Abstract
Aim:
Hypericin (Hyp) and its radio-derivatives have been investigated in animal models with ischemic heart diseases and malignancies for diagnostic and therapeutic purposes. Before radioiodinated Hyp (123I-Hyp or 131I-Hyp) can be considered as a clinically useful drug, vigorous evaluations on its chemotoxicity are necessary. In the present study, we examined the toxicity of a single dose of non-radioactive 127I-Hyp in normal mice for 24 h and 14 d.
Methods:
Studies were performed on 132 normal mice. 127I -Hyp at a clinically relevant dose of 0.1 mg/kg body weight and a 100-times higher dose of 10 mg/kg was intravenously injected into 40 mice. The safety aspects of clinical manifestations, serological biochemistry, and histopathology were assessed. In another 72 mice, 127I-Hyp was administered intravenously at assumed values to bracket the value of LD50. The rest 20 mice were used in the control groups.
Results:
At 24 h and 14 d following the injection of 127I -Hyp at either 0.1 or 10 mg/kg, all mice tolerated well without mortality or any observable treatment-related symptoms. No significant differences were found in blood biochemical parameters between the test and control groups. All organs presented normal appearances upon histopathological inspection. The value of LD50 of 127I-Hyp in mice through intravenous injection was 20.26 mg/kg, with the 95% confidence interval between 18.90 and 21.55 mg/kg.
Conclusion:
The current study reveals a broad safety range of 127I-Hyp, which not only supports the use of 123I-Hyp or 131I-Hyp in the necrosis targeting theragnostic strategy, but also serves as a valuable reference for exploring other possible applications for iodinated Hyp.
Keywords: Hypericin, iodinated compound, single dose toxicity, LD50, targeted anticancer therapy
Introduction
Hypericum perforatum L, commonly known as St John's Wort1, has been widely used as an herbal medicine since the time of ancient Greece2. This plant has attracted interest because of its wound-healing, bactericidal, anti-inflammatory, diuretic and sedative properties3,4. Hypericin (Hyp), a bioactive ingredient isolated from Hypericum perforatum, possesses the major pharmacological activities found in Hypericum perforatum. In addition to Hypericum perforatum, Hyp has also been found in some fungi and animal species5. Chemically, Hyp is a red-colored, polycyclic, polyaromatic quinine that structurally belongs to the naphthodianthrones class5,6. Hyp can either be isolated from the plant or semi-synthetically obtained from the anthraquinone derivative emodin7,8.
Hyp exhibits a wide range of pharmacologically interesting properties. Remarkable research and clinical applications include the antidepressant, antiviral, and antitumoral properties of Hyp5. In addition, Hyp has also been used for cancer photodynamic therapy (PDT)5,9. The potent photosensitizing property has made Hyp possibly the most powerful naturally occurring photosensitizer described thus far8. Although numerous pharmacological findings on the potential clinical uses of Hyp have been published, the preclinical efficacy concerning the above utilities still remains controversial10. During a recent contrast agent study, Ni et al further expanded the role of Hyp in medicine by revealing that the necrosis avidity of Hyp is superior to its tumor affinity6,11,12,13. Therefore, the necrosis avidity of radioiodinated Hyp, such as [123I]iodohypericin (123I-Hyp), has been used as a diagnostic tool for imaging ischemic myocardial infarction and therapeutic tumor necrosis11,12,13,14,15,16,17. Additionally, the potential use of 131I-Hyp for the treatment of cancer has been investigated with encouraging results, which have been demonstrated in rodent tumor models13,15.
Although Hyp is very promising and highly researched, phenomena associated with the use of Hyp and its derivatives, such as insolubilization, aggregation, fusion, and relative instability, could hinder the applications of Hyp. The relevance of the chemical and physical properties to the pharmaceutical application of Hyp has been discussed extensively7,18,19,20,21,22,23,24,25,26,27,28. However, specific clinical applications become more restricted when the focus is narrowed to those newly discovered activities. The toxicological properties of Hypericum perforatum have been tested in different animals for different clinical applications. The toxicity of Hypericum extract LI160, which contains Hyp, and its analog pseudohypericin have been tested orally, both acutely and over a period of 26 weeks in mice, rats, and dogs. The first intolerance reactions appeared at 900 mg·kg−1·d−1, and the LD50 (half lethal dose) was greater than 5000 mg/kg21. Fewer evaluations have been made regarding the toxicity of Hyp and any of its radioiodinated derivatives. Most of the previous studies with Hyp were performed either in vitro or under oral or intraperitoneal administration instead of intravenous (iv) injection, which has provided less valuable information about the novel applications of Hyp. Fox et al demonstrated that the iv application of Hyp was well tolerated by rhesus monkeys at a dose of 2 mg/kg, but a transient, severe photosensitivity rash occurred at 5 mg/kg20. In a phase I study, AIDS patients received Hyp intravenously at 30–40 mg twice weekly. Phototoxic reactions (facial pain and erythema) presented after direct exposure to sunlight within 8 weeks of treatment22. The LD50 of Hyp was reported to be greater than 23 600 mg/kg in a study that administered Hyp orally in mice23. Although some data appear to indicate low toxicity for St John's Wort or Hyp, previous preparations containing Hyp were largely unregulated and contained varying amounts of the drug24. However, data on the toxicity of a single iv dose of Hyp are extremely limited. The LD50 of Hyp via iv injection still remains absent, which is an essential index for characterizing and quantitatively predicting the acute toxicity effects of any chemical. Lack of the LD50 for Hyp might hinder the understanding and clinical progress of Hyp and its derivatives.
