Abstract
Overwhelming lung inflammation frequently occurs following exposure to both direct infectious and non-infectious agents, and is a leading cause of mortality world-wide. In that context, immunomodulatory strategies may be utilized to limit severity of impending organ damage. We sought to determine whether priming the lung by activating the immune system, or immunological priming, could accelerate resolution of severe lung inflammation. We assessed the importance of alveolar macrophages, regulatory T cells, and their potential interaction during immunological priming. We demonstrate that oropharyngeal delivery of low-dose lipopolysaccharide can immunologically prime the lung to augment alveolar macrophage production of interleukin-10 and enhance resolution of lung inflammation induced by a lethal dose of lipopolysaccharide or by pseudomonas bacterial pneumonia. Interleukin-10 deficient mice did not achieve priming and were unable to accelerate lung injury resolution. Depletion of lung macrophages or regulatory T cells during the priming response completely abrogated the positive effect of immunological priming on resolution of lung inflammation and significantly reduced alveolar macrophage interleukin-10 production. Finally, we demonstrated that oropharyngeal delivery of synthetic CpG-oligonucleotides elicited minimal lung inflammation compared to low-dose lipopolysaccharide, but nonetheless primed the lung to accelerate resolution of lung injury following subsequent lethal lipopolysaccharide exposure. Immunological priming is a viable immunomodulatory strategy used to enhance resolution in an experimental acute lung injury model with the potential for therapeutic benefit against a wide array of injurious exposures.
INTRODUCTION
Robust lung inflammation induced by infectious and non-infectious stimuli can lead to severe pathological states including acute respiratory distress syndrome (ARDS) and multi-organ failure with devastating, often lethal consequences. Adaptive immunomodulatory strategies to protect humans against severe injury represent potentially attractive options in susceptible hosts or during outbreaks of virulent disease (1), yet the possibility of maladaptive immune responses necessitates more thorough understanding of involved cellular mechanisms (2). In the lung, resident alveolar macrophages are crucial to the immune response, uniquely positioned as “first responders” designed to recognize and combat foreign antigen in the airspaces of the lower respiratory tract. Upon activation by toll-like receptor (TLR) or other pattern recognition receptor signaling, macrophages recruit additional pro-inflammatory immune cells including neutrophils, exudative macrophages, and Th1 lymphocytes to the lung and alveolar space as a critical part of the innate immune response (3-6). Once the pro-inflammatory milieu has recognized and removed foreign substances, resident and recruited macrophages undergo an active transition to a pro-resolution phenotype to dampen inflammation, initiate repair and restore the immune barrier (6-8).
Lipopolysaccharide (LPS; endotoxin), a component of the gram-negative bacteria cell wall, is recognized by mammalian Toll-Like Receptor 4-Lymphocyte Antigen 96 (TLR4-MD2) complex, and is abundantly expressed on the cell surface of macrophages and other antigen presenting cells (APCs) (9, 10). Often regarded as the prototypical danger signal - pattern recognition receptor response, LPS binding to membrane TLR4-MD2 activates transcription factors NF-κB and STAT1 by signaling through MyD88 and TRIF-dependent pathways respectively (11), leading to a myriad of pro-inflammatory signaling cascades. Repeated TLR4 stimulation by LPS results in a state of reduced inflammatory capacity known as endotoxin (LPS) tolerance (ET). ET has generally been ascribed to clinical scenarios involving sepsis and other sepsis-like states, in which circulating monocytes can be persistently exposed to endotoxin, resulting in ineffective TNF-α or other pro-inflammatory cytokine production with repeated LPS exposure (12-15). As a result, ET has been associated with worse outcomes including increased mortality and secondary infections (11, 15). In contrast to the systemic effects of sepsis where the majority of immune cells are exposed to the same circulating stimuli, recruited inflammatory macrophages may respond differently than resident macrophages to successive lung stimuli (16, 17).
Macrophages are an important source of interleukin-10 (IL-10), a potent immunomodulatory cytokine with diverse cellular production (18, 19). The primary biological function of IL-10 is to dampen inflammation, but other functions include modulating the proliferation and differentiation of immune cells including T cells, antigen presenting cells, and neutrophils (20), as well as non-immune cells such as keratinocytes, endothelial cells, and epithelial cells (10, 20, 21). IL-10 is produced by the TRIF-dependent TLR4 signaling pathway that dampens inflammation by induction of p50 NF-κB, STAT3, and SOCS3, all negative regulators of the TLR4 signaling cascade (11). Although IL-10 regulates several aspects of the macrophage response to LPS including type, magnitude, and duration, it is dispensable for development of endotoxin tolerance (22, 23). We and others have demonstrated that IL-10 contributes to resolution of inflammation in experimental acute lung injury models including bacterial pneumonia by limiting neutrophil recruitment and aiding in neutrophil removal (24-27), both critical for resolution. Phagocytosis of apoptotic neutrophils by both monocytes and macrophages further augments IL-10 macrophage production (6, 28). Independent of phagocytosis, ligation of the macrophage Fcγ receptor can also induce early IL-10 production with LPS co-stimulation (29). The role of IL-10 in immunological priming as an adaptive immunomodulatory mechanism against severe lung inflammation has not yet been defined.
Regulatory T cells (Tregs) are critical for active resolution of lung inflammation and repair. Tregs modulate innate immune cellular responses (30) including effects on macrophage phenotype and function in animals and humans (31-33). Specifically, Tregs blunt macrophage pro-inflammatory cytokine production, enhance their efferocytosis of apoptotic neutrophils, and transition them to an alternatively-activated phenotype (25, 32). Conversely, macrophages and circulating monocytes are critical for Treg induction, expansion, and suppressive function (34-37), but mechanisms mediating this interaction remain incompletely understood. We surmise that macrophages and Tregs are both necessary, and may act in concert, to regulate the immunological priming response and accelerate resolution of severe lung inflammation.
Our results demonstrate that activation of distinct pattern recognition receptors to induce minimal lung inflammation is sufficient to immunologically prime the lung and accelerate resolution of lung injury resulting from a severe injurious exposure. We found that both macrophages that produce IL-10 and Tregs are critical for immunological priming. Our results suggest the importance of immunological priming as a mechanism to improve host defense against a variety of direct insults to the lung, with significant therapeutic potential.
METHODS
Animals
Male C57BL/6 wild type (WT) mice (8-10 weeks old) and IL-10−/− mice (C57BL/6 background) were purchased from Jackson Laboratories (Bar Harbor, ME). Foxp3gfp and Foxp3DTR mice (B6.129(Cg)-Foxp3tm3Ayr/J) were gifts from Dr. Alexander Y. Rudensky (Memorial Sloan-Kettering Institute). Foxp3gfp reporter mice express an N-terminal GFP-Foxp3 fusion protein to further identify Foxp3+ Treg cells (38). Foxp3DTR mice express the human diphtheria toxin receptor (DTR) along with GFP, which have been fused to the 3’ untranslated region of the Foxp3 locus, and specific elimination of Foxp3+ Treg cells in vivo occurs through intraperitoneal (i.p.) administration of diphtheria toxin (DT) (39). Mice were housed at the Johns Hopkins University Asthma and Allergy Center, and experiments conducted under a protocol approved by the Johns Hopkins Animal Care and Use Committee.
Animal Preparation
For oropharyngeal (o.p.) delivery, mice were deprived of chow for 1-2 hours, anesthetized using inhaled isoflurane, followed by instillation of 1 mg/kg Escherichia coli lipopolysaccharide (O55:B5 Sigma L2880, diluted in 50 μL sterile water), 35 μg of class C CpG oligonucleotide (ODN 2395 Invivogen, diluted in 50 μL PBS), or respective vehicle controls. After witnessed aspiration, mice were returned to their cages and all exhibited immediate recovery. Following a priming period (5 or 7 days), we performed intratracheal (i.t.) delivery as before (25). Briefly, mice were anesthetized with intraperitoneal (i.p.) ketamine/acetylpromazine (150/2.5 mg/kg) before exposure of the trachea. Lipopolysaccharide (LPS) (3 or 7.5 μg/g mouse weight diluted in sterile water), Pseudomonas aeruginosa (PAO1) (1×106 CFU, ATCC, in 50 μL PBS) or respective vehicle controls were instilled intratracheally via a 20-gauge catheter. After 1, 3, or 5 days, groups of mice were anesthetized with i.p. ketamine/acetylpromazine and euthanized by exsanguination from the inferior vena cava. The lungs were perfused with 1 ml of phosphate-buffered saline (PBS), followed by bronchoalveolar lavage (BAL) of the right lung; the left lung was processed for histology. BAL samples were routinely cultured to assess for bacterial infection. For quantitative measures of bacteria, whole lungs were homogenized without prior lavage, and the lysates were diluted in PBS and streaked on agar plates. After 24 hours at 37 °C, colonies were counted.
