Abstract
Sublytic complement C5b-9 complexes can cause cell apoptosis, but the mechanism of glomerular mesangial cell (GMC) apoptosis mediated by these complexes has not been well defined. The activating transcription factor 3 (ATF3) gene is an immediate early gene for the cell to cope with a variety of stress signals and can promote apoptosis of some cells. In this study, ATF3 expression and cell apoptosis in GMCs induced by sublytic C5b-9 were measured, and then the effects of ATF3 gene over-expression or knockdown on GMC apoptosis induced by sublytic C5b-9 were examined at a fixed time. The results showed that both ATF3 expression and GMC apoptosis were markedly increased and ATF3 over-expression obviously increased sublytic C5b-9-induced GMC apoptosis, whereas ATF3 gene silencing had a significant opposite effect. Collectively, these findings indicate that upregulation of ATF3 gene expression is involved in regulating GMC apoptosis induced by sublytic C5b-9 complexes.
Keywords: sublytic C5b-9, apoptosis, glomerular mesangial cell (GMC), activating transcription factor 3 (ATF3)
Introduction
Mesangioproliferative glomerulonephritis (MsPGN), characterized by glomerular mesangial cell (GMC) proliferation and extracellular matrix accumulation, is a disease of high incidence in humans.1 Although GMC injury in human MsPGN involves complement system activation, especially formation of C5b-9 complexes, the factors regulating GMC lesions are not well known.2, 3 Rat MsPGN, namely Thy-1 nephritis (Thy-1N), is induced by the Thy-1 antibody binding to the corresponding antigen on the membrane of rat GMCs.4, 5 Early studies of Thy-1N have revealed that GMC injury is complement dependent and neutrophil independent.6, 7 Activation of the complement system can generate the complement C5b-9 complexes (i.e., membrane attack complex).8 The role of C5b-9 on the target cell is thought to be lytic or sublytic, involving the formation of trans-cellular transport on the membrane and resulting in cell lysis in the former and latter cases, causing a secondary cell injury through several inflammatory mediators or cytokines derived from GMCs after sublytic C5b-9 attack.9 Generally, the injury by C5b-9 to nucleated cells is almost non-lysis (sublytic) because the surfaces of the cells have many homologous restriction factors, such as complement receptor 1 (CR1)-related gene/protein y, CD59 and membrane cofactor protein, or the number of channels assembled on the surface of nucleated cells is limited.8, 9, 10 Previous experiments have shown that GMCs of rats with Thy-1N undergo changes in apoptosis, necrosis and secondary proliferation.11, 12 As for GMC apoptosis, there are many problems that remain unclear.13 Thus, the molecular mechanism of GMC apoptosis mediated by sublytic C5b-9 complexes is worth exploring.
Activating transcription factor 3 (ATF3) is a member of the ATF/cAMP response element binding (CREB) family of transcription factors.13, 14 Overwhelming evidence confirms that the ATF3 gene is activated in many tissues by a variety of stress signals, including proinflammatory cytokines, ischemia and hypoxia.15, 16 Several studies have revealed that ATF3 can induce apoptosis in ovarian cancer cells17, 18 and enhance etoposide- or camptothecin-induced apoptosis in HeLa cells.19 Moreover, our results of microarray analysis also demonstrated that ATF3 mRNA is increased 5.2-fold and 2.1-fold in the GMC apoptosis phase of Thy-1N rats and in cultured GMCs stimulated by sublytic C5b-9 attack, respectively (data not shown). Therefore, the purpose of the present study is to ascertain whether ATF3 expression can be upregulated in GMCs with sublytic C5b-9 stimulation and to determine the role of ATF3 in sublytic C5b-9-induced GMC apoptosis.
Materials and methods
Reagents
The polyclonal antibody against ATF3 (sc-188) and monoclonal antibody against β-actin (sc-47778) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The monoclonal antibody (ox-7) against rat Thy-1 antigen was provided by Santa Cruz. The horseradish peroxidase-conjugated secondary antibody was provided by Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The HBZY-1 rat GMC cell line was provided by the China Centre for Type Culture Collection (Wuhan, China). Fetal calf serum was from Gibco (Carlsbad, CA, USA). Normal human serum from several healthy adult donors was pooled and used as a source of serum complement. Heat-inactivated serum (HIS) was prepared by incubating the normal human serum at 56 °C for 30 min. Human complement C6-deficient serum (C6DS) was purchased from Sigma (Sigma, USA). The restriction enzyme SfiI and T4 DNA ligase were purchased from TaKaRa (Tokyo, Japan). The QIAprep Spin Miniprep kit was obtained from QIAGEN (Hilden, Germany). GenEscort III was supplied by Wisegen (Nanjing, China). Moloney murine leukemia virus (M-MLV) reverse transcriptase and oligo (dT)15 primer were from Promega (Madison, MI, USA). Chemiluminescence detection was from PerkinElmer Life Sciences (Boston, MA, USA). Hoechst 33342 was purchased from Beyotime Biotechnology Inc. (Nantong, China). Annexin-V/ allophycocyanin (APC) was purchased from Bender Medsystems (Vienna, Austria). Small hairpin RNA (shRNA) and PGCsi were obtained from Shanghai Genkan Biotechnology (Shanghai, China).
GMC culture and sublytic C5b-9 determination
Rat GMCs were cultured and maintained in modified Eagle's medium (MEM) supplemented with 10% fetal calf serum, 100 µg/ml penicillin and 100 µg/ml streptomycin, and used from passages 5 to 7 for experiments. The formation of complement sublytic C5b-9 complexes on the GMC membrane was identified as previously described.10, 13, 20 Briefly, the selection of the anti-Thy-1 antibody (Ab) and complement concentration used in the study was based on a checkerboard titration study to define the maximal amounts of anti-Thy-1 Ab and complement that could be used without producing rat GMC lysis.10, 20 To ensure that C5b-9 attack was insufficient to induce marked cell lysis, lactate dehydrogenase (LDH) was measured in the supernatants of cultured GMCs using the LDH assay kit. The lysis rate was expressed as a percentage of the change in the absorbance of the media compared to changes in absorbance of the media of Triton-lysed cells and media of untreated cells. Less than 5% LDH release from cells was regarded as a sublytic effect. The selection of anti-Thy-1 Ab and complement concentration used in our experiment was 5% anti-Thy-1 Ab and 4% normal human serum, which resulted in less than 5% LDH release.13 In addition, to demonstrate that the effects on GMCs following various treatments were in fact due to sublytic C5b-9 stimulation, GMCs were divided into five groups (five treatments), namely MEM, Thy-1 Ab, Thy-1 Ab+HIS, Thy-1 Ab+C6DS and sublytic C5b-9 groups.10 After time-course analysis of ATF3 expression upon attack of sublytic C5b-9, another human serum incubation time of 3 h was decided.
