Abstract
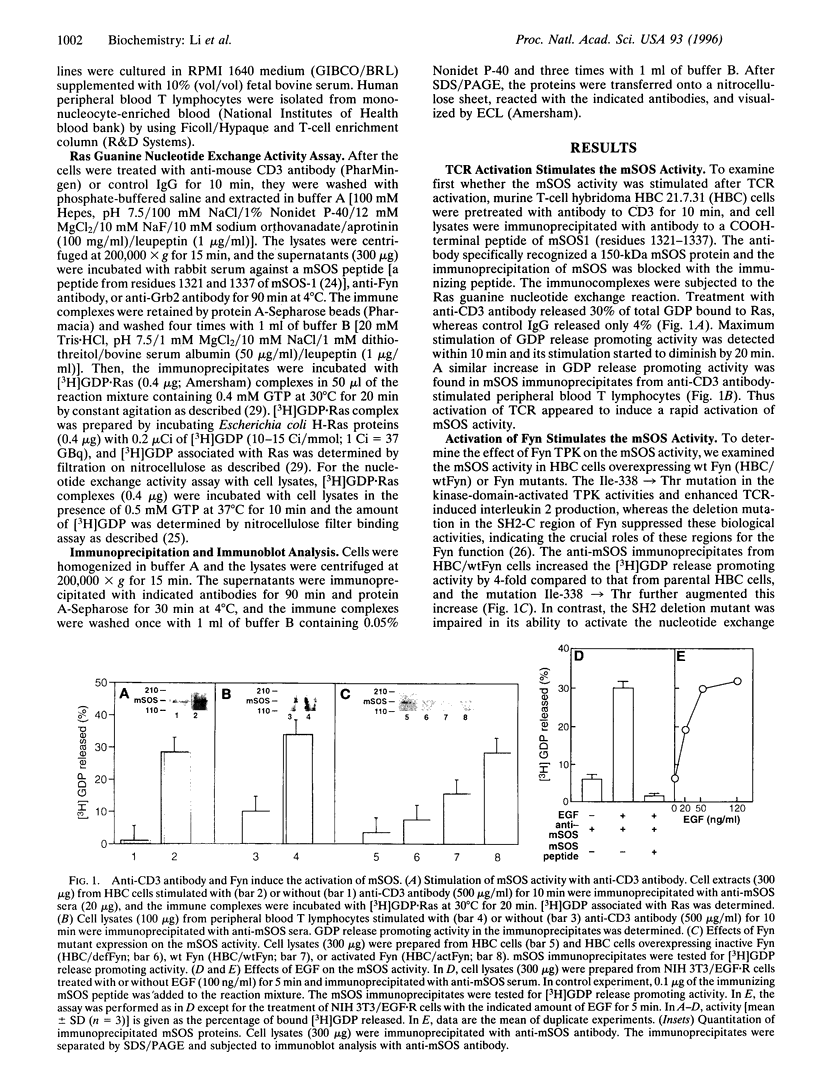
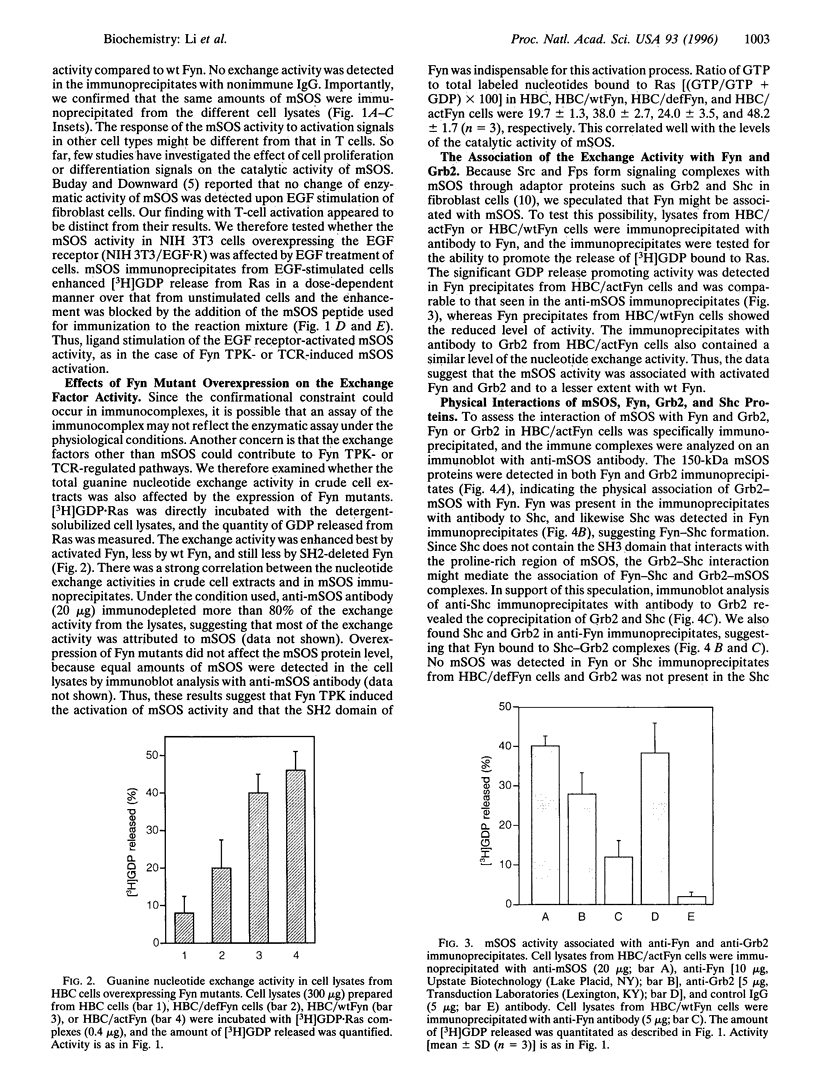
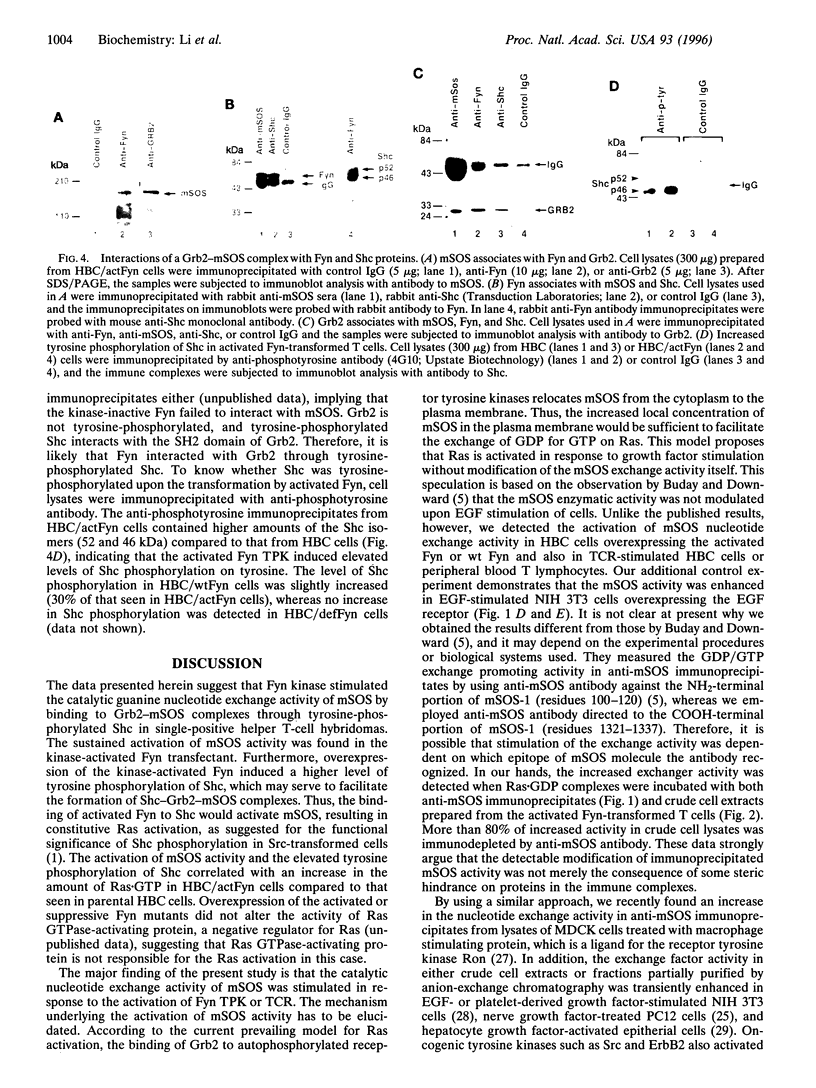
mSOS, a guanine nucleotide exchange factor, is a positive regulator of Ras. Fyn tyrosine protein kinase is a potential mediator in T-cell antigen receptor signal transduction in subsets of T cells. We investigated the functional and physical interaction between mSOS and Fyn in T-cell hybridoma cells. Stimulation of the T-cell antigen receptor induced the activation of guanine nucleotide exchange activity in mSOS immunoprecipitates. Overexpression of Fyn mutants with an activated kinase mutation and with a Src homology 2 deletion mutation resulted in a stimulation and suppression of the mSOS activity, respectively. The complex formations of Fyn-Shc, Shc-Grb2, and Grb2-mSOS were detected in the activated Fyn-transformed cells, whereas the SH2 deletion mutant of Fyn failed to form a complex with mSOS. Moreover, tyrosine phosphorylation of Shc was induced by the overexpression of the activated Fyn. These findings support the idea that Fyn activates the activity of mSOS bound to Grb2 through tyrosine phosphorylation of Shc. Unlike the current prevailing model, Fyn-induced activation of Ras might involve the stimulation of the catalytic guanine nucleotide exchange activity of mSOS.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N., Miceli M. C., Parnes J. R., Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991 Mar 7;350(6313):62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- Appleby M. W., Gross J. A., Cooke M. P., Levin S. D., Qian X., Perlmutter R. M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992 Sep 4;70(5):751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Aronheim A., Engelberg D., Li N., al-Alawi N., Schlessinger J., Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994 Sep 23;78(6):949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Bowtell D., Fu P., Simon M., Senior P. Identification of murine homologues of the Drosophila son of sevenless gene: potential activators of ras. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6511–6515. doi: 10.1073/pnas.89.14.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Cooke M. P., Abraham K. M., Forbush K. A., Perlmutter R. M. Regulation of T cell receptor signaling by a src family protein-tyrosine kinase (p59fyn). Cell. 1991 Apr 19;65(2):281–291. doi: 10.1016/0092-8674(91)90162-r. [DOI] [PubMed] [Google Scholar]
