Abstract
Rationale
The Na+/K+ ATPase (NKA) directly regulates intracellular Na+ levels, which in turn indirectly regulates Ca2+ levels by proximally controlling flux through the Na+/Ca2+ exchanger (NCX1). Elevated Na+ levels have been reported during heart failure, which permits some degree of reverse mode Ca2+ entry through NCX1, as well as less efficient Ca2+ clearance.
Objective
To determine if maintaining lower intracellular Na+ levels by NKA overexpression in the heart would enhance forward-mode Ca2+ clearance and prevent reverse-mode Ca2+ entry through NCX1 to protect the heart.
Methods and Results
Cardiac-specific transgenic mice overexpressing either the NKA-α1 or α2 were generated and subjected to pressure overload hypertrophic stimulation. We found that while increased expression of NKA-α1 had no protective effect, overexpression of NKA-α2 significantly decreased cardiac hypertrophy after pressure overload in mice at 2, 10 and 16 weeks of stimulation. Remarkably, total NKA protein expression and activity were not altered in either of these two transgenic models, as increased expression of one isoform led to a concomitant decrease in the other endogenous isoform. NKA-α2 overexpression, but not NKA-α1, led to significantly faster removal of bulk Ca2+ from the cytosol in a manner requiring NCX1 activity. Mechanistically, overexpressed NKA-α2 showed greater affinity for Na+ compared with NKA-α1, leading to more efficient clearance of this ion. Moreover, overexpression of NKA-α2, but not NKA-α1, was coupled to a decrease in phospholemman expression and phosphorylation, which would favor greater NKA activity, NCX1 activity and Ca2+ removal.
Conclusions
Our results suggest that the protective effect produced by increased expression of NKA-α2 on the heart after pressure overload is due to more efficient Ca2+ clearance because this isoform of NKA preferentially enhances NCX1 activity compared with NKA-α1.
Keywords: Hypertrophy, calcium signaling, cardiomyocyte, sodium, NKA, contractility
INTRODUCTION
Years of study have made it clear that Na+ entry and exit pathways play an important role in the pathogenesis of heart disease, as these systems are responsible not only for initiating the cardiac action potential (via voltage-gated Na+ channels) but also closely regulate the influx and efflux of Ca2+ through the Na+/Ca2+ exchanger (NCX1 in the heart) 1. NCX1 is an electrogenic exchanger that under normal conditions removes one Ca2+ ion in exchange for internalizing three Na+ ions. However, the direction and rate of NCX1-mediated countertransport is determined by membrane potential and relative concentrations of Na+ and Ca2+ inside and outside of the myocyte. Multiple studies have indicated that intracellular Na+ concentration ([Na+]i) is increased in numerous animal models of hypertrophy 2, as well as in human heart failure 3. This increased [Na+]i during cardiac disease is likely an adaptive mechanism to reduce Ca2+ extrusion via NCX1 to augment contractility and cardiac function. However, this increase in cytosolic Ca2+ also increases the propensity for arrhythmias and may activate Ca2+ dependent signaling pathways involved in the hypertrophic program and/or apoptosis 4.
Numerous studies have characterized mechanisms by which [Na+]i becomes elevated during cardiac disease, and they involve both entry and efflux pathways. Increased Na+ influx via both tetrodotoxin-sensitive Na+ channels and Na+/H+ exchanger (NHE1) have been demonstrated in a rabbit model of heart failure 5, 6 and in human heart failure 7-9. Overexpression of NHE1 in the murine heart resulted in increased [Na+]i, increased [Ca2+]i (likely due to decreased Ca2+ extrusion by NCX1), heart failure and premature death accompanied by arrhythmia, increased nuclear factor of activated T-cells (NFAT) translocation and elevated Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity resulting in exclusion of histone deactylase 4 (HDAC4) from the nucleus 10. In addition, the late component of voltage-gated Na+ channel activity (INaL) is enhanced in some models of heart failure 11, while inhibition of INaL in CaMKII transgenic animals can improve diastolic function and eliminate premature arrhythmogenic contractions in papillary muscle preparations 12.
The Na+/K+ ATPase (NKA) is the primary Na+ extrusion pathway in cardiac myocytes, consuming ATP to pump three Na+ ions out, in exchange for two K+ ions, which determines the driving force for Na+ entry into the myocyte. NKA is a heterodimer composed of an α subunit (α1 and α2 isoforms exist in the rodent heart) and β subunit (β1 is the only isoform in the heart) and is distributed both in the surface sarcolemma and t-tubules. NKA is functionally coupled to NCX1 in cardiac myocytes13 where even small changes in NKA activity may alter the local [Na+]i environment to modulate Ca2+ extrusion through NCX1 14-16. Accordingly, cardiac glycosides have been used for centuries to inhibit NKA activity, which during heart failure can blunt or reverse Ca2+ exit via NCX1 to enhance contractility 17. NKA activity is either downregulated 18 or unchanged 5 during heart failure, and can be further modulated by phosphorylation of the endogenous regulator protein phospholemman (PLM), which if hyperphosphorylated as shown in a rabbit model of heart failure enhances NKA activity 19. Alternatively reduced phosphorylation of PLM as shown in human heart failure 20 would lead to increased NKA inhibition.
The respective roles of the α1 and α2 subunits of NKA in the heart remain controversial. Early work suggested that α2 was directly coupled with NCX1 in t-tubules, while α1 regulated bulk Na+ levels in the cytosol 21. However, more recent work suggested that both isoforms are directly coupled to NCX113. Nevertheless, it appears that while the α1 subunit shows localization to both the surface sarcolemma and t-tubules, the α2 isoform is enriched up to five times more in t-tubules, suggesting that α2 may play a preferential role in generation of signaling domains within t-tubules that modulate NCX1 function 22. Supporting this notion is recent work demonstrating that partial inhibition of NKA-α2, but not NKA-α1 can increase Ca2+ transients, pointing to NKA-α2 as the isoform responsible for regulating NCX1 to control intracellular Ca2+ in the junctional space and subsequently affecting Ca2+ release from the SR 23.
Here we generated transgenic mice with cardiac-specific overexpression of the α1 or the α2 subunit of NKA to determine whether altered expression of a Na+ efflux protein could modulate the disease phenotype elicited by pressure overload in the heart. As suggested by previous reports 21, 24, we demonstrate that a true overexpression of either α isoform is impossible, since increased expression of one isoform results in compensatory down-regulation of the other endogenous α isoform. However, increased expression of the α2 isoform, which effectively results in replacement of α1 with α2, attenuated the hypertrophic response after transverse aortic constriction (TAC) and was associated with faster Ca2+ extrusion via NCX1. In addition, the protective effect of α2 overexpression is not mimicked by α1 overexpression, positioning NKA-α2 as the more important isoform affecting Ca2+ removal by NCX1 during disease.
