Abstract
Background
Exposure to World Trade Center (WTC) dust and fumes is associated with onset of asthma-like respiratory symptoms in rescue and recovery workers and exposed community members. Eosinophilic inflammation with increased lung and peripheral eosinophils has been described in subpopulations with asthma. We hypothesized that persistent asthma-like symptoms in WTC-exposed individuals would be associated with systemic inflammation characterized by peripheral eosinophils.
Methods
The WTC Environmental Health Center (EHC) is a treatment program for local residents, local workers, and clean-up workers with presumed WTC-related symptoms. Patients undergo a standardized evaluation including questionnaires and complete blood count. Between 9/2005–3/2009, 2461 individuals enrolled in the program and were available for analysis. Individuals with pre-existing respiratory symptoms or lung disease diagnoses prior to 9/11/2001 and current or significant tobacco use were excluded.
Results
1508 individuals met inclusion criteria. These patients had a mean age of 47 years, were mostly female (51%) and had a diverse race/ethnicity. Respiratory symptoms that developed after WTC dust/fume exposure and remained persistent included dyspnea on exertion (68%), cough (57%), chest tightness (46%), and wheeze (33%). A larger percentage of those with wheeze had elevated peripheral eosinophils compared to those without wheeze (21% vs. 13%, p<0.0001). Individuals with elevated peripheral eosinophils were more likely to have airflow obstruction on spirometry (16% vs. 7%, p = 0.0003).
Conclusions
Peripheral eosinophils were associated with wheeze and reduced lung function in a diverse WTC-exposed population. These data suggest that eosinophils may participate in lung inflammation in this population with symptoms consistent with WTC-related asthma.
Keywords: World Trade Center, World Trade Center Environmental Health Center, Lower Respiratory Symptoms, Spirometry, Eosinophil
Introduction
The destruction of the World Trade Center (WTC) towers on September 11, 2001 resulted in the massive release of dust, gas, and fumes with potential environmental and occupational exposures for thousands of individuals. Adverse health effects from these exposures are well described for rescue and recovery workers with work-related exposure, as well as for community members including local workers in the WTC towers and in surrounding commercial spaces, and residents of the surrounding buildings (1–9) (10–11). Community members, including over 360,000 local workers and over 57,000 residents south of Canal Street in lower Manhattan alone have been estimated to have had potential for dust and fume exposure (12). Exposure to the dust cloud from the collapsing buildings on 9/11/01 and exposure to resuspended dusts and ongoing fumes have been shown to be associated with new onset lower respiratory symptoms in local community members (13) (14).
Most studies of WTC-exposed populations demonstrate the presence of new onset and persistent lower respiratory tract symptoms (LRS) of cough, wheeze, dyspnea on exertion (DOE), or chest tightness. These symptoms have been described to persist at least five years after their exposure (7–8, 10, 13, 15). Most patients in screening or treatment programs for rescue workers or community members with potential for WTC dust/fume exposures have been described to have normal spirometry, with only one third displaying abnormalities on screening spirometry (3) (15). Airflow obstruction was identified in only a minority of individuals in these studies; a low forced vital capacity (FVC) was a more common abnormality (3, 15) (16). In a recent study of WTC-exposed community members, those with LRS had an increase in abnormal spirometry and oscillometry measurements compared to those without LRS symptoms; these lung function abnormalities were associated with WTC-dust/fume exposure (17). Many rescue workers and responders, as well as community members with LRS have persistent hyperresponsiveness (1–2) (9). Thus most patients have been given a diagnosis of reactive airways dysfunction, irritant-induced asthma, or asthma. However, not all patients clearly fit the diagnosis of asthma and in some patients sarcoidosis and other interstitial lung diseases have also been described, suggesting a variety of pathologic processes in WTC-exposed patients (18–20).
Few studies have examined biologic processes involved in WTC-related LRS. Rom et al. reported a case of acute eosinophilic pneumonia in a firefighter with symptoms that developed soon after the event (21). Fireman et al. reported an increase in both sputum eosinophils and neutrophils in WTC-exposed firefighters that persisted one year after the event (22). Elevated blood levels of pro-inflammatory cytokines, including granulocyte macrophage-colony stimulating factor, have been identifies in blood samples from firefighters obtained soon after the event (23).
The WTC Environmental Health Center (WTC-EHC) is a treatment program for self-referred community members (residents and individuals who worked in the area surrounding the WTC) as well as for individuals involved in the clean-up of the surrounding area (clean-up workers). These individuals undergo a standardized WTC-dust/fume exposure and clinical evaluation including a complete blood count. Because of the role of eosinophils in asthma, and the descriptions in the responder population, we hypothesized that new onset and persistent LRS in patients enrolled in the WTC EHC would be associated with an eosinophilic process reflected by elevated blood eosinophils.
Materials and Methods
Subjects
Patients were enrolled into the Bellevue WTC EHC as previously described (15). In brief, patients self-referred in response to program information distributed by community-based organizations and local news and advertisements. Enrollment criteria included exposure to WTC dust, gas, or fumes as a local worker, resident, or cleanup worker in southern Manhattan on or in the months after September 11, 2001 and the presence of a physical symptom that occurred or was exacerbated after September 11. Patients who enrolled over the 42 month period from September 2005 until March 2009 were included in the analysis.