- Davidson D., Chow L. M., Fournel M., Veillette A. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J Exp Med. 1992 Jun 1;175(6):1483–1492. doi: 10.1084/jem.175.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Bottaro D. P., Minichiello L., Auricchio A., Wong W. T., Segatto O., Di Fiore P. P. Identification and biochemical characterization of novel putative substrates for the epidermal growth factor receptor kinase. J Biol Chem. 1992 Mar 15;267(8):5155–5161. [PubMed] [Google Scholar]
- Fusaki N., Semba K., Katagiri T., Suzuki G., Matsuda S., Yamamoto T. Characterization of p59fyn-mediated signal transduction on T cell activation. Int Immunol. 1994 Aug;6(8):1245–1255. doi: 10.1093/intimm/6.8.1245. [DOI] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Graziani A., Gramaglia D., dalla Zonca P., Comoglio P. M. Hepatocyte growth factor/scatter factor stimulates the Ras-guanine nucleotide exchanger. J Biol Chem. 1993 May 5;268(13):9165–9168. [PubMed] [Google Scholar]
- Iwashima M., Irving B. A., van Oers N. S., Chan A. C., Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994 Feb 25;263(5150):1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Li B. Q., Kaplan D., Kung H. F., Kamata T. Nerve growth factor stimulation of the Ras-guanine nucleotide exchange factor and GAP activities. Science. 1992 Jun 5;256(5062):1456–1459. doi: 10.1126/science.1604323. [DOI] [PubMed] [Google Scholar]
- Li B. Q., Subleski M., Shalloway D., Kung H. F., Kamata T. Mitogenic activation of the Ras guanine nucleotide exchange factor in NIH 3T3 cells involves protein tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8504–8508. doi: 10.1073/pnas.90.18.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. Q., Wang M. H., Kung H. F., Ronsin C., Breathnach R., Leonard E. J., Kamata T. Macrophage-stimulating protein activates Ras by both activation and translocation of SOS nucleotide exchange factor. Biochem Biophys Res Commun. 