Abstract
Aim:
To investigate the potential of propofol in suppressing ventricular arrhythmias and to examine whether mitochondrial ATP-sensitive potassium channels are involved.
Methods:
Male Sprague-Dawley rats were pretreated with intravenous infusion of propofol (Prop), a selective mitochondrial KATP channel inhibitor 5-hydroxydecanoate (5-HD), propofol plus 5-HD (Prop+5-HD), a potent mitochondrial KATP channel opener diazoxide (DZ) or NS, respectively. The dosage of each drug was 10 mg/kg. The animals then underwent a 30 min-ligation of the left anterior descending artery. The severity of arrhythmias, the incidence of ventricular fibrillation (VF), and the time of the first run of ventricular arrhythmias were documented using an arrhythmia scoring system. Mitochondrial membrane potential (ΔΨm) was measured in freshly isolated rat cardiomyocytes with a fluorescence microscope.
Results:
The arrhythmia scores in the Prop and DZ group were 2.6(0-5) and 2.4(0-5), respectively, which were significantly lower than that in the control group [4.9(2-8)]. VF was not observed in both Prop and DZ groups. The first run of ventricular arrhythmias was significantly postponed in the Prop group (10.5±2.2 vs 7.3±1.9 min). Bracketing of propofol with 5-HD eliminated the anti-arrhythmic effect of propofol. In isolated rat cardiomyocytes, propofol (50 μmol/L) significantly decreased ΔΨm, but when propofol was co-administered with 5-HD, the effect on ΔΨm was reversed.
Conclusion:
Propofol preconditioning suppresses ischemia-induced ventricular arrhythmias in the rat heart, which are proposed to be caused by opening of mitochondrial KATP channels.
Keywords: propofol, cardiac ischemia, ventricular arrhythmias, ventricular fibrillation, mitochondrial KATP channels, 5-hydroxydecanoate, diazoxide, cardiomyocytes
Introduction
Propofol is a widely used anesthetic agent with some notable advantages including minimal side effects, controllable anesthetic state, and rapid onset and offset of drug effect. Apart from these anesthetic advantages, propofol has non-anesthetic effects such as organ protection against ischemia-reperfusion injury1,2. Propofol is usually used for patients with coronary artery disease. These patients are at high risk for myocardial ischemia, which is an important factor leading to vital ventricular arrhythmias. In addition, there were case reports indicating propofol's effects in the termination of supraventricular arrhythmias3,4, atrial fibrillation5, and recurrent ventricular arrhythmias6. During our clinical practice, we found that propofol could terminate refractory ventricular arrhythmia storm (unpublished data). Taken together, we hypothesize that propofol has the potential to stop or prevent arrhythmias.
ATP-sensitive potassium channels include mitochondrial ATP-sensitive potassium (KATP) channels and sarcolemmal KATP channels. In recent years, studies have demonstrated that mitochondrial KATP channels play an important role in mediating ischemia-induced ventricular arrhythmias7,8,9. The opening of mitochondrial KATP channels that have been blocked by the selective mitochondrial KATP channel inhibiter, 5-hydroxydecanoate (5-HD), could alleviate lethal ventricular arrhythmias. Agents such as sarafotoxin 6c10 and oxytocin 11 have been reported to inhibit ventricular arrhythmias by opening mitochondrial KATP channels. However, whether the inhibition of arrhythmia by propofol is based on the same mechanism is unknown.
In this study, we investigated the effects of propofol on ischemia-induced ventricular arrhythmias in vivo. Furthermore, we clarified whether mitochondrial KATP channels were involved.
Materials and methods
Animals and drugs
The experiments were performed according to the National Research Council's guidelines. Male Sprague-Dawley rats (weighing 200−250 g) were housed in cages under standardized conditions of 12 h light/dark cycles, 20−25°C ambient temperature, and 40%−60% humidity with free access to rat chow and water. The animals were made available by Laboratory Animal Centre, Shanghai First People's Hospital. The certificate number of the rats was SYXK (2009-0086).
Diazoxide, 5-HD, Evans blue, and collagenase type II were purchased from Sigma-Aldrich Chemical Co (St Louis, MO, USA). Propofol was purchased from Beijing Fresenius Kabi Co (Beijing, China). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazole-carbocyanide iodine (JC-1) was purchased from Beyotime Institute of Biotechnology (Nanjing, China). Other chemicals that were used were all of analytical reagent grade and commercially available. Diazoxide was dissolved in sodium hydroxide and diluted by 0.9% isotonic saline. Other drugs were dissolved in 0.9% isotonic saline when necessary.
