Abstract
Background
We have been investigating the molecular mechanisms by which trichloroethylene (TCE) might induce cardiac malformations in the embryonic heart. Previous investigations indicated that TCE disrupted expression of genes encoding proteins involved in regulation of intracellular Ca2+, [Ca2+]I, in cardiac cells, including Rynodine receptor isoform 2 (Ryr2), and Sarcoendoplasmatic reticulum Ca2+ ATPase, Serca2a. These observations are important in light of the notion that altered cardiac contractility can produce morphological defects.
Objectives
We hypothesized that the TCE-induced changes in gene expression of Ca2+-associated proteins resulted in altered Ca2+ response to natural agonists.
Methods
Real-time PCR and digital imaging microscopy were used in this study to characterize effects of TCE on gene expression and Ca2+ response to vasopressin in rat cardiac H9c2 myocytes.
Results
We observed a reduction in Serca2a and Ryr2 gene expression in response to 12 hr exposure to TCE. Longer TCE exposure (48hr) resulted in increased Ryr2 expression whereas Serca2a transcript levels remained lower. In addition, we found significant differences in Ca2+ response to vasopressin in cells treated with TCE doses as low as 10 parts per billion. Specific changes included a slower time to peak [Ca2+]i, lower peak [Ca2+]i and slower recovery to baseline [Ca2+]i.
Conclusions
Cell imaging and expression data analyses strongly indicate that exposure to TCE disrupts the ability of myocytes to regulate cellular Ca2+ fluxes. Time and dose-dependent changes in Serca2a and RyR2 gene expression are consistent with changes observed in calcium signaling. Perturbation of calcium signaling alters cardiac cell physiology and signal transduction and may hint to morphogenetic consequences in the context of heart development. These results, if confirmed in vivo, may help to explain the apparent cardio-specific toxicity of TCE exposure in the rodent embryo.
1. Introduction
Heart defects are the most common birth defect-related cause of infant death in the United States with nearly one-fifth of infant deaths annually (CDC 1998; NCHS 2004). There are multiple lines of evidence that point to environmental factors as possible causes of congenital heart malformations. Among these are chlorinated hydrocarbons, including trichloroethylene (TCE) which has been suggested as a cardiac teratogen in humans and animal models (Goldberg et al. 1990; Bove et al., 2002; Johnson et al. 1998; Loeber et al. 1988). Recent review articles (Hardin et al, 2005; Watson et al., 2006) criticized some of the epidemiological and rodent studies. Goldberg et al. (1990) studies were faulted for the selection of controls and patients, but a recent independent re-evaluation of the data largely supported the findings (Bove et al., 2002). Criticisms of the animal studies stress the inconsistent findings between species, low statistical power, and the use of high, environmentally unrealistic doses of TCE. Recently, several groups reported on possible mechanisms by which TCE may affect heart development in chick embryos (Drake et al., 2006a and b, Mishima et al., 2006) at more relevant doses in the ranges of 8ppb to 80ppm. Avian models provide an excellent model to study specific developmental processes common to most vertebrates and they are widely used to study heart development. We previously showed that the process of epithelial-mesenchymal transition, a process used to produce cardiac valve precursors in the heart, was inhibited ex vivo in the 50-250 ppm range (Boyer et al., 2000). Because TCE cardiac teratogenesis remains a highly controversial issue, studies elucidating the molecular and cellular mechanisms of its action may help to clarify some aspects of the controversy (e.g. effects of low versus high doses of TCE, cell-specific effects versus whole organism). Ou et al. (2003) used cultured bovine coronary endothelial cells (BCEC) to demonstrate that TCE disrupted the activity of the endothelial Nitric Oxide Synthase (eNOS) protein and endothelial cell function, suggesting a possible mechanism by which TCE may alter specific processes during heart development and contribute to heart malformations.
Similarly, previous molecular studies of the effects of TCE on rat embryonic hearts and a rat myocardial cell line in our laboratory showed that several molecules relevant to Ca2+ metabolism are perturbed. Collier et al. (2003) showed Serca2a mRNA expression to be reduced in rat embryo heart tissues collected at day 12 after daily maternal exposure to 100 ppm in drinking water. More recently, Ryr and CamK were identified in a microarray analysis of genes altered by exposure of a P19 mouse stem cell line to 1 ppm TCE in vitro (Selmin et al., 2008 submitted).
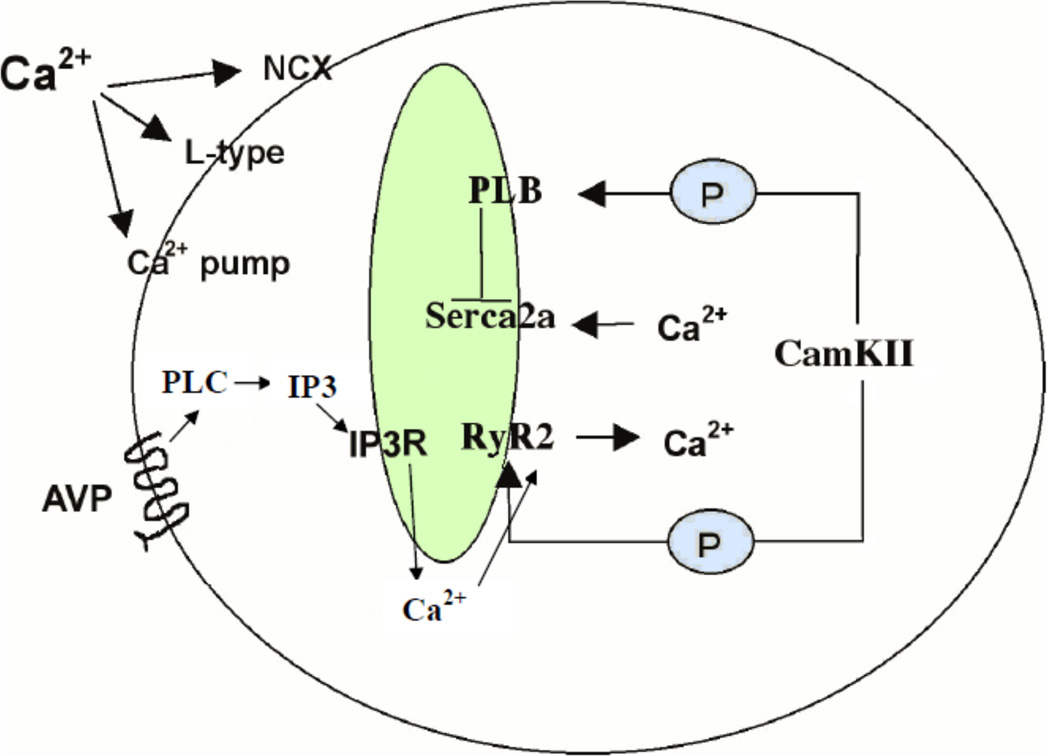
In cardiomyocytes, Ca2+ is a fundamental regulator of contractility and relaxation (Figure 1). A heart beat contraction is achieved when the sarcolemma L-type channels open and allow for Ca2+ to flow into the cytosol, activating RyR2 to release Ca2+ from the sarcoplasmic reticulum (SR) storage and thus increasing intracellular Ca2+ concentration ([Ca2+]i) around the myofibrils. The relaxation phase occurs when CamKIIγ phosphorylates phospholamban, resulting in inhibition of the sarcoplasmic reticulum Ca2+-ATPase (Serca2a) which allows Serca2a to quickly pump Ca2+ out of the cytosol and into the SR. This prepares Ca2+ stores (i.e., the SR) for the next contraction. Molecular defects in these Ca2+ channels or pumps can cause cardiac pathophysiologies due to a role in excitation-contraction (Prasad et al. 2004). Reduced expression of Serca2a protein is associated with impaired heart function and malformations (Ji et al. 2000; Lalli et al. 2001; Periasamy et al. 1999; Ver Heyen et al. 2001). Furthermore, targeted over-expression of the Serca2a gene in transgenic mouse hearts has been shown to alter cardiac contractility by increasing SR Ca2+ transport (Baker et al. 1998). Ca2+ signaling can be directly activated in primary cultured rat cardiomyocytes through application of extracellular VP (Chandrashekhar et al. 2003). Although application of VP results in an IP3-dependent release of Ca2+ from intracellular stores, many of the processes that contribute to [Ca2+]i regulation are shared with L-type channel initiated contractions.
