SUMMARY
Leishmania major is an etiological agent of cutaneous leishmaniasis. The parasite primarily infects immune sentinel cells, specifically macrophages and dendritic cells, in the mammalian host. Infection is receptor mediated and is known to involve parasite binding to cell surface protein complement receptor 3 (CR3, Mac-1, CD11b/CD18). Engagement of CR3 by various ligands inhibits production of interleukin-12 (IL-12), the cytokine that drives anti-leishmanial T helper 1-type immune responses. Likewise, L. major infection inhibits IL-12 production and activation of host macrophages. Our data indicate that in the absence of CR3, L. major-infected bone marrow-derived macrophages produce more IL-12 and nitric oxide compared to WT cells upon LPS stimulation. We therefore investigated multiple signaling pathways by which L. major may inhibit IL-12 transcription through CR3 ligation. We demonstrate that L. major infection does not elicit significant NFκB p65, MAPK, IRF-1, or IRF-8 activation in WT or CD11b deficient macrophages. Furthermore, infection neither inhibits LPS-induced MAPK or NFκB activation, nor blocks IFN-γ-activated IRF-1 and IRF-8. ETS-mediated transcription, however, is inhibited by L. major infection independently of CR3. Our data indicate that L. major mediated inhibition of IL-12 occurs through CR3 engagement, however the mechanism of inhibition is independent of NFκB, MAPK, IRF, and ETS.
Keywords: CR3, Leishmania major, Signal transduction, ETS
INTRODUCTION
Establishment and persistence within a vertebrate host requires that pathogens are able to avoid destruction by the host’s immune system. Occupying an intracellular niche is one mechanism employed to circumvent immune recognition, but necessitates that the intracellular pathogens be resistant to, or inhibit, the host-cell’s microbicidal mechanisms. This dilemma is particularly relevant to Leishmania species as these parasites reside primarily within macrophages (MP), cells whose function is to eliminate invading microorganisms and activate adaptive immunity.
Leishmania entry into host cells is receptor mediated. These parasites are able to engage numerous cell surface receptors (1), including scavenger receptor (CD163) (2), mannose receptor (3), Toll-like receptors 2, 3 (4), and 4 (5), Fc receptors (FcR) (6) and complement receptor 3 (CR3, Mac-1, CD11b/CD18) (7). Leishmania can bind CR3 directly (8) as well as through C3bi deposition on the parasite surface (9). Utilization of CR3 may allow Leishmania to evade innate immune responses such as production of reactive oxygen intermediates (10). Interestingly, ligation of CR3 via antibodies in the absence of any infection has been shown to inhibit interleukin-12 (IL-12) production and suppression of T helper 1 (Th1) immunity (11), suggesting that Leishmania parasites circumvent both innate and adaptive immunity through CR3 engagement. This model of receptor engagement to down-modulate host IL-12 mediated immunity may account for similar effects on MP function following infection with such diverse pathogens as viruses (12–14), fungi (15), bacteria (16), and other protozoa (17, 18).
Leishmania species alter MP and dendritic cell signaling events, cellular activation, and immune effector responses, making these cells a more hospitable niche. Leishmania infection of host MP down-regulates expression of MHCII (19) and the PKC substrate MRP (20), alters PKC localization and activation (21), dysregulates phagosomal actin accumulation (22), delays phagosome maturation (23), and represses cellular apoptosis (24). Leishmania species have been reported to inhibit ERK1/2 (25, 26), JAK1 (27) and interferon-gamma (IFN-γ) receptor (28) phosphorylation, and also activate the down-regulatory phosphatase SHP-1 (27, 29, 30). Of particular importance, Leishmania infection inhibits expression of IL-12 by MP (31–35), a predominant Th1 cytokine that drives IFN-γ mediated cell activation necessary to control intracellular organisms like Leishmania (36).
Here we investigated whether L. major utilizes CR3 to modulate cell signaling events that lead to IL-12 production. CR3 is a heterodimer composed of CD11b and CD18. IL-12 inhibition has been shown to be dependent on the presence of the CD18 chain of CR3 as L. major-infected WT MP inhibit IL-12 but such inhibition is absent in CD18 deficient MP (2). However, CD18 also couples with CD11a to form LFA-1 (37), and CD11c to form CR4 (38). Utilizing CD11b deficient (CD11b KO) MP, we demonstrate that in the absence of CD11b, L. major infection increases NO production and LPS-induced IL-12p40 transcription, implicating CR3 specifically as a regulator of anti-microbial immune responses. To explore pathways by which CR3 engagement by L. major leads to IL-12 inhibition, we assessed MAPK, NFκB, IRF, and ETS in WT and CD11b knockout mice.
MATERIALS AND METHODS
Mice and Parasites
WT Balb/c mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). CD11b KO mice were provided by Tanya Mayadas-Norton (Brigham and Women’s Hospital, Cambridge, MA) (39). Breeding lines of both WT and KO were generated from heterozygous matings. All animals were bred and housed at the University of Notre Dame’s Freimann Life Sciences Center (FLSC) according to IACUC standards. L. major strain Friedlin V1 (MHOM/IL/80/Friedlin) parasites were cultured at 26°C without CO2 in Medium 199 supplemented with 20% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 100 U/ml of penicillin, 100 ug/mL of streptomycin, 2 mM L-glutamine, (Cellgro Technologies, Manassass, VA), 40 mM HEPES, 0.1 mM adenine, 5 μg/ml hemin in 50% triethanolamine, 1 mg/ml biotin, and 2.2 mg/ml sodium bicarbonate (M199-C).
BMMP generation
Femurs from euthanized 6–8 week old female mice were flushed with RPMI 1640 media supplemented with 1% penicillin/streptomycin, 1% L-glutamine and 10% fetal bovine serum (RPMI-C). Pelleted cells were resuspended in ACK lysis buffer to remove RBC contamination, counted on a hemacytometer, and resuspended in 25% L929 cell supernatant (as a source of M-CSF) supplemented RPMI-C. Cultures were supplemented with fresh media on Days 3 and 5. On Day 6 media was changed to RPMI-C without supernatant supplement. Adherent cells were infected on Day 7.
Infection
Five day old stationary phase L. major cultures were enriched for metacyclic promastigotes via Ficoll gradient (40). Parasites or polystyrene microspheres (Bangs Laboratries, Inc., Fishers, IN) were washed and opsonized with 5% normal mouse serum (NMS) in HBSS prior to infection. MP were infected with 10 parasites per BMMP (multiplicity of infection (MOI) 10:1) for times indicated. Infections were monitored by Diff-Quick staining and manual counting via light microscopy and did not vary between WT and CD11b KO MP. Statistical analysis was performed using the Mann-Whitney test.