Diptheria toxin and clodronate liposome injections
Diphtheria toxin (List Biological Laboratories, Inc, Lot # 15043A1, diluted in PBS) was administered via i.p. injection on day −2 (50 μg/kg mouse) and day −1 (15 μg/kg) of the priming period prior to i.t. LPS as described (39). Mice harvested on day +5 also received a DT dose (15 μg/kg) on day +2 after i.t. LPS. Clodronate liposomes (Cl2MDP) or PBS liposomes (control) were prepared as described (8), followed by o.p. instillation (60 μL) on day −3, and i.p. injection (500 μl) on days −2 and −1 of the priming period.
Analysis of bronchoalveolar lavage (BAL)
BAL was obtained by cannulating the trachea with a 20-gauge catheter. The right lung was lavaged with two aliquots of 0.7 ml of calcium-free PBS except when noted. BAL was centrifuged at 700 × g for 10 min at 4°C. The cell-free supernatants were stored at −80°C until further analysis. The cell pellet was diluted in PBS, and total cell number was counted with a hemocytometer using trypan blue exclusion. Cell differentials (300 cells per sample) were counted on cytocentrifuge preparation with Diff-Quik stain (Baxter Diagnostics, McGaw Park, IL). Total protein was measured in the cell-free supernatant by the Lowry method (40).
Measurement of Cytokines
Tumor necrosis factor (TNF-α) or IL-10 levels were measured in BAL and cell culture supernatants by ELISA (R&D System, Minneapolis, MN).
Lung Histology and lung injury scoring
Lungs (n=5 per time point) were inflated to a pressure of 25 cmH2O using 1% low melting agarose (Invitrogen) for histologic evaluation by hemotoxylin and eosin staining (41). For lung injury scoring two blinded investigators analyzed the samples and determined levels of lung injury according to a semi-quantitative scoring system outlined below. All lung fields (×20 magnification) were examined for each sample. Quantification of histological lung injury was determined using the following scoring: 1- normal; 2- focal (<50% lung section) interstitial congestion and inflammatory cell infiltration; 3- diffuse (>50% lung section) interstitial congestion and inflammatory cell infiltration; 4- focal (<50% lung section) consolidation and inflammatory cell infiltration; 5- diffuse (>50% lung section) consolidation and inflammatory cell infiltration. The mean score was used for comparison between groups.
Flow Cytometry
For surface staining, cells were incubated with Fc Block-2.4G2 (BD Pharmingen) antibody to block Fcγ III/II receptors before staining with a specific antibody. The following antibodies were purchased from BD Pharmingen (San Diego, CA) and Biolegend (San Diego, CA): anti-Annexin V-PE, anti-7-AAD, anti-Ly6G-FITC, anti-Gr1-BV570, anti-CD11b-PETR, and anti-F4/80-APC-Cy7, along with relevant isotype antibodies. For our lymphocyte panel we used anti-CD4-Ax700, anti-CD25-APC-Cy7, anti-CD3-Pacblue, anti-CD8-PECF594. For intracellular staining of Foxp3, following Fc block and surface staining, cells were fixed and permeabilized with Foxp3 staining buffer (eBioscience, San Diego, CA), then stained with anti−Foxp3-APC mAbs (eBioscience). For intracellular staining of cytokines, following murine BAL (4 aliquots, 0.9 ml PBS + Golgi Plug (GP) (BD, protein transport inhibitor)), cells were isolated and resuspended (0.5×106 cells/ml) in RPMI/FCS/Pen/Strep/GP (unstimulated) or with additional Leukocyte Activation Cocktail (BD; PMA + Ionomycin+ Brefeldin A; 2 μl/ml, stimulated, to enhance intracellular cytokine signal) for 4 hours. Live-dead discrimination was performed with Fixable UV-excitable Blue Dead Cell Stain (Invitrogen). Cells were Fc blocked, surface stained for macrophage, neutrophil, and lymphocyte markers, and fixed/permeabilized (cytofix/cytoperm, BD pharmingen) and intracellular staining × 30 min for cytokines including anti-TNF-α-Percp, anti-IL-10-PE, anti-IL-6-APC, and anti-IFNγ-PECy7. Monocytes, alveolar macrophages, neutrophils, and lymphocytes were gated with characteristic forward scatter/side scatter using a FACSAria instrument, and CellDiva for data acquisition (Becton Dickinson, San Jose, CA), and FlowJo for analysis (Tree Star Inc, San Carlos, CA).
Statistical analysis
All values are reported as mean ± SEM. Parametric or nonparametric testing was performed as indicated. Markers of injury were compared using the Student t test or Mann-Whitney rank sum test. Pairwise comparisons were performed using the Student t test with Bonferroni correction. Baseline and pre- and post-treatment data within a group were compared using repeated measure one-way analysis of variance (Fisher's protected least significant difference test). The survival curve was established with Kaplan-Meier survival analysis. A p<0.05 was used as the cut-off point for significance.
RESULTS
LPS priming accelerates lung injury resolution
To begin to examine mechanisms by which the immune system can be reprogrammed or primed in preparation for severe and potentially lethal injury, we needed a route of delivery which safely and quickly activates the immune system. Using oropharyngeal instillation, we effectively delivered intrapulmonary agents in a reproducible manner without surgery or prolonged anesthesia as demonstrated using trypan blue dye (Supp. Fig. 1A) (42). We compared the response of WT mice to lipopolysaccharide (LPS, 1 mg/kg) administered by oropharyngeal (o.p.) versus intratracheal (i.t.) routes, the latter a more established method of inducing experimental lung inflammation (25, 40, 43), and followed mice for 7 days. Both routes of delivery induced significant weight loss (Supp. Fig. 1B) and signs of systemic injury with huddling and pilorection, significant increases in bronchoalveolar (BAL) protein (Supp. Fig. 1C), BAL total cell counts (Supp. Fig. 1D), and BAL neutrophils (>80% of total alveolar cells, not shown). WT mice challenged with o.p. LPS returned to baseline weight, BAL protein, and BAL cell count values by day 7. In contrast, WT mice challenged with i.t. LPS had persistent weight loss, persistent elevation of BAL protein and total cells at day 7. Notably, the alveolar cell profile of mice treated with o.p. LPS at day 7 was predominantly macrophages (85%), with significantly increased lymphocytes (10-15%) and fewer neutrophils (0-5%) compared to mice treated with i.t. LPS (not shown). These data suggest that o.p. delivery of LPS is a viable method to induce modest, self-limiting lung inflammation.
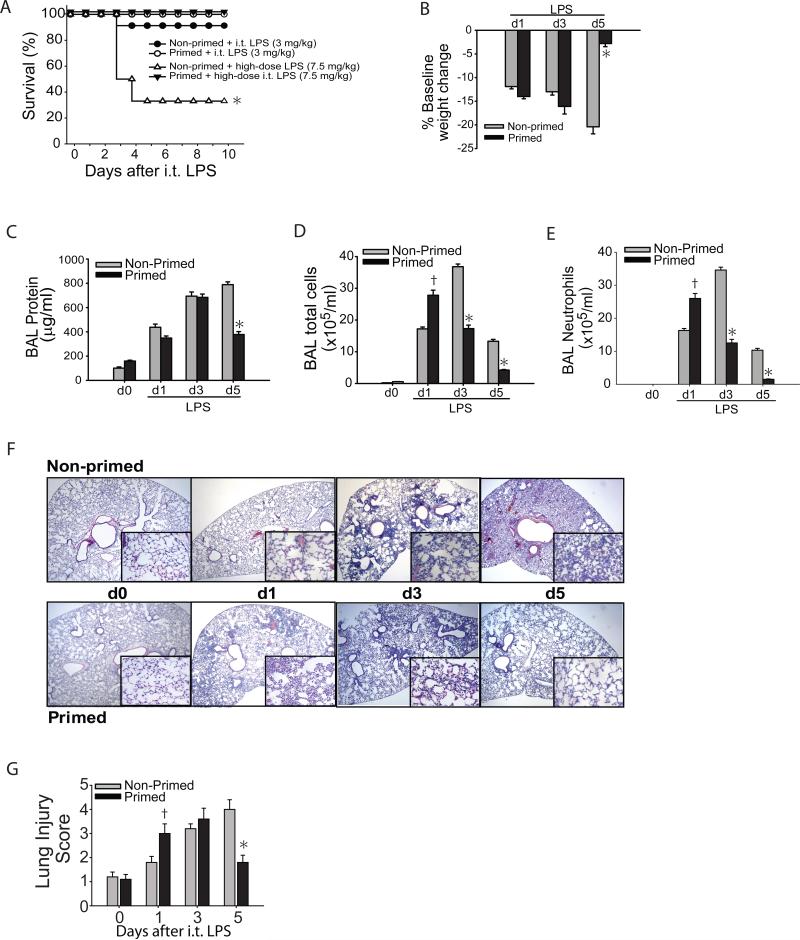
We sought to determine if immunological priming could protect mice from a subsequent more severe injury, in this case high dose i.t. LPS. We primed WT mice with o.p. LPS (1 mg/kg) or water (non-primed group). To determine whether immunological priming conferred mortality benefit, we then challenged LPS primed and non-primed mice with high dose i.t. LPS (7.5 mg/kg), more than twice our usual acute lung injury (ALI) LPS dose (25, 42), 7 days after priming. After high dose LPS, nearly 70% of the non-primed mice died, and surviving mice appeared ill even after 10 days (Fig. 1A). In marked contrast, none of LPS primed mice died, and all mice appeared healthy and recovered their baseline body weight by day 10 (not shown). Because survival was markedly reduced after high dose LPS in the non-primed group, we elected to use a lower i.t. LPS dose (3 mg/kg) to better discriminate the effects of priming on lung injury and resolution patterns as shown in the rest of Figure 1. After 7 days of “priming phase”, LPS (primed) and water (non-primed) treated animals received i.t. LPS (3 mg/kg), and were followed up to 5 days (Supp. Fig. 1E). In addition to reduced mortality at the 3 mg/kg LPS dose (Fig. 1A), mice in the primed group appeared healthier and had recovered their baseline body weight 5 days after i.t. LPS despite significant weight loss at days 1 and 3. Comparatively, mice in the non-primed group appeared ill, were less mobile, and at day 5 had a significant 20% weight loss from baseline (Fig. 1B). BAL protein, a marker of lung injury, was increased to similar levels in the non-primed and primed groups 1 and 3 days after i.t. LPS, but remained significantly elevated only in the non-primed group by day 5. Total alveolar cells (Fig. 1D) and alveolar neutrophils (Fig. 1E) were higher in the primed mice at day 1 after i.t. LPS, but significantly lower at days 3 and 5 when compared to non-primed mice. The pattern of histological changes in the lung was consistent with the pattern for BAL cells (Fig. 1F). Although interstitial thickening, cellular infiltration, and lung injury score (Fig. 1G) were worse in primed WT mice compared to non-primed mice at day 1, by day 5 only primed mice achieved resolution of histologic injury while non-primed mice remained severely injured.
