Abstract
Background
Both abnormal microvolt T-wave alternans (MTWA) and low peak VO2 predict poor outcome in heart failure. However, their independent predictive properties have not been assessed in large scale cohorts.
Methods
We performed an observational prospective cohort study of 303 consecutive patients referred for metabolic stress testing. All had an ejection fraction fl40% and were considered candidates for transplantation. Patients with defibrillators did not have MTWA collected by our exercise laboratory and therefore were not included in the analysis. The primary endpoint was a composite of all-cause death or UNOS 1 transplantation.
Results
During 2.8 years there were 34 deaths and 17 transplantations. Patients with abnormal MTWA had a higher event rate (31/136, 23%, vs. 20/167, 12%, unadjusted HR 1.90, 95% CI 1.90-3.33, P=.03). The association remained significant after adjustment for 3 clinical variables (1.89, 95% CI 1.05-3.39, P=.03). After adding peak VO2 to the model the association was no longer significant (adjusted HR 1.18, 95% CI 0.64-2.17, p=.60). After accounting for peak VO2 and 28 other confounders in a matched propensity analysis, MTWA was not predictive (propensity-matched HR 0.79, 95% CI 0.37-1.66, P=.53).
Conclusions
We confirm the association of abnormal MTWA with poor outcome amongst patients with impaired left ventricular systolic function. However, this association is markedly attenuated after accounting for peak VO2.
Introduction
Abnormal microvolt T-wave alternans (MTWA)1,2 and low peak oxygen consumption (peak VO2) 3 predict all-cause mortality in patients with impaired left ventricular systolic function. There are no large scale reports, however, that focus on how these powerful predictors of risk relate to each other as independent prognostic measures.
MTWA, a measure of beat-to-beat alternation in T-wave shape, amplitude, or timing 4, has been used to stratify susceptibility to ventricular arrhythmias and all-cause mortality in patients with impaired left ventricular systolic function 4,5. Two studies 6,7 have suggested that combining MTWA with peak VO2 to predict adverse outcomes in individuals with HF may improve the predictive ability of peak VO2 alone; however, each of these studies was limited by a low number of outcome events and restricted multivariable analysis. As was recently pointed out by Myles and colleagues 1, there is a need for larger observational studies of MTWA with robust multivariable models to address the incremental value of MTWA over established risk factors. Therefore, we examined the association between MTWA and peak VO2 in predicting all-cause mortality or UNOS Status 1 (urgent) cardiac transplantation in a relatively large cohort of patients with severely impaired left ventricular systolic function.
Methods
Study population
We performed an observational prospective cohort study of 303 consecutive outpatients referred for metabolic treadmill exercise testing at Cleveland Clinic between August 2002 and July 2006. As part of routine laboratory protocol, we measured MTWA on all patients undergoing metabolic exercise testing for heart failure evaluation and staging, except in patients who were in atrial fibrillation (software could not evaluate presence of MTWA in the setting of atrial fibrillation), had a left ventricular ejection fraction ≥40%, or had prior implantation of pacemaker or implantable cardioverter defibrillator (ICD). Patients with implanted devices did not have routine collection of MTWA for two reasons. Firstly, ventricular pacing was thought to obscure native MTWA.8,9 Secondly, at the time that these studies were done physicians generally viewed MTWA as a risk assessment tool for ventricular arrhythmic events (though this has since been disproven2,10). As such the stress laboratory made a clinical decision to systematically evaluate MTWA only in patients who did not have ventricular pacing or an ICD.
We also excluded patients who had: age <18 years, end-stage renal disease, cancer, congenital heart disease, history of prior cardiac transplantation, or absence of US Social Security number. In patients who had more than one metabolic exercise test, we considered only the initial study. We prospectively recorded data on a customized computer database. The study was approved by the institutional review board at the Cleveland Clinic, and the requirement for obtaining informed consent was waived.
