Abstract
Age-related thymic involution causes a decreased output of thymocytes from the thymus, thereby resulting in impairment of T cell-mediated immunity. While alterations in the T cell and non-haematopoietic stromal compartments have been described, the effects of thymic involution on thymic dendritic cells (DC) are not clearly known. Thymic DC play an essential role in shaping T cell-mediated immune responses by deleting self-reactive thymocytes to establish central tolerance and by inducing regulatory T-cell (Treg) development. It is therefore important to assess the prevalence of and alterations to thymic DC with age, as this may impact on their function. We assessed the numbers and proportions of the three distinct subsets of thymic DC in ageing mice, and showed that these subsets are differentially regulated. This is expected as thymic DC subsets have different origins of development. We further assessed the responses of thymic DC in a regenerative environment, such as that induced by sex-steroid ablation (SSA), and clearly showed that, consistent with global thymus regrowth, all three DC populations increased in numbers and regained their relative proportions to thymocytes after an initial lag period. These findings are important for the clinical translation of thymic regenerative approaches, and indicate that SSA facilitates the maintenance of critical processes such as negative selection and Treg induction through promoting thymic DC regeneration.
Keywords: ageing, dendritic cells, thymic regeneration
Introduction
The thymus has a central role in the deterioration of the immune system with age due to its natural involution.1 Some thymic decline is initially apparent from as early as the first year in humans, but then thymus undergoes more pronounced degeneration from puberty such that by ∼25 years of age, the thymus has decreased to approximately 50% of its size at birth progressing through to ∼10% capacity by the fifth and sixth decades.2 While the mechanisms of thymic involution have not been precisely defined, there is a clear correlation with the influence of sex steroids, the removal of which reverses thymic atrophy in animal models.1, 3, 4, 5, 6, 7, 8 The progressive decrease in thymus size with age is associated with a loss in thymic epithelial cells and a concomitant decrease in thymopoiesis9 leading to a reduced thymic output of naive T cells.8, 10, 11, 12 Although homeostatic proliferation ensures that the number of T cells in the periphery is maintained, the T-cell receptor repertoire is decreased due to greater clonal expansion of fewer thymic exported T cells.13, 14 The T-cell population becomes disproportionately high for memory T cells as the naive T cells become progressively exposed to environmental antigens.15, 16, 17 Thus, attrition of the thymus contributes to the impairment of T cell-mediated immunity seen in the aged population and in patients recovering from chemotherapy or suffering from immunoablative diseases such as HIV. Full immune recovery is dependent on high thymic output of naive T cells to replenish the peripheral pool.18 Consequently, there is considerable clinical interest in developing strategies to improve immune reconstitution, one of which is to regenerate the involuted aged or damaged thymus (reviewed in Ref. 19).
The inhibition of sex steroids has a dramatic impact on reversing the age-related degeneration of the thymus. Clinically, a reversible reduction in sex steroids is achieved by the agonist variants of luteinising hormone releasing hormone (reviewed in Ref. 20). In mouse models sex-steroid ablation (SSA) can be achieved through surgical or chemical castration (reviewed in Refs. 4 and 20, 21, 22), which in both cases results in the regeneration of the thymus and thymopoiesis, thereby increasing the number of naive T cells and providing a more diverse T-cell receptor repertoire in the periphery. Following SSA in male mice, there was an improvement in immune reconstitution in young (4–6 weeks), adult (3 months), middle-aged (9 months) and aged (18–24 months) mice in several immunocompromised models.1, 8, 23, 24 Increased proliferation was evident in early thymocyte subsets such that by 14 days post-castration, the aged thymus resembled a young thymus in cellularity.8, 23, 24, 25 SSA also induces changes outside the thymus with increases in immature cell types and lymphoid progenitors, such as haematopoietic stem cells and Lin−Sca-1+c-Kit+ cells, which are evident in the bone marrow. This leads to an increase in all immature B-cell subsets23, 24 and also likely contributes to the increase along the developmental pathway of thymocytes.8, 24, 26
Little is known about the response of dendritic cells (DC) in this environment. It is important to determine potential changes in the distribution or activation phenotype of DC as these cells play an important role in thymic education, particularly in negative selection and the induction of T regulatory cells (Tregs).27, 28 These processes could potentially be disrupted in the regenerative thymus with immunological consequences, particularly with regard to self-tolerance and autoimmunity. In the steady-state adult thymus, DC are a rare population of cells (around 0.5% of total thymus cells).29 Three major subsets of DC have been identified in the mouse thymus, a plasmacytoid DC (pDC) subset defined as CD11cintCD45RA+ and two conventional DC (cDC) subsets, namely, a major population defined as CD11c+CD45RA− signal-regulatory protein-α negative (Sirpα−)CD8α+CD11b− DC (CD8α+ cDC) and a minor population defined as CD11c+CD45RA−Sirpα+CD8α−CD11b+ DC (Sirpα+ cDC).29, 30, 31 The CD8α+ cDC develop in parallel with T-lineage cells from an intrathymic precursor and have been suggested to play a role in the selection of these developing thymocytes, whereas the Sirpα+ cDC enter the thymus from the bloodstream.29, 32, 33 Thymic cDC play an important role in negative selection.34, 35, 36, 37, 38 The Sirpα+ cDC may function to induce negative selection to peripheral antigens39 and the development of antigen-specific CD4+CD25+Foxp3+ Tregs from CD4+ thymocytes.28 The function of the pDC in the thymus is not clearly defined.
