Thanks to the dedicated involvement of the communities it serves, the National Heart, Lung, and Blood Institute (NHLBI) completed in 2007 development of a scientific working Strategic Plan to guide its activities and initiatives over the next 5–10 years.1 The plan outlines a cross cutting, approach that identifies areas where the NHLBI is well positioned to make major contributions through investigator-initiated research and through programs that enable and supplement investigator-initiated activities. Translational research ─ both from the bench to the bedside and from the bedside to the community ─ is strongly and specifically underscored throughout the plan. In this paper, we summarize NHLBI programs and efforts in translational research. Funding opportunities and early career advice for aspiring researchers have been presented previously in this series.2, 3
What is Translational Research?
The ultimate goal of biomedical research is to improve individual and public health by discovering new effective strategies for the maintenance of wellness and for the prevention and treatment of disease. The Institute of Medicine Clinical Research Roundtable identified two “translational blocks,” or obstacles, to the realization of tangible health benefits derived from original research.4 The first block, or T1, is “the transfer of new understandings of disease mechanisms gained in the laboratory into the development of new methods for diagnosis, therapy, and prevention and their first testing in humans.” The second block, or T2, is “the translation of results from clinical studies into everyday clinical practice and health decision making.” Some authorities divide T2 into two components: “T2” includes guideline development, meta-analyses, and systematic reviews, whereas T3 includes dissemination and implementation research.5 Some commentators have argued that NIH disproportionately funds T1 as compared to T2/T3, and that there is a need for increased national commitments to T2/T3 (from this point referred to as “T2”).5, 6 A recent survey of over 3000 biomedical researchers based at 50 universities that received relatively large sums of NIH support found that 9.1% of researchers primarily focus on T1 translation and 9.0% focus on T2.7 T2 researchers were more likely have no funding as compared to T1 or basic researchers (47% vs. 23% vs. 12%, respectively).7
The NIH recently demonstrated its strong interest in translational research through the launching of its Clinical and Translational Science Award (CTSA) program.8 Detailed descriptions of the program have been posted.9 Briefly, the NIH is funding 46 major academic centers with 5 goals in mind: building a national clinical and translational research capacity, providing opportunities for training and career development, enhancing consortium-wide collaborations, improving community health through research, and advancing T1 to accelerate the translation of basic science discoveries to clinical testing.10
The NHLBI’s Strategic Plan1 explicitly recognizes the critical value of both T1 and T2. Goal 2 of the Strategic Plan recognizes as an explicit challenge the need to “accelerate the translation of basic research findings into clinical studies”; Goal 3 calls for “an improved understanding of the processes involved in translating research into practice and use that understanding to enable improvements in public health.” NHLBI also recognizes that translation is not unidirectional. Discoveries made in the clinic or in populations often stimulate new ideas among bench laboratory researchers.
Connecting Basic and Clinical Research (T1)
The Strategic Plan’s clinical and translational research goals emphasize transmission of knowledge between basic and clinical research so that findings in one arena rapidly inform and stimulate research in others. Remarkable advances are being made in understanding the molecular, genetic, and cellular basis of disease, and opportunities now exist to uncover practical uses for this new knowledge. The application of this new understanding to the prevention and management of cardiovascular diseases will call for creative insights into possible relationships and implications. In particular, success in this arena will require a concerted effort to identify, measure, and validate targets and pathways that have been detected in basic studies.