MTWA and metabolic stress testing
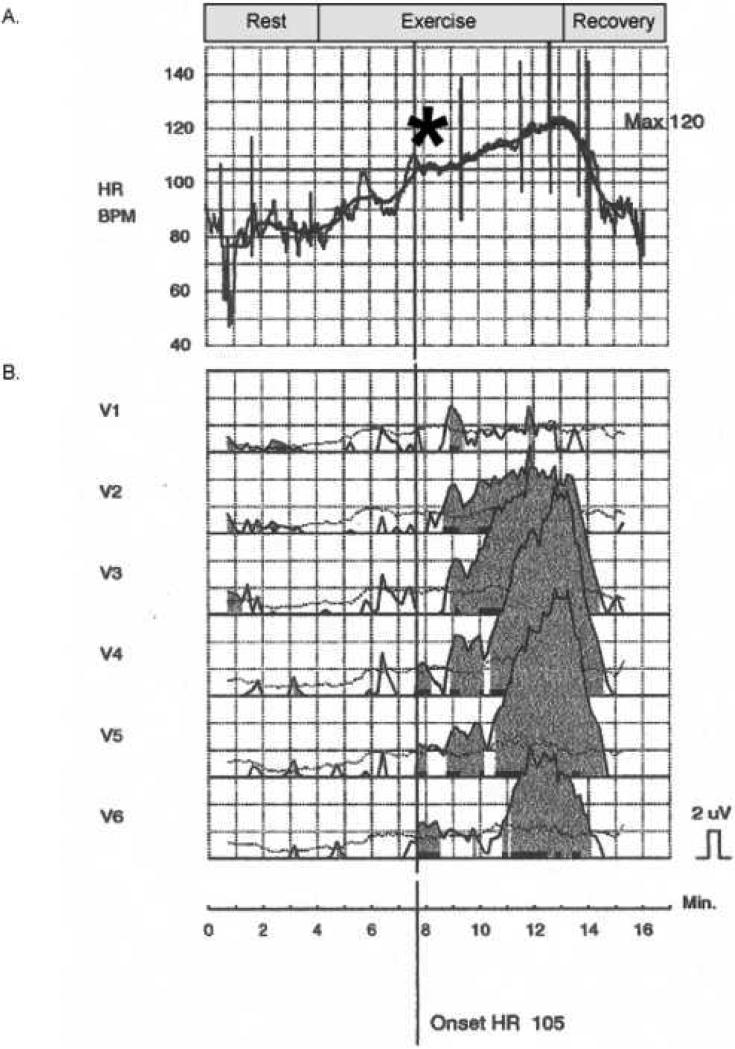
All patients underwent simultaneous metabolic stress testing and MTWA testing by treadmill exercise using high-resolution electrodes (Cambridge Heart, Bedford, Massachusetts). An example of the protocol in a patient who had abnormal MTWA is shown in Figure 1. MTWA measurements were collected throughout exercise and recovery, and automatically interpreted by the Heartwave system, version 1.34 (Cambridge Heart). To optimize MTWA collection an attempt was made with all patients to set the initial exercise intensity to achieve a heart rate of 105-110. Automatic MTWA interpretations were confirmed or over-read by trained board certified cardiologists, and classified according to conventional criteria 11. According to prior conventions 9,11-14, all comparisons were made between patients with normal (negative) and abnormal (positive or indeterminate) MTWA tests.
Figure 1.
Heart rate (HR) and MTWA tracings collected during a metabolic stress test. (A.) Intensity of exercise is increased slowly to achieve a target HR of 105-110 beats/min for a period of approximately 1-2 minutes (asterisk), during which MTWA are collected. Intensity is then increased per exercise protocol. Exercise ends with patient exhaustion. (B.) MTWA tracings (microvolt) in leads V1-V6. Dark shaded tracings represent appearance of MTWA. ‘Onset HR’ refers to the heart rate at which MTWA are first detected. Horizontal boxes represent 1 minute intervals.
Metabolic stress testing methods in our laboratory have been described previously 15. Before each test, a structured interview and chart review yielded data on demographics, left ventricular ejection fraction, medications, and various other clinic variables 15,16. Patients were instructed to take their cardiac medications per routine, including beta-blockers, prior to stress testing. Symptom-limited metabolic stress testing was performed using the Modified Naughton or Cornell protocols, and recorded on a MedGraphics cardiopulmonary system. Data on symptoms, rhythm, and blood pressure, were collected prospectively during each stage of exercise. Oxygen consumption (VO2) and other exercise and ventilatory measurements were recorded every 30 seconds during rest, exercise, and recovery. The ventilatory response to exercise was defined as the value of VE/VCO2 at peak exercise 16.
End points
The primary end point was a composite of all-cause mortality and UNOS Status 1 cardiac transplantation (whichever came first). All-cause mortality, a clinically relevant and totally unbiased end-point 17, was determined by the Social Security Death Index 18,19. Previously we have shown that this method has a sensitivity of 97% for detecting death 20. Urgent cardiac transplantation was defined as patients who were listed as United Network of Organ Sharing (UNOS) Status 1 (i.e. requiring continuous infusion of intravenous inotropes or mechanical circulatory support) 21. Patients who received a cardiac transplant while listed as UNOS Status 2 (n=6) were censored.
Statistical analyses
Differences between patients with and without abnormal MTWA were compared with the use of Student's t-test and Wilcoxon rank sum test for continuous variables, and the chi-square test for categorical variables. For descriptive purposes, we constructed Kaplan-Meier plots 22 of time free of a primary endpoint according to MTWA and peak VO2.