Luciferase reporter assays
Luciferase reporter constructs containing either 6 copies of the NFκB binding site (GGGAATTTC) or 5 copies of the ETS binding site (ACCGGAAGTT) of the TNFα promoter and a TATA box fused to a firefly luciferase reporter gene were generated as previously described (41) and subcloned into pcDNA3.1 (Invitrogen). The RAW 264.7 MP-like cell line was stably transfected with either NFκB-Luciferase-pcDNA3.1 or ETS-Luciferase-pcDNA3.1 using FuGene6 (Roche) following manufacturer’s recommended protocols. After transfection, the cells were washed and cultured in RPMI-C. Twenty-four hours later, the medium was replaced with a selection medium containing G418 (Invitrogen). The chosen clones were screened for luciferase reporter activity by treating with LPS; the clones exhibiting the greatest induction of luciferase in response to LPS stimulation were used in all subsequent studies. Cells were plated at 2×106 cells/well in 6-well plates, infected with NMS opsonized L. major, as described above. Cells were cultured in media or infected with a 10:1 ratio of parasites to MP overnight followed by LPS (100 ng/ml) stimulation for 8 hours. Luciferase activity was determined in triplicate via Luciferase assay (Promega, Corp. Madison, WI) according to the manufacturer’s instructions using a LMax II 384 luminometer (Molecular Devices, Corp., CA). Data was expressed as Normalized Luciferase Units with 100% defined as the highest value in each data set. Statistical analysis was performed on raw luciferase units using the Wilcoxon Signed Rank test.
MAPK and IkB Western Blot Analysis
BMMP whole cell lysates were prepared with lysis buffer containing 50mM Tris-HCl, 1% Igepal, 0.25% deoxycholic acid, 150mM NaCl, 1 mM each of PMSF, NaF, EDTA, and orthovanadate, and 1ug/ml each of aprotinin, leupeptin and pepstatin. 5×104 cell equivalents were loaded per well and lysates were separated on Novex 12% Tris-Glycine SDS mini-gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Membranes were probed with the following primary antibodies overnight at 4°C: mouse anti-pERK1/2, mouse anti-pp38, rabbit anti-p38, and mouse anti-IkB-α (Cell Signaling, Danvers, MA); rabbit anti-ERK1/2 (BioSource/Invitrogen, Carlsbad, CA); and mouse anti-GAPDH (Biogenesis/AbD Serotec, Raleigh, NC). Secondary antibodies were incubated for 1hr at room temperature with goat anti-mouse or goat anti-rabbit HRP-conjugated antibodies (Becton Dickinson, Franklin Lakes, NJ). All antibodies were used according to suppliers suggested dilutions. Membranes were developed with SuperSignal® West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) followed by exposure to X-ray film. To assess IkB-α degradation, densitometry was performed with a BioRad GS-800 densitometer and QuantityOne software. Optical density of IkB-α was normalized to GAPDH from each condition.
Nuclear translocation Western Blot Analysis
Nuclear extracts were prepared with NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce, Rockford, IL) via manufacturer’s protocol and were analyzed for translocation of transcription factors. Approximately 1×106 cell equivalents were analyzed as above. Membranes were probed with the following primary antibodies: rabbit anti-IRF-1, goat anti-ICSBP/IRF-8 (Santa Cruz, Santa Cruz, CA), and rabbit anti-Lamin A/C (Cell Signaling, Danvers, MA). Secondary antibodies used were goat anti-mouse and anti-rabbit HRP-conjugated antibodies described above and donkey anti-goat HRP (Santa Cruz, Santa Cruz, CA).
Immunofluorescence Staining
5×105 cells were seeded to sterile coverglasses. Cells were cultured in media or infected with a 10:1 ratio of parasites to MP overnight followed by LPS (100 ng/ml) stimulation. Cells were fixed with 2% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked with 1% BSA in PBS. Mouse anti-NFkB p65 mAb (Santa Cruz, Santa Cruz, CA) was used at 1:500. A goat anti-mouse IgG AlexaFluor 488 (Molecular Probes/Invitrogen, Carlsbad, CA) secondary Ab was used at 1:800. DAPI (Molecular Probes/Invitrogen, Carlsbad, CA) nuclear counterstain was used at 100 nM. Samples were viewed on a Leica DMIRE2 fluorescence microscope (Leica, Wetzlar, Germany) with OpenLab 3.1.3 software. Leishmania parasites were stained with rat anti-mouse C3 antibody followed by Cy3 conjugated anti-rat IgG staining (Abcam Inc., Cambridge, MA). Parasites were visualized using a Nikon Eclipse E400 microscope and overlays were generated using PhotoShop software or using a Beckman Coulter FC500 MPL flow cytometer.
Nitric Oxide Production
Nitrite concentrations in supernatants from cultures were analyzed with the Griess Reagent System (Promega, Madison, WI) according to manufacturer’s protocol using a SpectraMax Plate Reader (Molecular Devices, Sunnyvale, CA).
Quantitative RT-PCR
RNA was isolated from BMMP using the RNeasy Mini Kit (Qiagen, Valencia, CA) and was quantified on the ND-1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE). DNA contamination was removed from 1 ug RNA with 1 U DNase I in DNase buffer (20mM Tris-HCl pH 8.4, 2 mM MgCl and 50 mM KCl). DNA-free RNA was used to generate cDNA with random primers in the presence of 500 nM dNTP, 200 U of Superscript III reverse transcriptase, 40 U of RNaseOut, and 5 mM DTT (all reagents from Invitrogen, Carlsbad, CA). cDNA was analyzed via quantitative real-time PCR with the ABI PRISM 7700 sequence detector using 2x SYBR Green MasterMix (ABI Applied Biosystems, Foster City, CA) and 300 nM each of forward and reverse primers (IDT, Coralville, IA) for each reaction. Relative fold inductions of IL-12p40 (42), ETS-2 (5′-CGGCGCGATGAATGACTTTGGAAT-3′ and 5′-AGAAGGGAGCACAGCAAACAGAGA-3′), and PU.1 (5′-TGATGGAGAAGCTGATGGCTTGGA-3′ and 5′-TGCTTGGACGAGAACTGGAAGGTA-3′)were calculated using the ΔΔCt method using HPRT (42) or GAPDH (5′-TCAACAGCAACTCCCACTCTTCCA-3′ and 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′) for normalization and the uninfected, unstimulated control as the calibrator. Statistical significance was determined using Student’s t-test with a 95% confidence interval.