Several potentially fruitful areas for such bench-to-bedside translation are highlighted in the NHLBI plan. Regenerative biology, for instance, stands to benefit greatly from recent advances in stem cell biology and tissue engineering. As well, the evolving field of nanotechnology promises to play a key role in drug delivery and therapeutics, molecular imaging, diagnostics and biosensors, and tissue engineering and biomaterials. Biomedical research has generated volumes of data from advances in “-omic” technologies, and an overwhelming amount of new information can be expected from such developments as affordable individual genome sequencing and real-time metabolomics. The challenge will be to integrate, analyze, and widely share enormous quantities of complex genotypic and phenotypic data in order to realize their full potential.11
Our strong focus on bench-to-bedside translation is reflected in many activities under way (Table 1). We are conducting an in-depth reexamination of each of our programs of Specialized Centers of Clinically Oriented Research (SCCOR).12 The SCCOR provide rich environments in which findings from basic science drive clinical research directions and clinical needs, in turn, inform new basic investigations. However, they are funded only via periodic NHLBI requests for applications (RFAs) in specifically identified areas of scientific interest―we now support 15 awards as a result of solicitations on cardiac dysfunction, pediatric heart development and disease, and vascular injury, repair, and remodeling. Thus, access to these avenues for translational research is constrained. In our reassessment of the program we are looking for innovative ways to facilitate access to resources by investigators from many disciplines across the country and, in particular, to expand training opportunities in translational research.
Table 1.
Summary of NHLBI Translational Research Programs
| Title | Funding Announcement Web Link |
Number of Awards and Budget |
|---|---|---|
| Cardiac Translational Research Implementation Program (C-TRIP) (P20) | http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-10-001.html | NHLBI expects to commit $9M/year to support up to 12 two year Stage 1 grants and four subsequent Stage 2 grants. |
| NHLBI Pediatric Cardiac Genomics Consortium (U01) | http://grants1.nih.gov/grants/guide/rfa-files/RFA-HL-09-003.html | NHLBI expects to commit $25.4 M over six years. A total of 5 awards were funded. |
| Pediatric Heart Network | http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-05-010.htm | NHLBI committed $30M for a five year period. A total of 9 awards were funded. |
| NHLBI Progenitor Cell Biology Consortium Research Hubs (U01) | http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-09-004.html | NHLBI committed $70M for a six year period. A total of 17 awards were funded. |
| Cardiovascular Cell Therapy Research Network | http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-06-001.html | NHLBI committed $29.9M for 5 years to fund five clinical centers and one Data Coordinating Center. |
| Science Moving Towards Research Translation and Therapy (SMARTT) |
https://www.fbo.gov/index?s=opportunity&mode=form&id=eb9754c73b37ace85b11054a8e31b033&tab=core&_cview=1: https://www.fbo.gov/index?s=opportunity&mode=form&id=77f7424b4cd0d2f51d193a8779f7d731&tab=core&_cview=1 https://www.fbo.gov/index?s=opportunity&mode=form&id=b7cf90a712babadb5a05697ea302b8a0&tab=core&_cview=1 https://www.fbo.gov/index?s=opportunity&mode=form&id=687024bf044895f86c532dfe97ecab04&tab=core&_cview=1 |
NHLBI expects to commit $32 M for 6 years to fund 1 coordinating center and 3 production facilities. |
| Translating Basic Behavioral and Social Science Discoveries into Interventions to Reduce Obesity: Centers for Behavioral Intervention Development (U01) | http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-08-013.html | NHLBI committed $25 M for a five year period. A total of 5 awards were funded. |
| Demonstration and Education Research Grants (R18) | http://www.nhlbi.nih.gov/funding/policies/d&eguide.htm | Program renewed upon advice of NHBLI Council in 2009 |
| Interventions to Improve Hypertension Control in African Americans (U01) | http://grants.nih.gov/grants/guide/rfa-files/rfa-hl-04-007.html | NHLBI committed $17.5M for a five year period. A total of 5 awards were funded. |
| NHLBI Centers for Cardiovascular Outcomes Research (U01) |
http://grants.nih.gov/grants/guide/rfa-files/rfa-hl-10-008.html and http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-10-018.html |
NHLBI has committed $19.8M to fund 3 outcomes centers and one coordinating center |
| http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-10-018.html | ||
| Cardiovascular Research Network (U01) |
http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-07-011.html and http://www.cvrn.org |
NHLBI has committed $7.5M for a five year period. A total of 1 award was funded. Over $14 million for 7 ancillary grants have been awarded. |
Key websites:
http://www.nhlbi.nih.gov/health/dci/index.html - NHLBI Diseases and Conditions Index
http://www.nhlbi.nih.gov/recovery/index.htm - NHLBI Recovery Act Website
http://www.nhlbi.nih.gov/index.htm - NHLBI Public Website
http://report.nih.gov/index.aspx - Research Portfolio Online Reporting Tool (RePORT)
In line with these objectives, we developed a new initiative, the Cardiac Translational Research Implementation Program (C-TRIP)13, to supersede the SCCOR program in Cardiac Dysfunction and Disease, which is drawing to the end of its 10-year cycle. The goals of C-TRIP are to accelerate translation of promising new fundamental research discoveries for the treatment and prevention of heart failure and arrhythmias through well-designed clinical trials that demonstrate efficacy and safety. This Program has two stages: 1) Stage 1: 2 years of funding for pilot studies to plan phase 1 and 2 trials; and 2) Stage 2: 5 years of funding for Phase I and II trials.
