Abstract
The primate corpus luteum is a transient endocrine gland that differentiates from the ovulatory follicle midway through the ovarian (menstrual) cycle. Its formation and limited lifespan is critical for fertility, as luteal-derived progesterone is the essential steroid hormone required for embryo implantation and maintenance of intra-uterine pregnancy until the placenta develops. It is well-established that LH and the LH-like hormone, CG, are the vital luteotropic hormones during the menstrual cycle and early pregnancy, respectively. Recent advances, particularly through genome analyses and cellular studies, increased our understanding of various local factors and cellular processes associated with the development, maintenance and repression of the corpus luteum. These include paracrine or autocrine factors associated with angiogenesis (e.g., VEGF), and that mediate LH/CG actions (e.g., progesterone), or counteract luteotropic effects (i.e., local luteolysis; e.g., PGF2α). However, areas of mystery and controversy remain, particularly regarding the signals and events that initiate luteal regression in the non-fecund cycle. Novel approaches capable of gene “knockdown” or amplification”, in vivo as well as in vitro, should identify novel or underappreciated gene products that are regulated by or modulate LH/CG actions to control the functional lifespan of the primate corpus luteum. Further advances in our understanding of luteal physiology will help to improve or control fertility for purposes ranging from preservation of endangered primate species to designing novel ovary-based contraceptives and treating ovarian disorders in women. R01 HD020869, R01 HD042000, U54 HD018185, U54 HD055744, P51 OD011092, T32 HD007133, Bayer Schering Pharma AG.
Keywords: luteinization, luteolysis, LH-CG, luteotropic factors, luteolytic factors
I. Introduction
The corpus luteum is a transient endocrine gland that differentiates from the mature follicle after ovulation. Its formation and limited lifespan in the mammalian ovary is critical for fertility, as the corpus luteum produces progesterone, the essential steroid hormone permitting implantation of the embryo and sustaining intra-uterine pregnancy. This group recently provided an in depth review [1] on the history of research on the corpus luteum, including the vital discovery by Dr. Fraenkel [2] in the early 1900s of the endocrine function of the corpus luteum. The chapter reviews the remarkable species-differences in structure-function and control (via hormones and local factors) of the development (luteinization), functional lifespan, and regression of the corpus luteum. The current treatise, as part of the 2013 Ludwig Fraenkel Symposium, will focus on the endocrine and local control of the corpus luteum in primate species. It will feature knowledge gained from the senior author's 37 years of research on the corpus luteum of the rhesus macaque, an Old World Monkey whose ovarian/menstrual cycle is remarkably similar to that in women. The discussion will focus on recent advances at the molecular and cellular level as related to the physiologic control of the corpus luteum, with reference to other investigators who have contributed greatly to this field in the last decades, e.g., T. Zeleznik [3], H. Fraser [4]. Finally, areas of mystery and controversy will be identified where significant progress is still needed.
II. Periovulatory Processes Related to Luteinization
Unlike in typical rodent models, where coitus-induced secretion of PRL from the pituitary is required for luteal development [5], the mid-cycle surge of pituitary LH secretion is the sole hormonal stimulus required in primates for the cascade of events leading to further differentiation of the mature follicle into corpus luteum [3]. Luteal development is perceived as following, or at least concomitant with, the other major periovulatory events induced by the LH surge [6]: reinitiation of meiosis and cytoplasmic maturation of the enclosed oocyte, and subsequent ovulation and release of a fertilizable oocyte into the oviduct. Although investigators, including ourselves [7, 8], selectively studied certain genes and their products or cellular pathways, there were no reports of a genome-wide analysis of the dynamics of follicular activity in primates, including the periovulatory, luteinizing interval in the natural menstrual cycle. Therefore, in collaboration with colleagues at Bayer Schering Pharma AG (now Bayer Health Care) in Berlin, we systematically evaluated the changes in the transcriptome (mRNA levels) in the periovulatory follicle of rhesus monkeys during a Controlled Ovulation (COv) protocol [9].
The COv protocol [10] allows natural selection of the dominant follicle during the menstrual cycle, but then “clamps” pituitary gonadotropin secretion so that the endogenous LH surge can be replaced by exogenous hCG. This permits collection of the dominant follicle just prior to (Time 0; preovulatory follicle) and at precise intervals (12, 24 and 36 hr; periovulatory follicle) after hCG administration. Notably, at 36 hr post-hCG, half of the follicles had ruptured (ovulatory follicle). Total RNA preparations from individual follicles were hybridized on the Affymetrix Rhesus Macaque Total Genome Array. Levels and patterns of selected mRNAs were also quantified by real-time PCR (q-PCR) to further validate the data.
The microarray results, which are available for public use at NIH GEO (number GSE2276), displayed remarkable changes in mRNA levels for numerous genes, the patterns of which were typically confirmed by q-PCR analyses. Although the RNA preparations were prepared from the entire follicle, several transcripts displayed expression patterns expected for purported (based on the literature) theca (e.g., CYP17A), granulosa (CYP19A, FSHR), and cumulus (HAS2, TNFA1P6) cell-associated genes in the rodent or primate peri-ovulatory follicle. Although various patterns of mRNA levels were noted, from rapid (within 12 hr), sustained loss after hCG administration (e.g., FSHR) to an abrupt increase 36-hr post-hCG (e.g., aspartic acid protease PGA5), a number (n>300) of mRNAs displayed the intriguing pattern of (a) an initial change (often > 10-fold) in levels by 12 hrs post-hCG, with (b) a return to pre-hCG levels at 24-to-36 hrs in preovulatory follicle, followed by (c) another robust change, often greater than that at 12 hrs, at 36 hrs post-hCG in the ovulated follicle.
This biphasic pattern was observed for mRNAs from a diverse cohort of genes encoding steroidogenic enzymes (e.g., HSD3B2), proteolytic enzymes (e.g., MMP1 and 10) and local factors (e.g., AREG and EREG). Recall that the interval from onset of the ovulatory gonadotropin stimulus to follicle rupture is longer in macaques and women (~36 hr) compared to rodents (10-12 hrs), and includes LH-induced luteinization. We hypothesize that the initial change in many of these genes at 12 hr is essential for events related to cumulus expansion and oocyte maturation, thereby providing a cumulus-oocyte complex that can be released at ovulation. However, these gene products appear critical again by 24 hr later in processes associated with luteinization of the ovulatory follicle. Some of these genes are not surprising as they associate with renewed progestogenic activity (HSD3B2) and extracellular remodeling (MMPs, ADAMTSs) in the developing corpus luteum. However, the role(s) of others awaits investigation. For example, the EGF-related ligands EREG and AREG may serve as intermediates for LH-induced events, such as reinitiation of meiosis and cumulus-oocyte expansion [11] as reported in rodents, but a role for these factors in the ovulated, luteinizing follicle/developing corpus luteum is unknown.
Visual inspection of the microarray database allows one to consider possible activity of known genes that to date have not been clearly linked to periovulatory events. For example, mRNA levels for kisspeptin (KISS-1), a paracrine factor recently linked to control of GnRH neurons and hence hypothalamic-pituitary-gonadal function [12], displayed the biphasic pattern in the peri-ovulatory follicle described earlier (Figure 1). This finding supports the hypothesis that the kisspeptin-receptor system plays an early role in cumulus-oocyte maturation, as well as a later role in tissue remodeling related to ovulation and/or luteal development. Recent reports indicate that exogenous kisspeptin can promote oocyte maturation in vitro [13] and progesterone production by rat luteal cells during culture [14] but the physiologic role and its relevance to primate ovarian function or dysfunction (e.g., PCOS;)[15] awaits study.
Figure 1.
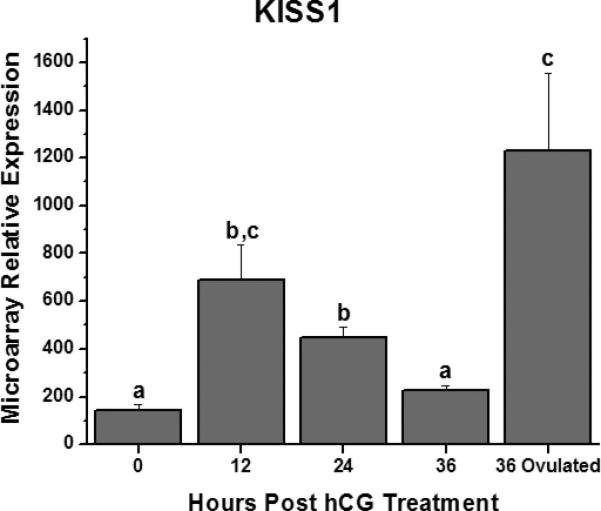
Relative levels of kisspeptin (KISS-1) mRNA in the macaque periovulatory follicle at selected intervals prior to (0 hr) and following administration of a hCG bolus in vivo. Follicles isolated at 36 hrs were either pre- or post-ovulation. Different letters denote significant differences between time points. See reference [9] for details.
