Abstract
Sucrose produces physical dependence and reinforcing effects in rats. We hypothesized that similar effects could be demonstrated in planarians, the earliest animal with a centralized nervous system. We used two assays, one that quantifies withdrawal responses during drug absence as a reduction in motility and another that quantifies reinforcing effects using a conditioned place preference (CPP) design. In withdrawal experiments, planarians exposed to sucrose (1%) for 60 min and then tested in water for 5 min displayed reduced motility compared to water controls. Acute or continuous sucrose (1%) exposure did not affect motility. CPP experiments used a biased design to capitalize upon planarians’ natural preference for the dark (pretest, sucrose conditioning in the light, posttest). Planarians conditioned with sucrose (1%) displayed a greater preference shift than sucrose-naïve planarians. Glucose (0.1, 1%), but not the non-digestible disaccharide lactulose (0.1, 1%), also produced a greater preference shift than water-exposed planarians. Development of sucrose-induced CPP was inhibited when sucrose (1%) conditioning was conducted in combination with dopamine receptor antagonists SCH 23390 (1 µM) or sulpiride (1 µM). These results suggest that rewarding and reinforcing effects of sugar are highly conserved across species and that planarians offer an invertebrate model to provide insight into the pharmacological effects of sucrose and related sweeteners.
Keywords: sucrose, sugar, planaria, dopamine, SCH 23390, sulpiride, withdrawal, place preference, dependence, invertebrate
1. Introduction
Discontinuation of sucrose consumption in some individuals can elicit withdrawal symptoms and craving that reinitiates sweet food intake, a deleterious cycle contributing to obesity and diabetes [6–7, 11, 14, 18, 28]. Rats exposed to sucrose display neuropharmacological effects resembling those of abused drugs, including withdrawal responses (e.g. anxiety, behavioral depression) and craving, locomotor and consummatory cross-sensitization, conditioned place preference (CPP), enhancement of extracellular dopamine in the nucleus accumbens, and changes in mesolimbic dopamine receptor expression [2–7, 10, 16–17, 29, 39, 45]. Based on the tenet that survival of all animals is dependent on a desire to procure food, we hypothesized that neuropharmacological effects of sucrose would be conserved in planarians, the phylogenetically simplest animal to have a body plan common to all vertebrates and most invertebrates [8, 33]. Planarians possess a centralized nervous system (cephalic ganglia and nerve cord processes) and multiple neurotransmitter systems, including glutamate, dopamine, serotonin, acetylcholine, and GABA [8, 12, 22, 33, 44]. Several classes of abused drugs produce mammalian-like behavioral responses in planarians, such as stereotyped activity, spontaneous withdrawal, behavioral sensitization, cross-sensitization, and CPP [20, 24–27, 30–38]. Using methodology analogous to that used for mammals, we now report that sucrose produces withdrawal responses and dopamine-sensitive place conditioning effects in planarians.
2. Methods
2.1. Animals and drugs
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA). Upon arrival in the laboratory, planarians were maintained in the aqueous solution provided by Carolina Biological Supply, acclimated to room temperature (21°C), and tested within 3 days of receipt. SCH 23390 ((R)-(+)-7-Chloro-8-hydroxy-3-methyl-1- phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride), sulpiride ((RS)-(±)-5-Aminosulfonyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide), and dopamine hydrochloride were obtained from Tocris Bioscience (St. Louis, MO, USA). Sucrose, D-glucose (dextrose), and lactulose were obtained from Sigma Aldrich (St Louis, MO, USA). Stock solutions were prepared daily in a vehicle of tap water containing AmQuel® water conditioner. Treatment solutions were diluted with tap water containing AmQuel® water conditioner (1 ml Amquel per 1 gallon of water).
2.2. Behavioral studies
2.2..1. Sucrose Withdrawal
Individual planarians were randomly removed from their home jars and placed for 60 min into a transparent petri dish (5.5 cm diameter) containing water or a 1% sucrose solution. Planarians from both groups were then removed and placed into another petri dish containing water or 1% sucrose for 5 min during which motility and headbops (“nodding”-like movements) were quantified. As previously described [30], motility counts were quantified as the number of gridlines crossed, or re-crossed, by placing the petri dish over graphing paper with gridlines spaced 0.5 cm apart. A reduction in motility following discontinuation of exposure to an addictive substance is a characteristic withdrawal response displayed by planarians [30, 33]. Experiments were also conducted with 0.1% sucrose.
2.2.2. Sucrose, glucose, and lactulose CPP
Because planarians display a natural preference for a dark environment, we used a biased, counterbalanced conditioning design to assess sucrose preference [37]. Dark and “ambient” light environments were created by covering half (top and bottom) of a petri dish containing water with black paper. Individual planarians were placed at the midline of the dish and given free access to both the light and dark sides of the dish. The time spent in the non-preferred setting (light) over a 5-min interval was determined (pretest). Planarians were then conditioned with sucrose (0, 0.1, 1, and 10 %) for 30 min in the non-preferred environment. Immediately following conditioning, planarians were placed at the midline of a petri dish containing water and allowed free access to the light and dark sides of the dish for 5 min. Time spent in the non-preferred environment was determined (posttest), and a preference score was calculated as the difference between the posttest and pretest times. Separate experiments were conducted with glucose (0, 0.1, and 1%), a metabolite of sucrose, and lactulose (0, 0.1, 1%), a synthetic non-digestible disaccharide that is used to treat constipation.
2.2.3. Dopamine receptor antagonist effects on sucrose CPP
Experiments were repeated as described for sucrose by itself with a couple of modifications. In experiments assessing development planarians were conditioned with sucrose (1%) by itself and in combination with either SCH23390 (1 µM) or sulpiride (1 µM). In experiments investigating expression planarians were conditioned with sucrose (1%) and then tested in the presence of SCH23390 (1 µM) or sulpiride (1 µM). Tables 1 and 2 display the experimental design for development and expression studies, respectively.
Table 1. Development Studies.
Experimental design for studies testing effects of dopamine D1 (SCH 23390, 1 µM) and D2 (sulpiride, 1 µM) receptor antagonists on the development of 1% sucrose place conditioning.
| Group | Pretest (5 min) | Conditioning (30 min) | Posttest (5 min) |
|---|---|---|---|
| Water | Water | Water | Water |
| Antagonist | Water | Antagonist | Water |
| Sucrose | Water | Sucrose | Water |
| Antagonist/Sucrose | Water | Antagonist/Sucrose | Water |
Table 2. Expression Studies.
