Abstract
Growth factors play an important role in supraspinatus tendon to bone healing. The objective of this study was to evaluate the temporal expression of eight different growth factors in tendon to bone healing in an animal model. We hypothesize that growth factors exhibit unique temporal profiles that correlate to specific stages in the acute process of the supraspinatus tendon. To test this hypothesis, rats underwent bilateral supraspinatus tendon detachment and repair. Animals were sacrificed at 1, 2, 4, 8 and 16 week time points. Immunohistochemical staining was done using antibodies for bFGF, BMP-12, BMP-13, BMP-14, COMP, CTGF, PDGF-B, and TGF-β1. Immunoassays showed an increase in the expression of all growth factors at 1 week followed by a return to control or undetectable levels by 16 weeks in both the insertion and midsubstance. Future studies will investigate the different impacts of growth factor expression in tendon to bone healing.
Introduction
Rotator cuff tears are one of the most common pathologies of musculoskeletal soft tissues although its underlying pathology and etiology are still not fully understood. Surgical repair is often indicated, but the re-tear rate remains quite high providing further impetus for studies to understand mechanisms of the healing process. Different growth factors have been shown to have specific roles in tendon healing. Growth factors are cell secreted proteins that regulate multiple cellular processes. However, little is known about the temporal expression of these factors in supraspinatus tendon to bone healing which might provide essential information for development of future, novel, targeted treatment modalities. Therefore, the objective of this study was to evaluate the temporal expression of eight different growth factors in supraspinatus tendon to bone healing in an established animal model of rotator cuff injury and repair.21, 25 We hypothesize that growth factors exhibit unique temporal profiles that correlate to specific stages in the injury and repair process of the supraspinatus tendon.25 Our specific hypotheses are: TGF-β1 will increase in the early inflammatory phase as well as later in association with the scarring process5, 9; PDGF-B will increase early; bFGF will remain at a moderate level throughout, consistent with the remodeling process24; BMP-12 will increase at later time points with predominance in the midsubstance6, 19, 27; and COMP and CTGF will increase later in the healing process in the insertion site.
Methods
Twenty male Sprague-Dawley rats (452±60g) underwent bilateral supraspinatus tendon detachment and repair as described.25 Postoperative cage activity was allowed. Studies were approved by the University of Pennsylvania IACUC. Animals were sacrificed at 1, 2, 4, 8 and 16 weeks post detachment and repair (n=4 each). Four additional rats received no treatment and served as a control group. At sacrifice, the supraspinatus tendon, muscle and its bony insertion were isolated, formalin fixed and decalcified in Cal-Ex II® (Formaldehyde 0.93–1.20M, Formic Acid 2.39–2.86M) for up to 3 days and embedded in paraffin. Histologic tissue sections from both shoulders of each animal were used for immunohistochemical analysis.
Immunohistochemical staining was performed using antibodies for bFGF, BMP-12, BMP-13, BMP-14, COMP, CTGF, PDGF-B and TGF-β1 and developed using a standard ABC/DAB method. After dehydration, sections were blocked with Peroxo-Block® to inhibit endogenous peroxidase, washed in PBS and incubated with polyclonal antibody (Table I). Detection of the antibody was conducted using SuperPicTure™ Polymer Detection kit (Zymed Labatories) and visualization of the antibody was accomplished by incubating slides in 3, 3′-diaminobenzidine (DAB) for 5–7 min. Between 4 and 8 histologic sections per antibody time point were evaluated. We originally planned to evaluate up to 8 sections for each, however, due to difficulties in processing and cutting the tendon to bone unit, a smaller number was used in some cases.
Table I.