Figure 1. Proteins in the cardiomyocyte Ca2+ Signaling Pathway.
A typical contraction in a mammalian cardiomyocyte is initiated by Ca2+ entry into the cytosol via L-type Ca2+ channels on the sarcolemma. This Ca2+ influx can directly cause release of Ca2+ from the sarcoplasmic reticulum via the ryanodine receptor (RyR2) channel on the sarcoplasmic reticulum (SR) resulting in an increased [Ca2+]i. The high levels of [Ca2+]i, in turn, trigger the myofilaments to contract and result in activation of CamKIIγ which can phosphorylates phospholamban to release its inhibition from Serca2a. Serca2a then pumps Ca2+ from the cytosol into the SR for the next cardiac cycle. Ca2+ signaling can also be initiated by vasopressin through the G-protein coupled AVP receptor to activate the IP3 pathway. Local production of IP3 results in release of Ca2+ form the SR via the IP3 receptor (IP3R) Ca2+ channel. The removal of cytosolic Ca2+ is also through Serca2a.
In this paper, we present evidence that expression of the Serca2a and Ryr2 transcript is repressed by TCE in a rat cardiomyocyte cell line (H9c2) consistent with our previous observations in vivo. We also show that the Ca2+ response to a natural agonist is altered by TCE, in both a dose- and time- dependent manner. These results suggest the toxic effects of TCE during embryonic cardiac differentiation might lead to development of congenital malformations in vivo via a dysregulated Ca2+ signaling pathway. The early requirement for cardiac function in normal development and the sensitivity of this process to perturbation of Ca2+ homeostasis may provide a working hypothesis to explore the apparent specificity of TCE as a cardiac teratogen in the developing rodent embryo.
2. Materials and Methods
TCE was purchased from Aldrich (Milwaukee, WI). All other reagents were obtained from Sigma Chemical Co. (St. Louis, MO), unless otherwise stated.
2.1. H9c2: Rat cardiac myocyte cell line
The rat cardiomyocyte cell line H9c2 was obtained from ATCC (CRL-1446). Cells were grown to 90% confluence in T-75 tissue culture flasks, in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% FBS and 3.3 g/L NaHCO3 at 10% CO2. TCE solutions were prepared fresh daily for each treatment by diluting the original TCE reagent in DMEM-10% FBS media to obtain a 1000 ppm stock solution. Air space within the original TCE reagent bottle and stock solution was flushed with nitrogen gas to reduce chemical breakdown. Medium was replaced every 24 hours. Control cultures (0 ppb) were incubated in a separate incubator to avoid any opportunity for volatile transfer.
For Real-Time PCR analysis, culture flasks were separated into treatment groups distinguished by TCE concentration: control, 10 ppb, 100 ppb, 1 ppm, 10 ppm; and time of TCE exposure: 12 and 48 hours. Total RNA was extracted from H9c2 cells using Trizol LE reagent (Invitrogen, Carlsbad, CA) and purified using RNeasy Mini Kits (Qiagen, Valencia, CA). Equal aliquots of total RNA were transcribed into cDNA using ISCRIPT supermix kit (Bio-Rad Laboratories, Hercules, CA), according to manufacturer protocols.
For calcium imaging analysis, cells were transferred to 15 mm glass coverslips (Carolina Biologicals, NC). Coverslips were placed in 25 mm wells in 12-well cell culture dishes (#3512, Corning Incorporated, Lowell, MA) and wells were filled with 1 ml of media. Each coverslip was separated into treatment groups: control, 10 ppb, 100 ppb, 1 ppm, 10 ppm. Within each group, cells were exposed to TCE for two different time periods: 18 and 54 hours. To account for protein synthesis, exposure time was increased by 6 hours, compared to the collection times for RNA.
2.2. Real-time PCR
cDNA concentrations from each treatment were measured by spectrophometry and equal amounts were used as templates for amplification. The Applied Biosystems Primer Express program was used to design primers and probes in regions specific to Serca2a (Gen Bank Accession Number gi 34872410), Ryr2 (Gen Bank Accession Number AF363960), and β-actin (GenBank Accession Number NM031144). Blast analyses of primers were performed against the rat genome to confirm their specificity. Efficiency of PCR amplification for each set of primers was determined using an external standard curve, generated using recombinant pCR2.1 plasmids containing amplicon fragments of the Serca2a, Ryr2 or β-actin genes. Real-time reactions were performed using QuantiTect Multiplex PCR kit (Qiagen, Valencia, CA) and run at a final volume of 25 µl. Each master mix consisted of the following: 12.5 µl QuantiTect Multiplex master mix, 2.5 µl Serca2a probe (2 µM), 1.0 ul reverse Serca2a primer (10 µM), 1.0 µl forward Serca2a primer (10 µM), 1.25 β-actin probe (2 µM), 0.5 ul forward β-actin primer (10 µM), 0.5ul reverse β-actin primer (10 µM), 2.0 µl of template cDNA, and 3.75 µl nuclease-free ddH2O. Ryr2 PCR was conducted by SYBR Green analysis using ITAQ SYBR Green supermix with ROX (Bio-rad Laboratories, Hercules, CA). Reactions were carried out using an ABI 7300 Real Time PCR system and software (Applied Biosystems, Foster City, CA). Analysis was performed in triplicate, using cDNA samples from two or three independent experiments to account for biological variances. Concentration of each experimental sample was determined using the linear regression obtained from the serial dilutions of the plasmid DNA standard. All samples were then normalized against β-actin. Expression levels in each treatment group were compared to untreated samples and expressed in fold change.
