Abstract
Background
Exacerbation of cutaneous wound infections and delayed wound closure are frequent complications seen in alcohol exposed subjects who sustain injuries. We previously reported that acute alcohol exposure alters the early dermal inflammatory phase of wound healing and also several parameters of the proliferative wound healing phase in wounds from ethanol-treated mice for several days or weeks after ethanol exposure. Hence, it is likely that the cumulative defects arising in the early phases of the wound healing process directly contribute to the increased complications observed in intoxicated patients at the time of injury.
Methods
C57BL/6 mice were given intraperitoneal ethanol (2.2g/kg body weight) or vehicle (saline) ethanol using our episodic binge ethanol exposure protocol (3 days ethanol, 4 days off, 3 days ethanol) to yield a blood alcohol concentration (B.A.C.) of 300 mg/dl at the time of wounding. Mice were subjected to six 3 mm full-thickness dorsal wounds and immediately treated topically with 10 μl of sterile saline (control) or diluted Staphylococcusaureus corresponding to 1 × 10^4 CFU/wound. Wounds were harvested at 24 hours post-injury to evaluate wound area, neutrophil and macrophage accumulation, and the protein levels of cytokines, interleukin (IL) IL-6, IL-1β, IL-6, and IL-10, and chemokines, macrophage inflammatory proteins (MIP), MIP-2 and MIP-1α, monocyte chemotactic protein-1 (MCP-1) and KC. The abundance and localization of cathelicidin-related antimicrobial peptide (CRAMP) and the kallikrein epidermal proteases (KLK5 and KLK7) were also determined.
Results
Compared to control mice, ethanol-treated mice exhibited delayed wound closure, decreased macrophage accumulation and impaired production of MIP-1α. Furthermore, skin from ethanol-treated mice demonstrated a reduction in the abundance of epidermal CRAMP and KLK7.
Conclusion
These findings suggest that ethanol exposure hinders several distinct components of the innate immune response, including phagocyte recruitment and chemokine/cytokine and AMP production. Together, these effects likely contribute to delayed wound closure and enhanced infection severity observed in intoxicated patients.
Keywords: Antimicrobial peptides, proteases, wound healing, macrophages, cathelicidin, inflammation
Introduction
In the United States, alcohol dependence and/or abuse affects an estimated 20-40% of hospitalized patients (Smothers et al., 2004, Gerke et al., 1997), and is an independent predictor of developing Hospital Associated Infections (HAI) (de Wit et al., 2012). Surgical site-infections (SSI) are a subset of HAI. SSI patients are more likely to need intensive care admission, are hospitalized for twice as long, have a higher rate of re-admission, and are twice as likely to die when compared to patients who do not sustain an SSI (Kirkland et al., 1999). Alcohol-abusing patients are 2.73 times more likely to develop SSIs (de Wit et al., 2012). Alcohol abuse has also been correlated with increased post-operative and cardiac complications (Bradley et al., 2011), as well as an average of 2.4 days longer length of hospital stay (de Wit et al., 2012). The need for improved post-operative wound care is critical, since an estimated 38 million surgical procedures were expected in the United States in 2012 (Surgery, 2008). Experimental models have demonstrated that a single ethanol exposure can have negative effects on tissue repair, including a dampened inflammatory response and impaired endothelial cell signaling necessary for vascular regeneration (Radek et al., 2005, Radek et al., 2007, Fitzgerald et al., 2007), that likely contribute to the observed delay in wound repair responses and morbidity in trauma patients (reviewed in (Radek et al., 2009)).
Binge alcohol consumption is a drinking pattern that results in extremely intoxicating blood alcohol concentrations (BACs) ranging from 300-500 mg/dl (Urso et al., 1981), and is combined with repeated withdrawal phases (Lundqvist et al., 1995). Weekend binge drinking by non-alcoholics is a commonly observed phenomenon in many cultures (Lundquvist et al., 1995), and this drinking pattern confers the highest risk factor for injury (Gmel et al., 2006). In America, the majority of binge drinkers consume alcohol an average of four times per month (Naimi et al., 2003), making it important to understand how repeated binge alcohol consumption, rather than a single acute dose, contributes to impaired immunological responses in the context of injury or infection.
Cutaneous wound healing is a dynamic process involving three overlapping phases: inflammation, proliferation, and remodeling. The elaborate coordination of events requires communication amongst multiple cell types and soluble factors, including cytokines and chemokines, to stimulate immune cell infiltration, keratinocyte proliferation, angiogenesis, and extracellular matrix renewal (Singer and Clark, 1999). Neutrophils and resident macrophages are critical for tissue repair and the prevention and clearance of infection during early wound healing (Leibovich and Ross, 1975). The inflammatory phase is characterized by platelet, neutrophil, and monocyte infiltration into the wound bed. This process is primarily mediated by coordinated waves of specific cytokine/chemokine production and is critical for progression of wound healing. These cytokine/chemokine waves initially begin with peaks of pro-inflammatory cytokines, including IL-6, IL-1β, and TNFα, within minutes to hours after initial wounding, which coincides with peaks of neutrophil and macrophage-specific chemokine production, including KC, MIP-2, MIP-1α, and MCP-1. These factors first recruit neutrophils, followed by extravasation of monocytes, which become activated to become macrophages by the specific cytokine/chemokine wound milieu (Gillitzer and Goebeler, 2001). Macrophages help to control the wound cytokine milieu by initially secreting pro-inflammatory cytokines (known as an M1 phenotype) and later secreting anti-inflammatory cytokines (M2 phenotype). This programmed M1/M2 switch facilitates the progression into the proliferative phase of wound healing (Daley et al., 2010). Compromised macrophage function or impaired tissue infiltration rates have been correlated with a prolonged inflammatory phase, inadequate wound closure and deficient restoration of normal cutaneous architecture (Mirza et al., 2009, Singer and Clark, 1999, Leibovich and Ross, 1975). Moreover, dysregulated cytokine/chemokine production and leukocyte recruitment has been associated with delayed wound closure, and was observed following ethanol exposure in animal models (Fitzgerald et al., 2007, Radek et al., 2008). Currently, the effect of ethanol on wound healing in the context of infection is unknown.