Exclusion criteria for this analysis included the existence of respiratory symptoms or disease prior to 9/11/2001 and active or significant past smoking history (≥ 5 pack-years). The study was approved by the New York University School of Medicine IRB and all patients who were analyzed signed consent.
Procedures
On enrollment in the program, patients were administered a multi-dimensional questionnaire that queried demographics; exposure to WTC dust via occupation, work or residence and symptoms, including their severity and temporal relationship relative to September 11, 2001. A physical examination, mental health screen, and blood test, including automated complete blood cell counts were performed. All individuals were initially referred for spirometry, which was performed in accordance with American Thoracic Society/European Respiratory Society standards on a Sensormedics spirometer (Yorba Linda, CA)(24). Lower respiratory tract symptoms (LRS) were defined as cough, chest tightness, dyspnea on exertion (DOE), daytime wheeze or nocturnal wheeze. “New onset” symptoms were defined to include those that began after 9/11/01. “Persistent symptoms” were defined to be present at a frequency ≥ 2 times each week in the month preceding entry into WTC EHC. Predicted values for forced vital capacity (FVC), and forced expiratory volume in one second (FEV1) were derived from NHANES III (25). Spirometry was categorized by spirometric pattern as normal, obstructed (FEV1/FVC < LLN FEV1/FVC) or low FVC (FEV1/FVC > LLN FEV1/FVC and FVC < LLN FVC) and these patterns were used for further analysis (26).
Data Analysis
We describe the population as a whole and after stratification by eosinophil percentage. Descriptive statistics of counts and proportions were calculated for categorical variables. Means and standard deviations were calculated for continuous variables. We performed univariate analysis using the chi-square test for categorical variables, the t-test or Wilcoxon rank-sum test for continuous variables. The percent eosinophils were dichotomized as high eosinophils if ≥ 4% (high eos) vs. low eosinophils if < 4% (low eos), to reflect elevated and normal values in the reference laboratory. Multivariate nominal logistic regression was performed to examine the association between the spirometry patterns and the eosinophil levels controlling for significant variables on univariate analysis (i.e., age and gender). P values less than 0.05 were considered statistically significant. For each individual analysis, individuals with missing values were excluded from the calculations. All statistical analyses were conducted using JMP, version 7. (SAS Institute Inc., Cary, NC,).
Results
Patient Characteristics
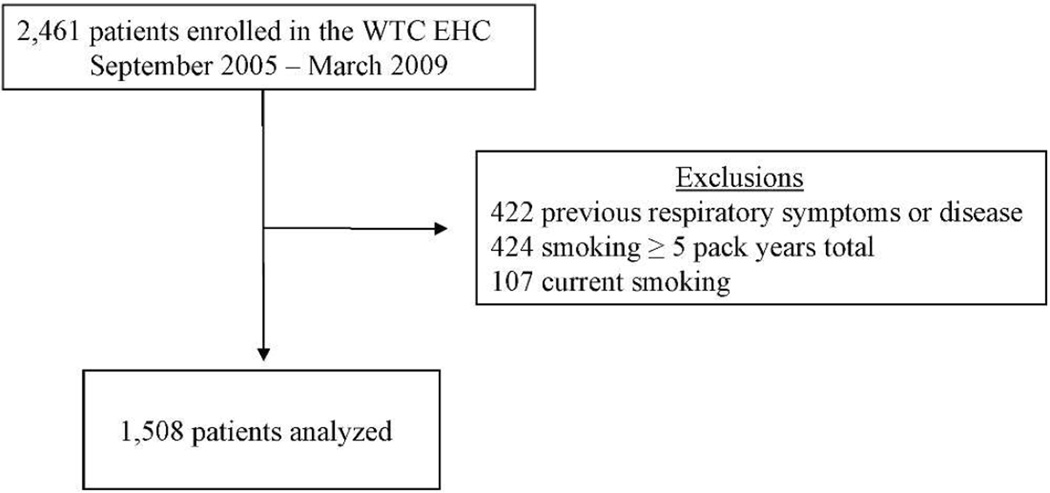
Two thousand four hundred and sixty one patients greater than 17 years of age were consecutively enrolled and signed consents between September 2005 and March 2009 (Figure 1). Nine hundred fifty-three patients were excluded for the presence of respiratory symptoms or respiratory diagnosis prior to September 11, 2001 (n = 422) or current or ≥ 5 pack-year tobacco history (n = 531). The baseline demographics of the eligible patients (n = 1,508) are shown in Table 1. Although nearly equal, there were more women (51%) than men. The mean age was 46.4 years, and the average body mass index (BMI) was 28.2. The group was racially and ethnically diverse. Many (42%) reported being in the initial dust cloud created as the buildings collapsed on 9/11.
Figure 1.
World Trade Center Environmental Health Center (WTC EHC) Population enrolled.
Table 1.
