Abstract
The PI3K-Akt pathway is a vital regulator of cell proliferation and survival. Alterations in the PIK3CA gene that lead to enhanced PI3K kinase activity have been reported in many human cancer types, including cancers of the colon, breast, brain, liver, stomach and lung. Deregulation of PI3K causes aberrant Akt activity. Therefore targeting this pathway could have implications for cancer treatment. The first generation PI3K-Akt inhibitors were proven to be highly effective with a low IC50, but later, they were shown to have toxic side effects and poor pharmacological properties and selectivity. Thus, these inhibitors were only effective in preclinical models. However, derivatives of these first generation inhibitors are much more selective and are quite effective in targeting the PI3K-Akt pathway, either alone or in combination. These second-generation inhibitors are essentially a specific chemical moiety that helps to form a strong hydrogen bond interaction with the PI3K/Akt molecule. The goal of this review is to delineate the current efforts that have been undertaken to inhibit the various components of the PI3K and Akt pathway in different types of cancer both in vitro and in vivo. Our focus here is on these novel therapies and their inhibitory effects that depend upon their chemical nature, as well as their development towards clinical trials.
Keywords: anticancer drug, kinase inhibitors, PI3K, Akt, clinical trials
Introduction
The phosphoinositol-3- kinase (PI3K) pathway was discovered in human cancers over 20 years ago as an enzymatic activity associated with a viral oncoprotein. This pathway has received much attention in the study of human cancer because it is important for the cell cycle, proliferation, growth, survival, protein synthesis and glucose metabolism1. PI3K pathway deregulation is the driver in approximately 30% of all cancers. This deregulation occurs via various genetic and epigenetic mechanisms and leads to a wide range of tumor types.
PI3K is a heterodimer with p85 regulatory and p110 catalytic subunits. The class IA PI3K consists of a p85α regulatory subunit and its truncated splice variants p50α and p55α, as well as p85β and p55γ and one of several p110 catalytic subunits (α, β, or δ)2,3. The regulatory subunits p85α, p50α, and p55α are encoded by the PIK3R1 gene. Evidence suggests that p85α is the most abundantly expressed regulatory isoform of PI3K, and p55α and p50α are two minor alternative splicing isoforms4,5. The p110 subunit is encoded by the PIK3CA gene and has three isoforms: α, β, and δ. The p110α isoform is the most common and important subunit in PI3K and the components of p110 include an N-terminal p85-binding domain (p85BD), a RAS binding domain (RBD), a protein-kinase-C homology-2 (C2) domain, a helical domain, and a C-terminal kinase domain. Class IB PI3K comprises p101 regulatory and p110γ catalytic subunits, which feature similar activity6. These two types of enzymes catalyze the phosphorylation of lipid substrate phosphatidylinositols such as PI(4)P and PI(4,5)P2 at their D3 position and the resulting product is PI-3,4,5-P3, which activates downstream signaling pathways through the phosphorylation of several kinases. These kinases include Akt and 3-phosphoinositide-dependent kinase (PDK) and appear to be involved in the regulation of cellular responses varying with the cell types and stimuli studied7.
The role of receptors in PI3K deregulation
The class I PI3K is activated by receptor tyrosine kinases (RTK). RTK activation results in the association of PI3K with the receptor through one or two SH2 domains in the adaptor unit binding to phosphotyrosine consensus motifs. Previously published data suggest that allosteric activation of the catalytic subunit of PI3K in this manner leads to PI-3,4,5-P3 production within a few seconds. The effect of polyphosphoinositide on cells is mediated through specific binding to at least two distinct protein-lipid binding domains, such as Fab-1, YGL023, Vps27, and EEA1 domain (FYVE) and pleckstrin homology (PH) domains. Proteins containing the latter domain are critical mediators for PI3K class IA-induced signaling. The protein serine/threonine kinase 30-phosphoinositide-dependent kinase1 (PDK1) and Akt/PKB are both critical for the transforming effects of deregulated PI3K activity. Ligand-dependent activation of protein tyrosine kinase receptors and receptors coupled with either G-proteins or integrins results in PI3K activation. Such activation may also occur independently of the receptor, as is the case in cells expressing constitutively active Ras. PI3K activation can also occur in the presence of some selected receptor families (eg, EGF and FGF) in cases where they are missing the consensus sequence of the p85 regulatory subunit, necessary for PI3K activation8.
ErbB receptor family and their role in PI3K/Akt activation
Among the surface receptor tyrosine kinases, the epidermal growth factor receptor (ErbB) family can also cause the deregulation of the PI3K pathway. The ErbB receptor family consists of the following four members: ErbB1/Her1, ErbB2/Neu/Her2, ErbB3/Her3, and ErbB4/Her4. ErbB ligand (EGF related peptide growth factor) binding leads to receptor dimerization followed by the activation of an intrinsic tyrosine residue that provides docking sites for PI3K. One unique characteristic of the ErbB receptor family is the ability to form both homodimers and heterodimers. Heterodimer formation leads to signal amplification as well as signal diversification. Interestingly, ErbB2 has the efficiency to form dimers independently of ligand availability, which attributes an important role in tumorigenesis in a large number of breast and ovarian cancers. The synergistic ability of ErbB2 to form dimers with most commonly ErbB3, as well as other ErbB receptors, has clear impact on abnormal cell proliferation as well as tumor progression. It has been shown that ErbB2-ErbB3 dimers are activators of the PI3K-Akt pathway9. Studies in breast cancer cells, primary breast tumors, and transgenic mice all indicate that ErbB2, when overexpressed, is constitutively associated with ErbB310. ErbB3 possesses seven phosphorylale tyrosine residues that act as binding sites for the SH2 domains of the p85 regulatory subunit of PI3K. Tumor cells overexpressing ErbB2 shows constitutive Akt activity11.
Insulin receptors and their role in PI3K/Akt activation
The insulin receptor (IR) and the type 1 insulin-like growth factor receptor (IGFR) evolved from a single ancestral receptor protein and have 70% homology with respect to protein structure. Both proteins are receptor tyrosine kinase that consist of two half-receptors, each comprising one extracellular alpha-subunit and one transmembrane beta-subunit that possess tyrosine kinase activity12.
Current data suggest that insulin resistance has a great impact on cancer progression. Insulin resistance that develops in cancer patients leads to increased levels of circulating insulin combined with IR overexpression. This phenomenon results in abnormal stimulation of the nonmetabolic effects of IR, such as cell survival, proliferation and migration. IR is activated by insulin, which is generally secreted from the pancreas.
IR has two isoforms: IR-A and IR-B. Although IR-B has a greater specificity and affinity for insulin, it plays a less important role in cancer progression than IR-A. Current evidence suggests that IR-A is overexpressed in many malignancies and signaling through IR-A results in more mitogenic effects than signaling through IR-B, which activates the metabolic signaling pathway. Recently, it was found that IR can activate metabolic pathways independently of IGF binding. The main activation mechanism of IR is via the RAS/Raf/MEK/MAPK pathway. The PI3K pathway is the second most common option for IR activation, which occurs via the activation of Akt and mTOR/p70S6K. After insulin binding to IR and subsequent activation of IRS1, activated PI3K catalyzes the production of PI-3,4,5-P3, which results in the phosphorylation of PDK1 and several downstream enzymes, including Akt and PKC. Based on cellular context, Akt can activate or inhibit several proteins involved in glucose and lipid metabolism (ie, GLUT4, PDE3B, Foxa2, GSK3, and AMPK), as well as in cell growth, division, and survival. IR-A has a greater affinity towards the IGFs, especially for IGF2, which is a primary cause of tumorigenesis in many cancer discussed below13.
As discussed above, increased levels of insulin in the portal circulatory system cause an increase in the expression of the growth receptor and an increase in IGF-I production.
Earlier evidence showed that higher level of IGF-I has been observed in colorectal, pre-menopausal breast cancer, and prostate cancer. In vivo studies using p53-deficient mice and LID mice with colon adenocarcinomas demonstrated that IGF-I deficiency reduces tumor growth. In addition to the growth-promoting effects of insulin and IGF-I, IGF-II overexpression has also been observed to play a role in tumor development. IGF-II is produced in the liver and many other tissues in adult humans. In contrast, in rats IGF-II expression decreases in postnatal life and is only expressed to a significant degree in the adult rat brain. In normal conditions, IGF-II expression is controlled by the paternal chromosome under the control of the differentially methylated region (DMR) associated with the H19 gene located upstream on chromosome 11. A loss of imprinting resulting from the methylation of the DMR on the maternal allele leads to overexpression of IGF-II, which has been observed in many tumor types14.
Insulin-like growth factor binding proteins (IGFBPs) play an important role in the stabilization of IGF-I and -II. Among the 6 IGFBP proteins, named IGFBP-1 to IGFBP-6, IGFBP-3 is the most predominant. These binding of proteins increase the circulating half-lives of IGF-I and IGF-II and protect them from further degradation. The main disadvantage of the IGFBPs is that although they increase the stability of IGF, they in turn reduce the availability of IGF for receptor binding14.
IGFR consists of two isoforms: IGF-1R and IGF-2R. These two isoforms, along with the insulin receptors, form the hybrid receptors IGF-1R/IR-A and IGF-1R/IR-B. All of the IGF receptors have significant homology, thus resulting in structural similarity and the possibility of signaling crosstalk. IGF-1R is a tetrameric receptor consisting of two α-subunits and two transmembrane β-subunits that are linked by disulfide bonds. The α-subunits are extracellular and bind IGF. Each transmembrane β-subunit contains an intracellular tyrosine kinase domain. Binding of IGF-I or IGF-II ligand to IGF-1R leads to the phosphorylation of three key tyrosine residues in the kinase domain, leading to the phosphorylation of downstream substrates. In addition, the phosphorylation of additional tyrosine residues in other areas of the β-subunit provide 'docking sites' that allow for the recruitment of adaptor proteins. Insulin receptor substrate family members are some of the many adaptor proteins that are known to have an important role in IGF-1R signaling15.
Ligand binding to IGF-1R causes its autophosphorylation and the tyrosine phosphorylation of IGF-IR substrates, especially the IR substrate 1 (IRS-1) and the Src and collagen-homology (SHC) protein. Tyrosine-phosphorylated IRS-1 and SHC bind different effector proteins (enzymes and/or adapters), inducing multiple signaling cascades, including several interconnecting pathways that control cell survival and proliferation. The critical survival pathway is activated by IGF-I stems from IRS-1. IRS-1 recruits and stimulates PI3K due to the presence of the same homology domain, which then transmits the signal to Akt12,15,16.
PDGF receptors and their role in PI3K/Akt activation
The PDGF family of signaling molecules regulates the growth and motility of connective tissue cells. PDGF is a dimer that consists of disulphide bonded homologous A and B-polypeptide chains. These A and B-polypeptides arrange themselves to form homodimers (PDGF-AA/BB) and heterodimers (PDGF-AB). PDGF isoforms act upon their target cells by binding to two structurally related protein tyrosine kinase receptors. PDGF receptors have two isoforms, α and β. The α-receptor binds both the A- and B-chains of PDGF, whereas the β-receptor binds only the B-chain. Due to these properties, the PDGF-AA and PDGF-BB homodimers typically tend to bind with the PDGF-αα and -ββ receptors, respectively. However, PDGF-AB binds only the β receptors. The α- and β-receptors for PDGF each contain five extracellular immunoglobulin domains and one intracellular tyrosine kinase domain with a characteristic inserted sequence17.
