The Inflammation and Host Response to Injury Large-Scale Collaborative Research program, funded by the National Institute of General Medical Sciences of the National Institutes of Health, is the first large-scale interdisciplinary program to attempt to solve the life-threatening problem of inflammation after major trauma or burn injury. One of the main goals of the project was to develop a large clinical database with baseline patient characteristics and well-defined outcomes that pertain to the host inflammatory response after injury. To minimize the impact of practice variation between participating sites, clinical guidelines were developed at the outset of the project, based on the best available evidence in concert with expert consensus.1 The resultant standards of practice guidelines have been provided to the clinical community to ensure transparency of the research initiative, highlight the applicability of these best practices, and to promote the ongoing development of consistent, high-quality care.
Recognizing the complexity and heterogeneity of the patient population, the investigators have additionally created a library of standardized diagnostic criteria for complications after traumatic injury. The method used to define these complications incorporated several key components. An expert panel of surgeons who were participating investigators in the Patient-Oriented Research Core was assembled to identify and enumerate the complications that arise after traumatic injury to be included in the study database. Definitions were based on the best available evidence and were clarified to attempt to unambiguously describe complications in such a manner that reliable data could be captured relative to that complication. Input into the consensus process was also obtained from trauma research nurses to modify the definitions to facilitate practical measurement of complications based on information available in the medical record. Although the primary intent was to maintain consistent reporting and quality assessment of treatment and outcomes among participating trauma centers, the compilation of these infectious and noninfectious complication definitions in a single source may serve as a useful reference for the clinician, as well as a framework for future research.
Surgical Site Infections
Surgical Site Infections2,3
A surgical site is any site in which an incision has been made or a procedure performed. As an example, an empyema occurring in a trauma patient without previous manipulation of the thorax is not a Surgical Site Infections (SSI), whereas it should be classified as an SSI if a chest tube was inserted or a video assisted thoracoscopic surgery or thoracotomy had been performed before the development of the infection. Classification of SSI is based on the anatomic depth of involvement, differentiated by superficial incisional, deep incisional, and organ or space locations (see below and Fig. 1). In the practical application of SSI definitions, our experience has been that problems may be encountered in the identification of SSI because there is frequently incomplete or ambiguous information in the medical record. The difficulty in retrospectively identifying this information from the medical record suggests that an initial and ongoing educational effort to document what is usually a clinical diagnosis is required to maximize identification and capture of this common postinjury complication.
Figure 1.
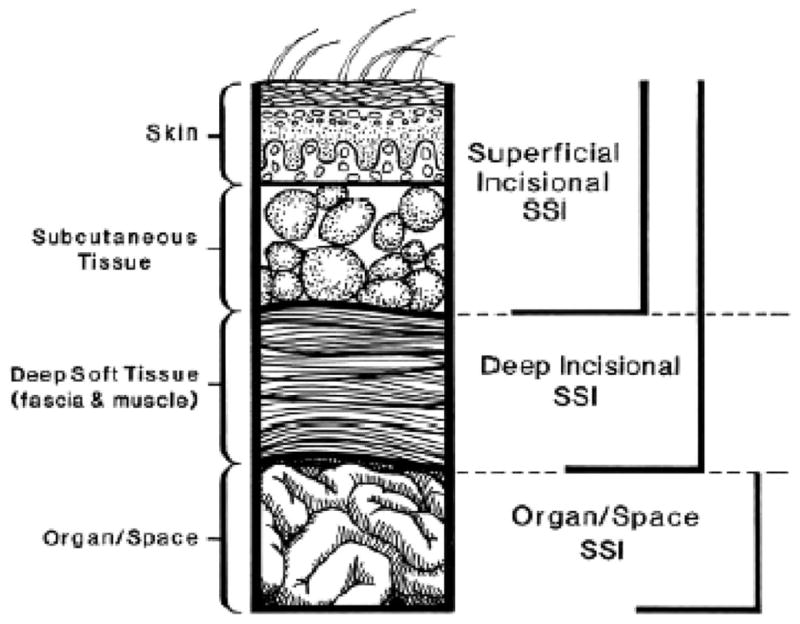
Schematic representation depicting classification of surgical site infections (SSI).
SSI Body Region, Date and Type of Organism
Confusion can easily arise with regard to how best to deal with multiple organisms or sites of infection. When two or more organisms are identified all pathogenic organisms should be identified and recorded. It is also possible for patients to have more than one SSI involving the same body region during the course of hospitalization. If so, the events are considered two separate SSI with different dates, different types, but the same region. When two types of SSI occur together in the same region, on the same date, only the deepest infection is recorded. Date of onset for SSI is the earliest date on which sufficient diagnostic criteria (discussed below) to define a SSI are present.
