, including heteromers of TRPC3/6/7 (Goel et al., 2002) and other subfamilies (Hofmann et al., 2002; Takai et al., 2004; Ambudkar et al., 2006). Addressing these points is essential to further extend our understanding on the self-limiting regulation by PI(4,5)P2 and DAG signals observed in TRPC3/6/7 channels.
Supplementary Material
Acknowledgments
We thank Masato Hirata (Kyushu University), Michael Schaefer (Leipzig University), and Moritoshi Sato (The university of Tokyo) for their helpful advice on the detection of PI(4,5)P2 and DAG. We thank to Yasuo Mori and Nozomi Ogawa (Kyoto University) for the critical reading of our manuscript. Miho Sumiyoshi (Fukuoka University) assisted with the molecular biological preparations.
This work was supported by Grants-in-Aid for Young Scientists from the Japan Society for the Promotion of Sciences and the Naito Foundation (both to M.X. Mori).
The authors declare no competing financial interests.
Author contributions: K. Itsuki and M.X. Mori conceived the study. K. Itsuki, H. Hase, and M.X. Mori performed the experiments and analyzed the data. Y. Okamura and R. Inoue provided guidance and support throughout. K. Itsuki, Y. Imai, Y. Okamura, R. Inoue, and M.X. Mori wrote the paper.
Sharona E. Gordon served as editor.
Footnotes
Abbreviations used in this paper:
- AVP
- arginine8 vasopressin
- CCh
- carbachol
- DAG
- diacylglycerol
- DrVSP
- Danio rerio voltage-sensing phosphatase
- FR
- Förster resonance energy transfer ratio
- FRET
- Förster resonance energy transfer
- HEK
- human embryonic kidney
- IP3
- inositol 1,4,5-trisphosphate
- M1R
- muscarinic type 1 receptor
- PA
- phosphatidic acid
- PHd
- Pleckstrin homology domain
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PIP5K
- phosphatidylinositol-4-phosphate-5-kinase
- PLC
- phospholipase C
- SPD
- self-limiting regulation by PI(4,5)P2–DAG signaling
- TRPC
- transient receptor potential classical/canonical
References
- Albert A.P., Piper A.S., Large W.A. 2005. Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes. J. Physiol. 566:769–780 10.1113/jphysiol.2005.090852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar I.S., Bandyopadhyay B.C., Liu X., Lockwich T.P., Paria B., Ong H.L. 2006. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 40:495–504 10.1016/j.ceca.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Beech D.J., Muraki K., Flemming R. 2004. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J. Physiol. 559:685–706 10.1113/jphysiol.2004.068734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet S.M., Monet M., Boulay G. 2010. Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J. Biol. Chem. 285:40534–40543 10.1074/jbc.M110.160051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet S.M., Monet M., Boulay G. 2011. The serine 814 of TRPC6 is phosphorylated under unstimulated conditions. PLoS ONE. 6:e18121 10.1371/journal.pone.0018121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B.L., Kimes B.W., Klier F.G. 1976. Development of a clonal myogenic cell line with unusual biochemical properties. J. Cell. Physiol. 88:255–275 10.1002/jcp.1040880302 [DOI] [PubMed] [Google Scholar]
- Brown S.A., Morgan F., Watras J., Loew L.M. 2008. Analysis of phosphatidylinositol-4,5-bisphosphate signaling in cerebellar Purkinje spines. Biophys. J. 95:1795–1812 10.1529/biophysj.108.130195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L.I., Markun D.R., Henderson K.K., Cribbs L.L., Byron K.L. 2006. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J. Pharmacol. Exp. Ther. 317:488–499 10.1124/jpet.105.095067 [DOI] [PubMed] [Google Scholar]
- Bunce C.M., French P.J., Allen P., Mountford J.C., Moor B., Greaves M.F., Michell R.H., Brown G. 1993. Comparison of the levels of inositol metabolites in transformed haemopoietic cells and their normal counterparts. Biochem. J. 289:667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Zakharian E., Borbiro I., Rohacs T. 2013. Interplay between calmodulin and phosphatidylinositol 4,5-bisphosphate in Ca2+-induced inactivation of transient receptor potential vanilloid 6 channels. J. Biol. Chem. 288:5278–5290 10.1074/jbc.M112.409482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E.J., Falkenburger B.H., Hille B. 2013. Quantitative properties and receptor reserve of the IP3 and calcium branch of Gq-coupled receptor signaling. J. Gen. Physiol. 141:521–535 10.1085/jgp.201210886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner J.F., Hatt H., Ramsey I.S. 2011. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J. Gen. Physiol. 137:271–288 10.1085/jgp.200910388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M.G., Alseikhan B.A., Peterson B.Z., Yue D.T. 2001. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 31:973–985 10.1016/S0896-6273(01)00438-X [DOI] [PubMed] [Google Scholar]