1995 Nov 2;216(1):110–118. doi: 10.1006/bbrc.1995.2598. [DOI] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- McGlade J., Cheng A., Pelicci G., Pelicci P. G., Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Quilliam L. A., Huff S. Y., Rabun K. M., Wei W., Park W., Broek D., Der C. J. Membrane-targeting potentiates guanine nucleotide exchange factor CDC25 and SOS1 activation of Ras transforming activity. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8512–8516. doi: 10.1073/pnas.91.18.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K. S., Lee K. K., Songyang Z., Cantley L. C., Burn P., Burakoff S. J. Interaction of Shc with the zeta chain of the T cell receptor upon T cell activation. Science. 1993 Nov 5;262(5135):902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Phillips A. F., Luong E. T., Klausner R. D. Association of the fyn protein-tyrosine kinase with the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4358–4362. doi: 10.1073/pnas.87.11.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993 Jun 25;260(5116):1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Stein P. L., Lee H. M., Rich S., Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992 Sep 4;70(5):741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Loeb D. M., Copeland T. D., Pawson T., Greene L. A., Kaplan D. R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994 Mar;12(3):691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Kuramochi S., Fusaki N., Nada S., Kawamura-Tsuzuku J., Matsuda S., Semba K., Toyoshima K., Okada M., Yamamoto T. Functional and physical interaction of protein-tyrosine kinases Fyn and Csk in the T-cell signaling system. J Biol Chem. 1993 Dec 25;268(36):27413–27419. [PubMed] [Google Scholar]
- Tsygankov A. Y., Bröker B. M., Fargnoli J., Ledbetter J. A., Bolen J. B. Activation of tyrosine kinase p60fyn following T cell antigen receptor cross-linking. J Biol Chem. 1992 Sep 15;267(26):18259–18262. [PubMed] [Google Scholar]
- Welham M. J., Duronio V., Leslie K. B., Bowtell D., Schrader J. W. Multiple hemopoietins, with the exception of interleukin-4, induce modification of Shc and mSos1, but not their translocation. J Biol Chem. 1994 Aug 19;269(33):21165–21176. [PubMed] [Google Scholar]