Abstract
People with Parkinson’s disease (PD) suffer from progressive impairment in their mobility. Locomotor and balance dysfunction that impairs mobility in PD is an important cause of physical and psychosocial disability. The recognition and evaluation of balance dysfunction by the clinician is an essential component of managing PD. In this review, we describe a framework for understanding balance dysfunction in PD to help clinicians recognize patients that are at risk for falling and impaired mobility.
Keywords: balance, gait, posture, Parkinson’s disease
Introduction
Falls in the elderly and in people with Parkinson’s disease (PD) have a multifactorial causation but balance impairment is a major contributor to this important problem.1 Falls and impaired mobility are frequent sources of disability and impact quality of life in individuals affected by PD. The resultant loss of independence and the costs of injuries significantly add to the healthcare expenditure associated with PD.2 Thus, the evaluation and early recognition of balance dysfunction by the clinician is an essential component of managing PD.
Balance control and postural control are often used interchangeably. Postural control aligns the body with respect to gravity, the support surface and the visual environment and stabilizes the center of mass (CoM) of the body relative to its base of support during daily activities.3
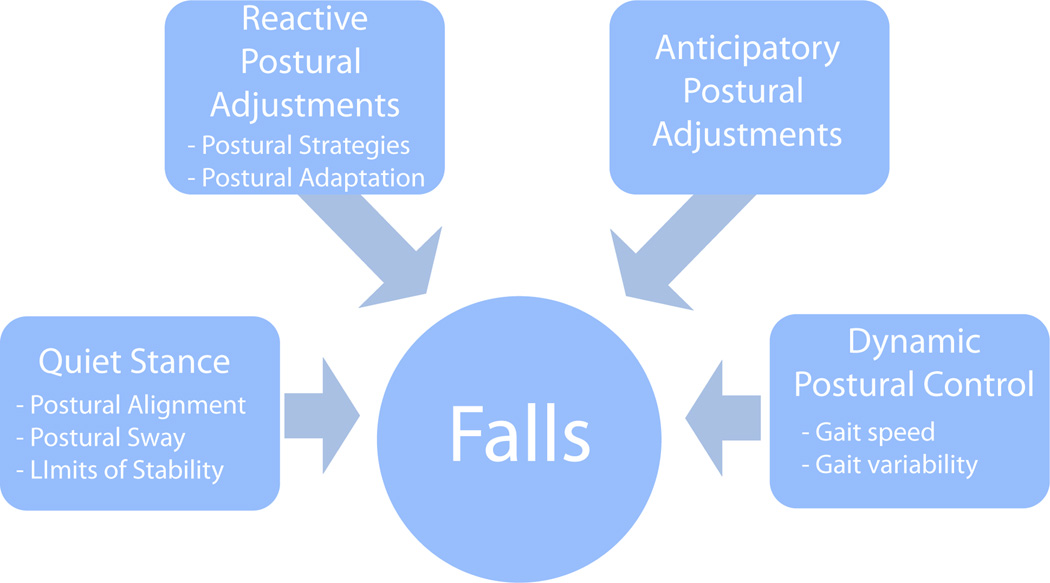
In this review, we describe a framework for understanding balance dysfunction in PD. As shown in Figure 1, balance dysfunction can be characterized by 4 main postural control systems: 1) balance during quiet stance, 2) reactive postural adjustments to external perturbations, 3) anticipatory postural adjustments in preparation for voluntary movements, 4) dynamic balance during movements such as walking. We also summarize the effects of cognitive dysfunction, levodopa and DBS on postural stability in PD.
Figure 1.
Framework for balance dysfunction. Summarized here are the four domains that contribute to postural stability including quiet stance, reactive postural adjustments, anticipatory postural adjustments and dynamic balance.
Balance during quite stance
Postural alignment
In order to achieve postural control, the body must be oriented with respect to gravity, the support surface, the environment and the task at hand.3 As PD progresses, patients stand with an increasingly narrow stance, stooped posture with rounding of the shoulders and flexion of the hips and knees, reflecting increased flexor tone.4 This classic stooped posture is the most common postural deformity observed in individuals with PD. Interestingly, when healthy people voluntarily assume this flexed posture, their postural stability especially in the backwards direction becomes impaired.56 The etiology of the postural misalignment in PD is not clear. Background muscle tone is larger than normal in people with PD, especially in flexor muscles, which could contribute to their flexed posture.4 A tilted or inaccurate internal representation of verticality could also result in postural alignment that is not aligned with gravity.3 This altered sense of verticality may relate to impaired proprioception and affect the position of the body center of mass over the base of support, making patients more vulnerable to falls.78 In addition to the characteristic flexed posture, up to one third of PD patients have deformities of their neck or trunk that may include camptocormia, anterocollis, Pisa syndrome and scoliosis.9–12 As reviewed by Doherty et al, many etiologies may exist for these more marked trunk abnormalities. Unpublished data from our laboratory indicates that abnormal postural alignment need not signify abnormal balance or postural responses.