Surgical preparation
Surgical preparation and techniques have been previously described in detail12. In brief, the rats were anesthetized with sodium pentobarbital (50 mg/kg, ip) and ventilated with a volume-controlled rodent respirator (Model ALC-V8, Shanghai Alcott Biotech Co, Ltd, Shanghai, China) at 60−70 strokes/min with a tidal volume of 3-6 mL. If necessary, additional sodium pentobarbital was infused to maintain anesthesia. The jugular vein was opened for drug administration. A polyethylene catheter was filled with heparinized saline (100 U/mL), connected to pressure transducers (Model PT-24, Institute for Transducer Research, Fudan University, Shanghai, China), and signals were sent to an amplifying monitor (Nanjing Meiyi Science and Technology Pty Ltd, Nanjing, China). The femoral artery was cannulated to monitor systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure (MAP). The electrocardiogram (lead II) was recorded using subcutaneous needle electrodes (Shanghai Medical Electronic Equipment Factory, Shanghai, China).
Thoracotomy was performed horizontally in the third intercostal space. A silk suture (6−0) was loosely placed around the left anterior descending (LAD) coronary artery near the origin. After a stabilization period of 10 min, drugs were administered according to group assignment. The rats were allowed 10 min for stabilization after all drugs were administered. The suture was then tightened for 30 min to produce acute artery occlusion. Myocardial infarction (MI) was confirmed by instant ST-segment elevation, visual inspection for cyanosis, and dyskinetic regional wall motion. During the experiment course, body temperature was maintained at 37°C using a heating pad.
Experiment protocols
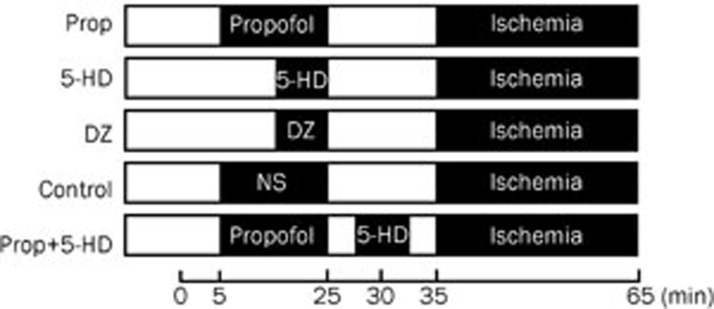
All animals underwent 30 min of coronary artery occlusion (in the sham group, LAD was isolated but not ligated). Rats were divided into the following 6 treatment groups: a sham-operated group (Sham, n=5), a propofol group (Prop, n=10), a propofol plus 5-HD group (Prop+5-HD, n=10), a 5-HD group (5-HD, n=10), a diazoxide group (DZ, n=10), and a control group (Control, n=10) (Figure 1). According to a previous study13 and our preliminary experiment, propofol was administered at a dose of 30 mg·kg−1·h−1 in this study. 5-HD14 and diazoxide15 were infused at a dose of 10 mg/kg.
Figure 1.
Experimental protocol. All rats experienced 30 min of ischemia. Propofol (30 mg·kg-1·h-1) was continuously infused for 20 min. In 5-HD and DZ group, 5-HD and diazoxide were infused 10 min before ischemia. In Prop+5-HD group, 5-HD was infused 5 min before LAD ligation.
Arrhythmia study
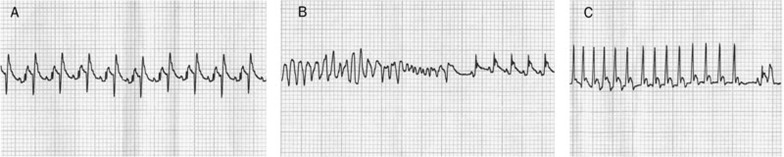
All types of arrhythmias were classified according to the Lambeth Conventions16 standard (Figure 2). An arrhythmia scoring system17 was used for quantitative comparison. The arrhythmias were scored as follows: 0 for no arrhythmia, 1 for occasional ventricular premature ectopic (VPB), 2 for frequent VPB when there were three or more occurring within 1 min, 3 for ventricular arrhythmia (VT, one or two episodes), 4 for VT (three to five episodes), 5 for VT (more than five episodes), 6 for ventricular fibrillation (VF, one or two episodes), 7 for VF (three to five episodes), 8 for VF (more than five episodes), and 9 for arrhythmia-induced death or VF sustained more than 2 min. The arrhythmia scores were estimated during the 30-min period of ischemia. When VF occurred, there was no attempt to restore sinus rhythm. The rat was pronounced dead once VF lasted for 2 min.