To date, little is known about the effects of ageing on the distinct DC populations found within the mouse thymus. It is important to characterise the age-related changes in cell number and relative incidence for each of the distinct thymic DC populations, as these DC populations mediate different functions in thymocyte development. It is also important to determine the changes induced within these DC populations in the regenerative thymus following SSA, as DC are important in the selection of regenerating thymocytes and maintaining immune balance.
Materials and methods
Mice
C57BL/6 mice were bred under specific pathogen-free conditions at the Walter and Eliza Hall Institute animal breeding facility or obtained from the University of Adelaide (Adelaide, SA, Australia) and the Animal Resource Centre (Perth, WA, Australia) and maintained at Mouseworks, Monash University (Melbourne, Vic., Australia). Young mice were defined as 8–12 weeks old, adult mice as 4–6 months old, middle-aged mice as 8–12 months old and aged mice as 16–24 months old.
Surgical castration
Male mice were anaesthetised and a small scrotal incision was made. The testes were sutured and removed, and then the wound was closed using surgical staples. Mice in surgical stress control groups underwent sham castration, wherein the same surgical procedure was followed but without removing the testes. Surgical procedures were performed according to the Animal Ethics Committee guidelines and approval from the Monash University Animal Welfare Committee.
DC enrichment
Cell preparations from spleen and thymus were performed as described in detail elsewhere.30, 40 Briefly, organs were finely minced and digested at room temperature in collagenase (type III, Worthington Biochemical Corp., Freehold, NJ, USA) and DNAse (grade II bovine pancreatic DNAseI, Boehringer Mannheim, Mannheim, Germany). EDTA (5 mM) was then added to improve the release of DC. DC-enriched light density fractions were then obtained by centrifugation of cells in Nycodenz of density 1.076 g/cm3 for thymus preparations and 1.077 g/cm3 for spleen preparations.
Flow cytometry analysis
Antibodies were purified and conjugated to fluorochromes as previously described.30, 40, 41 To analyse DC subsets, cells were labelled with anti-CD11c-PECy7, anti-CD45RA-PE, anti-CD8α-APCCy7 and anti-Sirpα-APC. Conventional DC were defined as CD11c+CD45RA− and further subdivided into the CD8α+ and Sirpα+ subsets. Plasmacytoid DC were defined as CD11cint and CD45RA+. The activation levels of the DC subsets were assessed by staining with anti-CD40-FITC, anti-CD80-FITC, anti-CD86-FITC, anti-MHC class II-FITC and anti-CD69-FITC. In the whole organ-cell preparations, B cells were analysed by staining with anti-CD19-FITC and anti-CD45R-APC, T cells were analysed by staining with anti-CD8α-FITC or anti-CD4-PE and Tregs were analysed by staining with both anti-CD4-PE and anti-CD25-APC. Propidium iodide was added in the final wash to differentiate dead cells. Flow cytometric analysis was performed on a LSR II flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