METHODS
cDNAs encoding rat NKA-α1 and ouabain-resistant rat NKA-α2 (gift from Dr. Jerry Lingrel, University of Cincinnati) were cloned into the murine α-myosin heavy chain (MHC) promoter expression vector and used to inject newly-fertilized oocytes to generate transgenic mice (FVB/N background). NFAT-luciferase transgenic mice were previously described 25. For cardiac pressure overload induction, mice aged 8-11 weeks were subjected to TAC or a sham surgical procedure, as previously described 25. Mouse ventricular cardiomyocytes were isolated as previously described26. For Na+ measurements, isolated myocytes were plated on laminin-coated coverslips and loaded with 10 μM SBFI-AM for 90-120 min (Invitrogen) as previously described 27. NKA activity was determined using an enzyme-linked assay measuring the rate of ADP production as linked to the rate of NADH fluorescence decrease in the absence or presence of 10 mM strophanthidin (Sigma Aldrich), as previously described 28. Results are presented in all cases as mean ± SEM. P-values less than 0.05 were considered significant.
RESULTS
Overexpression of Na+/K+ ATPase α2, but not α1, reduces hypertrophy after TAC
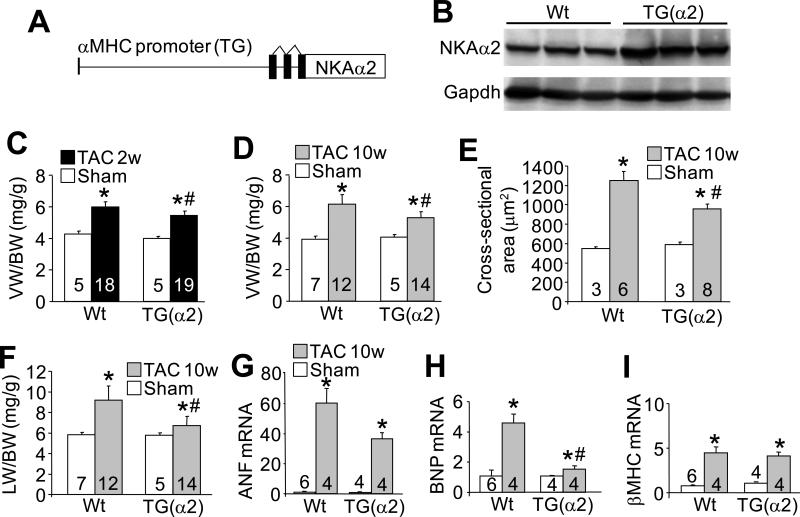
To determine the relative contribution of the α1 versus the α2 NKA isoform in the regulation of cytosolic Na+ levels and cardiac hypertrophy during disease, we created transgenic mice overexpressing each isoform using the α-MHC promoter (Figure 1A and 2A). We obtained one line of NKA-α2 transgenic mice that showed approximately 3-fold overexpression of protein in the heart relative to wild-type (Wt) levels (Figure 1B). Echocardiographic, gravimetric and histological analyses showed no baseline phenotype in NKA-α2 TG mice with aging, nor was survival affected (data not shown and Online Figure I). We next performed TAC surgery to induce pressure overload hypertrophy. After 2 weeks of TAC stimulation, hearts from the NKA-α2 transgenic animals showed a significant reduction in ventricle weight normalized to body weight compared with Wt controls subjected to TAC (Figure 1C and Online Figure II), which became even more apparent 10 weeks after TAC stimulation (Figure 1D). This reduction in cardiac hypertrophy in the transgenic mice after TAC correlated with reduced myocyte cross-sectional area (Figure 1E), reduced lung weight normalized to body weight (Figure 1F), a trend towards reduced mRNA expression of the hypertrophic marker atrial natriuretic factor (ANF) and a significant reduction in mRNA expression of brain natriuretic peptide (BNP) (Figure 1G-I).
Figure 1. NKA-α2 transgenic mice show less cardiac hypertrophy after pressure overload.
A, Schematic representation of the transgene used to drive NKA-α2 expression in the mouse heart. B, Immunoblot for NKA-α2 and GAPDH protein from Wt and NKA-α2 transgenic heart homogenates. C, Ventricular weight to body weight ratios measured from Wt and NKA-α2 transgenic hearts after 2 weeks of TAC or a sham surgery. D, Ventricular weight to body weight ratios measured from Wt and NKA-α2 transgenic hearts after 10 weeks of TAC or a sham surgery. E, Histological analysis of myocyte cross-sectional area using wheat germ agglutinin-TRITC stain of heart histological sections from Wt and NKA-α2 transgenic mice after 10 weeks of TAC or a sham surgery. F, Lung weight to body weight ratios measured from Wt and NKA-α2 transgenic mice after 10 weeks of TAC or a sham surgery. G-I, mRNA levels of atrial natriuretic factor (ANF), brain natriuretic peptide (BNP) and β-myosin heavy chain (β-MHC) measured via qPCR from Wt and NKA-α2 transgenic hearts after 10 weeks of TAC or a sham surgery. Wt controls were analysed after 10-12 weeks of sham surgery. For each experiment, number of mice analysed is given within the graph. *P<0.05 versus sham; #P<0.05 vs Wt TAC.
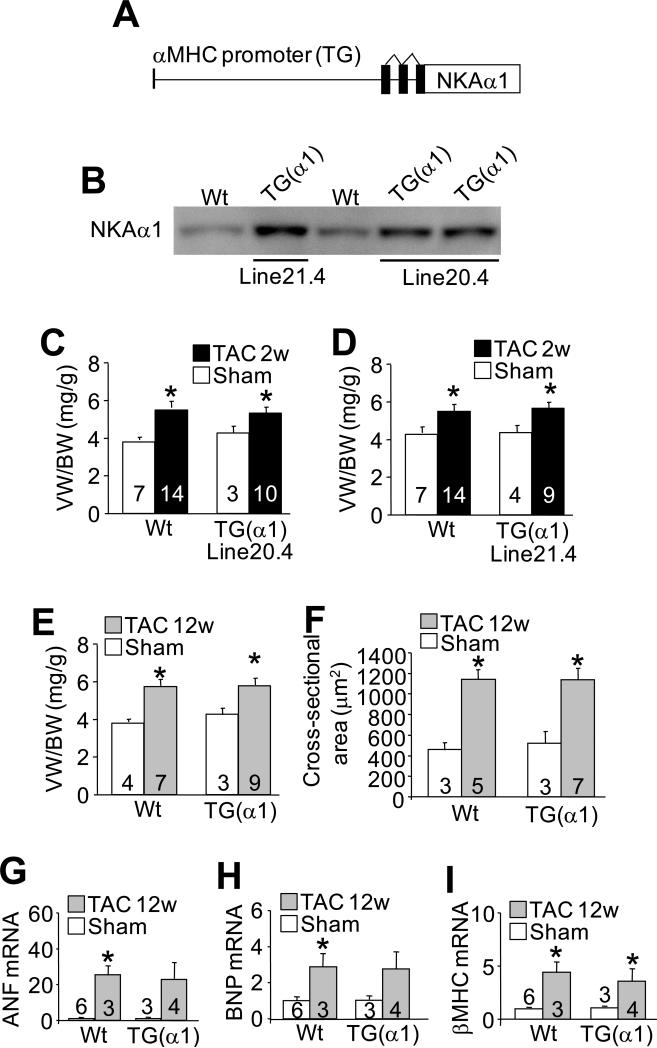
Figure 2. NKA-α1 transgenic mice show the same cardiac hypertrophic response after pressure overload as Wt mice.