With regard to 131I-Hyp, which was used in recent research15, the radio-toxicities of Iodine-131 have already been well defined in various clinical applications for many decades25,26,27,28. However, the chemotoxicity of 131I-Hyp still remains unknown. Therefore, a rigorous evaluation of the toxicity of non-radioactive iodinated Hyp (I-Hyp) would enable and promote the applications of either Hyp or its iodinated derivatives as potentially useful drugs. We conducted the present toxicity study with a single dose of I-Hyp in normal mice for a period of 24 h and 14 d. We paid special intention to providing the chemotoxicity information of the novel applications of I-Hyp in the recently developed necrosis-targeted anticancer theragnostic strategy and also a valuable reference safety profile for other possible applications.
Materials and methods
Preparation of non-radioactive iodinated hypericin
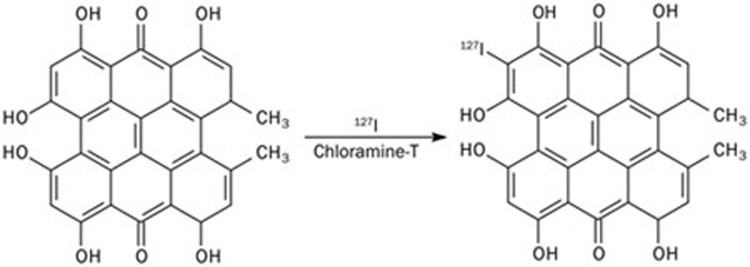
Hyp was purchased from Planta, Austria with a purity >98.6% HPLC (minimum 98.6%) (http://www.planta.at/hyper/hyper.htm). I-Hyp was synthesized by a standard electrophilic substitution reaction that combined Hyp and sodium iodide-127 in the presence of chloramine-T in acetonitrile at pH 7.4 for 30 min (Figure 1). For purification, the compound was precipitated using sodium chloride and removed by centrifugation. The purified I-Hyp was analyzed by reverse-phase high-performance liquid chromatography (RP-HPLC) and mass spectrometry (MS). The RP-HPLC was equipped with an RP C18 column (XterraTM C18, 5 μm, 4.6 mm×150 mm, Waters, Milford MA, USA) and a UV detector (254 nm). A mixture solvent consisting of acetonitrile-5 mmol/L NH4OAc (75:25, v/v) at a flow rate of 1 mL/min was used as the mobile phase. Mass spectrometry analysis was performed with a time of flight mass spectrometer equipped with an orthogonal ESI probe (Micromass LCT, Manchester, UK) in negative ion mode. Both techniques confirmed the presence of the compound. Then, the compound was re-dissolved in dimethylsulfoxide (DMSO) at a concentration of 8.8 mg/mL.
Figure 1.
Synthesis of nonradioactive iodinated Hyp (127I-Hyp). Being a polyphenolic polycyclic quinone, Hyp can be labeled with iodine-127 in the ortho position of a phenol group.
Animals
This animal experiment was approved by the institutional ethical committee on animal care and supply. Fifty healthy, normal CD-1 mice (Swiss, 25 male and 25 female) aged 6–8 weeks and weighing between 26–32 g (female) and 37–43 g (male) at the onset of dosing were used for the toxicity study. In addition, normal mice of both genders in equal number weighing approximately 25–30 g were subjected to LD50 studies (n=72) and to acute toxicity controls of DMSO (n=10). The females were nulliparous and nonpregnant. All mice were housed in polycarbonate cages with wood filing bedding, and food and water were given ad libitum. Each animal was marked on the tail with a different color by permanent marker pens. All mice were acclimatized to the laboratory conditions for 7 d prior to the initiation of dosing. Environmental conditions were maintained at a temperature of 22±2 °C with a relative humidity of 60%±10% and under an equal indoor daily light circle.
Experiments
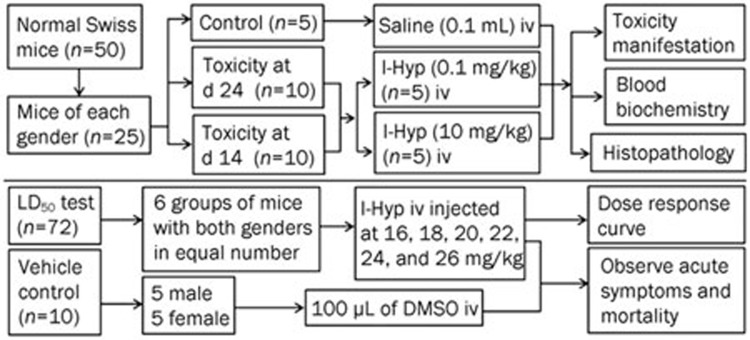
As illustrated in Figure 2, mice of the same sex were randomly assigned to the control group (n=5), the 24 h group (n=10) or the 14 d group (n=10). The latter two groups were further divided into low dose and high dose groups. Mice in the control group received 0.1 mL of normal saline solution, and the other groups received I-Hyp at either a low or a high dose. The low dose represents the recommended clinical dose (0.1 mg/kg body weight) that has been used in anticancer therapy14, and the high dose is 100-times greater than the low dose. The mice were anesthetized in an induction chamber with 2% isoflurane gas in a mixture of 20% oxygen and 80% room air at a flow rate of 2 L/min, and the anesthesia was maintained through a mask during the injection. All iv injections were performed via penile vein (male) or tail vein (female) by an experienced technician. The clinical manifestations, including mortality, change of body weight, general activity level, and food and water intake, were monitored. Blood samples were collected in all mice for standard clinical biochemistry analyses. After blood collection, all mice were euthanized. Internal organs such as the liver, heart, kidney and lungs were excised for gross inspection and histopathological study.