Significance was determined using the Mann–Whitney non-parametric test.
Results
Changes in DC subsets in ageing mice
The CD8α+ and Sirpα+ cDC subsets and the pDC (Figure 1a) were monitored to assess changes in their proportions and phenotypes in ageing mice. Mice were defined as young (8–12 weeks old), adult (4–6 months old), middle-aged (8–12 months old) and aged (16–24 months old). As expected, the cellularity of the thymus decreased markedly as the mice aged9 (Figure 1b). Accordingly, it was important to assess changes in DC populations in terms of their percentages (or proportions within the organ) as well as their total cell numbers.
Figure 1.
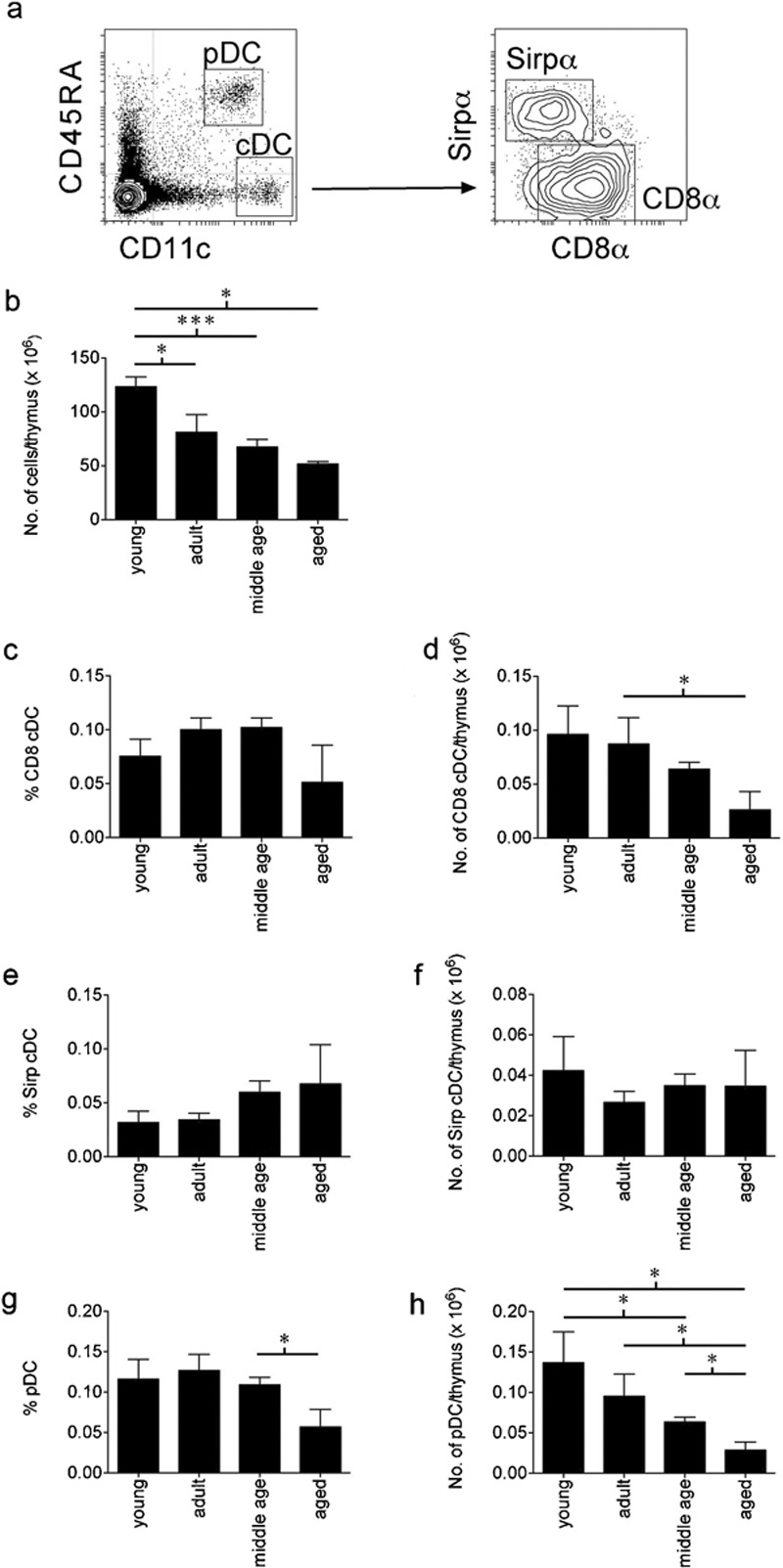
Changes in the cellularity and percentages of the thymic DC subsets within the ageing thymus. (a) The thymic DC subsets were defined by flow cytometric analysis as pDC or cDC based on expression of CD11c and CD45RA. The cDC were further defined as CD8α+ cDC or Sirpα+ cDC based on expression of Sirpα or CD8α. Thymuses of young (8–12 weeks), adult (4–6 months), middle-aged (8–12 months) and aged (16–24 months) C57BL/6 mice were assessed for (b) the total number of thymocytes, (c, e, g) the percentage of each DC subset comprising the total thymocyte population and (d, f, h) the numbers of cells within each DC subset. For each age group of mice, data are shown as the pooled results of several separate experiments where n=9 for young mice, n=10 for adult mice, n=25 for middle-aged mice and n=14 for aged mice. Data are represented as mean±SEM. cDC, conventional dendritic cells; DC, dendritic cells; pDC, plasmacytoid dendritic cells; Sirpα, signal-regulatory protein-α.
Although the total numbers of the CD8α+ cDC and pDC were reduced as the mice aged (Figure 1d and h), this parallelled the loss of total cells so there was no significant change in the proportion of these cells in the thymus of middle-aged mice (Figure 1c and g). However, in aged mice there appeared to be a marked reduction of CD8α+ cDC and pDC in both cell proportion and cell number. In contrast, the Sirpα+ cDC subset showed no change in the number of cells present in the ageing thymus, which translated into an increased proportion of these cells in middle-aged and aged mice (Figure 1e and f).
These three DC subsets are also present in mouse spleen, and potential changes in the DC populations due to ageing were also assessed in this organ. In the spleen the distribution of DC subsets differs from that of the thymus with Sirpα+ cDC representing the majority of cDC, and CD8α+ cDC representing the minority (Figure 2a). In contrast to the thymus, the spleen increases significantly in size as mice age (Figure 2b). Hence, although the CD8α+ cDC and pDC subsets did not change in cell numbers in ageing mice (Figure 2d and h), due to the increase in spleen cellularity they were present at a reduced proportion in aged mice (Figure 2c and g). Similarly, although a significant reduction in the proportion of Sirpα+ cDC subset was observed in aged mice, the cell number was only slightly reduced (Figure 2e and f).
Figure 2.