A, Schematic representation of the transgene used to drive NKA-α1 expression in the mouse heart. B, Immunoblot for NKA-α1 protein from the hearts of Wt animals and 2 transgenic lines. Line 21.4 (high line) expresses more NKA-α1 protein than line 20.4 (low line). C-D, Ventricular weight to body weight ratios measured from Wt and line 20.4 or line 21.4 NKA-α1 transgenic mice after 2 weeks of TAC or a sham surgery. E, Ventricle weight to body weight ratios measured from Wt and high-line NKA-α1 transgenic mice after 12 weeks of TAC or a sham surgery. F, Histological analysis of myocyte cross-sectional area using wheat germ agglutinin-TRITC stain of heart histological sections from Wt and high-line NKA-α1 transgenic mice after 12 weeks of TAC or a sham surgery. G-I, mRNA levels of atrial natriuretic factor (ANF), brain natriuretic peptide (BNP) and β-myosin heavy chain (β–MHC) measured via qPCR from Wt and high-line NKA-α1 transgenic hearts after 12 weeks of TAC or a sham surgery. Wt controls were analysed after 10-12 weeks of sham surgery. For each experiment, number of mice analysed is given within the graph. *P<0.05 versus sham; #P<0.05 vs Wt TAC.
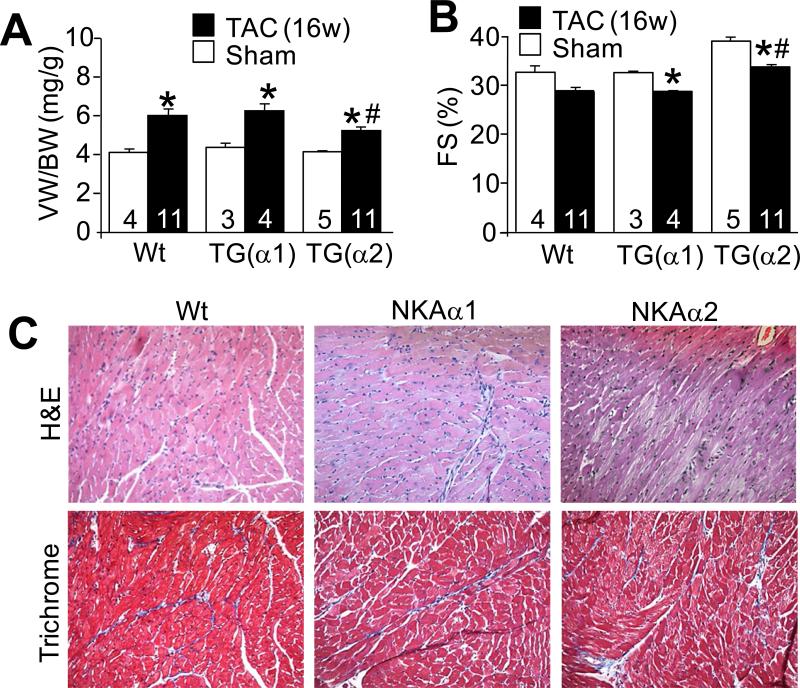
We also obtained 2 lines of NKA-α1 overexpressing transgenic mice that showed 2-3-fold overexpression of this ATPase isoform (Figure 2B). However, NKA-α1 transgenic mice failed to show any reduction in cardiac hypertrophy after 2 weeks of TAC stimulation compared with Wt mice (Figure 2C and 2D and Online Figure II). To extend these results we performed 12 weeks of TAC stimulation on the slightly higher-expressing NKA-α1 line (line 21.4), which also failed to show any reduction in cardiac hypertrophic remodeling as measured by ventricle weight normalized to body weight (Figure 2E), myocyte cross-sectional area (Figure 2F), and expression of the hypertrophic markers atrial natriuretic factor, brain natriuretic peptide and β–myosin heavy chain (Figure 2G-I). We also generated yet another separate cohort of Wt, NKA-α1 and NKA-α2 transgenic mice that were subjected to TAC surgery at the same time (exact same ages and sex) and followed for 2 weeks, which again showed attenuation of cardiac hypertrophy only in the NKA-α2 transgenic mice (Online Figure II). Direct comparison of NKA-α1 and NKA-α2 to Wt controls after even 16 weeks of TAC further confirmed these findings and demonstrated again that only overexpression of NKA-α2 could limit hypertrophy and remodeling as measured by echocardiography and gravimetry (Figure 3A,B). NKA-α2 TG mice were also protected from loss of cardiac ventricular performance compared with NKA-α1 and Wt hearts (Figure 3B). We also analysed the degree of intracellular fibrosis over 16 weeks of TAC stimulation but the α1 and α2 transgenic lines showed the same relative response as observed in Wt controls for this measure (Figure 3C). Thus, at multiple time points of TAC stimulation NKA-α2 TG mice were significantly protected from the full extent of the hypertrophic response and loss of ventricular function compared with Wt or NKA-α1 transgenic mice.
Figure 3. NKA-α2 transgenic mice are protected from hypertrophy after 16 weeks of pressure overload.
A, Ventricle weight to body weight ratio and B, echocardiographic assessment of fractional shortening (FS%) in Wt, high-line NKA-α1 transgenic or NKA-α2 transgenic mice after 16 weeks of TAC surgery or from 24 week-old baseline controls. C, Representative H&E and Masson's trichrome-stained histological sections from hearts of Wt, high-line NKA-α1 transgenic or NKA-α2 transgenic mice after 16 weeks of TAC surgery. Cardiac histology from corresponding baseline control animals is shown in Online Figure I.