Figure 2.
Flow diagram of toxicity experiments with I-Hyp in normal Swiss mice.
Toxicity at 24 h post exposure
Mice were designated to low-dose male, low-dose female, high-dose male and high-dose female groups. I-Hyp was iv administered at 0.1 and 10 mg/kg body weight in 100 μL of DMSO solution to the low- and high-dose groups, respectively. Observations were recorded systemically for morbidity, eating and drinking, skin changes, general activity level, sensitivity to the light, and respiratory movement. The results were compared with those of the control group.
Toxicity at 14 d post exposure
Mice were assigned to groups in the same manner as for studies at 24 h. I-Hyp was given at 0.1 and 10 mg/kg body weight in 100 μL of DMSO solution to the low-dose group and the high-dose group, respectively. The mice were monitored for 14 d for onset, severity, and reversibility of any toxicity as well as morbidity, general activity level, body weight, sensitivity to the light, skin changes, before being euthanized for clinical biochemistry blood tests and histopathology studies.
Clinical biochemistry blood tests
Blood samples were collected in non-heparinized tubes and were centrifuged at 3000 rounds per minute (1837×g) for 10 min. The serum was separated and analyzed with an IMAGE instrument (Beckman-Coulter) for the following parameters: urea, creatinine, albumin, alkaline phosphatase (ALP), aspartate-aminotransferase (AST), alanine-aminotransferase (ALT), gamma glutamyl transferase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), creatine kinase (CK), and lactate dehydrogenase (LDH).
Histopathological study
Histopathological studies of the organs were performed using a previously described method29. Briefly, the internal organs were fixed in 10% neutral buffered formalin for 24 h and dissected into tissue blocks. Samples were dehydrated in ethanol and cleared in benzene to remove the alcohol, and then the samples were infiltrated with molten paraffin at 50 °C and further embedded into a cubical block of paraffin24. The paraffin-embedded tissues were sectioned into 5-μm slices by microtome and stained with Hematoxylin and Eosin (H&E). The slides were examined under an optical microscope (Axioskop; Zeiss, Oberkochen, Germany) with magnifications at ×100 for histopathology, and photomicrographs were obtained using a digital system.
LD50 studies
Mice that were subjected to LD50 tests were randomly split into 6 groups of 12 mice with an equal number from each gender. Each mouse was anesthetized with isoflurane gas mixed with oxygen by a nose cone. Accordingly, six assumed doses of I-Hyp were iv administered as follows: 16, 18, 20, 22, 24, and 26 mg/kg in 100 μL of DMSO as solvent. The LD50 and 95% confidence interval for each gender and in combination with both genders were determined from weighted Probit analysis (Bliss and Finney) using SPSS30,31, and the corresponding dose response curves were generated based on the results. In addition, 10 mice of both genders in equal number (n=5) injected with 100 L of DMSO were used as solvent controls. All mice were observed initially for 2 h for acute symptoms of toxicity. Any mortality observed within 24 h was registered.
Statistical analysis
Numerical data were compared between the control group and the studied groups. The values were expressed as the mean±standard deviation (SD). Statistical analysis was carried out with the SPSS for Windows software package (version 16.0; SPSS, Chicago, IL, USA). One-way ANOVA was used to elucidate any significant differences between the treatment groups and the control groups. A significant difference was considered for P values less than 0.05.
Results
General conditions
The 50 mice used in the toxicity studies presented normal water and food intake during the study. There were no signs of clinical toxicity, significant changes in body-weight gain, or changes in food consumption. The mice tolerated the iv injection of I-Hyp and solvents without detectable side effects. No mice died of anesthesia. No observable clinical signs such as photophobia, weakness, aggression, restlessness, piloerection, tremors, loss of hair or appetite, diarrhea or ascites appeared in the studied mice.
Toxicity at 24 h post exposure
There was no mortality or any treatment-related signs or toxicity observed following the administration of either 0.1 or 10 mg/kg body weight of I-Hyp in both male and female mice within 24 h post injection.
Toxicity at 14 d post exposure
All mice survived the duration of the experiments. No alterations in photosensitivity, vivacity, or feces were noted in either the treatment or the control groups during the 2 week study. No changes in the pigmentation of the viscera were observed in any mice. Generally, male mice were heavier than females in both control and treated groups. The median body weight of all mice increased by 3.9%–7.6% during the 14 d follow up. No significant difference (P>0.05) in the mean body weight was observed between control and treated groups of the same gender.