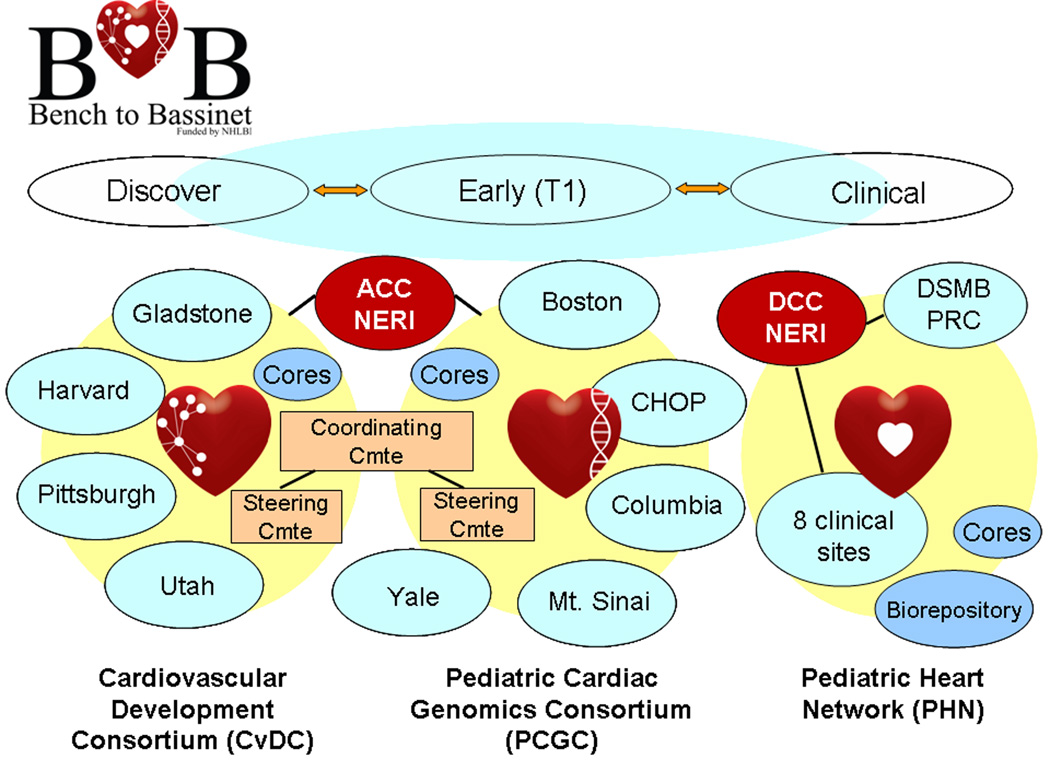
To accelerate translational research in the health and care of patients with congenital disease, we recently launched an innovative pediatric cardiovascular translational program, the “Bench to Bassinet Program.”14 This program will create a critical mass of collaborative research across three interacting consortia. (See Figure 1) The Cardiovascular Development Consortium will link four seasoned research teams in order to drill deeply into the details of the transcriptional regulatory networks that govern cardiac development, using complementary animal models. Investigators from five sites in the Pediatric Cardiac Genomics Consortium will recruit children into a common protocol to speed discovery of causative genes and to evaluate the effects of genetic variation on short- and long-term outcomes in congenital heart disease patients. These two Consortia will align with the Pediatric Heart Network15, a multi-center clinical research enterprise initiated by the NHLBI in 2001. All three programs will pursue their individual lines of research but will also interact with each other on multiple levels to ensure that important discoveries in one component will be rapidly evaluated and tested by the disciplines and technologies of the others.