Figure 2.
Electrocardiogram (ECG) recordings of rats. (A) Premature ventricular bigeminy; (B) Ventricular fibrillation reverted spontaneously to sinus rhythm; (C) Ventricular tachycardia. Recordings (25 mm/s chart speed) were made from one rat (5-HD group) by Lead II.
Determination of ischemic area
The heart was removed quickly and washed retrogradely from the aorta with 10 mL normal saline (0.9%), and 2 mL of 2% Evans blue was slowly injected retrograde from the aorta. Atria and the roots of the great vessels were removed. The remaining tissues were frozen at -70°C for 5 min. The heart was then cut into 2 mm-thick slices from apex to base. Because myocardial infarction is difficult to measure after only 30 min of coronary ligation, no attempt was made to separate ischemic from infarct tissue12. The ischemic area was determined by negative staining with Evans blue. Ischemic areas were not stained, and non-ischemic areas were colored blue. The ischemic zone was separated and weighed. The ischemic area was expressed as a percentage of the left ventricle of the slices.
Isolation of cardiomyocytes
Single adult ventricular myocytes isolated from rat hearts were obtained as previously described18. In brief, single ventricular myocytes were isolated from Sprague-Dawley rats (200−250 g) by enzymatic digestion. Hearts were excised and perfused retrograde via the aorta (8 mL/min) with oxygenated (100% O2) Krebs-Henseleit (KH) buffer containing (mmol/L) 118 NaCl, 4.8 KCl, 1.2 CaCl2, 37.5 NaHCO3, and 16.5 dextrose. The pH was equilibrated at 7.35. The hearts were then perfused with a Ca2+-free KH buffer containing collagenase type II (309 U/mL) for 20 min. The ventricles were minced and shaken in KH buffer. The resulting cellular digest was then washed, filtered, and re-suspended in phosphate-free HEPES-buffered saline (25°C) containing (mmol/L): 118 NaCl, 4.8 KCl, 1.2 MgCl2, 1.25 CaCl2, 11.0 dextrose, 25.0 HEPES, and 5.0 pyruvate, pH 7.35.
Mitochondrial inner membrane potential measurement
The changes of mitochondrial membrane potential (ΔΨm) were monitored with JC-1. Cells were stained with JC-1 for 15 min at 25°C. Propofol, propofol plus 5-HD, diazoxide, 5-HD, or 0.9% normal saline was added 10 min before the measurement. The observations were made with a fluorescence microscope (Eclipse TS100-F; Nikon, Tokyo, Japan). Five independent experiments were conducted, and each experiment had triplicates of the same treatment. To avoid bias in the analysis, 15 areas were randomly collected from the triplicates of the same treatment in each independent experiment. The ratio of aggregated JC-1 (red fluorescence) and monomeric JC-1 (green fluorescence) represented ΔΨm and was calculated after background subtraction. A decrease in this ratio represented depolarization of membrane potential, whereas an increase in the ratio was interpreted as an increase of the potential. The wavelengths of excitation and emission were 490 nm and 530 nm for the detection of the monomeric form of JC-1, and 525 nm and 590 nm were used to detect aggregations of JC-1.
Data analysis
For the statistical analysis of the incidence of ventricular arrhythmias, Fisher's exact probability test was applied. Arrhythmia scores were presented as the median with range and were compared with the Kruskal-Wallis test. Other continuous variables were expressed as the mean±standard deviation (SD) and compared using one-way analysis of variance, followed by the Tukey test. SAS software (version 7.0) was used for data analysis. A P value of less than 0.05 was considered statistically significant.
Results
Hemodynamic parameter, arterial blood gases and ischemia area
All animals survived the 30 min myocardial ischemia. The results depicted in Table 1 show hemodynamic changes and ischemic area. In the DZ group, MAP was lower before and during LAD ligation, compared with the control group (94±9 and 83±9 mmHg vs 122±8 and 110±7 mmHg, respectively; P<0.05). Administration of propofol did not appreciably affect this parameter. No significant difference in the value of heart rate among all the groups was observed. In addition, administration of diazoxide and propofol did not modify the size of ischemia when compared to the control group. There was no significant difference among all the groups in blood gas analyses during the course of experiments as shown in Table 2.