List of antibodies.
| Antibody (AB) | Antibody (AB) | Company | Dilution used |
|---|---|---|---|
| bFGF | Rabbit anti-basic fibroblast growth factor polyclonal AB | Chemicon | 1:600 |
| BMP-12 (=GDF-7, CDMP-3) | Bone Morphogenetic Protein 12 AB | Biogenesis | 1:800 |
| BMP-13 (=GDF-6, CDMP-2) | Bone Morphogenetic Protein 13 AB | Biogenesis | 1:50 |
| BMP-14 (=GDF-5, CDMP-1) | Anti-mouse growth differentiation factor 5 AB | R&D systems | 1:100 |
| COMP | Cartilage oligomeric matrix protein AB | Abcam | 1:200 |
| CTGF | Connective tissue growth factor AB | Abcam | 1:800 |
| PDGF-B | Platelet-derived growth factor, epitope specific rabbit AB | Lab Vision | 1:200 |
| TGF-β1 | Mouse anti-human transforming growth factor-β monoclonal antibody | Chemicon | 1:600 |
Images were acquired at 50x using a 5.0 megapixel camera (Qimaging). Specimen images were divided into insertion and midsubstance regions digitally using a calibrated pixel measurement tool (Photoshop). The insertion was defined as 0.5mm from the fibrocartilage and the midsubstance was the remainder of the tendon. Specimens were graded objectively using a custom DAB intensity measurement program (Matlab).10 Quartiles were produced using total target DAB intensity ranges, defined as the difference between the highest and lowest intensity value for each target. The minimum total target intensity range was set at 25.6/256 (10% of total range). Each quartile range was assigned a value of undetectable (−), low (+), moderate, (++), or high (+++), and the intensity for each group was graded accordingly. The limits of intensity, and hence the quartiles, were determined individually for each antibody using all the images for that given antibody. Therefore, we evaluated each antibody individually and did not compare directly across antibodies. Median intensity values for each group are reported.
No statistics were planned or performed. The methods used are semiquantitative and are used to descriptively evaluate local changes. Our results were consistent across specimens providing confidence in our results and conclusions.
Results
Immunoassays showed an increase in the expression of all growth factors at 1 week followed by a return to control or undetectable levels by 16 weeks in both the insertion and midsubstance. Of additional note in the insertion, COMP peaked at 1 week followed by a decrease in expression at 2 weeks. BMP-12 and CTGF were moderately expressed across all time points. Furthermore, both BMP-12 and PDGF-B were moderately expressed over time in the midsubstance. Results for PDGF at the insertion are not reported since the total target intensity range was below the set minimum. Complete results are provided in Table II and sample stained images are shown in Figures 1, 2, 3.
Table II.
Growth factor staining intensity (insertion/midsubstance).
| CA | 1 wk | 2 wk | 4 wk | 8 wk | 16 wk | |
|---|---|---|---|---|---|---|
| bFGF | +/+ | +++/+++ | ++/+++ | +/+ | ++/++ | +/− |
| BMP-12 | +/+ | +++/+++ | +/++ | +/+ | ++/++ | +/− |
| BMP-13 | +/− | ++/+++ | ++/+ | ++/+ | ++/+ | −/− |
| BMP-14 | +/++ | ++/+++ | +/+ | +/+ | +/++ | −/− |
| COMP | +/+ | +++/+++ | +/++ | ++/+ | +/+ | −/− |
| CTGF | +/+ | +++/++ | ++/++ | +/+ | +/++ | +/− |
| PDGF-B | undetectable/+ | undetectable/+++ | undetectable/++ | undetectable/+ | undetectable/+ | undetectable/+ |
| TGF-β1 | +/+ | ++/++ | ++/++ | −/+ | +/++ | −/− |
Figure 1.

Negative control (2° antibody staining only)
Figure 2.