Characteristics of the WTC EHC population with new onset and persistent lower respiratory symptoms as whole and stratified by eosinophil percentage
| Total | High Eos | Low Eos | P value** | |
|---|---|---|---|---|
| n = 1,508 | n = 216 | n = 1,192 | ||
| Gender, n (%) | ||||
| Male | 740 (49) | 127 (59) | 566 (47) | 0.002 |
| Female | 768 (51) | 89 (41) | 626 (53) | |
| Age, mean ± SE | 46.4 ± 0.3 | 44.7 ± 0.9 | 46.7 ± 0.4 | 0.03 |
| BMI, mean ±SE | 28.2 ± 0.2 | 28.0 ± 0.4 | 28.2±0.2 | 0.62 |
| Race/Ethnicity, n (%) | ||||
| Non-Hispanic White | 393 (26) | 47 (22) | 309 (26) | 0.70 |
| Non-Hispanic Black | 226 (15) | 31 (14) | 176 (15) | |
| Hispanic | 678 (45) | 108 (50) | 541 (45) | |
| Asian | 178 (12) | 25 (12) | 141 (12) | |
| No answer | 33 (2) | 5 (2) | 25 (2) | |
| Exposure category, n (%) | ||||
| Clean-up worker | 441(29) | 66 (31) | 361 (30) | NS |
| Rescue and Recovery | 82 (5) | 14 (6) | 65 (5) | |
| Resident | 289 (19) | 34 (16) | 231 (19) | |
| Local worker | 869 (37) | 81 (38) | 445 (37) | |
| Other | 126 (8) | 21 (10) | 89 (7) | |
| Dust cloud exposure, n (%) | ||||
| Yes | 622 (42) | 94 (45) | 494 (42) | NS |
| No | 853 (58) | 117 (55) | 673 (58) |
100 patients did not have phlebotomy performed; 1 subject missing exposure category; dust cloud data missing in 33 subjects
Univariate analysis with t-test or chi-square
The population was stratified into those with high eos (n = 216) and those with low eos (n = 1192). More males were in the high eos compared to low eos group (59 vs. 47% respectively, p = 0.002). The high eos group was slightly older than the low eos group (44.7 ± 0.9 years old vs. 46.7 ± 0.4, p = 0.03) but there was no significant difference in race/ethnicity or BMI. Type of exposure was not associated with either high or low eos group as there was no difference in initial WTC dust cloud exposure or when patients were categorized by type of potential exposure category (i.e. resident, local worker, clean-up worker). There was no difference in the results when absolute eosinophils per unit of volume (>500 eosinophils/microliter of blood) was used (data not shown).
Lower respiratory symptoms and eosinophils
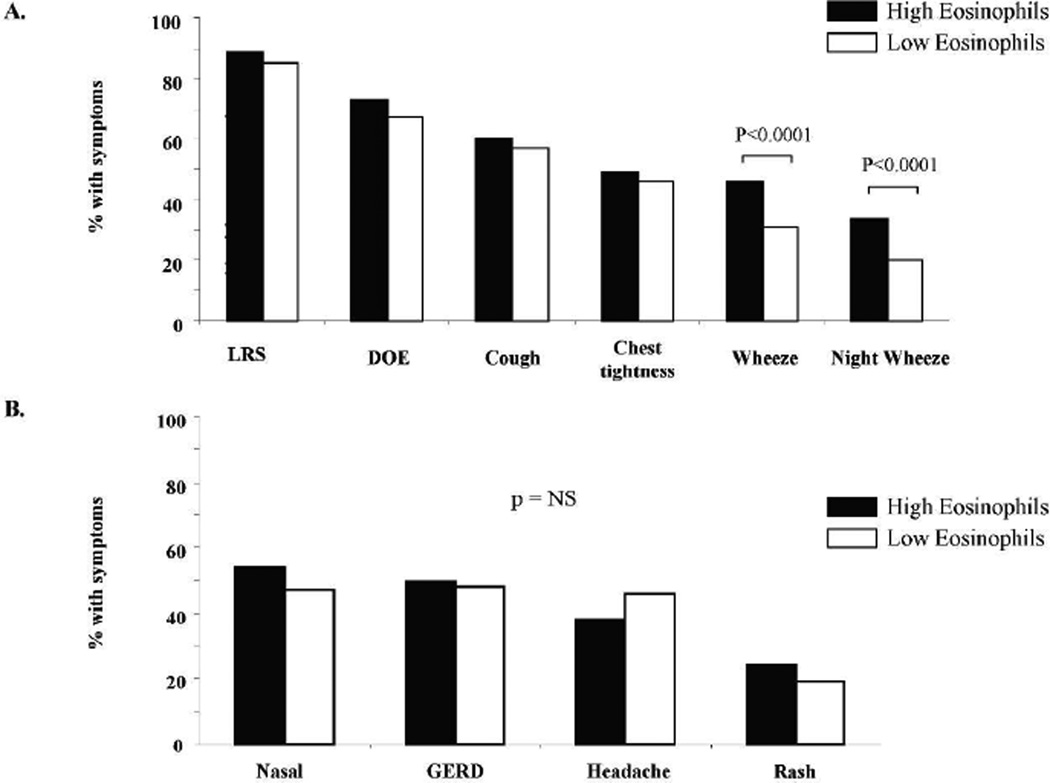
Most symptoms that patients reported upon entry into the WTC EHC related to the upper and lower respiratory tract. More than 80% of the population reported the presence of a new onset and persistent respiratory symptom. Dyspnea on exertion and cough were the most common symptoms (68% and 57% of the total group respectively), although many described chest tightness (46%) and wheeze (33%). The association of respiratory and non respiratory symptoms with the high and low eos groups is shown in Figure 2. Eosinophil level was not associated with presence of any LRS, however there was an association with a specific LRS since daytime and nocturnal wheeze was significantly increased in the high eos compared to the low eos group (daytime wheeze: 46% vs. 31%; nocturnal wheeze: 34% vs. 20 % respectively; p < 0.0001, Panel A). No significant difference between the high eos and low eos group was identified for other specific symptoms of DOE, cough, or chest tightness. Non LRS including nasal congestion, gastroesophageal reflux disease, headache and rash were not associated with high eos (Fig. 2, Panel B).
