Abstract
Aim:
To evaluate the antifibrotic effect of telmisartan, an angiotensin II receptor blocker, in bile duct-ligated rats.
Methods:
Adult Sprague-Dawley rats were allocated to 3 groups: sham-operated rats, model rats underwent common bile duct ligation (BDL), and BDL rats treated with telmisartan (8 mg/kg, po, for 4 weeks). The animals were sacrificed on d 29, and liver histology was examined, the Knodell and Ishak scores were assigned, and the expression of angiotensin-converting enzyme (ACE) and ACE2 was evaluated with immunohistochemical staining. The mRNAs and proteins associated with liver fibrosis were evaluated using RTQ-PCR and Western blot, respectively.
Results:
The mean fibrosis score of BDL rats treated with telmisartan was significantly lower than that of the model rats (1.66±0.87 vs 2.13±0.35, P=0.015). However, there was no significant difference in inflammation between the two groups, both of which showed moderate inflammation. Histologically, treatment with telmisartan significantly ameliorated BDL-caused the hepatic fibrosis. Treatment with telmisartan significantly upregulated the mRNA levels of ACE2 and MAS, and decreased the mRNA levels of ACE, angiotensin II type 1 receptor (AT1-R), collagen type III, and transforming growth factor β1 (TGF-β1). Moreover, treatment with telmisartan significantly increased the expression levels of ACE2 and MAS proteins, and inhibited the expression levels of ACE and AT1-R protein.
Conclusion:
Telmisartan attenuates liver fibrosis in bile duct-ligated rats via increasing ACE2 expression level.
Keywords: hepatic fibrosis, bile duct ligation, the Knodell and Ishak scoring system, angiotensin II, telmisartan
Introduction
Liver fibrosis is a common outcome of many chronic liver injuries. The normal liver structure, when distorted by scar tissue, may ultimately develop into overt cirrhosis. Despite significant efforts, there is still no effective therapy for hepatic fibrosis. The renin-angiotensin system (RAS) plays an important role in controlling liver fibrosis1,2,3. It is well known that the RAS influences cell differentiation, nutrition, and fibrosis, and it has been reported to play a role in heart and kidney disease4,5. Moreover, recent studies have revealed that the RAS plays an important role in the progression of many chronic liver diseases6,7. Furthermore, angiotensin II receptor blocker (ARB) modulation of the RAS to treat liver fibrosis has been reported in both animal and human studies8,9.
ARBs are largely regarded as a means to block vasoconstriction and inhibit the cell proliferation and fibrosis that are mediated by the angiotensin II type 1 receptor (AT1-R)10. Yang et al11 suggested that AT1-R play an important role in the development of fibrosis. An increase in hepatic transforming growth factor β1 (TGF-β1) and pro-inflammatory cytokine levels was attenuated in AT1-R knockout mice compared to WT mice11. Furthermore, Bataller et al12 demonstrated that increased systemic Angiotensin (Ang) II augments hepatic fibrosis and promotes inflammation, oxidative stress, and thrombogenic events. Telmisartan shows the greatest affinity for the AT1-R and exhibits the longest half-life of the ARBs that are currently available13. Moreover, telmisartan's lipophilicity and affinity for the liver suggests that it is especially suitable for hepatic indications13. The angiotensin-converting enzyme (ACE) 2/Ang (1–7) pathway is now regarded as an alternative pathway to that of the RAS4,14. Activation of Mas, the G-protein-coupled Ang (1–7) receptor, triggers vasodilation and antifibrotic signaling mechanisms in cardiac myocytes15. In this alternative pathway, ACE2 converts Ang II to Ang (1–7) and Ang I to Ang (1–9)16.
Enhanced ACE2 expression could counteract the relative increase in Ang II that occurs after blockade of the AT1-R and may therefore prevent excessive Ang II accumulation within the vessel wall. Here, we propose that telmisartan, a representative ARB, induces antifibrotic effects that are mediated by the ACE2/Ang (1–7)-Mas axis.
Materials and methods
Animals
Adult male Sprague-Dawley rats (250–300 g) (Vital River Laboratory Animal Technology Co, Ltd, Beijing, China) were used in this study. The common bile duct was doubly ligated with 4–0 silk and transected between the ligated sites. Sham-operated rats were operated on similarly, except the bile duct was not ligated or transected. Rats were housed at three per cage under a 12–12 h light–dark cycle and had free access to standard pellet food and tap water throughout the 4-week experiment. All procedures followed institutional guidelines for laboratory animals.