To account for potential confounding we used two approaches. We constructed Cox proportional hazards models, including as covariates peak VO2 as a continuous variable, age, gender, and history of coronary artery disease. We only included a limited number of covariates to avoid model over-fitting. Ejection fraction and symptomatic functional class were not included due to their limited variability in our cohort. Additionally, we have previously shown that ejection fraction was not an independent predictor of risk in a similar patient population 23. Possible nonlinear associations were tested with restricted cubic splines 24. The proportional hazards assumption was tested by scaled Schoenfeld residuals and inspection of hazard ratio plots over time 25. Model calibration was excellent, as assessed by constructing a plot of actual versus predicted survival in 100 bootstrap samples at 4 years 25. Model discrimination was assessed by calculating 100 bootstrapped estimates of the concordance index for time-to-event outcomes 25. This yielded a concordance index of 0.75, indicating a strong discrimination. The discriminative accuracy of this model was compared to that of smaller models containing MTWA alone, peak VO2 alone, and MTWA and peak VO2 in combination using the concordance index (c-index) 26,27.
In order to account for a large number of confounders without risking model over-fitting, we employed propensity score methods 28-30. We generated a propensity score using nonparsimonious multivariable logistic regression including all the variables listed in Table 1; this score is effectively the probability that any given patient would have an abnormal MTWA given the values of all other variables. The QRS width variable was log transformed due to its skewed distribution. This model yielded a c statistic of 0.79, indicating a strong ability to differentiate between those with normal and abnormal MTWA. We used a publicly available SAS macro 31 to match patients with abnormal MTWA to unique patients with normal MTWA according to their propensity scores 32. We performed a second Cox analysis within the matched cohort in which MTWA was adjusted for the propensity score.
Table 1.
Baseline Characteristics according to TWA findings
| Entire cohort | Propensity matched cohort | |||||
|---|---|---|---|---|---|---|
| Normal MTWA (n=167) | Abnormal MTWA (n=136) | P Value | Normal MTWA (n=76) | Abnormal MTWA (n=76) | P Value | |
| Demongraphics | ||||||
| Age, mean (SD), y | 49 (11) | 52 (11) | .02 | 51 (10) | 51 (12) | .88 |
| Body mass index, mean (SD), kg/m2 | 29 (6) | 29 (6) | .57 | 28 (5) | 30 (6) | .18 |
| Men, No. (%) | 97 (58) | 102 (75) | .002 | 50 (66) | 51 (67) | .86 |
| White, No. (%) | 141 (84) | 98 (72) | .009 | 62 (82) | 56 (74) | .24 |
| Clinical history, No. (%) | ||||||
| Diabetes | 21 (13) | 41 (30) | <.001 | 12 (16) | 15 (20) | .52 |
| Hypertension | 90 (54) | 75 (55) | .83 | 38 (50) | 38 (50) | NS |
| Current or prior smoker | 93 (56) | 86 (63) | .18 | 48 (63) | 46 (61) | .74 |
| History of coronary artery disease | 53 (32) | 55 (40) | .12 | 26 (34) | 24 (32) | .73 |
| History of Myocardial infarction | 36 (22) | 41 (30) | .09 | 16 (21) | 14 (18) | .68 |