2.3. Primers for Real-time PCR
The primers used for PCR measurement were: Serca2a (Acc. Number gi34872419) 96889F: 5’-TTCCAACATCCATCAACTAACCA-3’; 98678R”: 5’-TGGAAGATGTGTTGCTAACAACG-3’ Serca2a probe: 6FAM-ACTGGAGTAACCGCTTCCTAAACATTGCAGAA-TAMRA; Ryr2 (Acc. Number AF363960) F49:5’ACCGAGCAGGAGGAGTTGTTG ; R133:5’CCACCCGGTGATTCCCAAG; β-Actin (Acc. Number NM031144) 441F: 5’-CCAGATCATGTTTGAGACCTTCAA-3’ 527R: 5’-GTGGTACGACCAGAGGCATACA-3’ β-Actin probe 471 T: VIC-AGCCATGTACGTAGCCAT-MGBNFQ
2.4. Real-Time PCR Standard - Plasmid Construct Design
Fragments of the β-Actin, Serca2a and Ryr2 transcripts flanking their specific primers (see above) were isolated from H9c2 control cDNA. PCR products were separated on 1% TE-agarose gel to confirm the correct insert size, and sent to The University of Arizona Sequencing Core (DNA Sequencing Service, Tucson, AZ) for sequence verification. PCR products were sub cloned in pCR2.1 plasmid with the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Real-time standard curve linear regression copy concentrations were calculated from plasmid spectrophotometer readings.
2.5. Cell Imaging
H9c2 cells on glass coverslips were washed with a modified Hanks Balanced Saline Solution (HBSS: 1.3 mM CaCl2, 5.0 mM KCl, 0.3 mM KH2PO4, 0.5 mM MgCl2, 0.4 mM MgSO4, 137.9 mM NaCl, 0.3 mM Na2PO4 and 1% glucose additionally buffered with 25 mM HEPES, pH 7.4) and loaded for 45 min in 5 µM fura 2-AM (CalBiochem, La Jolla, CA). Cells were removed from fura2-AM loading solution and placed back into HBSS for at least 20 min before Ca2+ imaging. Fura 2 fluorescence was observed on an Olympus IX70 microscope with a 40X oil objective after alternating excitation at 340 and 380 nm by a 75 W Xenon lamp linked to a Delta Ram V illuminator (PTI Inc., NJ) and a gel optic line. Images of emitted fluorescence above 505 nm were recorded by an ICCD camera (PTI) and simultaneously displayed on a 21” vivitron color monitor. The imaging system was under software control (ImageMaster, PTI) and collected 340/380 ratios approximately every 0.6 sec. Intracellular Ca2+ concentration ([Ca2+]i) was calculated by ratiometric analysis of fura-2 fluorescence using equations originally published in (Grynkiewicz et al. 1985). A typical field of view contained between 8 and 17 cells (13.88 +/−2.04) for the 18hr treatment groups and between 10.17+/−2.04 for the 54 hr experiments. For each TCE treatment, 5 different fields were analyzed (except 18 hr 10ppmTCE, n=4) in two separate treatment days, and represent data from average of 60 cells. In each field, only those cells that increased [Ca 2+]i to > 100 nM within 10 seconds were considered “responsive”. On average, 85-90% of cells in each field were considered responsive to vasopressin and analyzed.
2.6. Statistical analysis
All Ca2+ data were compared with GraphPad Software (San Diego, CA) using ANOVA with Tukey’s multiple comparison post-test. A value of P < 0.05 was used to establish significant differences between samples. Figures are graphed ± standard error of the mean (SEM). Serca2a and Ryr2 expression data were analyzed by T-test. A value of P<0.05 was used to establish significance between control and each TCE-exposed group.
3. Results
3.1. TCE effects on Serca2a and Ryr2 expression in rat cardiomyocytes
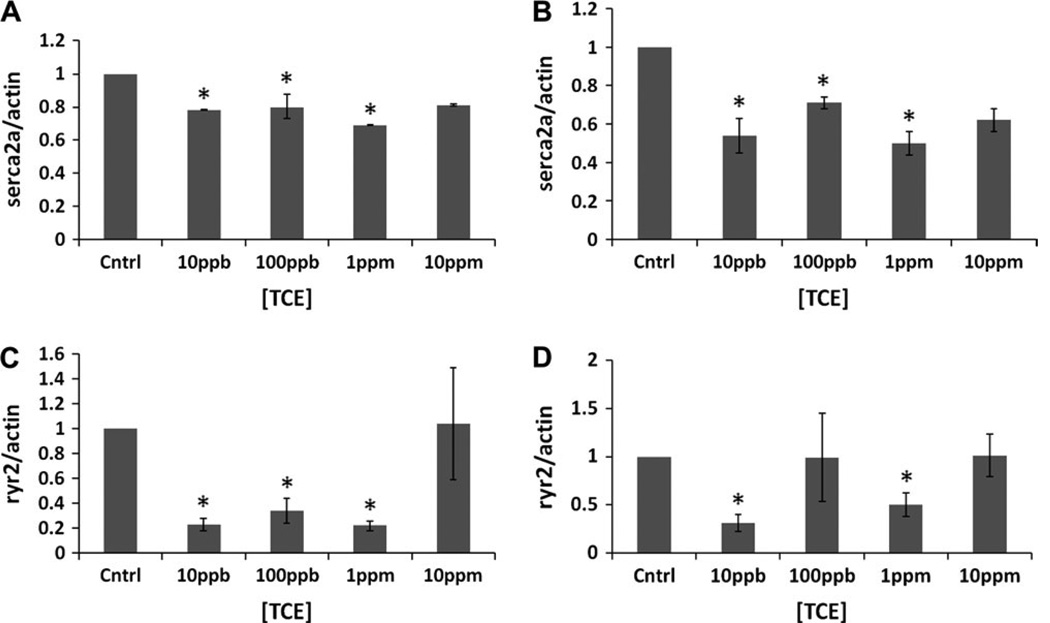
Real-time PCR was used to determine whether Serca2a and Ryr2 mRNA expression was perturbed by varied doses and times of TCE exposure. H9c2 cells were exposed to control (0 ppb), 10 ppb, 100 ppb, 1 ppm and 10 ppm TCE for 12 or 48 hours. After 12 hours (Figure 2A), TCE-exposed cells displayed significantly reduced Serca2a transcripts at 10 ppb, 100 ppb and 1ppm by 22%, 29% and 26%, respectively, compared to the controls. At 48 hours (Figure 2B), we observed overall a more pronounced inhibitory effect of TCE on Serca2a transcript levels. The most significant changes, compared to the control, were observed, again, at 10 ppb, 100 ppb and 1 ppm by 43%, 41% and 47%, respectively. At the highest dose tested, 10 ppm TCE, lower average transcript levels were observed but were not significant due to the variability of the cellular response. TCE had similar effects on Ryr2 mRNA at 12hr (Figure 2C), causing a reduced expression level at all doses of TCE tested, with the most dramatic (80% reduction) observed at 100ppb TCE. At 48 hr, (Figure 2D) we observed increased expression at 100ppb and 10ppm, 1 ppm did not show any change, and 10ppbTCE caused a 75% reduction.