Experimental design
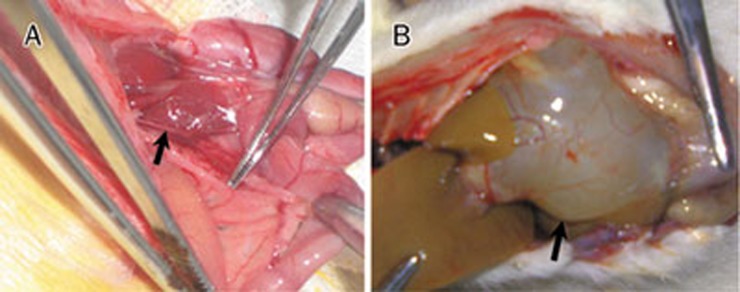
The animals were randomly distributed into three groups: group 1 (G1), sham-operated rats (n=10); group 2 (G2), bile duct ligation (BDL) without ARB (n=10); and group 3 (G3), BDL+8 mg/kg telmisartan daily (Bob YanGeHan Pharmaceutical Co, Shanghai, China) by gastric gavage for 4 weeks (n=10). The animals were sacrificed on d 29. Ballooning dilatation of the proximal common bile duct confirmed successful BDL (Figure 1B). Liver specimens were immediately snap-frozen and stored at −80 °C.
Figure 1.
(A) Identification of the common bile duct. (B) Ligation success was confirmed by assessing bile duct dilation 4 weeks after BDL.
Histological and immunohistochemical analysis
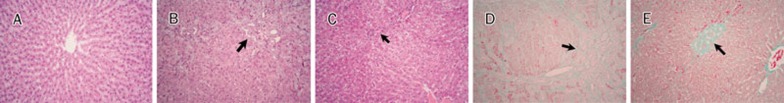
Liver sections (5 μm) were stained with hematoxylin and eosin (H&E) and Masson's trichrome. All histological examinations were blinded and performed by one pathologist. Histological changes were screened using the H&E-stained sections (Figure 2A–2C). The presence of fibrosis was more specifically evaluated in Masson's trichrome-stained sections (Figure 2D, 2E). Hepatic fibrosis was quantified using the Knodell and Ishak scoring system17: (1) absence of fibrosis; (2) fibrous portal expansion; (3) bridging fibrosis (portal-portal or portal-central linkage); and (4) cirrhosis. The following scores were applied to semi-quantify inflammatory infiltration: (0) none; (1) mild; (2) scattered mild; (3) inflammatory infiltration; (4) submassive; and (5) massive18. The numerical score is the result of multiplying the number of rats per grade by the grade, adding these products, and dividing by the total number of rats per group.
Figure 2.
H&E and Masson's trichrome. (A) (G1) Normal liver. (B and D) (G2) Liver sections stained with H&E and Masson's trichrome, respectively. Inflammatory infiltration, bile ductular proliferation, and fibrosis are shown. (C and E) (G3) Liver sections stained with H&E and Masson's trichrome, respectively. These samples showed obvious attenuation of inflammatory infiltration, bile ductular proliferation, and fibrosis (original magnification ×100).
Immunohistochemistry
Paraffin blocks were cut into 4-μm sections, deparaffinized in xylene, and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Tissue sections were incubated overnight at 4 °C with anti-ACE2 rabbit polyclonal antibody (diluted 1:500, Abcam, Hong Kong, China) or anti-ACE mouse monoclonal antibody (diluted 1:50, Abcam), washed, and incubated with a secondary antibody conjugated to PV-9000. After incubation with 3,3′-diaminobenzidine, the slides were counterstained with hematoxylin. The sections were dehydrated and covered with Permount (Thermo Fisher Scientific, Waltham, MA, USA).
Image Pro plus 6.0 (Media Cybernetics, Bethesda, MD, USA) was used to quantify antibody staining. The software was trained to select stained and unstained nuclei/cells based on color intensity and nuclear shape; brown staining was considered positive. The chromatic area and strength (light density values) of positive cells were calculated and represented as the percentage of total positively stained cells with integral light density values (integral optical density).