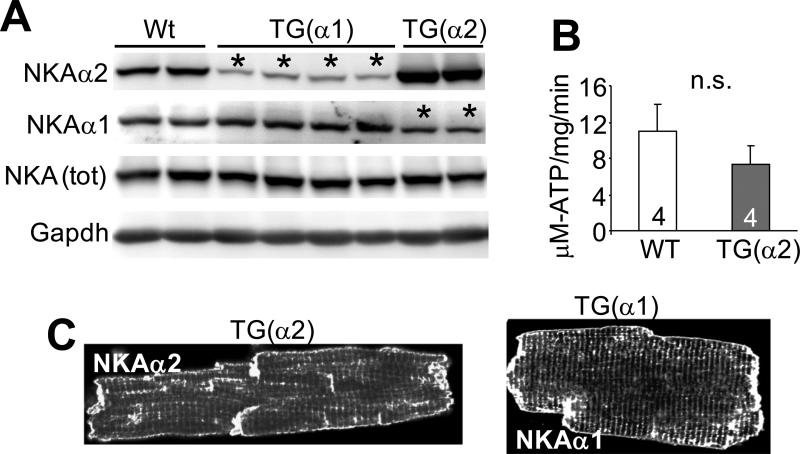
Total NKA protein levels are conserved during subtype overexpression
Previous work has suggested that a change in expression of a particular NKA isoform is balanced by altered expression of the other, such that a constant level of NKA protein is maintained 21, 24. In agreement with these previous observations, our Western blot experiments from whole-cell extracts of NKA-α1, NKA-α2 and Wt hearts demonstrated that overexpression of one NKA isoform elicited downregulation of the other isoform in a manner that preserved total levels of NKA (Figure 4A). Consistent with these observations, measurement of total ATPase activity in cardiac lysates from NKA-α2 transgenic and Wt hearts did not differ (Figure 4B). While other investigators have suggested a somewhat different subcellular localization of NKA-α2 versus NKA-α1 22, 29, it has been previously reported that both isoforms are expressed at the surface sarcolemma and t-tubules. Our findings agree with this, as we were unable to see a difference in NKA localization when comparing myocytes from each transgenic line by confocal microscopy, (Figure 4C). We also failed to observe any differences in localization of endogenous NKA-α1 versus α2 from isolated adult rat myocytes stained with the appropriate antibodies, as both protein isoforms populated the sarcolemma and T-tubule at what appeared to be the same relative ratios (data not shown).
Figure 4. Compensation between NKA-α1 and NKA-α2 in the hearts of transgenic mice.
A, Immunoblots for NKA-α1, NKA-α2, total NKA and GAPDH protein from Wt and transgenic heart homogenates. The asterisks show the lanes with reduced NKA protein expression due to compensation. B, NKA activity measured as strophanthidin-sensitive ATP turnover rate in crude homogenates from Wt and NKA-α2 transgenic hearts. C, Subcellular localization of NKA-α1 and NKA-α2 protein in NKA-α1 or NKA-α2 isolated transgenic myocytes, respectively, via immunofluorescence and confocal microscopy. For each experiment, number of mice analysed is given within the graph. *P<0.05 versus sham; #P<0.05 vs Wt TAC.
NKA overexpression does not alter Na+ content, but modulates Ca2+ efflux via an NCX1-dependent mechanism
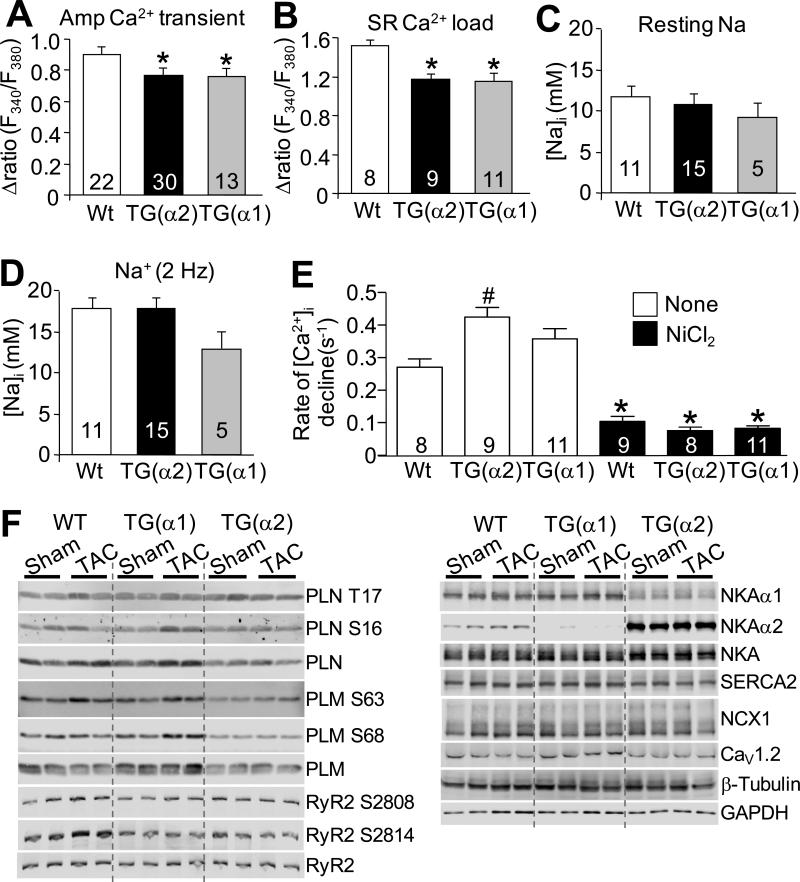
To determine the mechanism whereby NKA-α2 overexpression reduced cardiac hypertrophy and negative remodeling after TAC, we examined whether increased expression of either NKA isoform could alter Ca2+ handling. NKA is known to co-localize with NCX1 and several studies (and our unpublished work) have demonstrated co-immunoprecipitation 13, suggesting that NKA and NCX1 can co-associate as part of a complex to regulate Na+/Ca2+ countertransport. Interestingly, we found that increased expression of either NKA-α1 or NKA-α2 reduced the amplitude of the Ca2+ transient in myocytes isolated from these animals (Figure 5A), as well as total sarcoplasmic reticulum (SR) Ca2+ load (Figure 5B). Despite this, we found no change in baseline cytosolic Na+ levels in myocytes isolated from either NKA-α1 or NKA-α2 transgenic mice either at rest or after a 2 Hz pacing protocol (Figure 5C,D), which is consistent with our observations that increased expression of one NKA subtype resulted in reduction of the other isoform to maintain a constant level of total NKA protein and activity. However, we are uncertain why overexpression of either isoform causes a decrease in the amplitude of the Ca2+ transient or reduced sarcoplasmic reticulum Ca2+ load, even though total NKA activity is the same (see discussion).
Figure 5. Analysis of intracellular Ca2+ and Na+ handling in adult myocytes from NKA-α1 and NKA-α2 transgenic mice.