Table 1. Hemodynamics before and after 30 min of LAD ligation. All values are expressed as mean±SD. bP<0.05 vs the control group. Bpm, beat per minute; LAD, left anterior descending coronary artery.
| |
Ischemia area (% LV) |
Mean arterial pressure (mmHg) |
Heart rate (bpm) |
||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Control | 55±5 | 122±8 | 110±7 | 384±72 | 359±67 |
| Prop | 57±5 | 116±6 | 97±7 | 367±51 | 375±37 |
| Prop+5-HD | 55±7 | 115±7 | 95±11 | 351±30 | 328±61 |
| DZ | 56±5 | 94±9b | 83±9b | 337±35 | 323±45 |
| 5-HD | 54±5 | 117±9 | 106±9 | 407±28 | 376±32 |
| Sham | 0 | 117±10 | 114±15 | 357±40 | 370±11 |
Table 2. Arterial blood gas analyses in each group. There were no significant differences in pH, pO2, and pCO2 among all the groups. All values are expressed as mean±SD.
| |
|
pO2 (mmHg) |
pCO2 (mmHg) |
pH |
|||
|---|---|---|---|---|---|---|---|
| n | Before | After | Before | After | Before | After | |
| Control | 10 | 105±5 | 102±5 | 38±2 | 40±4 | 7.43±0.02 | 7.4±0.02 |
| Prop | 10 | 103±5 | 101±5 | 38±3 | 39±3 | 7.44±0.01 | 7.41±0.02 |
| Prop+5-HD | 10 | 105±8 | 103±8 | 38±4 | 40±4 | 7.43±0.03 | 7.38±0.05 |
| DZ | 10 | 107±6 | 105±6 | 39±4 | 40±4 | 7.41±0.03 | 7.4±0.03 |
| 5-HD | 10 | 106±4 | 104±5 | 40±4 | 40±3 | 7.42±0.02 | 7.4±0.02 |
| Sham | 5 | 104±2 | 104±4 | 39±3 | 40±4 | 7.43±0.02 | 7.42±0.02 |
Arrhythmia study
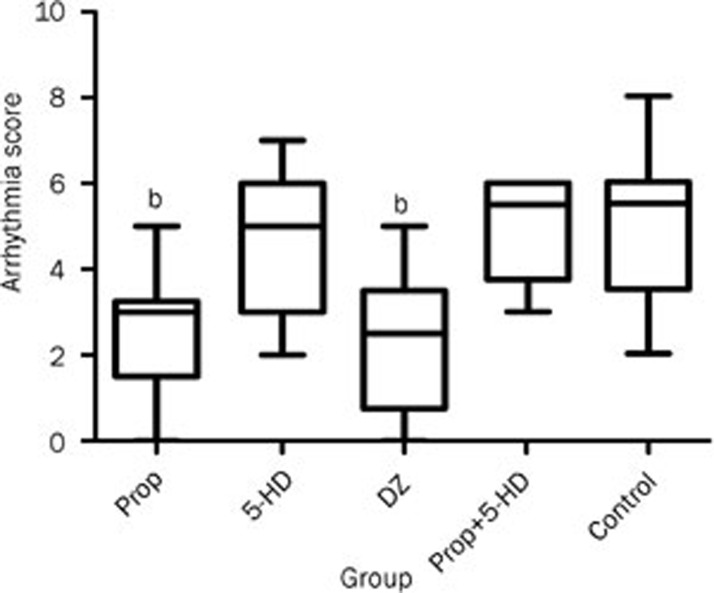
The administration of propofol prior to heart ischemia alleviated the severity of ventricular arrhythmias. The arrhythmia score in the Prop group was lower compared to the control group [3(0-5) vs 6(2-8); P<0.05]. Apart from the Prop group, the arrhythmia score in the DZ group was lower than the control group [3(0-5) vs 6(2-8); P<0.05]. 5-HD administered alone had no effect on the severity of arrhythmia when compared to the control group [6(2-7) vs 6(2-8); P>0.05]. However, bracketing of propofol with 5-HD blunted the anti-arrhythmic effect of propofol (Figure 3).
Figure 3.