Sway
During quiet stance, the center of mass is located within a base of support defined by the feet. However, the body is not entirely still, there is continuous movement of the center of mass termed “postural sway”. Postural sway represents a state of complex sensorimotor control loops that contribute to balance control.13
Sway can be measured by a force plate, that detects fluctuations of the center of pressure (CoP), or by accelerometers that detect fluctuations of the body center of mass (CoM).14 The variation in sway can be characterized by a number of variables such as sway area, velocity, frequency and maximum direction of sway.15
Depending on the size of the base of support, more or less postural sway can be tolerated. For example, less sway of the body CoM is tolerated when attempting to balance on one foot compared to standing with two feet far apart. Sway during quiet stance is increased with aging and more so in elderly people who fall.16 Sway is also increased in various neurological disease affecting sensory and motor systems including multiple sclerosis,17 cerebellar disease,18 peripheral neuropathies19 and stroke.20 Increased sway, particularly in the medio-lateral direction is associated with falls in a number of conditions, including PD.21–23
Postural sway can be abnormal in persons with PD, long before clinically evident balance impairment and prior to taking levodopa.14,24,25 In PD, postural sway tends to be of higher velocity, higher frequency, and larger in the lateral direction than in normal controls. Although closing the eyes while standing quietly increases sway in most people, postural sway with eyes closed is significantly different between patients with PD and age-matched controls.14,24
Standing balance is commonly thought to be an automatic process, largely independent of cortical control. However, performing another cognitive or motor task while standing, so called “dual-tasking,” increases postural sway area and velocity, especially in patients with PD.26This observation indicates that higher cortical function is important in maintaining balance even when standing quietly.27,26
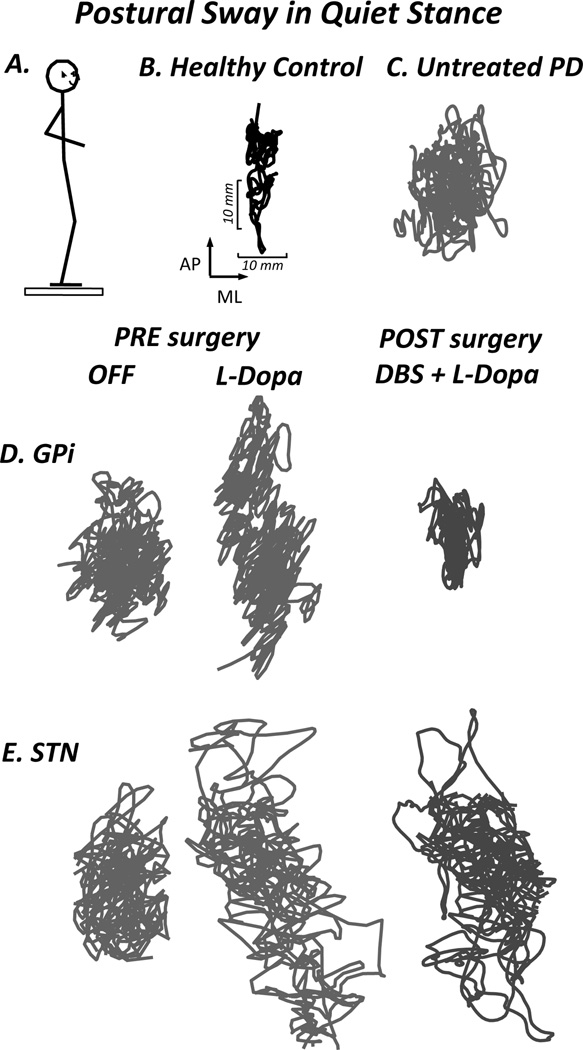
Treatment with levodopa increases postural sway in patients with advanced PD.28 Some of the increase in sway may be attributed to levodopa-induced dyskinesia.29 Levodopa-induced dyskinesia may, in fact, be part of the reason that PD patients fall when “ON.”30 Pallidotomy or deep brain stimulation (DBS) with electrodes targeting the globus pallidus interna (GPi) decreases sway, whereas more variable effects on sway are reported for electrodes in the subthalamic nucleus (STN) (Figure 2).31,25,32
Figure 2.
Postural sway during quiet stance. A. Subject standing on a foreceplate that measures fluctuations of CoP (postural sway), B. Health Control subject, C. Subject with early PD with large sway area prior to initiation of treatment, D. Subject before and after DBS in the GPi, demonstrating a reduction in sway area following surgery, E. Subject before and after DBS in the STN, showing no significant change following surgery. (St George, et al, 2011).
Sensory weighting
The ability to maintain a stable upright stance depends upon a complex integration of somatosensory, vestibular and visual stimuli with motor, premotor, and brainstem systems. In a healthy person, the somatosensory system contributes 70% to control of postural sway, whereas the vestibular and visual systems contribute 20% and 10% respectively.33 Sensory re-weighting is an adaptive process that changes dependence upon different sources of sensory input when the environmental conditions change. For example, when standing on an unstable surface with eyes closed, healthy individuals’ vestibular systems can contribute 100% to control postural sway.33 Although they generally have no problem standing with eyes closed, individuals with severe PD often have difficulty standing on an unstable surface with eyes closed.23 However, this does not necessarily mean that they have difficulty using vestibular information for balance.23 Instead, this may indicate an inability to rapidly change sensory weighting for different situations.34,35 In addition, people with PD have difficulty recognizing small changes in surface orientation which suggests reduced proprioception.36
Limits of Stability
The biomechanically determined limits of stability during stance are the maximal displacement of the body center of mass (CoM) in various directions without falling or having to take a step. The extent to which a person can lean toward these biomechanical limits of stability is also affected by the person’s confidence in their ability to return their CoM over their base of support. In PD, these perceived limits of stability are reduced, especially in the forward direction, and movement to the limits of stability are slower.37 The slowness of leaning may be related to fear of falling during this leaning task.38 Levodopa increases the perceived limits of stability and the speed with which they are reached.37 Dynamic limits of stability during walking take into account both the relative position and the velocity of the body center of mass over its base of support.39 Patients with PD actually fall faster than control subjects in response to an external perturbation; their body CoM gets closer to their limits of stability before they reverse CoM displacement with their reactive postural responses.40
Reactive (Automatic) Postural Adjustments
Reactive postural adjustments are movements to recover balance in response to an external perturbation of the body’s CoM. At times, the postural response to a perturbation may be due to a perception of instability rather than to actual disequilibrium.41 Reactive postural adjustments involving corrective stepping movements are tested by the pull test used in the Unified Parkinson’s Disease Rating Scale (UPDRS) or by the Push and Release test.42
Postural Strategies
Balance recovery can be achieved by various combinations of arm, hip, knee and ankle movements. In this review, we focus on three main strategies: 1) The ankle strategy consists of movement about the ankles starting with the distal muscles: tibialis anterior (in response to backward tilt) or gastrocnemius (in response to forward tilt). The ankle strategy is effective to regain upright stability when a person is on a firm surface and the perturbations are small.43 2) The hip strategy, which consists of quick movements about the hips and use of the arms to achieve upright balance, is a more conservative strategy that is commonly used by elderly subjects or in situations with a more precarious support surface. 3) The stepping strategy consists of taking a quick step to place the leg under the falling CoM, is usually accompanied by arm movements, and is the strategy that is used to recover from a large perturbation. Until recently, it was believed that stepping is used when the “in place” strategies above are not sufficient to regain upright balance.3 We now understand that stepping can occur early, when disturbances are small and before limits of stability have been reached. This strategy is also often accompanied by a reaching strategy, in which the arms make contact with a support surface to increase the base of support.44 The ankle and hip strategies have very short, 100 ms latencies, whereas the stepping strategy latency is longer (approximately 250 ms) and is more affected by voluntary intervention.44
Although the latency of ankle strategy postural responses is normal in PD, maximum force is reached more slowly and is reduced, especially when a subject is “OFF”.40 This bradykinetic postural response causes the CoM to be returned to within the limits of stability more slowly or not at all, putting the individual at risk for falls. Abnormal muscle activation with excessive co-contraction and short duration bursting is associated with these weak postural responses.40,45
Stepping responses for postural correction are late and smaller than normal in PD, as can be seen during the backward pull test of the UPDRS or push and release test.42,46 The protective steps in individuals with PD may not be large enough to arrest the movement of the CoM; thus requiring further steps producing propulsion (if the CoM is displaced forward) or retropulsion (if the CoM is displaced backwards). Whereas healthy individuals produce a compensatory step with no or one anticipatory postural response, PD subjects, especially those with freezing of gait, often have several anticipatory postural adjustments before stepping.46 This delays the step, is associated with “trembling of the knees” and increases their risk of falls.47 Similar to voluntary steps, the size of automatically triggered, protective stepping responses are improved by visual cues.48
Adaptation of Postural Responses
Postural responses rapidly adapt to changes in the support surface.49 For example, if a person is standing on a large, firm support surface, an ankle strategy is used to correct for perturbations of the center of mass. If the subject then switches to stand on a narrow support surface, the person immediately adopts a hip strategy because the ankle strategy is ineffective with a narrow base of support. Individuals with PD, however, not only have difficulty switching between strategies, but are also not able to appropriately scale the size their postural responses to the size of the perturbations.34,50,40. The inflexibility of postural responses when the biomechanical conditions change may reflect an important role of the basal ganglia that is disturbed in PD.34,51
Dual-tasking impairs postural responses and thereby balance recovery, especially in older people with neurological disorders like PD.52 The effects of dual-tasking appear to be on the later phases of in-place responses, reducing the generation of peak forces required for stabilization of the body.53 Dual-tasking also impairs stepping to regain balance (Table 1).54
Table 1.