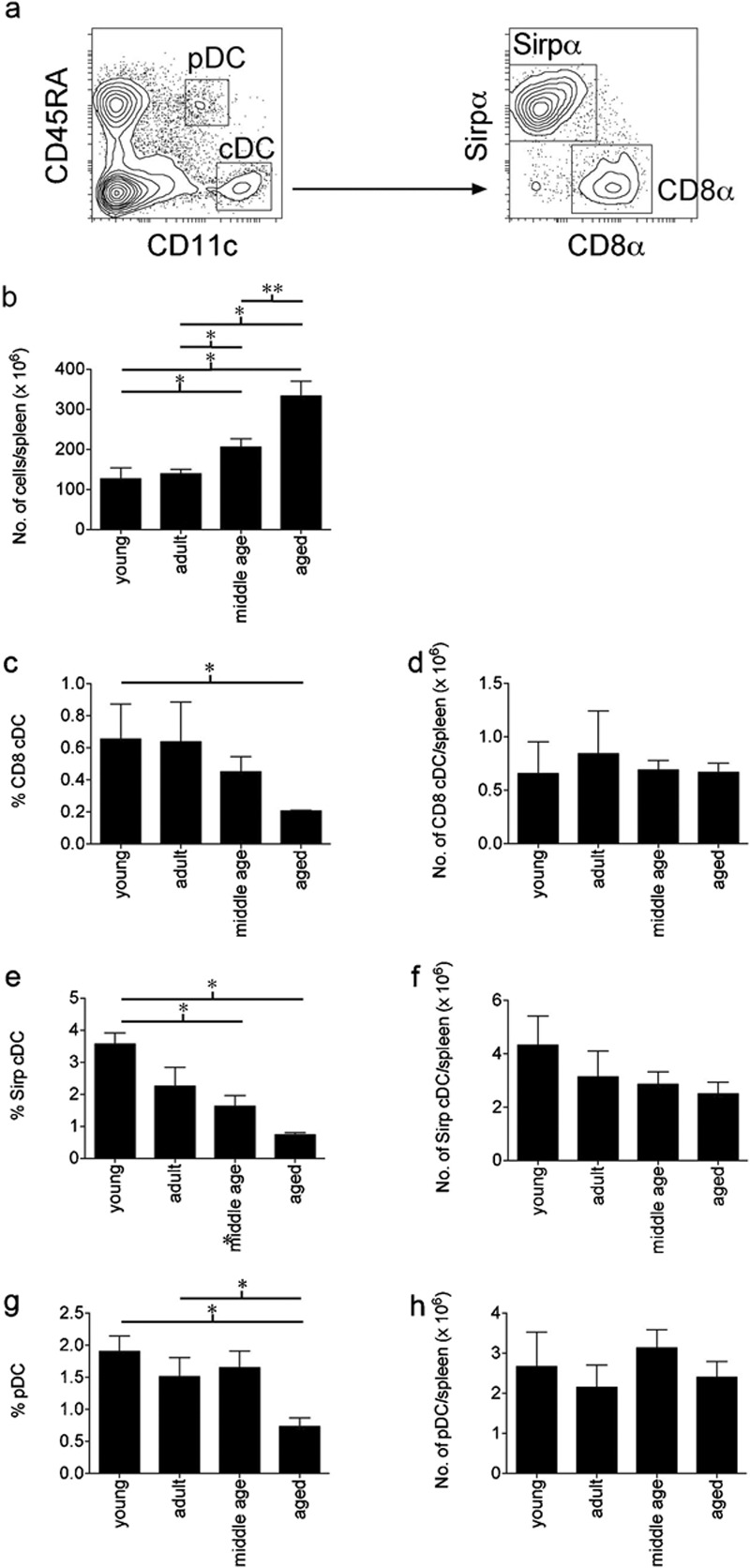
Changes in the cellularity and percentages of the splenic DC subsets within the ageing spleen. (a) The splenic DC subsets were defined by flow cytometric analysis as pDC or cDC based on expression of CD11c and CD45RA. The cDC were further defined as CD8α+ cDC or Sirpα+ cDC based on expression of Sirpα or CD8α. Spleens of young (8–12 weeks), adult (4–6 months), middle aged (8–12 months) and aged (16–24 months) C57BL/6 mice were assessed for (b) the total number of splenocytes, (c, e, g) the percentage of each DC subset comprising the total splenocyte population and (d, f, h) the numbers of cells within each DC subset. For each age group of mice, data are shown as the pooled results of several separate experiments where n=9 for young mice, n=10 for adult mice, n=25 for middle-aged mice and n=14 for aged mice. Data are represented as mean±SEM. cDC, conventional dendritic cells; DC, dendritic cells; pDC, plasmacytoid dendritic cells; Sirpα, signal-regulatory protein-α.
Furthermore, we determined the levels of surface expression on the DC subsets of molecules involved in DC activation and function, specifically the costimulatory molecules CD40, CD80 and CD86 and the antigen-presenting molecule MHC class II.42, 43, 44, 45 The CD8α+ cDC and Sirpα+ cDC subsets did not show an altered activation phenotype in young, adult and middle-aged mice, in either organ (data not shown). Although the thymic pDC did not change their activation phenotype, the splenic pDC subset demonstrated a more activated phenotype in middle-aged mice with upregulated expression of CD40, CD80, CD86 and MHC class II (Figure 3).
Figure 3.
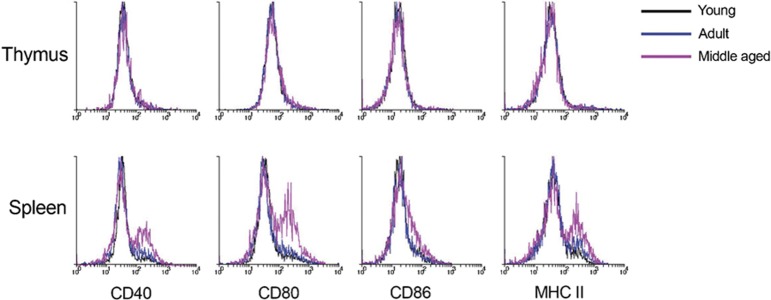
Expression levels of activation molecules and MHC class II on pDC in the thymus and spleen of ageing mice. DC-enriched light density fractions of splenic and thymic cell preparations from pooled organs were analysed by flow cytometric analysis. DC, dendritic cells; pDC, plasmacytoid dendritic cells.
All DC subsets increased in number during thymic regeneration
To examine whether DC populations can also be restored to the levels of young mice after surgical castration, the numbers of thymic and splenic DC populations were determined in castrated adult and middle-aged mice at 2, 4 and 6 weeks post-castration (Cx).
Increases in total thymic cellularity were assessed as an overall measure of regeneration. The cellularity of the thymuses of 5- and 10-month-old mice increased significantly for at least 6 weeks post-Cx, with a rapid increase seen at 2 weeks post-Cx (Figure 4a). Total organ cellularity observed in the control, sham-Cx mice was similar to that seen in age-matched wild-type mice (Figure 4a), indicating that stress associated with the sham-Cx operation had minimal impact on the thymus. Increased cellularity, to a smaller extent, was also seen in the spleens of castrated mice, and was sustained for at least 6 weeks post-Cx for mice of both age groups (Figure 4b).