Arrhythmia scores in each group. In the Prop and DZ groups, the scores were much lower than the control group. bP<0.05 compared to the control group.
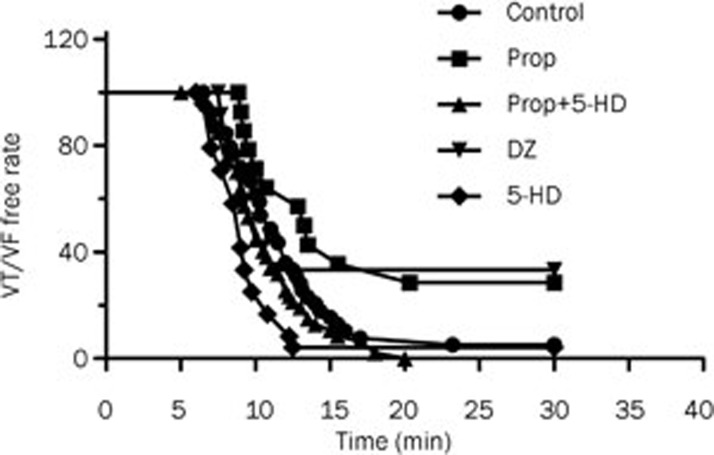
Ventricular arrhythmias were mostly observed 5 to 20 min after LAD ligation (Figure 4). A lower incidence of ventricular arrhythmias was observed in the propofol and diazoxide-treated rats compared to the control. There was no VF observed in the Prop and DZ groups during the course of the experiment. 5-HD did not affect the occurrence of VF. However, when 5-HD was administered 5 min after propofol, it eliminated the antiarrhythmic effect of propofol.
Figure 4.
VF/VT-free rate during 30 min of LAD ligation. In the Prop group, the first run of VT/VF was postponed compared to the control group.
In the propofol and diazoxide group, VT/VF occurred less frequently than the control group. VT/VF occurred 11 times in the propofol group and 17 times in the diazoxide group, which was significantly less when compared to the 57 occurrences in the control group. Frequency of occurrence of VT/VF in the 5-HD group (52 times) was almost as high as the control group. When 5-HD was administered after propofol, the antiarrhythmic effects of propofol were eliminated (56 times).
The time of the first episode of VT/VF
The first run of VT or VF was postponed when the animals were administered propofol (Figure 4). In the Prop group, the first run of VT or VF came later than that of the control group (10.5±2.2 min vs 7.3±1.9 min; P<0.05). However, diazoxide did not show similar effects. Although 5-HD did not affect the first run of VT or VF, it reversed the effect of propofol when administered after propofol. In the Prop+5-HD group, the onset of VT or VF was earlier than that of the Prop group (7.8±1.7 min vs 10.5±2.2 min; P<0.05).
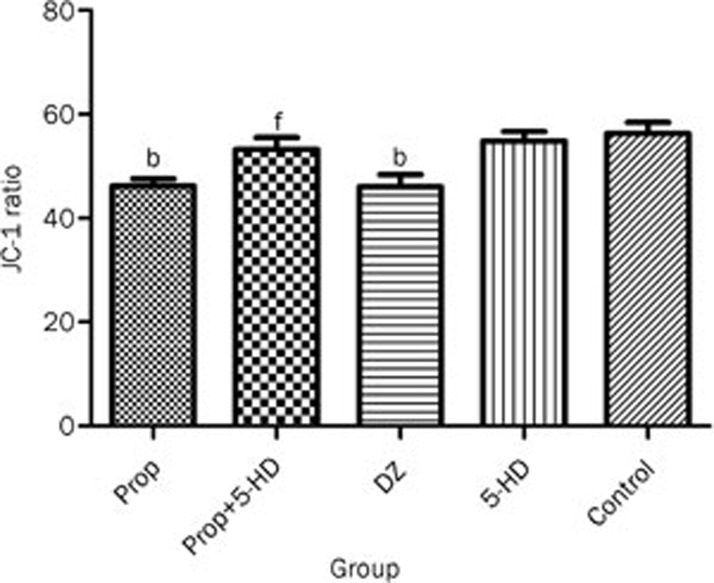
Changes in mitochondrial membrane potential
JC-1 ratio (J-aggregate/monomer) can reflect changes in ΔΨm. A decrease in this ratio represented depolarization of membrane potential, whereas an increase in the ratio was interpreted as a gain in the potential. Figure 5 shows the JC-1 ratio in five groups. 5-HD (100 μmol/L) had no effect on JC-1 ratio when compared to control (55±7 vs 56±8, P>0.05). Diazoxide (100 μmol/L) and propofol (50 μmol/L) can decrease JC-1 ratio (46±9 and 46±6 vs 56±8, P<0.01, respectively). However, when bracketing propofol with 5-HD, the propofol-induced loss of ΔΨm was reversed (P<0.01).