ATF3 expression vector construction
The full-length coding region of ATF3 cDNA was inserted into a pcDNA3.1 expression vector (a gift from Professor Jianming Li, Nanjing Medical University, Nanjing, China) to generate pcDNA3.1/ATF3. Briefly, first-strand cDNA was synthesized from total RNA of renal tissue of rat Thy-1N (3 h) using M-MLV reverse transcriptase. The ATF3 gene was amplified by polymerase chain reaction (PCR). Specific primer sequences were as follows: forward primer, 5′-AAGGCCAATCCGGCCATGATGCTTCAACATCCA-3′, and reverse primer, 5′-GGCCTCTAAGGCCTCACTCGGGAAGGGTGATGC-3′. PCR products and the pcDNA3.1 vector were digested with SfiI and ligated using T4 DNA ligase. The recombinant plasmids were amplified in Escherichia coli strain DH5α and purified with the QIAprep Spin Miniprep kit. The constructed plasmid was sequenced across both junctions to confirm the nucleotide sequence and the predicted orientation.13, 21
ATF3 shRNA vector generation
The nucleotide sequences of shRNAs targeting the rat ATF3 gene were designed and chemically synthesized by Genechem Co. Ltd (Shanghai, China). These segments of DNA were searched in the NCBI sequence bank using the BLAST program, which confirmed no match to any genes other than the target gene, verifying the specificity of the target region by the shRNAs. Five different nucleotide sequences of shRNAs against rat ATF3 were then cloned into the plasmid pGCsi-U6/Neo/green fluorescent protein (GFP), which contains a multiple cloning site for insertion of shRNA constructs to be driven by an upstream U6 promoter and a downstream cytomegalovirus promoter-GFP (marker gene) cassette. The resulting eukaryotic expression vectors containing rat ATF3 shRNAs were named ATF3 shRNAs 1–5, and their sequences were confirmed by sequence analysis.
GMC transfection and identification
Transient transfection of ATF3 expression plasmids or ATF3 shRNA expression plasmids into the cultured GMCs was conducted using GenEscort III according to the manufacturer's instructions. In brief, GMCs were seeded in a six-well culture dish with complete media and transfected at 80% confluence with pcDNA3.1/ATF3 (pATF3), mock control pcDNA3.1 or ATF3 shRNA/GFP using GenEscort III and incubated for 24, 36, 48 or 60 h. Then, the efficiency of shRNA transfection was detected by the fluorescence of GFP. Moreover, ATF3 protein expression in the GMCs transfected by pATF3 or ATF3 shRNAs after a fixed time was measured by western blot.
Reverse transcription (RT)-PCR
Cells were collected at indicated time points and RNA was prepared using Trizol reagent according to the manufacturer's protocol. A total of 1 μg RNA was used for the first-strand cDNA synthesis. RT was performed with M-MLV reverse transcriptase and oligo (dT)15 primer. The primers used for PCR amplification were as follows: ATF3 forward 5′-GCGCGGATCCATGATGCTTCAACATCCA-3′ and reverse 5′-GCGCAAGCTTTTAGCTCT GCAATGTTCCTTCTTTT-3′, β-actin primers forward 5′-TCCTGTGGCATCCA CGAAACT-3′ and reverse 5′-GAAGCATTTGCGGTGGACGAT-3′. The PCR reaction was performed at an annealing temperature of 56 °C for 25 cycles. PCR products were separated via 1.0% agarose gel electrophoresis and visualized by ethidium bromide staining. Ratios for IRF-1/β-actin mRNA were calculated for each sample.
Real-time PCR
The total RNA in various GMCs was extracted, and RT was performed using M-MLV reverse transcriptase. The quantification of ATF3 gene expression in the fixed time was performed by real-time PCR using an ABI PRISM 7300 sequence detection system. The following primers and fluorescence-labeled probes were used: for ATF3, forward primer 5′-CACCTTTGCCATCGGATGTCC-3′, reverse primer 5′-CTTTCCCGCCGCCTCCTT-3′, and FAM/TAMRA-labeled probe 5′-CGCTGGAGTCAGTCACCATCAACAACAG-3′ and for β-actin, forward primer 5′-TCACCCACACTGTGCCCATCTATGA-3′, reverse primer 5′-CATCGGAACCGCTCATTGCCGATAG-3′, and FAM/TAMRA-labeled probe: 5′-ACGCGCTCCCCCATGCCATCCTGCGT-3′. The relative level of the gene expression was obtained by calculating the ratio of threshold cycle (CT) numbers of the initial exponential amplification phase as determined by the sequence detection system for the specific target gene and β-actin using the following formula: 2 (normalized CT in control sample – normalized CT in stimulated sample). All measurements were performed in duplicate.
Western blot
After treatment, GMC proteins were extracted using radioimmunoprecipitation assay buffer, and the concentration was determined using the BCA kit. Then, 30 µg of protein was loaded and electrophoresed on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to 0.45-µm PVDF membrane, blocked in 5% skim milk and 0.5% Tween-20 in Tris-buffered saline, and incubated with the primary antibody (1:500 anti-ATF3 antibody, 1/1000 anti-β-actin antibody) followed by incubation with a 1/2500 dilution of horseradish peroxidase-conjugated secondary antibody. Bands were visualized by enhanced chemiluminescence detection and quantitated by densitometry scanning.
Immunohistochemistry
GMCs were cultured on plastic slides and divided into five treatments as previously described. The slides were washed in phosphate-buffered saline (PBS) and incubated for 1 h at 37 °C with anti-ATF3 antibody (diluted 1/100). After washing in PBS, the cells were incubated with horseradish peroxidase-conjugated secondary antibody for 30 min. The reaction was visualized using the chromogen 3′-diaminobenzidine and analyzed directly under a microscope.
Hoechst staining
Differently treated GMCs were washed once with PBS, fixed in 3% paraformaldehyde for 30 min, washed again and stained with Hoechst 33342 at a final concentration of 10 μM for 20 min. Cells were then washed twice with PBS and examined and immediately photographed under a fluorescence microscope (Nikon Corporation, Tokyo, Japan) at an excitation wavelength of 330–380 nm. Apoptotic nuclei of cells were assessed by counting the number of cells that displayed nuclear morphology changes, such as chromatin condensation and fragmentation.