Antimicrobial peptides (AMPs) are a critical component of innate immunity. They are typically small, cationic peptides produced by epithelia and innate immune cells, can be induced by injury or pathogen attack. and serve a critical role during cutaneous wound healing (reviewed in (Lai and Gallo, 2009)). AMPs directly combat microbes, initiate the wound healing processes by activating innate immune cells, and contribute to the generation and maintenance of the cutaneous permeability barrier. Impaired AMP activity can exacerbate the severity of chronic or non-healing wounds by influencing host resistance to infection (Nizet et al., 2001, Heilborn et al., 2003, Koczulla et al., 2003), wound closure (Heilborn et al., 2003), angiogenesis (Koczulla et al., 2003) and matrix restoration (Park et al., 2009), collectively demonstrating the significance of epidermal AMPs in the host response to injury and infection.
Several AMPs, including cathelicidin, are initially translated into pro-proteins and subsequently processed into smaller bioactive peptides by epidermal proteases. Such proteases include two members of the kallikrein (KLK) family of serine proteases, stratum corneum tryptic enzyme (SCTE/ KLK5) and stratum corneum chymotryptic protease (SCCE/KLK7) (Morizane et al., 2010, Yamasaki et al., 2006). The active forms of cathelicidin are human LL-37 and murine cathelicidin-related antimicrobial peptide (CRAMP). Aside from pathogen attack, LL-37/CRAMP is chemotactic for peripheral blood monocytes, macrophages, and neutrophils (De et al., 2000, Agerberth et al., 2000),. LL-37/CRAMP also has the capacity to activate phagocytes, mast cells, and epithelial cells (Scott et al., 2002), induce macrophage chemokine production and chemokine receptor expression (Scott et al., 2002), promotes adaptive immune responses (Kurosaka et al., 2005), alters MCP-1 production in LPS stimulated cultured epithelial cells (Scott et al., 2002),and promote angiogenesis (Koczulla et al., 2003), all of which are critical components of normal cutaneous wound repair.
In this manuscript, our goal was to determine if the delayed tissue repair of an infected cutaneous wound following ethanol exposure involves diminished AMP responses and impaired leukocyte recruitment. Although alcohol exposure and wound healing have been extensively studied independently, no investigations have directly evaluated the impact of ethanol exposure on infected wound healing (Radek et al., 2005, Fitzgerald et al., 2007, Radek et al., 2007), including ethanol-mediated defects in AMP production or leukocyte recruitment. Since S. aureus is a major cause of nosocomial infections, including SSIs (National Nosocomial Infections Surveillance, 2004), and since alcohol abusing patients are more likely to sustain an SSI (de Wit et al., 2012), we utilized a murine model of episodic binge ethanol exposure and excisional wounding with topical S. aureus infection. Our studies demonstrate that ethanol exposure has multi-factorial effects on innate immune responses, including altered macrophage infiltration kinetics, impaired production of the macrophage chemoattractant MIP-1α, the pro-inflammatory cytokine, IL-1β, and the anti-inflammatory cytokine, IL-10 and reduced production of the antimicrobial peptide CRAMP. We also observed that ethanol-treated mice had decreased levels of KLK7 within the epidermis, suggesting that ethanol exposure likely alters proteolytic processing of CRAMP to contribute to delayed wound closure and decreased macrophage accumulation within the wound bed.
Materials and Methods
Murine ethanol wound model
Eight week old, male C57BL/6 mice (Jackson Laboratories) were given 100μl ethanol (2.2 g/kg body weight) or vehicle (saline) by intraperitoneal injection using a well-documented episodic binge ethanol exposure protocol (3 days ethanol, 4 days off (no ethanol), 3 days ethanol), as previously described (Lauing et al., 2008). Thirty minutes after the last ethanol (B.A.C. of 300 mg/dl) or saline administration, mice were anesthetized and shaved to allow for six excisional, full-thickness dorsal wounds to be made using a 3mm biopsy punch (Acuderm, Fort Lauderdale, FL) as previously described (Fitzgerald et al., 2007, Radek et al., 2007, Radek et al., 2005). Staphylococcus aureus (Newman strain) was grown to an optical density of 600nm corresponding to 1 × 10^8 CFU/ml and then diluted 1:100 in tryptic soy broth. Immediately following excisional wounding, wounds were treated topically with 10 μl of sterile saline (control) or diluted S. aureus corresponding to 1 × 10^4 CFU/wound. Mice from each group were either sacrificed immediately following wound infection (to determine the baseline wound area) or were sacrificed 24 hours later. All experiments were performed between 8 and 9 am to avoid confounding factors related to circadian rhythms. All animal protocols were approved by the Loyola University Chicago Institutional Animal Care and Use Committee.
Determination of Blood Alcohol Content (BAC)
Whole blood was collected via cardiac puncture, incubated at room temperature for 20 minutes and then centrifuged at 3000 rpm at 4° C for 20 minutes. Serum was isolated and BAC was measured using the GM7 Micro-Stat Analyzer (Analox, London, UK). All animals had a B.A.C. of approximately 300 mg/dl at the time of injury.