A, Mean amplitude of Ca2+ transients measured from Wt, NKA-α2, and high-line NKA-α1 isolated transgenic cardiomyocytes paced at 0.5 Hz. B, Sarcoplasmic reticulum (SR) Ca2+ load measured from Wt, NKA-α2, and high-line NKA-α1 isolated transgenic cardiomyocytes via caffeine-induced Ca2+ release. C,D, [Na+]i measured from Wt, NKA-α2, and high-line NKA-α1 isolated transgenic cardiomyocytes under resting conditions (C), and during stimulation at 2 Hz (D). Number of myocytes analysed is given within the graph. *P<0.05 versus WT. E, Rate of [Ca2+]i decline after caffeine-induced depletion of SR stores measured from Wt, NKA-α2, and NKA-α1 transgenic myocytes either in the presence of 10 mM NiCl2 (to block NCX1) or in the absence of NiCl2 (control). *P<0.05 versus control (no nickel); #P<0.05 vs Wt of same treatment. F, Immunoblots of phosphoprotein and total protein levels for the indicated Ca2+ and Na+ handling proteins from cardiac homogenates of Wt, high-line NKA-α1 transgenic or NKA-α2 transgenic hearts after 16 weeks of TAC or sham surgery. For each experiment, the number of mice analyzed is given in the graph. Quantitation of the data are shown in Online Figure IV. *P<0.05 versus sham; #P<0.05 vs Wt TAC.
To examine the mechanism whereby NKA-α2 might be anti-hypertrophic, we more directly measured the effect of increased NKA-α2 on NCX1-mediated Ca2+ extrusion in isolated adult myocyte. We observed that the rate of [Ca2+]i decline after caffeine-mediated depletion of sarcoplasmic reticulum stores, which is the result of both NCX1 and SERCA2 activity, was significantly faster in adult myocytes from NKA–α2 transgenic animals compared with NKA-α1 and Wt control myocytes (no Ni2+), while inhibition of NCX1 by pre-treatment with 10 mM Ni2+ resulted in a slower [Ca2+]i decay that was almost entirely reflective of SERCA2 activity and was not significantly different between the three groups (Figure 5E). These results suggest that NCX1 activity is responsible for the increased rate of Ca2+ extrusion in NKA-α2 overexpressing adult myocytes, while α1 does not appear to have this effect. This enhanced profile of NCX1 activity coupled to NKA-α2 could be protective and possibly anti-hypertrophic if it more effectively reduced intracellular Ca2+ or some aspect of its global signalling due to rate of removal during relaxation (see discussion).
NKA-α2 overexpression does not alter hypertrophic signalling pathways but does alter rate of Na+ removal
Given that overexpression of NHE1, a Na+ entry pathway, results in heart failure accompanied by increased NFAT nuclear translocation and CaMKII activity10, we performed experiments to determine whether activation of these pro-hypertrophic signalling pathways was altered after TAC in hearts of NKA-α2 transgenic mice. However, we failed to identify any reduction in cardiac NFAT luciferase activity after TAC in NKA-α2 transgenic mice (Online Figure IIIA), nor was there a reduction in calcineurin activation as measured by calmodulin (CaM) co-immunoprecipitation with calcineurin B after TAC (Online Figure IIIB), or protein kinase Cα (PKCα) phosphorylation (data not shown). Similarly, assessment of CaMKII activity showed no difference between the NKA-α2 transgenic animals and Wt controls after 2 weeks of TAC stimulation (Online Figure IIIC). These results indicate that NKA-α2 overexpression is not acting in an anti-hypertrophic manner by affecting known Ca2+ regulated signalling pathways in the heart.
To probe more carefully into the mechanisms that might be responsible, or at least associated with the observed profile of altered Ca2+ and Na+ handling in the hearts of NKA-α2 transgenic mice we performed a series of Western blots for nodal ion handling proteins, both at baseline and after TAC stimulation. We analysed total phospholamban (PLN) protein levels and its phosphorylation status, ryanodine receptor 2 (RyR) levels and phosphorylation, sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2), NCX1, the α1c subunit of the L-type Ca2+ channel and PLM and its phosphorylation status (Figure 5F). We consistently observed that phosphorylation of PLM at both serine 63 and 68, as well as total PLM levels were reduced in hearts of NKA-α2 TG mice compared with Wt or NKA-α1 mice, both at baseline in sham animals as well as after TAC stimulation (Figure 5F). PLM is known to inhibit NKA activity as well as alter NCX1 activity, and its reduction in NKA-α2 transgenic hearts could be protective and anti-hypertrophic by increasing NKA activity/effectiveness, further adding to the ability of the myocyte to deal with Na+ and/or Ca2+ dysregulation that is often associated with hypertrophy and heart failure (see discussion). In addition, while total PLN levels appeared to be increased in both Wt and NKA-α1 hearts after TAC, no such increase was observed in NKA-α2 hearts, likely due to the need to preserve SR load in the face of enhanced NCX1-mediated Ca2+ extrusion. We also observed that the increased RyR2 phosphorylation at S2814 after TAC in the Wt animals did not occur in either the NKA-α1 or NKA-α2 transgenics after pressure overload. This agrees with our photometry data showing similar reductions in transient amplitude and reduction in SR load (Figure 5A,B) that could result in less CaMKII-mediated phosphorylation in the t-tubule/SR junctional space, however there appears to be no loss of total CaMKII activity any of the models after TAC (Online Figure IIIC).
The NKA-α2 isoform has greater Na+ affinity and pump activity
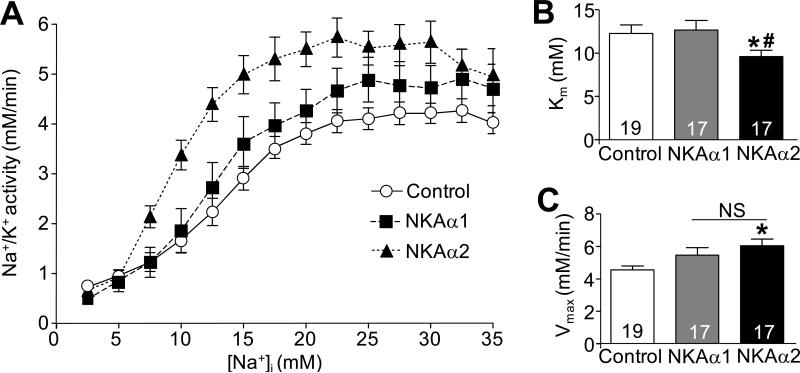
As demonstrated in Figure 5F, expression and phosphorylation of proteins such as PLN and PLM, which are important for controlling Na+ and Ca2+ handling are altered in NKA-α2 transgenic mice. To determine whether NKA-α2 and NKA-α1 functioned similarly in an uncompensated system we carefully analysed NKA activity in vivo in adult myocytes in which we overexpressed either the α1 or α2 isoform using adenoviral gene transfer (or a control β-galactosidase expressing virus), followed by analysis of Na+ pump activity as a function of [Na+]i. Using an antibody that recognizes both NKA–α1 and α2, overexpression between the two isoforms was equivalent in total protein levels achieved after adenoviral infection (data not shown). The data show that NKA-α2 overexpressing myocytes had significantly greater affinity for Na+ and a higher rate of activity compared with NKA-α1 overexpressing myocytes or endogenous activity in control infected myocytes (Figure 6A-C). These results suggest that the NKA-α2 isoform, which may be less regulated by PLM (see discussion) is more effective than NKA-α1 in pumping Na+ when expressed at high levels and should be more likely to maintain efficient forward mode NCX1 activity during cardiac disease states, which we hypothesize would be cardioprotective (see discussion).