Figure 1.
Organizational structure of the NHLBI Bench to Bassinet Program. Interactions between the Cardiovascular Development Consortium, the Pediatric Cardiac Genomics Consortium, and the Pediatric Heart Network are designed to facilitate bidirectional transmission of research along translational pathways from discovery to the clinic and back. ACC: Administrative Coordinating Center; CHOP: Children’s Hospital of Philadelphia; DSMB: Data and Safety Monitoring Board; NERI – New England Research Institutes; PRC – Protocol Review Committee. Based on figure from NHLBI14.
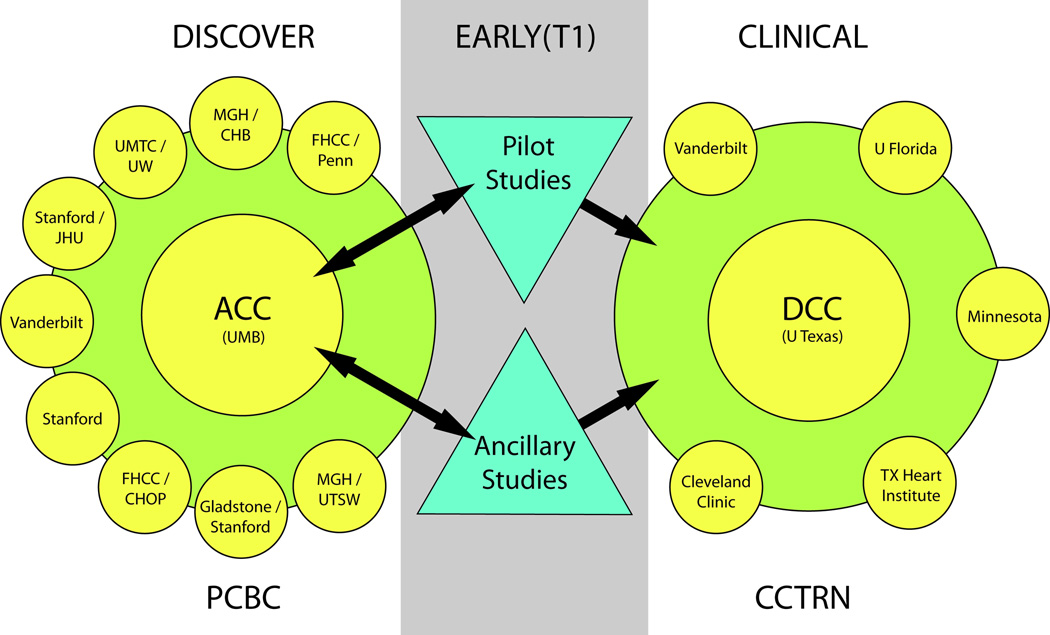
We have initiated the Progenitor Cell Biology Consortium 16 that will identify and characterize progenitor cell lines, direct the differentiation of stem and progenitor cells to desired cell fates, and develop new strategies to address the unique challenges presented by the transplantation of these cells. This Consortium will assemble multiple independent research projects, each with a multidisciplinary team of principal investigators, core research support facilities, and skills development components to establish synergistic virtual hubs focused on progenitor cell biology. The Consortium will complement the Cardiovascular Cell Therapy Research Network17 that was established by the NHLBI in 2006 to promote and accelerate clinical research in the evaluation of novel cell therapy treatment strategies for individuals with cardiovascular disease. (See Figure 2)
Figure 2.