The main event of PDGF activation is receptor dimerization. The close interaction between the kinase domains of the receptors causes phosphorylation in trans between the receptors, which is called autophosphorylation. The main purpose of this autophosphorylation is to regulate the catalytic activity of the kinase and provide a docking site for further downstream target molecule regulation. It has been observed that the tyrosine residues play the major role in the autophosphorylation process. In the case of the β-receptor, the phosphorylation site is Tyr857, which is located inside the kinase domain. This tyrosine is conserved in the α-receptor (Tyr849) and in almost all other tyrosine kinase receptors. Mutational analysis studies demonstrated that replacing the tyrosine with phenylalanine caused lower kinase activity. The involvement of various domains helps to a great extent in an intracellular system. These include the Src homology 2 (SH2) domains and the phosphotyrosine binding (PTB) domains that recognize phosphorylated tyrosine residues in specific environments, the SH3 domains that recognize proline-rich regions, the pleckstrin homology (PH) domains that recognize membrane phospholipids and the PDZ domains that recognize a C-terminal valine residue18. Current data suggest that more than 10 different SH2-domain-containing molecules tend to bind to different autophosphorylation sites in the PDGF α- and β-receptors. There are two types of molecules that bind to the SH2 domains of PDGF receptors: enzymes that have catalytic activity and adaptor molecules that help to bind other molecules. Phosphatidylinositol 3′-kinase is one such molecule that is activated by PDGF19.
PI3K activates PDGF by interacting with the SH2 domain of the p85 subunits. Two tyrosines with a methionine at the +3 position are present in the PDGF β-receptor (ie, Tyr740 and Tyr751) in the autophosphorylation sites, which helps to bind PI3K. Both of these tyrosines are conserved in the α-receptor, where they are also important for the binding of PI3K. The p85 subunit of PI3K has been shown to be phosphorylated at Tyr508 after binding to the PDGF β-receptor, but it is not clear if this has any functional relevance. However, the binding of p85 to a phosphorylated region of a protein has been shown to lead to a conformational change and increased enzymatic activity of the associated catalytic subunit19.
VEGF and its role in PI3K/Akt activation
Vascular endothelial growth factor (VEGF) is an endothelial cell (EC)-specific mitogen and chemotactic agent that is involved in wound repair, angiogenesis of ischemic tissue, tumor growth, microvascular permeability, vascular protection, and hemostasis. VEGF predominantly known as VEGF-A, belongs to the VEGF-PDGF supergene family. The other family members of the VEGF family include VEGF-B, VEGF-C, VEGF-D, and VEGF-E, which all show varying degrees of homology with VEGF-A. Alternative splicing of VEGF results in four isoforms containing 121, 165, 189, or 206 amino acids. The dominant form of VEGF 165 is a 45 kDa homodimeric glycoprotein that exists in a partly matrix and partly membrane bound form. The VEGF isoforms 189 and 206 are basic and mainly stay in the extracellular matrix. The VEGF 121 isoform is acidic and is secreted20.
The VEGF family of proteins binds to three receptor-type tyrosine kinases: Flt-1 (VEGF receptor-1), KDR/Flk-1 (VEGF receptor-2), and VEGFR-3. VEGFR-1 and -2 are normally expressed in vascular ECs, whereas VEGFR-3 is expressed in the lymphatic endothelium. Of the VEGF receptors, KDR/Flk-1 is believed to play the most important role in mediating EC proliferation, migration, and permeability21. VEGFR-1 is able to bind VEGF, VEGF-B, and placental growth factor. VEGFR-2 is activated primarily by VEGF, but proteolytically cleaved forms of VEGF-C and VEGF-D may also activate this receptor. VEGFR-3 is activated only by VEGF-C and VEGF-D22.
From gene targeting studies in mice, it has been proven that VEGF and VEGFR have remarkable effects on the development of the vascular system. Vegfr2-/- mice die between embryonic day 8.5 and 9.5 due to defects in the development of hematopoietic and endothelial cells, resulting in impaired vasculogenesis23.
The mechanism of VEGF activation involves receptor dimerization followed by autophosphorylation on several tyrosine residues and initiation of intracellular signaling pathways. These pathways are mediated by several effectors, which consist of docking sites composed of the phosphorylated tyrosine residues of the activated receptors. These interactions are mediated by Src homology 2 domains, phosphotyrosine-binding domains and other domains of the signaling proteins. Recent data demonstrate that VEGFR-2 is a major component of the VEGF mediated response in endothelial cells and it plays a critical role in signal transduction in both physiologic and pathologic angiogenesis.
Tyrosine 1175 in human VEGFR-2 has been identified as a major autophosphorylation site following VEGF binding. It serves as a docking site for phospholipase C-γ24, which indirectly mediates the activation of the mitogen activated protein kinase pathway that regulates cell proliferation. After VEGF stimulation, VEGFR-2 is phosphorylated at Tyr1175 and Src associates with the receptor, which results in the binding of the SH2 domain of Shb to the phosphorylated Tyr1175 in the C-terminal tail of VEGFR-2. Src phosphorylates Shb, which allows the subsequent activation of PI3K25. Previous studies demonstrated that Akt is required for the VEGF-mediated survival signal26. Thus, VEGF-mediated signaling acts through the PI3K-Akt pathway27.
The role of G-protein coupled receptors in the activation of PI3K class IA and IB
In previous sections of this review, it was discussed that the class I PI3K is activated by RTKs. However, recent data indicate that most class I PI3K subunits might be activated by G protein-coupled receptors (GPCRs), either directly through Gβγ protein subunits (in the case of p110β and p110γ) or indirectly, for example through Ras. The class IB regulatory subunits (p84 and p101) do not have SH2 domains and they link the single class IB PI3K catalytic subunit (p110γ) to GPCRs.
GPCRs are a superfamily of seven transmembrane spanning proteins that consist of Gα and Gβγ subunits. The activation of GPCRs involves the coupling of agonist-activated GPCRs to a wide variety of effector systems via their interaction with heterotrimeric guanine nucleotide binding proteins (G proteins). The binding of an agonist to a GPCR selects for a receptor conformation state that promotes the exchange of GDP for GTP on the G protein α-subunit, which is presumed to allow the dissociation of the G protein Gα- and Gβγ-subunits. Subsequently, the activated Gα- and Gβγ-subunits can either positively or negatively regulate the activities of effector enzymes and ion channels28.
Earlier it was discussed that class IB consists of p101 regulatory and p110γ catalytic subunits. Due to the absence of the p85 subunit, the p110γ catalytic subunit of the class IB PI3K does not form a complex with p85 but is instead activated directly by Gβγ (βγ subunits of GTP-binding protein). The tightly associated regulatory p101 subunit is not essential for Gβγ-induced activation of p110γ but rather plays a role in recruiting p110γ to the plasma membrane and determining substrate specificity. The non-catalytic p101 subunit is also known to determine the substrate specificity of p110γ. The molecular mechanisms of the Gβγ-induced activation of p110γ have been shown to involve the direct interaction of Gβγ with both the NH2- and COOH-terminal of p110γ29. These data suggest that the major isoform of PI3K that is transmitting the signal from GPCRs is p110γ. On the other hand, data suggest that GPCRs cause the activation of class IA PI3K in many cell lines, including hematopoietic cells. One of the possible mechanisms for this activation is that p110β, one of the catalytic subunits of class IA PI3Ks, is activated by Gβγ in cell-free systems. Other members of the class IA PI3Ks, p110α and p110δ, show no sensitivity to Gβγ. However, the structural basis for the Gβγ sensitivity of p110β has not been examined30.
PI3K deregulation by mutational approach
Deregulation of PI3K is most commonly due to the amplification of the PIK3CA gene. It has also been suggested that mutations in the PIK3R1 gene, which encodes the p85 subunit, lead to the activation of p11029. Activating somatic mutations in the p85α regulatory subunit of PI3K (PIK3R1) are prevalent in primary ovarian, colon, and breast cancer31. Interestingly, more than 80% of the mutations in the PIK3CA cluster have been found in two small conserved regions within the helical and kinase domains32. These include three hot spots that result in single amino acid substitutions. The first two hotspots are in exon eight and lead to E545K and E542K mutations in the helical domain. The third hotspot is located in exon twenty and produces a H1047R mutation in the kinase domain by replacing arginine. These first two mutations act cooperatively and relieve the inhibitory effect of p85 on p110α, and the third mutation allows for easier access to p110α in the membrane. Therefore, these mutations lead to increased kinase activity. The non-random distribution of these changes in highly conserved regions results in a series of cellular responses, including increased enzymatic function, enhanced downstream signaling elements, including Akt, and enhanced oncogenic transformation33,34.
Although PIK3CA mutations and ErbB overexpression are critical drivers of tumor development, mutations in the PTEN lipid phosphatase are also found to be major causes for PI3K activation in human cancer. The main substrate of PTEN is PIP3. PTEN dephosphorylates PIP3 at the D3 position and acts as a negative regulator of PI3K signaling. In addition, PTEN also acts as tumor suppressor gene. Mutation of PTEN leads to the formation of sporadic cancers, including glioblastoma, endometrial carcinoma, melanoma and bladder, lung and lymphatic cancer35.
The mechanisms of Akt activation, deregulation and oncogenesis
Akt is one of the downstream targets of PI3K. It is a serine/threonine kinase that is activated due to the formation of PIP3 by PI3K. PIP3 binds the PH domains of Akt and aids in the recruitment of Akt to the plasma membrane, which alters the conformation of Akt to allow for subsequent phosphorylation by PDK136. 3-Phosphoinositides help to increase the phosphorylation of this residue to achieve Akt activation. Akt is activated through the phosphorylation of Thr308, but phosphorylation of the Ser473 residue located at the hydrophobic C-terminal region is also required for full activation of the kinase8. Phosphorylation of Thr308 helps to increase the activation of Akt approximately 100-fold. However, full activation requires the phosphorylation of the Ser473 residue. A different hypothesis regarding Ser473 activation has been proposed for decades. However, recent data have shown that the kinases that aid in this activation are members of the PIKK (PI3 kinase-related kinase) superfamily (mammalian target of rapamycin complex 2 (mTORC2) and DNA-PK)37.
mTORC2 is composed of mTOR, rictor, mSIN1, and mLST8. The most important function of mTORC2 is the phosphorylation of Akt on Ser473. Studies suggest that targeting TSC2 deficient cells with a myristoylation sequence (myr-Akt) that is independent of the PI3K pathway and Sin1−/− MEFs, which completely lack mTORC2 activity, show a higher level of Ser473 phosphorylation. From this study, it can be concluded that recruiting Akt to the plasma membrane through either increased PI3K activity or membrane-targeting sequences is sufficient to stimulate phosphorylation at Ser473 under conditions of low or even no mTORC2 activity38.
The contribution of Ser473 phosphorylation in the regulation of Thr308 phosphorylation, Akt activity, and phosphorylation of downstream substrates is not yet fully understood. Initial studies using alanine mutants shows that Akt Thr308 and Ser473 can be phosphorylated independent of each other. Recent evidences confirm that mice deficient in the mTORC2 components rictor, mSIN1 or mLST8 have selective inhibition of Ser473 phosphorylation, leaving the majority of Thr308 phosphorylation intact. In contrast, RNA interference of rictor, which disrupts the mTORC2 complex, and small molecule inhibitors of mTOR decrease both Ser473 and Thr308 phosphorylation, suggesting that Ser473 phosphorylation can facilitate Thr308 phosphorylation39.
Akt activity is controlled by several molecules that help to dephosphorylate Akt. Among these negative regulators, PP2A (protein phosphatase 2A) regulates Akt activity by inducing Akt Thr308 dephosphorylation. The PH domain leucine-rich repeat protein phosphatase (PHLPP) suppresses Akt activity by dephosphorylating Akt at Ser473. FKBP51 (FK506 binding protein 51) helps maintain the interaction between PHLPP and Akt, serves as a scaffold for Akt and PHLPP and negatively regulates Akt phosphorylation at Ser473. Akt regulates itself like an autocrine system because its activity is controlled by phosphorylation and dephosphorylation cycles within the Akt molecules. Akt phosphorylation and activation is also regulated by promyelocytic leukemia protein (PML) tumor suppressor. PML forms a complex with PP2A and Akt and facilitates PP2A-mediated Akt dephosphorylation at Thr308. PML cooperates with the PTEN tumor suppressor to restrict prostate cancer development in an animal model of the disease40.