| History of coronary artery bypass graft surgery | 21 (13) | 26 (19) | .12 | 11 (14) | 10 (13) | .81 |
| History of percutaneous coronary intervention | 26 (16) | 21 (15) | .98 | 11 (14) | 10 (13) | .81 |
| Medication use, No. (%) | ||||||
| Aspirin | 95 (57) | 79 (58) | .83 | 46 (61) | 41 (54) | .41 |
| Beta-blockers | 148 (89) | 123 (90) | .61 | 68 (89) | 69 (91) | .79 |
| Angiotensin-convering enzyme inhibitors or Angiotensin II receptor blockers | 157 (94) | 123 (90) | .24 | 71 (93) | 72 (95) | .73 |
| Digoxin | 80 (48) | 98 (72) | <.001 | 52 (68) | 48 (63) | .49 |
| Diuretics | 122 (73) | 121 (89) | .001 | 61 (80) | 65 (86) | .39 |
| Statins | 56 (34) | 65 (48) | .012 | 30 (39) | 28 (37) | .74 |
| Calcium channel blockers | 6 (4) | 7 (5) | .51 | 5 (7) | 2 (3) | .25 |
| Cardiovascular assessment | ||||||
| Systolic blood pressure, mean (SD), mmHg | 111 (18) | 110 (19) | .64 | 111 (18) | 111 (17) | .71 |
| Diastolic blood pressure, mean (SD), mmHg | 75 (13) | 72 (12) | .09 | 75 (14) | 72 (12) | .35 |
| Left ventricular ejection fraction, mean (SD), % | 23 (8) | 20 (8) | <.001 | 21 (6) | 21 (9) | .35 |
| Peak Exercise | ||||||
| Heart rate | 142 (20) | 128 (27) | <.001 | 134 (17) | 137 (25) | .42 |
| Systolic blood pressure | 151 (27) | 142 (31) | .003 | 150 (30) | 148 (30) | .47 |
| Diastolic blood pressure | 79 (14) | 76 (13) | .13 | 78 (14) | 78 (13) | .68 |
| Peak oxygen consumption, mean (SD), ml/kg/min | ||||||
| Men | 23.7 (7.4) | 19.9 (6.6) | <.001 | 22.2 (7.0) | 22.5 (7.5) | .97 |
| Women | 18.7 (4.7) | 15.5 (3.7) | <.001 | 17.5 (2.8) | 16.3 (3.6) | .12 |
| Respiratory exchange ratio, mean (SD) | 1.06 (0.10) | 1.03 (0.11) | 0.025 | 1.04 (0.11) | 1.04 (0.11) | .85 |
| Ventilatory response to exercise, mean (SD) | 29.7 (8.2) | 32.7 (9.1) | .003 | 30.2 (7.5) | 30.9 (8.4) | .80 |
| Chronotropic Response, mean (SD) | 0.71 (0.19) | 0.58 (0.23) | <.001 | 0.63 (0.18) | 0.65 (0.21) | .44 |
| Frequent ventricular ectopic activity during recovery, number (%) | 11 (7) | 24 (18) | .003 | 6 (8) | 10 (13) | .29 |
| Heart rate recovery, mean (SD) | 15 (9) | 11 (10) | <.001 | 14 (8) | 14 (10) | .99 |
| QRS width, mean (SD), msec | 118 (29) | 123 (29) | .07 | 118 (30) | 119 (29) | .75 |
Data assembly and basic statistical comparisons were performed with Stata/SE version 9.2 (StataCorp). Propensity analysis was performed using Lori Parson's GREEDY 5→1 Digit Match Macro 31 on SAS version 9.1 (SAS, Inc.). Logistic regression, Kaplan-Meier calculations, Cox proportional hazards analyses, and model assessment were performed with Harrell's Design and Hmisc libraries 25 using R version 2.4.1 (www.r-project.org).
Results
Characteristics at baseline and during exercise
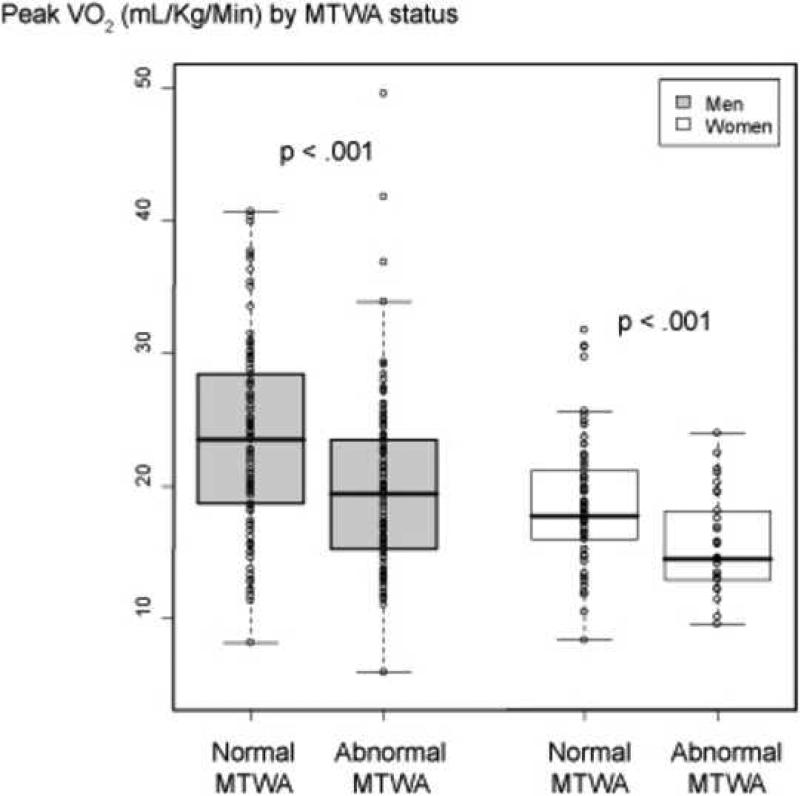
There were 303 patients eligible for analysis, of whom 136 (45%) had abnormal MTWA (84 positive tests and 51 indeterminate tests). Clinical and exercise data, according to MTWA status, are shown in Table 1. The median peak VO2 for all patients was 19.5 ml/kg/min (25th to 75th percentile of 15.4 ml/kg/min to 24 ml/kg/min). Women had lower peak VO2 than men 33. However, regardless of gender, the group with abnormal MTWA had significantly lower peak VO2 (Figure 2).