Figure 2. Dose-response analysis.
Real –time PCR analysis of Serca2a (2A and B) and Ryr2 (2C and D) transcripts was performed in H9c2 cells exposed to different doses of TCE (10 and 100 ppb, 1 and 10 ppm) for either 12 (A and C) or 48 hours (B and D). Fold expression at each dose was calculated against control (without TCE) as a ratio between Serca2a and B-actin. Graphs in A and B represent the average of three independent experiments. Each experiment point was tested in triplicate. [*] identify significant differences as determined by Student’s t-test (p < 0.05). Graphs in C and D represent the average of two independent experiments, each one was repeated at least two times.
3.2. TCE effects on Ca2+ signaling in rat cardiomyocytes
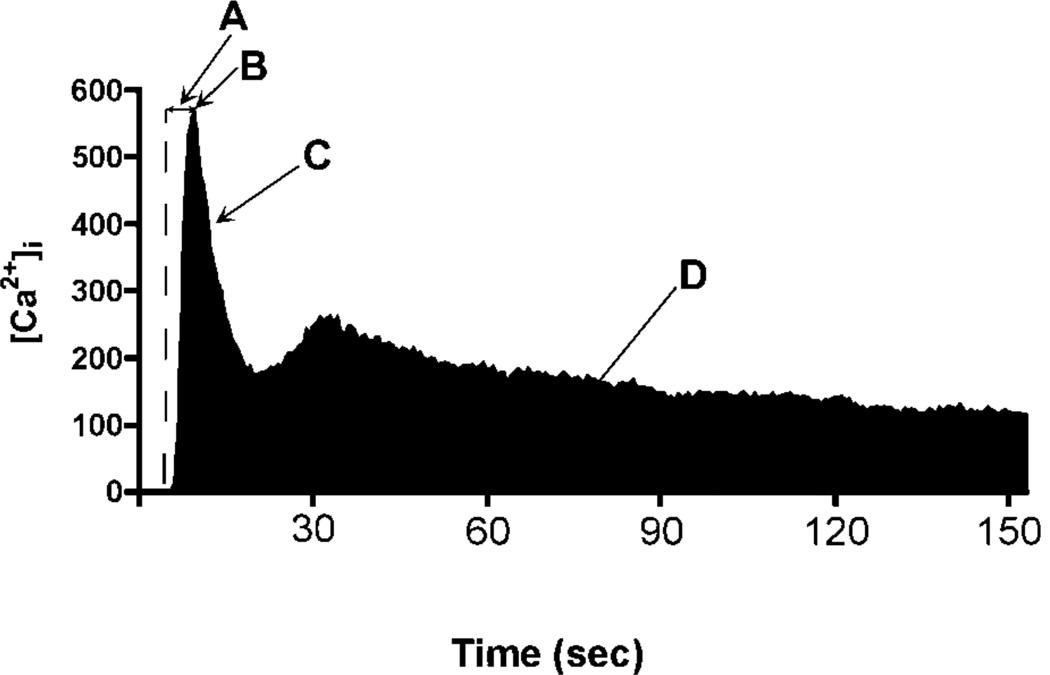
To evaluate whether the changes in Serca2a and Ryr2 transcript level produced by TCE had a measurable effect on Ca2+ signaling in cardiomyocytes, H9c2 cells exposed to varying concentrations of TCE were loaded with Fura2 dye, washed with 1 nM VP and [Ca2+]i changes over time were determined by ratio-imaging (Figures 3–5). A typical H9c2 [Ca2+]i response to VP is shown in Figure 3. Variations in response to TCE included several measurable events: rate of increase in [Ca2+]i in response to VP addition (3A); the peak [Ca2+]i response to VP (3B); the initial rate of recovery from the Ca2+ peak (3C); and the [Ca2+]i flux in response to VP (3D).
Figure 3. H9c2 cell Response to Vasopressin (VP).
A typical cell responding to a 1 nM VP increases [Ca2+]i (A) to reach a peak [Ca2+]i (B). [Ca2+]i then begins an exponential recovery phase until reaching a plateau of increased [Ca2+] (C), that may or may not show a second [Ca2+]i peak. The total Ca2+ response to agonist can be estimated by calculating the area under the Ca2+ trace (D, shaded black).
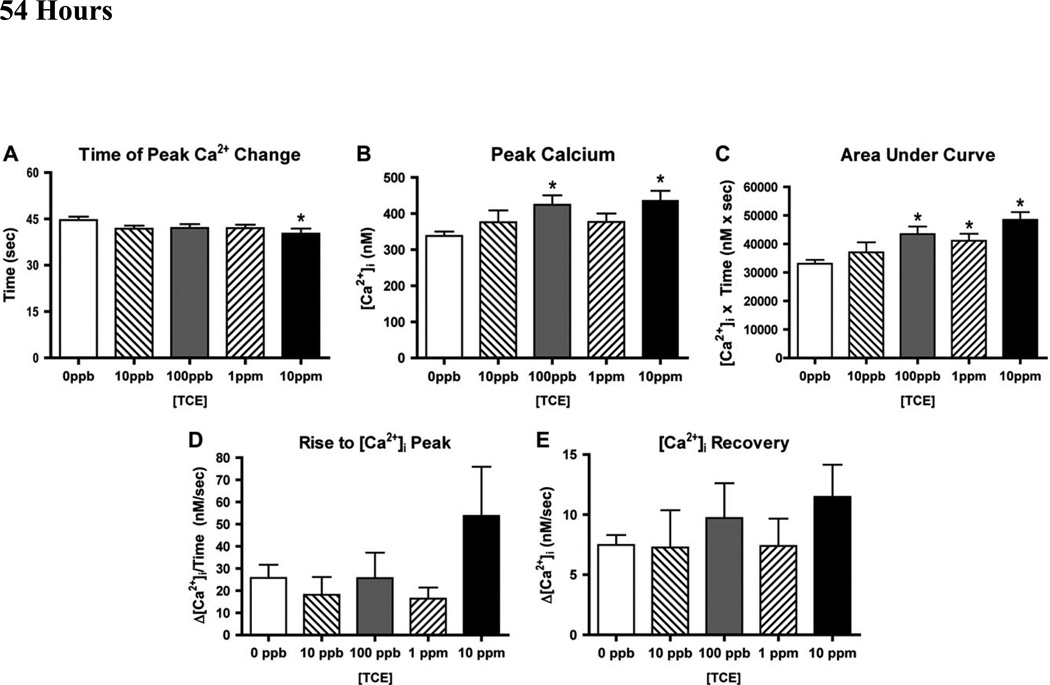
Figure 5. H9c2 Ca2+ Response to TCE after 54 hour.