Apoptosis detection by fluorescence-activated cell sorting
GMCs transfected with different plasmids were again stimulated with sublytic C5b-9 for 3 h. Then, the GMCs were harvested and washed twice with PBS, followed by re-suspending in binding buffer containing Annexin-V-APC and propidium iodide. The samples were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA). The percentage of apoptotic cells in a 10 000-cell cohort was determined by flow cytometry. Each sample was assayed in triplicate.
Statistical analysis
Data are presented as mean±SE. One-way analysis of variance was used to determine significant differences among groups with simultaneous multiple comparisons between groups by the Scheffe method. P<0.05 was considered significant. All experiments were repeated at least three times.
Results
ATF3 expression induced by sublytic C5b-9 complexes stimulation
To confirm whether upregulation of ATF3 gene expression is involved in stimulation of sublytic C5b-9, rat GMCs were treated with sublytic C5b-9 complexes for 40 min, 3 h, 6 h, 12 h and 24 h. The level of ATF3 mRNA and protein in the GMCs was measured using real-time PCR and western blot, respectively. The results showed that the mRNA and protein level of ATF3 increased at 40 min after sublytic C5b-9 attack, peaked at 3 h, and then was gradually reduced at 6 and 12 h (Figure 1a and b). To determine the effects of MEM, Thy-1 Ab and human serum and whether C6 is necessary for C5b-9 assembly on the membrane of GMCs, the cultured GMCs were treated with MEM, Thy-1 Ab, Thy-1 Ab+HIS, Thy-1 Ab+C6DS and sublytic C5b-9 for 3 h. ATF3 mRNA and protein in the above-mentioned groups were detected using the previously described methods. ATF3 expression increased at 3 h after exposure to sublytic C5b-9 compared with other control groups (Figure 1c and d). Moreover, the results of immunohistochemical staining showed that ATF3 protein in the sublytic C5b-9-treated group was markedly increased at 3 h and distributed largely in the nuclei of GMCs. In addition, other control groups did not show a positive reaction of ATF3 staining (Figure 1e). Together, these findings suggest that the upregulation of ATF3 expression is due to the assembly of C5b-9 on the GMC membrane, i.e., formation of sublytic C5b-9 complexes.
Figure 1.
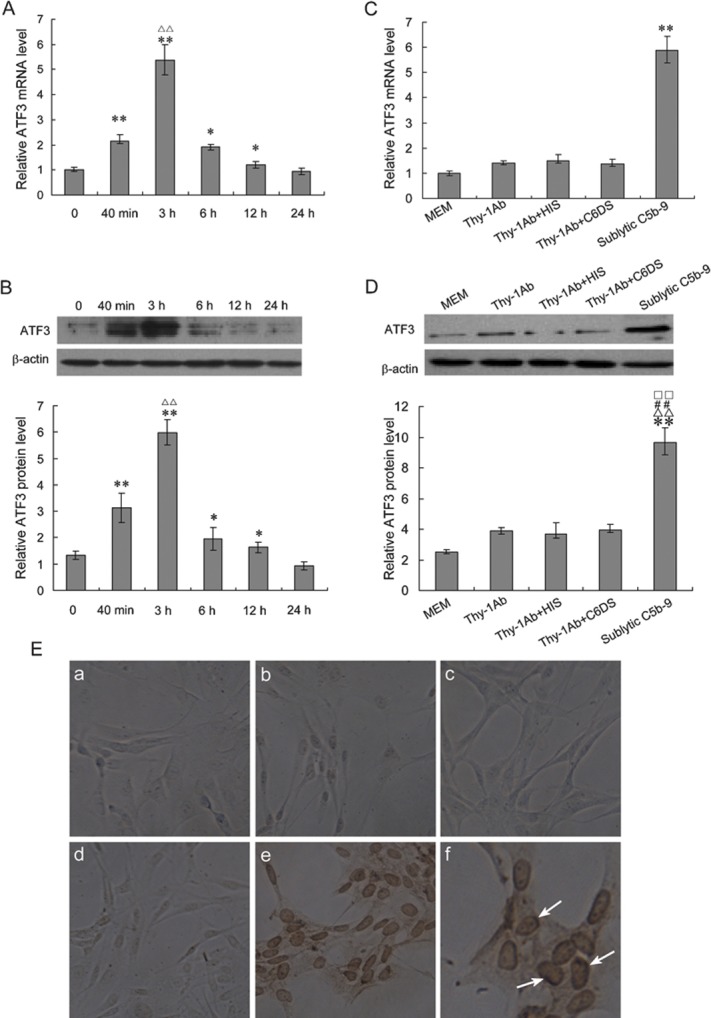
Expression pattern of rat ATF3 mRNA and protein. (a, b) The cultured rat GMCs were treated with sublytic C5b-9 for the indicated time points and analyzed by real-time PCR for ATF3 mRNA (a) or by western blot for ATF3 protein (b). The results showed that the levels of ATF3 mRNA and protein increased at 40 min after sublytic C5b-9 attack, peaked at 3 h, and then gradually reduced at 6 and 12 h (*P<0.05, **P<0.01 vs 0 h, ΔΔP<0.01 vs 40 min). (c, d) The cultured rat GMCs were treated with MEM, Thy-1 Ab, Thy-1 Ab+HIS, Thy-1 Ab+C6DS or sublytic C5b-9 for 3 h. Expression of ATF3 (mRNA and protein) was analyzed by real-time PCR (c) and western blot (d). β-actin was used as the loading control. All data represent averages and mean±SD from three independent experiments. The data showed that ATF3 expression increased at 3 h after exposure to sublytic C5b-9 compared with other control groups (**P<0.01 vs MEM, ΔΔP<0.01 vs Thy-1Ab, □□P<0.01 vs Thy-1 Ab+HIS, and # #P<0.01 vs Thy-1 Ab+C6DS). (e) Rat GMCs were cultured on plastic slides and treated with MEM (i), Thy-1 Ab (ii), Thy-1 Ab+HIS (iii), Thy-1 Ab+C6DS (iv) or sublytic C5b-9 (v) for 3 h then detected by immunohistochemistry using anti-ATF3 antibody and visualized under a microscope ((i)–(v), ×200; (vi), ×400; arrow, positive staining). The results showed that ATF3 protein in the sublytic C5b-9-treated group was markedly increased at 3 h and distributed largely in the nuclei of GMCs, while other control groups did not show a positive reaction of ATF3 staining. The images are representative of three independent experiments. Ab, antibody; ATF3, activating transcription factor 3; GMC, glomerular mesangial cell; HIS, heat-inactivated serum; MEM, modified Eagle's medium; PCR, polymerase chain reaction.