Figure 2.
Box and whisker plots of Peak VO2 by MTWA status. Circles represent individual patients.
MTWA and mortality or urgent cardiac transplantation
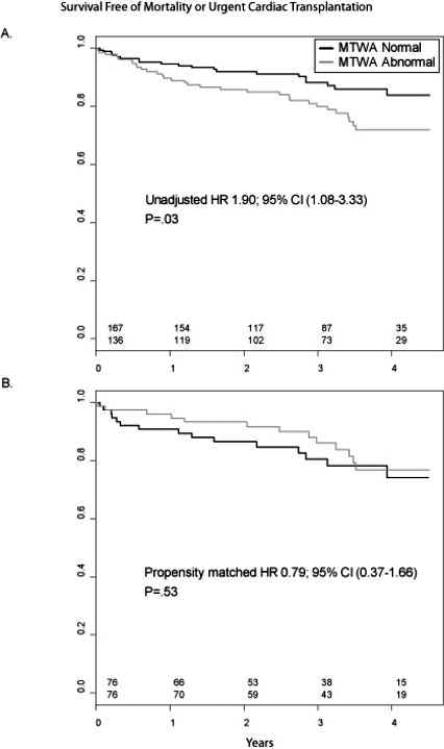
During a mean follow-up time of 2.8 years (range 0.6-4.5 years), there were 34 deaths and 17 UNOS Status 1 cardiac transplants. Abnormal MTWA had a significant unadjusted association with the composite end point (Figure 3A). There were multiple statistically significant differences between the normal and abnormal MTWA groups (Table 1) which may have accounted for this unadjusted finding (i.e., confounding). The patients in the abnormal MTWA group were older white males, had a higher prevalence of diabetes, and a higher use of digoxin, diuretics, and statins. Further this group had worse exercise test characteristics including lower peak exercise heart rate and blood pressure, worse peak oxygen consumption, poorer chronotropic response, and lower heart rate recovery.
Figure 3.
Kaplan-Meier plots relating MTWA status to survival. (A.) Unadjusted full cohort (n=303). (B.) Propensity matched cohort (n=152).
MTWA and peak VO2
Table 2 summarizes outcome based on MTWA status. In an unadjusted Cox analysis, MTWA was strongly associated with the outcome. After adjusting for three clinical variables (age, gender, history of CAD) the association remained statistically significant. This is consistent with findings of other studies of MTWA and outcomes 1,5. Finally, after adding peak VO2 to the model, the association between MTWA and the outcome was no longer statistically significant (HR 1.18 [95% CI 0.64 to 2.17], p=.60), implying that peak VO2 confounded the relationship.
Table 2.
Association of Microvolt T-Wave Alternans with Mortality or Urgent Cardiac Transplantation: Results of Proportional Hazards Analyses
| Model | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Unadjusted | 1.90 (1.08, 3.33) | .03 |
| Adjusted for clinical variables* | 1.89 (1.05, 3.39) | .03 |
| Adjusted for clinical variables* and peak VO2 | 1.18 (0.64, 2.17) | .60 |
| Propensity matched cohort^ | 0.79 (0.37, 1.66) | .53 |
Clinical variables included age, gender, and history of prior coronary artery disease
Fully adjusted for all 28 variables (see methods, and Table 1)
Table 3 summarizes the discriminative accuracy of multiple models containing MTWA and/or peak VO2. The c-index is a measure of discrimination analogous to the area under an ROC curve. The c-index for MTWA alone was 0.57, indicating its predictiveness was only slightly better than chance (0.5). The c-index for peak VO2 alone was 0.74. The combination of MTWA and peak VO2 yielded a c-index of 0.74, while the full model yielded only a slightly higher c-index of 0.75.
Table 3.