Wound size quantification
To assess wound closure, the pelt was removed and photographs were taken with a mounted Canon EOS Rebel XT camera. Using Photoshop, the number of pixels of each open wound (n=6 per mouse) was measured and averaged for each mouse. Mice were sacrificed immediately following wounding to obtain baseline wound size or were sacrificed 24 hours after injury (n =18, saline, n=12 ethanol). The open wound area was calculated using the formula: (# pixels open wound area at 24 hours) / (# pixels open wound area at baseline) * 100 = % open wound area. The experiment was performed twice and data is presented as mean % open wound area +/- SEM. Statistical analyses were performed using Student's t-test where p < 0.05 was considered significant.
Leukocyte quantification by IHF
Wound macrophages and neutrophils were quantified using a standard protocol for sequential immunohistochemical analyses. Frozen sequential sections (6μm) were fixed in 4% paraformaldehyde for 15 minutes, washed, and then incubated with blocking solution (10% bovine serum albumin (BSA)/3% normal goat serum in sterile phosphate buffered saline (PBS)) for 1 hour. For neutrophil detection, sections were incubated with 1° rat monoclonal anti-mouse-Gr-1 (Invitrogen, Carlsbad, CA) (1:200)) overnight at 4° C. Slides were washed and then incubated with goat anti-rat-Alexa Fluor 488 2° antibody (Invitrogen, Carlsbad, CA) (1:500) for 1 hour at room temperature. For macrophage detection, sections were then washed with PBS and incubated with 1° antibody (biotinylated monoclonal anti-mouse MOMA-2 (BMA Biomedicals (1:500)) for 1 hour, washed, and then incubated with 2° antibody (Streptavidin conjugated Alexa Fluor 495 (Invitrogen, Carlsbad, CA) (1:500). Slides were washed, dried and mounted using VectaShield mounting media with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Fluorescent microscopy was used at 40× magnification to count the number of positively stained neutrophils and macrophages in ten high-powered fields along the wound edge.
Mean fluorescent intensity (MFI) of MOMA-2 positive cells
Moma-2+ cells from our leukocyte quantification experiments (Gr-1/Moma-2 sequential IHF) were visualized using an Axiovert 200M fluorescence microscope and analyzed using the Axiovision 8.1 program (Carl Zeiss Microscopy, Jena, Germany). The densitometric mean of 10 Moma-2+ cells per section was determined as previously described (Karavitis et al., 2008), (n = 4-7 mice per group). Data is reported as the MOMA-2 densitometric mean +/- SEM. Statistical analyses were performed using Student's t-test where p < 0.05 was considered significant. Reported results are an average of 2 separate animal experiments.
Determination of cytokine and chemokine levels in skin wound homogenates
Wounds were individually homogenized in 1 ml of BioPlex cell lysis buffer and filtered, according to the manufacturer's instructions (BioRad, Hercules, CA). Cytokine and chemokine levels were determined using Enzyme linked immunosorbent assays (ELISA) for MCP-1, KC, MIP1-α, and MIP-2 (R&D Systems, Minneapolis, MN), or BioPlex multiplex bead array for IL-6, TNFα, IL-1β, and IL-10. Total protein was determined using the Bradford-based BioRad protein assay (BioRad, Hercules, CA) and ELISA/BioPlex results were normalized to total protein. Results were presented as the geometric mean cytokine/chemokine level (picograms of cytokine/chemokine per milligram of total protein) with 95% CIs. Experiments were performed twice, with a combined n= 12-17 mice per group. Statistical analyses were performed using the Student's t-test where p < 0.05 was considered significant.
Immunohistofluorescence (IHF) analysis of CRAMP, KLK5 and KLK7
6 μm thick frozen wound sections were analyzed. 1° rabbit anti-mouse CRAMP antibody (Abcam, Cambridge, MA) was used with Alexafluor488-donkey-anti-rabbbit IgG 2° antibody. 1° rabbit anti-human KLK5 or KLK7 antibodies (R&D Systems, Minneapolis, MN) were used with 2° Cy3-goat anti-rabbit-IgG. Rabbit IgG was used as a negative control. Slides were mounted with ProLong® Gold Antifade Reagent with DAPI (Invitrogen, Carlsbad, CA). Photographs were taken at 20× using an Evos Digital Inverted microscope and interpreted in a blinded manner. (n= 7-9 mice per group).
Results
We previously demonstrated that acute ethanol exposure delayed multiple components of the inflammatory and proliferative phase of wound repair in the absence of wound infection (Radek et al., 2007, Radek et al., 2005, Fitzgerald et al., 2007). In the current manuscript, our first goal was to identify wound healing defects in a model of multi-day episodic binge ethanol exposure (Lauing et al., 2008), in the presence of S. aureus wound infection. We first determined if binge ethanol exposure prior to wound infection would result in more prominent wounds after 24 hours compared to saline controls. We observed that wounds from ethanol-treated mice were larger than vehicle-treated controls (Figure 1A), which corresponded with a statistically significant increase in open wound area after 24 hours. Wounds from saline-treated control mice had 54% open wound area, whereas wounds from ethanol treated mice had 74% open wound area (Figure 1B).
Figure 1. Ethanol exposure increases the percentage of open wound area.