Sophisticated software programs can also be employed to relate mRNA expression patterns to gene ontologies and cellular pathways of activity. Such analyses revealed a biphasic pattern of HIF1α mRNA expression and of downstream HIF1α-regulated genes (e.g., VEGF, GLUT1) in the primate follicle at 12 hrs post-hCG and post-rupture at 36 hrs post-hCG [9]. The transcription factor HIF1α is regulated by oxygen concentrations; in hypoxic conditions, HIF1α exists in a de-hydroxylated state that leads to the formation of a HIF1α:β dimer, which in turn translocates into the nucleus and increases the expression of target genes such as VEGF [16]. Evidence from the mouse model suggests that hypoxia-inducible factors in the preovulatory follicle are critical for rupture [17], and LH/CG regulates HIF1α mRNA expression in human luteinizing granulosa cells [18]. Analyses of ontologies of gene products significantly impacted by controlled ovulation also identified a number of classical “immune pathways”, including cytokine receptor activity cytokine and growth factor binding and transducer activity, reinforcing the concept that immune processes (e.g., pro-inflammatory response) involving blood-derived immune cells or non-immune ovarian cells play a role in periovulatory events [19].
In summary, this database will be of great value in identifying novel or unappreciated genes involved in molecular and cellular pathways leading to periovulatory events in the primate follicle, including its luteinization and conversion into the early corpus luteum. One caveat is that currently only about 50% of the rhesus transcripts are annotated to specific genes (for discussion of limitations of microarray databases see [20]). Continued annotation of rhesus genes will permit the data to reach its full potential. A second caveat is that changes in mRNA levels do not necessarily correlate positively with levels of translated protein or protein activity (see next section, VEGF). Nevertheless, the transcriptome provides an initial perspective for prioritizing evaluation of proteins (the proteome) and their roles in luteal development. Finally, the database will be useful for comparisons of events within periovulatory follicles from various species, including women [21] and nonprimates [22], and how the results compare to those from distinct follicle compartment such as the theca and granulosa cell layers [23] and the cumulus-oocyte complex [24].
III. Angiogenesis and Luteal Structure-Function
One research area receiving considerable attention over the past decade is the development and maintenance of the luteal vasculature. The vascular changes associated with luteal development (here termed “angiogenesis”) and subsequent luteal regression (“angiolysis”) are remarkable [25]. However, the precise mechanisms whereby luteal neovascularization via angiogenesis (expansion of existing thecal vessels) or possibly vasculogenesis (e.g., from circulating vascular progenitor cells) forms the dense capillary network in differentiated luteal tissue are not well-defined. Much of the capillary expansion appears causally related to endothelial cell proliferation; the vast majority of dividing cells in the early developing corpus luteum are microvascular endothelial cells [26]. But there is some evidence that the vascular space increases somewhat in the primate corpus luteum through mid-luteal phase of the cycle. This may be related to maturation of the microvasculature, as they acquire endothelial support cells, or pericytes [27], but this important feature has received little attention especially in primates.
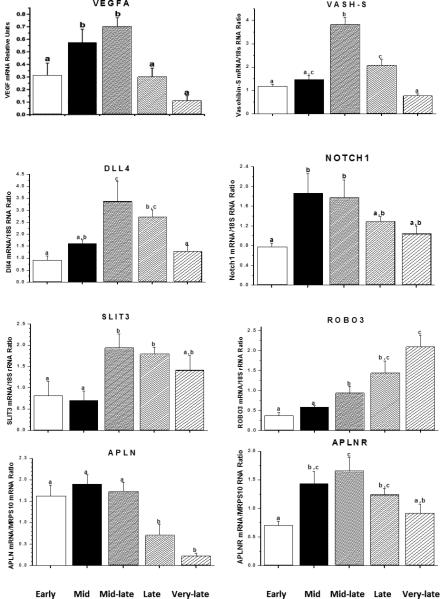
Advances in our understanding of the factors controlling luteal angiogenesis [4, 25] benefited from discoveries by investigators working in other areas, such as cancer or fetal development, where blood vessel development is vital. Notably, the family of vascular endothelial growth factors (VEGFs)[28], especially VEGF-A and its receptors FLT1 and KDR [29], are dynamically expressed in the corpus luteum of many species, including macaques. There are few studies to date on specific cell types, but it's generally hypothesized that VEGFA produced by luteinizing/luteal cells acts in a paracrine manner via VEGF receptors/co-receptors (e.g., neuropilins) [30] on microvascular endothelial cells to promote angiogenesis. Limited evidence suggests that the regulation of VEGFA production in the ovulatory, luteinizing follicle and corpus luteum differs from that in many tissues. It appears that the midcycle gonadotropin surge, via either transcriptional or post-transcriptional actions directly increases VEGF production in the primate luteinizing granulosa cell layer and developing corpus luteum [31, 32]. Moreover, local factors such as insulin-like growth factors (IGF)-1 and -2 may synergize with LH to promote VEGF production [32]. However, after this initiation of active VEGF expression, the typical stimulator of VEGF production in many tissues, i.e., hypoxia, appears to become a major regulator in primate luteal cells [33]. Nevertheless, the reason(s) or physiologic significance for the divergent patterns in VEGFA mRNA and protein levels during the functional lifespan of the primate corpus luteum [33], with mRNA exhibiting the biphasic pattern typical of circulating progesterone levels (Figure 2), but protein levels highest in the early luteal phase and declining thereafter, is unknown. Further research is required to understand the regulation and role of the various family members (VEGFA-D) and isoforms in the ovary.
Figure 2.
Relative levels of gene products (mRNAs) for selected local vascular factors and receptors in the macaque corpus luteum as a function of the stage of luteal phase during the menstrual cycle. Mid luteal phase corresponds to the developed corpus luteum. At mid-late luteal phase, the corpus luteum is on the verge of regression. Late and very late (menstruation) luteal phase corresponds to functional and structural regression, respectively. Data adapted from [37, 42, 107] see references for further details on tissue staging and collection.
Recent studies by three groups [4, 34, 35] established a critical role for VEGFA in primate follicular and luteal development and function. Local or systemic administration of VEGF antagonists to monkeys either at midcycle or during the early or midluteal phase indicates that VEGF is critical for: (a) ovulation and early luteinization in the mature follicle, (b) neovascularization and achievement of normal luteal function in the developing corpus luteum, and perhaps unexpectedly, (c) continued vascular integrity and progesterone production by the developed corpus luteum during the menstrual cycle. Collectively these data indicate that VEGF acts initially as an angiogenic factor, but subsequently as a trophic factor in the primate corpus luteum.
Preliminary studies indicate that other vascular factors act locally to influence luteal structure-function, perhaps to promote or control the actions of VEGF. For example, one scenario [36] suggests that the angiopoietins (ANGPTs) via the TEK receptor (which are expressed in the primate corpus luteum)[37] are essential for luteal development and function. A local tissue ratio favoring ANGPT1 (TEK agonist) over ANGPT-2 (TEK antagonist) may promote capillary growth and maturation. Focally, ANGPT2 may loosen cell-matrix interactions to allow VEGF-stimulated endothelial cell proliferation and capillary tube formation, whereas ANGPT1 recruits pericytes to stabilize and mature capillaries. In contrast, a ratio favoring ANGPT2 may destabilize vessels and cause capillary degeneration, especially in the relative absence of VEGF in immature vessels not supported by pericytes. Initial evidence [38] that intrafollicular injection of ANGPT2, but not ANGPT1, destroys the periovulatory follicle and resets the ovarian cycle in macaques suggest a critical role for the ANGPTs in the mature follicle and developing corpus luteum. However, further studies are needed to elucidate the regulation and role(s) of ANGPTs and their receptors in the corpus luteum.
Several other factors were recently identified that may modulate VEGF actions in tissues, including the corpus luteum. The concept of negative feedback regulation of VEGF emerged from the discovery of vasohibins (VASHs)[39] and led to the hypothesis that VASH-1 is a negative regulator of VEGF's angiogenic action in the corpus luteum [40]. Also, the discovery that the delta-like ligand 4 (DLL4) -Notch receptor system negatively regulates VEGF-mediated angiogenesis, led to experiments by Fraser and colleagues demonstrating that DLL4 neutralization during luteal development increased angiogenesis but markedly suppressed the progestogenic function and lifespan of the primate corpus luteum [41]. Clearly the ligand-receptor systems for these and other vascular regulators (e.g., SLIT3-ROBO3 [42], apelin-apelin receptor [42]) are expressed in the primate corpus luteum, in diverse patterns during the luteal lifespan (Figure 2). The challenge will be to discern the action(s) and regulation of each system, and their interactions with VEGF, during development, function and regression of the primate corpus luteum.
Finally, with the demonstration of specific molecular markers for lymphatic endothelial cells, we recently characterized a robust lymphatic in the primate corpus luteum [43]. Moreover, the lymphangiogenic factors VEGF-C and -D (FIGF) and their receptor FLT4 are dynamically expressed during the luteal lifespan. The lymphatic vasculature plays important roles, including fluid balance and transport of immune cells, in various organs which could be relevant to the corpus luteum, especially in larger species. Further studies on the “secondary circulatory system”, the lymphatics in the primate corpus luteum could be critical to understanding the physiology and pathophysiology of the ovary [44, 45].