Figure 5.
JC-1 ratios in each group. In the Prop and DZ groups, the JC-1 ratios were lower than in the control group. bP<0.05 compared to the control group. fP<0.01 vs Prop group.
Discussion
In this study, administration of propofol before ischemia protected rats from subsequent prolonged ischemia-induced, lethal, ventricular arrhythmias. Twenty minutes of propofol infusion lowered the severity of arrhythmia, decreased the incidence of VF, and postponed the first run of VT/VF. Flavoprotein fluorescence measurements also showed that propofol could potentially open mitochondrial KATP channels. These effects were eliminated by 5-HD, a highly selective mitochondrial KATP channel blocker. The findings indicated that the antiarrhythmic effect of propofol was mediated by mitochondrial KATP channels.
Single or multiple brief periods of ischemia have been shown to limit infarct size in the subsequent, sustained ischemia, which was termed as preconditioning19. Preconditioning was found initially to protect organs from the injury of ischemia and reperfusion. It was found that preconditioning could prevent ischemia-induced arrhythmias in subsequent studies. Recent studies showed that mitochondrial KATP channels may play an important role in ischemia-induced, ventricular arrhythmias7,8,9. Ischemia and diazoxide preconditioning could decrease the incidence and duration of ventricular arrhythmias in langendorff-perfused rat hearts. However, bracketing of diazoxide or ischemia with 5-HD exacerbated the severity of arrhythmia in the following 25 min of ischemia7. The decreased incidence of lethal ventricular arrhythmias due to opening of mitochondrial KATP channels has also been verified in rabbits8 and dogs9. Although it has been observed that mitochondrial KATP channels serve as central effectors in preconditioning, the actual mitochondrial proteins involved in this process are not well understood. Mitochondrial KATP channels displayed several characteristics similar to those of the sarcolemmal KATP channels in that both channels were reversibly inactivated by ATP applied to the matrix side and inhibited by glibenclamide20. The mitochondrial inner membrane is polarized by -180 mV, with the matrix side negative due to an H+ gradient generated by respiratory enzyme complexes20. When mitochondrial KATP channels open, the potential would dissipate. In our study, we used JC-1 as a marker to reflect the opening of the mitochondrial KATP channels. JC-1 could aggregate in normal mitochondria and present red fluorescence. On the contrary, green fluorescence represented the monomeric form of JC-1, appearing in the cytosol after mitochondrial membrane depolarization. Therefore, JC-1 can reflect a change in the mitochondrial membrane potential.
Previous studies showed that the heart rate and involved, ischemic zone size can influence susceptibility to ischemia-induced arrhythmias21,22. In our study, both factors were not affected by propofol. Therefore, the anti-arrhythmic effect of propofol was not mediated by its effect in controlling heart rate and ischemic area. According to a prior study, propofol could limit infarct size23, which seems contrary to our conclusion. Indeed, myocardial infarction is difficult to measure after only 30 min of coronary ligation12. Therefore, what we measured in this study was ischemic size but not infarct size. Diazoxide significantly decreased MAP and slightly lowered the heart rate, which may confer the antiarrhythmic effects of diazoxide. The main antiarrhythmic effect induced by diazoxide may be attributed to a temporal increase in reactive oxygen species production in the preconditioning phase7.