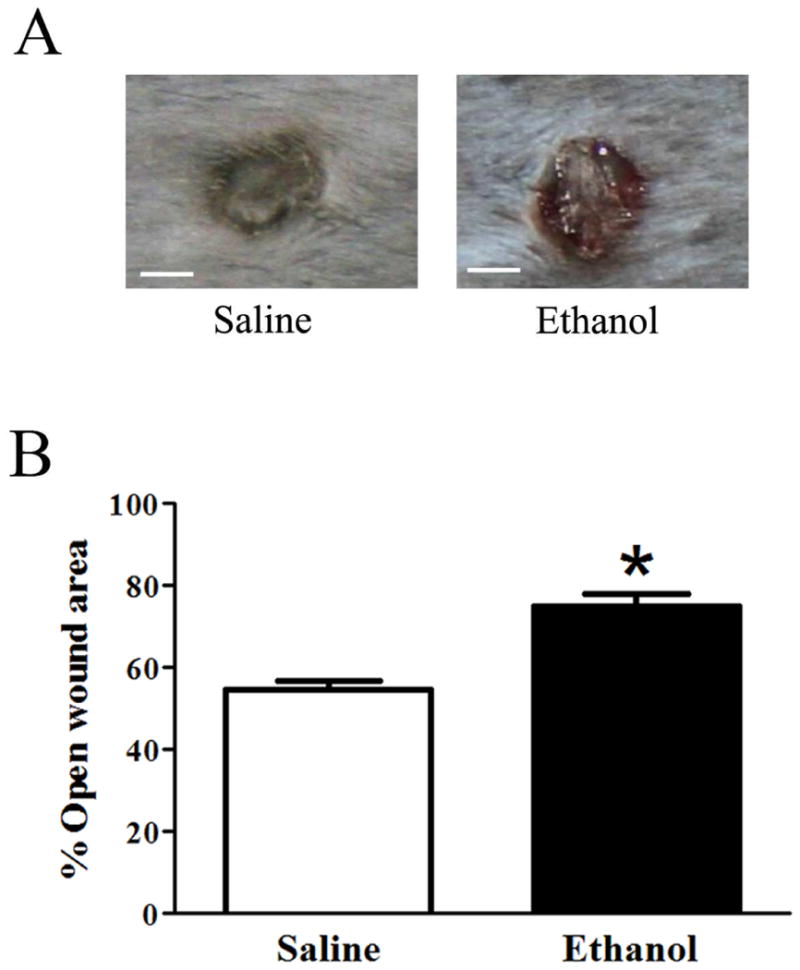
(A) Representative images of wounds from saline and ethanol-treated mice (n=18 per group). Scale bars = 1 cm. (B) Percentage of open wound area quantification. % open wound area = (average # pixels per wound 24hours after injury/ average # pixels per wound immediately following injury) *100. Data are represented as mean ± SEM. * p<0.0001 by Student's t-test.
Since we previously demonstrated that acute ethanol exposure impaired early stages of normal cutaneous wound healing (Fitzgerald et al., 2007), we performed IHF on sequential wound section to quantify the number of neutrophils and macrophages along the wound margin (Figure 2A). We found no difference in the number of Gr1+ cells, suggesting that episodic binge ethanol exposure did not alter neutrophil influx 24 hours after wound infection (Figure 2B, C). However, we observed a 57% decrease (p< 0.05) in the number of MOMA-2+ cells, where an average of 118 MOMA-2+ cells/ 10 high power fields were counted in saline treated control wounds compared to an average of 63 MOMA-2+ cells/10 high power fields in ethanol-treated mice (Figure 2B,D). To verify that the reduction in MOMA-2+ cells was specifically due to decreased macrophage infiltration and not from ethanol-induced down regulation of MOMA-2 expression on the cell surface, we determined the MFI of MOMA-2+ cells and observed no statistical difference between groups (Figure 2E).
Figure 2. Ethanol exposure decreases macrophage infiltration into infected wounds, but does not affect neutrophil infiltration.
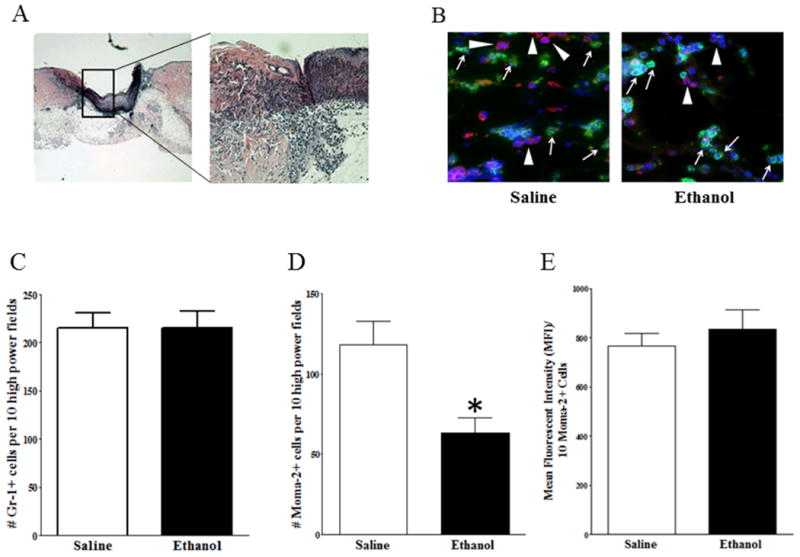
(A) H&E histological staining. Left image: Low-power magnification of skin sections. The boxed area indicates the wound margin. Right image: High-power magnification of the wound margin demonstrating large numbers of infiltrating cells. (B) IHF of wound margin from saline (n=9) or ethanol (n=5) treated mice. Arrows (→) indicate Gr-1+ neutrophils, stained green. Arrowheads (▶) indicate Moma-2+ macrophages, stained red. DAPI nuclear stain is blue. No antibody control did not show any staining (data not shown). (C) Quantification of Gr-1+ neutrophils. (D) Quantification of Moma-2+ macrophages. (E) Quantification of Mean Fluorescence Intensity of Moma-2+ cells. Data are represented as mean ± SEM. * = p < 0.05 by Student's t-test.
We next investigated whether ethanol exposure altered production of pro- and anti-inflammatory cytokines, and neutrophil or macrophage-recruiting chemokines in infected wounds. We observed similar levels of the potent neutrophil chemoattractants, MIP-2 and KC, in both treatment groups (Figure 3A, B). This finding was not surprising, since ethanol did not affect neutrophil accumulation in the wound bed. No difference in the production of MCP-1 was observed (Figure 3C). In contrast, ethanol suppressed the production of MIP-1α by 41% (p < 0.05) compared to saline-treated controls (Figure 3D). We observed that infected wounds from ethanol treated animals had 43% less IL-6 (p=0.0978) (Figure 3E), a 53% reduction in IL-1β (p < 0.05) (Figure 3F), but no difference in TNFα levels was observed (Figure 3G). Lastly, levels of the anti-inflammatory cytokine, IL-10 were reduced by 43% (p < 0.05) in wounds from ethanol-treated animals. Together, these findings suggest that ethanol treatment alters the cytokine/chemokine milieu in infected mouse wounds.