IV. Luteotropic Factors: Luteinizing Hormone (LH) and LH-induced Local Factors
Despite considerable controversy that extended into the 1980s [46], it is now generally accepted that following ovulation the corpus luteum of most primates (e.g., Old World monkeys to humans) is primarily if not solely dependent upon LH secreted from the anterior pituitary for its development and maintenance through the menstrual cycle. LH-depletion/neutralization by pharmacologic techniques beginning within 1 day of ovulation prevents normal development of the corpus luteum, whereas treatment at mid-luteal phase causes premature regression of the developed corpus luteum [47]. By mid luteal phase, pulses of LH are entrained to increases in progesterone secretion [48]. Evidence that GnRH antagonist treatment rapidly and completely suppresses circulating progesterone levels demonstrates the absolute requirement of primate luteal cells for LH exposure to maintain steroidogenic function.
Pituitary LH, as well as placental chorionic gonadotropin (CG), are members of the glycoprotein family of hormones containing a common α subunit non-covalently linked to a hormone-specific β subunit. Both LH and CG are ligands for the high-affinity LH-CG receptor, a member of the seven-transmembrane, rhodopsin-like, G-protein coupled receptor family [49]. G-protein coupled signaling through the LHCGR involves activation of adenylate cyclase, cyclic AMP production, and activation of cAMP-dependent protein kinases, which actively promotes steroidogenesis and chronically maintains luteal structure-function [50]. LH-CG can also activate cAMP-independent pathways [50, 51], but their role(s) during the functional lifespan of the primate corpus luteum await definition. Unlike in some species, in vitro studies suggest that LH-CG can act on both “small” and “large” luteal cells from monkeys to promote progesterone production [52]; thus steroidogenesis by large luteal cells is not “constitutive” as part of final luteal differentiation as proposed in the sheep corpus luteum. Studies of luteal cell types in primates are limited [52], as compared to rodents or domestic animals, but clearly display some unique features. Notably two luteal cell types can be distinguished in the macaque and human corpus luteum based on compartmentalization and steroid production [53, 54]. Typical luteal cells are found within the luteal parenchyma and appear to secrete primarily progesterone, whereas paraluteal cells are found around the exterior of the gland and along infoldings associated with the vascular and appear to produce androgens. Immunohistochemical staining for steroidogenic enzymes suggests that the “two-cell model” for estrogen production characteristic of the preovulatory follicle continues in the primate corpus luteum. It is not obvious that these two subpopulations correlate with the “small” and “large” luteal cells that can be separated by size, since the large luteal cell population from the macaque corpus luteum secretes both progesterone and estradiol in an LH-stimulated manner [52].
To discover LH-regulated gene products and cellular pathways in the primate corpus luteum, investigators have utilized differential display techniques or cDNA microarrays [55, 56]. In a recent study [55], we compared the transcriptome in the macaque corpus luteum before and three days after onset of GnRH antagonist treatment: (a) the mRNA levels of approximately 2% of the transcripts either increased or decreased ≥ 2-fold with LH deprivation, and (b) the great majority returned to control (pretreatment) levels if LH was co-administered with the antagonist. Notably, two of the pathways significantly altered by LH depletion/replacement, as noted by ontology abundance and z-scores, were those associated with steroid production (e.g., steroid biosynthetic processes, such as STARD4) and immune system/response (e.g., the negative regulator IL1RN). The findings identify LH-regulated processes that may be critical for controlling steroidogenesis as well as others likely involved in tissue/cell interactions that sustain luteal structure-function. Further studies are warranted to consider the proteome or protein activity related to the dynamics of the transcriptome, as well as to elucidate the critical roles of gene products and associated pathways. Likewise, more acute protocols are needed to evaluate the initial (e.g., early response genes) versus later processes regulated by LH, for example during exposure as well as following an LH pulse during the menstrual cycle. Such studies will further identify novel or underappreciated LH-regulated pathways and cell-cell interactions that are dynamic and regulate the development, function and lifespan of the primate corpus luteum.
LH-regulated local ligand-receptor systems
Global characterization of LH-dependent gene products in the ovulatory, luteinizing follicle and corpus luteum identified local ligand-receptor systems that likely mediate or modulate LH action and hence primate luteal structure-function. For example, mRNAs for the corticoptropin-releasing hormone (CRH)/urocortin (UCN)- receptor (R1,R2)-binding protein (CRHBP) system are dynamically expressed in the macaque corpus luteum [57], with UCN up-regulated and CRH-BP expression down-regulated, by LH. Consistent with LH down-regulation of CRH-BP expression, administration of a GnRH antagonist significantly increased CRH-BP mRNA levels; also levels were highest in luteal tissue at late luteal phase (i.e., luteolysis) of the menstrual cycle. When a general CRHR antagonist (astressin) was injected into the preovulatory follicle, ovulation occurred but circulating progesterone levels were significantly suppressed during the luteal phase and indices of cellular degeneration were observed in the corpus luteum of astressin-treated animals [57]. The data are consistent with the hypothesis that local CRH/UCN-R action promotes luteal development and function, whereas CRH-BP serves to check this action by sequestering CRH/UCN and predominates by late in the luteal lifespan. Many issues remain to be resolved at the tissue, cellular and molecular level regarding this putative local CRH/UCN-R-BP system and its role in cyclic ovarian function. There is evidence that this system exists in ovarian compartments of other species, including humans [58]. Moreover, its actions may vary between follicles and corpora lutea, or between ligand-R1 and –R2 systems [59, 60].
As another example, microanalyses of the transcriptome in the macaque, luteinizing follicle and corpus luteum during the menstrual cycle identified remarkable changes in mRNA levels for a number of proteins involved in prostaglandin (PG) synthesis, metabolism and cell signaling [9, 61]. Notably, increases in prostaglandin-endoperoxide synthase 2 (PTGS2), PGE synthase (PGES) and PGE receptor 2 (PTGER2) mRNAs occurred in the 12-36 hrs post-hCG administration leading up to follicle rupture in the COv model, which correlated with a rise in PGE2 levels in follicular fluid. Moreover, mRNA levels for these genes, as well as PTGER3, were high in luteal tissue from the early-to-mid luteal phase. Notably, levels for many these PGE2-related components declined by mid-late to late luteal phase, when prostaglandin F receptor (PTGFR) expression markedly increased. The data provide molecular evidence for the concept that a local PGE-R system serves as a local differentiation pathway in the ovulatory follicle and developing corpus luteum in primates [62]. Moreover, the shift in expression to favor PGF-R action as the luteal phase progresses may be critical for ending the luteal lifespan in primates. We recently reported the ability of PGE-R2 signaling to promote cyclic AMP production in and “expansion” of the cumulus-oocyte complex in macaques [63], and demonstrated PGE2 stimulation of cAMP and progesterone production in primate luteal cells [64, 65]. Moreover, chronic administration of a PTGER2 antagonist to monkeys markedly reduced their fertility [66]. However, a physiologic role for PGE2 and any of its receptor isoforms (PTGER1-4) in controlling the structure-function or lifespan of the primate corpus luteum remains unsubstantiated; in the above contraceptive trial, chronic PTGE2 antagonist treatment did not alter menstrual cyclicity or cycle length.
Perhaps the local factor that's received the most attention over the past decade as a possible mediator of LH's luteotropic actions is the steroid hormone, progesterone [47]. Since our discovery that the midcycle hCG bolus induces progesterone receptor (genomic PR) in luteinizing granulosa cells of macaques during controlled ovarian stimulation protocols [67], and that an inhibitor of 3β-hydroxy steroid synthesis (trilostane) in rhesus monkeys was prevented by concomitant administration of progestin [68], one focus of our research is to identify LH-dependent progesterone-regulated processes in the ovulatory, luteinizing follicle and corpus luteum in macaques. Considerable effort (e.g., in progesterone-receptor gene knockout mice)[69] focused on progestin's critical actions in the ovulatory process. However, evidence has also accrued that is consistent with Rothchild's [70] hypothesis that progesterone also acts as a local luteotropin in the developing corpus luteum. Steroid ablation and progestin replacement protocols in macaques, combined with techniques to clamp gonadotropin support (to rule out effects of steroid feedback on pituitary function), support a role for locally produced progesterone to promote luteal structure-function [71, 72], through specific actions involving luteal tissue remodeling (e.g., protease expression) [73] and sensitivity to other local factors [74]. Recent genome-wide analyses to discern between the effects of LH ablation-replacement versus steroid ablation-progestin replacement in the macaque corpus luteum [55] are providing valuable databases on gene products that are steroid-independent or steroid/progestin-regulated during luteal development and function. Further studies are needed to define the role(s) of these products in the control of the functional lifespan of the corpus luteum, and the involvement of the genomic progesterone receptor isoforms, PGRA and B. Likewise, the possible involvement of nongenomic progesterone receptors (e.g., membrane receptors, PGRMC1)[75, 76] warrant sturdy, based on reports of their survival/anti-apoptotic actions on granulosa and luteal cells [77].