RESULTS
Leishmania-infected CD11b deficient BMMP produce more IL-12p40 than infected WT BMMP upon LPS stimulation
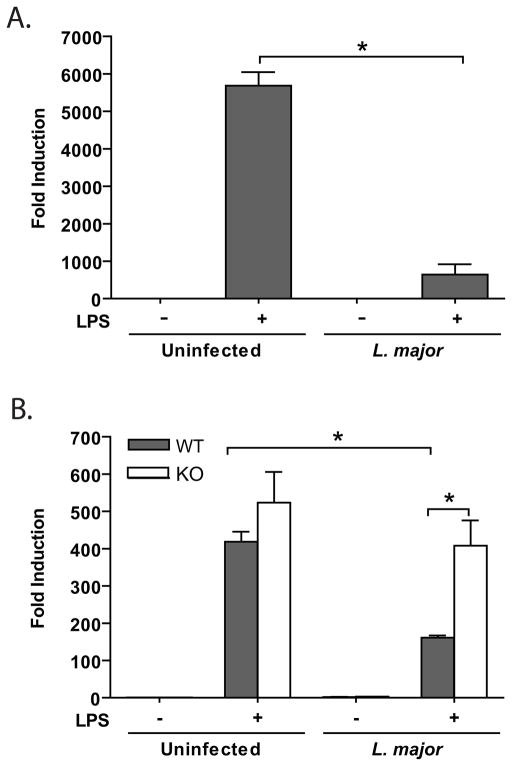
Previous reports have described inhibition of LPS-induced IL-12p40 (2, 11, 43, 44) mediated by CR3 binding of anti-CD18 and anti-CD11b antibodies (11), and serum-opsonized Leishmania parasites (2, 44). We investigated how L. major infection modulates cellular responses in the murine macrophage cell line RAW264.7 and the role that CD11b plays in L. major-mediated IL-12p40 expression in primary BMMP (Fig. 1). As expected, IL-12p40 transcripts were upregulated by LPS stimulation whereas infected RAW264.7 and BMMP expressed little IL-12p40. L. major infection significantly inhibited LPS-induced IL-12p40 expression in both RAW264.7 cells (Fig. 1A) and WT BMMP (Fig. 1B). Infection-induced inhibition of IL-12 was not observed in CD11b KO BMMP, suggesting that Leishmania utilization of CR3 inhibits production of IL-12 (Fig. 1B).
Figure 1.
CD11b is necessary for L. major-mediated inhibition of LPS-induced IL-12. RAW264.7 cells (A) and BMMP (B) were infected for 16 h with or without subsequent LPS stimulation (8 hr, 1ug/ml) after which cells were lysed and assayed for mRNA transcription by real-time PCR. Data was normalized to HPRT housekeeping gene and fold induction was calculated via ΔΔCt method using uninfected/unstimulated cultures as calibrators. One representative of three independent experiments performed in triplicate is presented. *p<0.05 via Student’s T-test.
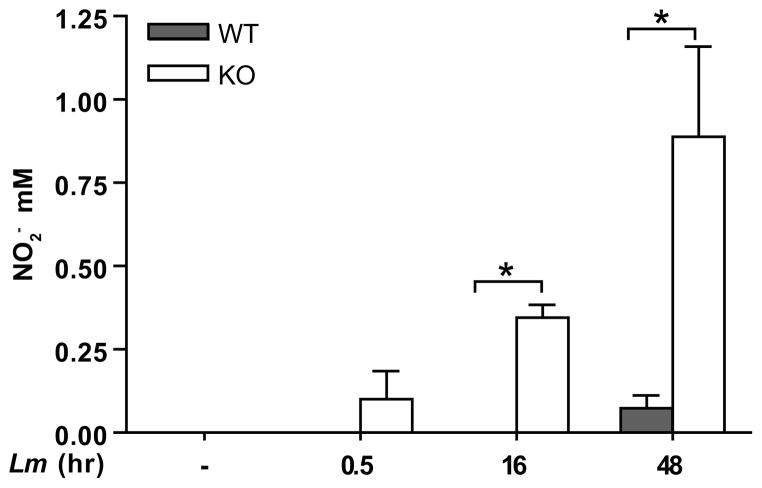
L. major interaction with CD11b mediates NO suppression
Activated MP produce NO, a reactive species that mediates intracellular parasite killing. We examined CD11b-mediated NO production by assaying L. major-infected BMMP culture supernatants; infected CD11b deficient MP produced approximately five fold more NO than their WT counterparts as late as 48 hr PI, indicating that CR3 ligation mediates the suppression of NO production (Fig. 2).
Figure 2.
L. major infection induces NO in the absence of CD11b. BMMP supernatants were collected after L. major infection (Lm) for the indicated times and assayed for NO2, via Griess reaction. One representative of three independent experiments performed in triplicate is presented. *p<0.05 via Student’s T-test.
CD11b KO and WT BMMP phagocytose opsonized and unopsonized parasites with equal efficiency
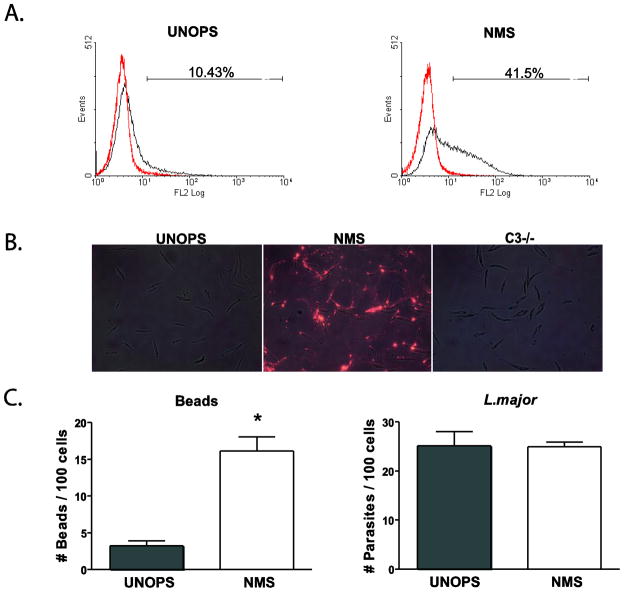
To assess if the phenotypic differences in IL-12 production detected between L. major-infected WT and CD11b KO MP were due to the ability of WT and CD11b KO MP to phagocytose L. major at different rates, we infected BMMP for various times. Both WT and CD11b KO BMMP phagocytosed either unopsonized (Fig. 3A) or opsonized (Fig. 3B) parasites with equal efficiency, indicating that Leishmania metacyclic promastigotes can enter BMMP irrespective of opsonization or the presence of CD11b. To ensure that the opsonization procedure deposited C3b on the surface of Leishmania, unopsonized and opsonized parasites were assessed by both flow cytometric analysis (Fig. 4A) and fluorescence microscopy (Fig. 4B) using antibodies specific for murine C3b. In both cases, only parasites opsonized with NMS were positively stained; parasites opsonized with serum collected from C3-deficient mice did not react with the antibody (Fig. 4B). Opsonization of polystyrene beads with NMS resulted in enhanced uptake, indicating that the opsonization procedure was effective; however, opsonization did not result in enhanced phagocytosis of Leishmania metacyclic promastigotes (Fig. 4C).