Superficial Incisional SSI
By definition, these infections occur within 30 days after the operation and involve only skin or subcutaneous tissue of the incision. In addition, at least one of four criteria should be present: (1) purulent drainage, with or without laboratory confirmation, from the superficial incision, (2) organisms isolated from an aseptically obtained culture of fluid or tissue from the incision, (3) at least one clinical sign or symptoms of infection: pain or tenderness, localized swelling, redness, or heat, and (4) clinical diagnosis of superficial incisional SSI by the surgeon or attending physician (e.g., need to open wound).
Conditions such as stitch abscess (minimal inflammation and discharge confined to the points of suture penetration), infected burn wound, or incisional SSI that extends into the fascial and muscle layers (refer Deep Incisional SSI) should not be described as superficial incisional SSI. Erythema or serous drainage at external fixator pin sites is common and does not constitute a superficial SSI. Although identification of hardware-associated infections is difficult, if purulence and positive site cultures are present, particularly when obtained on hardware removal, this is more consistent with a deep incisional SSI.
Deep Incisional SSI
Diagnosis of these infections must also occur within 30 days after the operation (or within 1 year if there was a surgical implant was placed at the index operation). By definition, this infection involves the deep soft tissues (e.g., fascial and muscle layers) of the incision, is related to the operation, and at least one of the following four criteria are present: (1) Purulent drainage emanates from the deep incision, but not from the organ or space component of the surgical site. (2) A deep incision spontaneously dehisces or is deliberately opened by a surgeon in patients with fever (temperature >38°C), localized pain or tenderness, unless the site is culture-negative. (3) An abscess or other evidence of infection involving the deep incision is found on direct examination, during re-operation, or by histopathologic or radiologic examination. (4) A surgeon or attending physician makes a clinical diagnosis of a deep incisional SSI. As a general rule, the morbidity of deep SSI is greater than superficial SSI, so if a patient has a SSI that involves both superficial and deep incision sites it should be described as a deep incisional SSI. In addition, an organ or space SSI (see below) that drains through the incision should be identified as a deep incisional SSI.
Organ or Space SSI
Infection is diagnosed within 30 days after the operation if no implant is left in place or within 1 year if an implant is in place and if the infection appears to be related to the operation and involves any part of the anatomy (e.g., organs or spaces) other than the incision, which was opened or manipulated during an operation. Additionally, at least one of the following criteria must be met: (1) purulent drainage from a drain that is placed through a stab wound into the organ or space, (2) organisms isolated from an aseptically obtained culture of fluid or tissue in the organ or space, (3) an abscess or other evidence of infection involving the organ or space that is found on direct examination, during reoperation, or by histopathologic or radiologic examination, and (4) clinical diagnosis of an organ or space SSI by a surgeon or attending physician. Note that if the area around a stab wound becomes infected, it is not an SSI. It is considered a skin or soft tissue infection, depending on its depth.
Other Healthcare-associated Infections
Healthcare-associated Infection Site, Date, and Organism
The date of infection refers to the date on which all criteria were met. Definitions of specific infections are as described below. For sites that have a bacterial threshold (e.g., bronchoalveolar lavage, urine), record only those infections that exceed the designated threshold. For all other sites, record all infections. Continue to use these rules when cultures reveal multiple pathogens, documenting all infections present according to criteria.
Pneumonia2,4
Diagnostic criteria for pneumonia remain contentious, in part because there is no “gold standard” definition. In some instances, the criteria differ depending on the diagnostic method of diagnosis; therefore, the method (invasive or noninvasive) should be recorded. Bacterial confirmation using invasive means (bronchoscopic alveolar lavage, bronchoscopic protected specimen brushing, or blind “mini-bronchoscopic alveolar lavage”) to obtain quantitative cultures is strongly encouraged for all ventilated patients and this approach is being used increasingly in many intensive care unit (ICU)s at this time.5 Use of the clinical pulmonary infection score may assist clinicians in identifying patients likely to have ventilator associated pneumonia; scores of 6 or greater, or a high clinical suspicion, should prompt invasive diagnostic tests of the lower respiratory tract. An algorithm depicting this procedure has been published previously as part of the standard operating procedures for the prevention, diagnosis, and treatment of ventilator associated pneumonia.6
Diagnosis of pneumonia is based on the presence of radiologic, clinical, and bacterial criteria as follows: (1) Radiologic criteria: new radiographic infiltrate that persists for at least 24 hours, (2) Clinical criteria (at least one): (a) temperature >38.5°C (or <35.0°C), (b) WBC >10,000/mm3 (or <3,000/mm3), (c) hypotension (systolic blood pressure <90 mm Hg or >25% drop in systolic blood pressure), (3) Bacterial confirmation by (at least one): (a) quantitative microbiologic cultures obtained by bronchoalveolar lavage yielding ≥104 CFU/mL or protected specimen brush >103 CFU/mL, (b) histopathologic examination of lung tissue shows abscess formation with intense polymorphonuclear neutrophil accumulation in bronchioles and alveoli or quantitative culture of lung parenchyma that shows ≥104 CFU/g tissue, and c) blood culture positive for bacterial pathogen identified in sputum or respiratory culture. Furthermore, these diagnostic criteria must be satisfied (present) within a 48-hour period. Considerably, less reliable bacterial criteria may be used to define pneumonia if the physician’s notes document that the patient has pneumonia and is being treated with antimicrobial therapy for this infection. These criteria are defined as pleural fluid culture with same organism identified in sputum or other respiratory culture, sputum Gram’s stain with ≥3 of one type of pathogenic bacteria, or heavy or moderate growth of one type of pathogenic bacteria on semi-quantitative sputum culture.