V. Extension of Luteal Structure-Function in Early Pregnancy
Although the cause (s) of luteolysis in primates during the menstrual cycle are unknown, it is generally believed that secretion of another LH-like hormone, chorionic gonadotropin (CG) by the implanting blastocyst and developing placenta “rescues” the corpus luteum in many primates, from Old World monkeys to humans, and extends luteal function in early pregnancy [78]. In some aspects, luteal function during early gestation is similar to that during menstrual cycle, e.g., the continued production of progesterone (and estrogens), albeit at perhaps a higher level. However, new functions may appear, such as the production of the non-steroidal hormone, relaxin. Since sequential treatment with exogenous estrogen and progesterone to mimic cyclic ovarian function leads to pregnancy and generally healthy offspring in women during fertility therapy [79], it is widely accepted that progesterone is the only luteal product required for initiation and maintenance of early pregnancy. However, recent evidence suggests that relaxin (RLN) of luteal origin acts on various tissues ranging from the embryo [80] and uterus [81] to the cardiovascular system [82], to optimize maternal-fetal function and maternal adaptations to pregnancy. Further studies are needed to assess the regulation and actions of relaxin, as a luteal hormone, during early pregnancy in primates.
For ethical and practical reasons, studies of the cellular and molecular changes in the human corpus luteum during CG rescue in early pregnancy are virtually nonexistent. However, there are some experiments where the effects of exogenous hCG on the corpus luteum of primate species, including women, were examined as a “simulated early pregnancy” (SEP) model [76]. Morphologically [83], there are changes in the luteal cells suggesting rapid use of steroid precursors (loss of lipid droplets) and increased production of secretory granules, consistent with greater secretion of steroids and relaxin. Remarkably, evidence to date suggests the increased hormonal production is not accompanied by a renewal of angiogenesis. Although microvascular cell proliferation remains low [26], this does not preclude other effects of CG or CG-regulated vascular factors on vessel structure-function (e.g., vascular permeability) that could influence luteal activity.
CG exposure results in broad, remarkable changes in luteal expression of mRNAs and proteins in the primate corpus luteum. In a recent study detailing the dynamics of the macaque luteal transcriptome (mRNAs) following 1,3, 6 and 9 days of CG during SEP [76], levels of 1192 transcripts changed compared to pretreatment (day 10 of the luteal phase). Cluster analysis indicated that samples from day 10 versus day 10 + hCG (1 day of treatment) were very dissimilar, with 292 mRNAs up-regulated and 127 down-regulated. As expected, a number of gene products were related to steroid production, with some being altered transiently (HSD3β2 unregulated at 1 day, then declined during CG exposure) and others chronically (CYP11A1, upregulated at 1 day and sustained). However, other gene products did not change until after more prolonged CG exposure; for example, RLN 1 mRNA levels did not increase until 3 days of CG exposure. Delayed responses may be related to actions of CG-induced local factors or down-regulation of CG-receptor signaling. Notably, CG exposure caused a transient re-expression of components of PGE-receptor signaling, reminiscent of the corpus luteum of the early luteal phase, but suppressed expression of genes encoding proteins associated with the immune system. Further studies on such databases (transcriptome, proteome) are needed to clarify the direct vs. indirect effects of CG that transiently prevent luteolysis and extend the structure-function of the primate corpus luteum in early pregnancy.
Since pituitary-derived LH continues to circulate, albeit at reduced pulse-frequency, during the mid-to-late luteal phase (i.e., time of implantation), it remains unclear why or how another LH-like hormone, i.e., CG, rescues the primate corpus luteum in early pregnancy. Two scenarios, which are not mutually exclusive, have been considered. The first is that the qualitative and quantitative changes in gonadotropin exposure, with CG presented in a non-pulsatile manner and levels rising over several days, promotes luteal cell activities beyond that provided by the intermittent LH pulses. Studies by Zeleznik [84] revealed that administration of increasing amounts of recombinant LH or hCG around the expected time of implantation, increases the functional lifespan of the macaque corpus luteum, thus supporting this scenario. Alternatively, the second scenario is that LH and CG, while both binding to same LH/CG receptor are structurally different enough to elicit different ligand-receptor signaling. This concept was initially proposed from differences in LHCGR movement and turnover following binding of ovine LH versus hCG in nonprimate target cells [85]. However, initial studies on primate granulosa cells [86] did not support the concept of differential receptor activation by hCG and hLH, based on the kinetics of progesterone production. Elegant studies using human cell systems recently identified differences in the potency and chronic actions of LH and CG to stimulate cyclic AMP and progesterone secretion, plus the activation of cell signaling pathways [87, 88]. For example, LH was more potent in ERK and AKT activation to promote cell proliferation and survival, whereas hCG was more potent in cyclic AMP and steroid production. Thus, LH may promote broader actions as needed during development of luteal structure-function in the early luteal phase of the menstrual cycle, whereas CG may specifically extend hormone (steroid and relaxin) production for a limited interval until the luteal-placental shift. Further studies are warranted that compare LH and CG action of primate target cells, and to determine whether luteal regression after the luteal-placental shift is comparable to that occurring at the end of the non-fecund cycle.
VI. Perspectives on Luteal Regression
The corpus luteum in primates ceases to function and structurally involutes, i.e., undergoes functional and structural regression or luteolysis, at one of two stages: (a) near the end of the non-fecund menstrual cycle, or (b) at the stage in gestation, called the luteal-placental shift, after which placental progesterone sustains pregnancy. There is evidence for distinguishing between the processes of functional and structural luteolysis, since the primate corpus luteum ceases to function (i.e., produce appreciable progesterone) by 3 days before menstruation at the end of the menstrual cycle [46], but appreciable luteal mass remains into the next follicular phase. However, even the functionally regressed corpus luteum retains some activity, based on evidence that exogenous gonadotropin treatment in the follicular phase can increase circulating progesterone levels [89]. It remains unknown if the processes causing regression of the corpus luteum of the cycle are comparable to those occurring in the corpus luteum of pregnancy. Evidence from other species suggests that luteal involution occurs slower in pregnancy [90], perhaps due to differences in the luteotropic or luteolytic milieu or changes in luteal cell populations, but data regarding luteal regression during pregnancy in primates is not available in large part due to ethical and logistic reasons, as lutectomy could/will terminate the pregnancy. It's intriguing though to note that Treloar [91] reported that despite “disappearance” of the macaque corpus luteum after the luteal-placental shift (as noted from the lack of luteal tissue at the site of dye injection), a corpus luteum was “rejuvenated” at the same site by parturition and appeared functional in lactation. This rarely cited evidence leads to intriguing questions, e.g.,: (a) in this era of stem cell/progenitor cell evaluation, does the corpus luteum contain cells that could re-establish functional luteal tissue, and (b) although PRL-like hormones are not considered a major luteotropin in primates, primate luteal tissue can express PRL receptor [92], so are there conditions such as the post-partum [46] when it serves a role?
A key limitation to our understanding of the primate corpus luteum, is the continuing mystery regarding the signal(s) that initiate luteolysis. What is known is that the onset of luteolysis is controlled differently from that in many nonprimate species, e.g., it is not initiated by a uterine luteolytic factor released if timely implantation is absent as hysterectomy does not alter the lifespan of the corpus luteum in Old World monkeys [93] or women [94]. This led Knobil [46] to propose that a “self-destruct” mechanism exists within the primate ovary/corpus luteum that controls the luteal lifespan. Two factors received attention as local signals, both of which lost favor but recently reappeared as possible “self-destruct” agents. First, unlike the corpus luteum of many species, primate luteal tissue produces significant amounts of estrogens. Evidence that intraluteal injections of estradiol caused premature luteolysis led to its proposed role as a primate luteolytic signal [46]. However, subsequent studies indicating that its luteolytic effect was indirect due to leakage into the circulation and negative feedback inhibition of LH secretion, as well as evidence [95] that primate luteal tissue did not appear to contain the “classical nuclear estrogen receptor” (now termed ERα), diminished interest. But more recent evidence that another nuclear receptor isoform, ERβ, is abundantly expressed in luteal tissue and appears down-regulated by progesterone [74], suggests that further studies of estrogen-receptor signaling and action in the primate corpus luteum are warranted. Second, although prostaglandin F2α (PGF2α) does not serve as a uterine luteolytic factor, primate luteal tissue produces PGF2α and its receptor in a dynamic fashion; therefore its local role in controlling luteolysis was proposed [96]. However, evidence [97] that intraluteal administration of a PG synthesis inhibitor caused, rather than prevented, luteolysis was difficult to reconcile with PGF2α as a luteolytic factor. After recent increases in our understanding of the molecular processes of PG synthesis, metabolism and signaling, further analyses [61] of the whole genome and cell pathways support the original concept that changes in the balance from luteotropic PG (e.g., PGE-R) to luteolytic PG (PGF-R) receptor signaling may control the functional lifespan of the corpus luteum.