GMC apoptosis induced by sublytic C5b-9 complexes attack
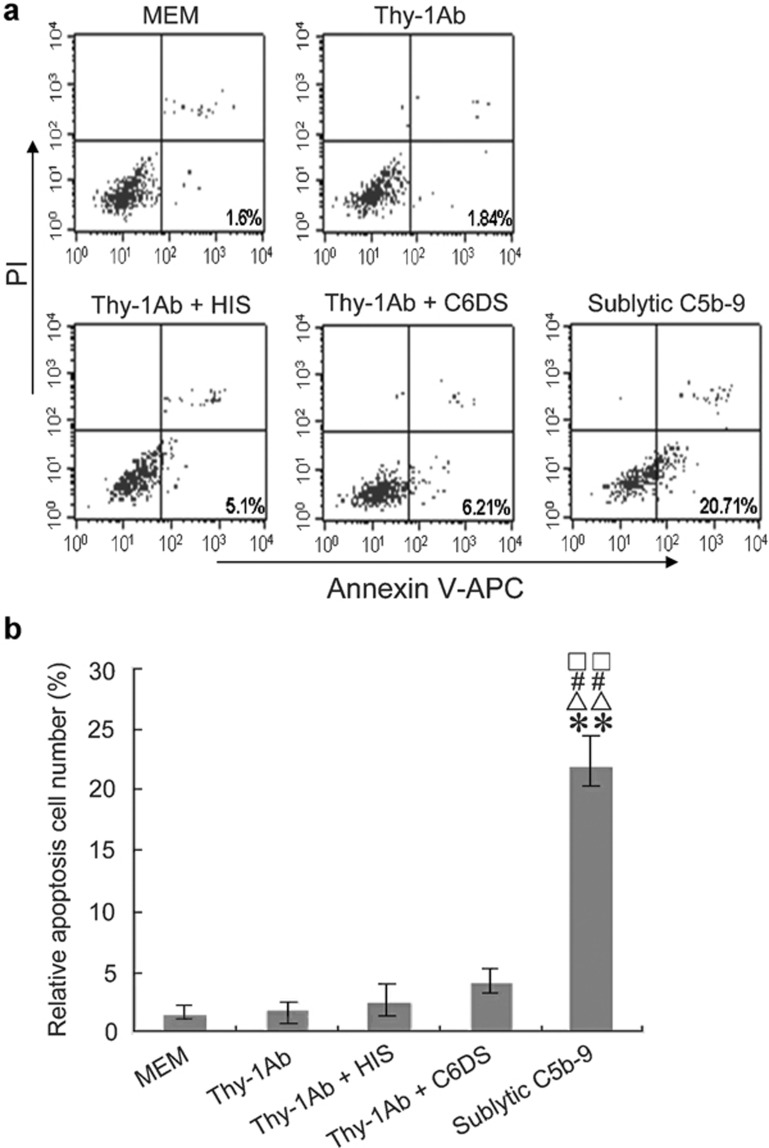
To demonstrate the role of sublytic C5b-9 complexes in GMC apoptosis, the cultured GMCs were divided into MEM, Thy-1 Ab, Thy-1 Ab+HIS, Thy-1 Ab+C6DS and sublytic C5b-9 groups. The percentage of GMC apoptosis in various groups at 3 h after different treatments was measured by flow cytometry. The results show that the percentage of GMC apoptosis increased significantly at 3 h after sublytic C5b-9 attack compared with other control groups (P<0.01), indicating that sublytic C5b-9 stimulation can indeed result in GMC apoptosis (Figure 2).
Figure 2.
Sublytic C5b-9 complexes induced apoptosis of rat GMCs in vitro. (a) The percentage of cultured rat GMC apoptosis in various groups at 3 h after different treatments was measured by flow cytometry. The images are representative of three independent experiments. (b) The data analysis showed that the percentage of GMC apoptosis increased significantly at 3 h after sublytic C5b-9 attack compared with other control groups (**P<0.01 vs MEM, △△P<0.01 vs Thy-1 Ab, □□P<0.01 vs Thy-1 Ab+HIS, # #P<0.01 vs Thy-1 Ab+C6DS). All data represent averages and mean±SD from three independent experiments. Ab, antibody; GMC, glomerular mesangial cell; HIS, heat-inactivated serum; MEM, modified Eagle's medium.