Expression levels
The ACE2, MAS, ACE, AT1-R, collagen type III (col III), and TGF-β1 expression levels were evaluated by real-time quantitative reverse-transcriptase polymerase chain reaction (RTQ-PCR). Liver tissue was homogenized, and total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To each sample, 0.2 mL of chloroform was added per 1 mL of TRIzol reagent before the mixtures were centrifuged for 15 min at 4 °C. RNA was precipitated by mixing 0.6 mL isopropyl alcohol per 1 mL of TRIzol reagent and incubating at room temperature for 10 min. RNA extracts were washed with 1 mL of 75% ethanol and centrifuged for 5 min. RNA purity and concentration were determined using an Ultrospec 3100 pro UV/Visible Spectrophotometer (Amersham Bioscience, Freiburg, Germany). Two-step PCR was carried out using an iCycler (Qiagen, Hilden, Germany). For the cDNA synthesis, 2 μL RNA, 2 μL reverse transcriptase buffer, 2.0 μL oligo d(T) primer, 0.8 μL dNTPs, 1.0 μL MultiScribe™ reverse transcriptase, and 1.0 μL RNase inhibitor were added to water to make a final volume of 20 μL (all from Life Technologies). The samples were incubated at 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min. The RTQ-PCR assay (ΔΔCt) for ACE, AT1-R, ACE2, MAS, col III, and TGF-β1 mRNA expression was performed using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems) and iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH was used to normalize each target mRNA using ΔCt (target Ct — GAPDH Ct) values. The data are presented by graphing two ΔCt values for each gene. The RTQ-PCR conditions were as follows: 2 μL cDNA, 10 μL 2×QuantiTect SYBR Green PCR Master Mix (Applied Biosystems), 1 μL forward primer, and 1 μL reverse primer were added to water to make a final volume of 20 μL. The thermal cycler conditions were 10 min at 95 °C, and 50 cycles of 30 s at 95 °C followed by 60 s at 60 °C and 15 s at 95 °C. The forward and reverse primer sequences are shown in Table 1.
Table 1. Primer sequences used to detect ACE2, MAS, ACE, AT1, col III, and TGF-β1.
| Gene name | Primer | Sequence |
|---|---|---|
| GAPDH | Forward | 5′-CTCAACTACATGGTCTACATGTTCCA-3′ |
| Reverse | 5′-CTTCCCATTCTCAGCCTTGACT-3′ | |
| ACE2 | Forward | 5′-GCCAGGAGATGACCGGAAA-3′ |
| Reverse | 5′-CTGAAGTCTCCATGTCCCAGATC-3′ | |
| MAS | Forward | 5′-CATCTCTCCTCTCGGCTTTGTG-3′ |
| Reverse | 5′-CCTCATCCGGAAGCAAAGG-3′ | |
| ACE | Forward | 5′-CACCGGCAAGGTCTGCTT-3′ |
| Reverse | 5′-CTTGGCATAGTTTCGTGAGGAA-3′ | |
| AT1 | Forward | 5′-CGGCCTTCGGATAACATGA-3′ |
| Reverse | 5′-CCTGTCACTCCACCTCAAAACA-3′ | |
| col III | Forward | 5′-TCCCCTGGAATCTGTGAA-3′ |
| Reverse | 5′-TGAGTCGAATTGGGGAGAAT-3′ | |
| TGF-β1 | Forward | 5′-AGAAGTCACCCGCGTGCTAA-3′ |
| Reverse | 5′-TCCCGAATGTCTGACGTATTG-3′ |
Western blot analysis of ACE2, MAS, ACE, and AT1-R
Protein was extracted from homogenized liver samples and assayed. Protein samples (50 μg) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (80 volts for 15 min in a 4% acrylamide stacking gel and 120 volts for 110 min in a 12% running gel) and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were then incubated in Tris-buffered saline (10 mmol/L Tris-HCl, and 250 mmol/L NaCl) containing 5% nonfat powdered milk and 0.1% Tween 20 for 1 h to block nonspecific sites before being incubated with primary antibody overnight at 4 °C in blocking solution. Mouse anti-Actin (1:1000, Millipore) was used as a loading control. The blots were washed and incubated with secondary antibody for 2 h at room temperature. Immunoreactivities were visualized using enhanced chemiluminescence kits (Pierce, Rockford, IL, USA). The films were scanned using a Kodak 120 digital imaging system (Rochester, NY, USA). The antibody dilutions were as follows: anti-ACE mouse monoclonal antibody (diluted 1:50, Abcam), rabbit anti-ACE2 polyclonal antibody (diluted 1:500), and rabbit anti-MAS polyclonal antibody (1:200, Alomone Labs Ltd, Jerusalem, Israel). Mouse monoclonal antibodies to AT1-R (diluted 1:400), TGF-β1 (diluted 1:500) and col III (diluted 1:500) were also used (all antibodies were from Abcam, unless noted otherwise).
Statistical analysis
All statistical analyses were performed using SPSS software version 12.0 (IBM, New York, NY, USA). Values are expressed as the mean±standard deviation (SD). The analysis was conducted using a nonparametric method with the Mann-Whitney U and Kruskal-Wallis H tests. For all of the tests, a P value <0.05 was considered to be statistically significant.
Results
Histological analysis
The mean fibrosis score of the telmisartan group was significantly lower than that of the BDL without ARB (G2) (P=0.015, Table 2, Figure 2D, 2E). By contrast, there was no significant difference in inflammation between the BDL without ARB (G2) and the BDL+8 mg/kg telmisartan daily (G3); both groups showed moderate inflammation (Table 2, Figure 2B, 2C).