Figure 2.
Comparison of symptoms in the high and low eosinophil groups. Percent of individuals in high and low eos groups with A: respiratory symptoms B: non-respiratory symptoms.
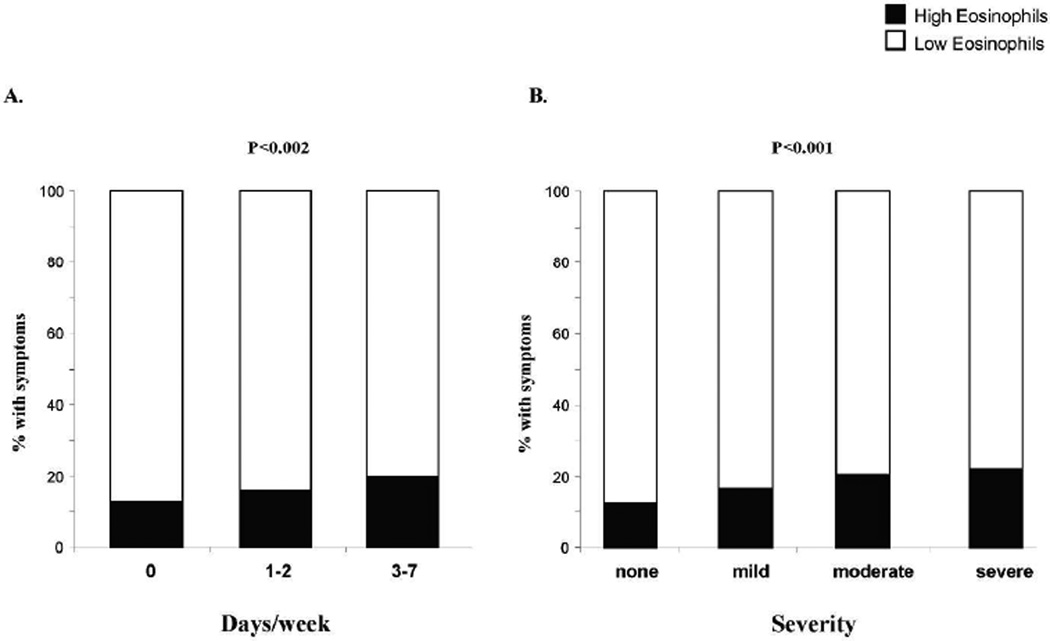
We evaluated whether there was an association of peripheral eosinophils with severity of asthma symptoms using frequency of symptoms or severity of symptoms described as mild, moderate or severe (Fig. 3). Patients with high eos were more likely to have an increase in wheezing frequency and wheeze severity with the percentage of high eos patients increasing from 16.7% in the mild group to 22.3% in the severe group (Figure 3: Panel B, p for trend < 0.001).
Figure 3.
Wheezing severity and eosinophil group. Percent of population with wheezing severity categorized as A: number of days/week with wheezing, and B: description of severity. p values <0.05 significant.
Spirometry and LRS symptoms
Spirometry results were available for 1,160 patients (Table 2). Mean values for FVC, FEV1 and FEV1/FVC were within normal limits for the total population (data not shown). As a whole, the majority of the population (75%) demonstrated a normal spirometry pattern with an obstructive pattern in 9% of the population. The presence of wheeze was noted in 35% of the study population, and was positively associated with an obstructive spirometry pattern (OR 1.92 p < 0.002). New onset and persistent DOE was noted in 68% of the population and was associated with a low FVC spirometry pattern (OR 1.54 p<0.02). Neither cough nor chest tightness discriminated between any lung function patterns, and thus was not included in subsequent analyses.
Table 2.
Presence of Wheezing and Spirometry Category
| Category | Total* | Wheeze yes | Wheeze no | OR† | P value** |
|---|---|---|---|---|---|
| n=1,160 | n = 405 | n = 755 | |||
| Spirometry pattern, n (%) | 0.004 | ||||
| Normal | 863 (74) | 281 (69) | 582 (77) | - | |
| Obstructed a | 99 (9) | 48 (12) | 51 (7) | 1.92 | 0.002 |
| Low FVC b | 198 (17) | 76 (19) | 122 (16) | 1.37 | 0.06 |
FEV1/FVC < LLN FEV1/FVC
FVC<LLN FVC and FEV1/FVC > LLN FEV1/FVC
unadjusted odds ratio (OR)
310 patients from the total population did not have spirometry adequate for evaluation
Univariate analysis with t-test or Chi-square
Spirometry and eosinophil levels
Spirometry results and eosinophil counts were available for 1,133 patients. There was no significant association between high eos and a reduction in specific spirometry measurements including FVC, FEV1 or FEV1/FVC. In contrast, as shown in Table 3, there was an association between high eos and an obstructed spirometry pattern (OR 2.4 p< 0.0003). A significant bronchodilator response (200 ml increase in FEV1 and 12% improvement in FEV1) was also associated with high eos compared to low eos group (34% vs. 24%, p = 0.01). The presence of high eos was not associated with a low FVC spirometry pattern.