Figure 6. Affinity of the Na+/K+-ATPase pump for Na+ is increased in myocytes with virus-mediated overexpression of NKA-α2 but not NKA-α1.
A, Na+/K+ ATPase pump rate as a function of intracellular Na+ loading in adult rat myocytes 24-34 hours after infection with an adenovirus expressing NKA-α1, NKA-α2, or β-galactosidase (control). B, Km and C, Vmax values for NKA-α1, NKA-α2, or β-galactosidase (control) -infected adult rat myocytes. For each experiment, number of myocytes analyzed is given within the graph. Data is from two separate myocyte isolations and viral infection experiments on two separate days. *P<0.05 versus control; #P<0.05 vs. NKA-α1 -infected myocytes.
DISCUSSION
Removal of Na+ from the cytosol of a cardiac myocyte is primarily accomplished through NKA-α1, which accounts for 88% of the NKA activity in the heart, while NKA-α2 accounts for the remaining 12% 22. Although these proteins share greater than 85% identity, published reports suggest that they are not completely redundant and likely have specialized functions. Indeed, NKA-α2 heterozygous gene-deleted mice are hypercontractile for cardiac function while NKA-α1 heterozygous gene-deleted mice are hypocontractile 21. While the reason for the profound phenotypic difference between these two heterozygous mouse models remains a mystery, especially since more recent studies suggest that both isoforms similarly interact with NCX1 13, the ultimate mechanism may relate to the observations we have made here: that NKA has greater affinity and pump activity for Na+, and that PLM levels are specifically reduced in NKA-α2 overexpressing hearts, effects that would tend to maintain proper Ca2+ handling in the face of disease insults. For example, transgenic mice expressing a mutant form of SERCA2 in the heart with greater Ca2+ affinity were protected from cardiac hypertrophy after TAC stimulation, presumably by better maintaining Ca2+ homeostasis during disease that would otherwise tend to secondarily lead to negative influences on the heart (i.e. increased neurohumoral drive) 30.
Previous observations suggest NKA-α1 and α2 differentially regulate cardiac contractility 21, and here we provide further evidence demonstrating that these two isoforms play fundamentally different roles in the heart; this time in relation to regulation of the cardiac hypertrophic response. Indeed, NKA-α1 transgenic showed a normal hypertrophic response after TAC surgery, while NKA-α2 transgenic mice were consistently less hypertrophic with less cardiac remodeling and signs of heart failure. The mechanism whereby increased NKA-α2 elicits such protection is intriguing, especially since overexpression of either NKA-α1 or α2 similarly reduces the Ca2+ transient amplitude and sarcoplasmic reticulum Ca2+ load. To determine whether NKA isoforms had differential effects on NCX1 activity, we utilized NiCl2, a well-described inhibitor of the exchanger 31. There was no difference in Ca2+ extrusion between control, NKA-α2 or NKA-α1 in the presence of Ni2+, suggesting no change in the rate of Ca2+ decay when NCX1 was inhibited and Ca2+ removal was primarily due to SERCA2 activity. However, when NiCl2 was absent, the rate of Ca2+ decay was significantly increased in NKA-α2, but not significantly different in NKA-α1 transgenic myocytes after caffeine-mediated sarcoplasmic reticulum Ca2+ release (Figure 5E), suggesting that the rate of Ca2+ extrusion through NCX1 is more under the influence of NKA-α2.
We reasoned that NKA-α2 could generate a selective reduction in [Na+]i that prevents the activation of pro-hypertrophic signalling pathways after TAC surgery by reducing resting [Ca2+]i in t-tubule microdomains, which cannot be currently measured. However, this hypothesis lacks an ultimate molecular effector, as there was no reduction in NFAT-luciferase activity, calcineurin/calmodulin interaction, or total CaMKII activity (Ca2+ dependent or Ca2+ independent, data not shown). These results suggest that normal hypertrophic signalling pathways are not differentially affected (reduced) in NKA-α2 transgenic hearts, and that the protection we observed was possibly associated with a preservation in Na+ and Ca2+ handling that would otherwise become dysregulated and lead to disease secondarily (i.e. increased β-adrenergic drive that attempts to maintain contractile function). Despa et al recently published more supportive evidence that NKA-α2 may have a selective effect on cardiac Na+ and Ca2+ handling compared with NKA-α1 23. They showed that selective inhibition of NKA-α2, but not NKA-α1 could increase Ca2+ transients and fractional Ca2+ release from the SR, even though intracellular Na+ was reduced to a similar extent by each isoform, likely due to the sensitizing effect of increased cleft Ca2+ on RyR2. Our results may ultimately indicate that enhanced local Na+ and Ca2+ removal elicited by NKA-α2 expression could subtly blunt alterations in abundance, post-translational modification and/or activity of proteins in the t-tubule/SR junctional space to work against remodeling of the E-C coupling process and at least partially improve cardiac function, even in the face of activated hypertrophic signaling pathways such as calcineurin/NFAT and CaMKII, and that has the effect of reducing cardiac hypertrophy after pressure overload.
Another profound change in NKA-α2 hearts was a dramatic reduction in PLM levels and phosphorylation. PLM is an inhibitor of NKA activity that functions in a manner analogous to PLN inhibition of SERCA2, such that it can generally inhibit NKA activity 32. Thus its reduction in NKA-α2 transgenic hearts would be predicted to lead to greater NKA activity, removing Na+ more efficiently. PLM phosphorylated at S68 also functions as an inhibitor of NCX1 33, 34, and overexpression of a PLM phosphomimetic protein resulted in arrhythmia and heart disease associated with loss of NCX1 activity 35. Therefore, the reduced phosphorylation of PLM we observed in our NKA-α2 transgenic mice, as well as the total reduction in PLM, may further increase NCX1 activity and enhance Ca2+ removal even during disease states that might otherwise lead to reverse mode Ca2+ influx due to intracellular elevations in Na+ (Online Figure V). Enhanced Ca2+ extrusion through NCX1 elicited by a steeper Na+ gradient and reduced PLM phosphorylation would, in effect, prime the heart with bolstered forward-mode activity prior to induction of pressure overload, contributing to a positive profile of inotropy and lusitropy that should be cardioprotective. For example, mice lacking the gene for PLN are hypercontractile with optimized Ca2+ cycling, and crossing these mice with other mouse models of heart failure prevented or rescued disease, likely by diminishing secondary neurohumoral signalling that is typically associated with reduced cardiac output and defects in Ca2+ cycling 36. Similarly, as stated above, overexpression of a mutant form of SERCA2 that enhances Ca2+ cycling in the hearts of transgenic mice produced less cardiac hypertrophy with pressure overload stimulation 30. Reciprocally, ventricle-specific deletion of the gene encoding NCX1 resulted in increased fibrosis and arrhythmias at baseline, and increased cardiac hypertrophy after pressure overload stimulation that resulted in the death of all animals within 3 weeks after surgery 37, a result that is consistent with less cardiac hypertrophy due to optimized NCX1 activity in our NKA-α2 transgenic mice. Thus, NKA-α2 overexpression could be cardioprotective, producing less pressure overload-induced hypertrophy, simply by optimizing Ca2+ handling and cardiac contractile performance (less need for neuroendocrine drive).