Discriminative Accuracy of Cox-Proportional Hazards Models
| Variables in Model | Concordance Index |
|---|---|
| MTWA | 0.57 |
| Peak VO2 | 0.74 |
| MTWA, Peak VO2 | 0.74 |
| MTWA, Peak VO2, and clinical variables * | 0.75 |
Clinical variables included age, gender, and history of prior coronary artery disease
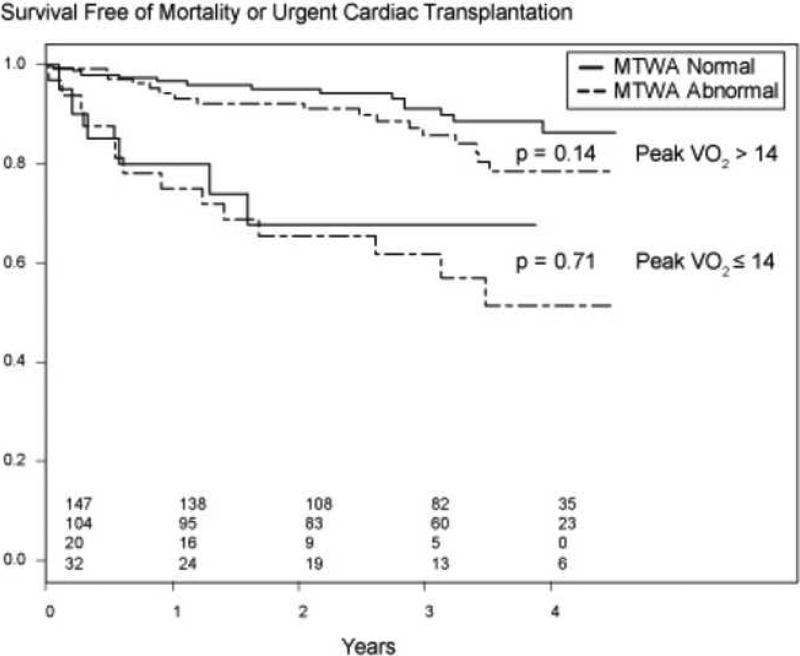
To test the prognostic contribution of MTWA to peak VO2 in a clinically meaningful manner we constructed a Kaplan-Meier plot of the variables with peak VO2 dichotomized at 14. Figure 4 demonstrates that MTWA status did not add incremental prognostic value to peak VO2. The cutoff of 14 was based on work by Mancini and colleagues 34.
Figure 4.
Kaplan-Meier plots relating peak VO2 to survival, stratified by MTWA status.
Propensity matched analysis
We were able to match successfully 76 patients with abnormal MTWA to 76 unique normal MTWA patients. The c-statistic for the logistic model was 0.80, indicating strong discrimination. There were no differences in baseline characteristics amongst the propensity matched patients (Table 1). In this cohort there was no significant association between MTWA status and outcome (HR 0.79 [95% CI 0.37 to 1.66], p=.53) (Table 2, Figure 3B).
Discussion
Main Findings
We report the largest study to date examining the association between MTWA, peak VO2, and outcome in patients with severely impaired left ventricular systolic function. Further, our cohort is amongst the largest MTWA studies to date 5. Our study design was unique in that patients had MTWA collected simultaneous to metabolic stress testing. Patients with abnormal MTWA had a significantly lower peak VO2. We found that abnormal MTWA predicted poor outcome wholly consistent with prior reports 1, though it is critically important to note that the patients with abnormal MTWA had multiple high risk characteristics as compared to those with normal MTWA, that may have accounted for this result. However, once we considered peak VO2, as well as other potential confounders, MTWA was no longer predictive. This suggests that peak VO2 confounds the association between abnormal MTWA and mortality or urgent cardiac transplantation. We believe our results are valid because we used multivariable methods as well as propensity analysis methods to carefully match patients in an effort to eliminate confounding as best as possible.
Indeterminate MTWA Tests
The pathophysiological basis for the association between abnormal MTWA and low peak VO2 is unclear. A possible link may be indeterminate MTWA tests. In a sub-study of the T-Wave Alternans in Congestive Heart Failure Study, Kaufman and colleagues 14 demonstrated that greater than 83% of indeterminate tests are due to either chronotropic incompetence (i.e. an inability raise HR > 105) or high levels of ventricular ectopy – both of which have been reported to be associated with low peak VO2 in patients with heart failure 23,35-37. In a supplementary analysis of our study we found that patients with indeterminate MTWA tests had lower median peak VO2 measurements than patients with positive MTWA tests (15.6 vs. 19.2, p<0.001). The association between indeterminate tests and outcome was not significant after adjustment for peak VO2, but extrapolation from this result is difficult due to the small sample sizes. The possibility that positive but not indeterminate MTWA tests may add incremental prognostic information to peak VO2 should be investigated in future studies.