Akt activation due to cellular stress
Akt activation often occurs due to the cellular stress response, which is independent of the PI3K pathway. Interestingly, each Akt subtype responds to different stimuli because the response of Akt to cellular stress differs slightly among the family. Stimulation of cells with H2O2, CdCl2, and NaAsO2 activates different Akt subtypes that show distinct responses. This cellular stress-mediated Akt activation occurs due to the association of Akt with Hsp27. By binding Akt, Hsp27 helps to alter the conformation of Akt, which is required for phosphorylation41. Recent data have shown that oxidative stress induced Akt activation, which leads to apoptosis though the increased expression of FOXO3a. This increased FOXO3a expression in turn increases the expression of its transcriptional targets Bim and p27kip142.
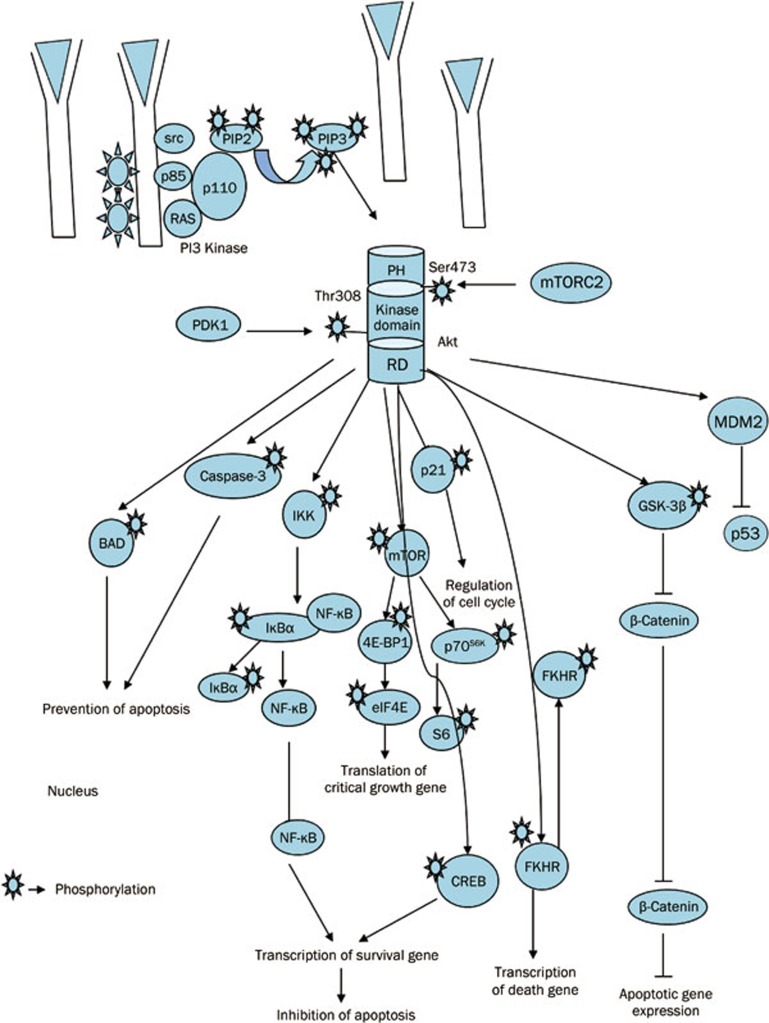
Deregulation of the Akt pathway occurs due to the genetic amplification of the Akt gene. The three isoforms of Akt that have been identified are Akt1, Akt2, and Akt3. These three isoforms are the protein products of the Akt1, Akt2, and Akt3 genes, respectively. It has been found that ovarian, breast, pancreatic, and gastric cancer can be caused by Akt gene amplification43,44. Akt activates a cascade of reactions through the phosphorylation of several molecular targets. Akt activates mTORC-1, a complex also modulated by ERK, which leads to the stimulation of protein synthesis and cell growth by regulating both the ribosomal p70S6 kinase (S6K) and the eukaryotic translation initiation factor 4E (eIF4E)45. Akt regulates cell cycle progression and events associated with them by modulating cell cycle inhibitors, such as p21, p27kip1, and GSK3, and cell cycle stimulators, such as c-myc and cyclin D1, by phosphorylation36. Akt also regulates apoptosis by inhibiting the translocation of the forkhead family of proteins from the cytoplasm to the nucleus. Inhibiting this translocation blocks transcription of the death genes FASL and BIM46,47, increases the transcription of survival genes by NF-κB and CREB transcription factor activation48,49, phosphorylates and inactivates the proapoptotic protein BAD50,51, and maintains mitochondrial integrity by activating hexokinase (Figure 1). Akt regulates many cellular processes, including differentiation, proliferation and transformation by phosphorylating GSK-352,53. Through the upregulation of the transcription repressor Snail, Akt modulates the induction of the epithelial-mesenchymal transition and tumor cell invasion. Akt/mTORC1 induces the expression of HIF-1 and VEGF, which are key elements involved in angiogenesis. Feedback inhibition mechanisms have also been identified in this complex pathway. One of the most notable examples that has clinical relevance is the ability of S6K to phosphorylate and inhibit IRS-1, an upstream member of the PI3K/Akt pathway54.
Figure 1.
Schematic representation of PI3K-AKT pathway and associated cellular activities. PDK2 is shown as mTORC2. Continuous lines represent direct AKT phosphorylation, leading to activation (arrow end) or inhibition (blunt end). PI3K kinase consists of p110 catalytic and p85 regulatory subunits. PI3K is activated by receptor tyrosine kinases. Once, activated PI3K converts phosphatidylinositol 3,4-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 helps to recruit PH domain binding protein like Akt to cell membrane and thus activates Akt. The recruitment of Akt to cell membrane leads to the conformational changes in Akt and helps PDK1 for phosphorylation at Thr308 residue. The complete activation of Akt requires phosphorylation of AKT at the hydrophobic C-terminal domain Ser473 residue. This phosphorylated AKT regulates apoptosis and various cellular activities by phosphorylating various downstream substrates.
PI3K inhibitors
The PI3K inhibitor LY294002 and wortmannin are first generation pan-PI3K inhibitors that act as ATP competitive manner. Wortmannin inhibits PI3K activity by binding covalently to a conserved lysine residue in the ATP binding site of the enzyme55. LY294002 is a flavonoid derivative and a reversible, ATP-competitive inhibitor that has an IC50 for recombinant PI3K in the low micromolar range56. Wortmannin and LY294002 both have anti-proliferative and apoptotic effects in vitro and in vivo56,57,58. Wortmannin and LY294002 are effective inhibitors of PI3K, but the use of these two compounds is limited to the preclinical level due to their instability in aqueous solutions, toxic side effects, poor pharmaceutical properties, and lack of selectivity for the oncogenic class I PI3K isoforms59 (Table 1).
Table 1. Chemical nature, mode of action and clinical stage of different PI3K inhibitors.
| PI3K inhibitor | Chemical nature | Target | Clinical trial | Reference |
|---|---|---|---|---|
| LY294002 | Flavonoid derivative | Pan PI3K | – | 57 |
| Wortmanin | Furansteroid | PI3K, Polo like kinase | – | 171 |
| SF1126 | Morpholino derivative | Pan PI3K, mTOR, DNA-PK | I | 61 |
| PX-866 | Viridian derivative | PI3K | II | Clinicaltrials.gov |
| GDC-0941 | Morpholino derivative | Pan PI3K, mTOR | II | 67 |
| GDC-0980 | Morpholino derivative | PI3K (p110α), mTOR | II | 74 |
| PKI-402 | Morpholino derivative with fused pyrimidines | Pan PI3K, mutant PI3K, mTOR | I | 76 |
| PKI-587 | Morpholino derivative with fused pyrimidines | Pan PI3K, mTOR | I | 78 |
| NVP-BKM120 | Bismorphalino derivative | Pan PI3K, VPS34, mTOR, DNA-PK, and PI4K | I | 80 |
| BGT-226 | Structure undisclosed | PI3K, mTOR | I/II | 96 |
| NVP-BEZ235 | Imidazoquinazoline derivative | Pan PI3K, mTOR | I/II | 84 |
| XL147 | Structure undisclosed | Class-I PI3K | I | 98 |
| XL765 | Structure undisclosed | Class-I PI3K, DNAPK, mTOR | II | 98 |
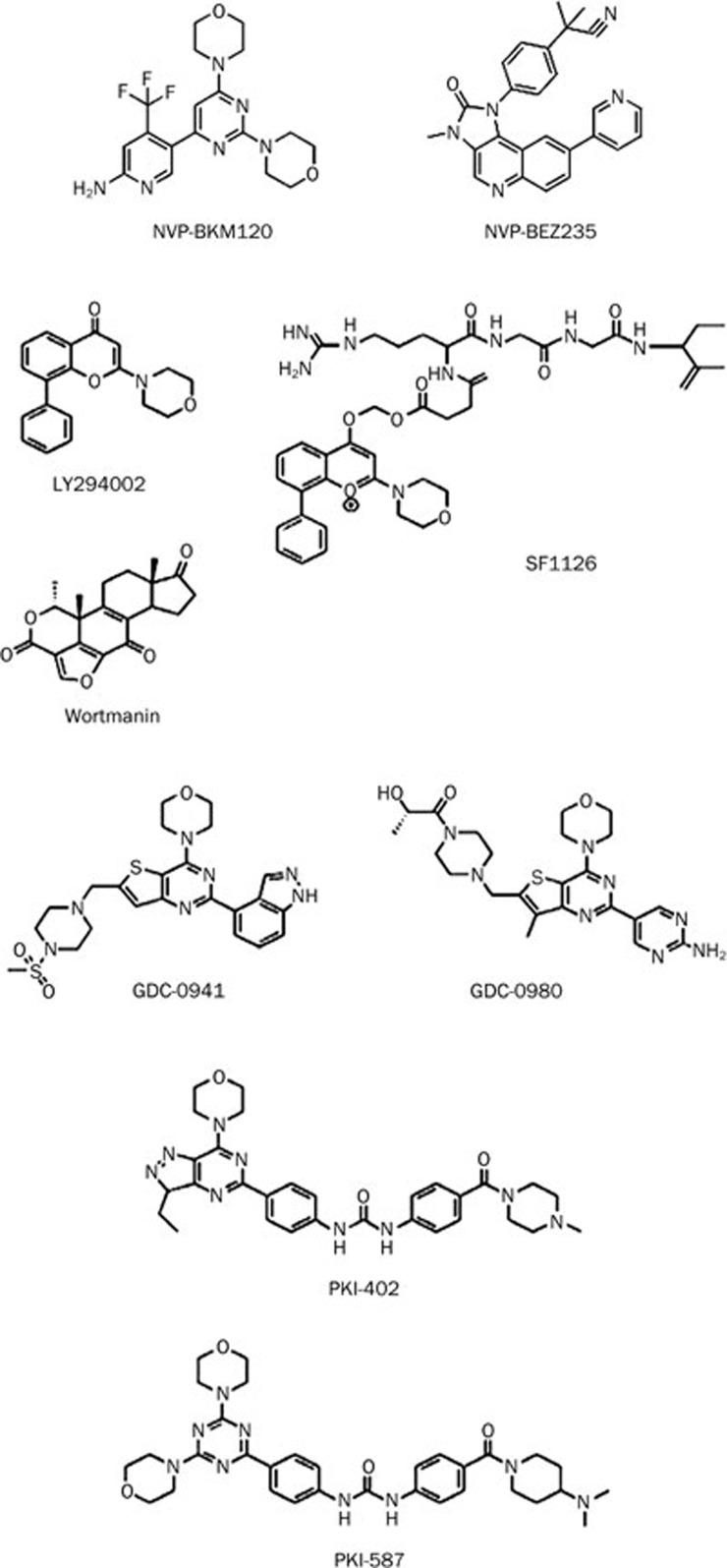
To overcome these pharmacological problems, including poor solubility and lack of selectivity, LY294002 was recently conjugated with an RGD-containing peptide. This conjugation of LY294002 to RGDs results in increased solubility and binding to specific integrins (primarily αvβ3 and α5β1) in the tumor compartment, which helps to target stromal endothelial and tumor cells, depending upon effect of LY294002 on the microenvironment. The anticancer effects of SF1126 (Figure 2) have been observed in a wide variety of in vitro and in vivo systems, including renal cell carcinoma, glioblastoma, neuroblastoma, and rhabdomyosarcoma, as well as in breast and prostate cancer xenografts60. Recent studies have found that SF1126, in combination with other chemotherapeutic agents, has a more profound anticancer effect. SF1126 in combination with trastuzumab synergistically inhibits the growth of HER2-overexpressing breast cancers, including those that are trastuzumab resistant61. SF1126, either alone or in combination with anthracyclines, has profound anticancer effects in neuroblastoma and tumors with a highly activated PI3K-Akt-MDM2 pathway (Table 2). SF1126 inhibits tumor growth by blocking the production of PIP2 and PIP3. Ultimately, SF1126 prevents the conversion of inactive Rac1-GDP to active Rac1-GTP, which deregulates actin cytoskeleton polymerization62.