Bloodstream Infections2
Bloodstream infections are generally diagnosed with little ambiguity based on the culture positive presence of bacteria in a sample of blood. Bacteriologic and clinical criteria listed below are required to make a diagnosis of bloodstream infection only if the organism identified is a common skin contaminant (e.g., Diptheroids, Bacillus spp., Propionobacterium spp., coagulase-negative Staphylococcus). Bacteriologic confirmation is based on the presence of a recognized skin pathogen from one or more blood cultures that is not related to an infection at another site. If a common skin contaminant as listed above is isolated, the organism must be cultured from at least two cultures within a 48-hour period. Clinical criteria to be satisfied include (at least one): (a) Temperature >38.5°C (or <35.0°C), (b) WBC >10,000/ mm3 (or <3,000/mm3), and (c) Hypotension (systolic blood pressure <90 mm Hg or >25% drop in systolic blood pressure). It should be noted that the primary infection responsible for positive bloodstream cultures should be indentified. Important etiologic information may be obtainable from identification of bacteria in the bloodstream. For example, isolation of gram-negative bacteria from the blood suggests the possibility of an abdominal, gastrointestinal, or urinary source.
Catheter-related Bloodstream Infections7
This infection is a diagnosis of exclusion defined by the presence of bacteremia or fungemia in a patient with a central venous catheter (CVC) in which there is no alternate source for bacteremia or fungemia except the catheter. To diagnose a catheter-related bloodstream infections, the patient must meet the following three criteria within a 48-hour period: (1) 1 or more positive blood cultures from a peripheral vein, (2) clinical criteria (at least one): (a) temperature >38.5°C (or <35.0°C), (b) WBC >10,000/mm3 (or <3000/mm3), (c) hypotension (systolic blood pressure <90 mm Hg or >25% drop in systolic blood pressure), (3) microbiologic evidence of catheter infection as demonstrated by one of the following: (a) positive semi-quantitative (>15 CFU/catheter segment) culture in which the same organism is isolated from the catheter and peripheral blood, (b) a positive quantitative (>103 CFU/catheter segment catheter) culture in which the same organism is isolated from the catheter and peripheral blood, (c) simultaneous quantitative blood cultures with a ≥5:1 ratio of the same bacterial species (CVC vs. peripheral), and (d) differential period of CVC culture versus peripheral blood culture positivity of >2 hours.
Urinary Tract Infections
Within a 48-hour period, urine culture must reveal >105 organisms per milliliter of urine in the setting of at least one of the following clinical criteria: (1) temperature >38.5°C, (2) WBC >10,000/mm3 or <3000/mm3 (3) urinary urgency, (4) dysuria, and (5) suprapubic tenderness.
Meningitis2
Diagnosed on the basis of positive bacterial or fungal cultures from cerebrospinal fluid. Common clinical symptoms suggestive of meningitis include headache, fever, neck stiffness, or altered mental status. One or more of these symptoms are present in 99% of ambulatory patients, however, critically injured patients in the ICU may not be able to provide reliable history or physical examination.
Sinusitis2
Requires positive bacterial or fungal cultures from either percutaneous or intra-operative aspirate of sinuses. Clinical symptoms or signs may include pain or tenderness over the affected sinus, tooth pain, or purulent nasal drainage. Opacification of sinuses on plain or computed tomography (CT) radiographs of the face may be suggestive, but culture of invasive specimens is required to make the diagnosis.
Endocarditis2
Along with microbiologic evidence of bloodstream infection, valvular vegetations must be demonstrated either on echocardiography or autopsy.