Fig. 1. Immunological priming accelerates lung injury resolution.
Following a 7-day priming period, primed and non-primed WT mice were assessed for survival after either 3 mg/kg or 7.5 mg/kg i.t. LPS (A). After either dose of i.t. LPS, survival over 10 days was determined in primed and non-primed WT mice (n=8-10 per time point). Primed and non-primed WT mice were assessed for body weight relative to baseline (B), bronchoalveolar (BAL) protein (C), BAL total cell counts (D) and BAL neutrophils (E) at intervals after i.t. LPS injury. (F) Histological sections were stained with H & E in primed and non-primed WT mice. Original magnifications x20; x100 (inserts). (G) Histopathological mean lung injury scores from x20 sections (n=4-6 animals per group per time point). Values expressed as mean ± SEM; * or † paired t-test against other group at same time point, p<0.05, * log-rank test for survival curve.
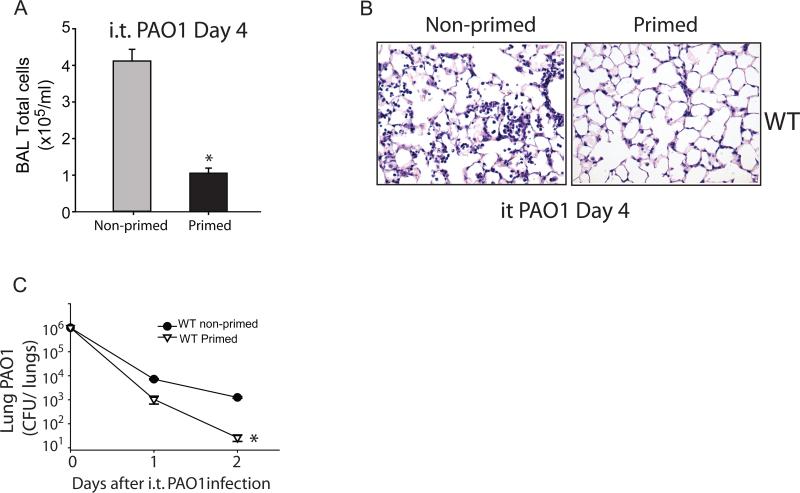
Next, we examined the effects of immunological priming on lung injury resolution in a live bacterial pneumonia model. WT mice were treated with i.t Pseudomonas aeruginosa (PAO1, 1×106 CFU) 7 days after being treated with o.p. 1 mg/kg LPS (primed) or water (non-primed), and assessed for parameters of lung injury resolution and bacterial clearance. Primed mice had significantly reduced total alveolar cell counts (Fig. 2A) and reduced histologic injury (Fig. 2B) at day 4, as well as significantly lower bacterial burden in whole lung at days 1 and 2 after i.t. PAO1 (Fig. 2C). Our studies suggest that priming animals with LPS accelerates resolution after non-infectious and infectious experimental lung injury.
Fig. 2. Immunological priming accelerates resolution from bacterial pneumonia.
Following an 7-day priming period, BAL total cells (A) and lung histology by H&E staining (B) were assessed in primed and non-primed WT mice at day 4 after i.t. PAO1 (1×106 CFU). Bacterial clearance was determined by measurement of whole lung PAO1 colony-forming units (CFU) in WT primed and non-primed mice on days 1 and 2 after i.t. PAO1 (C). Values expressed as mean ± SEM; *paired t-test against other group at same time point, p<0.05. (n=4-6 animals per group per time point)
Lung priming modulates the alveolar inflammatory milieu
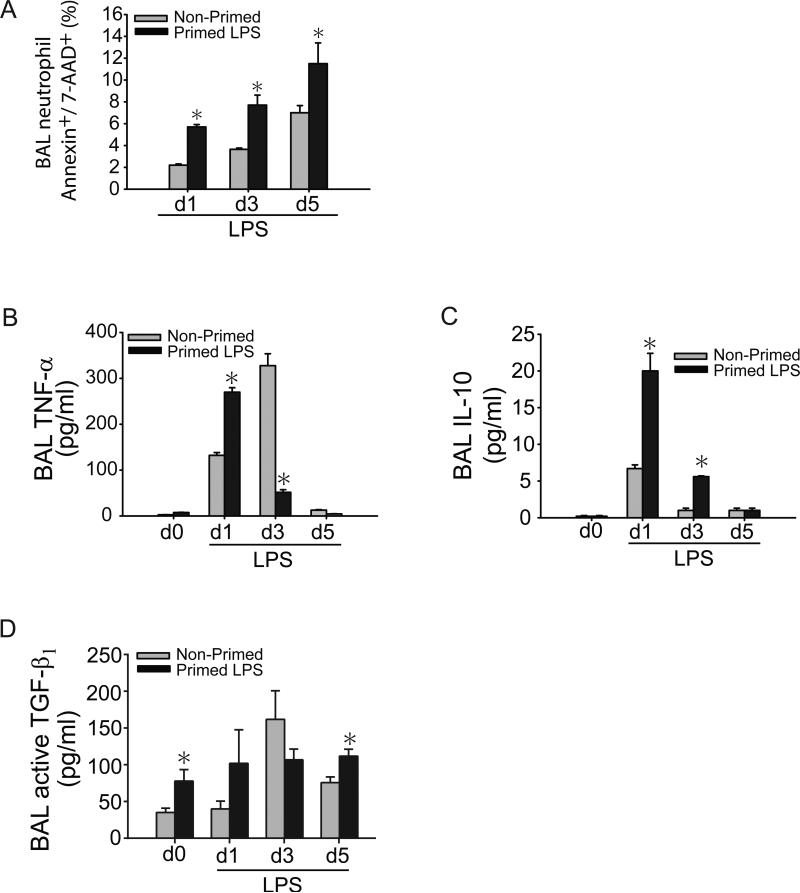
Alveolar neutrophils were significantly decreased in primed mice at days 3 and 5 after i.t. LPS. Given that apoptosis is a sine qua non for neutrophil removal and central to resolution of inflammation (44-47), we measured alveolar neutrophil apoptosis by annexin V/7-AAD staining. Neutrophil apoptosis was nearly two-fold higher at all measured intervals after i.t. LPS in the primed group compared to the non-primed group (Fig. 3A).
Fig. 3. Lung priming modulates the alveolar inflammatory milieu.