Figure 2.
Chemical structure of the PI3K inhibitors.
Table 2. Combinatorial effects of PI3K inhibitors on several cancers.
| PI3K inhibitors | Combination agents | Tumor types | Reference |
|---|---|---|---|
| SF1126 | Trastuzumab | HER-2 overexpressing breast cancer | 60 |
| Doxorubicin | Neuroblastoma | 61 | |
| GDC-0941 | Trastuzumab and pertuzumab | Her-2 specific breast cancer | 69 |
| Doxorubicin | Breast and ovarian cancer | 70 | |
| NVP-BEZ235 | AZD6244 | Melanoma | 82 |
| Gemcitabine | Pancreatic ductal adenocarcinoma | 172 | |
| Sorafenib | Renal cell carcinoma | 173 | |
| RAD001 | Non-small cell lung cancer | 174 | |
| Neuroendocrine tumors | 175 | ||
| GSK2126458 | Breast cancer | 176 | |
| Taxotere | Prostate cancer | 177 | |
| PD0325901 | Glioma | 178 | |
| Vincristine | Sarcoma | 92 | |
| XL147 | Erlotinib | Solid tumors | Bioportfolio.com |
| Trastuzumab or paclitaxel | Metastatic breast cancer | Exelixis.com | |
| XL765 | Temozolomide | Glioma | Exelixis.com |
| Erlotinib | Solid tumor |
Achievements in clinical trials
SF1126 has recently entered phase I clinical studies. The current phase I clinical trial of SF1126 in adults is at maximum dose 1, 110 mg/m2 per dose (40 mg/kg per dose) and shows minimal toxicity. SF1126 is in development in multiple phase I clinical trials as a single agent, and interim results have been presented recently from patients with solid tumors62 and multiple myelomas63. In an ongoing phase I dose escalation study in patients with solid tumors, SF1126 is administered weekly on days 1, 4 weekly by 90-min IV infusions in cycles of 4-week cycles. To date, 18 of 39 (46%) of dosed patients showed stable disease as the best response, with a median duration of 13 weeks (range 8 to 64+ weeks) and a mean duration of approximately 19 weeks. SF1126 is well tolerated, with the most common grade 1 adverse events being nausea, vomiting, diarrhea, fever, fatigue, chills, and pruritus. SF1126 is dual PI3K/mTOR inhibitor. However, SF1126 exerts its effects mainly as a PI3K inhibitor62.
Previous findings suggest that the morpholino group on the related quinazoline template of LY294002 was critical for the p110α inhibitory activity of LY294002. It was found that this morpholine oxygen helps in the hydrogen bonding interaction in the ATP pocket of both class of PI3K and mTOR59. Depending upon this phenomenon various analogs of LY294002 were discovered which interacts with PI3K and mTOR in a very similar manner63.
PX-866 is a semi-synthetic viridian derivative that potently and irreversibly inhibits PI3Kα, γ, and δ (IC50=5.5, 9.0, and 2.7 nmol/L, respectively). PX-866 shows both increased selectivity towards PI3Kβ (IC50>300 nmol/L) and improved stability and reduced hepatotoxicity, compared to wortmannin. The compound has also demonstrated antitumor activity in several human solid tumor models (eg, OvCar-3, HT-29, and A-549) when used alone (2 mg/kg, po) or in combination with chemotherapeutic or targeted anticancer agents (eg, paclitaxel or gefitinib)64. PX-866 has also exhibited inhibitory effects on human glioblastoma by inducing autophagy and exploiting the aberrant PTEN/PI3K expression65. One of the major side effects of PX-866 is hyperglycemia. The data demonstrate that administration of PX-866 to SCID mice causes hyperglycemia with a decreased glucose tolerance. Recent findings have shown ways to circumvent this problem. Pioglitazone, which is a peroxisome proliferator-activated receptor gamma agonist, prevents the hyperglycemia caused by PX-866 without affecting its antitumor activity66. PX-866 had entered phase I clinical trials in 2008 and is currently in phase II clinical trials for prostate cancer treatment.
GDC-0941 is a morpholino derivative (Figure 2) that is a potent, selective, and orally available Pan-PI3K and dual pan-PI3K/mTOR inhibitor, which has recently entered into phase I clinical trials. GDC-0941 has a prominent inhibitory effect against all four isoforms of PI3K, but it is the most effective against p110α and p110δ. GDC-0941 has selectivity against 228 kinases of the PI3K family. However, greater than 50% inhibition occurs in the case of only two enzymes, Flt3 (59% inhibition) and the human kinase TrkA (61% inhibition)67. It has been previously shown that GDC-0941 has inhibitory effects on U87MG, PC3, SKOV-3, IGROV-1, Detroit 562, HCT116, SNUC2B, and LoVo cell lines with very low IC50 values. GDC-0941 also inhibits U87MG glioblastoma and IGROV-1 human ovarian cancer xenograft models in athymic mice68. GDC-0941 either alone or in combination with trastuzumab and pertuzumab inhibits HER-2 specific breast cancer effectively in vitro and in vivo69 (Table 2). In combination with DNA damaging agents, GDC-0941 has inhibitory effects on breast and ovarian cancer cells70 (Table 2). The drug also shows remarkable inhibitory effects on B-cell lymphomas in PTEN/LKB1 deficient mice. GDC-0941 suppresses the phosphorylation of Akt at Ser473 and Thr308, as well as the phosphorylation of PRAS 40 and ribosomal S6 kinase, which resides in the downstream of the PI3K pathway. However, GDC-0941 does not have a significant effect on the phosphorylation of 4E-Bp1. It is still unclear whether PTEN status affects the activity GDC-0941 in B-cell lymphomas71. Recently, it has been shown that the metastatic abilities of thyroid cancer cells are suppressed by GDC-0941. The mechanism of this inhibition is solely dependent upon the hypoxia-inducible factor-1α (HIF-1α). It has also been suggested that the anti-proliferative effects of GDC-0941 depend on PTEN status in thyroid cancer72, but the mechanism is still unclear. Thus, the role of PTEN status in the effectiveness of GDC-0941 is distinct for different cancer types. Further investigations are required to reach definitive conclusions regarding the effect of PTEN status on GDC-0941 efficacy.
Another morpholino derivative that acts as dual PI3K/mTOR inhibitor and is going through phase II clinical trials is GDC-0980 (Figure 2). GDC-0980 is synthesized by substituting the indazole in GDC-0941 for a 2-aminopyrimidine73. This substitution also enhances the efficacy of mTOR inhibition. GDC-0980 has a very low IC50 (4.8 nmol/L for p110α/p85). The drug shows great potency against a wide array of cell lines, including breast, prostate, and lung cancers; however, it demonstrates less activity in melanoma and pancreatic cancers, which is consistent with KRAS and BRAF acting as resistance markers in these tumors74. GDC-0980 shows inhibitory effects against human xenograft models, including PC3 and MCF-7 neo/HER2 xenografts and xenografts with activated PI3K, loss of LKB1 or PTEN. The drug also elicited an exposure-related decrease in PD biomarkers74.
Current progress in clinical trials
In phase I trials, to date it has been found that twelve patients have been enrolled in four successive dose-escalation cohorts with daily dose levels of 2, 4, 8, and 16 mg of GDC-0980. The most frequently reported adverse events were nausea (25%), fatigue (50%), diarrhea (42%), and flatulence (25%). No drug-related grade 3 or higher events or DLT have been reported to date. Preliminary PK data revealed dose-proportional increases in Cmax and AUC. Preliminary PD data show >50% inhibition of pAkt levels assayed in PRP (relative to baseline) following a single dose of 8 mg or higher GDC-0980. Potential signs of antitumor activity have been observed in one mesothelioma patient (previously treated with radiation and cisplatin/pemetrexed). This patient had a decrease in target lesions of ∼32%, as measured by RECIST after one cycle of 8 mg GDC-0980 QD. A soft-tissue-sarcoma patient also had a decrease in tumor mean (SUVmax of ∼31%), as assessed by FDG-PET after 2 weeks of 16 mg GDC-0980 QD75. GDC-0941 and GDC-0980 will enter into phase 2 clinical trials with advanced and metastatic breast cancer patients (www.clinicaltrials.gov).
It has previously been reported that several morpholine group bearing fused pyrimidines, such as imidazolopyrimidines, pyrrolopyrimidines and triazolopyrimidines, act as PI3K/mTOR dual inhibitors. PKI-402 and PKI-587 are new additions to this group (Figure 2). PKI-402 is another dual PI3K/mTOR inhibitor that behaves in an ATP competitive manner. PKI-402 exerts inhibitory effects against all of the isoforms of PI3K, including the E545K and H1047R PI3K-α mutants and mTOR. Analyses of the inhibitory effects of PKI-402 against a panel of 236 human kinases showed a highly selective profile. However, only C-Raf and B-Raf (wild-type and V600E mutant) were inhibited by PKI-402 with a low IC50 value. PKI-402 has exhibited growth inhibition in vitro and in vivo against breast, brain, pancreas, and non-small cell lung cancer (NSCLC) cells76. The mechanism behind the inhibitory effects of PKI-402 was investigated in MDA-MB-361 cells. It was found out that PKI-402 inhibits MDA-MB-361 cell line growth in vitro and in vivo by inhibiting the phosphorylation of Akt at Ser473 and Thr308, as well as by inhibiting the downstream target molecules of Akt phosphorylation. PKI-402 induces apoptosis in MDA-MB-361 cells by a caspase-3 dependent pathway77.
PKI-587 has a similar in vitro and in vivo profile to PKI-402. PKI-587 has broad in vivo efficacy in the MDA-MB-361, BT474, H1975, U87MG, and HCT116 tumor models78. PKI-587 has shown great potency against EGFR- and HER2-inhibitor-resistant lung tumors. PKI-587 is less effective in HCT116 colon cancers bearing K-Ras and PIK3CA mutations. When combined with the MEK inhibitor PD0325901, PKI-587 shows enhanced efficacy in HCT116 cells in vitro and in vivo79.