Effects of pcDNA3.1/ATF3 or ATF3 shRNA transfection on ATF3 expression in the GMC apoptosis induced by sublytic C5b-9 attack
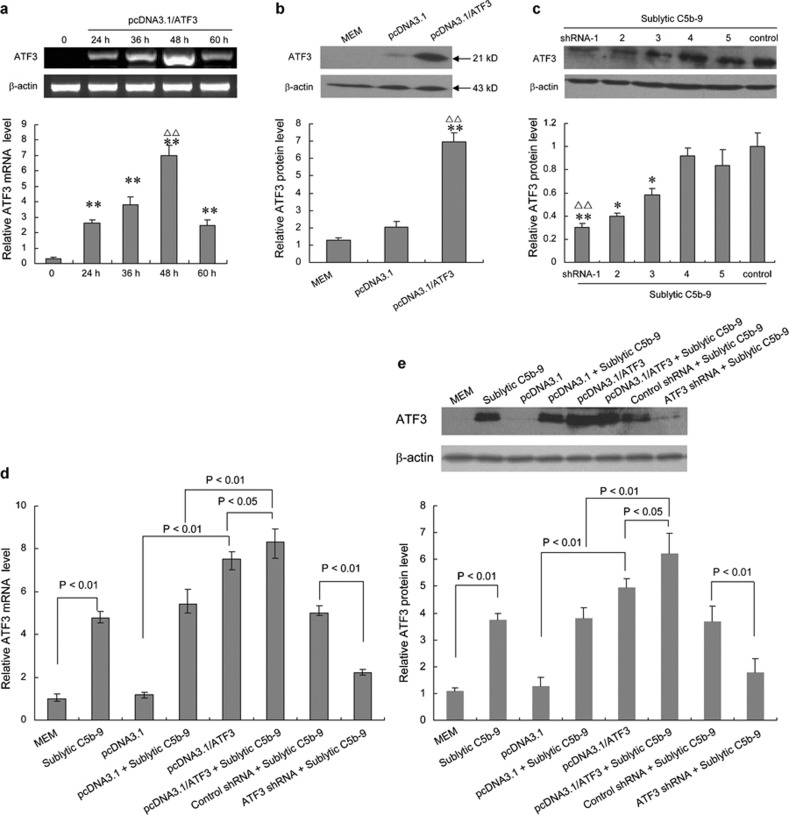
To ascertain the role of pcDNA3.1/ATF3 (pATF3) and ATF3 shRNA transfection in GMCs, the GMCs were first treated with pcDNA3.1/ATF3, pcDNA3.1 (control) or ATF3 shRNAs 1–5 for 48 h before sublytic C5b-9 stimulation again for 3 h. Then, to further explore the effects on ATF3 expression mediated by sublytic C5b-9, the GMCs were also divided into eight groups (MEM, sublytic C5b-9, pcDNA3.1, pcDNA3.1+sublytic C5b-9, pcDNA3.1/ATF3+sublytic C5b-9, control shRNA+sublytic C5b-9 and ATF3 shRNA+sublytic C5b-9). ATF3 expression in the GMCs of various treatments at the indicated time was assessed using RT-PCR or real-time PCR and western blot. The results showed that ATF3 mRNA increased markedly at 40 min and peaked at 48 h after transfection with pcDNA3.1/ATF3, and the level of ATF3 protein was also elevated significantly compared with transfection with pcDNA3.1 or treatment with MEM (Figure 3a and b). In addition, ATF3 expression in the GMCs transfected with ATF3 shRNA-1 plasmid for 48 h before sublytic C5b-9 stimulation for 3 h was obviously less than that of GMCs transfected with other ATF3 shRNAs (such as ATF3 2–5 shRNAs). Therefore, ATF3 shRNA-1 was chosen as the best ATF3-targeting gene in the subsequent experiments (Figure 3c). Correspondingly, the result of ATF3 protein detection revealed that ATF3 expression in the GMCs attacked by sublytic C5b-9 or transfected with pcDNA3.1/ATF3 was markedly increased (P<0.01 vs MEM or pcDNA3.1, respectively), and the level of ATF3 expression in the pcDNA3.1/ATF3+sublytic C5b-9 group was much higher than that of the pcDNA3.1/ATF3 group (P<0.05) or pcDNA3.1+sublytic C5b-9 group (P<0.01). However, ATF3 expression in the ATF3 shRNA+sublytic C5b-9 group was significantly less than that in the control shRNA+sublytic C5b-9 group (P<0.01; Figure 3d–e).
Figure 3.
ATF3 over-expression and knockdown in cultured rat GMCs. (a) The cultured rat GMCs were transfected with pcDNA3.1/ATF3 (pATF3), and at the indicated time points post-transfection, the cells were harvested and ATF3 mRNA levels were analyzed by RT-PCR. Semiquantitative analysis revealed that the ATF3 mRNA level in GMCs 48 h after transfection was significantly higher than that of other time points (**P<0.01 vs 0 h, △△P<0.01 vs 36 h). (b) The cultured rat GMCs were transfected with pcDNA3.1/ATF3 (pATF3) and pcDNA3.1 or MEM (control). At 48 h after transfection, the protein levels of ATF3 were measured by western blot. Semiquantitative analysis revealed that ATF3 protein was significantly increased in the pcDNA3.1/ATF3 transfection group (**P<0.01 vs MEM, △△P<0.01 vs pcDNA3.1). (c) shRNA for ATF3 was synthesized as described in Materials and Methods. shRNAs 1–5 were separately transfected into rat GMCs. After 48 h, the cells were treated with sublytic C5b-9 for another 3 h, and then cell lysates were examined by western blot. Semiquantitative analysis showed that ATF3 protein in the shRNA-1 transfection group was significantly decreased compared to other groups (*P<0.05, **P<0.01 vs control shRNA+sublytic C5b-9, △△P<0.01 vs ATF3 shRNA-2+sublytic C5b-9). (d, e) Rat GMCs were transfected with pcDNA3.1/ATF3, pcDNA3.1, shRNA (shRNA-1), or control shRNA (scrambled gene shRNA) for 48 h and then treated with or without sublytic C5b-9 for 3 h. The total RNA and protein from these cells were prepared, and the expression of ATF3 and β-actin was examined by real-time PCR (d) and western blot (e). The results showed that ATF3 mRNA in the GMCs attacked by sublytic C5b-9 or transfected with pcDNA3.1/ATF3 was markedly increased (P<0.01 vs MEM or pcDNA3.1, respectively), and the level of ATF3 expression in the pcDNA3.1/ATF3+sublytic C5b-9 group was much higher than that of the pcDNA3.1/ATF3 group (P<0.05) or pcDNA3.1+sublytic C5b-9 group (P<0.01), while ATF3 expression in ATF3 shRNA+sublytic C5b-9 was significantly less than in the control shRNA+sublytic C5b-9 group (P<0.01). The analysis of ATF3 protein expression in various groups revealed similar results as that of real-time PCR detection. All data are presented as mean±SD from three independent experiments. ATF3, activating transcription factor 3; GMC, glomerular mesangial cell; MEM, modified Eagle's medium; RT-PCR, reverse transcription polymerase chain reaction; shRNA, small hairpin RNA.