Figure 3.
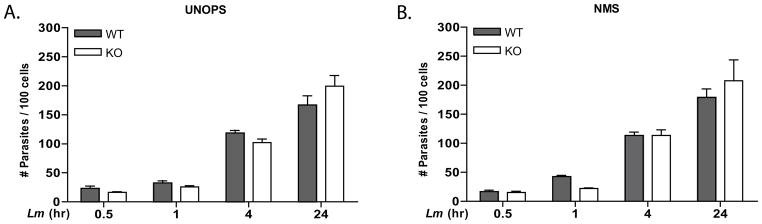
BMMP from WT or CD11b KO mice phagocytose L. major parasites with equal efficiency. BMMP were infected with unopsonized (A) or NMS opsonized (B) parasites at an MOI of 10:1 for the indicated times. Each time point was performed in triplicate and error bars represent standard deviation. *p<0.05. One representative of 3 independent experiments performed in triplicate is presented.
Figure 4.
Treatment of L. major metacyclic parasites with NMS leads to successful C3 opsonization but not to increased uptake. L. major metacyclic promastigotes were either unopsonized (UNOPS) (A,B) or opsonized with NMS (A,B) or C3 deficient mouse serum (C3−/−) (B) were left unstained or stained with a rat anti-mouse C3 antibody, followed by a Cy3 conjugated anti-rat IgG secondary staining. (A) Flow cytometric analysis of C3 stained (black) and secondary alone (red) stained parasites. % positive cells within the gate are indicated. (B) Visualization of stained parasites was performed using the Nikon Eclipse fluorescent microscope. Overlays of bright-field and fluorescent microscopy images where generated using the Adobe Photoshop software. (C) Phagocytic capacity of UNOPS or NMS opsonized L.major metacyclic parasites was evaluated in simultaneous assays with UNOPS or NMS opsonized polystyrene beads. WT BMMP were infected with parasites or beads at an MOI of 10:1 for 30 minutes. Each condition was performed in triplicate and error bars represent standard deviation. * p<0.05. One representative of 2–3 independent experiments performed in triplicate is presented.
L. major infection does not inhibit LPS-induced MAPK activation
MAPK signaling functions upstream of transcription factor activation and is known to be involved in numerous immune effector functions, including IL-12 (45, 46) and inducible nitric oxide synthase (47, 48) activation. We investigated whether L. major infection inhibits MAPK activation and assessed the role of this pathway in L. major signaling through CD11b (Fig. 5). BMMPs were infected with L. major, followed by stimulation with LPS and were analyzed for MAPK activation. While LPS stimulation resulted in ERK1/2 and p38 phosphorylation, infection did not elicit ERK1/2 or p38 activation in the presence or absence of CD11b. Furthermore, infection did not inhibit LPS-induced ERK1/2 or p38 phosphorylation. Identical results were observed in CD11b deficient BMMP, indicating that CD11b-mediated IL-12p40 inhibition in Leishmania-infected cells does not involve modulating MAPK signaling.
Figure 5.
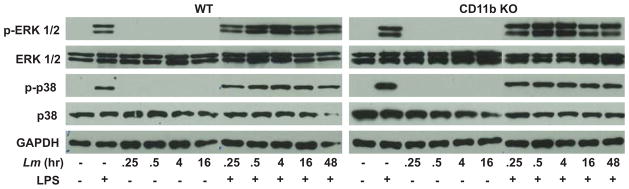
L. major does not inhibit LPS-induced MAPK activation. BMMP were infected for the indicated times with L. major (Lm) followed by LPS stimulation (30 min, 1 ug/ml). Cells were collected and analyzed by Western Blot using phospho-specific antibodies. GAPDH was assessed as a protein loading control. One representative of three independent experiments is presented.
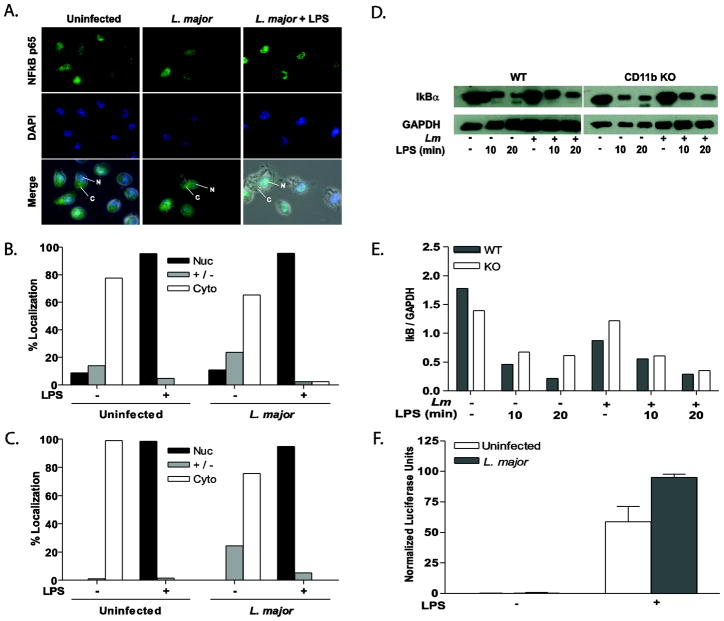
Leishmania infection does not inhibit NFκB activation
NFκB signaling is also known to play a role in immune signaling and activation. NFκB activation and subsequent nuclear translocation occurs in a two-step fashion. First, cytoplasmic IκB is phosphorylated and proteosomally degraded, allowing NFκB to enter the cell nucleus, bind to its promoter elements, and mediate transcription (49). Therefore, we examined the effects of CD11b and L. major infection on NFκB nuclear translocation, LPS-induced IκBα degradation, and NFκB-mediated transcription (Fig. 6). The vast majority of NFκB p65 is localized in the cytoplasm of resting cells, with infection only leading to a slight shift into the nucleus (Fig. 6A–C). In LPS-stimulated cells, NFκB p65 localized almost exclusively to the nucleus (approximately 96%); Leishmania infection of WT or CD11b KO BMMP did not block this translocation (Fig. 6A–C). As little as 10 minutes of LPS stimulation lead to rapid IκBα degradation in both WT and CD11b-deficient BMMP (Fig 6D & E). After sixteen hours of infection there was negligible IκBα degradation; however, L. major infection did not inhibit LPS-induced IκBα degradation in the presence or absence of CD11b, demonstrating that infection does not inhibit the IκB pathway.
Figure 6.