Organizational structure of the NHLBI Biological discovery in the Progenitor Cell Biology Consortium (PCBC) and NHLBI Cardiovascular Cell Therapy Research Network (CCTRN). The consortium is leveraged through Pilot studies and Ancillary/Collaborative studies, funded through set-aside funds administered by the Administrative Coordinating Center (ACC). These funds, using expedited peer review, can provide rapid early translation through pre-clinical models to help in the design of clinical studies in the Cardiovascular Cell Therapy Research Network (CCTRN). FHCC, Fred Hutchinson Cancer Center; Penn, University of Pennsylvania; MGH, Massachusetts General Hospital; CHB, Children’s Hospital, Boston; Stanford, Stanford University; JHU, Johns Hopkins University; UMTC, University of Minnesota, Twin Cities; UW, University of Wisconsin; Vanderbilt, Vanderbilt University; CHOP, Children’s Hospital of Philadelphia; Gladstone, J. David Gladstone Institutes; UTSW, University of Texas Southwestern Medical Center Dallas; UMB, University of Maryland, Baltimore.
In another effort to accelerate translational research, we will seek to provide the community access to resources and technologies that are otherwise unattainable by NIH investigators. A new program, Science Moving TowArds Reseach Translation and Therapy (SMARTT)18 will be starting in the fall of 2010 to assist in the translation to the clinic of new synthetic, natural, or biologic interventions for the treatment of heart, lung and blood diseases. This 6 year program will fund one coordinating center and 3 facilities to provide resources of preclinical and clinical-grade production and testing for biologics, non-biologics and small molecules in accordance with Good Manufacturing Practices (GMP), and in the conduct of pharmacology and toxicology studies. Regulatory assistance will be available to investigators. This Program will be similar to the Rapid Access to Interventional Development program of the National Cancer Institute, which provides contract services to expedite translation to the clinic of potential new therapeutic agents originating in academia.
NHLBI has taken a leading role in obesity research, recognizing that the rapid increase in obesity prevalence among Americans threatens to reverse population improvements in life expectancy and cardiovascular risk. The NHLBI is inaugurating a new program, “Translating Basic Behavioral and Social Science Discoveries into Interventions to Reduce Obesity,”19 that will fund 7 research teams to develop promising strategies for modifying behaviors that affect body mass. The program is funded in partnership with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the Office of Behavioral and Social Sciences Research (OBSSR). The program will focus on high-risk priority populations (including children, ethnic minorities, economically disadvantaged, and pregnant women) and will test a variety of individual, family, and community interventions.
Bridging Research and Practice (T2)
In its Goal 3, the NHLBI strategic plan1 highlights the need to enhance understanding of the processes involved in translating research into practice, which corresponds to what some authorities refer to as T35, and to use that understanding to enable improvements in public health and to stimulate further scientific discovery. There is a well-recognized “quality gap” in the United States, as evidenced, for example, in a 2003 report that found that only American adults receive only 55% of recommended services for a variety of chronic conditions.20 In cardiovascular disease, the situation is only slightly better: among adults with coronary artery disease, heart failure, and hypertension, 64–68% received recommended services.20 NHLBI recognizes that the processes by which evidence-based practices become widely adopted within routine care are themselves legitimate targets for research; this type of T2 research includes outcomes and health services research. (See Table 1) Focused behavioral and social science research may help uncover effective new approaches for communicating research findings to the public and for motivating and empowering individuals and communities to take charge of their health.