(A) Apoptosis (Annexin V+/7-AAD+) of BAL neutrophils from primed and non-primed WT mice on days 1, 3, or 5 after i.t. LPS was assessed by flow cytometry. BAL neutrophils were gated by characteristic granulocyte forward and side scatter, sub-gated for Gr-1+ to identify neutrophils, and then for AnnexinV/7-AAD, percentages for which are quantified in (A). BAL TNF-α (B), IL-10 (C), and active TGF-β1 (D) cytokine secretion were assessed at designated time points after i.t. LPS in primed and non-primed WT mice. Values expressed as mean ± SEM; *paired t-test against other group at same time point, p<0.05. (n=4-6 animals per group per time point)
To begin to understand potential mechanisms by which immunological priming could impact alveolar neutrophil abundance and accelerate lung injury resolution, we measured select BAL cytokines TNF-α, IL-10, and active TGF-β1. High BAL levels of TNF-α, a pro-inflammatory cytokine, can promote neutrophil apoptosis in experimental and human ARDS (48-50), and when present, denote a distinctly different phenotype than that which is induced by endotoxin tolerance (11). IL-10 is an anti-inflammatory, pro-repair cytokine, but can also reduce neutrophil burden at sites of inflammation by multiple mechanisms (6, 28, 51). BAL TNF-α was higher at 1 day after i.t. LPS in the primed group compared to the non-primed group (Fig. 3B), similar to the pattern observed with BAL protein and cell counts. As with the rapid decrease in BAL protein and cells after day 1 in primed mice, BAL TNF-α was significantly lower in primed mice at day 3 after i.t. LPS, but further elevated in non-primed mice. BAL IL-10 was 3.5 fold higher on day 1 after i.t. LPS in the primed group and remained significantly elevated compared to the non-primed group at day 3 (Fig. 3C). BAL TGF-β1 was significantly higher in primed mice before i.t. LPS (day 0) and after recovery from i.t. LPS (day 5) (Fig. 3D).
IL-10−/− mice do not benefit from immunological priming
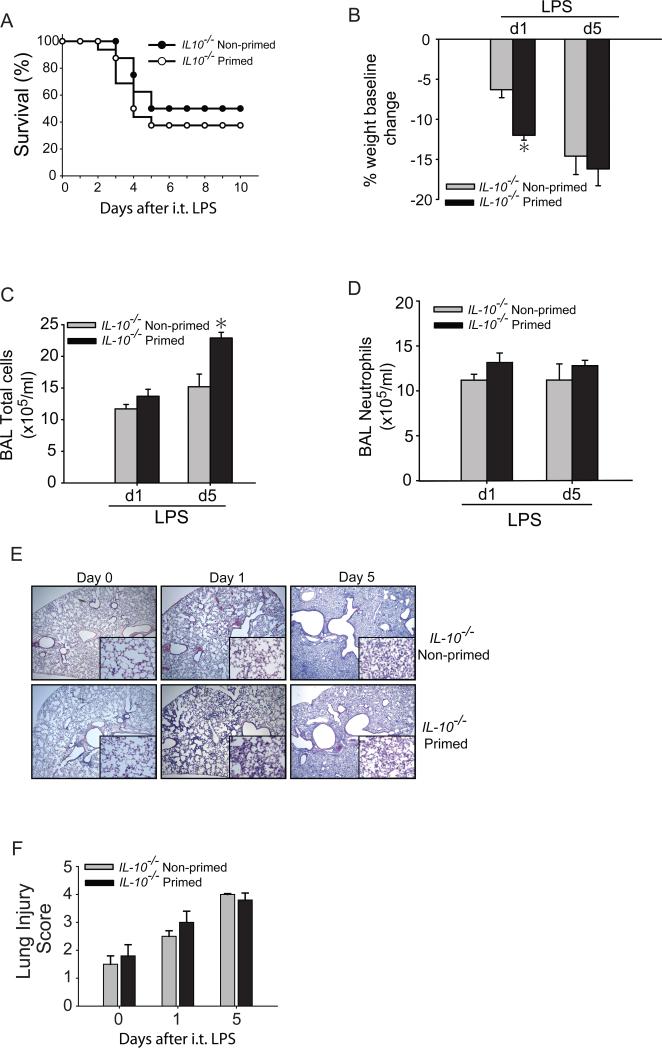
To determine if IL-10 plays a role in immunological priming, we attempted to prime IL-10−/− mice using o.p. LPS (1 mg/kg) compared to sterile water (non-primed) on day −7. IL-10−/− mice treated with o.p. LPS had returned to baseline weight by day 0 (we designated this group as primed), after which they were exposed to i.t. LPS (3 mg/kg) and compared to non-primed IL-10−/− mice exposed to i.t. LPS. In marked contrast to our results in WT mice (Fig.1A), primed IL-10−/− mice did not have a survival benefit compared to non-primed IL-10−/− mice, with 40-50% mortality in each group (Fig. 4A). Surviving mice from the primed IL-10−/− group were ill-appearing with persistent weight loss (Fig. 4B), sustained elevation of total BAL cells (Fig. 4C), BAL neutrophils (Fig. 4D), and persistent histologic injury (Figs. 4E, F). The pattern of these responses was similar to that seen in non-primed IL-10−/− mice and distinct from the pattern we observed in primed WT mice. These studies support an important role for IL-10 in the immunological priming response.
Fig. 4. IL-10−/− mice do not benefit from immunological priming.
(A) Survival was determined in primed and non-primed IL-10−/− mice. Primed and non-primed IL-10−/− mice were assessed for body weight relative to baseline (B), BAL total cell counts (C) or BAL neutrophils (D) at days 1 or 5 after i.t. LPS injury. (E) Histological sections were stained with H & E in primed and non-primed WT mice. Original magnifications x20; x100 (inserts). (F) Histopathological mean lung injury scores from x20 sections. Values expressed as mean ± SEM; *paired t-test against other group at same time point, p<0.05, (n=4-6 animals per group per time point). log-rank test for survival curve, n=8-10 in primed and non-primed groups.
Macrophages are a significant IL-10 source in the priming response
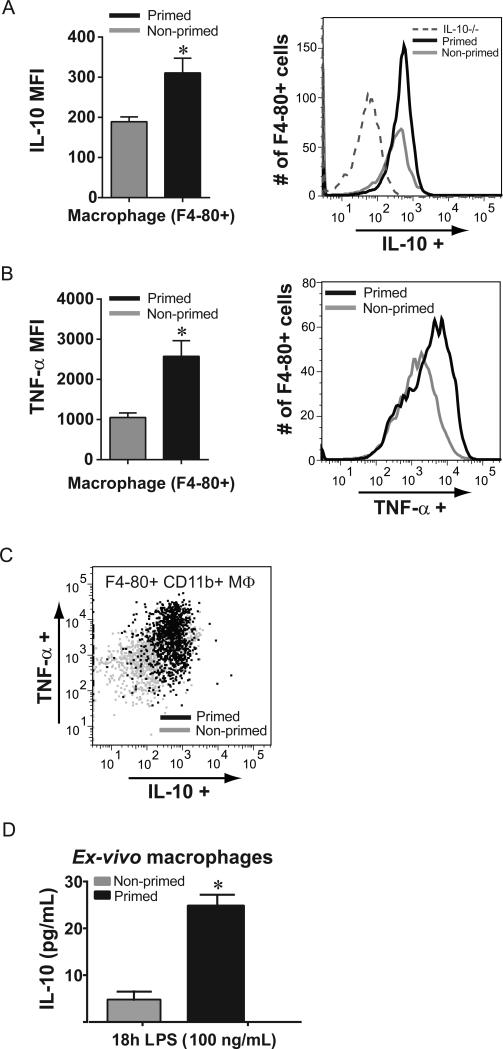
We sought to determine prominent cellular sources of IL-10 after lung priming with LPS. We focused on day 1 after i.t. LPS, at which point BAL IL-10 levels in primed mice were highest. BAL cells were collected from primed and non-primed WT mice 1 day following exposure to i.t. LPS, stimulated in vitro in the presence of a Golgi inhibitor, and stained for intracellular IL-10 expression. When assessed by flow cytometry, alveolar macrophages (F4-80+) isolated from primed WT mice produced significantly more IL-10 compared to macrophages isolated from non-primed WT mice (Fig. 5A); a representative histogram flow plot is shown and includes macrophages from primed IL-10−/− mice for comparison. CD4+ T lymphocytes were an additional cellular source of IL-10, but CD8+ T lymphocytes, CD4+Foxp3+ Tregs, and neutrophils were not a significant source of IL-10 (not shown). Alveolar macrophages from primed WT mice on day 1 after i.t. LPS also produced significantly more TNF-α compared to macrophages from non-primed WT mice (Fig. 5B). To further characterize differences between primed and non-primed macrophages, we assessed cytokine production among F4-80+CD11c+ or F4-80+CD11b+ alveolar macrophage subsets. We and others have determined that macrophage CD11b to be an acceptable marker of recruited or exudative macrophages (16, 52, 53). In contrast, CD11c expression among macrophages generally designates resident alveolar macrophages (54), but recruited macrophages also express CD11c at later time points with resolution of inflammation (53). In contrast to similar IL-10 and TNF-α production by CD11c+ cells in each group (not shown), CD11b+ macrophages from primed WT mice expressed more IL-10 and TNF-α than CD11b+ macrophages from non-primed WT mice (Fig. 5C).
Fig. 5. Macrophages are a significant IL-10 source after lung priming.