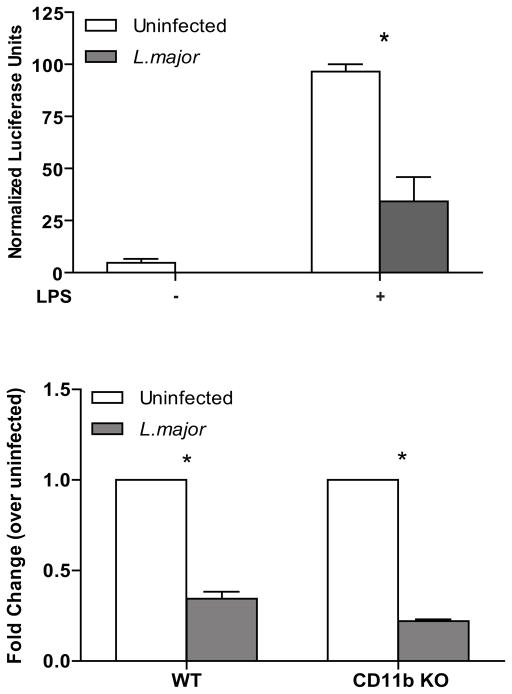
Leishmania infection does not inhibit LPS-induced NFκB activation. Representative immunofluorescence images of NFκB p65 (A) detail that infection of WT BMMP does not inhibit LPS-induced NFκB p65. WT (B) and CD11b KO (C) BMMP were seeded to glass coverslips and infected with L. major for 16 hr with or without LPS stimulation. Cells were fixed, antibody treated and DAPI stained to visualize NFκB p65 (green) and the cell nucleus (blue). Cytoplasm (C) and nuclei (N) are denoted by arrows. Nuclear (Nuc), cytoplasmic (Cyto), or intermediate (+/−) localization was quantified by counting at least 100 cells on each duplicate coverslip (B and C). Cells were collected and analyzed by Western Blot for IκB degradation (D). GAPDH was assessed as a protein loading control. Densitometry readings for IkB were normalized to GAPDH (E). One representative of three independent experiments is presented. (F) RAW 264.7 cells stably transfected with a NFκB luciferase reporter were infected with L. major for 16 hr followed by LPS stimulation for 8 hours. Samples were analyzed in triplicate. *p≤0.05 (Wilcoxon Matched Pair test; n=8) compared to unstimulated control. One representative of three independent experiments is presented.
To assess the transcriptional activity of NFκB promoter elements in L. major infected cells, we used RAW 264.7 MP, stably transfected with a NFκB reporter construct [NFκB-Luciferase-pcDNA3.1 (41)]. Infection alone in NFκB-Luciferase-pcDNA3.1 expressing cells did not activate the NFκB reporter construct. Furthermore, infection did not inhibit the LPS response (Fig. 6F).
IRF-1 and IRF-8 nuclear translocation are not disrupted by Leishmania infection
In addition to NFκB, the IFN-γ responsive transcription factors IRF-1 and IRF-8 also are known to play a role in the control of IL-12p40 promoter activity (50–53) and in expression of (54–56) and signaling through (56) Toll-like receptor-4. IFN-γ stimulation lead to translocation of both proteins from cytoplasm to the nucleus in both WT and CD11b deficient BMMP (Fig. 7). Infection of WT or CD11b KO BMMP with L. major was sufficient to induce IRF-8, but little IRF-1 nuclear translocation. IFN-γ stimulated nuclear translocation of both factors, however, was not inhibited by L. major infection (Fig. 7).
Figure 7.
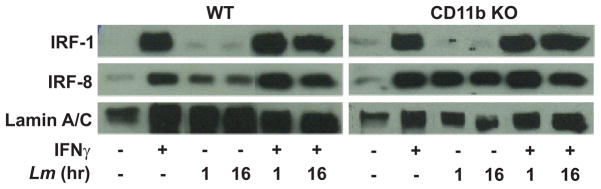
L. major infection does not inhibit IFN-γ-induced IRF-1 or IRF-8 nuclear translocation. WT and CD11b KO MP were infected for the indicated times with or without subsequent IFN-γ stimulation for 2 hr. Nuclear fractions were analyzed for presence of IRF-1 or IRF-8 by western blot analysis. Lamin A/C was assessed as a loading control. One representative of three independent experiments is presented.
ETS-mediated transcription is inhibited by Leishmania infection of MP
In addition to NFκB and IRF transcription factors, the IL-12p40 promoter is also regulated by ETS family members, including ETS-2 and PU.1 (57). To assess the transcriptional activity of ETS promoter elements in Leishmania-infected cells, we used RAW 264.7 MP, stably transfected with an ETS-Luciferase construct (ETS-Luciferase-pcDNA3.1) (41). L. major infection inhibited constitutive and induced ETS-mediated transcription (Fig. 8A). L. major infection also downregulated the mRNA expression of ETS-2 in WT and CD11b KO BMMP (Fig. 8B), indicating that L. major targets ETS-mediated transcription independently of signaling through CD11b.
Figure 8.
L. major infection inhibits ETS-mediated transcription. RAW 264.7 cells stably transfected with an ETS luciferase reporter were infected with L. major for 16 hr followed by LPS stimulation for 8 hours. Samples were analyzed in triplicate. *p≤0.05 (Wilcoxon Matched Pair test; n=7) compared to unstimulated control. One representative of three independent experiments is presented. B) WT and CD11b KO BMMP were infected 10:1 for 16 hrs, after which cells were lysed and assayed for ETS-2 mRNA expression by real-time PCR. Data was normalized to GAPDH and fold change was calculated by the ΔΔCt method, calibrated to uninfected samples. *p≤ 0.05 (Student’s paired t-test; n=3). Samples were analyzed in triplicate.
DISCUSSION
In this report we examine whether Leishmania parasites alter MP signal transduction and transcription factor activation in a CD11b-dependent manner to repress IL-12 and NO production upon cell stimulation. In agreement with previous studies (58) our data indicate that infection of MP with L. major metacyclic promastigotes alone does not induce production of IL-12. Furthermore, infection blocks LPS-induced IL-12 in RAW264.7 and WT BMMP, but this blockage is dependent on MP CD11b expression. IL-12 inhibition by L. major has previously been shown to be dependent on the presence of the CD18 chain of CR3, CR4, and LFA-1 (2). Here we definitively demonstrate a role for the CD11b chain, showing that CR3 ligation is responsible for the Leishmania-mediated inhibition of IL-12 and NO production. When CR3 is unavailable, L. major enters host cells via interactions with other receptors that do not lead to IL-12 inhibition.
While our IL-12 results are in agreement with that reported with CD18-deficient systems (2), our NO results differ. Our data indicate that CD11b deficient cells produce more NO than WT MP in response to L. major infection (Fig. 2). In contrast, NO production in CD18-deficient MP is decreased in response to L. major infection (2). Taken together, these data suggest that CR3 plays an inhibitory role in NO production and thus limits the immune response, whereas other CD18 containing receptors (e.g. LFA-1 or CR4) may promote NO production and the immune response.