Table 2. Evaluation of fibrosis and inflammatory infiltration.
| Group | Numerical score |
|
|---|---|---|
| Fibrosis | Inflammatory infiltration | |
| G1 | 0 | 0 |
| G2 | 2.13±0.35b | 1.38±0.52b |
| G3 | 1.66±0.87be | 1.66±0.70b |
Values are expressed as mean±SD.
bP=0.000 compared with sham-operated rats (G1).
eP=0.015 vs the BDL without ARB (G2).
Immunohistochemical analysis
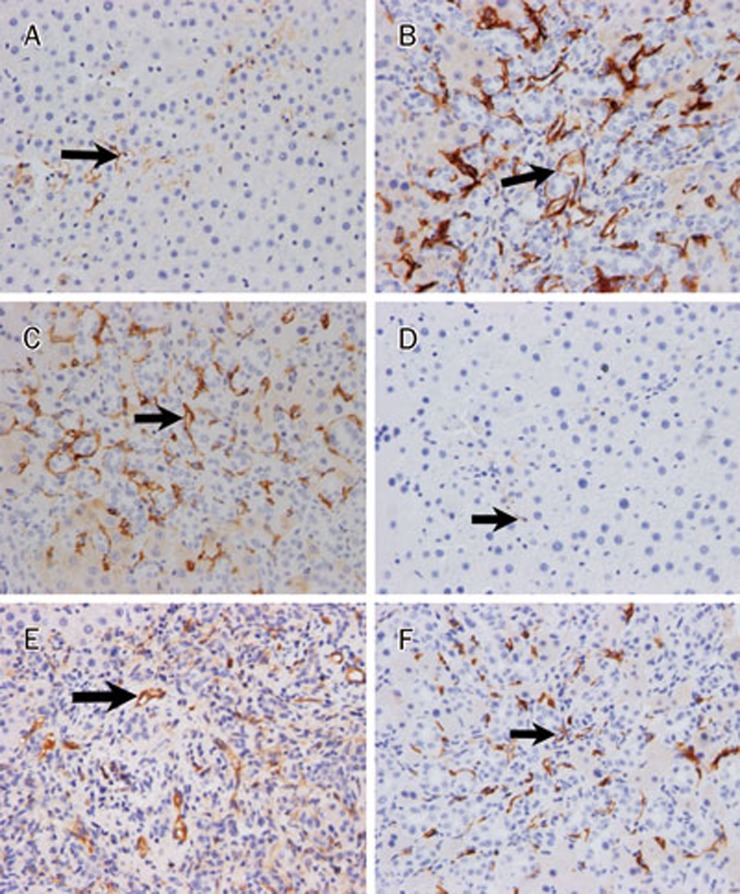
ACE2 expression was detected in a small percentage of cells from control livers, with expression restricted mainly to perivenular hepatocytes (Figure 3A). Hepatic ACE2 expression was markedly increased and exhibited a change in distribution from the hepatic lobule to the fibrous septae in the BDL without ARB (G2) (P<0.05, Table 3, Figure 3B). Both the number of cells and the staining intensity of cells expressing ACE2 (Figure 3C) were enhanced in livers from telmisartan-treated rats compared to the BDL without ARB (G2) (P<0.05, Table 3). The telmisartan-treated livers showed decreased ACE immunostaining compared to the BDL livers (P<0.05, Table 3, Figure 3D–3F).
Figure 3.
Immunohistochemical staining of ACE2 and ACE in rat liver. (A) Sham-operated rat liver tissue section incubated without ACE2 primary antibody. (B) ACE2 staining of rat liver tissue 4 weeks after BDL without ARB. (C) ACE2 staining of rat liver tissue after 4 weeks of BDL+8 mg/kg telmisartan daily. (D) Sham-operated rat liver tissue section incubated without ACE primary antibody. (E) ACE staining of rat liver tissue 4 weeks after BDL without ARB. (F) ACE staining of rat liver tissue after 4 weeks of BDL+8 mg/kg telmisartan daily (original magnification ×200).
Table 3. Immunohistochemical staining of ACE2 and ACE.
| Group | G1 (n=10) | G2 (n=10) | G3 (n=10) |
|---|---|---|---|
| ACE2 (%) | 2.7±0.6 | 31.1±6.6b | 12.3±3.0be |
| ACE (%) | 0.1±0.0 | 9.1±1.8b | 5.1±0.9be |
Values are expressed as mean±SD.
bP<0.05 compared with sham-operated rats (G1);
eP<0.05 compared with the BDL without ARB (G2).