Effects of ATF3 gene over-expression or knockdown on GMC apoptosis induced by sublytic C5b-9 attack
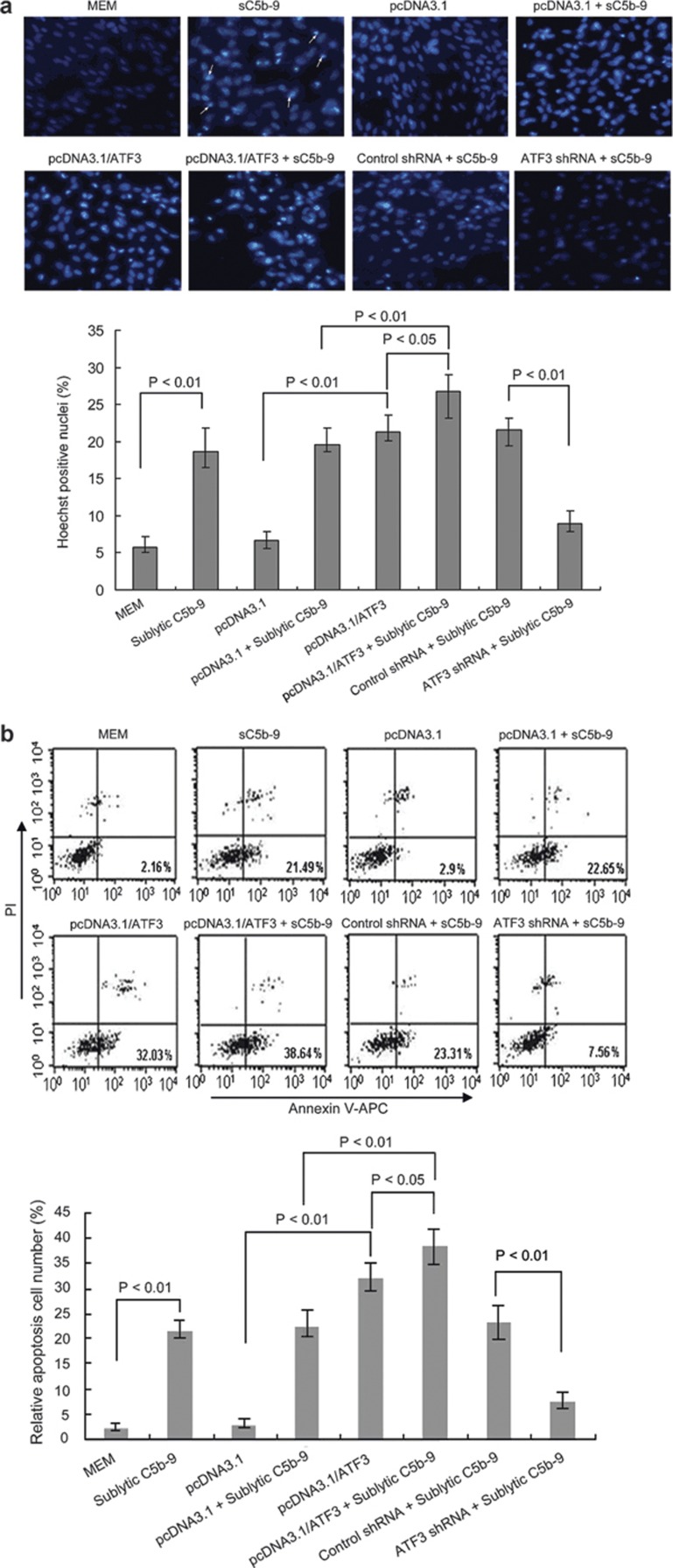
In order to further investigate the biological role of ATF3 in GMC apoptosis upon sublytic C5b-9 attack, GMCs were transfected with full-length ATF3 expression vector (pcDNA3.1/ATF3) or ATF3 shRNA for 48 h and then attacked with sublytic C5b-9 for 3 h. The groups of GMCs of different treatments were the same as the previously described groups (containing the same control groups). The apoptosis of GMCs in various groups was detected by Hoechst 33342 staining and AV-APC/propidium iodide (PI) analysis. The results showed that over-expression of ATF3 could induce GMC apoptosis and increase the percentage of GMC apoptosis mediated by sublytic C5b-9 attack. As shown in Figure 4a, apoptotic nuclei stained by Hoechst 33342 in the pcDNA3.1/ATF3 group or sublytic C5b-9 group were significantly higher than those in the MEM or pcDNA3.1 groups, respectively (P<0.01). Meanwhile, the apoptotic nuclei in the pcDNA3.1/ATF3+sublytic C5b-9 group were also higher than those in the pcDNA3.1/ATF3 (P<0.05) or pcDNA3.1+sublytic C5b-9 group (P<0.01). However, the positive nuclei in the ATF3 shRNA+sublytic C5b-9 group were significantly fewer than those in the control shRNA+sublytic C5b-9 group (P<0.01). In parallel, a similar change in apoptotic percentage in the GMCs with the previously mentioned treatments was found by fluorescence-activated cell sorting (Figure 4b). Collectively, these findings suggest that GMC apoptosis induced by sublytic C5b-9 attack is dependent, in part, on upregulation of ATF3 expression.
Figure 4.
ATF3 increased apoptosis of rat GMCs induced by sublytic C5b-9 (sC5b-9) attack. (a) The cultured rat GMCs were transfected with pcDNA3.1/ATF3, pcDNA3.1, shRNA, or control shRNA for 48 h and then treated with or without sublytic C5b-9 for 3 h. The cells were fixed in 3% paraformaldehyde for 30 min, washed, stained with Hoechst 33342 and examined under a fluorescence microscope. Apoptotic cells were identified as cells with condensed, disrupted nuclei (arrow, Hoechst staining, ×200). Apoptotic cells in at least five random fields were counted. The percentage of apoptotic nuclei was calculated with the following formula: 100×number of apoptotic/total counted cell number (%). At least 400 apoptotic cells were counted for the ATF3-positive staining groups. The data showed that Hoechst 33342-stained cells in the pcDNA3.1/ATF3 group or sublytic C5b-9 group were significantly higher than those in the MEM or pcDNA3.1 group, respectively (P<0.01). Meanwhile, Hoechst 33342-stained cells in the pcDNA3.1/ATF3+sublytic C5b-9 group were also higher than those in the pcDNA3.1/ATF3 (P<0.05) or pcDNA3.1+sublytic C5b-9 group (P<0.01). However, the positive cells in the ATF3 shRNA+sublytic C5b-9 group were less than that in the control shRNA+sublytic C5b-9 group (P<0.01). The result is representative of three independent experiments. (b) Differently treated GMCs were stained with Annexin V/APC and PI and analyzed by flow cytometry. Percentages of early apoptotic cells (Annexin V positive, PI negative) were calculated. In parallel, similar changes in the apoptosis percentage in the GMC groups as the above-mentioned treatments by Hoechst 33342 staining were found. The displayed result is representative of three independent experiments. APC, allophycocyanin; ATF3, activating transcription factor 3; GMC, glomerular mesangial cell; MEM, modified Eagle's medium; PI, propidium iodide; shRNA, small hairpin RNA.