In our study, propofol inhibited ischemia-induced, ventricular arrhythmias, which were mediated by mito-chondrial KATP channels. The exact mechanism involved was less clear. In cardiac myocytes, diazoxide activated mitochondrial KATP channels 2000 times more potently than surface KATP channels, and it exerted cardioprotective effects during ischemia. The PKC-mediated signal pathway has been proven to modify the opening of mitochondrial KATP channels through diazoxide24,25,26. Exposure to a PKC activator potentiated and accelerated the effect of diazoxide27. The opening of mitochondrial KATP channels by the PKC pathway provides a direct mechanistic link between the signal transduction of ischemic preconditioning and pharmacological cardioprotection targeted at mitochondrial KATP channels. In this study, both propofol and diazoxide showed anti-arrhythmic effects, and both could open mitochondrial KATP channels. However, propofol could postpone the first run of ventricular arrhythmias, but diazoxide could not. Therefore, the mechanism of opening mitochondrial KATP channels by propofol may be different from that of diazoxide. One of the examples of such different mechamisms is that although the PKC pathway plays an important role in preconditioning induced by propofol and diazoxide, the PKC isoform may be different. Diazoxide could induce translocation of PKC-epsilon as an upstream signaling molecule for mitochondrial KATP channels22,23. Although propofol causes an increase in PKC activity in rat ventricular myocytes, it caused translocation of PKC-epsilon to sites not associated with mitochondria16. Thus, the mechanisms involved in opening of mitochondrial KATP channels are not identical between propofol and diazoxide and need further study.
Intralipid is a solvent for propofol. Although studies have demonstrated that post-ischemic treatment with intralipid had cardioprotective effects against reperfusion injury28, previous studies12 and our preliminary results demonstrated that intralipid itself did not affect hemodynamics or ischemia-induced arrhythmias. Based on these results, we believe that the use of intralipid as a vehicle did not influence the effects of propofol during ischemic conditions.
In conclusion, propofol preconditioning could protect the heart from long, sustained, ischemia-induced, lethal arrhythmias. Activation of mitochondrial KATP channels might play an important role in this process. The mechanism by which mitochondrial KATP channel exerts its cardioprotective effect is poorly understood and needs further study. Lastly, our study should be interpreted with some degree of caution, particularly because it was performed using rat hearts, which may not be completely analogous to human heart tissue.
Author contribution
Qiang LIU and Jiang HONG were responsible for the study design and for writing the paper. Data analysis was conducted by Xiao-yu LI and Cheng QIAN. Qiang LIU and Rong CHEN conducted the experiments. Jun-yan YAO, Long-sheng SONG, Shao-wen LIU and Bao-gui SUN were senior advisors and provided valuable advice for this study and for writing the manuscript.
Acknowledgments
We thank Dr Tom XU from Toledo University, USA, for kindly polishing the language of this manuscript. The funding for this study is partly granted by National 973 Project (No 2007CB512008), the National Scientific Fund of China (No 30901392), Shanghai Jiao Tong University School of Medicine Scientific Research Fund, and the Shanghai First People's Hospital Scientific Fund (No 11B24).
References
- Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kong AL, Chen R, Qian C, Liu SW, Sun BG, et al. Propofol and arrhythmias: two sides of the coin. Acta Pharmacol Sin. 2011;32:817–23. doi: 10.1038/aps.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann R, Vettermann J. Change of ectopic supraventricular tachycardia to sinus rhythm during administration of propofol. Anesth Analg. 1992;75:1030–2. doi: 10.1213/00000539-199212000-00028. [DOI] [PubMed] [Google Scholar]
- Kannan S, Sherwood N. Termination of supraventricular tachycardia by propofol. Br J Anaesth. 2002;88:874–5. [PubMed] [Google Scholar]
- Miro O, de la Red G, Fontanals J. Cessation of paroxysmal atrial fibrillation during acute intravenous propofol administration. Anesthesiology. 2000;92:910. doi: 10.1097/00000542-200003000-00061. [DOI] [PubMed] [Google Scholar]
- Burjorjee JE, Milne B. Propofol for electrical storm; a case report of cardioversion and suppression of ventricular tachycardia by propofol. Can J Anaesth. 2002;49:973–7. doi: 10.1007/BF03016886. [DOI] [PubMed] [Google Scholar]