H9c2 cardiac cells were cultured with and without TCE for 54 hours. Cells were then stimulated with 1nM VP and evaluated for Ca2+ parameters described in Figure 3. A through E are as described in Figure 4. [*] denotes significant difference compared to 0 ppb as determined by ANOVA (p < 0.05).
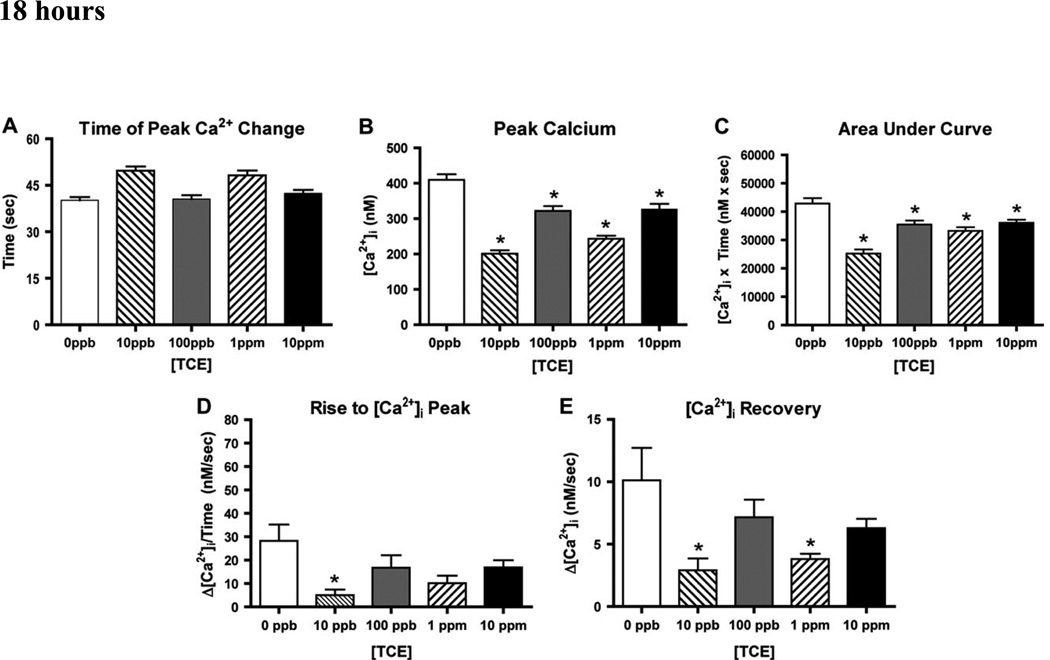
To approximate the intervals required for translation of transcripts measured in the previous experiments, we measured calcium fluxes in cells after 18 and 54 hours of TCE treatment. After 18 hours of TCE exposure, cellular response time to 1 nM VP were significantly delayed in cultures treated with 10ppb (~14 seconds +/−2.05) or 1ppm TCE (~11 seconds +/−2.04) (Fig. 4A). In contrast, the intervening 100 ppb and the higher 10 ppm TCE exposure cellular response times were not significantly different from the control. Peak [Ca]i were significantly reduced in all four TCE doses, with 10 ppb and 1 ppm showing the greatest reduction (Fig. 4B). In control experiments, peak [Ca2+]i averaged 400 nM, where 18 hours of TCE treatment significantly reduced this peak to 169 nM for 10 ppb, 313 nM for 100 ppb, 240 nM for 1ppm and 311 nM for 10 ppm.
Figure 4. H9c2 Ca2+ Response to TCE after 18 hours.
H9c2 cardiac cells were cultured in the absence of TCE (0 ppb) or with 10 ppb – 10 ppm TCE for 18 hours. Cells were then stimulated with 1nM VP and evaluated for Ca2+ parameters described in Figure 3. (A): The time (in seconds) required to reach the peak [Ca2+] was calculated for all treatments. (B): The peak [Ca2+] reached in each treatment group was compared to control unexposed cells (0ppb). (C): Area under the curve represents total [Ca2+] flux determined in 150 seconds, as illustrated in Figure 3. (D): Rise to peak represents the ratio between [Ca2+] determined at the beginning of the cell response to VP (time 0) and at the time when the peak was reached in each group. (E): “Calcium recovery” is the ratio between [Ca2+] measured at peak and at the return to baseline (slope C in Figure 3). [*] denotes significant difference determined by ANOVA (P<0.05); [**] denotes significant difference compared to 0 ppb as determined by Student’s t-test (p < 0.05).
The total change in [Ca2+]i after 1 nM VP can be approximated by evaluating the area under the Ca2+ trace of a single experiment (e.g., C in Fig. 3). Total [Ca2+]i change (Area under the curve, Fig. 4C) was significantly reduced with all TCE treatments after 1 nM VP. After exposure to our lowest dose, 10 ppb TCE, the largest decrease of 41% was observed in total [Ca2+]i. A loss of total [Ca2+]i was also observed for 100 ppb, 1 ppm and 10 ppm TCE by 17%, 22% and 16%, respectively.
From the information recorded in each [Ca2+]i peak, we calculated the slope to represent the changes of expulsion and intake of Ca2+ from the SR. The “Rise to peak” slope again showed a bi-modal effect from increasing doses of TCE with significant reductions at 10 ppb (82% decrease) and 1 ppm (64% decrease), (Figure 4D). We found a reduced “Calcium Recovery” following TCE exposure, although these changes reached significance in only the 10 ppb (71% decrease) and 1 ppm (62% decrease) treated samples (Figure 4E). Thus, while “Peak Calcium” and “Area Under the Curve” were significantly perturbed at all doses tested, measures of “Time to Peak’, “Rise to Peak” and “Calcium Recovery” were significantly altered only at the 10 ppb and 1 ppm TCE.
To evaluate further damage or recovery after extended exposure to TCE, we repeated Ca2+ evaluations in cultures treated with TCE for 54 hours (Figure 5). Under these conditions, no delays in the peak [Ca2+]i response time were observed at the lower TCE doses (10 ppb and 100 ppb), however, as TCE was raised to 1 and 10 ppm, a significant reduction in response time, 3.8 sec and 7.3 sec respectively, when compared to control was observed (Figure 5A). In contrast to the reduction seen at 18 hr, TCE longer exposures were found to increase both the peak change in [Ca2+]i (Figure 5B) and the total change in [Ca2+]i (Figure 5C). In control experiments, peak [Ca2+]i averaged 315 nM and 54 hours of TCE exposures significantly raised this peak to 362 nM for 100 ppb and 363 nM for 10 ppm. Total [Ca2+]i change graph shows a trend of increased [Ca2+]i with significance at 100 ppb, 1 ppm and 10 ppm TCE by 31%, 24% and 46%, respectively. The “Rise to Peak” (Fig. 5D) and “Calcium Recovery” (Fig. 5E) slopes showed the bi-modal response seen at 18 hr, but the 54 hr exposure treatments displayed considerable variability and the differences between the treatments and controls were not significant.