Figure 4.
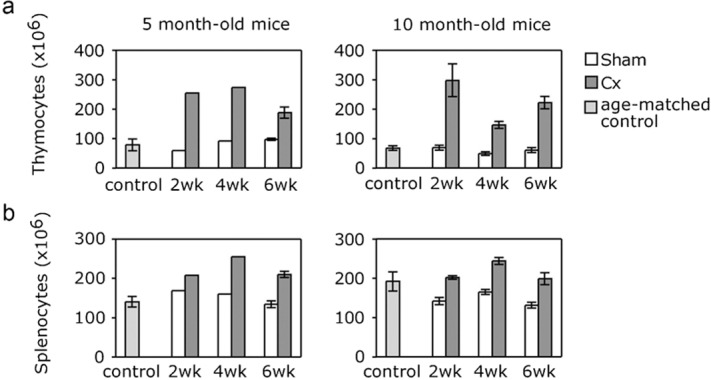
Increasing cell numbers in the regenerating thymus, and spleen, following surgical castration. Total cell numbers were determined for (a) thymus and (b) spleen at 2, 4 and 6 weeks post-castration, in male mice castrated or sham-castrated at 5 or 10 months of age. For 5-month-old mice data were determined from pooled samples of four organs, whereas for 10-month-old mice data were determined from individual samples (n=8) and represented as mean±SEM. As a control total organ cellularity for age-matched male mice was also included.
Importantly, the DC populations also responded to castration by increasing numbers, although the three thymic DC subsets had different kinetics of regeneration which was age-dependent. The two cDC subsets increased in numbers by more than twofold at 2 weeks post-Cx in both the adult and middle-aged mice, relative to sham-Cx mice (Figure 5a). However, by 4 weeks post-Cx, cell numbers of the cDC subsets were already decreased and were closed to sham-Cx levels at week 6 post-Cx. The increases in numbers of Sirpα+ and CD8α+ cDC at 2 weeks were not sufficient to maintain the proportions of these cells at sham-Cx levels in both adult and middle-aged mice, due to the significant increase in thymus cellularity (Figure 5b). The decrease in cDC proportion was maintained in adult mice until 4 weeks post-Cx, due to the elevated thymic cellularity seen in these mice, and the proportion of cDC subsets attained normal sham-Cx levels at 6 weeks post-Cx. In middle-aged mice the proportion of the cDC subsets returned to sham-Cx levels at 4 weeks post-Cx, yet the proportion of these cells in the thymus dropped well below normal levels at 6 weeks post-Cx.
Figure 5.
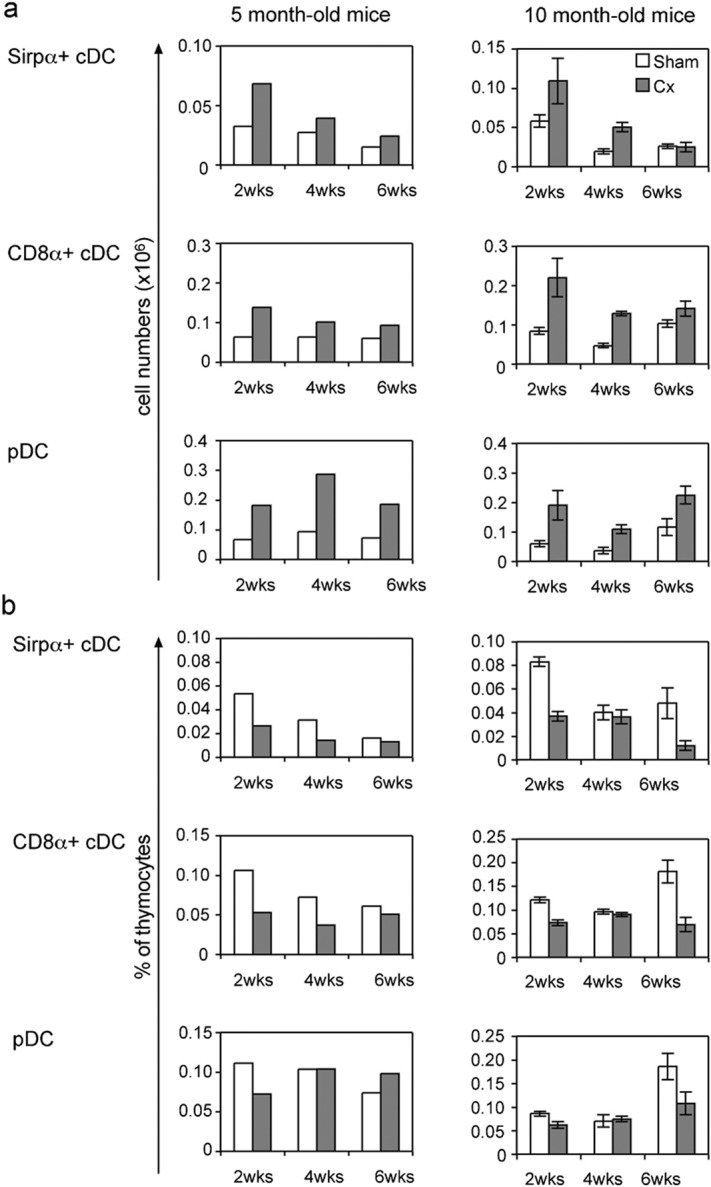
Changes in percentage and cell number of dendritic cell subsets within the thymus of castrated 5- and 10-month-old mice. The dendritic cell subsets are presented as (a) the total number of cells and (b) a percentage of thymocytes per thymus at 2, 4 and 6 weeks post-castration or sham castration. For 5-month-old mice data were determined from pooled samples of four organs, whereas for 10-month-old mice data were determined from individual samples (n=8) and represented as mean±SEM.