Alveolar cells from primed and non-primed WT mice were isolated at day +1 after i.t. LPS (3 mg/kg) challenge, and then restimulated, and stained for macrophage, neutrophil, and lymphocyte flow markers as well as intracellular cytokine production. IC IL-10 production (A) and IC TNF-α production (B) were quantified by mean fluorescence intensity (MFI) in F4-80+ alveolar macrophages collected from primed and non-primed WT mice; a representative flow cytometry histogram is shown for each, and for IL-10 includes alveolar macrophages from primed IL-10−/− mice (dashed line). (C) Among F4-80+ CD11b+ alveolar cells from primed (black) and non-primed (gray) mice, a dot plot demonstrating individual cell IC production demonstrates a predominant increase in dual cytokine production from primed alveolar cells. (D) Alveolar macrophages were isolated 7 days after o.p. LPS or o.p. water (control), and stimulated with LPS (100 ng/mL); IL-10 secretion was quantified by ELISA after 18 hours of stimulation. Values expressed as mean ± SEM; *paired t-test against other group at same time point, p<0.05. (n=4-5 animals or wells per group per time point)
To determine whether macrophages present in the alveolar space during the priming response (either recruited during priming or “resident” from before the priming response) could be a prominent source of IL-10, we isolated alveolar macrophages from mice on day 0 (7 days after exposure to o.p. LPS (primed) or water (non-primed)) and stimulated them with LPS (100 ng/mL) in culture. Alveolar macrophages from primed mice secreted nearly five-fold more IL-10 after 18 hours compared to alveolar macrophages from non-primed mice (Fig. 5D).
IL-10-producing alveolar macrophages are critical for immunological priming
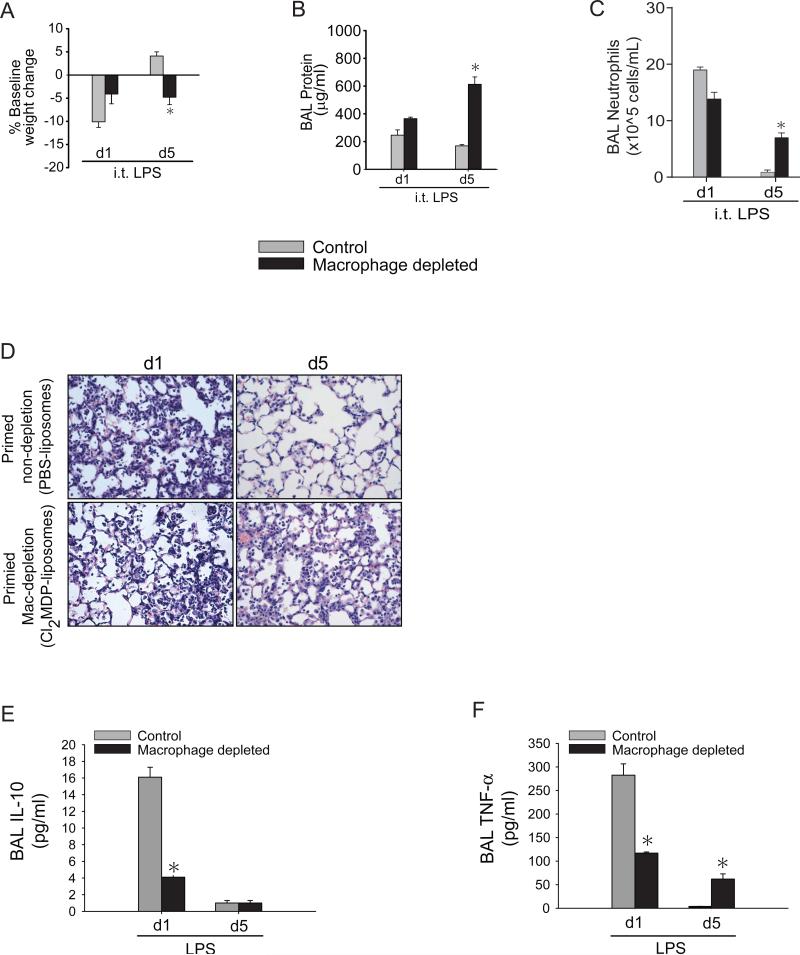
We were interested in defining macrophage contributions to the priming response. Specific tissue and systemic macrophage depletion is readily achieved using clodronate liposomes (42, 54). Mice were primed with o.p. LPS (1 mg/kg) on day −7 followed by o.p. clodronate liposomes (Cl2MDP) or PBS-liposomes (control) on day −3, and intraperitoneal (i.p.) Cl2MDP or PBS liposome on days −2 and −1 (Supp. Fig. 2A). Following i.t. LPS (3 mg/kg) on day 0, we assess lung injury parameters on days 1 and 5. We confirmed that alveolar macrophages were decreased by >90% at day 0 in the Cl2MDP-liposomes group compared to the PBS-liposomes group (not shown). Primed mice treated with PBS liposomes (control) had returned to baseline weight by day 5 after i.t. LPS and were significantly different from primed mice treated with Cl2MDP liposomes (macrophage depleted) that had persistent weight loss at day 5 (Fig. 6A). BAL protein (Fig. 6B) and BAL neutrophils (Fig. 6C) were similarly elevated in both groups on day 1 after i.t. LPS, but by day 5, only the control mice had reduced, near-normal levels of BAL protein and BAL neutrophils. Primed macrophage-depleted mice had persistent elevation of BAL protein and neutrophils at day 5. We observed a similar profile of lung injury by histological changes, where primed macrophage-depleted mice had sustained inflammatory cell infiltration and interstitial thickening compared to the primed control mice at day 5 (Fig. 6D). Day 1 BAL IL-10 (Fig. 6E) and TNF-α (Fig. 6F) increases were markedly abrogated in the primed macrophage-depleted mice, reaffirming that alveolar macrophages are a significant source of IL-10 and TNF-α in the priming response. Day 5 BAL TNF-α levels remained elevated in the macrophage-depleted group, correlating with other phenotypic markers that demonstrate persistent lung inflammation and injury. In macrophage-depleted mice, possible sources of alveolar TNF-α include neutrophils, CD4+ lymphocytes, and residual macrophages. In summary, macrophages that make IL-10 are critical for resolution of lung inflammation mediated by immunological priming.
Fig. 6. IL-10 producing alveolar macrophages are critical for immunological priming.
Primed mice treated with PBS-liposomes (control) and primed mice treated with CL2-MDP liposomes (macrophage depleted) were assessed for body weight relative to baseline (A), BAL total protein (B), BAL neutrophils (C) or histological damage (D) by H&E staining (x100 magnification) at days 1 or 5 after i.t. LPS injury. BAL IL-10 (E) and TNF-α from primed control and primed macrophage-depleted were measured at days 1 or 5 after i.t. LPS. Values expressed as mean ± SEM; *one-way ANOVA (A-C) or paired t-test (E-F) against other groups at same time point, p<0.05, (n=4-6 animals per group per time point)
Tregs are necessary for immunologic priming and alveolar macrophage IL-10 production
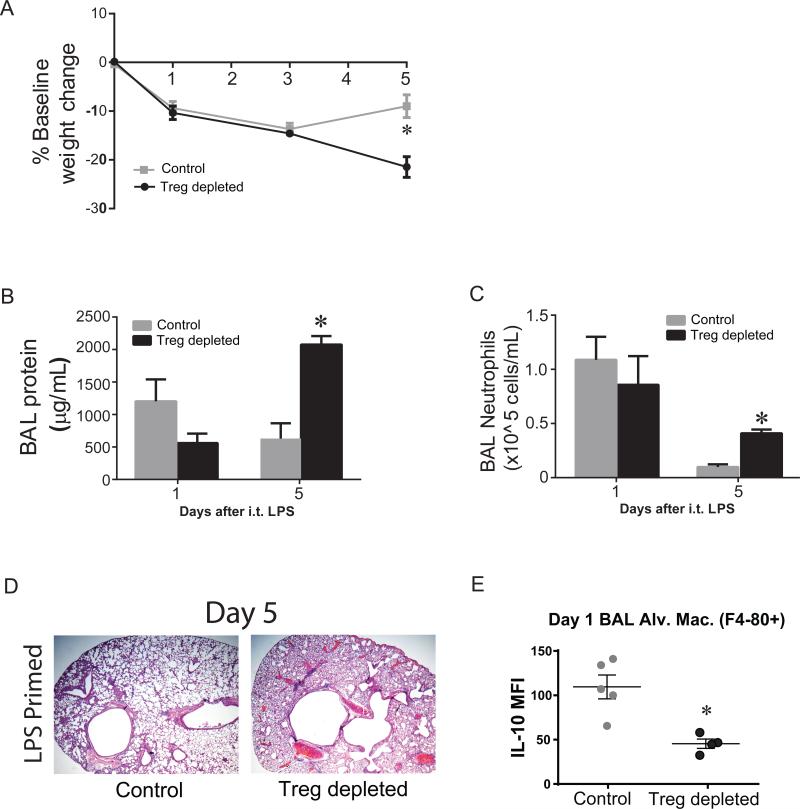
Regulatory T cells (Tregs) are critical for resolution of lung inflammation (25), in part through macrophage interaction (6). Alveolar Tregs are present after o.p. LPS most prominently in the latter half of the priming phase (not shown). We sought to determine whether Tregs are critical for immunological priming in our lung injury model. We primed Foxp3DTR and Foxp3gfp mice with 1 mg/kg o.p. LPS (Supp. Fig. 2B). On days −2, −1, and +2, LPS primed Foxp3DTR (Treg depleted) mice and LPS primed Foxp3gfp (control) mice were treated with Diphtheria toxin (DT) which successfully depleted >90% Tregs only in Foxp3DTR mice (Treg depleted, not shown). When we challenged mice with i.t. LPS (3 mg/kg) on day 0, both primed groups displayed similar systemic injury at day 1, but by day 5, only control mice were gaining weight back towards baseline (Fig. 7A). We assessed lung injury parameters on days 1 and 5 to focus on acute injury and resolution time points in this model. At day 1, mice in both primed groups were injured, with similar elevation of BAL protein (Fig. 7B) and BAL neutrophils (Fig. 7C). However, at day 5, only Treg depleted mice had significant lung injury manifest as increased BAL protein and BAL neutrophils, as well as severe histological damage (Fig. 7D) in comparison to primed control mice. To determine whether Treg depletion altered alveolar macrophage IL-10 production, we isolated and stimulated in vitro BAL cells from primed Treg depleted mice and primed control mice on day+1 after i.t. LPS and assessed intracellular IL-10 production by flow cytometry. The percentage and number of BAL macrophages (F4-80+) between groups was similar (not shown). BAL macrophages from primed control mice expressed significantly more IL-10 than macrophages from primed Treg depleted mice (Fig. 7E). In contrast, macrophage TNF-α, IL-6 and IFN-γ intracellular expression was similar between groups (not shown). In addition, the percentage of macrophages with dual expression of IL-10 and TNF-α was higher in the primed control group compared to the primed Treg depleted group (not shown), a pattern similar to what we observed previously in primed WT mice compared to non-primed WT mice in figure 5. Collectively, these data reinforce the importance of Tregs in resolution of lung injury after immunological priming and suggest that Tregs enhance macrophage IL-10 production.