Figure 3. Ethanol exposure specifically decreases the production of the chemokine, MIP1-α, the pro-inflammatory cytokine, IL-1β, and the anti-inflammatory cytokine, IL-10.
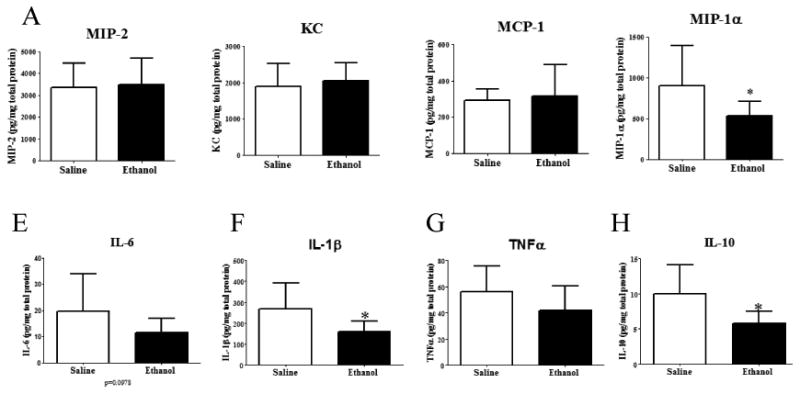
Chemokine levels were determined by ELISA. (A-D), Cytokine levels were determined by multiplex bead array (E-H). (A) MIP-2 levels in wounds from saline (n=13) or ethanol (n=12) treated mice. (B) KC levels in wounds from saline (n=13) or ethanol (n=12) treated mice. (C) MCP-1 levels in wounds from saline (n=13) or ethanol (n=11) treated mice. (D) MIP-1α levels in wounds from saline (n=18) or ethanol (n=17) treated mice. (E-H) (E) IL-6, (F) IL-1β, (G)TNFα, and (H) IL-10 levels in wounds from saline (n=18) or ethanol (n=16) treated mice. Data are represented as geometric mean with 95% Cl.* = p < 0.05 by Student's t-test.
Since the observed decrease in pro-inflammatory cytokines and MIP-1α likely contributes to reduced macrophage accumulation in the wound bed, we next elected to determine if other factors with known roles in monocyte/macrophage chemotaxis were down-regulated by ethanol exposure. To address this question, we performed IHF on wounds to determine if episodic binge ethanol exposure suppressed CRAMP production. We found that compared to saline-treated control mice, wounds from episodic binge ethanol-treated mice had dramatically reduced levels of CRAMP in the epidermis (Figure 4A,B), suggesting that ethanol exposure may impair keratinocyte production of CRAMP. Since we observed a decrease in epidermal CRAMP production in wounds from ethanol treated mice, we next determined if ethanol exposure altered the localization or abundance of two key epidermal proteases involved in CRAMP processing into its bioactive form, which include KLK5 and KLK7 (Ekholm et al., 2000). We observed that saline and ethanol-treated mice had similar levels of KLK5 (Figure 4C,D). In contrast, ethanol-exposed mice exhibited a robust reduction in the epidermal abundance of KLK7 (Figure 4E,F) compared to saline controls. However, no changes in KLK5 or KLK7 localization were seen. These findings suggest that the reduced abundance of epidermal CRAMP observed in ethanol-exposed mice may result from impaired proteolytic processing by KLK7.
Figure 4. Ethanol exposure decreases epidermal CRAMP and KLK7 production, but not KLK5.
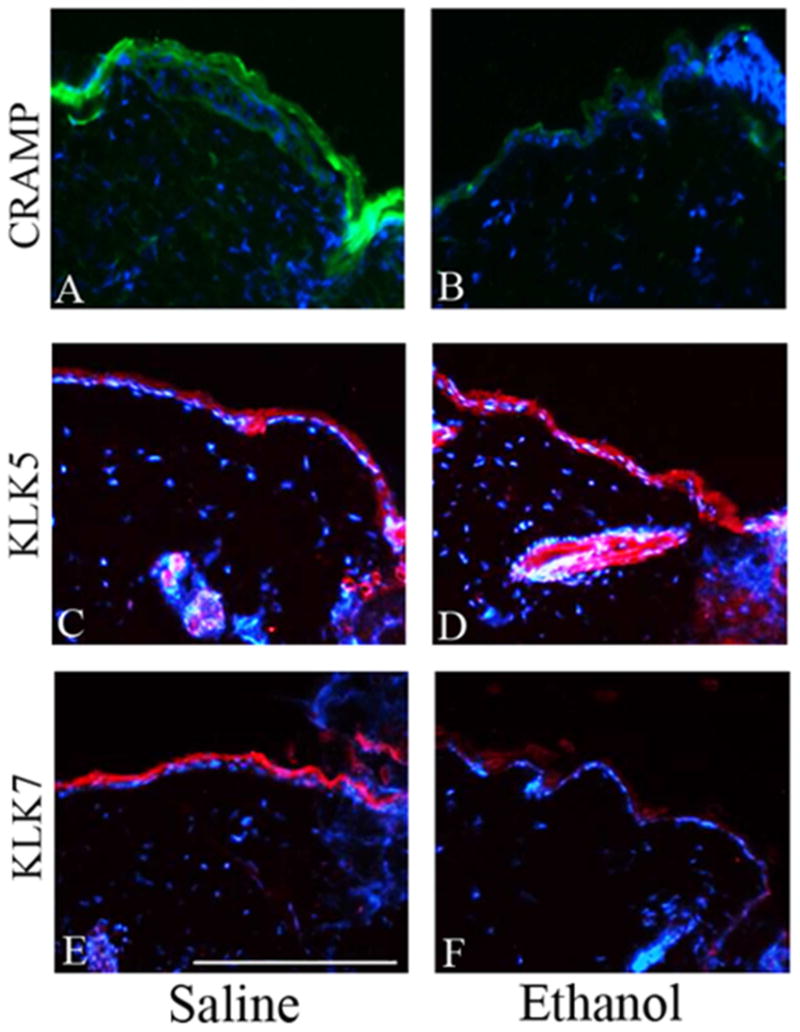
(A,B) IHF using rabbit-anti-CRAMP 1° antibody and Alexafluor 488-donkey-anti-rabbit 2° antibody. Whole rabbit sera negative control showed minimal background staining (data not shown). CRAMP levels in the epidermis are higher in saline-treated (A) mice as compared to ethanol-treated (B) mice. (C,D) IHF using goat anti-human KLK5 1° antibody and Cy3-donkey-anti-goat 2° antibody. Goat IgG control showed minimal background staining (data not shown). KLK5 levels in saline-treated (C) and ethanol-treated (D) mice. (E,F) IHF using goat anti-human KLK7 1° antibody and Cy3-donkey-anti-goat 2° antibody. Goat IgG control showed minimal background staining (data not shown). KLK7 levels in saline-treated (E) and ethanol-treated (F) mice. The wound edge is located on the right side of all images.