4. Discussion
4.1. Serca2a and Ryr2 expression
During development, the cardiac level of Serca2a mRNA is low and gradually increases, reaching its maximum in the adult stages (Moorman et al. 2000). As Serca2a transcript is relatively rare in the early stages of differentiation, small changes in expression levels, such as those determined in this study, are likely to be physiologically significant. In fact, relatively small decreases in Serca2 levels observed in diseased hearts (Mercadier et al. 1990) can make considerable differences in contractile function (Pieske et al. 1999). Reduced mRNA levels of Serca2a and PLB were significantly reduced in failing hearts of rabbits (Armoundas et al. 2007) and Serca2 heterozygous null mice have physiological deficits (Periasamy et al., 1999).
Western blotting and biochemical analysis of heart tissue from heterozygous Serca2a-null mutant mice showed that Serca2 mRNA was reduced by 45% while protein and maximal velocity of Ca2+ uptake into the sarcoplasmic reticulum were reduced by 35% (Periasamy et al. 1999). In H9c2 cells, the level of Serca2a mRNA is normally low, consistent with the notion that these cells represent immature cardiomyocytes. These cells were chosen as they provide a model for rat cardiomyocytes, and because they have been extensively used for studies concerning cardiac defects, disease, and toxicant exposure. In order to evaluate a model for acute versus chronic exposure to TCE, we chose to determine the mRNA expression levels of Serca2a and Ryr2 after 12 and 48 hours of exposure to various doses of TCE. These times were chosen based on time-course experiments showing that in H9c2 cells TCE effects on the Serca2a transcript were time-dependent (Selmin et al., unpublished observations).
An analysis of the kinetics of exposure in culture showed that when TCE is added to the media, 80% of the initial dose is lost within the first hour, due to TCE’s volatile nature (Mishima et al., 2006). In the present study the concentrations provided refer to initial TCE exposures, as decay in TCE is rapid enough that no measurable TCE concentration in the media can be determined accurately without compromising cell viability, especially when starting from low doses as 10ppb TCE.
The mRNA expression data show an overall inhibition by TCE on Serca2a mRNA, both at 12 and 48 hours. These results are consistent with previous reports indicating that reduced expression of Serca2a effects cardiac development and function (Haghighi et al. 2001; Hobai and O’Rourke, 2001). In particular, we observed that the lower concentrations of TCE used in the study (10 ppb-1 ppm) were effective in repressing Serca2a expression after 48 hours of exposure, whereas the highest dose (10 ppm) had no significant effect. The contrasting effects of low versus high doses are not unique to our findings. The same phenomenon was described by others comparing the effects of different doses of TCE on mesenchymal cell proliferation in avian embryonic hearts (Drake et al, 2006 a and b, Mishima et al, 2006, Boyer et al., 2000). Possible explanations for these findings include activation of TCE metabolizing enzymes occurring at the higher TCE doses, and/or different levels of these enzymes in specific cell types and developmental stages (Loeber et al., 1988; Golberg et al., 1992, Drake et al., 2006a and b). It is also important to note that other teratogens (eg. ethanol) have shown similar dose-dependent behavior (He et al., 2007). This observation that TCE does not produce a consistent dose-response curve contributes to the controversy regarding this solvent, but likely points to different affected substrates and multiple metabolites.
A reduced level of Serca2a and RyR2 expression at 12 hours is consistent with the results obtained by calcium imaging, specifically with the slow “Calcium recovery” and “Rise to Peak” showed by H9c2 cells exposed to TCE for 18 hours. In cardiac myocytes, Ryr2 proteins control the flux of calcium from the SR into the cytoplasm, where as calcium recovery inside the SR is controlled mainly by the activity of Serca2a. Conditions in which the number of Ryr2 and Serca2a complexes or their ability to function, are reduced, would likely produce the effects illustrated in Figure 4D and 4E. Therefore, it is tempting to speculate that Serca2a and Ryr2 expression and function are both inhibited at early exposure times. On the other hand, in conditions of chronic exposure to TCE, calcium release in the cytoplasm (5D) and its recovery (5E) appear to be improved, suggesting that cells may activate mechanisms to counteract the persistent high calcium concentrations in the cytosol, including an increased expression of Ryr2 and Serca2a, or that recovery has taken place as TCE has volatilized from the culture media.
4.2. Ca2+ response in H9c2 cells
The data presented in Figure 4 indicate that H9c2 cells exposed to TCE for an acute exposure time (18 hours) react slower and less efficiently to VP. In cardiomyocytes, RyR2 is essential to the SR Ca2+ release and amplitude of the peak [Ca2+]i, therefore, our results show that TCE alters RyR2 function. The real time PCR data suggest that this is accompanied by a loss of transcripts and the data probably reflect a loss of protein combined with turnover of pre-existing molecules. These results argue that acute exposure to TCE may significantly delay the heart beat cycle, at levels between 10 ppb and 1 ppm TCE (Figure 4A, D), where as 100 ppb and 10 ppm TCE produced minimal effect. Thus, acutely exposed cells have a reduced ability to expel and take in Ca2+ from the SR. While it is conceivable that TCE directly inhibits function of the RyR2 channels and Serca2a pump, we think it more likely that these changes reflect a loss of the two molecules as well as others. In subtractive hybridization and microarray studies, we found TCE to affect transcriptional regulation of a number of other molecules (Collier et al., 2003; Selmin et al., submitted). In agreement with our findings, Fu et al. (2006) found that mouse ES cell-derived cardiomyocytes lacking RyR2 showed a marked decrease in Ca2+ upstroke transients, twitch contractions, and prolonged time-to-peak. In other studies, it was reported that defective RyR2 channels can lead to irregular Ca2+ handling and this may represent a possible causative agent for heart failure and arrhythmias (Marks et al. 2002; Marx et al. 2000).
Conversely, after a chronic exposure (54 hours), cells were able to compensate or recover from the inefficiencies observed at 18 hours. In fact, the peak [Ca2+]i change and the total amount of Ca2+ flux inside the cells is greater, indicating that other players must now be involved in the overall maintenance and contracting abilities in the heart cells.