Fig. 7. Tregs are necessary for priming and alveolar macrophage IL-10 production.
Primed Foxp3gfp mice (control) and primed Foxp3DTR (Treg depleted) were assessed for body weight relative to baseline (A), BAL total protein (B), BAL neutrophils (C), or histological damage (D) by H&E staining (x2x magnification) at days 1 or 5 after i.t. LPS injury. All mice received i.p. DT injections (15 μg/kg) on days −2, −1, and +2 when harvested at day 5. (E) At day 1 after i.t. LPS, we determined intracellular production of IL-10 among F4-80+ BAL macrophages by flow cytometry. Values expressed as mean ± SEM, as well as individual values in E; *paired t-test against other primed group at same time point, p<0.05, (n=4-6 animals per group per time point)
Mild inflammation by CpG can induce immunological priming
We sought to determine whether immunological priming could be achieved using a priming agent which induced a milder lung inflammatory response compared to LPS. We used unmethylated CpG dinucleotides, a hallmark of microbial DNA sensed by TLR9, and mimicked by synthetic oligonucleotide containing CpG motifs (CpG). By activating an immune response sufficient to defend against a variety of bacterial, viral and parasitic pathogens (55), CpG has been used as a vaccine adjuvant in infectious disease and as an immunotherapeutic agent for oncologic illnesses. We treated WT mice with o.p. CpG (or an equal volume of o.p. PBS), followed their weights, and measured parameters of lung injury at peak weight loss (d3 after CpG) (Supp. Fig. 2). Mice primed with CpG had minimal weight change, and behaved similarly to mice treated with PBS (Supp. Fig. 2C). Furthermore, mice primed with CpG had only mild elevations of BAL protein (Supp. Fig. 2D) and BAL total cell count (Supp. Fig. 2E) compared to mice treated with PBS and much less than WT mice primed with LPS at comparable time points (Supp. Fig. 1B-D). Collectively, these data suggest that o.p. CpG induces only mild lung inflammation.
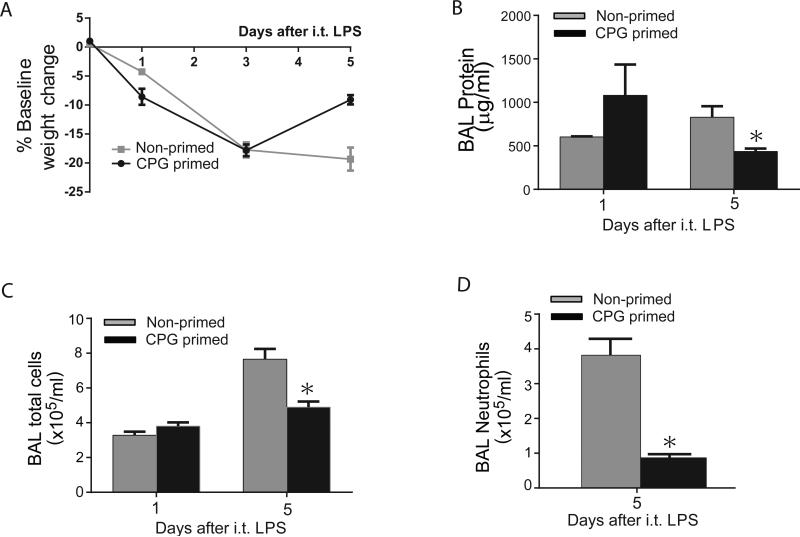
We then challenged WT mice with i.t. LPS (3 mg/kg) after o.p. CpG (Supp. Fig. 2F) and compared their response to o.p. PBS-treated mice (non-primed) for parameters of systemic and lung injury at days 1 and 5. WT mice primed with CpG had similar weight loss (Fig. 8A) and severity of lung injury to non-primed WT mice on day 1 after i.t. LPS (3 mg/kg) based on BAL protein (Fig. 8B) and total cell counts (Fig. 8C). By day 5, CpG primed mice were nearing baseline weight, and had significantly less BAL protein and lower total BAL cell counts than non-primed mice. Amongst BAL cells at day 5, we observed a marked four-fold decrease in neutrophils in the CpG primed group (Fig. 8D). In contrast to WT mice, IL-10−/− mice treated with CpG followed by i.t. LPS did not achieve accelerated lung injury resolution compared to non-primed IL-10−/− mice (not shown).
Fig. 8. Immunological priming is achieved with minimal inflammation.
WT mice were treated with o.p. PBS (Non-primed) or o.p. CpG (primed). 5 days later, mice were challenged with i.t. LPS (3 mg/kg), and were assessed for body weight relative to baseline (A), BAL total protein (B), BAL total cells (C), or BAL neutrophils (D) at days 1 or 5 after i.t. LPS injury. Values expressed as mean ± SEM; *paired t-test against other primed group at same time point, p<0.05, (n=4-6 animals per group per time point)
DISCUSSION
Our findings demonstrate that immunological priming renders the lung capable of robustly responding to a subsequent non-infectious or infectious severe exposure to improve survival and accelerate resolution from severe lung inflammation. IL-10 is critical for immunological priming in our model, and alveolar macrophages appear to be an important source of IL-10. Furthermore, macrophage production of IL-10 requires the presence of alveolar Tregs during the priming response, a finding from which we can infer the importance of cellular cross-talk to achieve resolution of lung inflammation and injury (25). Without either macrophages or Tregs, the benefits of immunological priming disappear.
In experimental acute lung injury models, IL-10 is reported to have somewhat diverse effects. IL-10 was protective at early time points after i.t. LPS-induced lung injury (56), during carrageenan-induced pleuritis (57), and in lung injury secondary to shock (58, 59). However, IL-10 was harmful in a Pseudomonas aeruginosa pneumonia model when overexpressed in the lung (60), and following sub-lethal influenza infection where antibody-mediated blockade of IL-10 improved susceptibility to a secondary pneumococcal pneumonia (61). While IL-10 is likely not the only mediator of immunological priming, the divergent conclusions between our work and that of others’ suggests that IL-10 may be most beneficial when its production is tightly regulated to counteract pro-inflammatory cascades. With its ability to dampen neutrophil recruitment and enhance neutrophil apoptosis, unopposed IL-10 may be harmful especially early in the inflammatory response when neutrophil presence is most critical. In our model, despite early increases in macrophage-derived IL-10 in primed mice, alveolar neutrophil numbers and overall lung inflammation were similar in both primed and non-primed groups at early time points. Concurrent increases in macrophage-derived TNF-α and potentially other unmeasured pro-inflammatory cytokines may oppose IL-10 effects to help preserve the early inflammatory response and may help explain why priming accelerated bacterial clearance and resolution of lung inflammation following pseudomonas infection in our model.