Table 3.
Presence of Eosinophils within each Spirometry Category
| Category | Total* | High Eos | Low Eos | OR† | P value |
|---|---|---|---|---|---|
| N = 1,133 | N = 174 | N = 959 | |||
| Spirometry pattern, n (%) | 0.002 | ||||
| Normal | 844 (75) | 116 (67) | 728 (76) | ||
| Obstructed a | 98 (9) | 27 (16) | 71 (7) | 2.4 | 0.0003 |
| Low FVC b | 191(17) | 31 (18) | 160 (17) | 1.22 | 0.04 |
FEV1/FVC < LLN FEV1/FVC
FVC<LLN FVC and FEV1/FVC > LLN FEV1/FVC
unadjusted odds ratio (OR)
PFT category missing in 275 subjects
Eosinophil counts missing in 100 subjects
Discussion
Many local residents, workers and individuals involved in the clean-up activities after the destruction of the WTC towers on September 11, 2001 were exposed to WTC dust, gases, and fumes. As has been described for firefighters and responders, community members have commonly presented with diverse LRS and heterogeneous lung function patterns (15–16, 27). We now report the association of a specific LRS and an obstructive lung function pattern with increased peripheral eosinophils suggesting a possible inflammatory mechanism.
The finding of an increase in peripheral eosinophils in symptomatic WTC-exposed patients is in accordance with the few descriptions of inflammatory responses in WTC-exposed residents. A case report of a firefighter, and a study of WTC-firefighters compared to Israeli firefighters both demonstrated eosinophils in the bronchoalveolar lavage and in induced sputum (21–22). Our study, using a peripheral biomarker, is consistent with these studies and suggests that in some patients with WTC-dust and fume exposure that occurred as a result from exposure as a local worker, resident or clean-up worker, an eosinophilic process may be present. The presence of eosinophils is consistent with the finding of elevated levels of GM-CSF in serum from the firefighter population (28). The persistence of elevated eosinophils and symptoms identified in this population 4–7 years after 9/11 suggests an ongoing process.
The presence of increased peripheral eosinophils was associated with specific symptoms of wheeze, but not DOE. Lower respiratory symptoms of wheeze have been suggested to have high sensitivity for asthma in epidemiologic studies, although they are not diagnostic of asthma (29). The finding of the association of elevated eosinophils with this symptom, but not with overall LRS or symptoms of cough or chest tightness suggests but does not prove, that eosinophils were associated with a more “asthma-like” syndrome in these patients.
Asthma is a heterogeneous disease with different phenotypes; occupational or irritant-induced asthma is considered one phenotype (30–31). Elevated eosinophils, either identified in induced sputum, bronchoalveolar lavage, or bronchial biopsy have been associated with many, but not all adults and children with asthma (32) (33). A “low Th2” lymphocyte/eosinophil and “high Th2“ lymphocyte/eosinophil asthmatic populations have also been suggested based on microarray studies of brushed bronchial epithelial cells (31). A recent multi-dimensional cluster analysis of common clinical factors and measurements distinguished seven clinical subphenotypes, three of which demonstrated an eosinophil predominance (30). In many asthmatics, eosinophilic inflammation has been associated with airway basement membrane remodeling, bronchial hyperreactivity, spirometric parameters of airflow obstruction, and physiologic and clinical improvements and fewer exacerbations after treatment with inhaled corticosteroids (34–40). Thus these studies highlight the importance of distinguishing specific phenotypes and underlying inflammatory types in asthma, including those potentially caused by environmental exposures.
Occupational asthma, one asthma phenotype, can be further categorized into reactive-airways dysfunction (RADS) and irritant-induced asthma. RADS involves the sudden onset of asthma symptoms and signs after a single brief, high-level exposure to an irritant, gas, or fume with resultant airflow obstruction and bronchial hyperresponsiveness (41–43). Irritant-induced asthma typically requires a longer exposure period, often with multiple low-moderate level exposures with a delayed onset of symptoms and signs of asthma which may last for years. Irritant-induced asthma has been suggested to result from direct airway epithelial damage with a subsequent release of pro-inflammatory mediators (44). A few case reports or retrospective cohort studies of irritant-induced asthma resulting from a variety of exposures suggest the presence of eosinophils although these findings have been inconsistent (44–50). The pattern of exposure, often delay in onset of symptoms, and presence of LRS has suggested the possible diagnosis of irritant induced asthma in many of the WTC-exposed community members and clean-up workers (4, 15, 51). In addition, the presence of airway hyperreactivity in very limited studies also supports this diagnosis (1–2, 4, 23, 52).
Our data, using blood eosinophils as a biologic marker, suggest an eosinophilic process in some patients with WTC exposure and symptoms. Interestingly eosinophils were most strongly associated with the specific symptoms of wheeze and an obstructive pattern on spirometry. These data suggest that WTC-related irritant-induced asthma may be associated with an eosinophilic process, whereas the mechanisms responsible for other LRS may differ.