Experimental design for studies testing effects of dopamine D1 (SCH 23390, 1 µM) and D2 (sulpiride, 1 µM) receptor antagonists on the expression of 1% sucrose place conditioning.
| Group | Pretest (5 min) | Conditioning (30 min) | Posttest (5 min) |
|---|---|---|---|
| Water/Water | Water | Water | Water |
| Water/Antagonist | Water | Water | Antagonist |
| Sucrose/Water | Water | Sucrose | Water |
| Sucrose/Antagonist | Water | Sucrose | Antagonist |
2.3. Data and statistical analysis
Group means (± S.E.M.) were compared by one-way ANOVA. In cases of a significant main effect, appropriate post-hoc tests (Dunnett’s or Bonferroni) were used to identify differences between individual groups. A Student’s t-test was used to compare pre-test and post-test times in CPP experiments testing different dose of sucrose. Planarians that preferred a light environment during pre-testing (15/275, or 5.5% of the total tested) were excluded from CPP data analysis. p < 0.05 was considered a statistically significant difference in all cases.
3. Results
3.1. Sucrose produces withdrawal responses (Fig. 1)
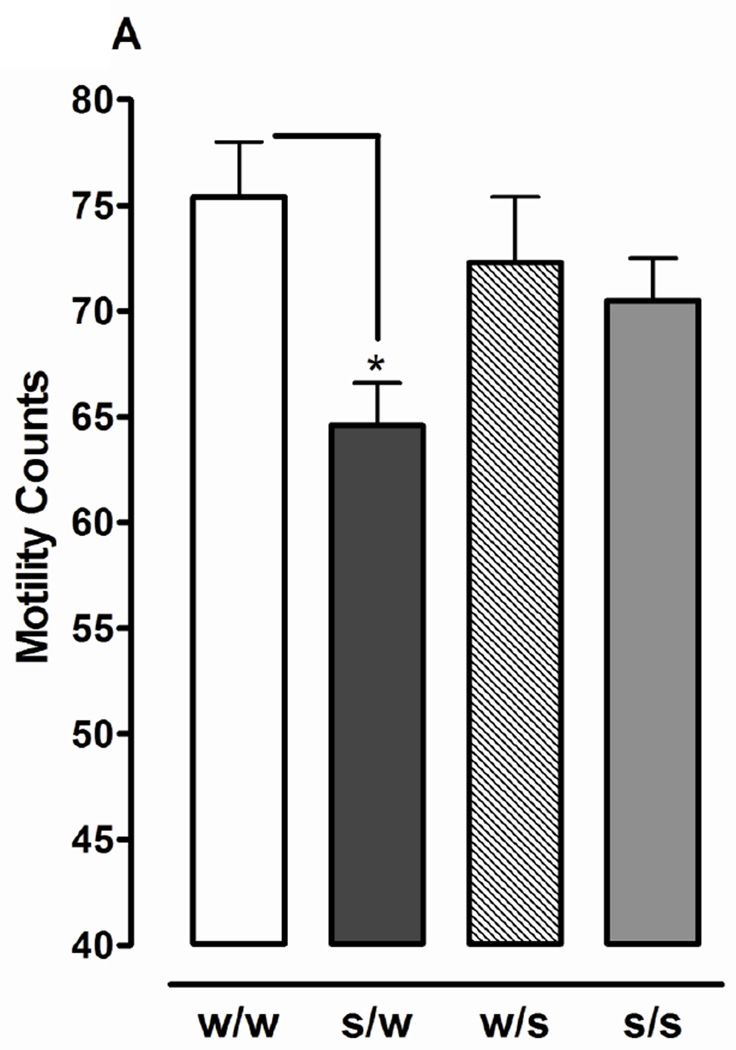
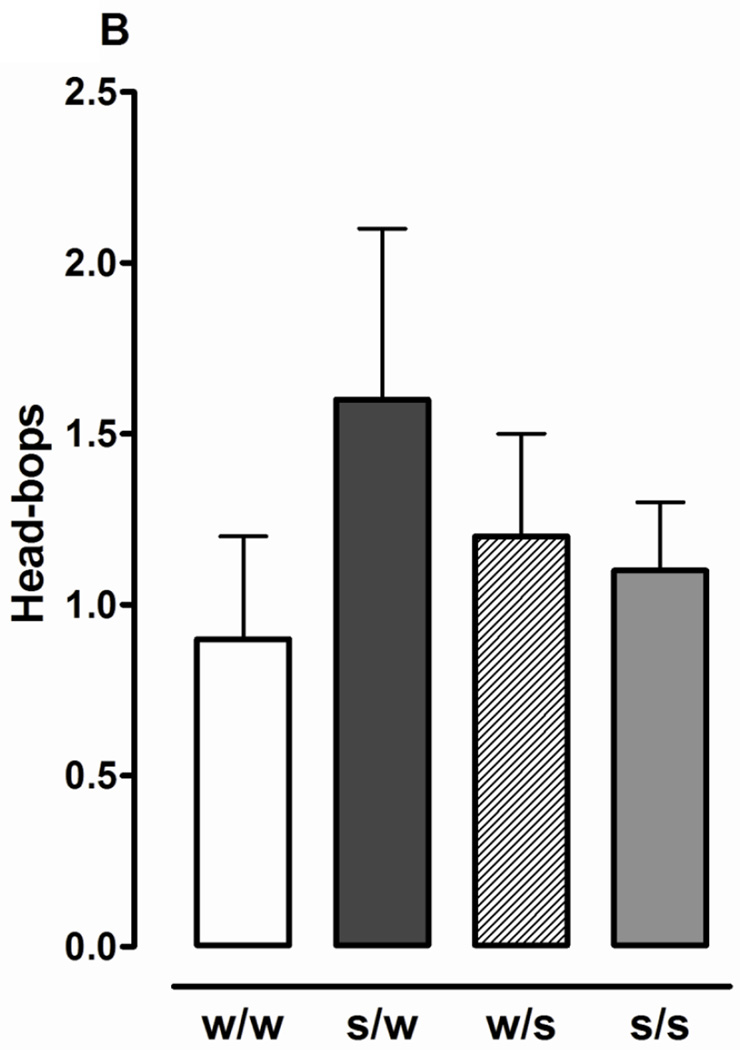
Fig. 1.
Sucrose produces withdrawal responses following discontinuation of exposure. Planarians exposed to sucrose (S) (1%) or water (W) for 60 min were removed and tested in S (1%) or W for 5 min. Data are presented as motility counts + S.E.M. (1A) or number of head-bops + S.E.M. (1B). N = 10 planarians/group. *p < 0.05 compared to W/W group.