Clinical biochemistry blood test
The results of the clinical biochemistry blood test in all studied groups are listed in Table 1. Comparisons were made between control groups and each treated group within the same gender. No significant differences were found in any comparisons.
Table 1. Effect of I-Hyp on biochemical parameters in mice. Mean±SD.
| Analyst | Units | Control | 24 h |
14 d |
||
|---|---|---|---|---|---|---|
| Low dose | High dose | Low dose | High dose | |||
| Male | ||||||
| Urea | mg/dL | 42.6±8.0 | 36.2±18.0 | 41.0±4.0 | 43.6±3.9 | 43.5±2.1 |
| Creatinine | mg/dL | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| Albumin | g/L | 29.1±3.2 | 25.4±8.7 | 22.0±7.1 | 28.7±1.0 | 28.9±1.0 |
| ALP | u/L | 239±27 | 171±150 | 212±37 | 236±12 | 237±11 |
| AST | u/L | 36.8±5.1 | 28.4±16.7 | 55.0±20.4 | 36.5±1.3 | 36.6±1.1 |
| ALT | u/L | 32.4±8.6 | 30.2±21.6 | 69.0±39.4 | 35.5±7.0 | 36.0±6.2 |
| GGT | u/L | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
| TBIL | mg/dL | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 |
| DBIL | mg/dL | 0.01±0.0 | 0.01±0.0 | 0.01±0.0 | 0.01±0.0 | 0.01±0.0 |
| CK | u/L | 55.8±20.6 | 67.8±42.9 | 68.4±17.3 | 59.0±9.2 | 57.6±8.6 |
| LDH | u/L | 261±88 | 246±215 | 286±76 | 178±4 | 238±89 |
| Female | ||||||
| Urea | mg/dL | 40.6±7.6 | 31.4±12.6 | 57.2±43.5 | 41.8±4.4 | 58.4±18.9 |
| Creatinine | mg/dL | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| Albumin | g/L | 32.5±2.2 | 26.8±6.2 | 22.6±9.8 | 32.1±1.3 | 34.1±2.2 |
| ALP | u/L | 237±31 | 200±127 | 181±114 | 232±44 | 228±42 |
| AST | u/L | 45.6±4.2 | 40.2±23.3 | 58.0±18.2 | 57.8±21.0 | 46.6±22.5 |
| ALT | u/L | 28.4±8.1 | 27.6±17.2 | 26.4±3.5 | 31.4±1.1 | 35.2±9.0 |
| GGT | u/L | 3.0±0.0 | 3.0±0.0 | 3.2±0.4 | 3.0±0.0 | 3.0±0.0 |
| TBIL | mg/dL | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 | 0.1±0.0 |
| DBIL | mg/dL | 0.01±0.0 | 0.01±0.0 | 0.01±0.0 | 0.01±0.0 | 0.01±0.0 |
| CK | u/L | 41.8±8.3 | 47.2±25.1 | 80.2±60.4 | 51.2±17.9 | 54.4±14.2 |
| LDH | u/L | 341±101 | 189±136 | 307±175 | 255±69 | 346±99 |
Histopathology
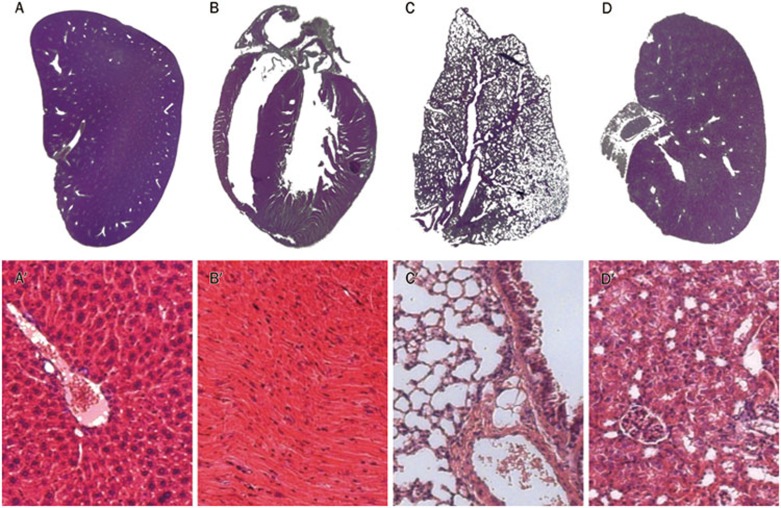
Macroscopic and microscopic photomicrographs of the histological sections of the liver, heart, lung, and kidney of the studied mice (collected from mice in the high dose group euthanized on d 14) are presented in Figure 3A, 3B, 3C, 3D, 3A', 3B', 3C', 3D'. All of the organs presented a normal appearance in both macroscopic and microscopic observations. No morphological or structural changes, including edema, necrosis, inflammation, or pigmentation, were observed within all organs in the histopathology study.
Figure 3.