There are several potential limitations to our study. Most patients had normal spirometry and we did not routinely perform methacholine testing. Thus we can only suggest a diagnosis of asthma. This was a study of a population affected by an unplanned disaster. As such, we do not have pre-existing lung function data and use self-report of symptoms. Thus we cannot confirm the attribution of symptoms to WTC exposures but use the combination of reported exposure, temporal association and pattern of symptoms, shown in previous epidemiologic studies, to suggest a likely cause and effect. The quantification of eosinophils performed in sputum and bronchoalveolar lavage has been suggested as a preferred biomarker in asthma (53). We were a large clinical program, and thus evaluated only peripheral eosinophils. Despite this, we detected an association of peripheral eosinophils and specific symptoms. The quantification of eosinophil counts and percentages was performed with automated systems, which may be less accurate than manual count but all patients were examined in the same manner, thus providing a technical bias that would have affected all patients.
In summary, these data suggest a potential inflammatory mechanism for WTC-related lung disease in a subpopulation of patients who present with symptoms consistent with an obstructive lung pattern and suggests a potential mechanism for lung damage. Conversely, in the remaining symptomatic individuals, a different pathophysiologic mechanism may be invoked. Further exploration with multimodality testing and additional clinical phenotyping will help clarify the mechanisms of disease in this considerable population of symptomatic individuals with a unique environmental and occupational exposure.
Conclusions
Peripheral eosinophils were associated with wheeze and reduced lung function in a diverse WTC-exposed population. These data suggest that eosinophils may participate in lung inflammation in this population with symptoms consistent with WTC-related asthma.
Acknowledgments
Grant Sponsor: City of New York, The Centers for Disease Control National Institute for Occupational Safety and Health; Grant Number: 1E11OH009630
Grant Sponsor: The National Institutes of Health; Grant Number: T32 ES007267
Footnotes
Declaration of Interest statement
The authors report no conflicts of interest.
Contributor Information
Angeliki Kazeros, Email: angeliki.kazeros@nyumc.org.
Ming-Tyh Maa, Email: mingtyhmaa@gmail.com.
Paru Patrawalla, Email: paru.patrawalla@nyumc.org.
Mengling Liu, Email: mengling.liu@nyumc.org.
Yongzhao Shao, Email: yongzhao.shao@nyumc.org.
Meredith Turetz, Email: meredith.turetz@nyumc.org.
Sam Parsia, Email: sam.parsia@nyumc.org.
Caralee Caplan-Shaw, Email: caralee.caplan-shaw@nyumc.org.
Linda Rogers, Email: linda.rogers@nyumc.org.
Joan Reibman, Email: joan.reibman@nyumc.org.
References
- 1.Banauch GI, Dhala A, Alleyne D, Alva R, Santhyadka G, Krasko A, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005 Jan;33(1 Suppl):S102–S106. doi: 10.1097/01.ccm.0000151138.10586.3a. [DOI] [PubMed] [Google Scholar]
- 2.Banauch GI, Dhala A, Prezant DJ. Pulmonary disease in rescue workers at the World Trade Center site. Curr Opin Pulm Med. 2005 Mar;11(2):160–168. doi: 10.1097/01.mcp.0000151716.96241.0a. [DOI] [PubMed] [Google Scholar]
- 3.Herbert R, Moline J, Skloot G, Metzger K, Baron S, Luft B, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect. 2006 Dec;114(12):1853–1858. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002 Sep 12;347(11):806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 5.Buyantseva LV, Tulchinsky M, Kapalka GM, Chinchilli VM, Qian Z, Gillio R, et al. Evolution of lower respiratory symptoms in New York police officers after 9/11: a prospective longitudinal study. J Occup Environ Med. 2007 Mar;49(3):310–317. doi: 10.1097/JOM.0b013e318032256e. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler K, McKelvey W, Thorpe L, Perrin M, Cone J, Kass D, et al. Asthma diagnosed after 11 September 2001 among rescue and recovery workers: findings from the World Trade Center Health Registry. Environ Health Perspect. 2007 Nov;115(11):1584–1590. doi: 10.1289/ehp.10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin S, Jones R, Reibman J, Bowers J, Fitzgerald EF, Hwang SA. Reported respiratory symptoms and adverse home conditions after 9/11 among residents living near the World Trade Center. J Asthma. 2007 May;44(4):325–332. doi: 10.1080/02770900701344181. [DOI] [PubMed] [Google Scholar]
- 8.Lin S, Reibman J, Bowers JA, Hwang SA, Hoerning A, Gomez MI, et al. Upper respiratory symptoms and other health effects among residents living near the World Trade Center site after September 11, 2001. Am J Epidemiol. 2005 Sep 15;62(6):499–507. doi: 10.1093/aje/kwi233. [DOI] [PubMed] [Google Scholar]