Among a subset of alveolar macrophages (F4-80+ CD11b+; Fig. 5C) derived from primed mice, the production of significantly more TNF-α and IL-10 on day 1 after re-challenge with i.t LPS suggests that distinct alveolar macrophage sub-populations may be present during the priming response. Recent work phenotyping lung macrophages during inflammation outlines complicated schema to fully characterize resident versus recruited lung macrophages (54, 62, 63). The current construct of our experimental model does not lend itself to definitive declaration of dynamic macrophage or dendritic cell subpopulations during the priming and post-injury resolution response. However, it is likely that our priming model induces sufficient inflammation to recruit bone-marrow derived macrophages to the alveolar space during the priming phase. As inflammation during the priming phase subsides, a percentage of recruited macrophages remain as new “resident” alveolar macrophages as others have shown (53). We hypothesize that the ability of this new “resident” alveolar macrophage population to produce significant levels of both TNF-α and IL-10 when challenged with a second inflammatory stimulus such as LPS may distinguish them from old “resident” macrophages that may exhibit endotoxin tolerance and produce less TNF-α after repeat LPS challenge (11, 23, 28). The new “resident” population may be distinguishable from additional inflammatory macrophages recruited to the alveolar space within the first day after i.t. LPS in that they produce higher levels of TNF-α but lower levels of IL-10. The concurrent production of TNF-α and IL-10 among primed macrophages may be an important and representative example of adaptive cellular reprogramming by alveolar macrophages that may not be fully recapitulated by exogenous delivery of anti-inflammatory cytokines such as IL-10 or TGF-β. In response to a subsequent severe insult, therefore, primed macrophages may simultaneously produce pro-inflammatory mediators critical for the initial immune response, and produce anti-inflammatory mediators necessary to modulate lung inflammation and hasten resolution.
We elected to focus our assessment of immune responses in the alveolar compartment of the lung, but cannot exclude the biological importance of the lung interstitium. When compared to our typical patterns of experimental lung injury and resolution (25), a distinguishing feature of the priming response was the increase in alveolar neutrophil apoptosis at all measured time points. Apoptosis and removal of neutrophils are critical for resolution of lung inflammation and intimately tied to the cytokine and cellular make-up of the alveolar space (44-46, 64), and thus provided us with additional rationale to focus our assessment within the alveolar compartment. We also cannot disregard the possible contribution of other immune cells such as IL-10-producing dendritic cells that can stimulate Tregs (65) or lung MDSC-like cells that can make IL-10 (66) in lung inflammatory models. With lavage, we typically do not recover significant numbers of dendritic cells from the bronchoalveolar spaces in our experimental lung injury models. It is conceivable that lung MDSC-like cells that express F4-80, CD11b, and Gr1 (low) are phenotypically similar to the CD11b subset of alveolar macrophages (F4-80+) we define to be a source of significant IL-10 production in our priming model. Lastly, our use of clodronate to specifically deplete macrophages, and not dendritic cells or neutrophils as others have shown (54), added specificity to the importance of macrophages in the priming response and as a critical source of IL-10.
Prior exposure of immune cells to endotoxin can induce a significantly diminished pro-inflammatory response to subsequent LPS exposure, known as endotoxin tolerance (ET) (67-71). The hallmark of ET is a marked downregulation of pro-inflammatory mediators such as TNF-α, IL-6, IL-12, IL-1β (72, 73)□ with concurrent increase in anti-inflammatory cytokines including IL-10, TGF-β and IL-1ra (74). Although priming with two interval exposures to LPS could be considered an example of in vivo endotoxin tolerance, there are major distinctions that suggest the observed phenotype might not be explained primarily by ET. First, following i.t. LPS in primed animals, we observed an initial robust lung inflammatory response notable for significant TNF-α secretion, prominent alveolar neutrophil influx, and increased histologic injury. Second, although crosstolerance or heterotolerance among TLR ligands can occur (75-77), the immunological priming response to accelerate lung injury resolution was also observed using a distinct TLR agonist, CpG, to prime the mice, and demonstrated similar early inflammatory lung injury patterns as with LPS priming. Third, the priming response evolved over a period of several days, not the 24-48 hour period usually associated with endotoxin tolerance (78, 79).
Our study generates several questions which we are actively pursuing in order to better understand lung priming-mediated protection. One, how do macrophages and Tregs communicate, if at all, to contribute to the priming response and hasten lung injury resolution? We are working to create an in vitro priming modeling system. We have shown that cell-cell contact was required for Tregs to modulate macrophage TNF-α production in an in vitro co-culture system (25), but did not determine whether Tregs modulate macrophage IL-10 production. We do not yet understand mechanisms mediating lung Treg recruitment during priming, or whether monocyte/macrophage populations contribute specifically to Treg recruitment. Elssner demonstrated the importance of caspase 3-regulated IL-16 secretion by blood monocytes (80). IL-16 is a potent Treg chemoattractant (81) and may be critical in our model for Treg recruitment during the priming response. Two, are there specific cellular proteins or pathways responsible for the priming effect? Our CpG data demonstrates that neither significant lung inflammation nor primary TLR4-based priming is required to accelerate lung injury resolution, but we have not yet compared downstream macrophage TLR signaling in each model. Three, does priming protect against other infectious models of lung injury? We are actively exploring the effects of priming on other prevalent pathogens such as pneumococcus and influenza. Four, do epigenetic, phenotypic, and functional differences exist between primed and non-primed macrophages? It is conceivable that macrophages from primed mice are able to produce other anti-inflammatory mediators such as TGF-β, IL-1ra, lipid mediators, or reprogram more effectively from M1 classically activated to M2 alternative activated or regulatory macrophages. Five, do macrophages from primed mice co-signal lymphocytes to modulate their proliferation and skewing more effectively? Lastly, are there additional cellular communications, for instance alveolar macrophage-epithelial or Treg-epithelial, which could contribute to the priming response? We have shown the importance of Th1 lymphocytes for prevention of fibrosis in a bleomycin model (82), from which we infer possible lymphocyte-epithelial interactions (83). Furthermore, the increase in BAL TGF-β we observed in primed mice prior to i.t. LPS may signify enhanced communication with airway and alveolar epithelial cells mediated by αψβ6 integrin (84).
We have developed immunological priming as a strategy to nonspecifically enhance the lung's mucosal immune responses and accelerate clearance of a wide range of pathogens to promote resolution and repair. This unconventional approach could be implemented rapidly in a large population, particularly in areas with high incidence of endemic infections such as influenza or in cases of new epidemics/ pandemics where a lack of sufficient or appropriate medicines and vaccines may preclude timely control of the epidemiological burden. Additionally, if longer lasting priming mediated protection can occur, supplementing current seasonal vaccines may be another area of potential clinical use. Using CpG, we have demonstrated induction of mild lung inflammation to be sufficient to elicit protection against a robust secondary insult, and anticipate translational applicability (85, 86).
Therapy for acute lung injury remains largely supportive. Measures to prevent high-risk patients from developing this often fatal syndrome have been largely disappointing. Moreover, emerging respiratory pathogen epidemics remain a huge concern and protecting individuals at risk is a tremendous challenge. Here we demonstrate a role for priming of alveolar macrophages leading to accelerated resolution of lung inflammation by non-infectious and infectious agents; applicability is enhanced by our ability to use a priming agent that induces only minimal lung inflammation. Understanding the cellular and molecular mechanisms of primed immune cells could lead to novel targets useful for resolution of lung inflammation.
Supplementary Material
Acknowledgements
Dr. Mark Soloski, Raeffello Cimbro, and Joe Crest for their assistance in the Johns Hopkins Bayview Flow Cytometry Core. James Watkins and Andre Robinson for expert assistance with tissue processing for histologic studies. Dr. Alexander Rudensky for his donation of Foxp3DTR and Foxp3gfp mice breeding pairs. Dr. N van Rooijen for use of clodronate liposomes for macrophage depletion.