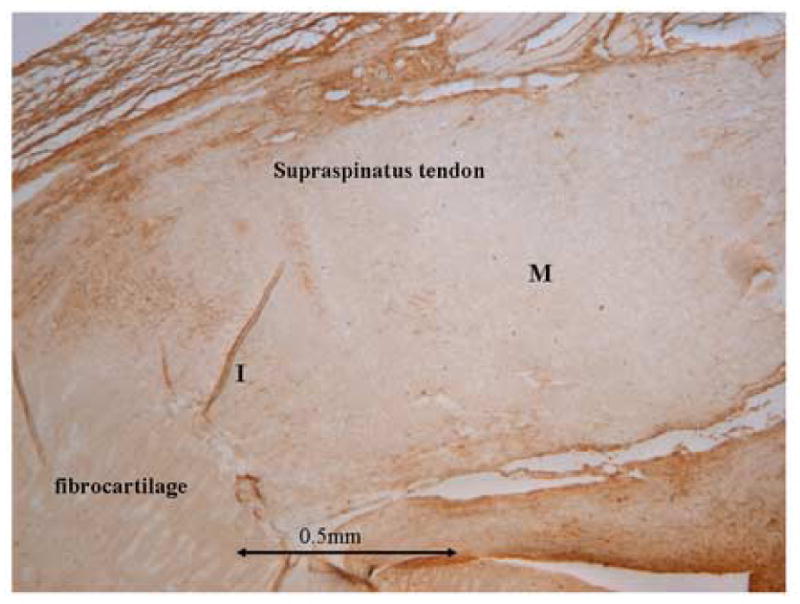
Representative stained image of one selected growth factor demonstrating the variation in expression. I = insertion, M = midsubstance. The insertion was defined as 0.5mm from the fibrocartilage and the midsubstance was the remainder of the tendon in all subsequent immunohistochemisty sub-figures.
Figure 3.

Representative stained images of selected growth factors at time points listed demonstrating the variation in expression. I = insertion, M = midsubstance.
Discussion
The objective of this study was to evaluate the temporal expression of eight different growth factors in supraspinatus tendon to bone healing. We hypothesized that growth factors exhibit unique temporal profiles that correlate to specific stages in the injury and repair process of the supraspinatus tendon. Our findings support this hypothesis as initially we found an increase in expression followed by a return to control or undetectable levels over time in all growth factors. This initial increase may be attributed to cellular degranulation and inflammatory processes in response to the injury/repair. Interestingly, most factors are relatively low at 4 weeks when the repair already has moderate quality and the tendon begins to integrate into bone. At the later time points, when remodeling and reorganization processes become more active, an increase in factor detection specific to the midsubstance occurs. This might be due to an increased loading environment at the tendon and insertion site as tissue heals over time. This finding supports the concept that mobilization and activity have an important impact on tendon to bone healing. At the latest time point, most growth factors returned to normal or were undetectable. This finding might be due to a relative quiescence at this time due to an increase in proliferation and synthetic activity in growth factor expression at the early time points.
The molecular processes of supraspinatus tendon to bone healing are still not fully understood. Since growth factors are important modulators of tissue growth and repair, evaluation of their roles in the healing process is important. Few studies have elaborated the temporal and local expression of different growth factors in tendon to bone healing in vivo. In this study, we used a clinically relevant animal model of tendon to bone healing to help elucidate the role of growth factors. Current fixation techniques of torn rotator cuff tendons in rats provide immediate stability of the tendon-bone unit; however, the healing process leads to the formation of a fibrous scar at the repair site and not fully restored tendon tissue. The following discussion specifically addresses each of the growth factors investigated in this study.
bFGF
Basic fibroblast growth factor (bFGF) has been shown to be involved in wound healing. Specifically, Chan et al showed that in vitro, supplementation of bFGF increases the proliferation of rat patellar tendon fibroblasts.2, 3 bFGF has also been shown to have a stimulatory effect on human rotator cuff tendon cells in vitro but to suppress collagen synthesis.23 Therefore, they hypothesize that early application of bFGf might have therapeutic effects on tendon healing. Recently, immunohistochemical expression of bFGF showed a peak on day 7 in a chronic supraspinatus injury in the rabbit and it was suggested that bFGF can be used to promote the healing process of a torn rotator cuff tendon.13 Our results support these findings of a peak of bFGF expression at 1 week, although in contrast to our hypothesis, in addition to an increase again at 8 weeks. This suggests an increase in tenocyte proliferation early and at late time points, a decrease in cell proliferation and initiation of the remodeling process.24
BMP
BMP-12 is a bone morphogenetic protein responsible for embryonic joint formation. Fu et al postulated that BMP-12 might play a role in tendon regeneration because studies show evidence of BMP-12 in active fibroblasts.7, 27 Since BMP-12 is reported in the literature to impact tendon healing and to correspond to GDF-5, Rickert et al used GDF-5 coated sutures in rat Achilles tendon repairs and showed improved healing.17 Our study showed BMP-12 expression that was present throughout the entire time and increased in the midsubstance at 8 weeks, supporting our hypothesis. Much less is known about BMP-13. However, BMP-13 is in the same sub-family as BMP-12 and is believed to act similarly. Nagakase et al showed CDMP-1, the corresponding factor for BMP-14, in torn human rotator cuff mainly at the torn edges of the tendon and at the bursal side.14 They concluded that rotator cuff cells synthesize CDMP-1 and that this is a modulating factor in tendon healing.