Influences of Levodopa (L-Dopa), DBS in the Subthalamic nucleus (STN) or Globus Pallidus Internus (GPi) and Dual Taking on Balance Systems
| L-Dopa | DBS | Dual Tasking |
||
|---|---|---|---|---|
| STN | GPi | |||
| Quiet Stance | ↑↓ | ↓ | ↑ | ↓ |
| Reactive Postural Adjustments | ↓ | ↓ | ↓ | ↓ |
| Anticipatory Postural Adjustments | ↑ | ↓ | ↓ | ↓ |
| Dynamic Balance | ↑ | ↑ | – | ↓ |
Upward arrows indicate improvement in the balance system, downward arrows indicate worsening of balance and arrows in both directions indicate that improvement and worsening can occur, depending upon stage of disease.
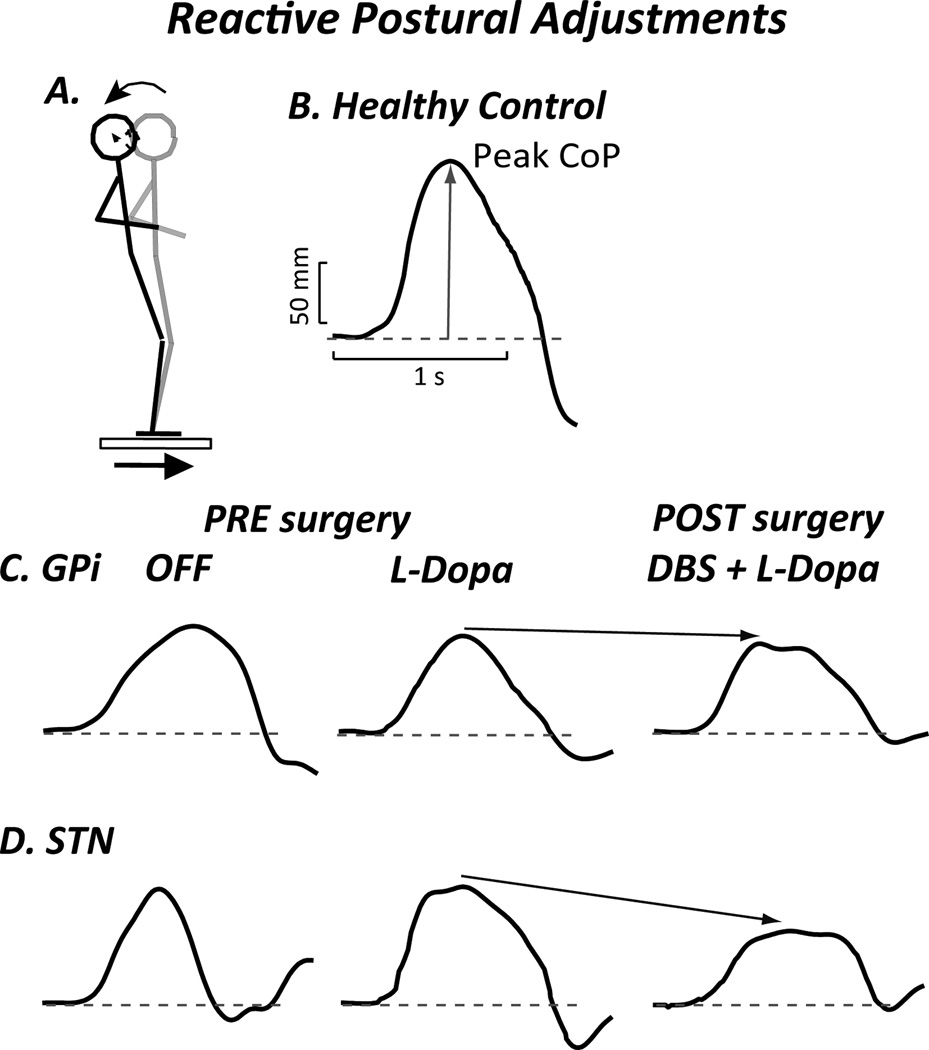
Reactive postural adjustments have a variable responses to levodopa; in some studies they are slightly improved55 and in other studies they may be significantly worsened.56 This variability in response to levodopa may depend upon the severity of PD in the study populations (Table 1). The effects of DBS on postural responses are also variable. In a recent study, reactive postural adjustments in the “OFF” medication/off DBS condition 6 months after surgery were worse than the OFF medication state before surgery for patients with electrodes in the STN; whereas electrodes placed in the GPi did not worsen reactive postural adjustment.55 These effects of DBS on reactive postural adjustments may be part of the reason that falls are increased after DBS in some reports (Figure 3).57,58
Figure 3.