Gene expression associated with fibrosis
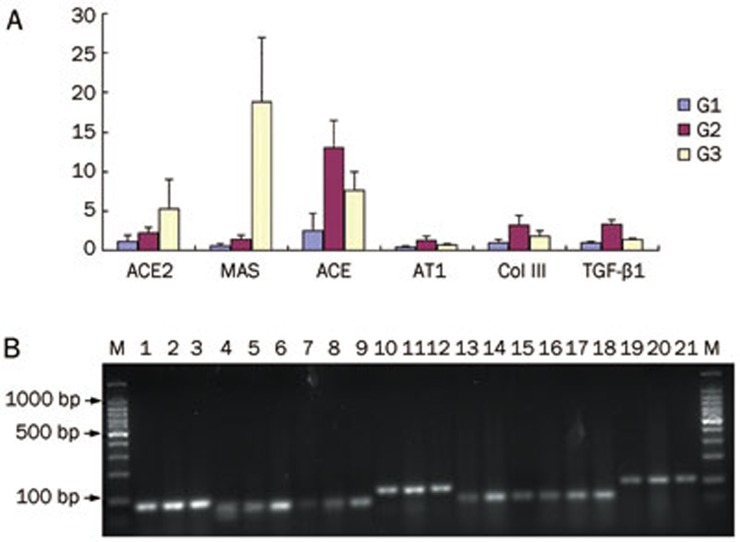
The ACE2 and MAS gene expression levels were significantly higher in the BDL+8 mg/kg telmisartan daily (G3) than that in the BDL without ARB (G2) (P<0.05, Table 4, Figure 4). By contrast, the ACE and AT1-R expression levels in the BDL without ARB (G2) were significantly higher than that in the other groups (P<0.05, Table 4, Figure 4). In addition, col III and TGF-β1 mRNA expression levels were significantly lower in the BDL+8 mg/kg telmisartan daily (G3) than that in the BDL without ARB (G2) (P<0.05, Table 4, Figure 4).
Table 4. Analysis of RTQ-PCR for ACE2, MAS, ACE, AT1-R, col III, and TGF-β1 mRNA expression.
| Group | G1 (n=10) | G2 (n=10) | G3 (n=10) |
|---|---|---|---|
| ACE2 | 1.06±0.83 | 2.28±0.67b | 5.20±3.77be |
| MAS | 0.61±0.28 | 1.45±0.52b | 18.77±8.12be |
| ACE | 2.49±2.23 | 12.96±3.48b | 7.55±2.39be |
| AT1 | 0.45±0.13 | 1.22±0.52b | 0.69±0.19be |
| Col III | 0.95±0.40 | 3.19±1.22b | 1.84±0.64be |
| TGF-β1 | 0.90±0.17 | 3.36±0.54b | 1.33±0.17be |
Values are expressed as mean±SD.
bP<0.05 compared with sham-operated rats (G1);
eP<0.05 compared with the BDL without ARB (G2).
Figure 4.
(A) Relative expression of hepatic ACE2, MAS, ACE, AT1-R, col III, and TGF-β1 by real-time PCR. Expression levels were normalized to GAPDH. (B) Representative DNA gel electrophoresis for quantitation of mRNA expression. Lane 1, 4, 7, 10, 13, 16, 19, sham-operated rats (G1); Lane 2, 5, 8, 11, 14, 17, 20, the BDL without ARB (G2); Lane 3, 6, 9, 12, 15, 18, 21, the BDL+8 mg/kg telmisartan group (G3); Lane 1 to 3, GADPH primer pair; Lane 4 to 6, ACE2 primer pair; Lane 7 to 9, Mas primer pair; Lane 10 to 12, ACE primer pair; Lane 13 to 15, AT1 primer pair; Lane 16 to 18, col III primer pair; and Lane 19 to 21, TGF-β1 primer pair.