- Falkenburger B.H., Jensen J.B., Hille B. 2010. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 135:99–114 10.1085/jgp.200910345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger B.H., Dickson E.J., Hille B. 2013. Quantitative properties and receptor reserve of the DAG and PKC branch of Gq-coupled receptor signaling. J. Gen. Physiol. 141:537–555 10.1085/jgp.201210887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth A.L., Remillard C.V., Yuan J.X. 2007. TRP channels in hypertension. Biochim. Biophys. Acta. 1772:895–906 10.1016/j.bbadis.2007.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A., Cheng J., Tauchi-Sato K, Takenawa T., Fujimoto T. 2009. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc. Natl. Acad. Sci. USA. 106:9256–9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N., Shapiro M.S. 2007. Regulation of ion transport proteins by membrane phosphoinositides. Nat. Rev. Neurosci. 8:921–934 10.1038/nrn2257 [DOI] [PubMed] [Google Scholar]
- Goel M., Sinkins W.G., Schilling W.P. 2002. Selective association of TRPC channel subunits in rat brain synaptosomes. J. Biol. Chem. 277:48303–48310 10.1074/jbc.M207882200 [DOI] [PubMed] [Google Scholar]
- Golebiewska U., Nyako M., Woturski W., Zaitseva I., McLaughlin S. 2008. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol. Biol. Cell. 19:1663–1669 10.1091/mbc.E07-12-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G.R., Fischer M.J., Anderson K.E., Holdich J., Koteci A., Balla T., Irvine R.F. 2012. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 337:727–730 10.1126/science.1222483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R.C. 2003. Regulation of TRP channels via lipid second messengers. Annu. Rev. Physiol. 65:735–759 10.1146/annurev.physiol.65.092101.142505 [DOI] [PubMed] [Google Scholar]
- Hartmann J., Dragicevic E., Adelsberger H., Henning H.A., Sumser M., Abramowitz J., Blum R., Dietrich A., Freichel M., Flockerzi V., et al. 2008. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 59:392–398 10.1016/j.neuron.2008.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W. 2007. Local PIP2 signals: when, where, and how? Pflugers Arch. 455:55–67 10.1007/s00424-007-0280-9 [DOI] [PubMed] [Google Scholar]
- Hill A.V. 1910. The possible effects of the aggregation of the molecules of haemoglobin on its oxygen dissociation curve. J. Physiol. 40:iv–vii [Google Scholar]
- Hirose K., Kadowaki S., Tanabe M., Takeshima H., Iino M. 1999. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 284:1527–1530 10.1126/science.284.5419.1527 [DOI] [PubMed] [Google Scholar]
- Hofmann T., Obukhov A.G., Schaefer M., Harteneck C., Gudermann T., Schultz G. 1999. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 397:259–263 10.1038/16711 [DOI] [PubMed] [Google Scholar]
- Hofmann T., Schaefer M., Schultz G., Gudermann T. 2002. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA. 99:7461–7466 10.1073/pnas.102596199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz L.F., Hirdes W., Suh B.C., Hilgemann D.W., Mackie K., Hille B. 2005. Phospholipase C in living cells: Activation, inhibition, Ca2+ requirement, and regulation of M current. J. Gen. Physiol. 126:243–262 10.1085/jgp.200509309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Itsuki K., Okamura Y., Inoue R., Mori M.X. 2012. A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. J. Physiol. 590:1101–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kuriyama H. 1993. Dual regulation of cation-selective channels by muscarinic and alpha 1-adrenergic receptors in the rabbit portal vein. J. Physiol. 465:427–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Jensen L.J., Shi J., Morita H., Nishida M., Honda A., Ito Y. 2006. Transient receptor potential channels in cardiovascular function and disease. Circ. Res. 99:119–131 10.1161/01.RES.0000233356.10630.8a [DOI] [PubMed] [Google Scholar]
- Irvine R.F., Schell M.J. 2001. Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2:327–338 10.1038/35073015 [DOI] [PubMed] [Google Scholar]
- Itsuki K., Imai Y., Okamura Y., Abe K., Inoue R., Mori M.X. 2012a. Voltage-sensing phosphatase reveals temporal regulation of TRPC3/C6/C7 channels by membrane phosphoinositides. Channels (Austin). 6:206–209 10.4161/chan.20883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsuki K., Imai Y., Okamura Y., Inoue R., Mori M.X. 2012b. PIP2 dynamics underlying muscarinic or vasopressin receptor-activated TRPC3, C6, and C7 currents. Biophys. J. 104:454a [Google Scholar]