Histological specimens of mouse tissues (A, Liver; B, Heart; C, Lung; and D, Kidney). All organs presented a normal appearance in macroscopic observations without edema, phlogosis, pigmentation and shape changes observed. Microscopically, the liver (A′) is composed of innumerable lobules, each of which is a hexagonal structure consisting of a central vein surrounded by radiating hepatocyte plates. Cardiac muscle cells (B′) are faintly striated, branching, mononucleated cells, which connect by means of intercalated disks to form a functional network. In lung tissue, foamy cells are observed in the pulmonary interstitium, and the alveolar walls appear normal (C′). Normal renal cortex and glomerular tufts were shown in kidney sections (D′).
LD50 studies
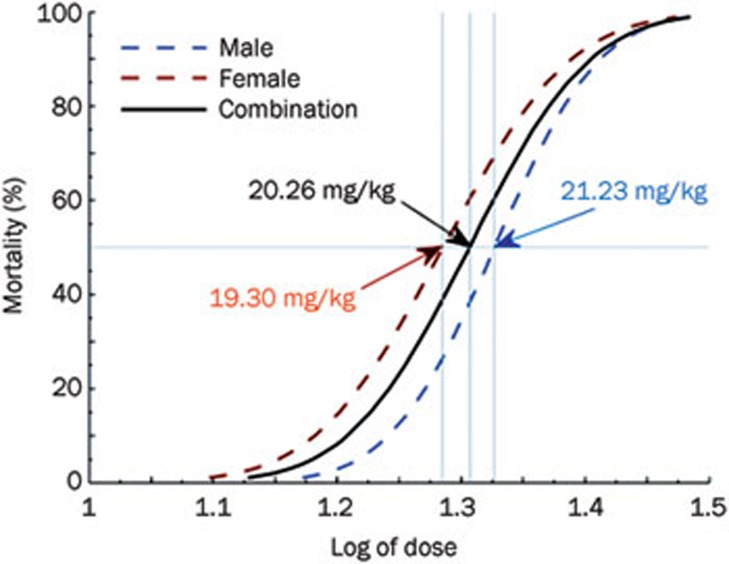
The results of the LD50 studies are listed in Table 2, and the corresponding dose response curve is shown in Figure 4. A transient or permanent respiratory depression was observed in mice at doses of 16 mg/kg and above. Most animals presented restlessness, aconuresis, clonicity hyperspasmia, opisthotonos, and dyspnea before death. The LD50 values of I-Hyp via iv injection in males, females, and in both genders in combination were found to be 21.23, 19.30, and 20.26 mg/kg, with the 95% confidence intervals of 19.35–23.21, 16.73–21.20, and 18.90–21.55 mg/kg, respectively.
Table 2. Acute LD50 tests of I-Hypericin of normal Swiss mice in male, female and both genders in combination, with DMSO as the solvent control. Groups of mice were iv injected with different doses of non-radioactively iodinated Hypericin (I-Hyp). In the vehicle control, 100 μL of DMSO injected into 10 mice did not reveal any observable side effects or animal death.
| Treatment (iv) | Gender | Dose (mg/kg) | Total No | No dead | Mortality (%) | Log of dose | LD50 (95% confidence interval) |
|---|---|---|---|---|---|---|---|
| I-Hyp | Male | 16.0 | 6 | 0 | 0.0 | 1.20 | 21.23 mg/kg (19.35–23.21) |
| 18.0 | 6 | 1 | 16.7 | 1.26 | |||
| 20.0 | 6 | 2 | 33.3 | 1.30 | |||
| 22.0 | 6 | 4 | 66.7 | 1.34 | |||
| 24.0 | 6 | 5 | 83.3 | 1.38 | |||
| 26.0 | 6 | 5 | 83.3 | 1.42 | |||
| Female | 16.0 | 6 | 1 | 16.7 | 1.20 | 19.30 mg/kg (16.73–21.20) | |
| 18.0 | 6 | 2 | 33.3 | 1.26 | |||
| 20.0 | 6 | 4 | 66.7 | 1.30 | |||
| 22.0 | 6 | 4 | 66.7 | 1.34 | |||
| 24.0 | 6 | 5 | 83.3 | 1.38 | |||
| 26.0 | 6 | 6 | 100.0 | 1.42 | |||
| Combination | 16.0 | 12 | 1 | 8.3 | 1.20 | 20.26 mg/kg (18.90–21.55) | |
| 18.0 | 12 | 3 | 25.0 | 1.26 | |||
| 20.0 | 12 | 6 | 50.0 | 1.30 | |||
| 22.0 | 12 | 8 | 66.7 | 1.34 | |||
| 24.0 | 12 | 10 | 83.3 | 1.38 | |||
| 26.0 | 12 | 11 | 91.7 | 1.42 | |||
| 100 μL of DMSO | Male & female | – | 10 | 0 | 0.0 | – | – |
Animals were observed initially for 2 h for acute symptoms of toxicity. Any mortality observed within 24 h was registered for analysis.
Figure 4.
Dose-response curves and the LD50 were generated based on the results of Probit analysis for males, females and for both genders in combination.
Discussion
Hyp and its radio-derivatives have been investigated in animal models with ischemic heart diseases and malignancies for diagnostic and therapeutic purposes11,14,15,18,32. Toxicity information is an indispensable part for exploring and integrating the targeting anticancer theragnostic strategy using radioiodinated Hyp13,15. We report in this study the toxic effects and the LD50 of I-Hyp via iv injection.