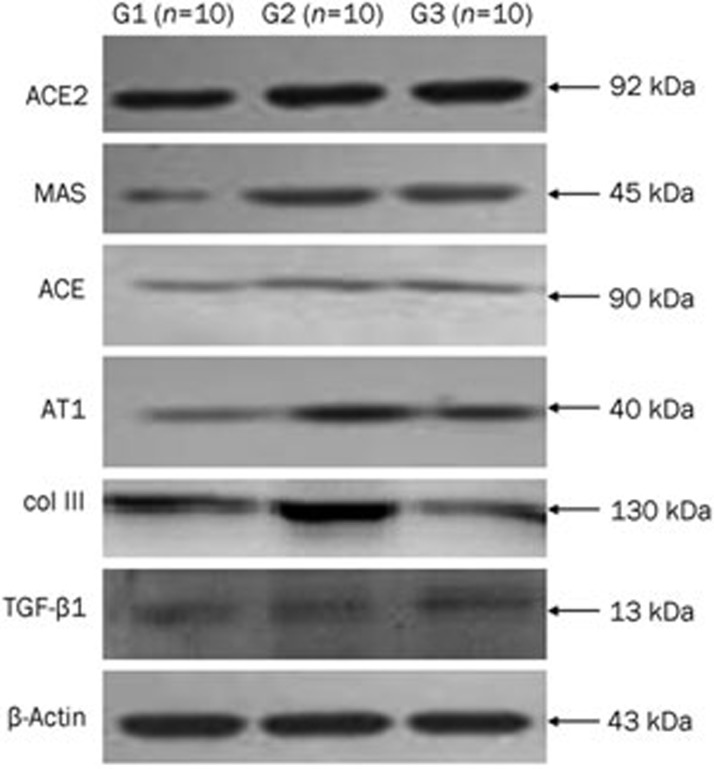
Western blot analysis of ACE2, MAS, ACE, AT1-R, col III, and TGF-β1
Western blot analysis revealed lower levels of ACE2, MAS, ACE, AT1-R, col III, and TGF-β1 protein in livers from sham-operated rats (G1) than that in BDL rat livers without ARB (G2). The MAS and ACE2 protein expression levels were significantly increased in the BDL+8 mg/kg telmisartan daily (G3) compared to those in the BDL without ARB (G2) (P<0.05, Table 5, Figure 5). However, the ACE, AT1-R, col III, and TGF-β1 protein expression levels were significantly lower in the BDL+8 mg/kg telmisartan daily (G3) than those in the BDL without ARB (G2) (P<0.05, Table 5, Figure 5).
Table 5. Effects of telmisartan on ACE2, MAS, ACE, AT1-R, col III, and TGF-β1 protein expression.
| Group | G1 (n=10) | G2 (n=10) | G3 (n=10) |
|---|---|---|---|
| ACE2 | 0.37±0.06 | 0.46±0.07b | 0.59±0.06be |
| MAS | 0.30±0.07 | 0.41±0.08b | 0.76±0.15be |
| ACE | 0.24±0.05 | 0.63±0.12b | 0.46±0.10be |
| AT1 | 0.24±0.04 | 0.60±0.13b | 0.44±0.09be |
| col III | 1.37±0.08 | 2.30±0.12b | 2.09±0.11be |
| TGF-β1 | 0.67±0.03 | 0.85±0.05b | 0.77±0.03be |
Values are expressed as mean±SD.
bP<0.05 compared with sham-operated rats (G1);
eP<0.05 compared with the BDL without ARB (G2).
Figure 5.
Expression of ACE2, MAS, ACE, and AT1 detected by Western blotting. Lane 1, sham-operated rats (G1); Lane 2, the BDL without ARB (G2); Lane 3, the BDL+8 mg/kg telmisartan daily (G3).
Discussion
Our results demonstrate that the progression of liver fibrosis in bile duct-ligated rats is attenuated by telmisartan, an ARB. However, there was no significant difference between the BDL without ARB group (G2) and the BDL+8 mg/kg telmisartan group (G3) with respect to inflammation, which is inconsistent with other studies19,20. One potential reason for this discrepancy may be related to species variation.
There is considerable animal model data supporting an antifibrotic role for the ACE2/Ang (1–7)-MAS axis of the RAS in hepatic fibrosis1,2,3,6,7,8. Therefore, we speculate that the RAS may be shifted away from profibrotic responses towards antifibrotic responses by the upregulation of a novel ACE2/Ang (1–7)-MAS axis in the liver. The current study demonstrates that telmisartan has antifibrotic effects that are associated with increased ACE2 expression levels.
With the progression of hepatic fibrosis in bile duct-ligated rats, hepatic ACE2 mRNA and protein expression levels increased and became more widely distributed. This observation is consistent with other studies in which ACE2 upregulation in fibrotic livers has been observed6,7. MAS expression levels closely paralleled the elevated ACE2 expression levels in rat liver, suggesting that increased ACE2 expression levels may favor the formation of Ang (1–7) and further amplify MAS expression levels. Moreover, this result suggests that changes in ACE2 and MAS expression may play an important role in augmenting Ang II levels during the resistance response.