- 9.Reibman J, Lin S, Hwang SA, Gulati M, Bowers JA, Rogers L, et al. The World Trade Center residents' respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect. 2005 Apr;113(4):406–411. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauer MP, Cummings KR, Hoen R. Long-term respiratory symptoms in World Trade Center responders. Occup Med (Lond) 2010 Mar;60(2):145–151. doi: 10.1093/occmed/kqp176. [DOI] [PubMed] [Google Scholar]
- 11.Samet JM, Geyh AS, Utell MJ. The legacy of World Trade Center dust. N Engl J Med. 2007 May 31;356(22):2233–2236. doi: 10.1056/NEJMp068287. [DOI] [PubMed] [Google Scholar]
- 12.Brackbill RM, Thorpe LE, DiGrande L, Perrin M, Sapp JH, 2nd, Wu D, et al. Surveillance for World Trade Center disaster health effects among survivors of collapsed and damaged buildings. MMWR Surveill Summ. 2006 Apr 7;55(2):1–18. [PubMed] [Google Scholar]
- 13.Farfel M, DiGrande L, Brackbill R, Prann A, Cone J, Friedman S, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health. 2008 Nov;85(6):880–909. doi: 10.1007/s11524-008-9317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maslow C, Friedman SM, Pillai PS, Reibman J, Berger KI, Goldring RM, Stellman SD, Farfel M. Chronic and acute exposures to the effects of the WTC disaster and lower respiratory symptoms: area residents and area workers. American Journal of Public Health. 2012 doi: 10.2105/AJPH.2011.300561. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reibman J, Liu M, Cheng Q, Liautaud S, Rogers L, Lau S, et al. Characteristics of a residential and working community with diverse exposure to World Trade Center dust, gas, and fumes. J Occup Environ Med. 2009 May;51(5):534–541. doi: 10.1097/JOM.0b013e3181a0365b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douwes J, Pearce N. Asthma and the westernization 'package'. Int J Epidemiol. 2002 Dec;31(6):1098–1102. doi: 10.1093/ije/31.6.1098. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SM, Maslow CB, Reibman J, Pillai PS, Goldring RM, Farfel MR, et al. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med. 2011 Sep 1;184(5):582–589. doi: 10.1164/rccm.201011-1909OC. [DOI] [PubMed] [Google Scholar]
- 18.Wu M, Gordon RE, Herbert R, Padilla M, Moline J, Mendelson D, et al. Case report: Lung disease in World Trade Center responders exposed to dust and smoke: carbon nanotubes found in the lungs of World Trade Center patients and dust samples. Environ Health Perspect. 2010 Apr;118(4):499–504. doi: 10.1289/ehp.0901159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caplan-Shaw C, Yee H, Rogers L, Abraham JL, Parsia SS, Naidich DP, Borczuk A, Moreira A, Shiau MC, Ko JP, Brusca-Augello G, Berger KI, Goldring RM, Reibman J. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas and fumes. Journal of Occupational Environmental Medicine. 2011 doi: 10.1097/JOM.0b013e31822fff60. in press. [DOI] [PubMed] [Google Scholar]
- 20.Izbicki G, Chavko R, Banauch GI, Weiden MD, Berger KI, Aldrich TK, et al. World Trade Center "sarcoid-like" granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007 May;131(5):1414–1423. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 21.Rom WN, Weiden M, Garcia R, Yie TA, Vathesatogkit P, Tse DB, et al. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002 Sep 15;166(6):797–800. doi: 10.1164/rccm.200206-576OC. [DOI] [PubMed] [Google Scholar]
- 22.Fireman EM, Lerman Y, Ganor E, Greif J, Fireman-Shoresh S, Lioy PJ, et al. Induced sputum assessment in New York City firefighters exposed to World Trade Center dust. Environ Health Perspect. 2004 Nov;112(15):1564–1569. doi: 10.1289/ehp.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolan A, Naveed B, Comfort AL, Ferrier N, Hall CB, Kwon S, et al. Inflammatory Biomarkers Predict Airflow Obstruction After Exposure to World Trade Center Dust. Chest. 2011 Oct 13; doi: 10.1378/chest.11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL. Office spirometry: does poor quality render it impractical? Chest. 1999 Aug;116(2):276–277. doi: 10.1378/chest.116.2.276. [DOI] [PubMed] [Google Scholar]
- 27.Brackbill RM, Hadler JL, DiGrande L, Ekenga CC, Farfel MR, Friedman S, et al. Asthma and posttraumatic stress symptoms 5 to 6 years following exposure to the World Trade Center terrorist attack. JAMA. 2009 Aug 5;302(5):502–516. doi: 10.1001/jama.2009.1121. [DOI] [PubMed] [Google Scholar]
- 28.Djukanovic R, Mann M, Rimmer J, Spackman D, Lau L, Church MK, et al. The effect of inhaled allergen on circulating basophils in atopic asthma. J Allergy Clin Immunol. 1992 Aug;90(2):175–183. doi: 10.1016/0091-6749(92)90069-e. [DOI] [PubMed] [Google Scholar]
- 29.Ravault C, Kauffmann F. Validity of the IUATLD (1986) questionnaire in the EGEA study. International Union Against Tuberculosis and Lung Disease. Epidemiological study on the Genetics and Environment of Asthma, bronchial hyperresponsiveness and atopy. Int J Tuberc Lung Dis. 2001 Feb;5(2):191–196. [PubMed] [Google Scholar]