In addition to optimizing cardiac contractile function, NKA-α2 enhancement could be cardioprotective by affecting one or more microdomains of Na+. For example, Na+ overload during ischemia results in damage to mitochondria and reduction in ATP production that can be ameliorated via inhibition of voltage-gated Na+ channels, NHE1 38, 39, and NCX1 40. A recent paper by Liu et al. further demonstrated that in a guinea pig aortic constriction model of heart failure, elevated [Na+]i results in decreased mitochondrial Ca2+ uptake, which the authors suggest can negatively impact mitochondrial energy production, diminish mitochondrial Ca2+ buffering, and reduce the ability of the cell to respond to reactive oxygen species41. Thus, augmented NKA-α2 expression in transgenic hearts may provide protection after TAC solely through a reduction in local [Na+]i. Our current data certainly strengthen the case that the NKA subtypes are not functionally redundant and further suggest that NKA-α2 controls Na+ with a more proximal influence on NCX1 activity, which positively impacts contractile parameters of the heart and imparts protection from pressure overload stimulation.
Supplementary Material
Novelty and Significance.
What Is Known?
The Na+/K+ ATPase (NKA) controls Na+ efflux from cardiac myocytes, a process that regulates Ca2+ homeostasis through the Na+/Ca2+ exchanger (NCX1).
There are two isoforms of NKA (α1 and α2) in the heart, of which the α2 subunit shows preferential localization to t-tubules and may generate a unique signalling domain.
Intracellular Na+ concentration is increased in many models of heart disease, and may play a role in disease progression.
What New Information Does This Article Contain?
The expression of NKA α subunit is regulated such that increased expression of one isoform leads to downregulation of the other isoform.
Increased expression of the α2 subunit reduces phospholemmen expression and phosphorylation and enhances Ca2+ efflux through forward-mode NCX1 activity.
Increased expression of the α2 subunit increases the afqfinity of NKA for Na+ compared with α1 overexpression.
This study was designed to examine whether increased expression of NKA, the primary Na+ efflux pathway in cardiac myocytes, could prevent the increase in intracellular Na+ associated with heart disease and mitigate hypertrophic remodelling. Our results show that upregulation of this pathway is protective and we provide new insights into the respective roles of the NKA isoforms during disease. We found that increased NKA-α2 (but not α1) expression reduced disease after pressure overload and enhanced Ca2+ removal via upregulation of forward-mode NCX1 activity. Additionally, increased NKA-α2 resulted in a profile that is predicted to more effectively remove Na+ from the myocyte, thus collectively providing a cardioprotective profile that resists dysregulation of Na+ and Ca2+ levels. These results reveal a unique role of the a2 subunit of NKA in mitigating cardiac hypertrophic remodelling during pressure overload.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the NIH (to J.D.M and D.M.B). J.D.M was also supported by the Howard Hughes Medical Institute. P.E was supported by the Austrian Science Fund (FWF, award J 2775-B12). R.N.C was supported by Grant Number F32HL097551 from the National Heart, Lung, and Blood Institute.
Nonstandard Abbreviations and Acronyms
- CaMKII
Ca2+/calmodulin-dependent kinase II
- MHC
myosin heavy chain
- NCX
Na+/Ca2+ exchanger
- NFAT
nuclear factor of activated T cells
- NHE1
Na+/H+ exchanger
- NKA
Na+/K+ ATPase
- PLN
phospholamban
- PLM
phospholemman
- SERCA2
sarco(endo)plasmic reticulum Ca2+ ATPase2
- TAC
transverse aortic constriction
- Wt
wildtype
Footnotes
See online supplement for expanded Materials and Methods.
DISCLOSURES
None.
REFERENCES
- 1.Bers DM, Despa S. Cardiac myocytes ca2+ and na+ regulation in normal and failing hearts. J Pharmacol Sci. 2006;100:315–322. doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- 2.Pogwizd SM, Sipido KR, Verdonck F, Bers DM. Intracellular na in animal models of hypertrophy and heart failure: Contractile function and arrhythmogenesis. Cardiovasc Res. 2003;57:887–896. doi: 10.1016/s0008-6363(02)00735-6. [DOI] [PubMed] [Google Scholar]
- 3.Pieske B, Maier LS, Piacentino V, 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 4.Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: Contractile versus signaling ca2+ in a cardiac myocyte. J Mol Cell Cardiol. 2012;52:317–322. doi: 10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular na(+) concentration is elevated in heart failure but na/k pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 6.Baartscheer A, Schumacher CA, van Borren MM, Belterman CN, Coronel R, Fiolet JW. Increased na+/h+-exchange activity is the cause of increased [na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res. 2003;57:1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- 7.Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama H, Gunasegaram S, Harding SE, Avkiran M. Sarcolemmal na+/h+ exchanger activity and expression in human ventricular myocardium. J Am Coll Cardiol. 2000;36:534–540. doi: 10.1016/s0735-1097(00)00730-0. [DOI] [PubMed] [Google Scholar]
- 9.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S. Activation of na+/h+ exchanger 1 is sufficient to generate ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res. 2008;103:891–899. doi: 10.1161/CIRCRESAHA.108.175141. [DOI] [PubMed] [Google Scholar]
- 11.Sossalla S, Maier LS. Role of ranolazine in angina, heart failure, arrhythmias, and diabetes. Pharmacol Ther. 2012;133:311–323. doi: 10.1016/j.pharmthera.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS. Diastolic dysfunction and arrhythmias caused by overexpression of camkiidelta(c) can be reversed by inhibition of late na(+) current. Basic Res Cardiol. 2011;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The alpha 1 isoform of na,k-atpase regulates cardiac contractility and functionally interacts and co-localizes with the na/ca exchanger in heart. J Biol Chem. 2004;279:54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 14.Lederer WJ, Niggli E, Hadley RW. Sodium-calcium exchange in excitable cells: Fuzzy space. Science. 1990;248:283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- 15.Swift F, Tovsrud N, Sjaastad I, Sejersted OM, Niggli E, Egger M. Functional coupling of alpha(2)-isoform na(+)/k(+)-atpase and ca(2+) extrusion through the na(+)/ca(2+)-exchanger in cardiomyocytes. Cell Calcium. 2010;48:54–60. doi: 10.1016/j.ceca.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Verdonck F, Mubagwa K, Sipido KR. [na(+)] in the subsarcolemmal ‘fuzzy’ space and modulation of [ca(2+)](i) and contraction in cardiac myocytes. Cell Calcium. 2004;35:603–612. doi: 10.1016/j.ceca.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Rahimtoola SH, Tak T. The use of digitalis in heart failure. Curr Probl Cardiol. 1996;21:781–853. doi: 10.1016/s0146-2806(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 18.Schwinger RH, Wang J, Frank K, Muller-Ehmsen J, Brixius K, McDonough AA, Erdmann E. Reduced sodium pump alpha1, alpha3, and beta1-isoform protein levels and na+,k+-atpase activity but unchanged na+-ca2+ exchanger protein levels in human heart failure. Circulation. 1999;99:2105–2112. doi: 10.1161/01.cir.99.16.2105. [DOI] [PubMed] [Google Scholar]