Prognosis
To our knowledge, there have been no large prior studies evaluating the prognostic value of abnormal MTWA and low peak VO2 side by side. Two small studies 6,7 (n=46, n=73) addressing this question had limited end points (n=7, n=8), thus significantly limiting their power to perform robust adjusted analyses. Our study had significantly more patients (n=303) and end points (n=51). In the recently published ALPHA study 38 examining the prognostic value of MTWA in patients with non-ischemic cardiomyopathy, only 69% of the patients had their peak VO2 measured. No attempt was made to adjust for this variable. Ours is the first study to report a lack of incremental prognostic value of abnormal MTWA beyond low peak VO2.
Selection bias?
The patients in this analysis did not have ICDs, raising the question whether this approach selected for lower risk individuals thus biasing our findings. Results from two recently published studies suggest that this may not be the case. In the MASTER trial 2 575 patients with impaired LV function (mean EF 24%) had MTWA testing prior to ICD implantation. After a mean follow-up of 2.1 years there was no association between a non-negative MTWA test result and ventricular arrhythmic events (HR 1.26, 95% CI [0.76 to 2.09], p=0.37), although mortality was significantly increased (HR 2.04, 95% CI [1.10 to 3.78], P=0.02). In the SCD-HeFT T-Wave Alternans substudy10 490 patients with heart failure and impaired LV function had MTWA testing and were then randomized to placebo, amiodarone, or ICD for a combined end point of sudden cardiac death, sustained ventricular tachycardia/fibrillation, or appropriate ICD discharge. During a mean follow-up of 30 months, there were no significant differences in the primary end point (HR 1.28, 95% CI [0.65 to 2.53], P=0.46), though there was a non-significant trend towards increased mortality. These findings of increased mortality risk without differential arrhythmic risk by MTWA status in patients with ICDs somewhat reduces the concern for selection bias in our analysis. To appropriately address this question future studies examining the association between MTWA and peak VO2 should enroll patients with ICDs.
Limitations
Our study has several limitations. It was an observational, single center experience, and did not include patients with ICDs. Prior reports have primarily demonstrated the utility of abnormal MTWA in predicting ventricular arrhythmias and sudden cardiac death. It may be that MTWA was not associated with the composite end point in our study because our population was at a relatively low risk for arrhythmic outcomes: low prevalence of ischemic heart disease, relatively young age, and no ICDs. Our findings should not be generalized to patients with ICDs as we did not study this population. Further, we used an end point that included urgent cardiac transplantation (approximately one third of the outcomes), which is a non-arrhythmic endpoint. Future studies of the association between MTWA and peak VO2 should include patients who are at higher risk for ventricular arrhythmias. It is unknown how many patients went on to receive an ICD after their initial stress test. We did not have data on cause of death or arrhythmic outcomes and as such could not account for these as an end-point.
We used a composite end point of death and UNOS 1 cardiac transplantation. The first is a clinical outcome while the second is a clinical decision. If the clinical decision of transplantation were influenced in any way by either the peak VO2 or MTWA outcomes then the associations we observed are biased. We feel however that this is unlikely as UNOS 1 status is assigned based on acute severe clinical deterioration rather than any prognostic tools used in clinically stable heart failure patients.
We used non-standard metabolic stress testing protocols to allow simultaneous collection of MTWA and peak VO2 information (Figure 1). As such, because of patient fatigue in the setting of a prolonged protocol, the measured peak VO2 may have been an underestimate of the true peak VO2.
Despite the size of our cohort and the relatively long follow-up time, we only noted 51 events, limiting our ability to perform robust analyses considering many covariates. We used propensity methods to overcome this 28, but this approach is limited by its focus on those covariates actually measured and recorded.
Conclusions
Despite these limitations, we found that among patients with severely impaired left ventricular systolic function the presence of abnormal MTWA was associated with low peak VO2 and predicted poor outcome by itself. However, MTWA no longer predicted poor outcome once peak VO2 was accounted for. Our findings are hypothesis generating. Further research is needed to determine what pathophysiological abnormalities link abnormal MTWA to impaired VO2.