Effects of different patterns of 1% sucrose exposure (acute, continuous, and discontinuation) on motility and stereotypical activity (headbops) are presented in Fig. 1. For motility experiments one-way ANOVA revealed a significant main effect [F(3, 44) = 3.387, p < 0.05]. Acute or (W/S) continuous (S/S) exposure did not produce motility counts that were significantly different than water controls (W/W) (p > 0.05). However, motility counts following spontaneous discontinuation of sucrose exposure (i.e. planarians treated with sucrose for 60 min and then tested in water) (S/W) were lower than those observed in water controls (W/W) and during acute (W/S) or continuous (S/S) sucrose exposure (p < 0.01). An increase in the number of headbops following discontinuation of sucrose exposure was not observed (p > 0.05) (Fig. 1A). Higher concentrations of sucrose (e.g., 10%) were not tested in withdrawal experiments because they suppressed motility upon acute exposure.
3.2. Sucrose and glucose, but not lactulose, produce CPP (Fig. 2)
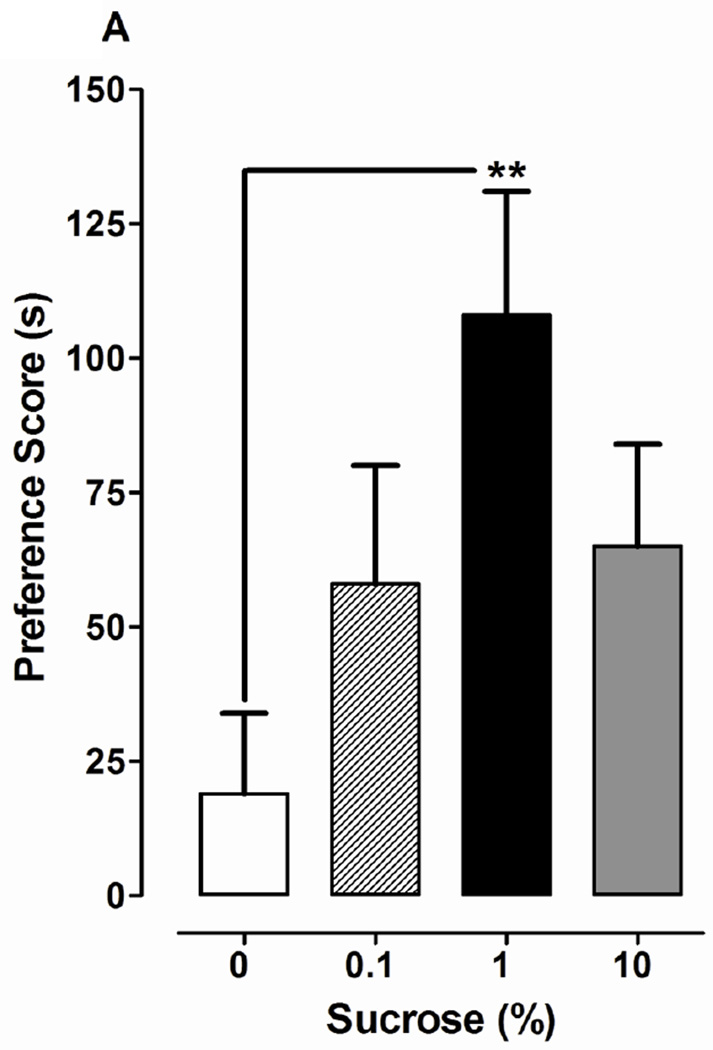
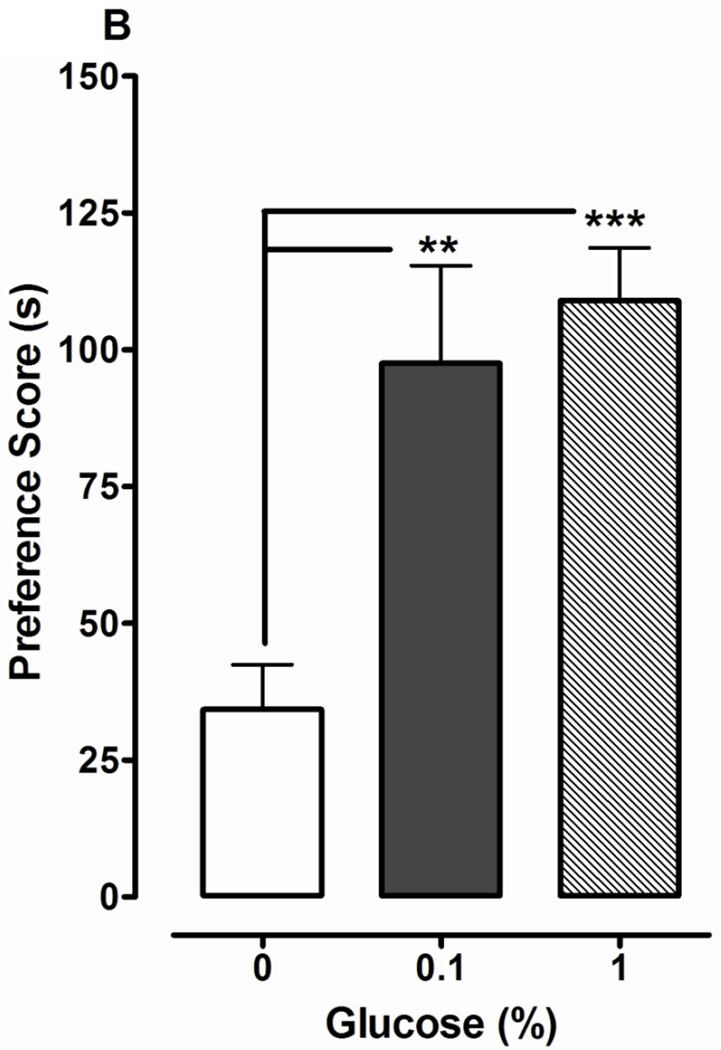
Fig. 2.