NVP-BKM120, a bismorpholino derivative, inhibits a range of PI3K deregulated cell lines from a variety of tumor types, including glioblastoma and ovarian, breast, and prostate cancer. NVP-BKM120 inhibits all four isoforms of PI3K with the following low IC50 values: 0.052 μmol/L (p110α), 0.166 μmol/L (p110β), 0.116 μmol/L (p110δ) and 0.262 nmol/L (p110γ). NVP-BKM120 has lower potency against class III and class IV PI3K. The biochemical activity of NVP-BKM120 was observed for the inhibition of VPS34, mTOR, DNAPK, and PI4K. The drug exerts antitumor activity against two models of PI3K/Akt pathway driven cancers: the A2780 ovarian carcinoma model and the U87MG glioma model, which carry a PTEN deletion80. It has also been observed that the inhibition of glioma cells by NVP-BKM120 depends upon the mutational status of p53. Wild-type p53 cells are more sensitive towards apoptosis than cells with mutated or deleted p53, which undergo mitotic catastrophe cell death81. NVP-BKM120 also enhances the efficacy of other antitumor agents in in vitro combination studies (Figure 2)82.
Another dual PI3K/mTOR inhibitor NVP-BEZ235, which is currently in phase I/II clinical trials, is the most effective small molecule inhibitor of PI3K to date. NVP-BEZ235 has an imidazoquinazoline moiety (Figure 2) that helps to mimic the H-bond interactions between the adenine moiety of ATP and the hinge region using several binding modes, which inhibits the binding of ATP83. NVP-BEZ235 is a pan-PI3K inhibitor that acts against all of the isoforms of PI3K and PI3K mutants. Previous studies have demonstrated the efficacy of NVP-BEZ235 as an antitumor agent in vitro and in vivo in glioblastoma, multiple myeloma, melanoma, lymphoma, sarcoma, breast, lung, and ovarian cancer models84,85,86,87,88,89,90,91,92,93. In the case of colon cancer, NVP-BEZ23 decreases cellular proliferation and causes sustained inhibition of mTORC1 and mTORC2 with a transient PI3K blockade with no subsequent effect on apoptosis either in vitro or in vivo in a GEM model. The effect of NVP-BEZ235 does not depend on the mutational status of PIK3CA in in vitro CRC cell lines. The GEM model also suggests that treatment with NVP-BEZ23 causes tumor regression and a decrease in tumor angiogenesis94. NVP-BEZ235 shows remarkable efficacy in a wide array of cancer types in combination with several chemotherapeutic agents (Table 2).
BGT226, a dual PI3K/mTOR inhibitor, is currently in phase I/II clinical trials for solid tumor, breast cancer, and Cowden syndrome patients (www.clinicaltrial.gov). It has shown efficacy in inhibiting estrogen-deprived ER positive breast cancer cell lines, multiple myelomas and head and neck cancers95,96,97. The modes of inhibition by BGT226 vary in different types of cancer cell lines. BGT226 inhibits breast cancer by an apoptosis dependent manner which is solely dependent upon the PIK3CA mutational status95. But, on the contrary, BGT226 inhibits head and neck cancer in an apoptosis-independent manner. Instead of apoptosis, BGT226 induces autophagy, which is regulated by upregulation of the microtubule-associated protein light chain 3B-II and p62 degradation97.
XL-147 (structure not disclosed) is a low-molecular-weight, potent, orally bioavailable PI3K inhibitor that recently went into phase II clinical trials for advanced or recurrent endometrial cancer (www.clinicaltrials.gov). XL-147 generally inhibits the class I PI3K family of lipid kinases with a nanomolar range of IC50 values98. XL-147 is active against class I PI3Ks (IC50=39, 383, 23, and 36 nmol/L for p110α, β, γ, and δ, respectively) without inhibiting the kinase activity of Vps34 (IC50=6975 nmol/L), DNA-PK, or mTOR (IC50>15 000 nmol/L)98,99. XL-147 acts in an ATP competitive and reversible manner98. Preclinical studies suggest that XL-147 has a remarkable inhibitory effect on MCF-7 breast cancer cells and A549 lung adenocarcinoma cells98. The drug also induces striking tumor regression in breast, lung, ovarian, prostate, and glioma tumors100. In combination with several chemotherapeutic agents, XL147 has also shown tumor growth inhibition (Table 2).
XL-765 is a dual PI3K/mTOR inhibitor with IC50s in the low nanomolar range (IC50=39, 113, 9, and 43 nmol/L for p110α, β, γ, and δ, respectively). Unlike XL-147, XL-765 also inhibits DNA-PK (IC50=150 nmol/L) and mTOR (IC50=157 nmol/L)101,102. Recent studies suggest that XL-765, in combination with autophagy-inducing agents, shows increased antitumor activity in pancreatic adenocarcinomas103. The combination of XL-765 and other chemotherapeutic agents is now undergoing a screening process and entering into clinical trials (Table 2). The clinical trial data suggest that there is inter-patient variability, with terminal half-life values that range from 2.5 to 8 d for XL-147 and 2 to 15 h for XL-765. Preliminary signs of exposure-dependent pharmacodynamic modulations (changes in plasma insulin levels) are observed at 30 mg/60 mg for XL-147 and 15 mg/30 mg bid for XL-765100.
Akt inhibitors
The role of Akt in tumor initiation and progression has been discussed above. Overexpression and activation of Akt are often associated with resistance of tumors to chemotherapy or radiotherapy104,105,106. Current studies with a dominant negative Akt suggest that introducing a dominant negative Akt reduces Akt activity. These data suggest that Akt activity causes resistance against drug and radiation therapy107,108. Introduction of pan-Akt or Akt isoform-specific inhibitors exhibit great efficacy in inhibiting tumor growth.
Alkyl-phospholipids (APLs) are a heterogeneous group of unnatural lipids that have been shown to have anticancer activity. APLs are made up of two aliphatic side chains that are linked to glycerol phosphocholine by either an ether or thio-ether bond. Unlike the other anti-cancer agents described, APLs inhibit cancer by targeting cell membranes rather than DNA109,110. The rapid turnover and nonessential glycerol moiety of APLs restrict their use as anticancer agents. To overcome this problem, next generation molecules called alkylphosphocholines (APCs) have been synthesized.
APCs are derived from APLs by the removal of the glycerol group, which leads to increased stability of the compounds. This allows the APCs to integrate into the lipid bilayer of the cell membrane by accumulating in the lipid rafts where they disrupt the natural balance and metabolism of phospholipids, which in turn leads to alterations in membrane-signaling pathways that are associated with apoptosis111. Examples of anticancer APCs include the prototypic compound edelfosine and the novel drug octadecyl-(1,1-dimethyl-piperidinio-4-yl)-phosphate (D-21266, Perifosine), which affect apoptotic signaling. Studies on malignantly transformed hematopoietic cell lines showed that APCs reduce the levels of phosphorylated Akt (Figure 3)112.
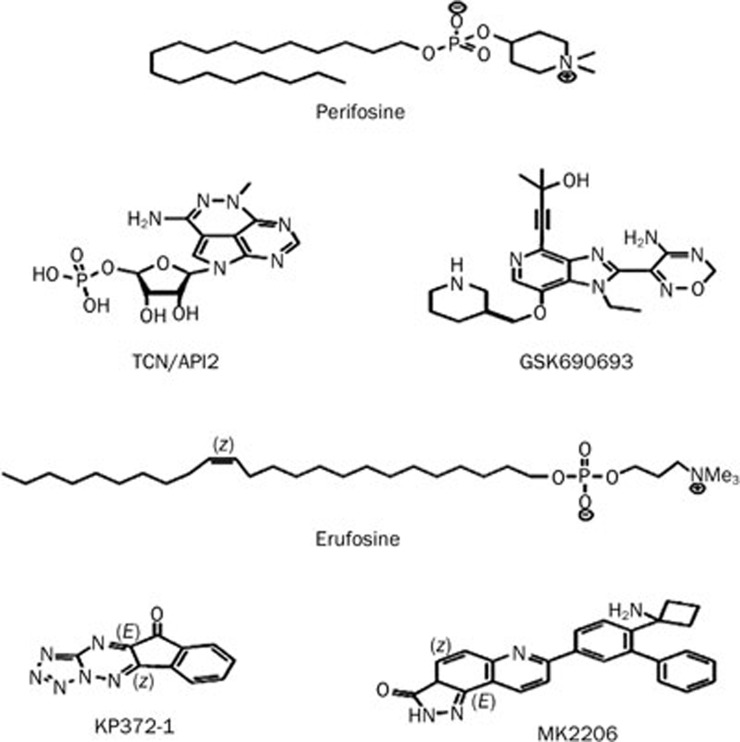
Figure 3.
Chemical structure of Akt inhibitors.
Perifosine is considered to be one of the first generation of Akt inhibitors. It is a alkylphospholipid derived from the compound miltefosine, which is also an APL that has antitumor activity to a lesser extent113 (Figure 3). Mechanistically, Perifosine exhibits antitumor activity by preventing Akt translocation to the cell membrane114. The drug has demonstrated antitumor effects in neuroblastoma, ovarian cancer, Waldenstrom's macroglobulinemia, medulloblastoma, kidney cancer, endometrial cancer, prostate cancer, myelogenous leukemia, multiple myeloma, squamous cell carcinoma, breast cancer, sarcoma, lung cancer, hepatocellular carcinoma, and other solid tumors115,116,117,118,119,120,121,122,123,124,125,126,127. With its success in preclinical models, Perifosine reached phase I and II trials for the treatment of only solid tumors, Waldenstrom's macroglobulinemia, and sarcoma127,128,129. In cases of relapsed or refracted Waldenstrom's macroglobulinemia, Perifosine resulted in at least a minimal response in 35% and stable disease in 54% of patients but had major side effects, including cytopenias and gastrointestinal toxicities. Despite its promising performance in the preclinical models, Perifosine monotherapy does not show efficacy in clinical trials. Disappointingly, it has been showing poor results in advanced or metastatic breast cancer, head and neck cancer, pancreatic adenocarcinoma, and metastatic melanoma patients. Recently, preclinical data have informed the attempts to use Perifosine for combination therapy (Table 3), Perifosine has had clinical success when used in combination regimens in metastatic colorectal cancer, relapsed or refractory multiple myeloma and glioma130,131,132. Perifosine in combination with paclitaxel leads to Akt/mTORC1 inhibition, along with a marked increase in ceramide and ROS generation and an increase in AMPK and JNK that results in apoptosis133. Perifosine induces caspase-dependent apoptosis and downregulates P-glycoprotein expression in multidrug-resistant human T-acute leukemia cells by a JNK-dependent mechanism. In multiple myeloma, the drug phosphorylates Rb134 and downregulates β-catenin and survivin135. Perifosine has been given Fast Track status by the FDA in these tumors and is currently in phase III clinical trials.