Discussion
Multiple components of the innate immune response could be affected by ethanol exposure during wound infection, including leukocyte chemotaxis, cytokine production, bacterial recognition, immune cell signaling, and AMP production. We report for the first time that episodic binge ethanol exposure diminishes macrophage infiltration during early wound repair, which parallels a reduction in IL-1β, MIP-1α, IL-10 and CRAMP. We previously demonstrated that a single dose of acute ethanol dampened neutrophil activation and reduced the production of the neutrophil chemoattractants, MIP-2 and KC, as well as the pro-inflammatory cytokine IL-1β within 12 hours following skin wounding (Fitzgerald et al., 2007). Here, we demonstrate that in the context of wound infection, multiple days of binge ethanol exposure appear to have more robust effect on macrophages, by altering macrophage infiltration kinetics and the production of the macrophage chemoattractant, MIP-1α, as well as reducing both pro- and anti-inflammatory cytokine production 24 hours following post wound infection. Since we previously demonstrated that a single ethanol exposure altered later phases of wound healing, including re-epithelialization (Day 2), reduced collagen deposition (Day 7), and decreased wound vascularity (Days 7, 10, and 14) (Radek et al., 2005), it is evident that the cumulative consequences of ethanol exposure on wound repair are not transient. Thus, these alterations can promote wound complications that may arise days after cutaneous injury, including wound infection.
During wound healing, impaired macrophage function and altered rates of macrophage infiltration/duration have been correlated with a prolonged inflammatory phase, delayed wound closure, and poor restoration of the extracellular matrix (Singer and Clark, 1999, Mirza et al., 2009). We observed that infected wounds from ethanol-treated mice contained 53% fewer macrophages compared to saline-treated controls, which likely contributes to the enlarged wound size in episodic binge ethanol-exposed mice. Ethanol exposure both decreased pro-inflammatory cytokine (IL-1β) and CRAMP levels, which would presumably reduce the efficacy of pathogen clearance. Human cathelicidin (LL-37) was previously found to synergistically enhance the IL-1β-mediated induction of several cytokines in human mononuclear cells, including IL-6 and IL-10 (Yu et al., 2007). These data suggest that the reduction in epidermal CRAMP observed in our studies may synergistically suppress IL-1β-mediated production of wound IL-6 and IL-10 by infiltrating macrophages and neutrophils in our murine wound model by a complex mechanism that warrants further investigation. In parallel, separate studies determined that IL-1β had the capacity to stimulate both KC and MIP1α in keratinocyte and fibroblasts cell lines (Hu et al., 2010a), while an IL-1β antagonist administered in vivo decreased the production of KC and MIP1α in murine wounds (Hu et al., 2010b). These data further highlight the role of IL-1β as a critical regulator of the sustained pro-inflammatory wound macrophage phenotype, particularly in models of impaired wound healing (Mirza et al., 2013). In the current study, we speculate that ethanol exposure prior to wounding decreases IL-1β production, which in turn blocks the production of key cytokines and chemokines necessary for macrophage recruitment and activation. This notion is supported by a recent study demonstrating that ethanol dose-dependently suppressed the production of mature IL-1β induced by inflammasome activators in macrophages (Nurmi et al., 2013), indicating a potential role for an IL-1β antagonist as a therapeutic to reverse macrophage and innate immune dysfunction in wounds sustained in intoxicated trauma patients.
In the present study, ethanol exposure prior to wounding also reduced IL-10 levels, which is a key factor for progressing the wound healing process from the inflammatory to the proliferative phase (Eming et al., 2007). Epidermal cells and infiltrating mononuclear cells serve as a key source of IL-10 in wounds, which may contribute to the normal biphasic response of IL-10 at 24 and 72 hours post-wounding (Sato et al., 1999). This indicates a regulatory function for IL-10 in the phase-specific recruitment of macrophages to the wound bed, which is impaired in infected wounds following ethanol exposure.