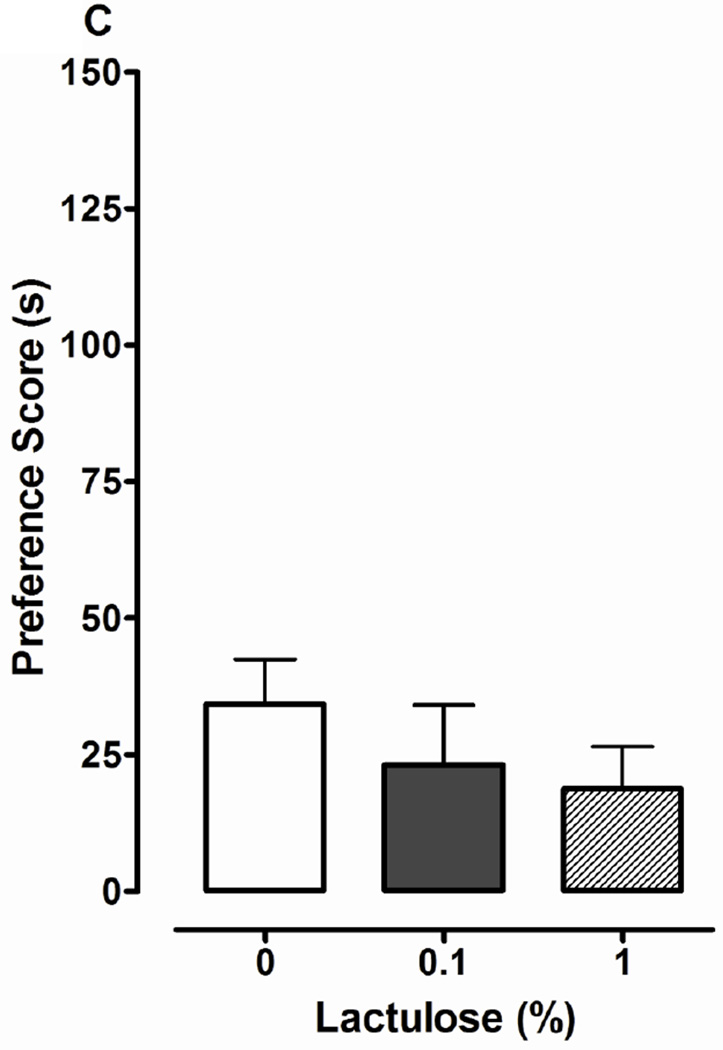
Sucrose and glucose, but not lactulose, produce CPP. Data are presented as the mean preference score (s) + S.E.M. (difference between post-conditioning and pre-conditioning times) from planarians in which (A) sucrose (SUC) (0, 0.1, 1, 10%), (B) glucose (0, 0.1, 1%) or (C) lactulose (0, 0.1, 1%) was paired with ambient light during the conditioning phase. N = 8–10 planarians per group. **p < 0.01 compared to W in the respective experiments.
One-way ANOVA indicated a significant main effect in experiments testing effects of sucrose (0, 0.1, 1 and 10%) on environmental preference [F(3, 44) = 3.326, p < 0.05]. Planarians conditioned with 1% sucrose displayed a greater preference shift (108 ± 23 s) than water controls (19 ±15 s) (p < 0.01). For planarians in the 1% sucrose group, the time spent in the non-preferred environment (light) was enhanced following sucrose conditioning (155 ± 26 s posttest versus 48 ± 15 s pretest) (p < 0.05, Student’s t-test within subjects comparison). Conditioning with lower (0.1%) or higher (10%) sucrose concentrations produced an enhanced preference shift relative to water controls, but the effects did not reach statistical significance (p > 0.05). For glucose experiments (Fig. 2B), one-way ANOVA indicated a significant main effect on environmental preference [F(2, 21) = 10.13, p < 0.001]. Compared to water controls (34 ± 8 s), planarians conditioned with 0.1% (98 ± 18 s, p < 0.01) or 1% (109 ± 10 s, p < 0.001) glucose displayed significantly greater preference shifts (Fig 2B). For lactulose experiments (Fig. 2C), a significant main effect on environmental preference was not observed [F(2, 21) = 0.77, p > 0.05]. Planarians conditioned with dopamine (100 µM) also displayed significant preference shift relative to water controls (data not shown).
3.3. Development of sucrose CPP is inhibited by dopamine receptor antagonists (Fig. 3)
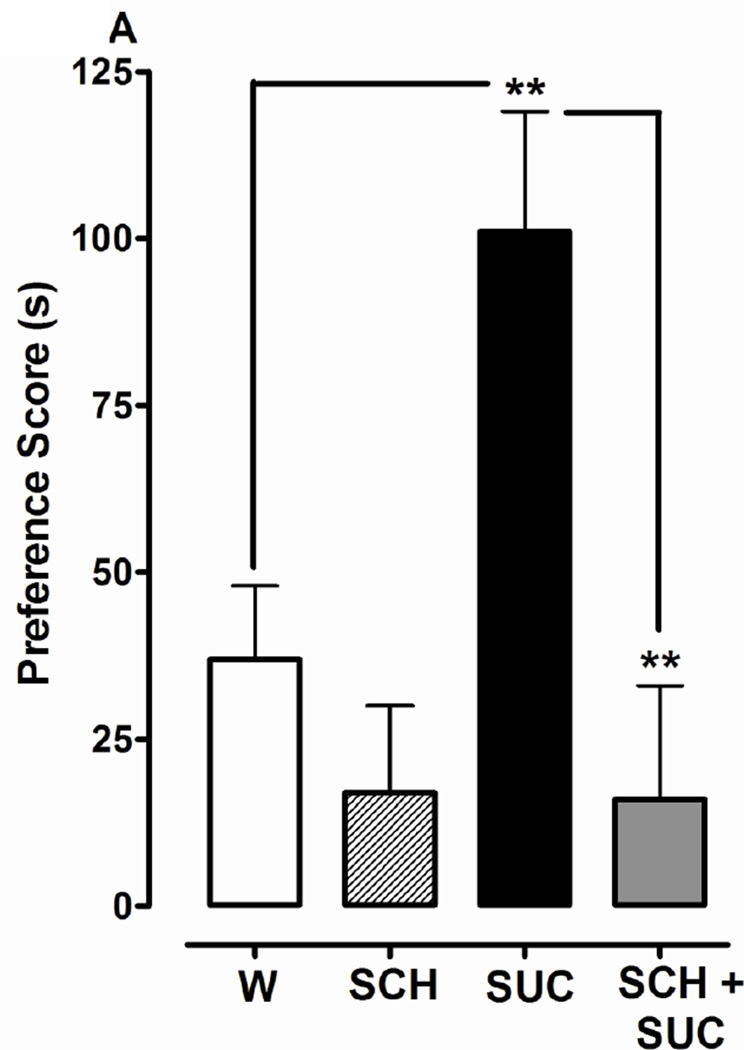
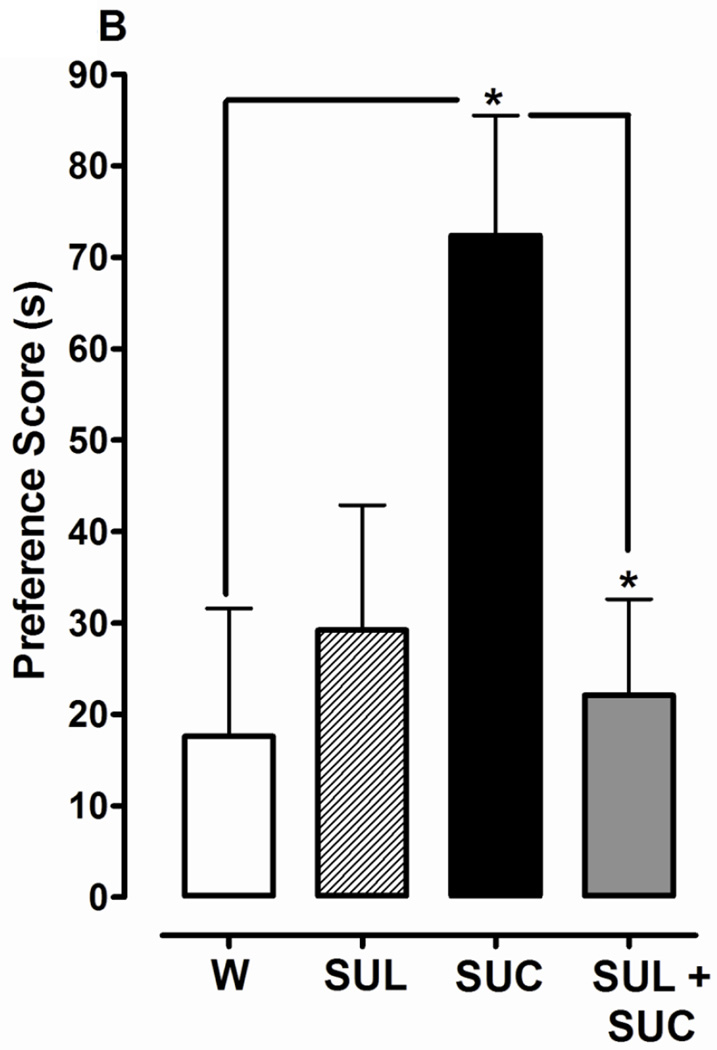
Fig. 3.
Dopamine D1 or D2 receptor antagonists significantly inhibit development of CPP induced by 1% sucrose (SUC). 3A) D1 antagonist effects: CPP was determined in planarians that were conditioned with water (W), SCH, SUC, or SCH + SUC in the ambient light. 3B) D2 antagonist effects: CPP was determined in planarians that were conditioned with water (W), SUL, SUC, or SUL + SUC in the ambient light. Data are presented as the mean preference score (s) + S.E.M. (difference between post-conditioning and pre-conditioning times). N = 10 planarians per group. **p < 0.01, *p < 0.05 compared to W or SUC.