Alterations in intracellular Ca2+ homeostasis is thought to be a result of altered Ca2+ handling proteins working together, i.e. Serca2a and RyR2. In heart failure, Ca2+ uptake in the SR is diminished by Serca2a protein loss (Arai et al. 1993; Hasenfuss et al. 1994; Lehnart et al. 1998), by functional upregulation of the Serca2a inhibitor, PLB (Reiken et al. 2003), or RyR2 gene down-regulation (Go et al. 1995). Such an impact on the loss of SR function may be recovered by upregulation of NCX protein expression. A microarray project, from our lab, found that a transcript for the sodium/calcium exchanger protein (Slc17a6) was found to be induced 3-fold in TCE exposed cells (Selmin et al., submitted). This idea is also supported by Armoundas et al. (2007) who showed that failing hearts with functionless Serca2a rely on NCX to remove cytosolic calcium. In another study of failing hearts, protein levels of RyR, Serca2a and PLB were significantly downregulated while NCX protein was significantly upregulated (Lehnart et al. 2004). Alternatively, mitochondria may use a phosphate uptake mechanism to pump Ca2+ into the matrix when the cytosol has high levels of Ca2+ for an extended period of time. This may cause a calcium phosphate precipitate in the mitochondria matrix leading to calcification and damage to the mitochondria (Opie, 2004).
4.3. Dose Response to TCE
One of the recurring issues in evaluating TCE as a cardiac teratogen has been the dose - response relationship (Dugard, 2000; Hardin et al. 2005). The National Research Council (2006) in its recent assessment of the developmental toxicity of TCE noted that studies in the rat model showed an unusually flat dose response curve. The data presented here show a dose sensitivity that is lower than the majority of previous studies. In our own earlier studies we had noted an in vitro inhibition of epithelial-mesenchymal transition by cardiac valve precursors in the range 50-250 ppm of TCE (Boyer et al., 2000). This dose is consistent with a loss of valvular mesenchyme in vivo seen by Mishima et al. (2006) in the 10-80 ppm range. A loss of mesenchyme would be expected to produce valvular insufficiency and membranous septal defects as seen in exposed populations (Goldberg et al. 1990).
The present data are consistent with recent reports by Drake et al. (2006a; 2006b) showing that in ovo exposure to doses between 8 and 800 ppb produced a proliferation of cardiac mesenchymal cells in the cardiac cushions and a reduced aortic flow in developing chick embryos. Increased cellular proliferation would likely be relevant to the etiology of the pulmonary stenosis noted in the Goldberg et al. (1990) study. While attributed to a valvular stenosis, the reduction in aortic flow noted by Drake et al. (2006b) would also be consistent with a reduction in muscle contractility produced by a loss of calcium signaling as seen here. We note that reduced blood flow in the developing embryo is sufficient to produce morphological defects in cardiac structure (Groenedijk el al. 2004). Although we used an in vitro system, our data, if confirmed in vivo, are supportive of the idea that the toxic activities of TCE are due to the presence of multiple targets in cardiac tissue and perhaps differential effects of TCE metabolites. Thus the spectrum of cardiac defects noted in the study by Goldberg et al. (1990) may be indicative of varied exposures in the community as well as differential susceptibilities. Further studies using animal models and epidemiological data are warranted in order to prove or disprove this hypothesis.
In summary, our studies identify a major signal transduction pathway in cardiac muscle cells that is altered by exposure to low doses of TCE in a rat cardiac H9c2 myocyte cell culture model. As cardiac contraction during early embryonic development is critical for normal heart morphology, the data suggest a basis for the particular sensitivity of the heart to TCE as a teratogen. Calcium fluxes are used as a second messenger mechanism in other cellular processes including the regulation of cardiac cushion formation (Runyan et al. 1990), but there may be less sensitivity to calcium perturbation in other developmental processes. Though agonism of PPARalpha by TCE has been implicated in TCE-mediated carcinogenesis (see table 4.7 in NRC, 2006), perturbation of calcium signaling in a variety of cells may also be relevant. Our ongoing studies will be examining the apparent regulation of transcription by low levels of TCE.
References
- Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993 Feb;72(2):463–469. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- Armoundas AA, Rose J, Aggarwal R, Stuyvers BD, O’Rourke B, Kass DA, et al. Cellular and molecular determinants of altered Ca2+ handling in the failing rabbit heart: primary defects in SR Ca2+ uptake and release mechanisms. Am J Physiol Heart Circ Physiol. 2007 Mar;292(3):H1607–H1618. doi: 10.1152/ajpheart.00525.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DL, Hashimoto K, Grupp IL, Ji Y, Reed T, Loukianov E, et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ Res. 1998;83:1205–1214. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- Boyer AS, Finch WT, Runyan RB. Trichloroethylene inhibits development of embryonic heart valve precursors in vitro. Toxicol Sci. 2000;53:109–117. doi: 10.1093/toxsci/53.1.109. [DOI] [PubMed] [Google Scholar]
- Bove F, Shim Y, Zeitz P. Drinking water contaminants and adverse pregnancy outcomes: a review. Environ Health Perspect. 2002 Feb;110(Suppl 1):61–74. doi: 10.1289/ehp.02110s161. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Center for Disease Prevention and Control) Trends in Infant Mortality Attributable to Birth Defects -- United States, 1980–1995. 1998 http://www.cdc.gov/mmwr/preview/mmwrhtml/00054921.htm.
- Chandrashekhar Y, Prahash AJ, Sen S, Gupta S, Roy S, Anand IS. The role of arginine vasopressin and its receptors in the normal and failing rat heart. J Mol Cell Cardiol. 2003 May;35(5):495–504. doi: 10.1016/s0022-2828(03)00053-1. 2003. [DOI] [PubMed] [Google Scholar]
- Collier JM, Selmin O, Johnson PD, Runyan RB. Trichloroethylene effects on gene expression during cardiac development. Birth Defects Res A Clin Mol Teratol. 2003;67:488–495. doi: 10.1002/bdra.10073. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Lough J, Hu N, Smith SM. Trichloroethylene exposure during cardiac valvuloseptal morphogenesis alters cushion formation and cardiac hemodynamics in the avian embryo. Environ Health Perspect. 2006a;114:842–847. doi: 10.1289/ehp.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Hu N, Smith SM, Lough J. Cardiogenic effects of trichloroethylene and trichloroacetic acid following exposure during heart specification of avian development. Toxicol Sci. 2006b Nov;94(1):153–162. doi: 10.1093/toxsci/kfl083. [DOI] [PubMed] [Google Scholar]
- Dugard PH. Effects of trichloroethylene (TCE) on an in vitro chick atrioventricular canal culture. Toxicol Sci. 2000;56:437–438. doi: 10.1093/toxsci/56.2.437. [DOI] [PubMed] [Google Scholar]