Reactive (Automatic) Postural Adjustment. Although the latency is normal, maximum force is reduced and reached more slowly in subjects with PD, especially when OFF. A. Subject standing on forceplate that is translated forward, resulting in backwards body displacement. B. Center of pressure (CoP) displacement associated with dorsiflexion torque in a healthy control subject. C. CoP displacement in a subject with DBS in the GPi illustrates how levodopa and DBS surgery decrease postural response. D. CoP displacement in subject with DBS in STN shows how postural response decreases significantly following surgery.
Anticipatory Postural Adjustments
Anticipatory postural adjustments (APA) are postural movements that precede voluntary actions when upright, such as raising an arm or taking a step. The APA, in preparation for stepping, moves the body CoM off of the stepping leg and then forward to allow the other leg to be un-weighted so that it can step. APAs function to preserve balance in anticipation of internally-generated perturbations and assist in force generation for the voluntary movement, e.g.; when opening a heavy door.
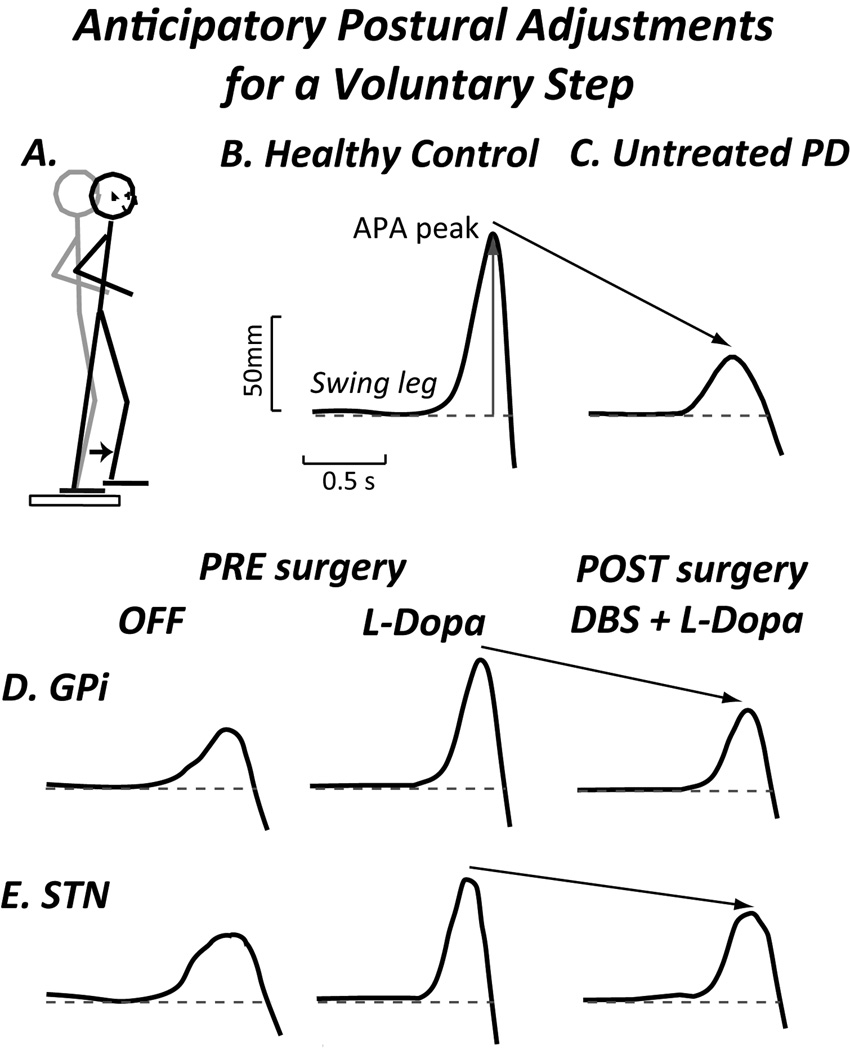
In PD, the magnitude of the APAs for self-initiated walking is reduced, resulting in longer latencies or time until the stepping foot leaves the ground (Figure 4).59 A lateral postural assist reverses this delay in the APA and the step.60 Sensory cueing, leading to externally-initiated gait, increases the magnitude of the APA and allows the swing leg to leave the support surface more quickly.61 Levodopa also increases the size of APA.61 DBS in either the STN or GPi decrease the size of APAs after surgery, compared to before surgery (Table 1).62
Figure 4.
Lateral anticipatory Postural Adjustments (APA) in preparation for a voluntary step. A. Subject standing on a forceplate stepping forward. B. Healthy control subject. C. Early, untreated PD shows smaller APA and longer latency before stepping foot leaves the ground. D. Example of APA in subject with DBS in the GPi illustrates how levodopa increases APA but after DBS surgery APA decreases, even with levodopa. E. Example of subject with DBS in STN shows how levodopa increases APA, but APA decreases following surgery.
Individuals with PD who have freezing of gait (FoG) are generally not actually frozen (akinetic) but rather exhibit “trembling of the knees” that resembles multiple APAs in preparation for stepping.63,64,65,66,47 The knee trembling is the basis for speculation that there may be a problem with coupling the APA with a voluntary movement (a step).47 There is much evidence that the postural adjustments that precede voluntary movements are programmed separately and attached to the voluntary movement later in the process of carrying out the movement.67 When walking, the normal frequency of leg movements is about 1 HZ; whereas trembling movements of the knees occurs at 3 HZ or greater. This trembling, when captured with accelerometers on the legs, is a method to quantify freezing.66,68,69
Dynamic Balance during Walking
Walking poses a large challenge to balance as it requires appropriate foot placement and axial control of lateral and forward stability to control a constantly shifting CoM. With each step, the CoM is not only shifting from side to side to successively un-weight alternate legs, but it is also moving forward beyond the anterior limits of stability.70 This forward instability is arrested by a step that is placed in front of the CoM to keep a person from falling forward. A failure to take a large enough step to arrest the forward movement of the CoM requires additional steps to maintain balance. If the succeeding steps are too small to arrest the forward movement of the CoM, festination or propulsion occurs. If this occurs on backing up, it is termed retropulsion. Propulsion and retropulsion are a frequent cause of falls in advanced PD.