One-way ANOVA revealed a significant main effect for experiments testing the effect of SCH 23390 (1 µM) on development of the preference shift produced by 1% sucrose conditioning [F(3, 44) = 7.095, p < 0.001] (Fig. 3A). Planarians conditioned with sucrose by itself again displayed a greater preference shift than water controls (101 ± 18 s versus 37 ± 11 s, respectively) (p < 0.01). However, when planarians were conditioned with a combination of sucrose and SCH 23390, the preference shift was less than that observed following conditioning with sucrose by itself (16 ± 17 s versus 101 ± 18 s, respectively) (p < 0.01). One-way ANOVA revealed a significant main effect for experiments testing the effect of sulpiride (1 µM) on the development of place conditioning produced by 1% sucrose conditioning [F(3, 44) = 3.809, p < 0.05] (Fig. 3B). Planarians conditioned with sucrose by itself displayed a greater preference shift compared to water controls (73 ± 13 s versus 17 ± 14 s, respectively) (p < 0.05) and planarians conditioned with a combination of sucrose and sulpiride (73 ± 18 s versus 22 ± 10 s, respectively) (p < 0.05). Preference shifts produced by conditioning with SCH 23390 (Fig. 3A) or sulpiride (Fig. 3B) were not different than water controls (p > 0.05).
3.4. Expression of sucrose CPP is not affected by dopamine receptor antagonists (Fig. 4)
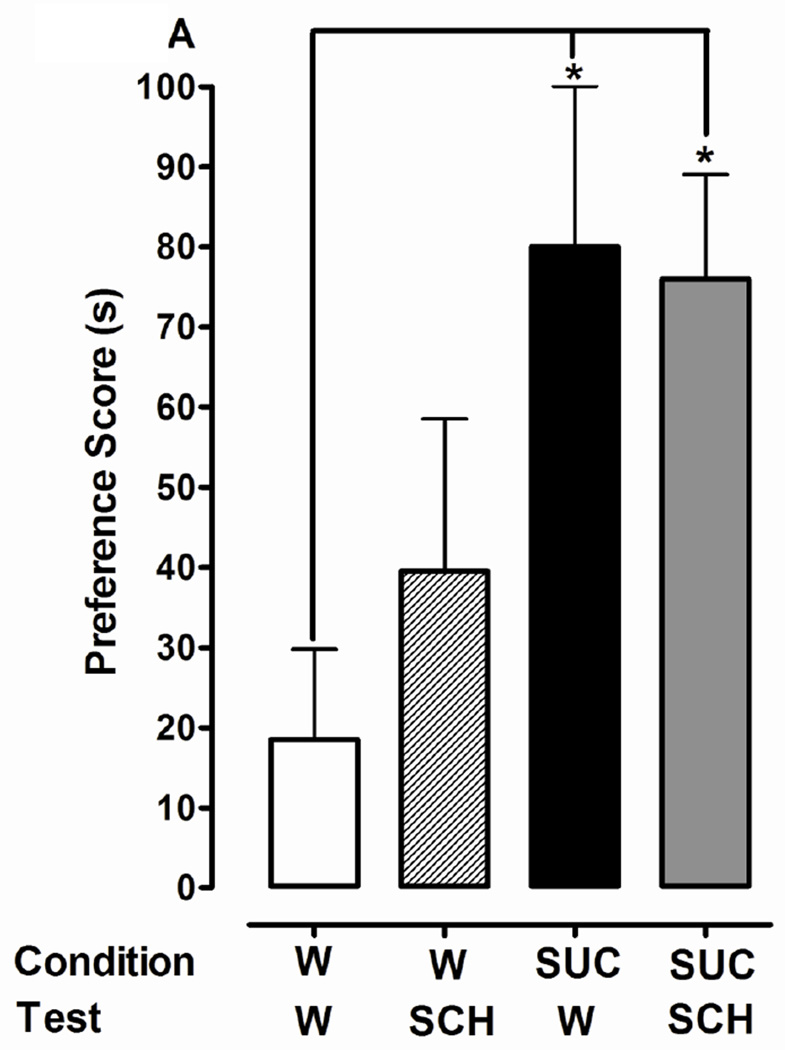
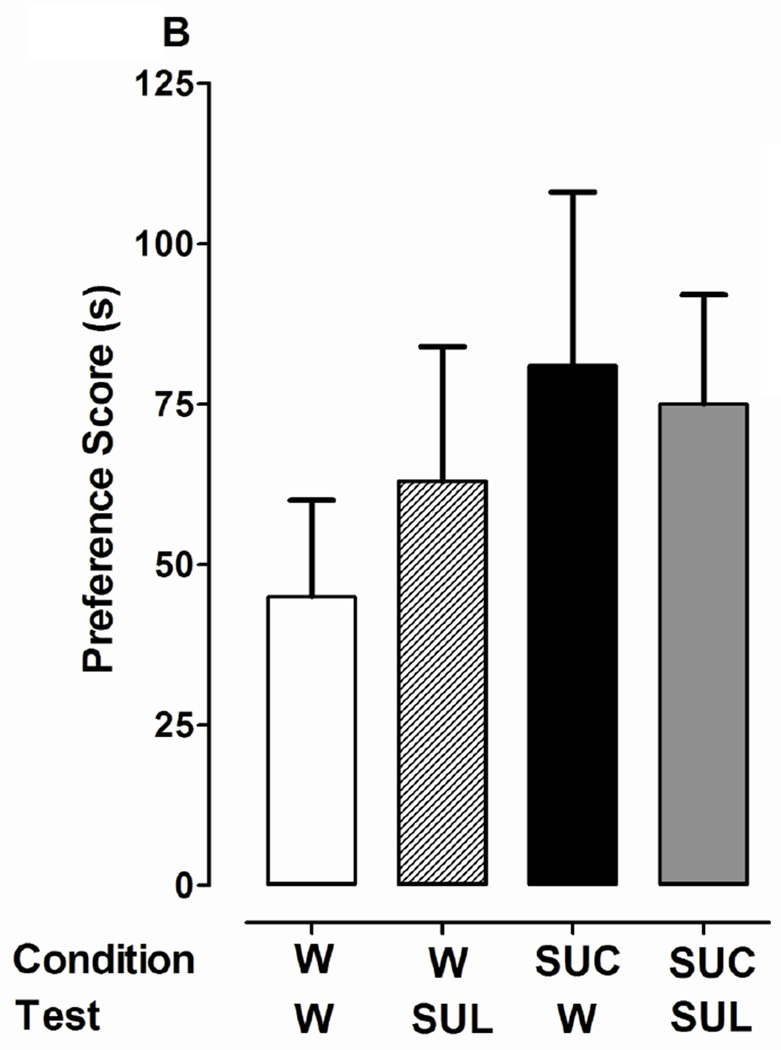
Fig. 4.
Dopamine D1 or D2 receptor antagonists do not affect expression of CPP induced by 1% sucrose (SUC). 4A) D1 antagonist effects: Following a pre-test in drug-naïve planarians, conditioning with water (W) or sucrose (SUC) was conducted in the ambient light. A post-test was conducted in which planarians from both groups (W and SUC) were exposed to W or SCH. 4B) D2 antagonist effects: Experiments were conducted as described in Panel A except that SUL was used instead of SCH. Data are presented as the mean preference score (s) + S.E.M. (difference between post-conditioning and pre-conditioning times). N = 10 planarians per group. *p < 0.05 compared to respective W/W group.
One-way ANOVA indicated a significant main effect for experiments testing the effect of SCH 23390 (1 µM) on expression of 1% sucrose-induced place conditioning [F(3, 44) = 3.315, p < 0.05] (Fig. 4A). A greater preference shift was observed in planarians conditioned with sucrose than in water controls (80 ± 20 s versus 18 ± 11 s, respectively) (p < 0.05). However, preference shifts observed in planarians that were conditioned with sucrose and then tested in water (80 ± 20 s) or SCH 23390 (76 ± 13 s) were not significantly different (p > 