The number of cells in the pDC subset also increased following castration in adult and middle-aged mice, even at 6 weeks post-Cx in contrast to cDC cell numbers which were reduced almost to sham-Cx levels by this time (Figure 5a). Similar to the cDC subsets, the pDC did not initially proliferate to the same extent as thymocytes resulting in a drop in proportion at 2 weeks post-Cx which returned to normal levels at 4 weeks post-Cx in both adult and middle-aged mice (Figure 5b). The elevated pDC numbers in adult mice resulted in an increased proportion of these cells at 6 weeks post-Cx, whereas in middle-aged mice, pDC proportions were significantly reduced when the numbers of thymocyte increased at 6 weeks post-Cx.
The regenerative process did not alter the activation phenotype of any of the thymic DC subtypes, as determined by expression levels of CD40, CD80, CD86, CD69 and MHC class II, at 2, 4 or 6 weeks post-Cx (data not shown).
In the spleen of Cx mice, in comparison to sham-Cx mice, the numbers of CD8α+ cDC, Sirpα+ cDC and pDC remained relatively unchanged (data not shown). However, as the spleen increased in cellularity post-Cx, the percentages of all three DC subsets appeared to be slightly reduced at 2, 4 and 6 weeks post-Cx. Similar to the thymic DC, the splenic DC subsets were not altered in activation phenotype during the regenerative process induced by castration (data not shown).
Changes in other cell subsets in mice undergoing thymic regeneration
We monitored changes in T-lineage cells and other cell types within the regenerating thymuses of both adult and middle-aged mice at 2, 4 and 6 weeks post-Cx. T-lineage cells were the dominant cell type at all stages so their numbers reflected the total cell counts in the expanding thymus (Figure 4a). The development of thymocytes appeared to proceed normally as the profiles of CD4+8+, CD4−8−, CD4+8− and CD4−8+ thymocytes were similar to those of the young steady-state thymus (data not shown). In the spleen there was an increase in the numbers of CD4+ and CD8+ T cells at 4 and 6 weeks post-Cx (data not shown), indicating that by this time significant numbers of mature T-lineage cells were exported from the thymus to the periphery. These results agree with previous studies,8, 46 showing that castration-induced thymic regeneration results in a significant increase in T-cell development within the thymus and emigration into the periphery.
B cells also increased markedly in numbers in the regenerating thymus, although they were maintained at a reduced relative proportion. In the spleen, B cells increased significantly in numbers and percentages from 2 to 6 weeks post-Cx (data not shown), which was consistent with a previous study showing that castration of aged mice results in increases in B-cell progenitors and peripheral B cells, which are recent bone marrow emigrants.47
Discussion
The ageing process has differential effects on the relative prevalence of CD8α+ cDC, Sirpα+ cDC and pDC in the thymus, although all types of DC do decline in absolute numbers with age. We have shown that the CD8α+ cDC and pDC are maintained at the same relative proportions compared to T-lineage cells in the thymus until middle age, after which their percentage decreases. Similarly, in the spleen there is a steady trend for these cells to decrease in relative proportion with age. However, while the Sirpα+ cDC decrease in proportion in the spleen, they increase in proportion in the ageing thymus. Previous studies have not investigated the changes in DC subsets in the ageing mouse thymus, whereas in the ageing human thymus it has been reported that there is a decrease in total thymic DC numbers,48, 49 but no change in their relative proportion.49 Although in these studies the different subsets of human thymic DC were not defined, in general they are consistent with the results from our studies, where the predominant DC population in the thymus, the CD8α+ cDC, also decreases in numbers but not in proportion until after middle age.
Although little is known about the mechanisms involved in the changes in dendritic cell numbers and proportions in the ageing thymus, these changes are likely to be mediated through their different precursors, which may become defective in differentiation within the ageing animal. The CD8α+ cDC subset arises intrathymically from an early thymic precursor population defined as CD4loLin−CD25−c-Kithi (reviewed in Ref. 50) which maintains DC and T-cell potentials within the thymic environment.32, 51, 52, 53, 54, 55 There is a reduced number and frequency of early thymic precursors in aged mice56 and in addition, their capacity to produce thymocytes is decreased.56 Given that we saw a reduction in the number of CD8α+ cDC in the ageing thymus, in proportion to the decrease in numbers of thymocytes, this indicates that as mice age, the reduced numbers of early thymic precursors still produce a normal ratio of thymocyte to DC. However, in aged mice the reduction in number of CD8α+ cDC relative to thymocytes may be due to a deregulated differentiation capacity of these precursors, or due to a shorter lifespan of the DC. The different effects of ageing on the CD8α+ cDC and Sirpα+ cDC subsets in the thymus may reflect the different origins of precursor since the Sirpα+ (CD11b+) cDC are believed to be derived directly from the bloodstream.28, 57 Therefore, the age-related increase in the frequency of these cells within the thymus may be due to extrathymic factors, such as alterations within their bone marrow precursors or the number of these cells in the circulation which have access to the thymus, or due to thymus-specific factors, such as altered migration into the thymus or available space in the thymus.