COMP
COMP is a mediator in tendon growth. For instance, Smith et al have shown increased COMP in young equine tendons.19 Recent studies from Södersten et al showed COMP expression increased with maturity and training and they hypothesize that COMP is important for structural integrity of the tendon by binding to collagen fibers.20 In human tendon cultures, Recklies et al showed that COMP is inducible with TGF-β and that COMP is highly expressed in inflammatory synovial tissue.16 It might be important, that a certain level of COMP be expressed and that over-expression might harm the cellular structures providing injury to the site.
CTGF
CTGF increases cartilage proliferation and differentiation as shown by Fukunaga et al.8 Schultze et al showed declining expression of CTGF with differentiation of mesenchymal stem cells into chondrocytes, emphasizing the importance of CTGF in developing skeleton.18 CTGF mediates TGF-β1 induced fibroblast collagen synthesis and may be an important factor in healing.
PDGF-B
PDGF-B increases collagen synthesis has a proliferative effect and induces TGF- β1.22 PDGF has previously been shown to increase type I collagen synthesis in human periodontal ligament cells in vitro.15 Furthermore, Hildebrand et al reported that injections of PDGF in torn rabbit medial collateral ligaments improved biomechanical behaviour.11 Our results coincide with type I collagen synthesis at early time points which may be triggered through an initial high PDGF expression.
TGF- β1
Previous studies have shown that TGF- β1 activates IGF-1, affects fibroblast activity and has an impact on functional recovery in the healing phase. Additionally, it has an important role in collagen production, angiogenesis, restoration of gliding surfaces and adhesion formation.4, 12 Early high levels of TGF-β1 might coincide with excessive scar formation and may remain high due to healing with scar as reported by Tsubone et al.26 Anaguchi et al reported that after injection of TGF- β1, strength increased in patellar tendons with full thickness tears in rabbits.1 Characteristics of TGF- β1 in rat supraspinatus tendon to bone healing after acute injury and repair was previously shown by Galatz et al.9 In that study, TGF-β1 was found to be localized to the scar tissue, the insertion site and the articular surface. Levels in the healing tissue were low in the first days after repair and reached a distinct peak at 10 days post-surgery. Our results show a similar early increase at 1 and 2 weeks in both the insertion and midsubstance. We also saw an increase at 8 weeks specific to the midsubstance. This change may correlate with the scarring process, however, at later time points it was no longer detectable.
Limitations
As with any animal model, there are some limitations to address, one of which is the inherent variation in any in vivo model. Additionally, this rat supraspinatus animal model does not perfectly match the human condition of a chronic rotator cuff tear, however, it does yield useful information, particularly in the acute setting and in questions related to tendon to bone healing. Lastly, we acknowledge the limitations of immunohistochemistry which does not allow for quantification of the results although it does allow for localization of the tendon into an insertion site and tendon midsubstance. Finally, our ability to detect small differences in growth factor expression was limited by the sensitivity of the immunohisochemical analysis.
Conclusion
The objective of this study was to evaluate the temporal expression of eight different growth factors in supraspinatus tendon to bone healing. We hypothesized that growth factors exhibit unique temporal profiles that correlate to specific stages in the injury and repair process of the supraspinatus tendon. Our findings support this hypothesis as initially we found an increase in expression followed by a return to control or undetectable levels over time in all growth factors.
The significance of this work is to provide a characterization of the series of growth factors evaluated which are believed to be important in rotator cuff healing. Based on this study, future therapeutic strategies could be created to improve tendon to bone healing in humans by application of specific growth factors clinically.