Similar to a person’s perceived limits of stability while standing, gait speed may be a self-imposed compensatory measure related to the balance confidence a person feels while walking.71 Gait speed decreases with age and has been shown to correlate with falls.72 But this slowing may be appropriate; even a healthy, young person slows their gait if walking under conditions where balance is threatened, such as on a slippery floor.
In patients with PD, gait speed is decreased, stride length shortened and double support time (time with both feet on the ground) is increased whereas cadence (steps per minute) remains unchanged and at times, increased.73,74 These changes in gait may reflect bradykinesia, poor balance or fear of falling (FoF) as the patient compensates to prevent falls.71 Reduced trunk rotation and reduced and asymmetrical arm swing are some of the earliest signs of gait dysfunction in untreated PD.75 Lack of arm swing, itself, could impair dynamic balance while walking.76 The challenges to balance during walking are increased by turning or walking around obstacles. Even in the early stages of PD, individuals have turning deficits. Turns are executed more slowly and with multiple steps.75,77,78 The slowing of turns while walking is often where the first abnormalities of gait in PD become apparent in the clinic.
Gait variability is the stride-to-stride variation in stride length, stride time, double support time or step width, measured as the standard deviation or coefficient of variation of the average for each measure.79,80,81 Increased gait variability is associated with falls in the elderly and in PD, suggesting it may result from foot placements needed to correct perturbations to balance.82,83 Gait variability is independent of fear of falling and may, in fact, be the best predictor of falls in the elderly.71 It is speculated that variability in stride length and stride time may reflect dysfunction of locomotor pattern generators; and that variability in double-support time and width of steps reflect neural balance circuits.79,84
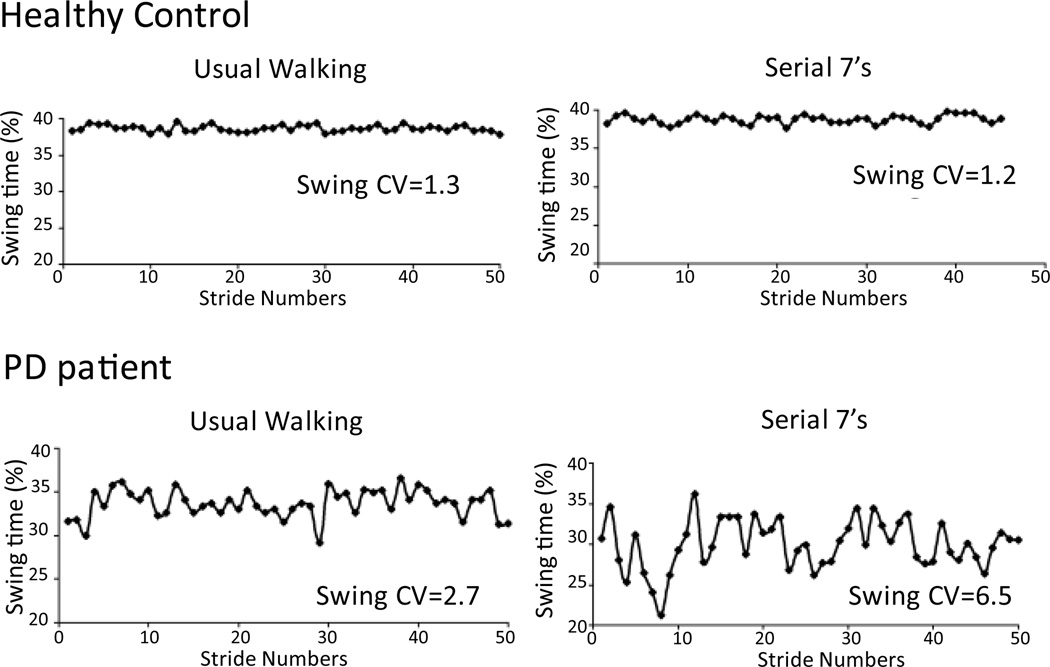
Dual-tasking increases gait variability in older adults and this is particularly marked for elderly with cognitive impairment.83 In PD subjects, dual-tasking decreases gait speed and increases gait variability.85 (Figure 5) Interestingly, this increase (worsening) in variability correlates with impaired executive dysfunction and not with memory impairment in PD subjects.85
Figure 5.
Swing time (as a % of gait cycle) series in a healthy control and in a PD patient under usual walking condition and dual task condition (subtracting backward by 7s). Coefficient of variation (CV=SD/mean) is reported for each condition. (Adapted from Yogev G. et al., European Journal of Neuroscience, 2005)
Both levodopa and DBS increase gait speed.78,86,87 Levodopa also reduces gait variability, presumably enhancing dynamic stability.88,89 DBS in the STN plus levodopa further reduced gait variability.90,87 The reduction in gait variability with DBS is in contrast to the observed increase in falling associated with DBS in some studies.57
Cognitive Function for Balance and Gait
For balance control to be functional in complex daily activities, balance systems need to be modulated by higher cortical centers to adapt posture to the changing environment and goals of a person. This dependence of balance upon cortical control and attention is evident by the degradation of performance of all balance systems with dual tasking (Table 1). For example, asking a patient with PD to maintain their balance while performing serial 7s, increases their postural sway, impairs their postural responses, reduces their anticipatory postural adjustments, and increases their gait variability and slowing.91 The greater impairment of balance control while dual-tasking in PD suggests automatic control is reduced so more conscious attention is required.53
Executive function is increasingly recognized as important for balance control and locomotion.92,93 These studies suggest that the medial frontal cortex may play an important role in inhibiting inappropriate postural motor behavior and promoting set-switching which is essential to postural responses. The observation that cortical function is important to balance opens another potential therapeutic window. Preliminary evidence indicates that elderly people can improve measures of balance with various forms of cognitive therapy.94,52,95
Fear of falling (FoF) occurs more frequently in PD than healthy individuals of similar age, leads to activity restriction and significantly impacts quality of life.96,97 In healthy people FoF or a postural threat, such as standing on a high surface, results in reduced postural sway area, increased sway frequency associated with increased muscle co-activation, reduced anticipatory postural adjustments, and use of a hip, instead of an ankle strategy for postural correction.98 FoF also changes gait patterns by slowing gait speed and increasing duration of double support.99
Conclusion
Identifying patients that are at risk for falling is an essential component of managing PD. Balance control, as examined by sway while standing quietly, responses to perturbations while standing, anticipatory postural responses preceding step initiation and variability of gait are abnormal in PD and have been linked to a propensity to fall.