The ratio of CD8α+ cDC to thymocytes may affect the efficiency of negative selection in the thymus. Indeed, the lifespan of the DC has been shown to be similar to the intrathymic lifespan of the T-cell lineages,32 thereby indicating that T cells and CD8α+ cDC develop in parallel from the same precursor in the thymus and the DC can mediate negative selection of developing thymocytes. In this study we have shown that the thymocyte to DC ratio remains constant in young to middle-aged mice, so there is potentially no loss of function for negative selection. It was only in aged mice that the proportion of CD8α+ cDC was reduced. These observations are consistent with the findings that the ability to negatively select autoreactive T cells remains intact in 20- to 24-month-old mice, but potentially autoreactive T cells escape deletion in the very aged (30- to 36-month-old) mice.58, 59 This may be due to a reduction in the percentage of thymic DC as we observed.
We demonstrated that as mice age there is no alteration in the expression level of the costimulatory molecules CD40, CD80 and CD86, or the antigen-presenting molecule, MHC class II, on the conventional DC subsets in thymus or spleen, nor on the thymic plasmacytoid DC. However, changes in expression of these molecules on splenic pDC were evident. This suggests that the functions of thymic cDC in aged mice are not altered, whereas such functions mediated by pDC may be affected.
Importantly, we have clearly shown that castration-induced regeneration of the thymus results in an increase in the numbers of all three DC subsets within the thymus. This extends our previous studies, which evaluated the effectiveness of castration on haematopoietic stem-cell transplantation, and demonstrated a significant increase in the numbers of donor-derived DC (defined as CD11chigh and/or MHC class II+) within the thymus at 4 weeks following castration.8, 23, 24 These results have positive implications for the clinical application of this thymic regeneration approach, indicating that the functions mediated by each DC subset will be maintained in the regenerating thymus, and the induction of autoimmune disease through failure of negative selection of self-reactive thymocytes or Treg induction should be avoided. Indeed, it has been shown that autoimmunity does not develop in castrated mice as far as 12 months post-castration (A. Chidgey, unpublished). Thus, the lag in the proportional increase of DC subsets seen at 2 weeks post-castration in both adult and middle-aged mice, and maintained until 4 weeks in the adult mice, which is due to the overwhelming increase of T cells in the regenerative thymus, does not necessarily appear to impact negatively on T-cell regulation in the long term. It is possible that the DC to thymocyte ratio is sufficient to maintain the integrity of T-cell regulation during the lag phase, or in the event that autoreactive thymocytes escape into the periphery during this period, under regulatory mechanisms in the peripheral control of these cells. In line with this, we also observed an increase in the number of Tregs and enhanced Treg function post-Cx (Chidgey et al., manuscript in preparation), suggesting that the induction and maintenance of Treg cells by DC were efficient in these mice.
Overall, our study demonstrated that parallelling the decline in thymocytes, numbers of thymic DC decrease with ageing. The castration-induced regeneration of the aged thymus increased the number of all DC populations, despite with different kinetics, the implication of which is yet to be determined, but is consistent with the reconstruction of a normal thymus which does not predispose to pathological conditions. In particular the parallel regeneration of thymocytes and thymic DC may be important to ensure that the regenerated thymocytes can be selected properly to prevent the development of autoimmunity. Further functional studies of the regenerated DC will be required to confirm this.
Acknowledgments
We thank J. Homann for performing the castration operations and animal husbandry. L. Wu, K. Shortman and S. van Dommelen are supported by a grant from the Australian Stem Cell Centre. L. Wu and K. Shortman are supported by fellowships and grants from the Australian National Health and Medical Research Council.
References
- Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabarra B, Andrianarison I. Ultrastructural study of thymic microenvironment involution in aging mice. Exp Gerontol. 1996;31:489–506. doi: 10.1016/0531-5565(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Guarcello V, Morale MC, Bartoloni G, Raiti F, Palumbo G, Jr, et al. Luteinizing hormone-releasing hormone (LHRH) agonist restoration of age-associated decline of thymus weight, thymic LHRH receptors, and thymocyte proliferative capacity. Endocrinology. 1989;125:1037–1045. doi: 10.1210/endo-125-2-1037. [DOI] [PubMed] [Google Scholar]
- Greenstein BD, Fitzpatrick FT, Adcock IM, Kendall MD, Wheeler MJ. Reappearance of the thymus in old rats after orchidectomy: inhibition of regeneration by testosterone. J Endocrinol. 1986;110:417–422. doi: 10.1677/joe.0.1100417. [DOI] [PubMed] [Google Scholar]