- Jardín I., Redondo P.C., Salido G.M., Rosado J.A. 2008. Phosphatidylinositol 4,5-bisphosphate enhances store-operated calcium entry through hTRPC6 channel in human platelets. Biochim. Biophys. Acta. 1783:84–97 10.1016/j.bbamcr.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Jensen J.B., Lyssand J.S., Hague C., Hille B. 2009. Fluorescence changes reveal kinetic steps of muscarinic receptor–mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels. J. Gen. Physiol. 133:347–359 10.1085/jgp.200810075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Guzman-Hernandez M.L., Balla T. 2011. A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev. Cell. 21:813–824 10.1016/j.devcel.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Schernthaner M., Maechler H., Kappe C.O., Glasnov T.N., Hoefler G., Braune M., Wittchow E., Groschner K. 2013. A TRPC3 blocker, ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (Pyr3), prevents stent-induced arterial remodeling. J. Pharmacol. Exp. Ther. 344:33–40 10.1124/jpet.112.196832 [DOI] [PubMed] [Google Scholar]
- Kosenko A., Kang S., Smith I.M., Greene D.L., Langeberg L.K., Scott J.D., Hoshi N. 2012. Coordinated signal integration at the M-type potassium channel upon muscarinic stimulation. EMBO J. 31:3147–3156 10.1038/emboj.2012.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y., Hofmann T., Montell C. 2007. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol. Cell. 25:491–503 10.1016/j.molcel.2007.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon M.A., Ferguson K.M., Schlessinger J. 1996. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 85:621–624 10.1016/S0092-8674(00)81022-3 [DOI] [PubMed] [Google Scholar]
- Lemonnier L., Trebak M., Putney J.W., Jr 2008. Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 43:506–514 10.1016/j.ceca.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M.V., Pak W.L. 1981. Light-induced pigment granule migration in the retinular cells of Drosophila melanogaster. Comparison of wild type with ERG-defective mutants. J. Gen. Physiol. 77:155–175 10.1085/jgp.77.2.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew L.M. 2007. Where does all the PIP2 come from? J. Physiol. 582:945–951 10.1113/jphysiol.2007.132860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis D.E., Jin T., Lupyan D., Rosenhouse-Dantsker A. 2007. Phosphoinositide-mediated gating of inwardly rectifying K+ channels. Pflugers Arch. 455:83–95 10.1007/s00424-007-0276-5 [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Nakanishi Y., Walsh E.J., Wilson D.P., Welsh D.G., Cole W.C. 2006. Heteromultimeric TRPC6-TRPC7 channels contribute to arginine vasopressin-induced cation current of A7r5 vascular smooth muscle cells. Circ. Res. 98:1520–1527 10.1161/01.RES.0000226495.34949.28 [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Murray D. 2005. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 438:605–611 10.1038/nature04398 [DOI] [PubMed] [Google Scholar]
- Minke B. 2010. The history of the Drosophila TRP channel: the birth of a new channel superfamily. J. Neurogenet. 24:216–233 10.3109/01677063.2010.514369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monet M., Francoeur N., Boulay G. 2012. Involvement of phosphoinositide 3-kinase and PTEN protein in mechanism of activation of TRPC6 protein in vascular smooth muscle cells. J. Biol. Chem. 287:17672–17681 10.1074/jbc.M112.341354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M.X., Imai Y., Itsuki K., Inoue R. 2011. Quantitative measurement of Ca2+-dependent calmodulin-target binding by Fura-2 and CFP and YFP FRET imaging in living cells. Biochemistry. 50:4685–4696 10.1021/bi200287x [DOI] [PubMed] [Google Scholar]
- Okada T., Inoue R., Yamazaki K., Maeda A., Kurosaki T., Yamakuni T., Tanaka I., Shimizu S., Ikenaka K., Imoto K., Mori Y. 1999. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 274:27359–27370 10.1074/jbc.274.39.27359 [DOI] [PubMed] [Google Scholar]
- Okamura Y., Murata Y., Iwasaki H. 2009. Voltage-sensing phosphatase: actions and potentials. J. Physiol. 587:513–520 10.1113/jphysiol.2008.163097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Nayak S.K., Campo B., Walker J.R., Hogenesch J.B., Jegla T. 2005. Illumination of the melanopsin signaling pathway. Science. 307:600–604 10.1126/science.1105121 [DOI] [PubMed] [Google Scholar]