Our data indicate that telmisartan has a significant antifibrotic effect in the liver of bile duct-ligated rats. Interestingly, a previous study showed that telmisartan inhibits hepatic stellate cells (HSC) activation and proliferation. Schuppan et al suggested that telmisartan may be a promising drug for non-alcoholic steatohepatitis (NASH)-related liver fibrosis13. We observed that the expression of ACE2 and MAS levels were upregulated by telmisartan. Moreover, the inhibition of ACE activity could encumber degradation of Ang (1–7) to Ang (1–5), as has been suggested7. Ang (1–7) expression would be enhanced by ARB-mediated inhibition, which in turn could promote MAS expression. Additionally, RTQ-PCR and Western blot results demonstrated that ACE expression moved in the opposite direction of ACE2 expression in animals treated with telmisartan. That is, whereas ACE2 expression levels increased after telmisartan administration, ACE expression levels decreased at the same time. This result suggests that the local RAS is activated in BDL rats and that inhibition of the RAS with telmisartan could inhibit the progression of fibrosis. These findings are consistent with previous reports in which the ACE2/Ang (1–7)-Mas axis plays a key role during liver fibrosis6,7.
In addition, telmisartan has been shown to inhibit HSC activation and proliferation by downregulating TGF-β1 and TIMP-1 and 2, and increasing MMP-13 expression levels21. In an animal model of NASH, another AT1-R antagonist (losartan) inhibited angiotensin II-induced proliferation and TGF-β1 expression in activated HSCs22. We observed that TGF-β1 and col III mRNA expression levels were attenuated in the BDL+8 mg/kg telmisartan daily. Based on these findings, we postulate that telmisartan administration may be an efficient therapeutic strategy for fibrosis in patients with cholestatic liver diseases.
The accumulation of ACE2 in the liver may be responsible for the enhanced Ang II degradation that weakens Ang II accumulation following ARB treatment. This study is consistent with others that have shown that telmisartan evokes ACE2 upregulation in human liver disease and have antifibrotic activity in bile duct-ligated rats6,7. Sukumaran et al suggested that telmisartan provided beneficial protection against heart failure in rats, at least partly by suppressing inflammation, oxidative stress, and endoplasmic reticulum stress, as well as through modulation of the ACE2/Ang (1–7)-Mas axis23. Moreover, kidney ACE2 was shown to be downregulated in a mouse model of early chronic kidney disease24, whereas AT1-R antagonism of early radiation-induced changes in microglial activation or neurogenesis in normal rat brains has not been reported25. This discrepancy may be relevant to differences in disease conditions, the tissues involved, or the variety of ARB drugs tested.
We presume that ACE2 amplification stimulates Ang II degradation and that a decrease in ACE lessens the formation of Ang II. Moreover, Ang (1–7) could be converted to the inactive peptide fragment Ang (1–5) by the degradation of ACE, and ARBs are known to promote Ang (1–7) expression levels26. Furthermore, a decrease of ACE expression levels could offer additional protective effects for hepatic fibrosis through the increased expression of MAS levels. This would explain the reduced degradation of Ang (1–7) due to reduced ACE expression levels after the administration of telmisartan.
In conclusion, this study demonstrates that telmisartan, an ARB, significantly ameliorates hepatic fibrosis by increasing ACE2 expression levels. The accumulation of ACE2 in the liver could play an important role in the regulation of the intrahepatic RAS system by promoting the degradation of Ang II to Ang (1–7) because ACE2 acts in a counter regulatory manner to ACE in promoting Ang II degradation. Increasing ACE2 expression levels in the liver by attenuating Ang II accumulation may be responsible for the antifibrotic and hepatoprotective effects associated with ARBs, such as telmisartan.
Author contribution
Cheng-hong YIN proposed the study; En-tong YI, Rui-xia LIU, and Yan WEN performed the experiments; En-tong YI collected and analyzed the data; and En-tong YI and Rui-xia LIU wrote the first draft. All of the authors contributed to the design and interpretation of the study and to the final draft.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (81073131/H2814).