- Windmill KF, Meade BJ, Lee VW. Effect of prepubertal gonadectomy and sex steroid treatment on the growth and lymphocyte populations of the rat thymus. Reprod Fertil Dev. 1993;5:73–81. doi: 10.1071/rd9930073. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick FT, Kendall MD, Wheeler MJ, Adcock IM, Greenstein BD. Reappearance of thymus of ageing rats after orchidectomy. J Endocrinol. 1985;106:R17–R19. doi: 10.1677/joe.0.106r017. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- Steffens CM, Al-Harthi L, Shott S, Yogev R, Landay A. Evaluation of thymopoiesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naive phenotypes. Clin Immunol. 2000;97:95–101. doi: 10.1006/clim.2000.4938. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Uldrich AP, Sutherland JS, Gill J, Miller JF, Godfrey DI, et al. Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med. 2002;8:469–476. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- Mosley RL, Koker MM, Miller RA. Idiosyncratic alterations of TCR size distributions affecting both CD4 and CD8 T cell subsets in aging mice. Cell Immunol. 1998;189:10–18. doi: 10.1006/cimm.1998.1369. [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, et al. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, et al. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Kurashima C, Utsuyama M, Kasai M, Ishijima SA, Konno A, Hirokawa K. The role of thymus in the aging of Th cell subpopulations and age-associated alteration of cytokine production by these cells. Int Immunol. 1995;7:97–104. doi: 10.1093/intimm/7.1.97. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, et al. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- Chidgey AP, Seach N, Dudakov J, Hammett M, Boyd RL. Strategies for reconstituting and boosting T cell-based immunity following haemotopoietic stem cell transplantation: pre-clinical and clinical approaches. Semin Immunopathol. 2008;30:457–477. doi: 10.1007/s00281-008-0140-5. [DOI] [PubMed] [Google Scholar]
- Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol. 2008;252:122–138. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K. Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mech Ageing Dev. 1989;47:175–185. doi: 10.1016/0047-6374(89)90030-4. [DOI] [PubMed] [Google Scholar]
- Wei XY, Zhang JK, Li J, Chen SB. Effect of bilateral testicular resection on thymocyte and its microenvironment in aged mice. Asian J Androl. 2001;3:271–275. [PubMed] [Google Scholar]
- Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, et al. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Viselli SM, Shults K, Stelzer G, Kovacs WJ. Induction of immature thymocyte proliferation after castration of normal male mice. Endocrinology. 1994;134:107–113. doi: 10.1210/endo.134.1.8275924. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, et al. Dendritic cells in the thymus contribute to T regulatory cell induction. Proc Natl Acad Sci USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Lahoud MH, Proietto AI, Gartlan KH, Kitsoulis S, Curtis J, Wettenhall J, et al. Signal regulatory protein molecules are differentially expressed by CD8-dendritic cells. J Immunol. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- Wu L, Vremec D, Ardavin C, Winkel K, Suss G, Georgiou H, et al. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur J Immunol. 1995;25:418–425. doi: 10.1002/eji.1830250217. [DOI] [PubMed] [Google Scholar]
- Donskoy E, Foss D, Goldschneider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J Immunol. 2003;171:3568–3575. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- Brocker T. The role of dendritic cells in T cell selection and survival. J Leukoc Biol. 1999;66:331–335. doi: 10.1002/jlb.66.2.331. [DOI] [PubMed] [Google Scholar]
- Anderson G, Partington KM, Jenkinson EJ. Differential effects of peptide diversity and stromal cell type in positive and negative selection in the thymus. J Immunol. 1998;161:6599–6603. [PubMed] [Google Scholar]
- Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Abe M, Shirai T, Fukao K, Nakauchi H. Reconstitution ratio is critical for alloreactive T cell deletion and skin graft survival in mixed bone marrow chimeras. J Immunol. 1995;155:5631–5636. [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Kumanogoh A, Wang X, Lee I, Watanabe C, Kamanaka M, Shi W, et al. Increased T cell autoreactivity in the absence of CD40–CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001;166:353–360. doi: 10.4049/jimmunol.166.1.353. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, et al. Distinct roles for the OX40–OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Mancini C, Brunelli R, Leter G, Doria G. Differential effects of gonadectomy on thymic stromal cells in promoting T cell differentiation in mice. Mech Ageing Dev. 1995;81:107–117. doi: 10.1016/0047-6374(95)01589-r. [DOI] [PubMed] [Google Scholar]
- Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED. Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol. 2001;13:553–558. doi: 10.1093/intimm/13.4.553. [DOI] [PubMed] [Google Scholar]
- Nakahama M, Mohri N, Mori S, Shindo G, Yokoi Y, Machinami R. Immunohistochemical and histometrical studies of the human thymus with special emphasis on age-related changes in medullary epithelial and dendritic cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58:245–251. doi: 10.1007/BF02890079. [DOI] [PubMed] [Google Scholar]
- Varas A, Sacedon R, Hernandez-Lopez C, Jimenez E, Garcia-Ceca J, Arias-Diaz J, et al. Age-dependent changes in thymic macrophages and dendritic cells. Microsc Res Tech. 2003;62:501–507. doi: 10.1002/jemt.10411. [DOI] [PubMed] [Google Scholar]
- Wu L. T lineage progenitors: the earliest steps en route to T lymphocytes. Curr Opin Immunol. 2006;18:121–126. doi: 10.1016/j.coi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HQ, Lu M, Ikawa T, Masuda K, Ohmura K, Minato N, et al. T/NK bipotent progenitors in the thymus retain the potential to generate dendritic cells. J Immunol. 2003;171:3401–3406. doi: 10.4049/jimmunol.171.7.3401. [DOI] [PubMed] [Google Scholar]
- Lu M, Tayu R, Ikawa T, Masuda K, Matsumoto I, Mugishima H, et al. The earliest thymic progenitors in adults are restricted to T, NK, and dendritic cell lineage and have a potential to form more diverse TCRbeta chains than fetal progenitors. J Immunol. 2005;175:5848–5856. doi: 10.4049/jimmunol.175.9.5848. [DOI] [PubMed] [Google Scholar]
- Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisi GM, Tsiagbe VK, Russo C, Basch RS, Thorbecke GJ. Evaluation of presence and functional activity of potentially self-reactive T cells in aged mice. Int Immunol. 1996;8:387–395. doi: 10.1093/intimm/8.3.387. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintial R, Baccala R, Balderas RS, Theofilopoulos AN. V beta gene repertoire in the aging mouse: a developmental perspective. Int Rev Immunol. 1995;12:27–40. doi: 10.3109/08830189509056700. [DOI] [PubMed] [Google Scholar]