Levodopa and DBS improve some measures of balance but worsen others. Medical and surgical therapies may affect measures in opposing directions, violating the clinical rule that levodopa predicts the response to DBS (Table 1). The differences in the effects of levodopa and of DBS in STN and GPi suggest that balance is mediated by several distinct circuits and is only partly under the control of dopaminergic influenced motor circuits. Exploring stimulation in different sites in the CNS, such as the pedunculopontine nucleus, or examining the effects of other neurotransmitter systems, such as the cholinergic system, are important for furthering our knowledge of balance dysfunction in PD.
Acknowledgments
Funding sources for this study:
Support from the PADRECC fellowship, National Institute of Aging, National Parkinson Foundation, National Institute of Neurological Disorders and Stroke, Ceregene.
Financial Disclosures (for preceding 12 months):
Bernadette Schoneburg has received support for this study from PADRECC fellowship
Martina Mancini post-doctoral work has been funded by a NIH/NINDS Challenge Grant (RC1 NS068678 Horak (PI) until September 2011), and NIH/NIA Merit Award (R37, AG006457, Horak PI, from September 2011 to March 2012); currently funded by Continuous monitoring of Turning in Patients with Parkinson’s disease (R41 NS07608801-01 Horak (co-PI) and Feasibility of relating continuous monitoring of turning mobility to falls and executive function (ORCATECH Pilot Grant, Mancini PI)
Fay Horak has the following research support: R37 -NIH/NIA MERIT - Peripheral & Central Postural Disorders in the Elderly, 1R41 -STTR from NINDS - Continuous Monitoring of Turning in Patients with Parkinson's Disease, 1 R41 - STTR from NIH/CHHD/NCMRR - A Short Instrumented Test of Mobility for Neurological Disorders, 1 R01 -NIH/NCI -Preventing Falls After Cancer: Tai Chi vs. Strength Training, The Parkinson Alliance - Instrumented Balance and Gait Measures to Evaluate Effects of DBS Surgery, National Multiple Sclerosis Society -Rehabilitation Research Training in Postural Control of Multiple Sclerosis. She is also on the board of directors of APDM
John Nutt has grant and research support from the National Parkinson’s Foundation, NIH and Ceregene. He serves as a consultant for Neuroderm Ltd, Merck, Elan Pharmaceuticals, Lundbeck Inc, ONO Pharma, SynAgile Crop, Prexa Inc and US World Med. He has received honoraria from the American Academy of Neurology
Footnotes
- Fay Horak and OHSU have significant financial interests in APDM, a company that might have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Oversight Council.
- John Nutt serves as a consultant for Neuroderm Ltd, Merck, Elan Pharmaceuticals, Lundbeck Inc, ONO Pharma, SynAgile Crop, Prexa Inc and US World Med. He has received honoraria from the American Academy of Neurology.
- Bernadette Schoneburg: Writing of the first draft; review and critique.
- Martina Mancini: review and critique.
- Fay Horak: review and critique.
- John Nutt: review and critique.
References
- 1.Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson's disease: a systematic review. Parkinsons Dis. 2013;2013:906274. doi: 10.1155/2013/906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloem BR, Hausdorff JM, Visser JE, et al. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 3.Horak FB, Macpherson JM. Handbook of Physiology. New York: Oxford University Press; 1996. Postural orientation and equilibrium: Interaction and coordination; pp. 255–292. [Google Scholar]
- 4.Burleigh A, Horak F, Nutt J, et al. Levodopa reduces muscle tone and lower extremity tremor in Parkinson's disease. Can J Neurol Sci. 1995;22:280–285. doi: 10.1017/s0317167100039470. [DOI] [PubMed] [Google Scholar]
- 5.Bloem BR, Beckley DJ, van Dijk JG. Are automatic postural responses in patients with Parkinson's disease abnormal due to their stooped posture? Exp Brain Res. 1999;124:481–488. doi: 10.1007/s002210050644. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs JV, Dimitrova DM, Nutt JG, et al. Can stooped posture explain multidirectional postural instability in patients with Parkinson's disease? Exp Brain Res. 2005;166:78–88. doi: 10.1007/s00221-005-2346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaugoyeau M, Viel S, Assaiante C, et al. Impaired vertical postural control and proprioceptive integration deficits in Parkinson's disease. Neuroscience. 2007;146:852–863. doi: 10.1016/j.neuroscience.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter MG, Bloem BR. Postural control in Parkinson patients: a proprioceptive problem? Exp Neurol. 2011;227:26–30. doi: 10.1016/j.expneurol.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006;21:1856–1863. doi: 10.1002/mds.21058. [DOI] [PubMed] [Google Scholar]
- 10.Benatru I, Vaugoyeau M, Azulay JP. Postural disorders in Parkinson's disease. Neurophysiol Clin. 2008;38:459–465. doi: 10.1016/j.neucli.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson's disease. Lancet Neurol. 2011;10:538–549. doi: 10.1016/S1474-4422(11)70067-9. [DOI] [PubMed] [Google Scholar]
- 12.Tassorelli C, Furnari A, Buscone S, et al. Pisa syndrome in Parkinson's disease: clinical, electromyographic, and radiological characterization. Mov Disord. 2012;27:227–235. doi: 10.1002/mds.23930. [DOI] [PubMed] [Google Scholar]
- 13.Horak FB, Macpherson JM. Postural Orientation and Equilibrium. In: Shepard J, Rowell L, editors. Handbook or Physiology. Section 12. Oxford University Press; 1996. [Google Scholar]
- 14.Mancini M, Horak FB, Zampieri C, et al. Trunk accelerometry reveals postural instability in untreated Parkinson's disease. Parkinsonism Relat Disord. 2011;17:557–562. doi: 10.1016/j.parkreldis.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocchi L, Chiari L, Cappello A, et al. Identification of distinct characteristics of postural sway in Parkinson's disease: a feature selection procedure based on principal component analysis. Neurosci Lett. 2006;394:140–145. doi: 10.1016/j.neulet.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Ickenstein GW, Ambach H, Kloditz A, et al. Static posturography in aging and Parkinson's disease. Front Aging Neurosci. 2012;4:20. doi: 10.3389/fnagi.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porosinska A, Pierzchala K, Mentel M, et al. Evaluation of postural balance control in patients with multiple sclerosis - effect of different sensory conditions and arithmetic task execution. A pilot study. Neurol Neurochir Pol. 2010;44:35–42. doi: 10.1016/s0028-3843(14)60405-9. [DOI] [PubMed] [Google Scholar]