Discussion
Rat Thy-1N is an acknowledged animal model for studying human MsPGN.1, 22 Administration of anti-Thy-1 antibody, which binds to the Thy-1 antigen on the membrane of rat GMCs, can activate the complement system, leading to GMC injury.23, 24 It has been reported that GMC lesions of Thy-1N is complement dependent, but neutrophil independent,6, 24 and pathologic changes in apoptosis/necrosis and proliferation of GMCs appear in rat Thy-1N.12 Because GMC apoptosis in the early stage of Thy-1N is considered a contributor to the initiation of Thy-1N, recent published studies have focused on GMC apoptosis in the early phase of rat Thy-1N.11, 13 Nevertheless, the molecular mechanism responsible for GMC apoptosis of Thy-1N is currently not well elucidated.
It is well known that cell apoptosis is usually associated with the expression of apoptosis-related genes, including some transcription factor genes, i.e., ATF3.25, 26 ATF3 is a member of the ATF/CREB subfamily of the basic region leucine zipper family.27 Emerging evidence has demonstrated that ATF3 expression can be induced by a variety of physiological stimuli and pathologic stress signals.28, 29 Early literature has shown that ATF3 could play a role in cell apoptosis or proliferation, and the diversity in the final readouts is most likely determined by the context of the cells.30, 31, 32
Our previous studies have demonstrated that GMC apoptosis, necrosis and proliferation initially involved in Thy-1N development,7, 10, 13 and ATF3 expression in the apoptotic GMCs of Thy-1N and the cultured GMCs attacked with sublytic C5b-9 was increased by microarray analysis (data not shown). In light of earlier reports suggesting that GMC injury in Thy-1N is complement dependent (especially C5b-9 complexes) and sublytic C5b-9 is generally regarded as the principal mediator of GMC lesions in Thy-1N,6, 13 in the present study, ATF3 was selected as a candidate gene to first explore its expression in the GMC apoptosis mediated by sublytic C5b-9 stimulation in vitro.
Given that upregulation of ATF3 expression was revealed by microarray and to further confirm these results, the levels of both ATF3 mRNA and protein in the GMCs attacked with sublytic C5b-9 were measured by real-time PCR, western blot and immunohistochemistry. The results showed that ATF3 expression in the GMCs after sublytic C5b-9 exposure was markedly increased, and the number of GMCs upon sublytic C5b-9 attack was also elevated at the same time, suggesting that sublytic C5b-9 complexes could indeed induce ATF3 production and GMC apoptosis.
It has been reported that complement C5b-9 complexes in sublytic concentrations can induce a time-dependent activation of mitogen-activated protein kinase extracellular signal-regulated kinase and result in aortic smooth muscle cell or endothelial cell proliferation.33, 34 Sublytic C5b-9 assembly on the plasma membrane was also able to activate Janus kinase 1, signal transducer and activator 3 and signal transducer and activator 4 in endothelial cells.34 Our experiments have revealed that sublytic C5b-9 can trigger activation of the PI3-k/Akt signal pathway in GMCs, causing activation of some transcription factors, i.e., early growth response-1 (data not shown), and release of inflammatory mediators (i.e., nitric oxide) or cytokines (i.e., tumor-necrosis factor-α.10, 21 Because ATF3 is a nuclear transcription factor induced by stress signals,28, 35, 36 upregulation of ATF3 expression in the cultured GMCs may be due to the activation of some signal pathways via sublytic C5b-9 stimulation.
As for the role of ATF3 expression in GMC apoptosis induced by sublytic C5b-9 attack, our studies confirmed that over-expression of ATF3 protein in the GMCs transfected with the pcDNA3.1/ATF3 plasmid could markedly enhance sublytic C5b-9-induced GMC apoptosis. By contrast, knockdown of the ATF3 gene with specific ATF3 shRNA in advance could significantly decrease the percentage of GMC apoptosis in response to sublytic C5b-9 attack. Taken together, these results show that sublytic C5b-9 complexes can indeed induce GMC apoptosis, which is partially dependent on upregulation of ATF3 gene expression, suggesting that ATF3 may be an important factor in regulating GMC apoptosis of rats with Thy-1N.
Our and other studies7, 11, 13 have demonstrated that GMC undergoes apoptosis, necrosis and proliferation during the progression of Thy-1N, and sublytic C5b-9 complexes not only cause GMC apoptosis or necrosis7, 13 but also result in GMC proliferation.10, 21 The different changes in GMC damage may be associated with the dose and time of complement C5b-9 complex stimulation or cell age, and activity and number of homologous restriction factors on the GMC surface. In addition, the fragments of some GMCs that undergo apoptosis may be a stimulus to other undamaged GMCs and lead to the release of inflammatory mediators or cytokines from GMCs or infiltrating macrophage phagocytosis, causing secondary cell proliferation. Thus, it is possible that inhibition of GMC apoptosis in rats with Thy-1N, i.e., knockdown of the ATF3 gene, would decrease GMC secondary injury of Thy-1N, but further studies in vitro and in vivo are needed to demonstrate this in the future.
Acknowledgments
The authors thank Dr Jianmin Li (Nanjing Medical University, Nanjing, China) for providing the pcDNA3.1 plasmid. This work was supported by grants from the National Natural Science Foundations of China (No. 30772016), the Ministry of Education of China (No. 20060312005), and Jiangsu Provincial Department of Education (No. KJB310004) as well as Nanjing Medical University (No. 08NMUZ002 and No. 09JC007).