The NHLBI has for many years been a leader in this arena, facilitating the transfer of health information to physicians, patients, and the general public through support of implementation R18 (formerly known as “demonstration and education (D&E)” research grants21 and through health education programs. Implementation research takes interventions that are generally accepted as beneficial and addresses their extension or adaptation to specific populations or settings, testing a variety of educational approaches, behavioral techniques, and environmental or organizational strategies. The study populations can come from a variety of settings, e.g., people living in geographically defined areas; employees in work sites; school children; patients and health-care providers in physicians’ offices, ambulatory care clinics, or health maintenance organizations; and other community settings. Examples of recently reported NHLBI-funded implementation research projects include the Activity Counseling Trial22, the Rapid Early Action for Coronary Treatment (REACT) trial23, a trial of case management for reducing cardiovascular risk in low-income ethnic-minority neighborhoods24, and a trial demonstrating the value of pharmacist collaboration for achieving blood pressure control.25
NHLBI is or has recently completed supporting research programs that are specifically targeted towards improved implementation. Because poor adherence is a major obstacle to therapeutic success, the Institute funded 13 randomized trials to test innovative but practical interventions to improve adherence in disadvantaged populations.26 Another NHLBI program is funding 11 randomized trials testing approaches to improve provider adherence to evidence-based cardiovascular guidelines. A third program is funding 5 randomized trials that test approaches to improve hypertension control in African Americans.27 NHLBI is about to inaugurate an ambitious program to fund centers in cardiovascular outcomes research, with each center expected to conduct at least two major projects and to provide high-level training to young cardiovascular outcomes researchers.28
Since 2007, the NHLBI has been funding a large-scale Cardiovascular Research Network (CVRN)29, which brings together researchers and databases from 15 integrated health plans that are members of the NIH Health Maintenance Organization Research Network (HMORN). The Network has access to the electronic health records over 11 million people. Three primary projects are analyzing characteristics, management and outcomes of patients with hypertension, atrial fibrillation, and newly implanted cardioverter-defibrillators. In the ICD project, NHLBI is partnering with the Agency for Healthcare Research and Quality (AHRQ) and the American College of Cardiology to assess long-term outcomes and in particular to determine rates and determinants of ICD shocks. The electronic data infrastructure and organization of the CVRN offer potential for a broad array of future translational and reverse-translational research opportunities, including high-throughput mega-epidemiology technology projects, robust observational comparative effectiveness research, and large simple trials.
We are in the process of an ambitious effort to develop comprehensive, evidence-based, integrated guidelines to assist primary care physicians in helping adult patients reduce their risk of cardiovascular diseases. The integrated approach will focus on all cardiovascular risk factors to reflect the complicated clinical scenarios that patients and physicians typically face. Expert panels are being convened to review available scientific evidence and update existing guidelines for the prevention, detection, evaluation, and treatment of high cholesterol, hypertension, and overweight/obesity. An important goal of both the integrative guidelines and the updates is to improve implementation, especially among high-risk and minority communities.
A Final Word
The NHLBI is firmly committed to pursuing excellent science both to advance scientific knowledge and to improve the public health, and we believe that a strong emphasis on translation is essential to fulfilling our mission. Ensuring that the public benefits from the nation’s investment in biomedical research is, and has always been, our highest priority.
Acknowledgements
The authors are grateful to Ms. Catherine Burke and Mr. Dennis Stanley for their invaluable assistance preparing the Table and Figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
None.
References
- 1. [Accessed November 27, 2009];NHLBI Strategic Plan. Available at: http://www.nhlbi.nih.gov/about/strategicplan/.
- 2.Sumandea CA, Balke CW. Funding opportunities for investigators in the early stages of career development. Circulation. 2009;119:1320–1327. doi: 10.1161/CIRCULATIONAHA.107.752691. [DOI] [PubMed] [Google Scholar]
- 3.Bettmann M. Choosing a research project and a research mentor. Circulation. 2009;119:1832–1835. doi: 10.1161/CIRCULATIONAHA.107.752683. [DOI] [PubMed] [Google Scholar]
- 4.Sung NS, Crowley WF, Jr, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Korn A, Rimoin D. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 5.Westfall JM, Mold J, Fagnan L. Practice-based research--"Blue Highways" on the NIH roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 6.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 7.Zinner DE, Campbell EG. Life-science research within US academic medical centers. JAMA. 2009;302:969–976. doi: 10.1001/jama.2009.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed November 27, 2009];Clinical and Translational Science Awards. Available at: http://www.ncrr.nih.gov/clinical_research_resources/clinical_and_translational_science_awards/.