- Fu JD, Li J, Tweedie D, Yu HM, Chen L, Wang R, et al. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J. 2006 Jan;20(1):181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995 Feb;95(2):888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SJ, Lebowitz MD, Graver EJ, Hicks S. An association of human congenital cardiac malformations and drinking water contaminants. J Am Coll Cardiol. 1990;16:155–164. doi: 10.1016/0735-1097(90)90473-3. [DOI] [PubMed] [Google Scholar]
- Goldberg SJ, Dawson BV, Johnson PD, Hoyme HE, Ulreich JB. Cardiac teratogenicity of dichloroethylene in a chick model. Pediatr Res. 1992 Jul;32(1):23–26. doi: 10.1203/00006450-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Groenendijk BC, Hierck BP, Gittenberger-De Groot AC, Poelmann RE. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn. 2004 May;230(1):57–68. doi: 10.1002/dvdy.20029. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haghighi K, Schmidt AG, Hoit BD, Brittsan AG, Yatani A, Lester JW, et al. Superinhibition of sarcoplasmic reticulum function by phospholamban induces cardiac contractile failure. J Biol Chem. 2001;276(26):24145–24152. doi: 10.1074/jbc.M102403200. [DOI] [PubMed] [Google Scholar]
- Hardin BD, Kelman BJ, Brent RL. Trichloroethylene and dichloroethylene: a critical review of teratogenicity. Birth Defects Res A Clin Mol Teratol. 2005;73:931–955. doi: 10.1002/bdra.20192. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994 Sep;75(3):434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- He L, Marecki JC, Serrero G, Simmen FA, Ronis MJJ, Badger TM. Dose-Dependent Effects of Alcohol on Insulin Signaling:Partial Explanation for Biphasic Alcohol Impact on Human Health. Molecular Endocrinology. 2007;21(10):2541–2550. doi: 10.1210/me.2007-0036. [DOI] [PubMed] [Google Scholar]
- Hobai IA, O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- Ji Y, Lalli MJ, Babu GJ, Xu Y, Kirkpatrick DL, Liu LH, et al. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem. 2000;275:38073–38080. doi: 10.1074/jbc.M004804200. [DOI] [PubMed] [Google Scholar]
- Johnson PD, Dawson BV, Goldberg SJ. A review: trichloroethylene metabolites: potential cardiac teratogens. Environ Health Perspect. 1998;106(Suppl 4):995–999. doi: 10.1289/ehp.98106s4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PD, Goldberg SJ, Mays MZ, Dawson BV. Threshold of trichloroethylene contamination in maternal drinking waters affecting fetal heart development in the rat. Environ Health Perspect. 2003;111:289–292. doi: 10.1289/ehp.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli MJ, Yong J, Prasad V, Hashimoto K, Plank D, Babu GJ, et al. Sarcoplasmic reticulum Ca(2+) atpase (SERCA) 1a structurally substitutes for SERCA2a in the cardiac sarcoplasmic reticulum and increases cardiac Ca(2+) handling capacity. Circ Res. 2001;89:160–167. doi: 10.1161/hh1401.093584. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Schillinger W, Pieske B, Prestle J, Just H, Hasenfuss G. Sarcoplasmic reticulum proteins in heart failure. Ann N Y Acad Sci. 1998 Sep 16;853:220–230. doi: 10.1111/j.1749-6632.1998.tb08270.x. Review. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004 May;1015:144–159. doi: 10.1196/annals.1302.012. Review. [DOI] [PubMed] [Google Scholar]
- Loeber CP, Hendrix MJ, Diez De Pinos S, Goldberg SJ. Trichloroethylene: a cardiac teratogen in developing chick embryos. Pediatr Res. 1988 Dec;24(6):740–744. doi: 10.1203/00006450-198812000-00018. [DOI] [PubMed] [Google Scholar]
- Marks AR, Priori S, Memmi M, Kontula K, Laitinen PJ. Involvement of the cardiac ryanodine receptor / calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J Cell Physiol. 2002;190:1–6. doi: 10.1002/jcp.10031. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mercadier JJ, Lompre AM, Duc P, Boheler KR, Fraysse JB, Wisnewsky C, Allen PD, Komajda M, Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990 Jan;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima N, Hoffman S, Hill EG, Krug EL. Chick embryos exposed to trichloroethylene in an ex ovo culture model show selective defects in early endocardial cushion tissue formation. Birth Defects Res A Clin Mol Teratol. 2006;76:517–527. doi: 10.1002/bdra.20283. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Schumacher CA, de Boer PA, Hagoort J, Bezstarosti K, van den Hoff MJ, et al. Presence of functional sarcoplasmic reticulum in the developing heart and its confinement to chamber myocardium. Dev Biol. 2000;223:279–290. doi: 10.1006/dbio.2000.9752. [DOI] [PubMed] [Google Scholar]
- NCHS (National Center for Health Statistics) Deaths: Final Report for 2004. 2004 http://www.cdc.gov/nchs/products/pubs/pubd/hestats/finaldeaths04/finaldeaths04.htm.
- NRC (National Research Council) Assessing the Human Health Risks of Trichloroethylene: Key scientific Issues. Washington, DC: National Academies Press; 2006. [Google Scholar]
- Opie LH. Heart Physiology: From Cell to Circulation. 4th Ed. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- Ou J, Ou Z, McCarver DG, Hines RN, Oldham KT, Ackerman AW, Pritchard KA., Jr Trichloroethylene Decreases Heat Shock Protein 90 Interactions with Endothelial Nitric Oxide Synthase: Implications for Endothelial Cell Proliferation. Toxicological Sciences. 2003;73:90–97. doi: 10.1093/toxsci/kfg062. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, et al. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+- ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999;274:2556–2562. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999 Jul 9;85(1):38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun. 2004 Oct 1;322(4):1192–1203. doi: 10.1016/j.bbrc.2004.07.156. Review. [DOI] [PubMed] [Google Scholar]
- Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D’Armiento J, Burkhoff D, et al. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J Biol Chem. 2003 Jan 3;278(1):444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- Runyan RB, Potts JD, Sharma RV, Loeber CP, Chiang JJ, Bhalla RC. Signal transduction of a tissue interaction during embryonic heart development. Cell Regul. 1990 Feb;1(3):301–313. doi: 10.1091/mbc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmin O, Thorne PA, Caldwell PT, Johnson PD, Runyan RB. Effects of trichloroethylene and its metabolite trichloroacetic acid on the expression of vimentin in the rat H9c2 cell line. Cell Biol Toxicol. 2005;21:83–95. doi: 10.1007/s10565-005-0124-3. [DOI] [PubMed] [Google Scholar]
- Tshori S, Gilon D, Beeri R, Nechushtan H, Kaluzhny D, Pikarsky E, Razin E. Transcription factor MITF regulates cardiac growth and hypertrophy. J Clin Invest. 2006 Oct 2;116(10):2673–2681. doi: 10.1172/JCI27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ver Heyen M, Heymans S, Antoons G, Reed T, Periasamy M, Awede B, et al. Replacement of the muscle-specific sarcoplasmic reticulum Ca(2+)-ATPase isoform SERCA2a by the nonmuscle SERCA2b homologue causes mild concentric hypertrophy and impairs contraction-relaxation of the heart. Circ Res. 2001;89:838–846. doi: 10.1161/hh2101.098466. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jacobson CF, Williams AL, Howard WB, DeSesso JM. Trichloroethylene-contaminated drinking water and congenital heart defects: a critical analysis of the literature. Reprod Toxicol. 2006 Feb;21(2):117–147. doi: 10.1016/j.reprotox.2005.07.013. 2006. Review. [DOI] [PubMed] [Google Scholar]