- Petersson M.E., Yoshida M., Fransén E.A. 2011. Low-frequency summation of synaptically activated transient receptor potential channel-mediated depolarizations. Eur. J. Neurosci. 34:578–593 10.1111/j.1460-9568.2011.07791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott E.D., Julius D. 2003. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 300:1284–1288 10.1126/science.1083646 [DOI] [PubMed] [Google Scholar]
- Ramsey I.S., Delling M., Clapham D.E. 2006. An introduction to TRP channels. Annu. Rev. Physiol. 68:619–647 10.1146/annurev.physiol.68.040204.100431 [DOI] [PubMed] [Google Scholar]
- Sinnecker D., Schaefer M. 2004. Real-time analysis of phospholipase C activity during different patterns of receptor-induced Ca2+ responses in HEK293 cells. Cell Calcium. 35:29–38 10.1016/S0143-4160(03)00169-6 [DOI] [PubMed] [Google Scholar]
- Smedlund K., Tano J.Y., Vazquez G. 2010. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ. Res. 106:1479–1488 10.1161/CIRCRESAHA.109.213314 [DOI] [PubMed] [Google Scholar]
- Soboloff J., Spassova M., Hewavitharana T., He L.P., Luncsford P., Xu W., Venkatachalam K., van Rossum D., Patterson R.L., Gill D.L. 2007. TRPC channels: integrators of multiple cellular signals. Handbook Exp. Pharmacol. 179:575–591 10.1007/978-3-540-34891-7_34 [DOI] [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2002. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 35:507–520 10.1016/S0896-6273(02)00790-0 [DOI] [PubMed] [Google Scholar]
- Takai Y., Sugawara R., Ohinata H., Takai A. 2004. Two types of non-selective cation channel opened by muscarinic stimulation with carbachol in bovine ciliary muscle cells. J. Physiol. 559:899–922 10.1113/jphysiol.2004.065607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauseef M., Knezevic N., Chava K.R., Smith M., Sukriti S., Gianaris N., Obukhov A.G., Vogel S.M., Schraufnagel D.E., Dietrich A., et al. 2012. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J. Exp. Med. 209:1953–1968 10.1084/jem.20111355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvilovskyy V.V., Zholos A.V., Aberle T., Philipp S.E., Dietrich A., Zhu M.X., Birnbaumer L., Freichel M., Flockerzi V. 2009. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 137:1415–1424 10.1053/j.gastro.2009.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufret-Vincenty C.A., Klein R.M., Hua L., Angueyra J., Gordon S.E. 2011. Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 286:9688–9698 10.1074/jbc.M110.192526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart G., Meyenberg K., Risselada H.J., Amin H., Willig K.I., Hubrich B.E., Dier M., Hell S.W., Grubmüller H., Diederichsen U., Jahn R. 2011. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 479:552–555 10.1038/nature10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal J., Habets R., Várnai P., Balla T., Jalink K. 2001. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J. Biol. Chem. 276:15337–15344 10.1074/jbc.M007194200 [DOI] [PubMed] [Google Scholar]
- van Rossum D.B., Patterson R.L., Sharma S., Barrow R.K., Kornberg M., Gill D.L., Snyder S.H. 2005. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 434:99–104 10.1038/nature03340 [DOI] [PubMed] [Google Scholar]
- Venkatachalam K., Montell C. 2007. TRP channels. Annu. Rev. Biochem. 76:387–417 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin J.D., Zhang J., Tsien R.Y., Newton A.C. 2003. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell Biol. 161:899–909 10.1083/jcb.200302125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N., Sydykov A., Kalwa H., Storch U., Fuchs B., Mederos y Schnitzler M., Brandes R.P., Grimminger F., Meissner M., Freichel M., et al. 2012. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 3:649 10.1038/ncomms1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winks J.S., Hughes S., Filippov A.K., Tatulian L., Abogadie F.C., Brown D.A., Marsh S.J. 2005. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J. Neurosci. 25:3400–3413 10.1523/JNEUROSCI.3231-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Sun B., Du J., Yang W., Chen H.C., Overton J.D., Runnels L.W., Yue L. 2011. Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci. Rep. 1:146 10.1038/srep00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin Y., Lukacs V., Cao C., Rohacs T. 2011. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J. Physiol. 589:6007–6027 10.1113/jphysiol.2011.220228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias D.A., Violin J.D., Newton A.C., Tsien R.Y. 2002. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 296:913–916 10.1126/science.1068539 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.