- 19.Bossuyt J, Ai X, Moorman JR, Pogwizd SM, Bers DM. Expression and phosphorylation of the na-pump regulatory subunit phospholemman in heart failure. Circ Res. 2005;97:558–565. doi: 10.1161/01.RES.0000181172.27931.c3. [DOI] [PubMed] [Google Scholar]
- 20.El-Armouche A, Wittkopper K, Fuller W, Howie J, Shattock MJ, Pavlovic D. Phospholemman-dependent regulation of the cardiac na/k-atpase activity is modulated by inhibitor-1 sensitive type-1 phosphatase. FASEB J. 2011;25:4467–4475. doi: 10.1096/fj.11-184903. [DOI] [PubMed] [Google Scholar]
- 21.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the na,k-atpase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell. 1999;3:555–563. doi: 10.1016/s1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 22.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac na+/k+-atpase alpha1 and alpha2 subunits in t-tubule and surface sarcolemmal membranes. Cardiovasc Res. 2007;73:92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Despa S, Lingrel JB, Bers DM. Na(+)/k)+)-atpase alpha2-isoform preferentially modulates ca2(+) transients and sarcoplasmic reticulum ca2(+) release in cardiac myocytes. Cardiovasc Res. 2012;95:480–486. doi: 10.1093/cvr/cvs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifford RJ, Kaplan JH. Regulation of na,k-atpase subunit abundance by translational repression. J Biol Chem. 2009;284:22905–22915. doi: 10.1074/jbc.M109.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/nfat coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Chang B, Blair NS, Sargent M, York AJ, Robbins J, Shull GE, Molkentin JD. Plasma membrane ca2+-atpase isoform 4 antagonizes cardiac hypertrophy in association with calcineurin inhibition in rodents. J Clin Invest. 2009;119:976–985. doi: 10.1172/JCI36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on na/k pump function in cardiac myocytes. Circ Res. 2005;97:252–259. doi: 10.1161/01.RES.0000176532.97731.e5. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard TJ, Parvatiyar M, Bullard DP, Lynch RM, Lorenz JN, Paul RJ. Transgenic mice expressing na+-k+-atpase in smooth muscle decreases blood pressure. Am J Physiol Heart Circ Physiol. 2007;293:H1172–1182. doi: 10.1152/ajpheart.00279.2007. [DOI] [PubMed] [Google Scholar]
- 29.McDonough AA, Zhang Y, Shin V, Frank JS. Subcellular distribution of sodium pump isoform subunits in mammalian cardiac myocytes. Am J Physiol. 1996;270:C1221–1227. doi: 10.1152/ajpcell.1996.270.4.C1221. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama H, Otsu K, Yamaguchi O, Nishida K, Date MO, Hongo K, Kusakari Y, Toyofuku T, Hikoso S, Kashiwase K, Takeda T, Matsumura Y, Kurihara S, Hori M, Tada M. Cardiac-specific overexpression of a high ca2+ affinity mutant of serca2a attenuates in vivo pressure overload cardiac hypertrophy. FASEB J. 2003;17:61–63. doi: 10.1096/fj.02-0474fje. [DOI] [PubMed] [Google Scholar]
- 31.Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung JY, Zhang XQ, Song J, Gao E, Rabinowitz JE, Chan TO, Wang J. Phospholemman: A novel cardiac stress protein. Clin Transl Sci. 2010;3:189–196. doi: 10.1111/j.1752-8062.2010.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J, Zhang XQ, Ahlers BA, Carl LL, Wang J, Rothblum LI, Stahl RC, Mounsey JP, Tucker AL, Moorman JR, Cheung JY. Serine 68 of phospholemman is critical in modulation of contractility, [ca2+]i transients, and na+/ca2+ exchange in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;288:H2342–2354. doi: 10.1152/ajpheart.01133.2004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XQ, Ahlers BA, Tucker AL, Song J, Wang J, Moorman JR, Mounsey JP, Carl LL, Rothblum LI, Cheung JY. Phospholemman inhibition of the cardiac na+/ca2+ exchanger. Role of phosphorylation. J Biol Chem. 2006;281:7784–7792. doi: 10.1074/jbc.M512092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song J, Gao E, Wang J, Zhang XQ, Chan TO, Koch WJ, Shang X, Joseph JI, Peterson BZ, Feldman AM, Cheung JY. Constitutive overexpression of phosphomimetic phospholemman s68e mutant results in arrhythmias, early mortality, and heart failure: Potential involvement of na+/ca2+ exchanger. Am J Physiol Heart Circ Physiol. 2012;302:H770–781. doi: 10.1152/ajpheart.00733.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorn GW, 2nd, Molkentin JD. Manipulating cardiac contractility in heart failure: Data from mice and men. Circulation. 2004;109:150–158. doi: 10.1161/01.CIR.0000111581.15521.F5. [DOI] [PubMed] [Google Scholar]
- 37.Jordan MC, Henderson SA, Han T, Fishbein MC, Philipson KD, Roos KP. Myocardial function with reduced expression of the sodium-calcium exchanger. J Card Fail. 2010;16:786–796. doi: 10.1016/j.cardfail.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawyer DB, Suter TM, Apstein CS. The sting of salt on an old, but open, wound--is na(+) the cause of mitochondrial and myocardial injury during ischemia/reperfusion? J Mol Cell Cardiol. 2002;34:699–702. doi: 10.1006/jmcc.2002.2030. [DOI] [PubMed] [Google Scholar]
- 39.Iwai T, Tanonaka K, Inoue R, Kasahara S, Kamo N, Takeo S. Mitochondrial damage during ischemia determines post-ischemic contractile dysfunction in perfused rat heart. J Mol Cell Cardiol. 2002;34:725–738. doi: 10.1006/jmcc.2002.2002. [DOI] [PubMed] [Google Scholar]
- 40.Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the na+-ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res. 2005;97:916–921. doi: 10.1161/01.RES.0000187456.06162.cb. [DOI] [PubMed] [Google Scholar]
- 41.Liu T, O'Rourke B. Enhancing mitochondrial ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.