- Matejikova J, Kucharska J, Pinterova M, Pancza D, Ravingerova T. Protection against ischemia induced ventricular arrhythmias and myocardial dysfunction conferred by preconditioning in the rat heart: involvement of mitochondrial KATP channels and reactive oxygen species. Physiol Res. 2009;58:9–19. doi: 10.33549/physiolres.931317. [DOI] [PubMed] [Google Scholar]
- Das B, Sarkar C. Is the sarcolemmal or mitochondrial KATP channel activation important in the antiarrhythmic and cardioprotective effects during acute ischemia/reperfusion in the intact anesthetized rabbit model. Life Sci. 2005;77:1226–48. doi: 10.1016/j.lfs.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Vegh A, Parratt JR. The role of mitochondrial KATP channels in antiarrhythmic effects of ischaemic preconditioning in dogs. Br J Pharmacol. 2002;137:1107–15. doi: 10.1038/sj.bjp.0704966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Sarkar C, Shankar PR. Pretreatment with sarafotoxin 6c prior to coronary occlusion protects against infarction and arrhythmias via cardiomyocyte mitochondrial KATP channel activation in the intact rabbit heart during ischemia/reperfusion. Cardiovasc Drugs Ther. 2007;21:243–51. doi: 10.1007/s10557-007-6031-5. [DOI] [PubMed] [Google Scholar]
- Alizadeh AM, Faghihi M, Sadeghipour HR, Mohammadghasemi F, Imani A, Houshmand F, et al. Oxytocin protects rat heart against ischemia-reperfusion injury via pathway involving mitochondrial ATP-dependent potassium channel. Peptides. 2010;31:1341–5. doi: 10.1016/j.peptides.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Hirata N, Kanaya N, Kamada N, Kimura S, Namiki A. Differential effects of propofol and sevoflurane on ischemia induced ventricular arrhythmias and phosphorylated connexin 43 protein in rats. Anesthesiology. 2009;110:50–7. doi: 10.1097/ALN.0b013e318190b537. [DOI] [PubMed] [Google Scholar]
- Taheri S, Halsey MJ, Liu J, Eger EI, Koblin DD, Laster MJ. What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs. Anesth Analg. 1991;72:627–34. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Hsu AK, Nagase H, Gross GJ. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J Pharmacol Exp Ther. 2000;294:451–7. [PubMed] [Google Scholar]
- Gonca E, Bozdogan O. Both mitochondrial KATP channel opening and sarcolemmal KATP channel blockage confer protection against ischemia/reperfusion-induced arrhythmia in anesthetized male rats. J Cardiovasc Pharmacol Ther. 2010;15:403–11. doi: 10.1177/1074248410372925. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–55. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Yang CS, Tsai PJ, Chou ST, Niu YL, Lai JS, Kuo JS. The roles of reactive oxygen species and endogenous opioid peptides in ischemia induced arrhythmia of isolated rat hearts. Free Radic Biol Med. 1995;18:593–8. doi: 10.1016/0891-5849(94)00153-b. [DOI] [PubMed] [Google Scholar]
- Wickley PJ, Ding X, Murray PA, Damron DS. Propofol-induced activation of protein kinase C isoforms in adult rat ventricular myocytes. Anesthesiology. 2006;104:970–7. doi: 10.1097/00000542-200605000-00013. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Yamada M. Mitochondrial ATP-sensitive K+ channels, protectors of the heart. J Physiol. 2010;588:283–6. doi: 10.1113/jphysiol.2009.179028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc Res. 1998;39:194–215. doi: 10.1016/s0008-6363(98)00083-2. [DOI] [PubMed] [Google Scholar]
- Bernier M, Curtis MJ, Hearse DJ. Ischemia-induced and reperfusion-induced arrhythmias: importance of heart rate. Am J Physiol. 1989;256:H21–31. doi: 10.1152/ajpheart.1989.256.1.H21. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Kokita N, Namiki A. Propofol attenuates ischaemia-reperfusion injury in the rat heart in vivo. Eur J Anaesthesiol. 2008;25:144–51. doi: 10.1017/S0265021507001342. [DOI] [PubMed] [Google Scholar]
- Garg V, Hu K. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. Am J Physiol Heart Circ Physiol. 2007;293:H322–32. doi: 10.1152/ajpheart.01035.2006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirai K, Ashraf M. Activation of mitochondrial ATP-sensitive K+ channel for cardiac protection against ischemic injury is dependent on protein kinase C activity. Circ Res. 1999;85:731–41. doi: 10.1161/01.res.85.8.731. [DOI] [PubMed] [Google Scholar]
- Kim MY, Kim MJ, Yoon IS, Ahn JH, Lee SH, Baik EJ, et al. Diazoxide acts more as a PKC-epsilon activator, and indirectly activates the mitochondrial KATP channel conferring cardioprotection against hypoxic injury. Br J Pharmacol. 2006;149:1059–70. doi: 10.1038/sj.bjp.0706922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, O'Rourke B, Marbán E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–4. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- Rahman S, Li J, Bopassa JC, Umar S, Iorga A. Phosphorylation of GSK-3β mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology. 2011;115:242–53. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]