The dogmatic theory for Leishmania entry is that serum opsonization of promastigotes augments parasite entry via binding to complement receptors (59). A deeper look into the literature, however, reveals that generally serum opsonization leads to a small enhancement of promastigote attachment, with most studies only assessing early time points (≤ 1 hr.) (3, 7, 60–62). While it is true that Leishmania species are readily complement-opsonized and these parasites can bind to CR3 (9), the extent to which L. major promastigotes interact simultaneously with other receptors remains in question. Our data clearly demonstrate that both unopsonized and C3-opsonized metacyclic promastigotes can readily enter MP in the absence of CD11b (Fig. 3). CD11b is, however, playing a role in L. major inhibition of IL-12 and NO production. Both IL-12 (63) and NO (64) are essential for controlling L. major infection. Accordingly, CD11b deficient mice have been shown to exhibit decreased lesion size and progression (65).
We examined signaling pathways as a potential means by which L. major infection may be inhibiting IL-12 production as observed in WT BMMP. NFκB (66, 67), IRF (68, 69), ETS (57), and MAPK (43, 70) factors are known to play an important role in IL-12p40 transcriptional control. As LPG is thought to bind CR3 directly (8) and is the major C3bi acceptor (9) we hypothesized that Leishmania infection modulates MAPK signaling through CR3. Our data indicate, however, that L. major infection does not activate ERK1/2 or p38 in the presence or absence of CD11b. The MAPK pathway is particularly relevant as it has been reported that activation of ERK1/2 inhibits IL-12 production (43, 70). In addition, there is evidence suggesting that Leishmania infection can modulate MAPK signaling, activating or inhibiting depending on the Leishmania species, life-cycle stage, and host cell (26, 70–75).
Our examination of the NFκB pathway illustrates that Leishmania infection, CD11b-mediated or otherwise, has only a modest activating effect (Fig. 6). L. major amastigotes activate NFκB in promonocytic human cell line U937 cells and human monocytes (76). In contrast, our data indicate that L. major metacyclic promastigote infection induces minimal to no activation of NFκB p65 in both WT and CD11b deficient BMMP. Interestingly, L. donovani promastigotes also fail to degrade IκB and activate NFκB (73). Leishmania infection does not block MAPK or NFκB activation. Leishmania-infected cells treated with LPS following infection exhibit no decrease in p38 or ERK1/2 phosphorylation (Fig. 5) or nuclear translocation of NFκB or upstream IκB degradation (Fig. 6), indicating that neither initial parasite-CR3 contact (and subsequent receptor-propagated signaling) nor established infection disrupts MAPK or NFκB signaling in infected macrophages.
In addition to NFκB, a large transcriptional complex containing, IRF-1, IRF-8, ETS-2 and PU.1 binds the IL-12p40 promoter, functioning primarily in response to IFN-γ and LPS (57). Mutations in the either the NFκB or ETS promoter elements abolishes IL-12p40 promoter activity in response to IFN-γ and LPS (66). IRF-1 was not activated upon L. major infection. However, IFN-γ-induced IRF-1 or IRF-8 nuclear translocation was not inhibited (Fig. 7). Interestingly, Leishmania infection inhibited LPS-induced ETS activation (Fig. 8A), and furthermore down-regulated the expression of ETS-2 (Fig. 8B). Previous studies have shown that MAPK signaling is important in regulating the phosphorylation of ETS transcription factors, including ETS-2 and PU.1 (77); however, as Leishmania infection does not inhibit MAPK activation under the conditions studied (Fig. 5), it is unlikely that Leishmania targets ETS-phosphorylation by blocking MAPK signaling. Rather, L. major infection inhibits the production of ETS-2. The 29 known ETS family transcription factors can modulate transcriptional activity alone, but often synergize with other factors to activate or repress transcription. ETS-2 synergizes with PU.1 at the ETS site in the IL-12p40 promoter (78). L. major infection did not significantly modulate PU.1 expression (data not shown), indicating a role for ETS-2 specifically in the inhibition of ETS-mediated transcription with respect to IL-12p40.
While we have yet to uncover the intracellular signaling mechanisms that mediate CD11b-dependent inhibition of macrophage activation by Leishmania, we have identified a novel CD11b-independent mechanism of Leishmania immune evasion, the downregulation of ETS-mediated transcription. It is likely that Leishmania parasites utilize multiple mechanisms to evade immune responses and may require both CR3 engagement and inhibition of ETS to inhibit IL-12.
Acknowledgments
We thank Freimann Life Science Center at the University of Notre Dame for excellent animal care. We are grateful to Dr. Tanya Mayadas-Norton (Brigham and Women’s Hospital and Harvard Medical School) for the use of the CD11b KO mice. This work was supported by grants to M.A.M. from the National Institutes of Health (NIH) (#RO1AI056242) and American Heart Association (#0435333Z). C.C. was supported by a training grant from the NIH (#T32AI07030).
References
- 1.Antoine JC, Prina E, Courret N, Lang T. Leishmania spp: on the interactions they establish with antigen-presenting cells of their mammalian hosts. Adv Parasitol. 2004;58:1–68. doi: 10.1016/S0065-308X(04)58001-6. [DOI] [PubMed] [Google Scholar]
- 2.Schonlau F, Scharffetter-Kochanek K, Grabbe S, Pietz B, Sorg C, Sunderkotter C. In experimental leishmaniasis deficiency of CD18 results in parasite dissemination associated with altered macrophage functions and incomplete Th1 cell response. Eur J Immunol. 2000;30:2729–2740. doi: 10.1002/1521-4141(200009)30:9<2729::AID-IMMU2729>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell JM, Ezekowitz RA, Roberts MB, Channon JY, Sim RB, Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol. 2006;36:411–420. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 5.Kropf P, Freudenberg MA, Modolell M, et al. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infection and immunity. 2004;72:1920–1928. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woelbing F, Kostka SL, Moelle K, et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosser DM, Edelson PJ. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. Journal of immunology. 1985;135:2785–2789. [PubMed] [Google Scholar]
- 8.Talamas-Rohana P, Wright SD, Lennartz MR, Russell DG. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. Journal of immunology. 1990;144:4817–4824. [PubMed] [Google Scholar]
- 9.Dominguez M, Torano A. Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection. J Exp Med. 1999;189:25–35. doi: 10.1084/jem.189.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balestieri FM, Queiroz AR, Scavone C, Costa VM, Barral-Netto M, de Abrahamsohn IA. Leishmania (L) amazonensis-induced inhibition of nitric oxide synthesis in host macrophages. Microbes Infect. 2002;4:23–29. doi: 10.1016/s1286-4579(01)01505-2. [DOI] [PubMed] [Google Scholar]