References
- Barratt J, Feehally J, Smith AC. Pathogenesis of IgA nephropathy. Semin Nephrol. 2004;24:197–217. doi: 10.1016/j.semnephrol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Turrnberg D, Cook HT. Complement and glomerulonephritis, new insight. Curr Opin Nephrol Hypertens. 2005;14:223–228. doi: 10.1097/01.mnh.0000165887.75501.24. [DOI] [PubMed] [Google Scholar]
- Stangou M, Alexopoulos E, Pantzaki A, Leonstini M, Memmos D. C5b-9 glomerular deposition and tubular α3β1-integrin expression are implicated in the development of chronic lesions and predict renal function outcome in immunoglobulin A nephropathy. Scand J Urol Nephrol. 2008;42:373–380. doi: 10.1080/00365590801943241. [DOI] [PubMed] [Google Scholar]
- Wang H, Jiang XM, Xu JH, Xu J, Tong JX, Wang YW. The profile of gene expression and role of nuclear factor kappa B on glomerular injury in rats with Thy-1 nephritis. Clin Exp Immunol. 2008;152 3:559–567. doi: 10.1111/j.1365-2249.2008.03654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki M. The presence of Thy1.1 antigen in rat glomerular mesangial cells. Allergy. 1980;29:816–819. [Google Scholar]
- Brandt J, Pippin J, Schulze M, Hänsch GM, Alpers CE, Johnson RJ, et al. Role of the complement membrane attack complex (C5b-9) in mediating experimental mesangiopreoliferative glomerulonephritis. Kidney Int. 1996;49:335–343. doi: 10.1038/ki.1996.50. [DOI] [PubMed] [Google Scholar]
- Wang Y, He Q, Qin H, Xu J, Tong J, Gao L. The complement C5b-9 complexes induced injury of glomerular mesangial cells in rats with Thy-1 nephritis by increasing nitric oxide synthesis. Life Sci. 2006;79:182–192. doi: 10.1016/j.lfs.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Niculecu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24:191–195. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- Soane L, Cho HJ, Niculescu F. C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J Immunol. 2001;167:2305–2311. doi: 10.4049/jimmunol.167.4.2305. [DOI] [PubMed] [Google Scholar]
- Gao LJ, Qiu W, Wang Y, Xu W, Xu J, Tong J. Sublytic complement C5b-9 complexes induce thrombospondin-1 production in rat glomerular mesangial cells via PI3-k/Akt, association with activation of latent transforming growth factor-β1. Clin Exp Immunol. 2006;144:326–334. doi: 10.1111/j.1365-2249.2006.03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Ohashi R, Sugisaki Y, et al. Complement-mediated killing of mesangial cells in experimental glomerulonepphritis, cell death by a combination of apoptosis and necrosis. Nephron. 2000;86:152–160. doi: 10.1159/000045734. [DOI] [PubMed] [Google Scholar]
- Xu JH, Qiu W, Wang YW, Xu J, Tong JX, Gao LJ, et al. Gene expression profile and overexpression of apoptosis-related genes (NGFI-B and Gadd 45γ) in early phase of Thy-1 nephritis model. Cell Tissue Res. 2006;326:159–168. doi: 10.1007/s00441-006-0214-4. [DOI] [PubMed] [Google Scholar]
- Qiu W, Che N, Feng X, Xia M, Wang H, Zhao D, et al. Apoptosis of glomerular mesangial cells induced by sublytic C5b-9 complexes in rats with Thy-1 nephritis is dependent on Gadd45γ upregulation. Eur J Immunol. 2009;39:3251–3266. doi: 10.1002/eji.200939264. [DOI] [PubMed] [Google Scholar]
- Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, and regulation. J Biol Chem. 1996;271:1695–1701. doi: 10.1074/jbc.271.3.1695. [DOI] [PubMed] [Google Scholar]
- Li D, Yin X, Zmuda EJ, Wolford CC, Dong X, White MF, et al. The repression of IRS2 gene by ATF3, a stress-inducible gene, contributes to pancreatic beta-cell apoptosis. Diabetes. 2008;57:635–644. doi: 10.2337/db07-0717. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Sugiura H, Mitobe M, Tsuchiya K, Shirota S, Nishimura S, et al. ATF3 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:217–224. doi: 10.1681/ASN.2005111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Yuan Z, Song B, Li D, Ma C, Hu C, et al. Activating transcription factor 3 up-regulated by c-Jun NH(2)-terminal kinase/c-Jun contributes to apoptosis induced by potassium deprivation in cerebellar granule neurons. Neuroscience. 2008;151:771–779. doi: 10.1016/j.neuroscience.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- Downward J. Cell biology: metabolism meets death. Nature. 2003;424:896–897. doi: 10.1038/424896a. [DOI] [PubMed] [Google Scholar]
- Couser WG, Pippin JW, Shankland SJ. Complement (C5b-9) induces DNA synthesis in rat GMCs in vitro. Kidney Int. 2001;59:905–912. doi: 10.1046/j.1523-1755.2001.059003905.x. [DOI] [PubMed] [Google Scholar]
- Gao L, Zhang Y, Qiu W, Xu W, Feng X, Ren J, et al. Effects of PI3-k/Akt short hairpin RNA on proliferation, fibronectin production and synthesis of thrombospondin-1 and transforming growth factor-beta1 in glomerular mesangial cells induced by sublytic C5b-9 complexes. Cell Prolif. 2009;42:83–93. doi: 10.1111/j.1365-2184.2008.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill AM, Ballardie FW. Mesangial autoantigens in IgA nephropathy: matrix synthesis and localization. J Lab Clin Med. 2006;147:301–309. doi: 10.1016/j.lab.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Wilson CB. Complement dependence of antibody-induced mesangial cell injury in the rat. J Immunol. 1987;138:3758–3765. [PubMed] [Google Scholar]
- Bohana-Kashtan O, Ziporen L, Donin N, Kraus S, Fishelson Z. Cell signals transduced by complement. Mol Immunol. 2004;41:583–597. doi: 10.1016/j.molimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Fan F, Jin S, Amundson SA, Tong T, Fan W, Zhao H, et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21:7488–7496. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J Biol Chem. 2008;283:29795–29801. doi: 10.1074/jbc.M802515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Dewille JW, Hai T. A potential dichotomous role of ATF3, an adaptive-response gene, in cancer development. Oncogene. 2008;27:2118–2127. doi: 10.1038/sj.onc.1210861. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CG, Woods A, Underhill TM, Beier F. The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription. BMC Mol Biol. 2006;378:53–62. doi: 10.1186/1471-2199-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Liu YC, Shyu KG, Wang DL. Acute hypoxia to endothelial cells induces activating transcription factor 3 (ATF3) expression that is mediated via nitric oxide. Atherosclerosis. 2008;201:281–288. doi: 10.1016/j.atherosclerosis.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Mungrue IN, Pagnon J, Kohannim O, Gargalovic PS, Lusis AJ. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J Immunol. 2009;182:466–476. doi: 10.4049/jimmunol.182.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30:73–80. doi: 10.1385/IR:30:1:073. [DOI] [PubMed] [Google Scholar]
- Fosbrink M, Niculescu F, Rus H. The role of C5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol Res. 2005;31:37–46. doi: 10.1385/IR:31:1:37. [DOI] [PubMed] [Google Scholar]
- Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen- activated protein kinase. Biochem J. 2007;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi L, Fareh M, Aberdam E, Kitajima S, Simpson F, Wicking C, et al. ATF3 and p15PAF are novel gatekeepers of genomic integrity upon UV stress. Cell Death Differ. 2009;16:728–737. doi: 10.1038/cdd.2009.2. [DOI] [PubMed] [Google Scholar]