In a recent preliminary study, 131I-Hyp was used at a radioactive dose of 300 MBq/kg to target necrotic malignancies for clearing residual viable tumor cells15. The amount of Hyp used to form the equivalent radioactive dose was approximately 0.1–0.2 mg/kg of body weight. Thus, the studied doses of 0.1 mg/kg and 10 mg/kg body weight cover all of the possible ranges for its new applications.
Hyp is generally well tolerated, and the side effects mainly include photosensitivity, photodermatitis, constipation, mood or mental changes, dizziness or fatigue, muscle tremors, decreased sexual libido33. A previous study revealed that Hyp is well tolerated at a systemic dose of 40 mg/kg in mice34, while severe dose-limiting photosensitivity presenting as a skin rash was reported in another study with iv injection of Hyp at a single dose of 5 mg/kg in rhesus monkeys20. This discrepancy might be due to the following: 1) there is a higher photo-tolerance in mice than in rhesus monkeys; or 2) Hyp is more tolerable when injected intraperitoneally than intravenously. In the present study, none of the mice showed obvious toxic manifestations after a single dose of I-Hyp at 0.1 mg/kg and 10 mg/kg body weight, which might have been caused by the photochemical property change after the substitution of iodine onto the intact Hyp structure to form I-Hyp. Severe toxicity and a loss of appetite may be accompanied by a loss of body weight, but the mice in the 14-d studies gained weight at the same rate as the control groups during the 2 weeks of follow-up, which reflects no toxicity or less toxicity at the studied doses.
The results of the blood biochemical tests with obvious deviations from the normal control were chosen for discussion. A low level of serum albumin normally indicates diseases such as liver dysfunction, nephritic syndrome, genetic variations, and malignancies. However, decreased albumin levels are not observed in acute liver failure until several weeks before the impaired albumin production drops the serum albumin level35. In this study, the slightly decreased serum albumin in some cases of the acute phase high dose group cannot represent substantial organ damage but might indicate a temporary depletion of serum albumin due to protein binding of I-Hyp for blood-borne transportation and body distribution after iv administration. ALT and AST are two liver enzymes that are associated with hepatocellular injury. ALT is a cytosolic enzyme specific for liver, and AST is also present in the myocardium, skeletal muscle, brain, and kidneys. The serum levels of ALT and AST were both slightly elevated in the male high-dose group of the 24 h study, which may be caused by a transient hepatotoxicity of liver cells. As recommended by the 2002 American Gastroenterological Association guidelines for any hepatotoxic drug, only a single ALT and AST elevation of three times the upper limit of the normal range was considered as a reason for discontinuation of treatment36. The slight increase of ALT and AST levels does not impose an argument for ending treatment with I-Hyp. The slight increase of blood urea in the female groups at the dose of 10 mg/kg of body weight may suggest a subtle disturbance of kidney function. Further evidence is needed to address the renal toxicity at higher doses. However, renal toxicity should not be present at a clinically relevant dose. There were no obvious changes observed in other parameters such as GGT, TBIL, and DBIL, CK, and LDH. Gross examination of internal organs and histopathological studies from all of the mice revealed no detectable cell death or abnormalities.
The studies of single dose I-Hyp helped to not only verify any toxicity at the acute phase of 24 h but also to evaluate the morphological and physiological changes of organs and the tendency of body weight to change at the sub-acute phase of 14 d. I-Hyp at either a clinically relevant dose or 100-times the clinical dose was proven to be either low or non-toxic in the studies. The LD1 value of I-Hyp was more than 130 times that of the recommended therapeutic dose that was used in targeting cancer therapy, which suggests a negligible chemotoxicity of I-Hyp and a wide safety range for its clinical use.
Despite the long history of its medicinal applications, few toxicological studies with purified Hyp have been conducted. For the first time to our knowledge, we performed toxicity studies for I-Hyp, and the results present strong evidence of acceptable safety and low toxicity at a single dose range from 0.1 to 10 mg/kg body weight within 24 h and 14 d post exposure in mice of both genders. The current study with I-Hyp not only provides supportive toxicity information for its use in necrosis-targeting anticancer theranostic strategy15,37 but might also set a valuable reference for other utilities of Hyp and its radioiodinated derivatives.
Author contribution
Study concept and design: Jun-jie LI, Marlein Miranda CONA and Yi-cheng NI; experimental performance: Jun-jie LI, Marlein Miranda CONA, Yuan-bo FENG, Feng CHEN, Guo-zhi ZHANG, Xue-bin FU, and Yi-cheng NI; literature search and manuscript drafting or editing: Jun-jie LI, Marlein Miranda CONA, Yuan-bo FENG, Feng CHEN, and Yi-cheng NI; manuscript revision for intellectual content: Jun-jie LI, Marlein Miranda CONA, Yuan-bo FENG, Feng CHEN, Guo-zhi ZHANG, Xue-bin FU, Uwe HIMMELREICH, Raymond OYEN, Alfons VERBRUGGEN, and Yi-cheng NI; approval of final version of submitted manuscript: Jun-jie LI, Marlein Miranda CONA, Yuan-bo FENG, Feng CHEN, Guo-zhi ZHANG, Xue-bin FU, Uwe HIMMELREICH, Raymond OYEN, Alfons VERBRUGGEN, and Yi-cheng NI.