0.05). For experiments testing the effect of sulpiride (1 µM) on the expression of 1% sucrose-induced place conditioning, a significant main effect was not detected [F(3, 44) = 0.5986, p > 0.05] (Fig. 4B). The lack of overall significance was because the preference score in the water control group was greater than previously observed. Nevertheless, for the groups conditioned with sucrose, posttests conducted in water (81 ± 27 s) and sulpiride (75 ± 17 s) yielded similar preference scores.
4. Discussion
The present study investigated pharmacological effects of a naturally-occurring reward, viz., sucrose, in planarians. Experiments revealed that planarians displayed abstinence-induced withdrawal responses following abrupt discontinuation of sucrose exposure that were not evident during acute or continuous sucrose exposure. Sucrose produced a conditioned place preference (CPP) that was inhibited by dopamine D1 or D2 receptor antagonists. Drugs of abuse (cocaine, amphetamines, opioids, nicotine, benzodiazepines, and substituted cathinones) from different pharmacological classes elicit spontaneous withdrawal responses in planarians [24–25, 30–38]. Characteristic withdrawal responses include reduced motility, headbops, headswings (head rotation in the absence of gliding while the tail is fixed to the bottom of the dish), tailtwists (bending of the tail tip) and corkscrews (spiral rotation while floating or swimming) [25]. Our laboratory quantifies planarian withdrawal using the endpoint of reduced motility, a response that is specific to addictive substances and independent of pH or osmolarity changes [30–35, 37]. We demonstrated here that planarians also display withdrawal responses to a natural reward (sucrose) that are spontaneous and do not require precipitation with a receptor antagonist. A sucrose withdrawal syndrome is observed in rats and is most intense following precipitation by administration of the opioid receptor antagonist naloxone [4]. For example, following intermittent access to sucrose, rats injected with naloxone exhibit somatic withdrawal responses (e.g. teeth chattering, head shakes, forepaw tremor), anxiogenic behavior, and behavioral depression [10]. Rats spontaneously removed from sucrose-rich diets display hypothermia and aggression [15, 45]. A comparison of findings from rat and planarian studies suggests that sucrose withdrawal, while detectable in both species, is more sensitive in planarians. Future studies will probe underlying mechanisms, such as involvement of kappa opioid receptors, which are preferentially expressed in planarians relative to mu and delta opioid receptors [27].