- 18.Diener HC, Dichgans J, Bacher M, et al. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol. 1984;57:134–142. doi: 10.1016/0013-4694(84)90172-x. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet CT, Ray C. Peripheral neuropathy may not be the only fundamental reason explaining increased sway in diabetic individuals. Clin Biomech (Bristol, Avon) 2011;26:699–706. doi: 10.1016/j.clinbiomech.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Manor B, Hu K, Zhao P, et al. Altered control of postural sway following cerebral infarction: a cross-sectional analysis. Neurology. 2010;74:458–464. doi: 10.1212/WNL.0b013e3181cef647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell SL, Collins JJ, De Luca CJ, et al. Open-loop and closed-loop postural control mechanisms in Parkinson's disease: increased mediolateral activity during quiet standing. Neurosci Lett. 1995;197:133–136. doi: 10.1016/0304-3940(95)11924-l. [DOI] [PubMed] [Google Scholar]
- 22.Matinolli M, Korpelainen JT, Korpelainen R, et al. Postural sway and falls in Parkinson's disease: a regression approach. Mov Disord. 2007;22:1927–1935. doi: 10.1002/mds.21633. [DOI] [PubMed] [Google Scholar]
- 23.Frenklach A, Louie S, Koop MM, et al. Excessive postural sway and the risk of falls at different stages of Parkinson's disease. Mov Disord. 2009;24:377–385. doi: 10.1002/mds.22358. [DOI] [PubMed] [Google Scholar]
- 24.Stylianou AP, McVey MA, Lyons KE, et al. Postural sway in patients with mild to moderate Parkinson's disease. Int J Neurosci. 2011;121:614–621. doi: 10.3109/00207454.2011.602807. [DOI] [PubMed] [Google Scholar]
- 25.Nantel J, McDonald JC, Bronte-Stewart H. Effect of medication and STN-DBS on postural control in subjects with Parkinson's disease. Parkinsonism Relat Disord. 2012;18:285–289. doi: 10.1016/j.parkreldis.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Morris M, Iansek R, Smithson F, et al. Postural instability in Parkinson's disease: a comparison with and without a concurrent task. Gait Posture. 2000;12:205–216. doi: 10.1016/s0966-6362(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 27.Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson's disease: a posturographic study. Mov Disord. 2003;18:652–658. doi: 10.1002/mds.10418. [DOI] [PubMed] [Google Scholar]
- 28.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:267–274. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung KA, Lobb BM, Nutt JG, et al. Objective measurement of dyskinesia in Parkinson's disease using a force plate. Mov Disord. 2010;25:602–608. doi: 10.1002/mds.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburn A, Stack E, Pickering RM, et al. A community-dwelling sample of people with Parkinson's disease: characteristics of fallers and non-fallers. Age Ageing. 2001;30:47–52. doi: 10.1093/ageing/30.1.47. [DOI] [PubMed] [Google Scholar]
- 31.Bronte-Stewart HM, Minn AY, Rodrigues K, et al. Postural instability in idiopathic Parkinson's disease: the role of medication and unilateral pallidotomy. Brain. 2002;125:2100–2114. doi: 10.1093/brain/awf207. [DOI] [PubMed] [Google Scholar]
- 32.Colnat-Coulbois S, Gauchard GC, Maillard L, et al. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76:780–787. doi: 10.1136/jnnp.2004.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually-induced human postural sway. In: Woollacott M, Horak F, editors. Posture and Gait: Control Mechanisms. Eugene, Oregon: University of Oregon Press; 1992. pp. 272–275. [Google Scholar]
- 34.Bronstein AM, Hood JD, Gresty MA, et al. Visual control of balance in cerebellar and parkinsonian syndromes. Brain. 1990;113(Pt 3):767–779. doi: 10.1093/brain/113.3.767. [DOI] [PubMed] [Google Scholar]
- 35.Chong RK, Horak FB, Woollacott MH. Time-dependent influence of sensorimotor set on automatic responses in perturbed stance. Exp Brain Res. 1999;124:513–519. doi: 10.1007/s002210050647. [DOI] [PubMed] [Google Scholar]
- 36.Konczak J, Corcos DM, Horak F, et al. Proprioception and motor control in Parkinson's disease. J Mot Behav. 2009;41:543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 37.Mancini M, Rocchi L, Horak FB, et al. Effects of Parkinson's disease and levodopa on functional limits of stability. Clin Biomech (Bristol, Avon) 2008;23:450–458. doi: 10.1016/j.clinbiomech.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franchignoni F, Martignoni E, Ferriero G, et al. Balance and fear of falling in Parkinson's disease. Parkinsonism Relat Disord. 2005;11:427–433. doi: 10.1016/j.parkreldis.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Pai, YC and Patton J. Center of mass velocity-position predictions for balance control. J Biomech. 1997;30:347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- 40.Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- 41.Macpherson JM, Horak FB. Posture. In: Kandel E, editor. Neuroscience. 2013. pp. 935–959. [Google Scholar]
- 42.Jacobs JV, Tran V, Nutt JG, et al. Push and release: an alternative clinical test of postural instability for patients with Parkinson's disease. J Neurol. 2006 doi: 10.1007/s00415-006-0224-x. [DOI] [PubMed] [Google Scholar]
- 43.Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 44.Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the "change-in-support" strategy. Phys Ther. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- 45.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- 46.King LA, St George RJ, Carlson-Kuhta P, et al. Preparation for compensatory forward stepping in Parkinson's disease. Arch Phys Med Rehabil. 2010;91:1332–1338. doi: 10.1016/j.apmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs JV, Nutt JG, Carlson-Kuhta P, et al. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs JV, Horak FB. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp Brain Res. 2007;179:29–42. doi: 10.1007/s00221-006-0763-5. [DOI] [PubMed] [Google Scholar]
- 49.Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 50.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 51.Chong RK, Horak FB, Woollacott MH. Parkinson's disease impairs the ability to change set quickly. J Neurol Sci. 2000;175:57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 52.Segev-Jacubovski O, Herman T, Yogev-Seligmann G, et al. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurother. 2011;11:1057–1075. doi: 10.1586/ern.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 54.Maki BE, McIlroy WE. Cognitive demands and cortical control of human balance-recovery reactions. J Neural Transm. 2007;114:1279–1296. doi: 10.1007/s00702-007-0764-y. [DOI] [PubMed] [Google Scholar]
- 55.St George RJ, Carlson-Kuhta P, Burchiel KJ, et al. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J Neurosurg. 2012;116:1347–1356. doi: 10.3171/2012.2.JNS11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bloem BR, Beckley DJ, van Dijk JG, et al. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord. 1996;11:509–521. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 57.Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.St George RJ, Nutt JG, Burchiel KJ, et al. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75:1292–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burleigh-Jacobs A, Horak FB, Nutt JG, et al. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 60.Mille ML, Johnson HM, Martinez KM, et al. Acute effects of a lateral postural assist on voluntary step initiation in patients with Parkinson's disease. Mov Disord. 2007;22:20–27. doi: 10.1002/mds.21139. [DOI] [PubMed] [Google Scholar]