Acknowledgments
This work was partially supported by the grants awarded by FWO Vlaanderen ZWAP/05/018; Geconcerteerde Onderzoeksactie of the Flemish Government, OT project (OT/06/70); the KU Leuven Molecular Small Animal Imaging Center MoSAIC (KUL EF/05/08); the center of excellence In vivo Molecular Imaging Research (IMIR) of KU Leuven; the IWT SBO 'Imagine' (SBO80017) and an EU project Asia-Link CfP 2006-EuropeAid/123738/C/ACT/Multi-Proposal No 128-498/111. The corresponding author, Yi-cheng NI, is currently a Bayer Lecture Chair holder.
References
- Hölzl J, Ostrowski E. Analysis of the Essential Compounds of Hypericum perforatum. Planta Med. 1986;(6):531. doi: 10.1055/s-2007-969321. [DOI] [PubMed] [Google Scholar]
- Tammaro F, Xepapadakis G. Plants used in phytotherapy, cosmetics and dyeing in the Pramanda district (Epirus, North-West Greece) J Ethnopharmacol. 1986;16:167–74. doi: 10.1016/0378-8741(86)90087-5. [DOI] [PubMed] [Google Scholar]
- Crak C, Radusiene J, Janulis V, Ivanauskas L. Secondary metabolites in Hypericum perfoliatum: variation among plant parts and phenological stages. Bot Helv. 2007;117:29–36. [Google Scholar]
- Diasa ACP, Seabrab RM, Andradeb PB, Ferreresc F, Fernandes-Ferreiraa M. Xanthone biosynthesis and accumulation in calli and suspended cells of Hypericum androsaemum. Plant Sci. 2000;150:93–101. [Google Scholar]
- Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int J Mol Sci. 2010;11:562–94. doi: 10.3390/ijms11020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marysael T, Ni Y, Lerut E, de Witte P. Influence of the vascular damaging agents DMXAA and ZD6126 on hypericin distribution and accumulation in RIF-1 tumors. J Cancer Res Clin Oncol. 2011;137:1619–27. doi: 10.1007/s00432-011-1032-y. [DOI] [PubMed] [Google Scholar]
- Kubin A, Wierrani F, Burner U, Alth G, Grünberger W. Hypericin — the facts about a controversial agent. Curr Pharm Des. 2005;11:233–53. doi: 10.2174/1381612053382287. [DOI] [PubMed] [Google Scholar]
- Agostinis P, Vantieghem A, Merlevede W, de Witte PA. Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol. 2002;34:221–41. doi: 10.1016/s1357-2725(01)00126-1. [DOI] [PubMed] [Google Scholar]
- Head CS, Luu Q, Sercarz J, Saxton R. Photodynamic therapy and tumor imaging of hypericin-treated squamous cell carcinoma. World J Surg Oncol. 2006;4:87. doi: 10.1186/1477-7819-4-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin A, Wierrani F, Burner U, Alth G, Grünberger W. Hypericin — the facts about a controversial agent. Curr Pharm Des. 2005;11:233–53. doi: 10.2174/1381612053382287. [DOI] [PubMed] [Google Scholar]
- Ni Y, Huyghe D, Verbeke K, de Witte PA, Nuyts J, Mortelmans L, et al. First preclinical evaluation of mono-[123I]iodohypericin as a necrosis-avid tracer agent. Eur J Nucl Med Mol Imaging. 2006;33:595–601. doi: 10.1007/s00259-005-0013-2. [DOI] [PubMed] [Google Scholar]
- Ni Y, Bormans G, Chen F, Verbruggen A, Marchal G. Necrosis avid contrast agents: functional similarity versus structural diversity. Invest Radiol. 2005;40:526–35. doi: 10.1097/01.rli.0000171811.48991.5a. [DOI] [PubMed] [Google Scholar]
- Li J, Chen F, Cona MM, Feng Y, Himmelreich U, Oyen R, et al. A review on various targeted anticancer therapies. Target Oncol. 2012;7:69–85. doi: 10.1007/s11523-012-0212-2. [DOI] [PubMed] [Google Scholar]
- Fonge H, Vunckx K, Wang H, Feng Y, Mortelmans L, Nuyts J, et al. Non-invasive detection and quantification of acute myocardial infarction in rabbits using mono-[123I]iodohypericin microSPECT. Eur Heart J. 2008;29:260–9. doi: 10.1093/eurheartj/ehm588. [DOI] [PubMed] [Google Scholar]
- Li J, Sun Z, Zhang J, Shao H, Cona MM, Wang H, et al. A dual-targeting anticancer approach: soil and seed principle. Radiology. 2011;260:799–807. doi: 10.1148/radiol.11102120. [DOI] [PubMed] [Google Scholar]
- Van de Putte M, Wang H, Chen F, de Witte PA, Ni Y. Hypericin as a marker for determination of tissue viability after intratumoral ethanol injection in a murine liver tumor model. Acad Radiol. 2008;15:107–13. doi: 10.1016/j.acra.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Van de Putte M, Wang H, Chen F, De Witte PA, Ni Y. Hypericin as a marker for determination of tissue viability after radiofrequency ablation in a murine liver tumor model. Oncol Rep. 2008;19:927–32. [PubMed] [Google Scholar]