Thus the scenario detailed by Hamburger [98] several years ago can be updated to include a number of other changes in luteotropic vs. luteolytic factors as a function of age of the primate corpus luteum (Figure 3). As the balance tips from luteotropic to luteolytic signaling, the functional lifespan of the corpus luteum ends and menstruation occurs. It is well established that in the early luteal phase, LH pulses [48] are more frequent and luteal cells are most responsive to LH in terms of cAMP and P production. By mid-late luteal phase, there are fewer LH pulses and luteal cells are less responsive to LH; e.g., the dose-response curve for LH stimulation of cAMP is shifted [99]. The decreased sensitivity to LH is likely due to LHCGR desensitization, not down-regulation, as luteal LHCGR content does not decline until P levels decrease [100]. Thus LHCGR desensitization appears early as part of functional regression, whereas receptor down-regulation may manifest later primarily as part of structural regression. The decline in luteal sensitivity to LH appears critical, since studies by Hutchison [101] and Duffy [102] et al. demonstrated that sustaining LH levels either by promoting endogenous LH pulses or administering exogenous LH, did not prolong the luteal lifespan in macaques.
Figure 3.
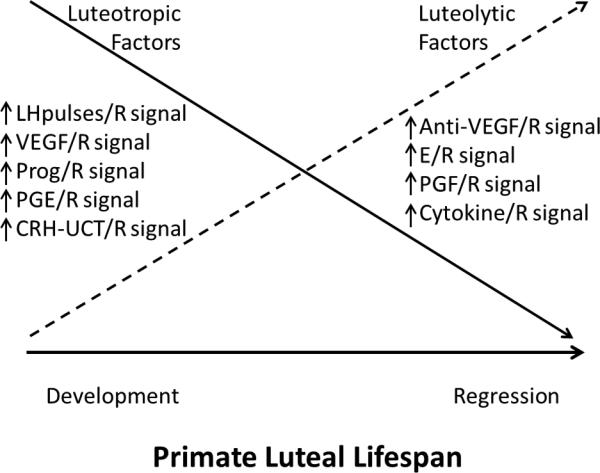
Schematic of proposed changes in the balance between luteotropic and luteolytic signals in the primate corpus luteum during its lifespan in the non-fecund menstrual cycle.
Coincident, and perhaps as a consequence of reduced LH support/sensitivity, the levels of local luteotropic factors and/or their receptors, and hence receptor signaling appear to decline as the corpus luteum of the cycle ages. For example, the ratio of VEGFA 165:121 isoforms declines after luteal development and the levels of VEGFA protein are markedly reduced by mid-late luteal phase at the onset of luteal regression [33]. Given the role of VEGF in maintaining the luteal vasculature [4], and perhaps non-vascular actions, one may speculate that the level of VEGF-R signaling is no longer sufficient by mid-luteal phase to meet its luteotropic role. Likewise, by mid-late luteal phase, progesterone secretion is entrained to the intermittent LH pulses such that the corpus luteum is producing a milieu of sequential intervals of progesterone repletion and depletion [48]. Moreover, the number of progesterone receptor-positive cells [95] and the ratio of PRA:B isoforms [103] in the corpus luteum diminish with age. As noted earlier [61], transcriptome studies established that the expression of pathways promoting PGE synthesis and receptor signaling were highest in the developing corpus luteum and diminished by the mid (e.g., PTGER3), mid-late (PTGS2) and late (PTGES) luteal phase.
Conversely, and perhaps as the result of local negative feedback loops or declining suppression by luteotropic factors, the levels of local luteolytic factors and or their receptors, and hence receptor signaling appear to increase as the corpus luteum ages. As noted in the earlier section on angiogenesis, expression of a number of vascular factors increases, some as proposed negative feedback regulators of VEGF-A (e.g., vasohibins, Figure 2), that may serve to check VEGF action or destabilize vessels (e.g., ANGPT2)[37] . Also, based on steroid depletion/progestin replacement studies [74], the intervals of progesterone repletion between pulses may promote ERβ expression and estrogen (which continues production) in later stages of the menstrual cycle. Likewise the intermittent progesterone pulses or decline in progesterone levels may remove its “immunosuppressive actions” and permit migration of immune cells into the corpus luteum and cytokine production that portends luteal regression in many species [104, 105]. Finally, the shift to PGFR expression [92], along with continued if not increased PGF synthase activity may promote PGF-receptor signaling in primate luteal tissue by mid-late luteal phase of the cycle. One can envision a local luteolytic effect of luteal PGF2α that is comparable to that proposed in domestic animals where the uterine PGF2α signal promotes intraluteal PGF2α production to amplify its luteolytic actions in the corpus luteum [106].
VII. Summary and future perspectives
The past three decades provided quantum increases in our understanding of the processes that promote luteal development, (e.g., angiogenesis), the cellular components of the corpus luteum, the control of steroidogenesis and the mechanisms of LH and CG action as luteotropic hormones. Nevertheless, key issues need to be resolved, such as the roles of local luteotropic and luteolytic factors in controlling the structure-function and lifespan of the corpus luteum during the menstrual cycle. Is there indeed, a factor or sequelae of factors that initiate luteolysis, and can we distinguish those involved in functional versus structural regression? Recent advances in genome analyses are permitting detection of dynamic changes in gene products as a function of luteal lifespan (Figure 4), that appear regulated by gonadotropin hormones (LH, CG) or local factors (e.g., progesterone). The stage is set for identification of gene products (via transcriptome, proteome analyses), perhaps novel or underappreciated, that control primate luteal activity and lifespan. A clear limitation to date has been the difficulty in selectively manipulating individual gene products to ascertain their function; pharmacologic approaches to ablate and replace substances are limited by their specificity and delivery. However, gene “knock-down” approaches, such as the direct or viral-mediated delivery of siRNAs into the luteinizing follicle or corpus luteum, offer promise. The ovarian primatologist faces a plethora of intriguing problems to further understand this unique gland, the corpus luteum, which is essential for fertility. Further advances are needed to improve or control fertility for such purposes as preserving endangered primate species, designing novel ovary-based contraceptive, and treating ovarian disorders in women's health
Figure 4.
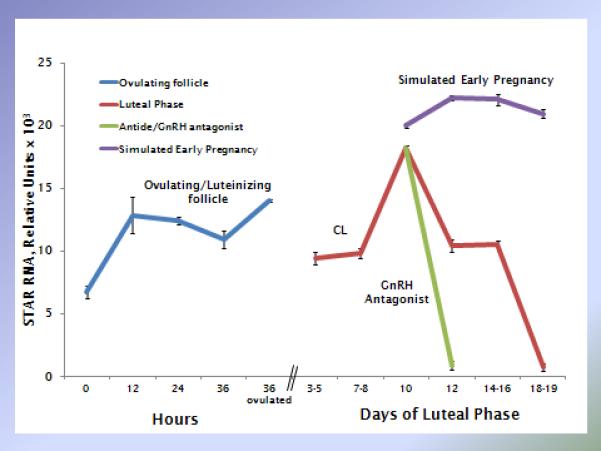
Example of the dynamic expression of a specific gene product (STAR mRNA, relative units) during the lifespan of the macaque corpus luteum, plus the effects of LH depletion during the menstrual cycle and hCG administration during simulated early pregnancy. Similar data analyses can be generated for any number of genes of interest from the publically available NCBI GEO databases (e.g., GSE2276, GSE10367, GSE25335). Data adapted from [9, 76, 92].
Acknowledgements
The expertise and support of members of the Division of Comparative Medicine in animal care, protocol performance and surgical procedures was critical to the success of described nonhuman primate studies at ONPRC. Likewise, the expertise and services of those in the research support cores, including the Endocrine Technology Support Laboratory, the Imaging and Morphology Core, the Molecular and Cellular Biology Core, the Assisted Reproductive Technologies Core at ONPRC, plus the Affymetrix Microarray Core, OHSU is greatly appreciated. Thanks to Ms. Traci Lea for preparation of this manuscript.
The authors’ research as summarized in this review was supported by several federal (NIH, USA) grants, including R01 HD020869 (to R.L.S.) R01 HD042000 (to J.D.H.), NICHD-supported cooperative research centers in infertility and reproduction (SCCPIR U54 HD018185) and contraceptive development and research (CDRC U54 HD055744), plus a research contract from Schering Female AMPPA (now Bayer Schering Pharma AG, Berlin, Germany). All animals and facilities for animal research were provided by ONPRC (P51 OD011092). Two Fellows (C.V.B, R.L.B) received federal training grant support (T32 HD007133).
Contributor Information
Richard L. Stouffer, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, 505 NW 185th Ave, Beaverton, OR, 97006, U.S.A., and Department of Obstetrics & Gynecology, College of Medicine, Oregon Health & Sciences University, 3181 SW Sam Jackson Park Road, Mail code KPV7C, Portland, Oregon, 97239, U.S.A., stouffri@ohsu.edu, 1-503-690-5366
Cecily V. Bishop, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, 505 NW 185th Ave, Beaverton, OR, 97006, U.S.A., bishopc@ohsu.edu, 1-503-533-2422
Randy L. Bogan, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, 505 NW 185th Ave, Beaverton, OR, 97006, U.S.A., boganr@ohsu.edu, 1-503-614-3720
Fuhua Xu, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, 505 NW 185th Ave, Beaverton, OR, 97006, U.S.A., xuf@ohsu.edu, 1-503-690-5369.