- 11.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. Journal of Infections Disease. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 13.Karp CL, Wysocka M, Wahl LM, et al. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 14.Smith A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102:2877–2884. doi: 10.1182/blood-2002-10-3152. [DOI] [PubMed] [Google Scholar]
- 15.Tang N, Liu L, Kang K, et al. Inhibition of monocytic interleukin-12 production by Candida albicans via selective activation of ERK mitogen-activated protein kinase. Infection and immunity. 2004;72:2513–2520. doi: 10.1128/IAI.72.5.2513-2520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai S, Rajaram MV, Curry HM, Leander R, Schlesinger LS. Fine Tuning Inflammation at the Front Door: Macrophage Complement Receptor 3-mediates Phagocytosis and Immune Suppression for Francisella tularensis. PLoS pathogens. 2013;9:e1003114. doi: 10.1371/journal.ppat.1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher BA, Denkers EY. Mechanism of entry determines the ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infection and immunity. 2002;70:5216–5224. doi: 10.1128/IAI.70.9.5216-5224.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Sumita K, Feng C, et al. Down-regulation of IL-12 p40 gene in Plasmodium berghei-infected mice. Journal of immunology. 2001;167:235–241. doi: 10.4049/jimmunol.167.1.235. [DOI] [PubMed] [Google Scholar]
- 19.Kwan WC, McMaster WR, Wong N, Reiner NE. Inhibition of expression of major histocompatibility complex class II molecules in macrophages infected with Leishmania donovani occurs at the level of gene transcription via a cyclic AMP-independent mechanism. Infection and immunity. 1992;60:2115–2120. doi: 10.1128/iai.60.5.2115-2120.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corradin S, Mauel J, Ransijn A, Sturzinger C, Vergeres G. Down-regulation of MARCKS-related protein (MRP) in macrophages infected with Leishmania. J Biol Chem. 1999;274:16782–16787. doi: 10.1074/jbc.274.24.16782. [DOI] [PubMed] [Google Scholar]
- 21.Olivier M, Brownsey RW, Reiner NE. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci U S A. 1992;89 :7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm A, Tejle K, Magnusson KE, Descoteaux A, Rasmusson B. Leishmania donovani lipophosphoglycan causes periphagosomal actin accumulation: correlation with impaired translocation of PKCalpha and defective phagosome maturation. Cell Microbiol. 2001;3:439–447. doi: 10.1046/j.1462-5822.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- 23.Scianimanico S, Desrosiers M, Dermine JF, Meresse S, Descoteaux A, Desjardins M. Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cell Microbiol. 1999;1:19–32. doi: 10.1046/j.1462-5822.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 24.Akarid K, Arnoult D, Micic-Polianski J, Sif J, Estaquier J, Ameisen JC. Leishmania major-mediated prevention of programmed cell death induction in infected macrophages is associated with the repression of mitochondrial release of cytochrome c. Journal of leukocyte biology. 2004;76:95–103. doi: 10.1189/jlb.1001877. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Bhattacharyya S, Das S, et al. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasitic survival. Mol Cell Biochem. 2001;223:47–60. doi: 10.1023/a:1017996609928. [DOI] [PubMed] [Google Scholar]
- 26.Martiny A, Meyer-Fernandes JR, de Souza W, Vannier-Santos MA. Altered tyrosine phosphorylation of ERK1 MAP kinase and other macrophage molecules caused by Leishmania amastigotes. Mol Biochem Parasitol. 1999;102:1–12. doi: 10.1016/s0166-6851(99)00067-5. [DOI] [PubMed] [Google Scholar]
- 27.Blanchette J, Racette N, Faure R, Siminovitch KA, Olivier M. Leishmania-induced increases in activation of macrophage SHP-1 tyrosine phosphatase are associated with impaired IFN-gamma-triggered JAK2 activation. Eur J Immunol. 1999;29:3737–3744. doi: 10.1002/(SICI)1521-4141(199911)29:11<3737::AID-IMMU3737>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 28.Ray M, Gam AA, Boykins RA, Kenney RT. Inhibition of interferon-gamma signaling by Leishmania donovani. J Infect Dis. 2000;181:1121–1128. doi: 10.1086/315330. [DOI] [PubMed] [Google Scholar]
- 29.Forget G, Siminovitch KA, Brochu S, Rivest S, Radzioch D, Olivier M. Role of host phosphotyrosine phosphatase SHP-1 in the development of murine leishmaniasis. Eur J Immunol. 2001;31:3185–3196. doi: 10.1002/1521-4141(200111)31:11<3185::aid-immu3185>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Nandan D, Reiner NE. Leishmania donovani engages in regulatory interference by targeting macrophage protein tyrosine phosphatase SHP-1. Clin Immunol. 2005;114:266–277. doi: 10.1016/j.clim.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron P, McGachy A, Anderson M, et al. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-kappaB signaling pathway. Journal of immunology. 2004;173:3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 33.Carrera L, Gazzinelli RT, Badolato R, et al. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. Journal of immunology. 2000;165:364–372. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- 35.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. Journal of immunology. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 37.Lub M, van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 38.Kuypers TW, Roos D. Leukocyte membrane adhesion proteins LFA-1, CR3 and p150,95: a review of functional and regulatory aspects. Res Immunol. 1989;140:461–486. doi: 10.1016/0923-2494(89)90114-4. [DOI] [PubMed] [Google Scholar]
- 39.Coxon A, Rieu P, Barkalow FJ, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 40.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 41.Lee SB, Schorey JS. Activation and mitogen-activated protein kinase regulation of transcription factors Ets and NF-kappaB in Mycobacterium-infected macrophages and role of these factors in tumor necrosis factor alpha and nitric oxide synthase 2 promoter function. Infection and immunity. 2005;73:6499–6507. doi: 10.1128/IAI.73.10.6499-6507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, McDowell MA. Uninfected mosquito bites confer protection against infection with malaria parasites. Infection and immunity. 2007;75:2523–2530. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo X, Liu L, Tang N, et al. Inhibition of monocyte-derived dendritic cell differentiation and interleukin-12 production by complement iC3b via a mitogen-activated protein kinase signalling pathway. Exp Dermatol. 2005;14:303–310. doi: 10.1111/j.0906-6705.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 44.Weinheber N, Wolfram M, Harbecke D, Aebischer T. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur J Immunol. 1998;28:2467–2477. doi: 10.1002/(SICI)1521-4141(199808)28:08<2467::AID-IMMU2467>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Kang BY, Chung SW, Cho D, Kim TS. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem Pharmacol. 2002;63:1901–1910. doi: 10.1016/s0006-2952(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 46.Kim L, Del Rio L, Butcher BA, et al. p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. Journal of immunology. 2005;174:4178–4184. doi: 10.4049/jimmunol.174.7.4178. [DOI] [PubMed] [Google Scholar]