- 61.Burleigh-Jacobs A, Horak FB, Nutt JG, et al. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 62.Rocchi L, Carlson-Kuhta P, Chiari L, et al. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease. J Neurosurg. 2012;117:1141–1149. doi: 10.3171/2012.8.JNS112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagisawa N, Ueno E, Takami M. Frozen gait of Parkinson's disease and vascular parkinsonism - A study with floor reaction forces and EMG. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Tokoyo: Japan.Scientific.Societies.Press; 1991. pp. 291–304. [Google Scholar]
- 64.Hausdorff JM, Balash Y, Giladi N. Time series analysis of leg movements during freezing of gait in Parkinson's diesease: akinesia, rhyme or reason? Physica A. 2003;321:565–570. [Google Scholar]
- 65.Schaafsma JD, Balash Y, Gurevich T, et al. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur J Neurol. 2003;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- 66.Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson's disease. J Neurosci Methods. 2008;167:340–348. doi: 10.1016/j.jneumeth.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 68.Delval A, Snijders AH, Weerdesteyn V, et al. Objective detection of subtle freezing of gait episodes in Parkinson's disease. Mov Disord. 2010;25:1684–1693. doi: 10.1002/mds.23159. [DOI] [PubMed] [Google Scholar]
- 69.Mancini M, Priest KC, Nutt JG, et al. Quantifying freezing of gait in Parkinson's disease during the instrumented timed up and go test. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1198–1201. doi: 10.1109/EMBC.2012.6346151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter DA, Patla AE, Frank JS, et al. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]
- 71.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 72.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 73.Morris ME, Iansek R, Matyas TA, et al. Ability to modulate walking cadence remains intact in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:1532–1534. doi: 10.1136/jnnp.57.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Svehlik M, Zwick EB, Steinwender G, et al. Gait analysis in patients with Parkinson's disease off dopaminergic therapy. Arch Phys Med Rehabil. 2009;90:1880–1886. doi: 10.1016/j.apmr.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 75.Zampieri C, Salarian A, Carlson-Kuhta P, et al. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyns P, Bruijn SM, Duysens J. The how and why of arm swing during human walking. Gait Posture. 2013 doi: 10.1016/j.gaitpost.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Carpinella I, Crenna P, Calabrese E, et al. Locomotor function in the early stage of Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15:543–551. doi: 10.1109/TNSRE.2007.908933. [DOI] [PubMed] [Google Scholar]
- 78.Hong M, Earhart GM. Effects of medication on turning deficits in individuals with Parkinson's disease. J Neurol Phys Ther. 2010;34:11–16. doi: 10.1097/NPT.0b013e3181d070fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gabell A, Nayak US. The effect of age on variability in gait. J Gerontol. 1984;39:662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- 80.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J Biomech. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schaafsma JD, Giladi N, Balash Y, et al. Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 83.Lamoth CJ, van Deudekom FJ, van Campen JP, et al. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil. 2011;8:2. doi: 10.1186/1743-0003-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 85.Yogev G, Giladi N, Peretz C, et al. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 86.Ferrarin M, Rizzone M, Bergamasco B, et al. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson's disease. Exp Brain Res. 2005;160:517–527. doi: 10.1007/s00221-004-2036-5. [DOI] [PubMed] [Google Scholar]
- 87.Hausdorff JM, Gruendlinger L, Scollins L, et al. Deep brain stimulation effects on gait variability in Parkinson's disease. Mov Disord. 2009;24:1688–1692. doi: 10.1002/mds.22554. [DOI] [PubMed] [Google Scholar]
- 88.Schaafsma JD, Giladi N, Balash Y, et al. Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 89.Bryant MS, Rintala DH, Hou JG, et al. Gait variability in Parkinson's disease: influence of walking speed and dopaminergic treatment. Neurol Res. 2011;33:959–964. doi: 10.1179/1743132811Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shivitz N, Koop MM, Fahimi J, et al. Bilateral subthalamic nucleus deep brain stimulation improves certain aspects of postural control in Parkinson's disease, whereas medication does not. Mov Disord. 2006;21:1088–1097. doi: 10.1002/mds.20905. [DOI] [PubMed] [Google Scholar]
- 91.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson's disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719. doi: 10.1155/2012/918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vandenbossche J, Deroost N, Soetens E, et al. Conflict and freezing of gait in Parkinson's disease: support for a response control deficit. Neuroscience. 2012;206:144–154. doi: 10.1016/j.neuroscience.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 94.Van OK, Frank JS, Horak FB. Practice-related improvements in posture control differ between young and older adults exposed to continuous, variable amplitude oscillations of the support surface. Exp Brain Res. 2009;199:185–193. doi: 10.1007/s00221-009-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yogev-Seligmann G, Giladi N, Brozgol M, et al. A training program to improve gait while dual tasking in patients with Parkinson's disease: a pilot study. Arch Phys Med Rehabil. 2012;93:176–181. doi: 10.1016/j.apmr.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Rahman S, Griffin HJ, Quinn NP, et al. On the nature of fear of falling in Parkinson's disease. Behav Neurol. 2011;24:219–228. doi: 10.3233/BEN-2011-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord. 2003;18:496–502. doi: 10.1002/mds.10396. [DOI] [PubMed] [Google Scholar]
- 98.Adkin AL, Frank JS, Carpenter MG, et al. Fear of falling modifies anticipatory postural control. Exp Brain Res. 2002;143:160–170. doi: 10.1007/s00221-001-0974-8. [DOI] [PubMed] [Google Scholar]
- 99.Reelick MF, van Iersel MB, Kessels RP, et al. The influence of fear of falling on gait and balance in older people. Age Ageing. 2009;38:435–440. doi: 10.1093/ageing/afp066. [DOI] [PubMed] [Google Scholar]