Jon D. Hennebold, Division of Reproductive & Developmental Sciences, Oregon National Primate Research Center, 505 NW 185th Ave, Beaverton, OR, 97006, U.S.A., and Department of Obstetrics & Gynecology, College of Medicine, Oregon Health & Sciences University, 3181 SW Sam Jackson Park Road, Mail code KPV7C, Portland, Oregon, 97239, U.S.A., henneboj@ohsu.edu, 1-503-614-3720
References
- 1.Stouffer RL, Hennebold JD. Structure, Function and Regulation of the Corpus Luteum. In: Plant TM, Zeleznik A, editors. Knobil and Neill's Physiology of Reproduction. Third Ed. Elsevier Academic Press; San Diego: 2013. In Press. [Google Scholar]
- 2.Fraenkel L. Die function des corpus luteum. Arch Gynaekol. 1903(20):461–466. [Google Scholar]
- 3.Zeleznik AJ, Benyo DF. Control of follicular development, corpus luteum function, and the recognition of pregnancy in higher primates. In: Neill JD, Knobil E, editors. Physiology of Reproduction. 2nd Ed. Academic Press; St Louis: 2006. [Google Scholar]
- 4.Fraser HM, Duncan WC. SRB Reproduction, Fertility and Development Award Lecture 2008. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod Fertil Dev. 2009;21(3):377–392. doi: 10.1071/rd08272. [DOI] [PubMed] [Google Scholar]
- 5.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 6.Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12:297–307. doi: 10.2741/2065. [DOI] [PubMed] [Google Scholar]
- 7.Chaffin CL, Hess DL, Stouffer RL. Dynamics of periovulatory steroidogenesis in the rhesus monkey follicle after ovarian stimulation. Hum Reprod. 1999;14(3):642–649. doi: 10.1093/humrep/14.3.642. [DOI] [PubMed] [Google Scholar]
- 8.Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod. 2007;22(5):1247–1252. doi: 10.1093/humrep/del519. [DOI] [PubMed] [Google Scholar]
- 9.Xu F, Stouffer RL, Muller J, Hennebold JD, Wright JW, Bahar A, Leder G, Peters M, Thorne M, Sims M, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17(3):152–165. doi: 10.1093/molehr/gaq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18(11):2257–2263. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
- 11.Peluffo MC, Ting AY, Zamah AM, Conti M, Stouffer RL, Zelinski MB, Hennebold JD. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. 2012;27(8):2430–2437. doi: 10.1093/humrep/des158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George JT, Seminara SB. Kisspeptin and the hypothalamic control of reproduction: lessons from the human. Endocrinology. 2012;153(11):5130–5136. doi: 10.1210/en.2012-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saadeldin IM, Koo OJ, Kang JT, Kwon DK, Park SJ, Kim SJ, Moon JH, Oh HJ, Jang G, Lee BC. Paradoxical effects of kisspeptin: it enhances oocyte in vitro maturation but has an adverse impact on hatched blastocysts during in vitro culture. Reprod Fertil Dev. 2012;24(5):656–668. doi: 10.1071/RD11118. [DOI] [PubMed] [Google Scholar]
- 14.Peng J, Tang M, Zhang BP, Zhang P, Zhong T, Zong T, Yang B, Kuang HB. Kisspeptin stimulates progesterone secretion via the Erk1/2 mitogen-activated protein kinase signaling pathway in rat luteal cells. Fertil Steril. 2013;99(5):1436–1443. doi: 10.1016/j.fertnstert.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Jeon YE, Lee KE, Jung JA, Yim SY, Kim H, Seo SK, Cho S, Choi YS, Lee BS. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2013;75(4):268–274. doi: 10.1159/000350217. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama K. Cellular signal transduction of the hypoxia response. J Biochem. 2009;146(6):757–765. doi: 10.1093/jb/mvp167. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150(7):3392–3400. doi: 10.1210/en.2008-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Driesche S, Myers M, Gay E, Thong KJ, Duncan WC. HCG up-regulates hypoxia inducible factor-1 alpha in luteinized granulosa cells: implications for the hormonal regulation of vascular endothelial growth factor A in the human corpus luteum. Mol Hum Reprod. 2008;14(8):455–464. doi: 10.1093/molehr/gan040. [DOI] [PubMed] [Google Scholar]
- 19.Richards JS, Liu Z, Shimada M. Immune-like mechanisms in ovulation. Trends Endocrinol Metab. 2008;19(6):191–196. doi: 10.1016/j.tem.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Bishop CV, Bogan RL, Hennebold JD, Stouffer RL. Analysis of microarray data from the macaque corpus luteum; the search for common themes in primate luteal regression. Mol Hum Reprod. 2011;17(3):143–151. doi: 10.1093/molehr/gaq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoroddsen A, Dahm-Kahler P, Lind AK, Weijdegard B, Lindenthal B, Muller J, Brannstrom M. The water permeability channels aquaporins 1-4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J Clin Endocrinol Metab. 2011;96(4):1021–1028. doi: 10.1210/jc.2010-2545. [DOI] [PubMed] [Google Scholar]
- 22.Richards JS. Genetics of ovulation. Semin Reprod Med. 2007;25(4):235–242. doi: 10.1055/s-2007-980217. [DOI] [PubMed] [Google Scholar]
- 23.McCord LA, Li F, Rosewell KL, Brannstrom M, Curry TE. Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat. Biol Reprod. 2012;86(3):78. doi: 10.1095/biolreprod.111.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors in cumulus oocyte complexes: novel evidence for innate immune-like functions during ovulation. Mol Endocrinol. 2006;20(12):3228–3239. doi: 10.1210/me.2006-0194. [DOI] [PubMed] [Google Scholar]
- 25.Hazzard TM, Stouffer RL. Angiogenesis in ovarian follicular and luteal development. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(6):883–900. doi: 10.1053/beog.2000.0133. [DOI] [PubMed] [Google Scholar]
- 26.Christenson LK, Stouffer RL. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology. 1996;137(1):367–374. doi: 10.1210/endo.137.1.8536637. [DOI] [PubMed] [Google Scholar]
- 27.Redmer DA, Doraiswamy V, Bortnem BJ, Fisher K, Jablonka-Shariff A, Grazul-Bilska AT, Reynolds LP. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol Reprod. 2001;65(3):879–889. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 29.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28(9):488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 30.Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc Med. 2002;12(1):13–19. doi: 10.1016/s1050-1738(01)00140-2. [DOI] [PubMed] [Google Scholar]
- 31.Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999;5(12):1115–1121. doi: 10.1093/molehr/5.12.1115. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Chequer JC, Stouffer RL, Hazzard TM, Patton PE, Molskness TA. Insulin-like growth factors-1 and -2, but not hypoxia, synergize with gonadotropin hormone to promote vascular endothelial growth factor-A secretion by monkey granulosa cells from preovulatory follicles. Biol Reprod. 2003;68(4):1112–1118. doi: 10.1095/biolreprod.102.011155. [DOI] [PubMed] [Google Scholar]
- 33.Tesone M, Stouffer RL, Borman SM, Hennebold JD, Molskness TA. Vascular endothelial growth factor (VEGF) production by the monkey corpus luteum during the menstrual cycle: isoform-selective messenger RNA expression in vivo and hypoxia-regulated protein secretion in vitro. Biol Reprod. 2005;73(5):927–934. doi: 10.1095/biolreprod.105.039875. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab. 2001;86(2):768–772. doi: 10.1210/jcem.86.2.7181. [DOI] [PubMed] [Google Scholar]
- 35.Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67(4):1305–1312. doi: 10.1095/biolreprod67.4.1305. [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277(5322):48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 37.Hazzard TM, Christenson LK, Stouffer RL. Changes in expression of vascular endothelial growth factor and angiopoietin-1 and -2 in the macaque corpus luteum during the menstrual cycle. Mol Hum Reprod. 2000;6(11):993–998. doi: 10.1093/molehr/6.11.993. [DOI] [PubMed] [Google Scholar]
- 38.Xu F, Stouffer RL. Local delivery of angiopoietin-2 into the preovulatory follicle terminates the menstrual cycle in rhesus monkeys. Biol Reprod. 2005;72(6):1352–1358. doi: 10.1095/biolreprod.104.037143. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y. The vasohibin family: a novel family for angiogenesis regulation. J Biochem. 2013;153(1):5–11. doi: 10.1093/jb/mvs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirasuna K, Kobayashi A, Nitta A, Nibuno S, Sasahara K, Shimizu T, Bollwein H, Miyamoto A. Possible action of vasohibin-1 as an inhibitor in the regulation of vascularization of the bovine corpus luteum. Reproduction. 2012;143(4):491–500. doi: 10.1530/REP-11-0465. [DOI] [PubMed] [Google Scholar]