- 9. [Accessed December 23, 2009];Clinical and Translational Science Awards: Advancing Scientific Discoveries Nationwide to Improve Health (Progress Report 2006–2008) Available at: http://www.ncrr.nih.gov/clinical_research_resources/clinical_and_translational_science_awards/publications/2008_ctsa_progress_report.pdf.
- 10. [Accessed December 23, 2009];NCRR Fact Sheet: Clinical and Translational Science Awards. 2009 Fall; Available at: http://www.ncrr.nih.gov/publications/pdf/ctsa_factsheet.pdf.
- 11.Guttmacher AE, Nabel EG, Collins FS. Why data-sharing policies matter. Proc Natl Acad Sci U S A. 2009;106:16894. doi: 10.1073/pnas.0910378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Accessed November 27, 2009];Program Description Specialized Centers of Clinically Oriented Research (SCCOR) Available at: http://www.nhlbi.nih.gov/funding/policies/sccor_desc.htm.
- 13.Cardiac Translational Research Implementation Program (C-TRIP) (P20) [Accessed November 27, 2009]; Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-10-001.html.
- 14.NHLBI Pediatric Cardiovascular Translation Consortium. [Accessed November 27, 2009]; Available at: http://www.nhlbi.nih.gov/funding/inits/faq-ptc.htm.
- 15.Pediatric Heart Network. [Accessed November 27, 2009]; Available at: http://www.pediatricheartnetwork.org/.
- 16. [Accessed November 27, 2009];NHLBI Awards $170 Million to Fund Stem Cell Research. Available at: http://www.nih.gov/news/health/oct2009/nhlbi-07.htm.
- 17.Cardiovascular Cell Therapy Research Network. [Google Scholar]
- 18. [Accessed November 27, 2009];Science Moving TowArds Research Translation and Therapy (SMARTT) - Pharmacology/Toxicology Center. Available at: https://www.fbo.gov/index?s=opportunity&mode=form&tab=core&id=77f7424b4cd0d2f51d193a8779f7d731&_cview=1&cck=1&au=&ck=.
- 19. [Accessed November 27, 2009];Translating Basic Behavioral and Social Science Discoveries into Interventions to Reduce Obesity: Centers for Behavioral Intervention Development (U01) Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-08-013.html.
- 20.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, Kerr EA. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348:2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed November 27, 2009];Research Demonstration and Dissemination Grants (R18) Available at: http://grants.nih.gov/grants/guide/pa-files/PA-07-017.html.
- 22.Effects of physical activity counseling in primary care: the Activity Counseling Trial: a randomized controlled trial. JAMA. 2001;286:677–687. doi: 10.1001/jama.286.6.677. [DOI] [PubMed] [Google Scholar]
- 23.Luepker RV, Raczynski JM, Osganian S, Goldberg RJ, Finnegan JR, Jr, Hedges JR, Goff DC, Jr, Eisenberg MS, Zapka JG, Feldman HA, Labarthe DR, McGovern PG, Cornell CE, Proschan MA, Simons-Morton DG. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: The Rapid Early Action for Coronary Treatment (REACT) Trial. JAMA. 2000;284:60–67. doi: 10.1001/jama.284.1.60. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Berra K, Haskell WL, Klieman L, Hyde S, Smith MW, Xiao L, Stafford RS. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009;169:1988–1995. doi: 10.1001/archinternmed.2009.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, Chrischilles EA, Franciscus CL, Xu Y. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed November 27, 2009];Overcoming Barriers to Treatment Adherence in Minorities and Persons Living in Poverty. Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-01-005.html.
- 27. [Accessed November 27, 2009];Interventions to Improve Hypertension Control in African Americans. Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-04-007.html.
- 28.NHLBI Centers for Cardiovascular Outcomes Research. [Accessed November 27, 2009]; Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-10-008.html.
- 29. [Accessed November 27, 2009];Cardiovascular Research Network Home Page. Available at: http://www.cvrn.org/.