Extravasation of monocytes from the circulation into an infected wound also requires sufficient chemotactic signals. Thus, the observed reduction in both in MIP-1α and IL-1β is a likely mechanism for the reduction in macrophages in wound from ethanol-exposed mice, which would limit monocyte extravasation into the wound bed (Chavakis et al., 2009). MIP-1α is also critical for promoting wound healing, as mice treated with anti-MIP-1α neutralizing antiserum demonstrated delayed wound repair (DiPietro et al., 1998), suggesting the reduced MIP-1α levels observed in infected mouse wounds from ethanol treated animals directly contributes to the diminished macrophage accumulation within the wound bed and delayed wound closure. Future studies will be necessary to characterize the divergent mechanisms which specifically target wound macrophages following ethanol exposure.
Many in vivo and in vitro model systems suggest that ethanol exposure generates a state of oxidative stress via ethanol metabolism (El-Assal et al., 2004, Kurose et al., 1997). Metabolites generated from the breakdown of ethanol induce tissue damage and can be detrimental to wound healing, in part, through the production of reactive oxygen species (ROS) (Molina et al., 2002, Shukla et al., 2001). However, in response to infection, keratinocyte cells produce a substantial amount of ROS for direct pathogen killing. In the stratum corneum of the epidermis, excessive oxidative stress caused by ROS production can alter epidermal pH which disrupts the permeability barrier and increase the progression of wound infection (Grange et al., 2009). A minor increase in skin pH can have several deleterious effects by increasing serine protease activity, leading to degradation of antimicrobial peptides, elevated cytokine activation, and impaired permeability and antimicrobial barriers (reviewed in (Elias, 2005)). Epidermal pH may alter the activity of KLK proteases, since activation of another epidermal chymotrypsin-like serine protease, chymase, is inhibited by ROS in cultured NHEKs (Firth et al., 2008). Moreover, IL-1β activation, which requires proteolytic processing of its biologically inactive precursor, occurs through a pH-dependent increase in the serine protease activity of KLK7 in the epidermis (Nylander-Lundqvist and Egelrud, 1997). Our current finding that ethanol decreased KLK7 levels in the epidermis suggests one possible mechanism for the decrease in IL-1β in infected wounds.
Kallikreins also regulate the physical and innate antimicrobial barriers in the epidermis (Yamasaki et al., 2006). Reduced epidermal CAMP production has been associated with the prevalence and severity of eczema-related skin infections (Ong et al., 2002). Our data that infected mouse wounds from ethanol treated animals have decreased levels of KLK7 suggests that intoxication at the time of injury may directly contribute to delayed wound healing.
Researchers have gained significant insight into several key aspects of the wound healing process by utilizing the murine excisional wound healing model, but it should be noted that a few limitations exist with this model. Mouse wounds close primarily through contraction, whereas human wounds close mostly through re-epithelialization (Wong et al., 2011) (Tranquillo and Murray, 1993). Furthermore, murine wound repair processes occur more rapidly with respect to the duration of each phase compared to human wound healing (Tranquillo and Murray, 1993). This suggests that the kinetics of leukocyte recruitment are likely different between humans and mice. However, the work presented herein demonstrates that several conserved factors involved in early in the wound healing process are altered by episodic binge ethanol exposure.. Such factors include macrophage recruitment, chemokine/cytokine production, and AMP regulation, factors which are conserved as part of the human wound healing process. Aberrant innate immune responses, such as these, may contribute to the increased rates of infection, morbidity, and mortality in patients intoxicated at the time of cutaneous injury.
In conclusion, our research suggests that episodic binge ethanol intoxication prior to skin wound infection impairs the normal macrophage inflammatory response and epidermal AMP production. These defects likely limit the transition into the proliferative phase of the normal wound healing process to increase morbidity in intoxicated patients.
Acknowledgments
Research reported in the publication was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the National Institutes of Health (NIH) under award numbers P30AA19373 (EJK), R01AA012034 (EJK), T32AA013527 (EJK), and F32AA021636 (BJC). Work was also supported by and the Marian and Ralph C Falk Medical Research Trust (KAR and EJK). The authors would also like to thank Luis Ramirez and Huzefa Husain for technical assistance, and Jim Sinacore, PhD for assistance with statistical analyses.
References
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- Bradley KA, Rubinsky AD, Sun H, Bryson CL, Bishop MJ, Blough DK, Henderson WG, Maynard C, Hawn MT, Tonnesen H, Hughes G, Beste LA, Harris AH, Hawkins EJ, Houston TK, Kivlahan DR. Alcohol screening and risk of postoperative complications in male VA patients undergoing major non-cardiac surgery. Journal of general internal medicine. 2011;26:162–169. doi: 10.1007/s11606-010-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thrombosis and haemostasis. 2009;102:191–197. doi: 10.1160/TH08-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. Journal of leukocyte biology. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M, Goldberg S, Hussein E, Neifeld JP. Health care-associated infections in surgical patients undergoing elective surgery: are alcohol use disorders a risk factor? Journal of the American College of Surgeons. 2012;215:229–236. doi: 10.1016/j.jamcollsurg.2012.04.015. [DOI] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. The Journal of experimental medicine. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. The Journal of clinical investigation. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm IE, Brattsand M, Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? The Journal of investigative dermatology. 2000;114:56–63. doi: 10.1046/j.1523-1747.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1:205–211. [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. The Journal of investigative dermatology. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermohlen O, Davidson JM, Krieg T, Roers A. Accelerated wound closure in mice deficient for interleukin-10. The American journal of pathology. 2007;170:188–202. doi: 10.2353/ajpath.2007.060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth JD, Uitto VJ, Putnins EE. Mechanical induction of an epithelial cell chymase associated with wound edge migration. The Journal of biological chemistry. 2008;283:34983–34993. doi: 10.1074/jbc.M801975200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DJ, Radek KA, Chaar M, Faunce DE, DiPietro LA, Kovacs EJ. Effects of acute ethanol exposure on the early inflammatory response after excisional injury. Alcoholism, clinical and experimental research. 2007;31:317–323. doi: 10.1111/j.1530-0277.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- Gerke P, Hapke U, Rumpf HJ, John U. Alcohol-related diseases in general hospital patients. Alcohol and alcoholism. 1997;32:179–184. doi: 10.1093/oxfordjournals.alcalc.a008252. [DOI] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. Journal of leukocyte biology. 2001;69:513–521. [PubMed] [Google Scholar]