- 41.Fraser HM, Hastings JM, Allan D, Morris KD, Rudge JS, Wiegand SJ. Inhibition of delta-like ligand 4 induces luteal hypervascularization followed by functional and structural luteolysis in the primate ovary. Endocrinology. 2012;153(4):1972–1983. doi: 10.1210/en.2011-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu F, Stouffer RL. Dynamic expression of apelin and its receptor in the primate preovulatory follicles and corpus luteum during the menstrual cycle. Biol Reprod. 2012;83(Suppl 1) Abstract #169. [Google Scholar]
- 43.Xu F, Stouffer RL. Existence of the lymphatic system in the primate corpus luteum. Lymphat Res Biol. 2009;7(3):159–168. doi: 10.1089/lrb.2009.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown HM, Robker RL, Russell DL. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology. 2010;151(11):5446–5455. doi: 10.1210/en.2010-0629. [DOI] [PubMed] [Google Scholar]
- 45.Shirasuna K, Nitta A, Sineenard J, Shimizu T, Bollwein H, Miyamoto A. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest Anim Endocrinol. 2012;43(2):198–211. doi: 10.1016/j.domaniend.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Knobil E. On the regulation of the primate corpus luteum. Biol Reprod. 1973;8:246–258. [Google Scholar]
- 47.Stouffer RL. Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update. 2003;9(2):99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- 48.Ellinwood WE, Norman RL, Spies HG. Changing frequency of pulsatile luteinizing hormone and progesterone secretion during the luteal phase of the menstrual cycle of rhesus monkeys. Biol Reprod. 1984;31(4):714–722. doi: 10.1095/biolreprod31.4.714. [DOI] [PubMed] [Google Scholar]
- 49.Bousfield GR, Jia L, Ward DN. Gonadotropins: Chemistry and Biosynthesis. In: Neill JD, Knobil E, editors. Physiology of Reproduction. 2nd Ed. Academic Press; St. Louis: 2006. [chapter30] [Google Scholar]
- 50.Hunzicker-Dunn M, Mayo K. Gonadotropin signaling in the ovary. In: Neill JD, Knobil E, editors. Knobil and Neill's Physiology of Reproduction. 2nd Ed. Academic Press; St. Louis: 2006. [chapter14] [Google Scholar]
- 51.Houmard BS, Guan Z, Stokes BT, Ottobre JS. The effects of gonadotropin on the phosphatidylinositol pathway in the primate corpus luteum. Mol Cell Endocrinol. 1994;104(1):113–120. doi: 10.1016/0303-7207(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 52.Stouffer RL. The functions and regulation of cell populations comprising the corpus luteum during the ovarian cycle. In: Leung PCK, Adashi EY, editors. The Ovary. Elsevier Science; San Diego: 2004. [Google Scholar]
- 53.Sasano H, Suzuki T. Localization of steroidogenesis and steroid receptors in human corpus luteum. Classification of human corpus luteum (CL) into estrogen-producing degenerating CL, and nonsteroid-producing degenerating CL. Semin Reprod Endocrinol. 1997;15(4):345–351. doi: 10.1055/s-2008-1068372. [DOI] [PubMed] [Google Scholar]
- 54.Sanders SL, Stouffer RL, Brannian JD. Androgen production by monkey luteal cell subpopulations at different stages of the menstrual cycle. J Clin Endocrinol Metab. 1996;81(2):591–596. doi: 10.1210/jcem.81.2.8636273. [DOI] [PubMed] [Google Scholar]
- 55.Bishop CV, Hennebold JD, Stouffer RL. The effects of luteinizing hormone ablation/replacement versus steroid ablation/replacement on gene expression in the primate corpus luteum. Mol Hum Reprod. 2009;15(3):181–193. doi: 10.1093/molehr/gap005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Priyanka S, Jayaram P, Sridaran R, Medhamurthy R. Genome-wide gene expression analysis reveals a dynamic interplay between luteotropic and luteolytic factors in the regulation of corpus luteum function in the bonnet monkey (Macaca radiata). Endocrinology. 2009;150(3):1473–1484. doi: 10.1210/en.2008-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu J, Xu F, Hennebold JD, Molskness TA, Stouffer RL. Expression and role of the corticotropin-releasing hormone/urocortin-receptor-binding protein system in the primate corpus luteum during the menstrual cycle. Endocrinology. 2007;148(11):5385–5395. doi: 10.1210/en.2007-0541. [DOI] [PubMed] [Google Scholar]
- 58.Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010;85(1):33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Yata A, Nakabayashi K, Wakahashi S, Maruo N, Ohara N, Maruo T. Suppression of progesterone production by stresscopin/urocortin 3 in cultured human granulosa-lutein cells. Hum Reprod. 2009;24(7):1748–1753. doi: 10.1093/humrep/dep063. [DOI] [PubMed] [Google Scholar]
- 60.Dinopoulou V, Partsinevelos GA, Mavrogianni D, Anagnostou E, Drakakis P, Makrigiannakis A, Chrousos GP, Loutradis D. The effect of CRH and its inhibitor, antalarmin, on in vitro growth of preantral mouse follicles, early embryo development, and steroidogenesis. Endocrinology. 2013;154(1):222–231. doi: 10.1210/en.2012-1838. [DOI] [PubMed] [Google Scholar]
- 61.Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Prostaglandin synthesis, metabolism, and signaling potential in the rhesus macaque corpus luteum throughout the luteal phase of the menstrual cycle. Endocrinology. 2008;149(11):5861–5871. doi: 10.1210/en.2008-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dennefors BL, Sjogren A, Hamberger L. Progesterone and adenosine 3′,5′-monophosphate formation by isolated human corpora lutea of different ages: influences of human chorionic gonadotropin and prostaglandins. J Clin Endocrinol Metab. 1982;55(1):102–107. doi: 10.1210/jcem-55-1-102. [DOI] [PubMed] [Google Scholar]
- 63.Peluffo M, Murphy M, Zelinski M, Lindenthal B, Stouffer R. Cumulus-oocyte expansion in rhesus macaques: A critical role for prostaglandin E2 (PGE2) and the PGE2 receptor subtype-2 (PTGER2). Biol Reprod. 2011;84(Suppl 1) Abstract #O-146. [Google Scholar]
- 64.Molskness TA, VandeVoort CA, Stouffer RL. Stimulatory and inhibitory effects of prostaglandins on the gonadotropin-sensitive adenylate cyclase in the monkey corpus luteum. Prostaglandins. 1987;34(2):279–290. doi: 10.1016/0090-6980(87)90250-4. [DOI] [PubMed] [Google Scholar]
- 65.Stouffer RL, Nixon WE, Hodgen GD. Disparate effects of prostaglandins on basal and gonadotropin-stimulated progesterone production by luteal cells isolated from rhesus monkeys during the menstrual cycle and pregnancy. Biol Reprod. 1979;20(4):897–903. doi: 10.1095/biolreprod20.4.897. [DOI] [PubMed] [Google Scholar]
- 66.Peluffo MC, Stouffer RL, Stanley J, Hennebold JD, Zelinski MB, Lindenthal B. Contraceptive trial testing a prostaglandin E2 receptor (EP2) antagonist in female monkeys. Fertil Steril. 2012;98(3):S334. [Google Scholar]
- 67.Chandrasekher YA, Melner MH, Nagalla SR, Stouffer RL. Progesterone receptor, but not estradiol receptor, messenger ribonucleic acid is expressed in luteinizing granulosa cells and the corpus luteum in rhesus monkeys. Endocrinology. 1994;135(1):307–314. doi: 10.1210/endo.135.1.8013365. [DOI] [PubMed] [Google Scholar]
- 68.Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci U S A. 1996;93(5):1897–1901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conneely OM. Progesterone receptors and ovulation. Handb Exp Pharmacol. 2010;(198):37–44. doi: 10.1007/978-3-642-02062-9_3. [DOI] [PubMed] [Google Scholar]
- 70.Rothchild I. The regulation of the mammalian corpus luteum. Recent Prog Horm Res. 1981;37:183–298. doi: 10.1016/b978-0-12-571137-1.50009-8. [DOI] [PubMed] [Google Scholar]
- 71.Duffy DM, Hess DL, Stouffer RL. Acute administration of a 3 beta-hydroxysteroid dehydrogenase inhibitor to rhesus monkeys at the midluteal phase of the menstrual cycle: evidence for possible autocrine regulation of the primate corpus luteum by progesterone. J Clin Endocrinol Metab. 1994;79(6):1587–1594. doi: 10.1210/jcem.79.6.7989460. [DOI] [PubMed] [Google Scholar]
- 72.Duffy DM, Stouffer RL. Gonadotropin versus steroid regulation of the corpus luteum of the rhesus monkey during simulated early pregnancy. Biol Reprod. 1997;57(6):1451–1460. doi: 10.1095/biolreprod57.6.1451. [DOI] [PubMed] [Google Scholar]