Consistent with prior studies demonstrating that sucrose produces CPP in rats [1, 13, 21] and conditioned preference effects (e.g. a positive Proboscis Extension Response) in Drosophila [9, 19, 42], our experiments revealed that sucrose conditioning produced an environmental preference shift in planarians. To enable the environment to act as a conditioned stimulus, time spent in the non-preferred environment (ambient light) was determined prior to sucrose exposure and this environment was then paired with sucrose during conditioning. It was hypothesized that planarians would prefer, or avoid, the conditioned environmental stimulus depending on the rewarding or aversive properties of the paired substance (sucrose). Experiments revealed that planarians conditioned with sucrose displayed a preference shift, i.e.they developed a greater preference for the light environment that was paired with sucrose despite their natural preference for the dark. The shift in preference was not directly proportional to sucrose concentration. A median concentration (1 %) produced the greatest shift whereas relatively lower (0.1 %) and higher (10 %) concentrations were less efficacious. The inverted u-shaped response with sucrose in the CPP experiments is intriguing. The observation that a concentration of 1% sucrose produces both withdrawal responses and a preference shift in planarians suggests that this concentration elicits rewarding effects (i.e.planarians are experiencing rewarding effects in the presence of sucrose and aversive effects upon removal of sucrose). In addition, the highest concentration (10%) that was tested in CPP experiments also reduced motility upon acute exposure, suggesting that it may have produced toxic effects in planarians that would negate or counter an expected preference shift.
It is interesting to note that conditioning with glucose, but not with the synthetic non-digestible disaccharide lactulose, also produced an environmental preference shift in planarians. Glucose is formed from the hydrolytic cleavage of sucrose by β-fructosidase in microvilli of the intestinal brush border in mammals. Evidence that both sucrose and glucose produced preference shifts in planarians suggests that the rewarding effects of sucrose were primarily mediated by glucose and that pathways of sucrose metabolism in planarians and mammals share commonalities. A lack of knowledge about taste, smell, and digestive processes in planarians precludes extensive speculation about how interactions between their appetite and reward systems may have shaped the sucrose and glucose preference shifts. The digestive system of planarians is comprised of a mouth and gastrovacular cavity connected by a pharynx, and their natural diet consists of segmented worms and dead fish [33]. It is known that chemoreceptors concentrated in the auricles at the side of the planarian head respond to gustatory and olfactory stimuli and that olfactory/taste signals received in the head region are conveyed in the main lobes of the brain [23]. Future studies will be aimed at linking reward and appetite pathways in planarians to the pharmacological effects of sucrose and related sweeteners.
Sucrose preference in planarians was dependent on an active dopamine system. In vertebrates dopamine mediates reinforcing effects of natural and drug rewards [2–7, 43], and a comparable function in planarians is suggested by findings observed here. Dopamine receptor antagonists inhibited the development, but not expression, of sucrose CPP in planarians, suggesting that dopamine signaling has different roles across the stages of sucrose reinforcement. The most obvious interpretation is that enhanced dopamine signaling is required for the development of the reinforcing properties of sucrose but that non-dopaminergic systems, such as glutamate, GABA, or opioid, are more important for the expression and/or maintenance of those properties. Dopamine receptors in planarians have been distinguished by pharmacological and biochemical criteria [26–27]. For example, dopamine D1 and D2 receptor agonists produce an increase in cAMP levels that is antagonized by dopamine subtype-specific antagonists. In the present experiments dopamine receptor subtype-specific effects were not discriminated as antagonists from the D1 and D2 receptor classes similarly inhibited development of preference. Dopamine receptors in planarians have been distinguished by pharmacological and biochemical criteria [26–27]. For example, dopamine D1 and D2 receptor agonists produce an increase in cAMP levels that is antagonized by dopamine subtype-specific antagonists. Excluding pharmacokinetic effects of SCH 23390 or sulpiride, or chemical antagonism of sucrose by the antagonists, the simplest explanation for the efficacy of the antagonists in the present experiments is that active dopamine D1 and D2 receptors are required for sucrose to produce CPP in planarians. In this model, sucrose exposure during conditioning increases extracellular dopamine, through enhancing release or disrupting uptake or catabolism. In turn, this increases dopamine transmission at D1 and D2 receptors that manifests as CPP during which planarians engage in sucrose-seeking behaviors. Rodent CPP studies with sucrose have also demonstrated that nonselective dopamine receptor antagonists (e.g. cis(z)-flupentixol, alpha-flupenthixol), as well as lesions of the dopaminergic innervation of the nucleus accumbens, reduce sucrose preference [1, 21].
A limitation of the planarian assay is the inability to screen for a neophobic response to sucrose. Rodents typically display a lack of preference for sugar-containing solutions upon initial exposure that is interpreted as a neophobic response that is also associated with other stimuli [21, 40]. Because rodents lack a vomiting reflex and cannot interrupt digestion following the consumption of a substance, neophobia is protective against the intake of potentially toxic foods. In the case in which consumption of a novel food produces dysphoria, the animal develops conditioned place aversion to the food [41]. In the absence of toxic or dysphoric effects the subsequent effects of the food would produce rewarding effects manifested as CPP. Our observation that sucrose produces a preference shift following a single conditioning session suggests its rewarding properties are relatively rapid in onset. The concentration of sucrose that elicited CPP did not produce signs of toxicity but did cause withdrawal effects following discontinuation of exposure. Nonetheless, an initial neophobic effect of sucrose cannot be discounted because assays comparable to those used in rodents to quantify sucrose consumption and choice (e.g. versus water through a licking response) are not available for planarians.
5. Conclusion
Sucrose, similar to drugs of abuse, produced withdrawal responses and reinforcing effects in planarians [24–25, 30–38]. Rodent studies using dopamine receptor antagonists, gene knockout approaches, and neuroanatomical lesions indicate that natural and drug rewards produce changes in dopamine transmission that mediate their reinforcing properties [43]. The present evidence that place conditioning effects of sucrose develop only in the presence of an active dopamine system suggests that mechanisms underlying the reinforcing efficacy of table sugar are highly conserved. It can also be postulated that planarians may provide an invertebrate model to study behavioral and neurochemical effects of sucrose and a pre-mammalian screen to identify medications to decrease adverse effects associated with excessive sugar consumption.
Research Highlights.