References
- Pereira RM, Dos Santos RA, Teixeira MM, Leite VH, Costa LP, da Costa Dias FL, et al. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of angiotensin-(1–7) J Hepatol. 2007;46:674–81. doi: 10.1016/j.jhep.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327–38. doi: 10.1111/j.1440-1746.2008.05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–6. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Chaves P, Cerqueira R, Pintalhao M, Leite-Moreira AF. New pathways of the renin-angiotensin system: the role of ACE2 in cardiovascular pathophysiology and therapy. Expert Opin Ther Targets. 2010;14:485–96. doi: 10.1517/14728221003709784. [DOI] [PubMed] [Google Scholar]
- Dejima T, Tamura K, Wakui H, Maeda A, Ohsawa M, Kanaoka T, et al. Prepubertal angiotensin blockade exerts long-term therapeutic effect through sustained ATRAP activation in salt-sensitive hypertensive rats. J Hypertens. 2011;29:1919–29. doi: 10.1097/HJH.0b013e32834a5a46. [DOI] [PubMed] [Google Scholar]
- Huang ML, Li X, Meng Y, Xiao B, Ma Q, Ying SS, et al. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol. 2010;37:e1–6. doi: 10.1111/j.1440-1681.2009.05302.x. [DOI] [PubMed] [Google Scholar]
- Lubel JS, Herath CB, Tchongue J, Grace J, Jia Z, Spencer K, et al. Angiotensin-(1–7), an alternative metabolite of the renin-angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat. Clin Sci (Lond) 2009;117:375–86. doi: 10.1042/CS20080647. [DOI] [PubMed] [Google Scholar]
- Moreno M, Gonzalo T, Kok RJ, Sancho-Bru P, van Beuge M, Swart J, et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942–52. doi: 10.1002/hep.23419. [DOI] [PubMed] [Google Scholar]
- Corey KE, Shah N, Misdraji J, Abu Dayyeh BK, Zheng H, Bhan AK, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int. 2009;29:748–53. doi: 10.1111/j.1478-3231.2009.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- Yang L, Bataller R, Dulyx J, Coffman TM, Gines P, Rippe RA, et al. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol. 2005;43:317–23. doi: 10.1016/j.jhep.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Bataller R, Gabele E, Parsons CJ, Morris T, Yang L, Schoonhoven R, et al. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology. 2005;41:1046–55. doi: 10.1002/hep.20665. [DOI] [PubMed] [Google Scholar]
- Schuppan D, Gorrell MD, Klein T, Mark M, Afdhal NH. The challenge of developing novel pharmacological therapies for non-alcoholic steatohepatitis. Liver Int. 2010;30:795–808. doi: 10.1111/j.1478-3231.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- Iwai M, Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs ACE2-angiotensin-(1–7)-Mas receptor axis. Hypertens Res. 2009;32:533–6. doi: 10.1038/hr.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–6. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- Li N, Zimpelmann J, Cheng K, Wilkins JA, Burns KD. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1–7 by rat proximal tubules. Am J Physiol Renal Physiol. 2005;288:F353–62. doi: 10.1152/ajprenal.00144.2004. [DOI] [PubMed] [Google Scholar]
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Aoyama H, Ohnishi S, Imawari M. Protective effects of fibronectin in galactosamine-induced liver failure in rats. Hepatology. 1986;6:1334–9. doi: 10.1002/hep.1840060619. [DOI] [PubMed] [Google Scholar]
- Kim MY, Baik SK, Park DH, Jang YO, Suk KT, Yea CJ, et al. Angiotensin receptor blockers are superior to angiotensin-converting enzyme inhibitors in the suppression of hepatic fibrosis in a bile duct-ligated rat model. J Gastroenterol. 2008;43:889–96. doi: 10.1007/s00535-008-2239-9. [DOI] [PubMed] [Google Scholar]
- Karimian G, Mohammadi-Karakani A, Sotoudeh M, Ghazi-Khansari M, Ghobadi G, Shakiba B. Attenuation of hepatic fibrosis through captopril and enalapril in the livers of bile duct ligated rats. Biomed Pharmacother. 2008;62:312–6. doi: 10.1016/j.biopha.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Jin H, Yamamoto N, Uchida K, Terai S, Sakaida I. Telmisartan prevents hepatic fibrosis and enzyme-altered lesions in liver cirrhosis rat induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2007;364:801–7. doi: 10.1016/j.bbrc.2007.10.083. [DOI] [PubMed] [Google Scholar]
- Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Shirai Y, et al. Combination treatment of angiotensin II type I receptor blocker and new oral iron chelator attenuates progression of nonalcoholic steatohepatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1094–104. doi: 10.1152/ajpgi.00365.2010. [DOI] [PubMed] [Google Scholar]
- Sukumaran V, Veeraveedu PT, Gurusamy N, Yamaguchi K, Lakshmanan AP, Ma M, et al. Cardioprotective effects of telmisartan against heart failure in rats induced by experimental autoimmune myocarditis through the modulation of angiotensin-converting enzyme-2/angiotensin 1–7/mas receptor axis. Int J Biol Sci. 2011;7:1077–92. doi: 10.7150/ijbs.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ACE2 and angiotensin-(1–7) in a mouse model of early chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1523–32. doi: 10.1152/ajprenal.00426.2009. [DOI] [PubMed] [Google Scholar]
- Conner KR, Forbes ME, Lee WH, Lee YW, Riddle DR. AT1 receptor antagonism does not influence early radiation-induced changes in microglial activation or neurogenesis in the normal rat brain. Radiat Res. 2011;176:71–83. doi: 10.1667/rr2560.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, et al. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1–7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387–95. doi: 10.1016/j.jhep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]