Table 3. Combinatorial effects of Akt inhibitors on cancer and progression into the clinical trial.
| AKT inhibitors | Combination agent | Tumor type | Clinical trial | Reference |
|---|---|---|---|---|
| Perifosine | Capecitabine | Metastatic colorectal cancer | I | 130 |
| Bortezomib | Multiple myolema | I | 131 | |
| Bortezomib+dexamethasone | Relapsed/refractory multiple myeloma | II | 131 | |
| CCI-779 | Glioma | I | 132 | |
| Erufosine | Etoposide | Acute myeloid leukemia | I | 141 |
| Ionizing radiation | Prostate cancer | I | 141 | |
| MK2206 | AZD6244 | Colorectal cancer | II | 166 |
| Gefitinib | Glioma | I | 168 |
Erufosine, another APC compound, was introduced in late 1990. However, its lack of efficiency on the leukemia cell line has restricted its use136. Later, Erufosine was improved upon with a longer (22-carbon) chain containing cis-13–14 double bonds (Figure 3). Erufosine shows less hemolytic toxicity upon intravenous application and its cholinomimetic properties are less pronounced compared to perifosine137. It has been previously reported that Erufosine induced apoptosis in human astrocytoma and glioblastoma cell lines in vitro and affected rat C6 glioma tumor growth in vivo138,139. Erufosine induces apoptosis in glioma tumor by ROS generation and inhibits the mitochondrial respiratory chain, especially F1F0-ATP-synthase140. Erufosine, either alone or in combination with other chemotherapeutic agents and ionic radiation, induces apoptosis in different kinds of cancer141 (Table 3). Erufosine combined with etoposide and cytarabine acts additively in acute myeloid leukemia142. Erufosine causes cell death in acute myeloid leukemia by JNK 1/2 activation and ERK 1/2 dephosphorylation, leading to apoptosis143. Cytotoxic effects of Erufosine on chronic lymphocytic leukemia (CLL) and multiple myeloma have also been observed. However, depending upon Akt phosphorylation status, the mode of apoptosis induction is different144,145.
KP372-1, a triazinones derivative (Figure 3), inhibits proliferation and induces apoptosis in thyroid cancer, glioblastoma, squamous cell cancer, and acute myelogenous leukemia cell lines146,147,148,149. It is also a PDK1 and FLT3 inhibitor that causes the inhibition of PKB activation through Ser473 phosphorylation, as well as downstream target phosphorylation, which reflects the non-selective mode of action. Recently KP372-1 was incorporated into nanoparticles and used in targeted drug-delivery systems150.
Triciribine (TCN/API-2) is a small-molecule Akt inhibitor that was invented in the early 1980 and 1990s. Despite showing efficacy in inhibiting the growth of several cancer cell lines, Triciribine was not widely used due to its poor performance in clinical trials151. This poor performance was due to the drug's minimal efficacy and poor toxicity profile, including hyperglycemia and hypertriglyceridemia at high doses. Recently, Triciribine was rediscovered from a chemical library of 1992 compounds in the NCI Diversity Set as an agent that was capable of inhibiting the growth of Akt2-transformed cell lines but not the parental NIH-3T3 cell line. The drug induces cell death by suppressing the phosphorylation of Akt on both Ser473 and Thr308 residue152. Triciribine suppresses epidermal growth factor-induced kinase activity and phosphorylation of all three isoforms of Akt and results in EGF-mediated Akt recruitment to the plasma membrane. However, Triciribine does not inhibit Akt kinase activity in vitro and prevents the phosphorylation of Akt in intact cells153. After entering the cell, Triciribine is converted into the active metabolite TCN-P by adenosine kinase154. TCN-P binds with the PH domain of Akt and inhibits PIP3 to bind with it.
Causes for inclusion in clinical trials
One of the main problems for cancer therapeutics is immunoevasion, which occurs when the immune system cannot control tumor growth. The main cause of immunoevasion is supposed to be activation of Akt. TCN modulates immunoevasion efficiently in combination with the E7-specific vaccine155. TCN and TCN-P are currently being tested in clinical trials. They have been showing positive results in soft tissue sarcoma, colorectal cancer, tonsillar carcinoma and cervical squamous cell carcinoma patients156,157.
GSK690693 is a novel ATP competitive low-nanomolar pan-Akt kinase inhibitor (Figure 3) that belongs to the aminofurazan family, and it has IC50 values of 2, 13, and 9 nmol/L for Akt1, Akt2, and Akt 3, respectively158. It is highly selective towards Akt and 13 other kinases. Most of these kinases belong to the AGC kinase family, including the Akt kinases159. GSK690693 is found to be sensitive against the sixty-two hematologic tumor cell lines, including ALL, AML, CLL, CML, Burkitt's lymphoma, non-Hodgkin's lymphoma and Hodgkin's lymphoma cell lines, and induces apoptosis and growth inhibition160. Recent studies using preclinical models suggest that GSK690693 had its greatest efficacy in delaying tumor progression in tumors and tumor derived cell lines with a high degree of Akt activation, including those with myristoylated constitutively active Akt or with PTEN loss161. However, in vivo testing of preclinical models suggests that GSK690693 exerts a moderate activity in solid tumor and ALL xenograft models162.
MK2206 is an orally active allosteric Akt inhibitor (Figure 3). It shows equal potency toward two isoforms of Akt, purified recombinant human Akt1 (IC50, 5 nmol/L) and Akt2 (IC50, 12 nmol/L), but it is approximately five-fold less potent against human Akt3 (IC50, 65 nmol/L)163. MK2206 shows great potency against several cancer cell lines harboring PIK3CA mutations164,165. In addition, MK-2206, in combination with other chemotherapeutic agents, exerts a greater potency against non-small cell lung cancer, glioma, breast, and ovarian carcinoma in both in vivo and in vitro models (Table 3)166,167,168. MK-2206 helps in restoring the efficacy of sorefenib to induce apoptosis in sorefenib-resistant hepatocellular carcinomas169. MK-2206 is now in phase I clinical trials and is demonstrating efficacy with good tolerance levels170.
Conclusion
There is an impressive and increasing armamentarium of targeted agents that can inhibit the key components of the PI3K-Akt pathway and many of these drugs are already in clinical trials. As discussed above, deregulation of these signaling cascades by mutation or overexpression of several receptors leads to aberrant activation of these intracellular cascades. This phenomenon is a frequent event in human tumors and provides unique opportunities for therapeutic approaches. Epidemiological data provide information regarding the different components and their roles in the PI3K-Akt pathway, which has allowed for the identification and development of clinical candidates that can control the irregular activation of these pathways. Currently, one of the biggest challenges for researchers designing kinase inhibitors is to identify structural features around the ATP-binding site of the targeted enzyme. Modern kinase inhibitors that are going into clinical trials demonstrate that small molecule inhibitors are highly successful in inducing anticancer effects. This is due to the strong interaction between the inhibitors and groups in the domain adjacent to the ATP-binding site. Despite the lack of complete specificity, with the exception of the PI3K/Akt inhibitors that are among the most selective inhibitors reported to date, the compounds in clinical trials have shown significant in vivo antitumor activities with no side effects in preclinical models. It is still unclear whether the selectivity of PI3K/Akt inhibitors, the potential therapeutic benefit, less side effects, greater drug tolerance, and concomitant inhibition of other off-targets and downstream targets will be beneficial in the treatment of cancer. It is imperative that clinical reagents with maximum efficacy and minimum lethal dose and side effects be carefully chosen. This will allow therapies to be made swiftly available for all suitable candidates. We can only hope that novel and safe targeted anticancer agents will come from these drug discovery efforts171,172,173,174,175,176,177,178.
Acknowledgments
This study was supported by grants from the Department of Biotechnology and the Department of Science and Technology, the University Grant Commission (UGC), India.
References
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Hernandez C, Pimentel B, Carrera AC. The p85 regulatory subunit controls sequential activation of phosphoinositide 3-kinase by Tyr kinases and Ras. J Biol Chem. 2002;277:41556–62. doi: 10.1074/jbc.M205893200. [DOI] [PubMed] [Google Scholar]
- Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, et al. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–9. [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda–Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–64. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3′-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994;13:2831–41. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–70. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–47. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–8. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Toretsky JA, Scher D, Helman LJ. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;22:6589–97. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J, et al. Over-expression of PDGFR-beta promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway. PLoS One. 2012;7:e30503. doi: 10.1371/journal.pone.0030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH. Simultaneous induction of stimulatory and inhibitory signals by PDGF. FEBS Lett. 1997;410:17–21. doi: 10.1016/s0014-5793(97)00318-9. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9:2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- Abid MR, Guo S, Minami T, Spokes KC, Ueki K, Skurk C, et al. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–22. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–6. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–78. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist K, Cross MJ, Rolny C, Hagerkvist R, Rahimi N, Matsumoto T, et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004;279:22267–75. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–54. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, et al. The p110beta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110gamma. Proc Natl Acad Sci U S A. 2008;105:8292–7. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3:1221–4. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Curr Opin Cell Biol. 2009;21:256–61. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–22. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–27. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9:487–97. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, et al. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–8. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- van Gorp AG, Pomeranz KM, Birkenkamp KU, Hui RC, Lam EW, Coffer PJ. Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res. 2006;66:10760–9. doi: 10.1158/0008-5472.CAN-06-1111. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–5. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BA, Roux PP, Gerber SA, MacKeigan JP, Blenis J, Gygi SP. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc Natl Acad Sci U S A. 2005;102:667–72. doi: 10.1073/pnas.0409143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–9. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Ferkey DM, Kimelman D. GSK-3: new thoughts on an old enzyme. Dev Biol. 2000;225:471–9. doi: 10.1006/dbio.2000.9816. [DOI] [PubMed] [Google Scholar]
- Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–14. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Castaneda CA, Cortes-Funes H, Gomez HL, Ciruelos EM. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev. 2010;29:751–9. doi: 10.1007/s10555-010-9261-0. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–33. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzker R, Rommel C. Phosphoinositide 3-kinases as targets for therapeutic intervention. Curr Pharm Des. 2004;10:1915–22. doi: 10.2174/1381612043384402. [DOI] [PubMed] [Google Scholar]
- Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957–63. [PubMed] [Google Scholar]
- Fan QW, Specht KM, Zhang C, Goldenberg DD, Shokat KM, Weiss WA. Combinatorial efficacy achieved through two-point blockade within a signaling pathway — a chemical genetic approach. Cancer Res. 2003;63:8930–8. [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011;16:325–31. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbay T, Durden DL, Liu T, O'Regan RM, Nahta R. In vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cells. Cancer Chemother Pharmacol. 2010;65:697–706. doi: 10.1007/s00280-009-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce SK, Findley HW, Prince C, Dasgupta A, Cooper T, Durden DL. The PI-3 kinase-Akt-MDM2-survivin signaling axis in high-–risk neuroblastoma: a target for PI-3 kinase inhibitor intervention. Cancer Chemother Pharmacol. 2011;68:325–35. doi: 10.1007/s00280-010-1486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M, Kaizawa H, Moritomo H, Koizumi T, Ohishi T, Okada M, et al. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg Med Chem. 2006;14:6847–58. doi: 10.1016/j.bmc.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Ihle NT, Williams R, Chow S, Chew W, Berggren MI, Paine-Murrieta G, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–72. [PubMed] [Google Scholar]
- Koul D, Shen R, Kim YW, Kondo Y, Lu Y, Bankson J, et al. Cellular and in vivo activity of a novel PI3K inhibitor, PX-866, against human glioblastoma. Neuro Oncol. 2010;12:559–69. doi: 10.1093/neuonc/nop058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle NT, Lemos R, Schwartz D, Oh J, Halter RJ, Wipf P, et al. Peroxisome proliferator-activated receptor gamma agonist pioglitazone prevents the hyperglycemia caused by phosphatidylinositol 3-kinase pathway inhibition by PX-866 without affecting antitumor activity. Mol Cancer Ther. 2009;8:94–100. doi: 10.1158/1535-7163.MCT-08-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GD-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–32. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther. 2009;8:1725–38. doi: 10.1158/1535-7163.MCT-08-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao E, Zhou W, Lee-Hoeflich ST, Truong T, Haverty PM, Eastham-Anderson J, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–56. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, et al. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci Transl Med. 2010;2:48ra66. doi: 10.1126/scitranslmed.3000630. [DOI] [PubMed] [Google Scholar]
- Garcia–Martinez JM, Wullschleger S, Preston G, Guichard S, Fleming S, Alessi DR, et al. Effect of PI3K- and mTOR-specific inhibitors on spontaneous B-cell follicular lymphomas in PTEN/LKB1-deficient mice. Br J Cancer. 2011;104:1116–25. doi: 10.1038/bjc.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows N, Babur M, Resch J, Ridsdale S, Mejin M, Rowling EJ, et al. GDC-0941 inhibits metastatic characteristics of thyroid carcinomas by targeting both the phosphoinositide-3 kinase (PI3K) and hypoxia-inducible factor-1alpha (HIF-1alpha) pathways. J Clin Endocrinol Metab. 2011;96:E1934–43. doi: 10.1210/jc.2011-1426. [DOI] [PubMed] [Google Scholar]
- Sutherlin DP, Bao L, Berry M, Castanedo G, Chuckowree I, Dotson J, et al. Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J Med Chem. 2011;54:7579–87. doi: 10.1021/jm2009327. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–36. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- Dolly S.A first-in-human, phase l study to evaluate the dual PI3K/mTOR inhibitor GDC-0980 administered QD in patients with advanced solid tumors or non-Hodgkin's lymphoma. ASCO Annual Meeting 2010.