Acknowledgments
This work was supported by the SGO, ResOrtho, and the National Institutes of Health through the Penn Center for Musculoskeletal Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anaguchi Y, Yasuda K, Majima T, Tohyama H, Minami A, Hayashi K. The effect of transforming growth factor-beta on mechanical properties of the fibrous tissue regenerated in the patellar tendon after resecting the central portion. Clin Biomech (Bristol, Avon) 2005;20(9):959–65. doi: 10.1016/j.clinbiomech.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Chan BP, Chan KM, Maffulli N, Webb S, Lee KK. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clin Orthop Relat Res. 1997;(342):239–47. [PubMed] [Google Scholar]
- 3.Chan BP, Fu SC, Qin L, Rolf C, Chan KM. Supplementation-time dependence of growth factors in promoting tendon healing. Clin Orthop Relat Res. 2006;448:240–7. doi: 10.1097/01.blo.0000205875.97468.e4. [DOI] [PubMed] [Google Scholar]
- 4.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–55. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005;23(1):84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. Faseb J. 1999;13(13):1774–86. [PubMed] [Google Scholar]
- 7.Fu SC, Wong YP, Chan BP, Pau HM, Cheuk YC, Lee KM, et al. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2003;72(26):2965–74. doi: 10.1016/s0024-3205(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga T, Yamashiro T, Oya S, Takeshita N, Takigawa M, Takano-Yamamoto T. Connective tissue growth factor mRNA expression pattern in cartilages is associated with their type I collagen expression. Bone. 2003;33(6):911–8. doi: 10.1016/j.bone.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24(3):541–50. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 10.Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561–8. doi: 10.1016/j.jbiomech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, et al. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med. 1998;26(4):549–54. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- 12.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg [Am] 2002;27(4):615–20. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, et al. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15(3):371–7. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Nakase T, Sugamoto K, Miyamoto T, Tsumaki N, Luyten FP, Inui H, et al. Activation of cartilage-derived morphogenetic protein-1 in torn rotator cuff. Clin Orthop Relat Res. 2002;(399):140–5. doi: 10.1097/00003086-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Ojima Y, Mizuno M, Kuboki Y, Komori T. In vitro effect of platelet-derived growth factor-BB on collagen synthesis and proliferation of human periodontal ligament cells. Oral Dis. 2003;9(3):144–51. doi: 10.1034/j.1601-0825.2003.02906.x. [DOI] [PubMed] [Google Scholar]
- 16.Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998;41(6):997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19(2):115–26. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- 18.Schutze N, Noth U, Schneidereit J, Hendrich C, Jakob F. Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun Signal. 2005;3(1):5. doi: 10.1186/1478-811X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith RK, Gerard M, Dowling B, Dart AJ, Birch HL, Goodship AE. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: a proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet J Suppl. 2002;(34):241–4. doi: 10.1111/j.2042-3306.2002.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 20.Sodersten F, Ekman S, Eloranta ML, Heinegard D, Dudhia J, Hultenby K. Ultrastructural immunolocalization of cartilage oligomeric matrix protein (COMP) in relation to collagen fibrils in the equine tendon. Matrix Biol. 2005;24(5):376–85. doi: 10.1016/j.matbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5(5):383–92. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 22.Spindler KP, Murray MM, Detwiler KB, Tarter JT, Dawson JM, Nanney LB, et al. The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res. 2003;21(2):245–9. doi: 10.1016/S0736-0266(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 23.Takahasih S, Nakajima M, Kobayashi M, Wakabayashi I, Miyakoshi N, Minagawa H, et al. Effect of recombinant basic fibroblast growth factor (bFGF) on fibroblast-like cells from human rotator cuff tendon. Tohoku J Exp Med. 2002;198(4):207–14. doi: 10.1620/tjem.198.207. [DOI] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg [Am] 2005;30(3):441–7. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125(1):106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 26.Tsubone T, Moran SL, Amadio PC, Zhao C, An KN. Expression of growth factors in canine flexor tendon after laceration in vivo. Ann Plast Surg. 2004;53(4):393–7. doi: 10.1097/01.sap.0000125501.72773.01. [DOI] [PubMed] [Google Scholar]
- 27.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100(2):321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]