- 47.Chan ED, Riches DW. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280:C441–450. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- 48.Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res. 2006;84:1037–1046. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- 49.Chen LF, Greene WC. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med. 2003;81:549–557. doi: 10.1007/s00109-003-0469-0. [DOI] [PubMed] [Google Scholar]
- 50.Holtschke T, Lohler J, Kanno Y, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 51.Maruyama S, Sumita K, Shen H, et al. Identification of IFN regulatory factor-1 binding site in IL-12 p40 gene promoter. Journal of immunology. 2003;170:997–1001. doi: 10.4049/jimmunol.170.2.997. [DOI] [PubMed] [Google Scholar]
- 52.Masumi A, Tamaoki S, Wang IM, Ozato K, Komuro K. IRF-8/ICSBP and IRF-1 cooperatively stimulate mouse IL-12 promoter activity in macrophages. FEBS Lett. 2002;531:348–353. doi: 10.1016/s0014-5793(02)03556-1. [DOI] [PubMed] [Google Scholar]
- 53.Wu CY, Maeda H, Contursi C, Ozato K, Seder RA. Differential requirement of IFN consensus sequence binding protein for the production of IL-12 and induction of Th1-type cells in response to IFN-gamma. Journal of immunology. 1999;162:807–812. [PubMed] [Google Scholar]
- 54.Maratheftis CI, Giannouli S, Spachidou MP, Panayotou G, Voulgarelis M. RNA interference of interferon regulatory factor-1 gene expression in THP-1 cell line leads to Toll-like receptor-4 overexpression/activation as well as up-modulation of annexin-II. Neoplasia. 2007;9:1012–1020. doi: 10.1593/neo.07640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nhu QM, Cuesta N, Vogel SN. Transcriptional regulation of lipopolysaccharide (LPS)-induced Toll-like receptor (TLR) expression in murine macrophages: role of interferon regulatory factors 1 (IRF-1) and 2 (IRF-2) J Endotoxin Res. 2006;12:285–295. doi: 10.1179/096805106X118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J, Kong HJ, Li H, et al. IRF-8/interferon (IFN) consensus sequence-binding protein is involved in Toll-like receptor (TLR) signaling and contributes to the cross-talk between TLR and IFN-gamma signaling pathways. J Biol Chem. 2006;281:10073–10080. doi: 10.1074/jbc.M507788200. [DOI] [PubMed] [Google Scholar]
- 57.Ma X, Neurath M, Gri G, Trinchieri G. Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J Biol Chem. 1997;272:10389–10395. doi: 10.1074/jbc.272.16.10389. [DOI] [PubMed] [Google Scholar]
- 58.Jayakumar A, Widenmaier R, Ma X, McDowell MA. Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp-infected macrophages. J Parasitol. 2008;94:84–93. doi: 10.1645/GE-1153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stafford JL, Neumann NF, Belosevic M. Macrophage-mediated innate host defense against protozoan parasites. Crit Rev Microbiol. 2002;28:187–248. doi: 10.1080/1040-840291046731. [DOI] [PubMed] [Google Scholar]
- 60.Da Silva RP, Hall BF, Joiner KA, Sacks DL. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. Journal of immunology. 1989;143:617–622. [PubMed] [Google Scholar]
- 61.Rosenthal LA, Sutterwala FS, Kehrli ME, Mosser DM. Leishmania major-human macrophage interactions: cooperation between Mac- 1 (CD11b/CD18) and complement receptor type 1 (CD35) in promastigote adhesion. Infection and immunity. 1996;64:2206–2215. doi: 10.1128/iai.64.6.2206-2215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infection and immunity. 1988;56:363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattner F, Di Padova K, Alber G. Interleukin-12 is indispensable for protective immunity against Leishmania major. Infection and immunity. 1997;65:4378–4383. doi: 10.1128/iai.65.11.4378-4383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukbel RM, Patten C, Jr, Gibson K, Ghosh M, Petersen C, Jones DE. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Am J Trop Med Hyg. 2007;76:669–675. [PubMed] [Google Scholar]
- 65.Carter CR, Whitcomb JP, Campbell JA, Mukbel RM, McDowell MA. Complement receptor 3 deficiency influences lesion progression during Leishmania major infection in BALB/c mice. Infection and immunity. 2009;77:5668–5675. doi: 10.1128/IAI.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gri G, Savio D, Trinchieri G, Ma X. Synergistic regulation of the human interleukin-12 p40 promoter by NFkappaB and Ets transcription factors in Epstein-Barr virus- transformed B cells and macrophages. J Biol Chem. 1998;273:6431–6438. doi: 10.1074/jbc.273.11.6431. [DOI] [PubMed] [Google Scholar]
- 67.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half- site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giese NA, Gabriele L, Doherty TM, et al. Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J Exp Med. 1997;186:1535–1546. doi: 10.1084/jem.186.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- 70.Feng GJ, Goodridge HS, Harnett MM, et al. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. Journal of immunology. 1999;163:6403–6412. [PubMed] [Google Scholar]
- 71.Balaraman S, Singh VK, Tewary P, Madhubala R. Leishmania lipophosphoglycan activates the transcription factor activating protein 1 in J774A. 1 macrophages through the extracellular signal-related kinase (ERK) and p38 mitogen-activated protein kinase. Mol Biochem Parasitol. 2005;139:117–127. doi: 10.1016/j.molbiopara.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Boggiatto PM, Jie F, Ghosh M, et al. Altered dendritic cell phenotype in response to Leishmania amazonensis amastigote infection is mediated by MAP kinase, ERK. The American journal of pathology. 2009;174:1818–1826. doi: 10.2353/ajpath.2009.080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prive C, Descoteaux A. Leishmania donovani promastigotes evade the activation of mitogen-activated protein kinases p38, c-Jun N-terminal kinase, and extracellular signal-regulated kinase-1/2 during infection of naive macrophages. Eur J Immunol. 2000;30:2235–2244. doi: 10.1002/1521-4141(2000)30:8<2235::AID-IMMU2235>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 74.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. Journal of immunology. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Z, Zhang X, Darrah PA, Mosser DM. The regulation of Th1 responses by the p38 MAPK. Journal of immunology. 2010;185:6205–6213. doi: 10.4049/jimmunol.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guizani-Tabbane L, Ben-Aissa K, Belghith M, Sassi A, Dellagi K. Leishmania major amastigotes induce p50/c-Rel NF-kappa B transcription factor in human macrophages: involvement in cytokine synthesis. Infection and immunity. 2004;72:2582–2589. doi: 10.1128/IAI.72.5.2582-2589.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tootle TL, Rebay I. Post-translational modifications influence transcription factor activity: a view from the ETS superfamily. Bioessays. 2005;27:285–298. doi: 10.1002/bies.20198. [DOI] [PubMed] [Google Scholar]
- 78.Gallant S, Gilkeson G. ETS transcription factors and regulation of immunity. Archivum immunologiae et therapiae experimentalis. 2006;54:149–163. doi: 10.1007/s00005-006-0017-z. [DOI] [PubMed] [Google Scholar]