- 73.Young KA, Stouffer RL. Gonadotropin and steroid regulation of matrix metalloproteinases and their endogenous tissue inhibitors in the developed corpus luteum of the rhesus monkey during the menstrual cycle. Biol Reprod. 2004;70(1):244–252. doi: 10.1095/biolreprod.103.022053. [DOI] [PubMed] [Google Scholar]
- 74.Duffy DM, Chaffin CL, Stouffer RL. Expression of estrogen receptor alpha and beta in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology. 2000;141(5):1711–1717. doi: 10.1210/endo.141.5.7477. [DOI] [PubMed] [Google Scholar]
- 75.Peluso JJ. Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med. 2007;25(3):198–207. doi: 10.1055/s-2007-973432. [DOI] [PubMed] [Google Scholar]
- 76.Bishop CV, Satterwhite S, Xu L, Hennebold JD, Stouffer RL. Microarray analysis of the primate luteal transcriptome during chorionic gonadotrophin administration simulating early pregnancy. Mol Hum Reprod. 2012;18(4):216–227. doi: 10.1093/molehr/gar073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab. 2009;94(7):2644–2649. doi: 10.1210/jc.2009-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stouffer RL, Hearn JP. Endocrinology of the transition from menstrual cyclicity to establishment of pregnancy in primates. The Endocrinology of Pregnancy. 1998:35. [Google Scholar]
- 79.Gupta P, Banker M, Patel P, Joshi B. A study of recipient related predictors of success in oocyte donation program. J Hum Reprod Sci. 2012;5(3):252–257. doi: 10.4103/0974-1208.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.VandeVoort CA, Mtango NR, Latham KE, Stewart DR. Primate preimplantation embryo is a target for relaxin during early pregnancy. Fertil Steril. 2011;96(1):203–207. doi: 10.1016/j.fertnstert.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vodstrcil LA, Tare M, Novak J, Dragomir N, Ramirez RJ, Wlodek ME, Conrad KP, Parry LJ. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 2012;26(10):4035–4044. doi: 10.1096/fj.12-210567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R267–275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Booher C, Enders AC, Hendrickx AG, Hess DL. Structural characteristics of the corpus luteum during implantation in the rhesus monkey (Macaca mulatta). Am J Anat. 1981;160(1):17–36. doi: 10.1002/aja.1001600103. [DOI] [PubMed] [Google Scholar]
- 84.Zeleznik AJ. In vivo responses of the primate corpus luteum to luteinizing hormone and chorionic gonadotropin. Proc Natl Acad Sci U S A. 1998;95(18):11002–11007. doi: 10.1073/pnas.95.18.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niswender GD, Roess DA, Sawyer HR, Silvia WJ, Barisas BG. Differences in the lateral mobility of receptors for luteinizing hormone (LH) in the luteal cell plasma membrane when occupied by ovine LH versus human chorionic gonadotropin. Endocrinology. 1985;116(1):164–169. doi: 10.1210/endo-116-1-164. [DOI] [PubMed] [Google Scholar]
- 86.Molskness TA, Zelinski-Wooten MB, Hild-Petito SA, Stouffer RL. Comparison of the steroidogenic response of luteinized granulosa cells from rhesus monkeys to luteinizing hormone and chorionic gonadotropin. Biol Reprod. 1991;45(2):273–281. doi: 10.1095/biolreprod45.2.273. [DOI] [PubMed] [Google Scholar]
- 87.Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, Pignatti E, Simoni M. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One. 2012;7(10):e46682. doi: 10.1371/journal.pone.0046682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Becker J, Walz A, Daube S, Keck C, Pietrowski D. Distinct responses of human granulosa lutein cells after hCG or LH stimulation in a spheroidal cell culture system. Mol Reprod Dev. 2007;74(10):1312–1316. doi: 10.1002/mrd.20696. [DOI] [PubMed] [Google Scholar]
- 89.Castracane VD, Stevens V, Knickerbocker J, Powell J, Randolph M, Gimpel T. Late luteal rescue in the baboon (Papio cynocephalus). Hum Reprod Update. 1998;4(4):383–388. doi: 10.1093/humupd/4.4.383. [DOI] [PubMed] [Google Scholar]
- 90.O'Shea JD, Wright PJ. Regression of the corpus luteum of pregnancy following parturition in the ewe. Acta Anat (Basel) 1985;122(2):69–76. doi: 10.1159/000145985. [DOI] [PubMed] [Google Scholar]
- 91.Treloar OL, Wolf RC, Meyer RK. The corpus luteum of the rhesus monkey during late pregnancy. Endocrinology. 1972;91(3):665–668. doi: 10.1210/endo-91-3-665. [DOI] [PubMed] [Google Scholar]
- 92.Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol. 2008;22(5):1260–1273. doi: 10.1210/me.2007-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castracane VD, Moore GT, Shaikh AA. Ovarian function of hysterectomized Macaca fascicularis. Biol Reprod. 1979;20(3):462–472. doi: 10.1095/biolreprod20.3.462. [DOI] [PubMed] [Google Scholar]
- 94.Ranney B, Abu-Ghazaleh S. The future function and fortune of ovarian tissue which is retained in vivo during hysterectomy. Am J Obstet Gynecol. 1977;128(6):626–634. doi: 10.1016/0002-9378(77)90208-3. [DOI] [PubMed] [Google Scholar]
- 95.Hild-Petito S, Stouffer RL, Brenner RM. Immunocytochemical localization of estradiol and progesterone receptors in the monkey ovary throughout the menstrual cycle. Endocrinology. 1988;123(6):2896–2905. doi: 10.1210/endo-123-6-2896. [DOI] [PubMed] [Google Scholar]
- 96.Auletta FJ, Flint AP. Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocr Rev. 1988;9(1):88–105. doi: 10.1210/edrv-9-1-88. [DOI] [PubMed] [Google Scholar]
- 97.Sargent EL, Baughman WL, Novy MJ, Stouffer RL. Intraluteal infusion of a prostaglandin synthesis inhibitor, sodium meclofenamate, causes premature luteolysis in rhesus monkeys. Endocrinology. 1988;123(5):2261–2269. doi: 10.1210/endo-123-5-2261. [DOI] [PubMed] [Google Scholar]
- 98.Hamberger L, Hahlin M, Hillensjo T, Johanson C, Sjogren A. Luteotropic and luteolytic factors regulating human corpus luteum function. Ann N Y Acad Sci. 1988;541:485–497. doi: 10.1111/j.1749-6632.1988.tb22285.x. [DOI] [PubMed] [Google Scholar]
- 99.Eyster KM, Ottobre JS, Stouffer RL. Adenylate cyclase in the corpus luteum of the rhesus monkey. III. Changes in basal and gonadotropin-sensitive activities during the luteal phase of the menstrual cycle. Endocrinology. 1985;117(4):1571–1577. doi: 10.1210/endo-117-4-1571. [DOI] [PubMed] [Google Scholar]
- 100.Cameron JL, Stouffer RL. Gonadotropin receptors of the primate corpus luteum. II. Changes in available luteinizing hormone- and chorionic gonadotropin-binding sites in macaque luteal membranes during the nonfertile menstrual cycle. Endocrinology. 1982;110(6):2068–2073. doi: 10.1210/endo-110-6-2068. [DOI] [PubMed] [Google Scholar]
- 101.Hutchison JS, Nelson PB, Zeleznik AJ. Effects of different gonadotropin pulse frequencies on corpus luteum function during the menstrual cycle of rhesus monkeys. Endocrinology. 1986;119(5):1964–1971. doi: 10.1210/endo-119-5-1964. [DOI] [PubMed] [Google Scholar]
- 102.Duffy DM, Stewart DR, Stouffer RL. Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab. 1999;84(1):342–349. doi: 10.1210/jcem.84.1.5362. [DOI] [PubMed] [Google Scholar]
- 103.Duffy DM, Wells TR, Haluska GJ, Stouffer RL. The ratio of progesterone receptor isoforms changes in the monkey corpus luteum during the luteal phase of the menstrual cycle. Biol Reprod. 1997;57(4):693–699. doi: 10.1095/biolreprod57.4.693. [DOI] [PubMed] [Google Scholar]
- 104.Brannstrom M, Friden B. Immune regulation of corpus luteum function. Semin Reprod Endocrinol. 1997;15(4):363–370. doi: 10.1055/s-2008-1068374. [DOI] [PubMed] [Google Scholar]
- 105.Walusimbi SS, Pate JL. Physiology and Endocrinology Symposium: role of immune cells in the corpus luteum. J Anim Sci. 2013;91(4):1650–1659. doi: 10.2527/jas.2012-6179. [DOI] [PubMed] [Google Scholar]
- 106.Atli MO, Bender RW, Mehta V, Bastos MR, Luo W, Vezina CM, Wiltbank MC. Patterns of gene expression in the bovine corpus luteum following repeated intrauterine infusions of low doses of prostaglandin F2alpha. Biol Reprod. 2012;86(4):130. doi: 10.1095/biolreprod.111.094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu F, Hurliman AK, Stouffer RL. Dynamic Expression of the SLIT-ROBO Pathway in the Periovulatory Follicle and Corpus Luteum in Primates. Biol Reprod. 2010;83(Suppl 1) Abstract #216. [Google Scholar]