- Gmel G, Bissery A, Gammeter R, Givel JC, Calmes JM, Yersin B, Daeppen JB. Alcohol-attributable injuries in admissions to a swiss emergency room--an analysis of the link between volume of drinking, drinking patterns, and preattendance drinking. Alcoholism, clinical and experimental research. 2006;30:501–509. doi: 10.1111/j.1530-0277.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- Grange PA, Chereau C, Raingeaud J, Nicco C, Weill B, Dupin N, Batteux F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009;5:e1000527. doi: 10.1371/journal.ppat.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. The Journal of investigative dermatology. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, Dill D, Shi X, Qiao Y, Yeomans D, Carvalho B, Angst MS, Clark JD, Peltz G. The role of interleukin-1 in wound biology. Part I: Murine in silico and in vitro experimental analysis. Anesthesia and analgesia. 2010a;111:1525–1533. doi: 10.1213/ANE.0b013e3181f5ef5a. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, Dill D, Shi X, Qiao Y, Yeomans D, Carvalho B, Angst MS, Clark JD, Peltz G. The role of interleukin-1 in wound biology. Part II: In vivo and human translational studies. Anesthesia and analgesia. 2010b;111:1534–1542. doi: 10.1213/ANE.0b013e3181f691eb. [DOI] [PubMed] [Google Scholar]
- Karavitis J, Murdoch EL, Gomez CR, Ramirez L, Kovacs EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2008;28:413–422. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. The Journal of clinical investigation. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. Journal of immunology. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T, Ebinuma H, Kato S, Ishii H. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25:368–378. doi: 10.1053/jhep.1997.v25.pm0009021949. [DOI] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends in immunology. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–656. doi: 10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. The American journal of pathology. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Lundqvist C, Alling C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment. Alcohol and alcoholism. 1995;30:737–748. [PubMed] [Google Scholar]
- Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62:2579–2587. doi: 10.2337/db12-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, McClain C, Valla D, Guidot D, Diehl AM, Lang CH, Neuman M. Molecular pathology and clinical aspects of alcohol-induced tissue injury. Alcohol Clin Exp Res. 2002;26:120–128. [PubMed] [Google Scholar]
- Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. The Journal of investigative dermatology. 2010;130:1297–1306. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA : the journal of the American Medical Association. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- National Nosocomial Infections Surveillance S. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. American journal of infection control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Nurmi K, Virkanen J, Rajamaki K, Niemi K, Kovanen PT, Eklund KK. Ethanol Inhibits Activation of NLRP3 and AIM2 Inflammasomes in Human Macrophages-A Novel Anti-Inflammatory Action of Alcohol. PloS one. 2013;8:e78537. doi: 10.1371/journal.pone.0078537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander-Lundqvist E, Egelrud T. Formation of active IL-1 beta from pro-IL-1 beta catalyzed by stratum corneum chymotryptic enzyme in vitro. Acta dermato-venereologica. 1997;77:203–206. doi: 10.2340/0001555577203206. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. The New England journal of medicine. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Park HJ, Cho DH, Kim HJ, Lee JY, Cho BK, Bang SI, Song SY, Yamasaki K, Di Nardo A, Gallo RL. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843–850. doi: 10.1038/jid.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek KA, Kovacs EJ, DiPietro LA. Matrix proteolytic activity during wound healing: modulation by acute ethanol exposure. Alcoholism, clinical and experimental research. 2007;31:1045–1052. doi: 10.1111/j.1530-0277.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- Radek KA, Kovacs EJ, Gallo RL, DiPietro LA. Acute ethanol exposure disrupts VEGF receptor cell signaling in endothelial cells. American journal of physiology. Heart and circulatory physiology. 2008;295:H174–184. doi: 10.1152/ajpheart.00699.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek KA, Matthies AM, Burns AL, Heinrich SA, Kovacs EJ, Dipietro LA. Acute ethanol exposure impairs angiogenesis and the proliferative phase of wound healing. American journal of physiology. Heart and circulatory physiology. 2005;289:H1084–1090. doi: 10.1152/ajpheart.00080.2005. [DOI] [PubMed] [Google Scholar]
- Radek KA, Ranzer MJ, Dipietro LA. Brewing complications: the effect of acute ethanol exposure on wound healing. Journal of leukocyte biology. 2009;86:1125–1134. doi: 10.1189/jlb.0209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochemical and biophysical research communications. 1999;265:194–199. doi: 10.1006/bbrc.1999.1455. [DOI] [PubMed] [Google Scholar]
- Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. Journal of immunology. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Sun GY, Gibson Wood W, Savolainen MJ, Alling C, Hoek JB. Ethanol and lipid metabolic signaling. Alcoholism, clinical and experimental research. 2001;25:33S–39S. doi: 10.1097/00000374-200105051-00006. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Archives of internal medicine. 2004;164:749–756. doi: 10.1001/archinte.164.7.749. [DOI] [PubMed] [Google Scholar]
- Surgery U. Executive Summary 2008 [Google Scholar]
- Tranquillo RT, Murray JD. Mechanistic model of wound contraction. The Journal of surgical research. 1993;55:233–247. doi: 10.1006/jsre.1993.1135. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life sciences. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical approaches to create murine models of human wound healing. Journal of biomedicine & biotechnology. 2011;2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, Rehaume L, Hancock RE. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. Journal of immunology. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]