- Dehnhardt CM, Venkatesan AM, Delos Santos E, Chen Z, Santos O, Ayral-Kaloustian S, et al. Lead optimization of N-3-substituted 7-morpholinotriazolopyrimidines as dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors: discovery of PKI-402. J Med Chem. 2010;53:798–810. doi: 10.1021/jm9014982. [DOI] [PubMed] [Google Scholar]
- Mallon R, Hollander I, Feldberg L, Lucas J, Soloveva V, Venkatesan A, et al. Antitumor efficacy profile of PKI-402, a dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor. Mol Cancer Ther. 2010;9:976–84. doi: 10.1158/1535-7163.MCT-09-0954. [DOI] [PubMed] [Google Scholar]
- Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, et al. Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem. 2010;53:2636–45. doi: 10.1021/jm901830p. [DOI] [PubMed] [Google Scholar]
- Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res. 2011;17:3193–203. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- Burger MT, Pecchi S, Wagman A, Ni ZJ, Knapp M, Hendrickson T, et al. Identification of NVP-BKM120 as a potent, selective, orally bioavailable class I PI3 kinase inhibitor for treating cancer. ACS Med Chem Lett. 2011;2:774–9. doi: 10.1021/ml200156t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul D, Fu J, Shen R, Lafortune TA, Wang S, Tiao N, et al. Antitumor activity of NVP-BKM120 — a selective pan class 1 PI3 Kinase inhibitor showed differential forms of cell death based on P53 status of glioma cells. Clin Cancer Res. 2012;18:184–95. doi: 10.1158/1078-0432.CCR-11-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz SA, Jilaveanu LB, Zito C, Camp RL, Rimm DL, Conrad P, et al. Vertical targeting of the phosphatidylinositol-3 kinase pathway as a strategy for treating melanoma. Clin Cancer Res. 2010;16:6029–39. doi: 10.1158/1078-0432.CCR-10-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer F, Maira SM, Furet P, Garcia-Echeverria C. Imidazo[4,5-c]quinolines as inhibitors of the PI3K/PKB-pathway. Bioorg Med Chem Lett. 2008;18:1027–30. doi: 10.1016/j.bmcl.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D, et al. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res. 2011;17:2373–84. doi: 10.1158/1078-0432.CCR-10-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–10. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin DW, Ooi M, Delmore J, Negri J, Hayden P, Mitsiades N, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69:5835–42. doi: 10.1158/0008-5472.CAN-08-4285. [DOI] [PubMed] [Google Scholar]
- Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, Cmiljanovic V, et al. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res. 2009;7:601–13. doi: 10.1158/1541-7786.MCR-08-0366. [DOI] [PubMed] [Google Scholar]
- Baumann P, Mandl–Weber S, Oduncu F, Schmidmaier R. The novel orally bioavailable inhibitor of phosphoinositol-3-kinase and mammalian target of rapamycin, NVP–BEZ235, inhibits growth and proliferation in multiple myeloma. Exp Cell Res. 2009;315:485–97. doi: 10.1016/j.yexcr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Konstantinidou G, Bey EA, Rabellino A, Schuster K, Maira MS, Gazdar AF, et al. Dual phosphoinositide 3-kinase/mammalian target of rapamycin blockade is an effective radiosensitizing strategy for the treatment of non-small cell lung cancer harboring K-RAS mutations. Cancer Res. 2009;69:7644–52. doi: 10.1158/0008-5472.CAN-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, et al. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 2010;16:530–40. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- Bhatt AP, Bhende PM, Sin SH, Roy D, Dittmer DP, Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–63. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper J, Richardson MP, Wang WV, Richard LG, Chen W, Coffee EM, et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model of PIK3CA wild-type colorectal cancer. PLoS One. 2011;6:e25132. doi: 10.1371/journal.pone.0025132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CG, Ma CX, Crowder RJ, Guintoli T, Phommaly C, Gao F, et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011;13:R21. doi: 10.1186/bcr2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Schneider L, Mandl-Weber S, Oduncu F, Schmidmaier R. Simultaneous targeting of PI3K and mTOR with NVP-BGT226 is highly effective in multiple myeloma. Anticancer Drugs. 2012;23:131–8. doi: 10.1097/CAD.0b013e32834c8683. [DOI] [PubMed] [Google Scholar]
- Chang KY, Tsai SY, Wu CM, Yen CJ, Chuang BF, Chang JY. Novel phosphoinositide 3-Kinase/mTOR dual inhibitor, NVP-BGT226, displays potent growthiinhibitory activity against human head and neck cancer cells in vitro and in vivo. Clin Cancer Res. 2011;17:7116–26. doi: 10.1158/1078-0432.CCR-11-0796. [DOI] [PubMed] [Google Scholar]
- Shapiro GI, Edelman G, Calvo E, Aggarwal SK, Laird AD.Targeting aberrant PI3K pathway signaling with XL147, a potent, selective, and orally bioavailable PI3K inhibitor Proc 97th Annu Meet AACR 2007. Apr 14-18; Los Angeles, CA. Abstract C205.
- Foster P.Potentiating the antitumor effects of chemotherapy with the selective PI3K inhibitor XL147; 19th AACR-NCI-EORTC Meeting; San Francisco, CA. 2007
- Garcia-Echeverria C. Protein and lipid kinase inhibitors as targeted anticancer agents of the Ras/Raf/MEK and PI3K/PKB pathways. Purinergic Signal. 2009;5:117–25. doi: 10.1007/s11302-008-9111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird D.XL765 targets tumor growth, survival, and angiogenesis in preclinical models by dual inhibition of PI3K and mTOR (abstract B250). EORTC meeting 2007.
- Patnaik A, LoRusso P, Tabernero JA, Laird D, Aggarwal S, Papadopoulos K.Biomarker development for XL765, a potent and selective oral dual inhibitor of PI3K and mTOR currently being administered to patients in a phase I clinical trial (abstract B265). EORTC Meeting 2007.
- Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K, et al. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med (Berl) 2011;89:877–89. doi: 10.1007/s00109-011-0774-y. [DOI] [PubMed] [Google Scholar]
- Winograd-Katz SE, Levitzki A. Cisplatin induces PKB/Akt activation and p38 (MAPK) phosphorylation of the EGF receptor. Oncogene. 2006;25:7381–90. doi: 10.1038/sj.onc.1209737. [DOI] [PubMed] [Google Scholar]
- Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–32. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–17. [PubMed] [Google Scholar]
- Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Biological activities, mechanisms of action and biomedical prospect of the antitumor ether phospholipid ET-18-OCH(3) (edelfosine), a proapoptotic agent in tumor cells. Curr Drug Metab. 2002;3:491–525. doi: 10.2174/1389200023337225. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Fernandez-Luna JL, Gajate C, Martin-Martin B, Benito A, Martinez-Dalmau R, et al. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (Edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-X(L) Cancer Res. 1997;57:1320–8. [PubMed] [Google Scholar]
- van der Luit AH, Vink SR, Klarenbeek JB, Perrissoud D, Solary E, Verheij M, et al. A new class of anticancer alkylphospholipids uses lipid rafts as membrane gateways to induce apoptosis in lymphoma cells. Mol Cancer Ther. 2007;6:2337–45. doi: 10.1158/1535-7163.MCT-07-0202. [DOI] [PubMed] [Google Scholar]
- Zaharieva MM, Konstantinov SM, Pilicheva B, Karaivanova M, Berger MR. Erufosine: a membrane targeting antineoplastic agent with signal transduction modulating effects. Ann N Y Acad Sci. 2007;1095:182–92. doi: 10.1196/annals.1397.022. [DOI] [PubMed] [Google Scholar]
- Hilgard P, Klenner T, Stekar J, Nossner G, Kutscher B, Engel J. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. Eur J Cancer. 1997;33:442–6. doi: 10.1016/s0959-8049(97)89020-x. [DOI] [PubMed] [Google Scholar]
- Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–103. [PubMed] [Google Scholar]
- Li Z, Tan F, Liewehr DJ, Steinberg SM, Thiele CJ. In vitro and in vivo inhibition of neuroblastoma tumor cell growth by AKT inhibitor perifosine. J Natl Cancer Inst. 2010;102:758–70. doi: 10.1093/jnci/djq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Lu Y, Poradosu E, Yu Q, Yu S, Hall H, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–31. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- Leleu X, Jia X, Runnels J, Ngo HT, Moreau AS, Farag M, et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110:4417–26. doi: 10.1182/blood-2007-05-092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Fillmore HL, Kadian R, Broaddus WC, Tye GW, Van Meter TE. The alkylphospholipid perifosine induces apoptosis and p21–mediated cell cycle arrest in medulloblastoma. Mol Cancer Res. 2009;7:1813–21. doi: 10.1158/1541-7786.MCR-09-0069. [DOI] [PubMed] [Google Scholar]
- Porta C, Figlin RA. Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney cancer, and the therapeutic potential of phosphatidylinositol-3-kinase/Akt inhibitors. J Urol. 2009;182:2569–77. doi: 10.1016/j.juro.2009.08.085. [DOI] [PubMed] [Google Scholar]
- Engel JB, Honig A, Schonhals T, Weidler C, Hausler S, Krockenberger M, et al. Perifosine inhibits growth of human experimental endometrial cancers by blockade of AKT phosphorylation. Eur J Obstet Gynecol Reprod Biol. 2008;141:64–9. doi: 10.1016/j.ejogrb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Floryk D, Thompson TC. Perifosine induces differentiation and cell death in prostate cancer cells. Cancer Lett. 2008;266:216–26. doi: 10.1016/j.canlet.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–62. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Lahusen T, Sy T, Sausville EA, Gutkind JS, Senderowicz AM. Perifosine, a novel alkylphospholipid, induces p21 (WAF1) expression in squamous carcinoma cells through a p53-independent pathway, leading to loss in cyclin-dependent kinase activity and cell cycle arrest. Cancer Res. 2002;62:1401–9. [PubMed] [Google Scholar]
- Bailey HH, Mahoney MR, Ettinger DS, Maples WJ, Fracasso PM, Traynor AM, et al. Phase II study of daily oral perifosine in patients with advanced soft tissue sarcoma. Cancer. 2006;107:2462–7. doi: 10.1002/cncr.22308. [DOI] [PubMed] [Google Scholar]
- Elrod HA, Lin YD, Yue P, Wang X, Lonial S, Khuri FR, et al. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–38. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- Fei HR, Chen G, Wang JM, Wang FZ. Perifosine induces cell cycle arrest and apoptosis in human hepatocellular carcinoma cell lines by blockade of Akt phosphorylation. Cytotechnology. 2010;62:449–60. doi: 10.1007/s10616-010-9299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink SR, Schellens JH, Beijnen JH, Sindermann H, Engel J, Dubbelman R, et al. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother Oncol. 2006;80:207–13. doi: 10.1016/j.radonc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Ghobrial IM, Roccaro A, Hong F, Weller E, Rubin N, Leduc R, et al. Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia. Clin Cancer Res. 2010;16:1033–41. doi: 10.1158/1078-0432.CCR-09-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowling M, Blackstein M, Tozer R, Bramwell V, Dancey J, Dore N, et al. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs. 2006;24:435–9. doi: 10.1007/s10637-006-6406-7. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Nemunaitis J, Vukelja SJ, Hagenstad C, Campos LT, Hermann RC, et al. Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2011;29:4394–400. doi: 10.1200/JCO.2011.36.1980. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Wolf J, Jakubowiak A, Zonder J, Lonial S, Irwin D, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011;29:4243–9. doi: 10.1200/JCO.2010.33.9788. [DOI] [PubMed] [Google Scholar]