Sucrose elicits abstinence-induced withdrawal responses in planarians.
Sucrose and glucose produce conditioned place preference in planarians.
Lactulose does not produce CPP in planarians
Dopamine D1 and D2 antagonists inhibit development of sucrose preference in planarians.
Reinforcing properties of sucrose are conserved across species.
Acknowledgements
This study was supported by NIDA center grant P30 DA013429. The authors thank Timothy Shickley, Ph.D., for suggesting Planaria as a test model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agmo A, Galvan A, Talamantes B. Reward and reinforcement produced by drinking sucrose: two processes that may depend on different neurotransmitters. Pharmacol Biochem Behav. 1995;52:403–414. doi: 10.1016/0091-3057(95)00128-j. [DOI] [PubMed] [Google Scholar]
- 2.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–362. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2010;55:734–737. doi: 10.1016/j.appet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avena NM, Gold MS. Food and addiction - sugars, fats and hedonic overeating. Addiction. 2011;106:1214–1215. doi: 10.1111/j.1360-0443.2011.03373.x. [DOI] [PubMed] [Google Scholar]
- 7.Avena NM, Bocarsly ME, Hoebel BG. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol. 2012;829:351–365. doi: 10.1007/978-1-61779-458-2_23. [DOI] [PubMed] [Google Scholar]
- 8.Buttarelli FR, Pellicano C, Pontieri FE. Neuropharmacology and behavior in planarians: translations to mammals. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:399–408. doi: 10.1016/j.cbpc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Chabaud MA, Devaud JM, Pham-Delègue MH, Preat T, Kaiser L. Olfactory conditioning of proboscis activity in Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:1335–1348. doi: 10.1007/s00359-006-0160-3. [DOI] [PubMed] [Google Scholar]
- 10.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 11.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson KS, Panula P. gamma-Aminobutyric acid in the nervous system of a planarian. J Comp Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- 13.Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73:229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- 14.Fortuna JL. The obesity epidemic and food addiction: clinical similarities to drug dependence. J Psychoactive Drugs. 2012;44:56–63. doi: 10.1080/02791072.2012.662092. [DOI] [PubMed] [Google Scholar]
- 15.Galic MA, Persinger MA. Voluminous sucrose consumption in female rats: increased "nippiness" during periods of sucrose removal and possible oestrus periodicity. Psychol Rep. 2002;90:58–60. doi: 10.2466/pr0.2002.90.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005 Jan 21;1031(2):194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann N Y Acad Sci. 1989;575:171–191. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim YC, Lee HG, Han KA. Classical reward conditioning in Drosophila melanogaster. Genes Brain Behav. 2007;6:201–207. doi: 10.1111/j.1601-183X.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 20.Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11:2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Hernández J, Lanuza E, Martínez-García F. Lesions of the dopaminergic innervation of the nucleus accumbens medial shell delay the generation of preference for sucrose, but not of sexual pheromones. Behav Brain Res. 2012;226:538–547. doi: 10.1016/j.bbr.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zoolog Sci. 2005;22:535–546. doi: 10.2108/zsj.22.535. [DOI] [PubMed] [Google Scholar]
- 24.Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur J Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- 27.Passarelli F, Merante A, Pontieri FE, Margotta V, Venturini G, Palladini G. Opioiddopamine interaction in planaria: a behavioral study. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:51–55. doi: 10.1016/s0742-8413(99)00048-1. [DOI] [PubMed] [Google Scholar]
- 28.Peters A. Does sugar addiction really cause obesity? Front Neuroenergetics. 2011;3:11. doi: 10.3389/fnene.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur J Pharmacol. 2001;430:143–145. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- 31.Raffa RB, Dasrath CS, Brown DR. Disruption of a drug-induced choice behavior by UV light. Behav Pharmacol. 2003;14:569–571. doi: 10.1097/00008877-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Raffa RB, Desai P. Description and quantification of cocaine withdrawal signs in Planaria. Brain Res. 2005;1032:200–2002. doi: 10.1016/j.brainres.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 33.Raffa RB, Rawls SM. A model for drug action and abuse. Landes Bioscience, Austin, TX. 2008 [Google Scholar]
- 34.Raffa RB, Stagliano GW, Ross G, Powell JA, Phillips AG, Ding Z, Rawls SM. The kappaopioid receptor antagonist nor-BNI inhibits cocaine and amphetamine, but not cannabinoid (WIN 52212-2), abstinence-induced withdrawal in planarians: an instance of 'pharmacologic congruence'. Brain Res. 2008;1193:51–56. doi: 10.1016/j.brainres.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawls SM, Cavallo F, Capasso A, Ding Z, Raffa RB. The beta-lactam antibiotic ceftriaxone inhibits physical dependence and abstinence-induced withdrawal from cocaine, amphetamine, methamphetamine, and clorazepate in planarians. Eur J Pharmacol. 2008;584:278–284. doi: 10.1016/j.ejphar.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Rawls SM, Karaca F, Madhani I, Bhojani V, Martinez RL, Abou-Gharbia M, Raffa RB. β-lactamase inhibitors display anti-seizure properties in an invertebrate assay. Neuroscience. 2010;169:1800–1804. doi: 10.1016/j.neuroscience.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: clues from planarians. Drug Alcohol Depend. 2011;118:274–279. doi: 10.1016/j.drugalcdep.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur J Pharmacol. 2008;583:170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiatelike effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Stewart CN, Reidinger RF Jr. Disparity between formation of conditioned flavor aversions and neophobia during grooming in rats and mice. Physiol Behav. 1984;32:955–959. doi: 10.1016/0031-9384(84)90285-3. [DOI] [PubMed] [Google Scholar]
- 41.Swerdlow NR, van der Kooy D, Koob GF, Wenger JR. Cholecystokinin produces conditioned place-aversions, not place-preferences, in food-deprived rats: evidence against involvement in satiety. Life Sci. 1983;32:2087–2093. doi: 10.1016/0024-3205(83)90096-6. [DOI] [PubMed] [Google Scholar]
- 42.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyas CA, Rawls SM, Raffa RB, Shackman JG. Glutamate and aspartate measurements in individual planaria by rapid capillary electrophoresis. J Pharmacol Toxicol Methods. 2010;63:119–122. doi: 10.1016/j.vascn.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Wideman CH, Nadzam GR, Murphy HM. Implications of an animal model of sugar addiction, withdrawal and relapse for human health. Nutr Neurosci. 2005;8(5 – 6):269–276. doi: 10.1080/10284150500485221. [DOI] [PubMed] [Google Scholar]