Work supported by NHLBI HL089346 and the Johns Hopkins Bayview Scholars Program (LSK), R00HL103793 (FRD), AHA FTF7280014 and YCSA 110587 (NRA), HL010342 (MRH)
References
- 1.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun. 2011;79:3576–3587. doi: 10.1128/IAI.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meldrum DR, Cleveland JC, Jr., Moore EE, Partrick DA, Banerjee A, Harken AH. Adaptive and maladaptive mechanisms of cellular priming. Ann Surg. 1997;226:587–598. doi: 10.1097/00000658-199711000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Muhl H. Macrophages in inflammation and its resolution. Front Immunol. 2012;3:324. doi: 10.3389/fimmu.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bem RA, Farnand AW, Wong V, Koski A, Rosenfeld ME, van Rooijen N, Frevert CW, Martin TR, Matute-Bello G. Depletion of resident alveolar macrophages does not prevent Fas-mediated lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L314–325. doi: 10.1152/ajplung.00210.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty DE, Downey GP, Worthen GS, Haslett C, Henson PM. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Invest. 1988;59:200–213. [PubMed] [Google Scholar]
- 6.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 8.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med. 1989;170:499–509. doi: 10.1084/jem.170.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 10.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–194. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 13.Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–177. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 14.Draisma A, Bemelmans R, van der Hoeven JG, Spronk P, Pickkers P. Microcirculation and vascular reactivity during endotoxemia and endotoxin tolerance in humans. Shock. 2009;31:581–585. doi: 10.1097/SHK.0b013e318193e187. [DOI] [PubMed] [Google Scholar]
- 15.Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Crit Care Med. 2009;37:1261–1267. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- 16.Tighe RM, Liang J, Liu N, Jung Y, Jiang D, Gunn MD, Noble PW. Recruited exudative macrophages selectively produce CXCL10 after noninfectious lung injury. Am J Respir Cell Mol Biol. 2011;45:781–788. doi: 10.1165/rcmb.2010-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 18.Asadullah K, Sabat R, Friedrich M, Volk HD, Sterry W. Interleukin-10: an important immunoregulatory cytokine with major impact on psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:185–192. doi: 10.2174/1568010043343886. [DOI] [PubMed] [Google Scholar]
- 19.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 20.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 21.Smith DR, Kunkel SL, Burdick MD, Wilke CA, Orringer MB, Whyte RI, Strieter RM. Production of interleukin-10 by human bronchogenic carcinoma. Am J Pathol. 1994;145:18–25. [PMC free article] [PubMed] [Google Scholar]
- 22.Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 24.Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR, Virella-Lowell IL. IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 2010;17:567–576. doi: 10.1038/gt.2010.28. [DOI] [PubMed] [Google Scholar]
- 25.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 27.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 29.Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 30.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Ma H, Qiu L, Li L, Cao Y, Ma J, Zhao Y. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 89:130–142. doi: 10.1038/icb.2010.70. [DOI] [PubMed] [Google Scholar]
- 32.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akilov OE, Wu MX, Jin Y, Zhou Z, Geskin LJ, Falo LD, Hasan T. Vaccination with photodynamic therapy-treated macrophages induces highly suppressive T-regulatory cells. Photodermatol Photoimmunol Photomed. 2011;27:97–107. doi: 10.1111/j.1600-0781.2011.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boenisch O, Lopez M, Elyaman W, Magee CN, Ahmad U, Najafian N. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4(+) T cells and requires the presence of monocytes. Am J Transplant. 2012;12:856–866. doi: 10.1111/j.1600-6143.2011.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, Duan K, Ma H, Niu Z, Peng J, Zhao Y. An instructive role of donor macrophages in mixed chimeras in the induction of recipient CD4(+)Foxp3(+) Treg cells. Immunol Cell Biol. 2011;89:827–835. doi: 10.1038/icb.2011.65. [DOI] [PubMed] [Google Scholar]
- 37.Coleman MM, Ruane D, Moran B, Dunne PJ, Keane J, Mills KH. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am J Respir Cell Mol Biol. 2013;48:773–780. doi: 10.1165/rcmb.2012-0263OC. [DOI] [PubMed] [Google Scholar]
- 38.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal NR, D'Alessio FR, Tsushima K, Files DC, Damarla M, Sidhaye VK, Fraig MM, Polotsky VY, King LS. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 298:L371–381. doi: 10.1152/ajplung.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halbower AC, Mason RJ, Abman SH, Tuder RM. Agarose infiltration improves morphology of cryostat sections of lung. Lab Invest. 1994;71:149–153. [PubMed] [Google Scholar]
- 42.D'Alessio FR, Tsushima K, Aggarwal NR, Mock JR, Eto Y, Garibaldi BT, Files DC, Avalos CR, Rodriguez JV, Waickman AT, Reddy SP, Pearse DB, Sidhaye VK, Hassoun PM, Crow MT, King LS. Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase. J Immunol. 2012;189:2234–2245. doi: 10.4049/jimmunol.1102606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31:S184–188. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 45.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 46.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maus U, Rosseau S, Knies U, Seeger W, Lohmeyer J. Expression of pro-inflammatory cytokines by flow-sorted alveolar macrophages in severe pneumonia. Eur Respir J. 1998;11:534–541. [PubMed] [Google Scholar]
- 49.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, 2nd, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 50.van den Berg JM, Weyer S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–473. [PubMed] [Google Scholar]
- 51.Turina M, Hoth JJ, Turpen RM, Scott MJ, Cheadle WG. Alveolar interleukin-10 regulates neutrophil apoptosis in severely traumatized patients. J Trauma. 2007;63:733–739. doi: 10.1097/01.ta.0000240112.35246.ae. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal NR, D'Alessio FR, Eto Y, Chau E, Avalos C, Waickman AT, Garibaldi BT, Mock JR, Files DC, Sidhaye V, Polotsky VY, Powell J, Horton M, King LS. Macrophage A2A adenosinergic receptor modulates oxygen-induced augmentation of murine lung injury. Am J Respir Cell Mol Biol. 2013;48:635–646. doi: 10.1165/rcmb.2012-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184:547–560. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of Myeloid Cell Subsets in Murine Lungs Using Flow Cytometry. Am J Respir Cell Mol Biol. 2013;49:180–190. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krieg AM. Antiinfective applications of toll-like receptor 9 agonists. Proc Am Thorac Soc. 2007;4:289–294. doi: 10.1513/pats.200701-021AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spight D, Zhao B, Haas M, Wert S, Denenberg A, Shanley TP. Immunoregulatory effects of regulated, lung-targeted expression of IL-10 in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;288:L251–265. doi: 10.1152/ajplung.00122.2004. [DOI] [PubMed] [Google Scholar]
- 57.Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di Paola R, Genovese T, De Sarro A, Caputi AP. Absence of endogenous interleukin-10 enhances the evolution of acute lung injury. Eur Cytokine Netw. 2002;13:285–297. [PubMed] [Google Scholar]
- 58.Kobbe P, Schmidt J, Stoffels B, Chanthaphavong RS, Bauer AJ, Pape HC. IL-10 administration attenuates pulmonary neutrophil infiltration and alters pulmonary iNOS activation following hemorrhagic shock. Inflamm Res. 2009;58:170–174. doi: 10.1007/s00011-009-8116-z. [DOI] [PubMed] [Google Scholar]
- 59.Kobbe P, Stoffels B, Schmidt J, Tsukamoto T, Gutkin DW, Bauer AJ, Pape HC. IL-10 deficiency augments acute lung but not liver injury in hemorrhagic shock. Cytokine. 2009;45:26–31. doi: 10.1016/j.cyto.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Sun L, Guo RF, Newstead MW, Standiford TJ, Macariola DR, Shanley TP. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am J Respir Cell Mol Biol. 2009;41:76–84. doi: 10.1165/rcmb.2008-0202OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, Goldman M, Jansen HM, Lutter R, van der Poll T. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 62.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow Cytometric Analysis of the Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am J Respir Cell Mol Biol. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Schreiner J, Kretschmer D, Klenk J, Otto M, Buhring HJ, Stevanovic S, Wang JM, Beer-Hammer S, Peschel A, Autenrieth SE. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J Immunol. 2013;190:3417–3426. doi: 10.4049/jimmunol.1202563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, Ray A, Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2012;6:189–199. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol. 2009;130:7–15. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 69.Cross AS. Endotoxin tolerance-current concepts in historical perspective. J Endotoxin Res. 2002;8:83–98. doi: 10.1179/096805102125000227. [DOI] [PubMed] [Google Scholar]
- 70.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9:101–107. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- 72.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 73.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 74.Mages J, Dietrich H, Lang R. A genome-wide analysis of LPS tolerance in macrophages. Immunobiology. 2007;212:723–737. doi: 10.1016/j.imbio.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 76.Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “heterotolerance” on NF-kappa B signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- 77.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 78.Blackwell TS, Blackwell TR, Christman JW. Induction of endotoxin tolerance depletes nuclear factor-kappaB and suppresses its activation in rat alveolar macrophages. J Leukoc Biol. 1997;62:885–891. doi: 10.1002/jlb.62.6.885. [DOI] [PubMed] [Google Scholar]
- 79.Blackwell TS, Blackwell TR, Christman JW. Impaired activation of nuclear factor-kappaB in endotoxin-tolerant rats is associated with down-regulation of chemokine gene expression and inhibition of neutrophilic lung inflammation. J Immunol. 1997;158:5934–5940. [PubMed] [Google Scholar]
- 80.Elssner A, Doseff AI, Duncan M, Kotur M, Wewers MD. IL-16 is constitutively present in peripheral blood monocytes and spontaneously released during apoptosis. J Immunol. 2004;172:7721–7725. doi: 10.4049/jimmunol.172.12.7721. [DOI] [PubMed] [Google Scholar]
- 81.McFadden C, Morgan R, Rahangdale S, Green D, Yamasaki H, Center D, Cruikshank W. Preferential migration of T regulatory cells induced by IL-16. J Immunol. 2007;179:6439–6445. doi: 10.4049/jimmunol.179.10.6439. [DOI] [PubMed] [Google Scholar]
- 82.Collins SL, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. Pulmonary vaccination as a novel treatment for lung fibrosis. PLoS One. 2012;7:e31299. doi: 10.1371/journal.pone.0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Garibaldi BT, D'Alessio FR, Mock JR, Files DC, Chau E, Eto Y, Drummond MB, Aggarwal NR, Sidhaye V, King LS. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 48:35–43. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 85.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008;3:27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.