- Pitter KL, Galban CJ, Galban S, Tehrani OS, Li F, Charles N, et al. Perifosine and CCI 779 co-operate to induce cell death and decrease proliferation in PTEN-intact and PTEN-deficient PDGF-driven murine glioblastoma. PLoS One. 2011;6:e14545. doi: 10.1371/journal.pone.0014545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Yu T, Li J. Co-administration of perifosine with paclitaxel synergistically induces apoptosis in ovarian cancer cells: more than just AKT inhibition. Cancer Lett. 2011;310:118–28. doi: 10.1016/j.canlet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Berger MR, Tsoneva I, Konstantinov SM, Eibl H. Induction of apoptosis by erucylphospho-N,N,N-trimethylammonium is associated with changes in signal molecule expressionand location. Ann N Y Acad Sci. 2003;1010:307–10. doi: 10.1196/annals.1299.054. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Catley L, Raje N, Chauhan D, Podar K, Mitsiades C, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138:783–91. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- Konstantinov SM, Eibl H, Berger MR. Alkylphosphocholines induce apoptosis in HL-60 and U-937 leukemic cells. Cancer Chemother Pharmacol. 1998;41:210–6. doi: 10.1007/s002800050730. [DOI] [PubMed] [Google Scholar]
- Nieto-Miguel T, Gajate C, Mollinedo F. Differential targets and subcellular localization of antitumor alkyl-lysophospholipid in leukemic versus solid tumor cells. J Biol Chem. 2006;281:14833–40. doi: 10.1074/jbc.M511251200. [DOI] [PubMed] [Google Scholar]
- Erdlenbruch B, Jendrossek V, Marx M, Hunold A, Eibl H, Lakomek M. Antitumor effects of erucylphosphocholine on brain tumor cells in vitro and in vivo. Anticancer Res. 1998;18:2551–7. [PubMed] [Google Scholar]
- Jendrossek V, Erdlenbruch B, Hunold A, Kugler W, Eibl H, Lakomek M. Erucylphosphocholine, a novel antineoplastic ether lipid, blocks growth and induces apoptosis in brain tumor cell lines in vitro. Int J Oncol. 1999;14:15–22. doi: 10.3892/ijo.14.1.15. [DOI] [PubMed] [Google Scholar]
- Veenman L, Alten J, Linnemannstons K, Shandalov Y, Zeno S, Lakomek M, et al. Potential involvement of F0F1-ATP(synth)ase and reactive oxygen species in apoptosis induction by the antineoplastic agent erucylphosphohomocholine in glioblastoma cell lines : a mechanism for induction of apoptosis via the 18 kDa mitochondrial translocator protein. Apoptosis. 2010;15:753–68. doi: 10.1007/s10495-010-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner J, Ruiner CE, Handrick R, Eibl HJ, Belka C, Jendrossek V. The Akt-inhibitor Erufosine induces apoptotic cell death in prostate cancer cells and increases the short term effects of ionizing radiation. Radiat Oncol. 2010;5:108. doi: 10.1186/1748-717X-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiegl M, Lindner LH, Juergens M, Eibl H, Hiddemann W, Braess J. Erufosine, a novel alkylphosphocholine, in acute myeloid leukemia: single activity and combination with other antileukemic drugs. Cancer Chemother Pharmacol. 2008;62:321–9. doi: 10.1007/s00280-007-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli AM, Papa V, Tazzari PL, Ricci F, Evangelisti C, Chiarini F, et al. Erucylphosphohomocholine, the first intravenously applicable alkylphosphocholine, is cytotoxic to acute myelogenous leukemia cells through JNK- and PP2A-dependent mechanisms. Leukemia. 2010;24:687–98. doi: 10.1038/leu.2010.32. [DOI] [PubMed] [Google Scholar]
- Yosifov DY, Todorov PT, Zaharieva MM, Georgiev KD, Pilicheva BA, Konstantinov SM, et al. Erucylphospho-N,N,N-trimethylpropylammonium (erufosine) is a potential antimyeloma drug devoid of myelotoxicity. Cancer Chemother Pharmacol. 2011;67:13–25. doi: 10.1007/s00280-010-1273-5. [DOI] [PubMed] [Google Scholar]
- Konigs SK, Pallasch CP, Lindner LH, Schwamb J, Schulz A, Brinker R, et al. Erufosine, a novel alkylphosphocholine, induces apoptosis in CLL through a caspase-dependent pathway. Leuk Res. 2010;34:1064–9. doi: 10.1016/j.leukres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Samudio IJ, Zhang W, Estrov Z, Pelicano H, Harris D, et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–46. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- Mandal M, Younes M, Swan EA, Jasser SA, Doan D, Yigitbasi O, et al. The Akt inhibitor KP372-1 inhibits proliferation and induces apoptosis and anoikis in squamous cell carcinoma of the head and neck. Oral Oncol. 2006;42:430–9. doi: 10.1016/j.oraloncology.2005.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul D, Shen R, Bergh S, Sheng X, Shishodia S, Lafortune TA, et al. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol Cancer Ther. 2006;5:637–44. doi: 10.1158/1535-7163.MCT-05-0453. [DOI] [PubMed] [Google Scholar]
- Mandal M, Kim S, Younes MN, Jasser SA, El-Naggar AK, Mills GB, et al. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899–905. doi: 10.1038/sj.bjc.6602595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xi RG, Huang J, Li D, Wang XB, Wu LJ. Roles of PI3-K/Akt pathways in nanoparticle realgar powders-induced apoptosis in U937 cells. Acta Pharmacol Sin. 2008;29:355–63. doi: 10.1111/j.1745-7254.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Feun LG, Savaraj N, Bodey GP, Lu K, Yap BS, Ajani JA, et al. Phase I study of tricyclic nucleoside phosphate using a five-day continuous infusion schedule. Cancer Res. 1984;44:3608–12. [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17:1795–804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G, Basseches PJ, Kroschel DM, Richardson RL, O'Connell MJ, Kvols LK. Disposition of tricyclic nucleoside-5′-monophosphate in blood and plasma of patients during phase I and II clinical trials. Cancer Treat Rep. 1986;70:359–62. [PubMed] [Google Scholar]
- Noh KH, Kang TH, Kim JH, Pai SI, Lin KY, Hung CF, et al. Activation of Akt as a mechanism for tumor immune evasion. Mol Ther. 2009;17:439–47. doi: 10.1038/mt.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilcher RB, Haas CD, Samson MK, Young JD, Baker LH. Phase I evaluation and clinical pharmacology of tricyclic nucleoside 5′-phosphate using a weekly intravenous regimen. Cancer Res. 1986;46:3147–51. [PubMed] [Google Scholar]
- Feun LG, Blessing JA, Barrett RJ, Hanjani P. A phase II trial of tricyclic nucleoside phosphate in patients with advanced squamous cell carcinoma of the cervix. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1993;16:506–8. doi: 10.1097/00000421-199312000-00010. [DOI] [PubMed] [Google Scholar]
- Heerding DA, Rhodes N, Leber JD, Clark TJ, Keenan RM, Lafrance LV, et al. Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase. J Med Chem. 2008;51:5663–79. doi: 10.1021/jm8004527. [DOI] [PubMed] [Google Scholar]
- Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–74. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- Levy DS, Kahana JA, Kumar R. AKT inhibitor, GSK690693, induces growth inhibition and apoptosis in acute lymphoblastic leukemia cell lines. Blood. 2009;113:1723–9. doi: 10.1182/blood-2008-02-137737. [DOI] [PubMed] [Google Scholar]
- Altomare DA, Zhang L, Deng J, Di Cristofano A, Klein-Szanto AJ, Kumar R, et al. GSK690693 delays tumor onset and progression in genetically defined mouse models expressing activated Akt. Clin Cancer Res. 2010;16:486–96. doi: 10.1158/1078-0432.CCR-09-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol H, Morton CL, Gorlick R, Kolb EA, Keir ST, Reynolds CP, et al. Initial testing (stage 1) of the Akt inhibitor GSK690693 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010;55:1329–37. doi: 10.1002/pbc.22710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–85. doi: 10.1210/jc.2010-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JA, Golden B, Yan L, Carroll WR, Helman EE, Rosenthal EL. Disruption of the AKT pathway inhibits metastasis in an orthotopic model of head and neck squamous cell carcinoma. Laryngoscope. 2011;121:2359–65. doi: 10.1002/lary.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One. 2010;5:e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, et al. Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. Clin Cancer Res. 2011;17:2852–62. doi: 10.1158/1078-0432.CCR-10-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang Y, Zhang L, Ren X, Huber-Keener KJ, Liu X, et al. MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther. 2012;11:154–64. doi: 10.1158/1535-7163.MCT-11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337:155–61. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang N, Wu J, Dai W, Rosenblum JS. Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects. J Biol Chem. 2007;282:2505–11. doi: 10.1074/jbc.M609603200. [DOI] [PubMed] [Google Scholar]
- Awasthi N, Yen PL, Schwarz MA, Schwarz RE. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J Cell Biochem. 2012;113:784–91. doi: 10.1002/jcb.23405. [DOI] [PubMed] [Google Scholar]
- Roulin D, Waselle L, Dormond-Meuwly A, Dufour M, Demartines N, Dormond O. Targeting renal cell carcinoma with NVP-BEZ235, a dual PI3K/mTOR inhibitor, in combination with sorafenib. Mol Cancer. 2011;10:90. doi: 10.1186/1476-4598-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Li Y, Yue P, Owonikoko TK, Ramalingam SS, Khuri FR, et al. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS One. 2011;6:e20899. doi: 10.1371/journal.pone.0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann K, Ruden J, Brand S, Goke B, Lichtl J, Spottl G, et al. Compensatory activation of Akt in response to mTOR and Raf inhibitors — a rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Lett. 2010;295:100–9. doi: 10.1016/j.canlet.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Leung E, Kim JE, Rewcastle GW, Finlay GJ, Baguley BC. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol Ther. 2011;11:938–46. doi: 10.4161/cbt.11.11.15527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A, Elliott J, Salamone RJ, Kim S, Aimone LJ, Walker JR, et al. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin Cancer Res. 2010;16:5692–702. doi: 10.1158/1078-0432.CCR-10-1601. [DOI] [PubMed] [Google Scholar]
- Robinson JP, Vanbrocklin MW, Lastwika KJ, McKinney AJ, Brandner S, Holmen SL. Activated MEK cooperates with Ink4a/Arf loss or Akt activation to induce gliomas in vivo. Oncogene. 2011;30:1341–50. doi: 10.1038/onc.2010.513. [DOI] [PMC free article] [PubMed] [Google Scholar]