Acknowledgments
Theresa Guy and the exercise physiologists at Cleveland Clinic stress lab for their meticulous, consistent, and tireless work
Funding: National Heart, Lung, and Blood Institute grant CAN# 8324207
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Myles RC, Jackson CE, Tsorlalis I, Petrie MC, McMurray JJ, Cobbe SM. Is microvolt T-wave alternans the answer to risk stratification in heart failure? Circulation. 2007;116:2984–91. doi: 10.1161/CIRCULATIONAHA.107.699918. [DOI] [PubMed] [Google Scholar]
- 2.Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, Brown ML, Pu W, Benditt DG. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52:1607–15. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Mancini D, LeJemtel T, Aaronson K. Peak VO(2): a simple yet enduring standard. Circulation. 2000;101:1080–2. doi: 10.1161/01.cir.101.10.1080. [DOI] [PubMed] [Google Scholar]
- 4.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–81. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 5.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005;46:75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 6.Sarzi Braga S, Vaninetti R, Laporta A, Picozzi A, Pedretti RF. T wave alternans is a predictor of death in patients with congestive heart failure. Int J Cardiol. 2004;93:31–8. doi: 10.1016/s0167-5273(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 7.Baravelli M, Fantoni C, Rogiani S, Farina S, Anza C, Caltabiano V, Forzani T, Salerno-Uriarte JA. Combined prognostic value of peak O(2) uptake and microvolt level T-wave alternans in patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 2007;121:23–9. doi: 10.1016/j.ijcard.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS, Fontaine JM. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT., Jr. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–9. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 10.Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, Lee KL, Bardy GH. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118:2022–8. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol. 2002;13:502–12. doi: 10.1046/j.1540-8167.2002.00502.x. [DOI] [PubMed] [Google Scholar]
- 12.Hohnloser SH, Ikeda T, Bloomfield DM, Dabbous OH, Cohen RJ. T-wave alternans negative coronary patients with low ejection and benefit from defibrillator implantation. Lancet. 2003;362:125–6. doi: 10.1016/s0140-6736(03)13865-2. [DOI] [PubMed] [Google Scholar]
- 13.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. J Am Coll Cardiol. 2003;41:2220–4. doi: 10.1016/s0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman ES, Bloomfield DM, Steinman RC, Namerow PB, Costantini O, Cohen RJ, Bigger JT., Jr. “Indeterminate” microvolt T-wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;48:1399–404. doi: 10.1016/j.jacc.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation. 2005;111:2313–8. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 16.Robbins M, Francis G, Pashkow FJ, Snader CE, Hoercher K, Young JB, Lauer MS. Ventilatory and heart rate responses to exercise : better predictors of heart failure mortality than peak oxygen consumption. Circulation. 1999;100:2411–7. doi: 10.1161/01.cir.100.24.2411. [DOI] [PubMed] [Google Scholar]
- 17.Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–20. doi: 10.1016/s0735-1097(99)00250-8. [DOI] [PubMed] [Google Scholar]
- 18.Curb JD, Ford CE, Pressel S, Palmer M, Babcock C, Hawkins CM. Ascertainment of vital status through the National Death Index and the Social Security Administration. Am J Epidemiol. 1985;121:754–66. doi: 10.1093/aje/121.5.754. [DOI] [PubMed] [Google Scholar]
- 19.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4:233–7. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. Jama. 2000;284:1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 21.United Network For Organ Sharing [Apr 19, 2007];Policy 3.7: Organ Distribution: Allocation of Thoracic Organs. 2006 Dec 14; http://www.unos.org/PoliciesandBylaws2/policies/pdfs/policy_9.pdf.
- 22.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assn. 1958:457–481. [Google Scholar]
- 23.O'Neill JO, Young JB, Pothier CE, Lauer MS. Severe frequent ventricular ectopy after exercise as a predictor of death in patients with heart failure. J Am Coll Cardiol. 2004;44:820–6. doi: 10.1016/j.jacc.2004.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE., Jr. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer-Verlag; New York, NY: 2001. [Google Scholar]
- 26.Harrell FE, Jr., Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247:2543–6. [PubMed] [Google Scholar]
- 27.Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95:634–5. doi: 10.1093/jnci/95.9.634. [DOI] [PubMed] [Google Scholar]
- 28.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–5. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 29.D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 31.Parsons L. [June 29, 2008];Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Available at: http://www2.sas.com/proceedings/sugi26/p214-26.pdf.
- 32.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: A propensity analysis. Jama. 2001;286:1187–94. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]
- 33.Hsich E, Chadalavada S, Krishnaswamy G, Starling RC, Pothier CE, Blackstone EH, Lauer MS. Long-term prognostic value of peak oxygen consumption in women versus men with heart failure and severely impaired left ventricular systolic function. Am J Cardiol. 2007;100:291–5. doi: 10.1016/j.amjcard.2007.02.096. [DOI] [PubMed] [Google Scholar]
- 34.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr., Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 35.Lauer MS, Mehta R, Pashkow FJ, Okin PM, Lee K, Marwick TH. Association of chronotropic incompetence with echocardiographic ischemia and prognosis. J Am Coll Cardiol. 1998;32:1280–6. doi: 10.1016/s0735-1097(98)00377-5. [DOI] [PubMed] [Google Scholar]
- 36.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. Jama. 1999;281:524–9. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 37.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–90. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 38.Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, Libero L, Pettinati G, Molon G, Curnis A, Occhetta E, Morandi F, Ferrero P, Accardi F. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. J Am Coll Cardiol. 2007;50:1896–904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]