- Marysael T, Bauwens M, Ni Y, Bormans G, Rozenski J, de Witte P.Pretargeting of necrotic tumors with biotinylated hypericin using 123I-labeled avidin: evaluation of a two-step strategy Invest New Drugs 2011. doi: 10.1007/s10637-011-9778-2 [DOI] [PubMed]
- Van de Putte M, Ni Y, De Witte PA. Exploration of the mechanism underlying the tumor necrosis avidity of hypericin. Oncol Rep. 2008;19:921–6. [PubMed] [Google Scholar]
- Fox E, Murphy RF, McCully CL, Adamson PC. Plasma pharmacokinetics and cerebrospinal fluid penetration of hypericin in nonhuman primates. Cancer Chemother Pharmacol. 2001;47:41–4. doi: 10.1007/s002800000188. [DOI] [PubMed] [Google Scholar]
- Leuschner J.Gutachten zur experimentellen Toxikologie von Hypericum-Extract LI 160Berlin: Lichtwer Pharma GmbH. 1995
- Gulick RM, McAuliffe V, Holden-Wiltse J, Crumpacker C, Liebes L, Stein DS, et al. Phase I studies of hypericin, the active compound in St. John's wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258. Ann Intern Med. 1999;130:510–4. doi: 10.7326/0003-4819-130-6-199903160-00015. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liang J, Luo Y, Wang X, Zhu Y, Shang R, et al. Acute toxicity tests of extractum hypericin. Chin J Vet Drug. 2005;39:19–20. [Google Scholar]
- Stowell RE. Effect on tissue volume of various methods of fixation, dehydration, and embedding. Biotechnic & Histochemistry. 1941;16:67–83. [Google Scholar]
- Watanabe N, Yokoyama K, Kinuya S, Shuke N, Shimizu M, Futatsuya R, et al. Radiotoxicity after iodine-131 therapy for thyroid cancer using the micronucleus assay. J Nucl Med. 1998;39:436–40. [PubMed] [Google Scholar]
- Narra VR, Howell RW, Harapanhalli RS, Sastry KS, Rao DV. Radiotoxicity of some iodine-123, iodine-125 and iodine-131-labeled compounds in mouse testes: implications for radiopharmaceutical design. J Nucl Med. 1992;33:2196–201. [PubMed] [Google Scholar]
- Shapiro B, Sisson JC, Wieland DM, Mangner TJ, Zempel SM, Mudgett E, et al. Radiopharmaceutical therapy of malignant pheochromocytoma with [131I]metaiodobenzylguanidine: results from ten years of experience. J Nucl Biol Med. 1991;35:269–76. [PubMed] [Google Scholar]
- Order SE, Stillwagon GB, Klein JL, Leichner PK, Siegelman SS, Fishman EK, et al. Iodine 131 antiferritin, a new treatment modality in hepatoma: a Radiation Therapy Oncology Group study. J Clin Oncol. 1985;3:1573–82. doi: 10.1200/JCO.1985.3.12.1573. [DOI] [PubMed] [Google Scholar]
- Vincek V, Nassiri M, Nadji M, Morales AR. A tissue fixative that protects macromolecules (DNA, RNA, and protein) and histomorphology in clinical samples. Lab Invest. 2003;83:1427–35. doi: 10.1097/01.lab.0000090154.55436.d1. [DOI] [PubMed] [Google Scholar]
- Finney DJ.Probit AnalysisCambridge, England, Cambridge University Press1952
- Finney DJ, Stevens WL. A table for the calculation of working probits and weights in probit analysis. Biometrika. 1948;35:191–201. [PubMed] [Google Scholar]
- Song S, Xiong C, Zhou M, Lu W, Huang Q, Ku G, et al. Small-animal PET of tumor damage induced by photothermal ablation with 64Cu-bis-DOTA-hypericin. J Nucl Med. 2011;52:792–9. doi: 10.2967/jnumed.110.086116. [DOI] [PubMed] [Google Scholar]
- http://www.livestrong.com/article/107004-hypericin-side-effects/
- Luksiene Z, de Witte PA. Hypericin as novel and promising photodynamic therapy tool: studies on intracellular accumulation capacity and growth inhibition efficiency. Medicina (Kaunas) 2003;39:677–82. [PubMed] [Google Scholar]
- Nagao Y, Sata M. Serum albumin and mortality risk in a hyperendemic area of HCV infection in Japan. Virol J. 2010;7:375. doi: 10.1186/1743-422X-7-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onusko E. Statins and elevated liver tests: what's the fuss. J Fam Pract. 2008;57:449–52. [PubMed] [Google Scholar]
- Li J, Cona MM, Chen F, Feng Y, Yu J, De witte P, et al. Exploring theranostic potentials of radioiodinated hypericin in rodent necrosis models. Theranostics. 2012;2:1010–9. doi: 10.7150/thno.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]