- 30.Bradding P, Green RH. Subclinical phenotypes of asthma. Curr Opin Allergy Clin Immunol. 2010 Feb;10(1):54–59. doi: 10.1097/ACI.0b013e32833489a9. [DOI] [PubMed] [Google Scholar]
- 31.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009 Sep 1;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002 Jul;57(7):643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A Large Subgroup of Mild-to-Moderate Asthma Is Persistently Noneosinophilic. Am J Respir Crit Care Med. 2012 Mar 15;185(6):612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 35.Djukanovic R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992 Mar;145(3):669–674. doi: 10.1164/ajrccm/145.3.669. [DOI] [PubMed] [Google Scholar]
- 36.Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J Allergy Clin Immunol. 2000 Apr;105(4):752–759. doi: 10.1067/mai.2000.105319. [DOI] [PubMed] [Google Scholar]
- 37.Kampe M, Stalenheim G, Janson C, Stolt I, Carlson M. Systemic and local eosinophil inflammation during the birch pollen season in allergic patients with predominant rhinitis or asthma. Clin Mol Allergy. 2007;5:4. doi: 10.1186/1476-7961-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petsky HL, Kynaston JA, Turner C, Li AM, Cates CJ, Lasserson TJ, et al. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2007;(2):CD005603. doi: 10.1002/14651858.CD005603.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Ward C, Pais M, Bish R, Reid D, Feltis B, Johns D, et al. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002 Apr;57(4):309–316. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meijer RJ, Postma DS, Kauffman HF, Arends LR, Koeter GH, Kerstjens HA. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy. 2002 Jul;32(7):1096–1103. doi: 10.1046/j.1365-2222.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 41.Brooks SM. Occupational asthma. Toxicol Lett. 1995 Dec;82–83:39–45. doi: 10.1016/0378-4274(95)03466-8. [DOI] [PubMed] [Google Scholar]
- 42.Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest. 1985 Sep;88(3):376–384. doi: 10.1378/chest.88.3.376. [DOI] [PubMed] [Google Scholar]
- 43.Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome. Case reports of persistent airways hyperreactivity following high-level irritant exposures. J Occup Med. 1985 Jul;27(7):473–47. [PubMed] [Google Scholar]
- 44.Quirce S, Gala G, Perez-Camo I, Sanchez-Fernandez C, Pacheco A, Losada E. Irritant-induced asthma: clinical and functional aspects. J Asthma. 2000 May;37(3):267–274. doi: 10.3109/02770900009055449. [DOI] [PubMed] [Google Scholar]
- 45.Chang-Yeung M, Lam S, Kennedy SM, Frew AJ. Persistent asthma after repeated exposure to high concentrations of gases in pulpmills. Am J Respir Crit Care Med. 1994 Jun;149(6):1676–1680. doi: 10.1164/ajrccm.149.6.8004329. [DOI] [PubMed] [Google Scholar]
- 46.Gautrin D, Boulet LP, Boutet M, Dugas M, Bherer L, L'Archeveque J, et al. Is reactive airways dysfunction syndrome a variant of occupational asthma? J Allergy Clin Immunol. 1994 Jan;93(1 Pt 1):12–22. doi: 10.1016/0091-6749(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 47.Lemiere C, Efthimiadis A, Hargreave FE. Occupational eosinophilic bronchitis without asthma: an unknown occupational airway disease. J Allergy Clin Immunol. 1997 Dec;100(6 Pt 1):852–853. doi: 10.1016/s0091-6749(97)70286-0. [DOI] [PubMed] [Google Scholar]
- 48.Malo JL, L'Archeveque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med. 2009 May 15;179(10):923–928. doi: 10.1164/rccm.200810-1550OC. [DOI] [PubMed] [Google Scholar]
- 49.Moscato G, Pala G, Perfetti L, Frascaroli M, Pignatti P. Clinical and inflammatory features of occupational asthma caused by persulphate salts in comparison with asthma associated with occupational rhinitis. Allergy. 2010 Jun 1;65(6):784–790. doi: 10.1111/j.1398-9995.2009.02288.x. [DOI] [PubMed] [Google Scholar]
- 50.Takeda N, Maghni K, Daigle S, L'Archeveque J, Castellanos L, Al-Ramli W, et al. Long-term pathologic consequences of acute irritant-induced asthma. J Allergy Clin Immunol. 2009 Nov;124(5):975–981. e1. doi: 10.1016/j.jaci.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 51.de la Hoz RE, Shohet MR, Chasan R, Bienenfeld LA, Afilaka AA, Levin SM, et al. Occupational toxicant inhalation injury: the World Trade Center (WTC) experience. Int Arch Occup Environ Health. 2008 Feb;81(4):479–485. doi: 10.1007/s00420-007-0240-x. [DOI] [PubMed] [Google Scholar]
- 52.Weiden MD, Ferrier N, Nolan A, Rom WN, Comfort A, Gustave J, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010 Mar;137(3):566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai CK, Djukanovic R, Wilson JW, Wilson SJ, Britten KM, Howarth PH, et al. Effect of inhaled platelet-activating factor on bronchial inflammation in atopic non-asthmatic subjects. Int Arch Allergy Immunol. 1992;99(1):84–90. doi: 10.1159/000236339. [DOI] [PubMed] [Google Scholar]