Cholecystitis (Acalculous or Calculous)
Diagnosis may be made on pathologic confirmation of acute cholecystitis or based on ultrasound evidence of acute cholecystitis in conjunction with at least one clinical criteria: temperature >38.5°C or WBC >10,000/mm3 or WBC <3,000/mm3.
Empyema2
To meet the criteria for diagnosis of empyema, positive bacterial or fungal culture of fluid or tissue must be obtained from the pleural space, requiring insertion of chest tube, percutaneous drainage or thoracoscopy or thoracotomy for evacuation and drainage.
Pseudomembranous Colitis8
Also referred to as Clostridium difficile colitis, diagnosis requires at least one of the following: (1) pseudomembranes identified at lower gastrointestinal endoscopy, (2) pathologic confirmation of pseudomembranous colitis, (3) C. difficile toxin detected in stool. The presence of C. difficile colitis should be strongly suspected in ICU patients with unexplained watery diarrhea who have recently received antibiotics. However, diarrhea may be absent in patients with severe C. difficile colitis (for example, due to paralytic ileus or toxic megacolon), and therefore, the diagnosis should be considered in the setting of unexplained fever or high white blood cell counts.
Non-Infectious Complications
Acute Lung Injury9
Requires all the following criteria to be met within a 24-hour period: (1) acute onset bilateral infiltrates on chest radiograph, (2) PaO2/FiO2 <300 regardless of positive end-expiratory pressure, and (3) no evidence of left atrial hypertension (pulmonary capillary wedge pressure [PCWP] ≤ 18 if measured) or no evidence of congestive heart failure in the absence of a pulmonary artery catheter (PAC). If a PAC is in place, there must be evidence that the PCWP was ≤ 18 for at least 12 consecutive hours during the 24-hour assessment block.
Acute Respiratory Distress Syndrome9
Requires all the following criteria to be met within a 24-hour period: (1) acute onset bilateral infiltrates on chest radiograph, (2) PaO2/FiO2 <200 regardless of positive end-expiratory pressure, and (3) no evidence of left atrial hypertension (PCWP ≤18 if measured) or no evidence of congestive heart failure in the absence of a PAC. If a PAC is in place, there must be evidence that the PCWP was ≤18 for at least 12 consecutive hours during the 24-hour assessment block.
Fat Embolism Syndrome
Diagnosis requires presence of long bone fracture with subsequent development of at least 1 major and 3 minor or 2 major and 2 minor criteria (as defined below) within 48 hours of admission or within 24 hours of fixation of femur, fibula, tibia, or humerus.10,11 Major criteria include: (1) Petechial rash, (2) central nervous system depression as defined by confusion, drowsiness, or coma not evident at admission, and (3) respiratory signs: PaO2/FiO2<300 or bilateral diffuse patchy infiltrates on chest radiograph. Minor criteria include (1) heart rate >120 bpm, (2) temperature >39.4°C, (3) retinal changes (e.g., fat or petechiae), (4) jaundice, (5) anuria or oliguria (<30 ml urine per hour), (6) thrombocytopenia (>50% decrease from admission platelet count), (7) sudden, >20% decrease in hemoglobin or hematocrit, and (8) fat globules found in urine or blood. In the rare setting of patent foramen ovale and massive fat embolism, pulmonary hypertension can result in direct right to left shunting and account for the systemic manifestations of fat embolism.12
Cardiac Arrest13
Sudden cessation of cardiac activity, including development of pulseless electrical activity, after arrival to emergency department.
Myocardial Infarction14
Acute, irreversible myocardial injury is documented by both enzymatic and electrocardiographic changes. These include abnormal increase in CK-MB or troponin and new, serial T-wave, S-T segment, or Q wave electrocardiography abnormalities.
Cerebral Infarction15
New neurologic deficit not present on admission which is sudden or rapid in onset, lasts more than 24 hours or until death, and is confirmed as an infarction by CT or magnetic resonance imaging.
Deep Venous Thrombosis
Venous thrombosis in the extremities or pelvis is confirmed by autopsy, venogram, or duplex scan or other non-invasive vascular evaluation.
Pulmonary Embolus
Diagnosis requires evidence of pulmonary embolus on angiography or CT scan or on the basis of a moderate-or high-probability ventilation or perfusion radionuclide scan.
Rhabdomyolysis
Criteria for diagnosis of rhabdomyolysis include serum myoglobin >10,000 ng/mL or creatine kinase >5,000 U/l in the setting of at least one of the following: (1) positive urinary myoglobin (qualitative or quantitative), (2) urine that is positive for blood on dipstick (thus positive for heme) with no red blood cells present, and (3) renal dysfunction not explained by other insult.
Abdominal Compartment Syndrome
Requires opening of abdominal cavity for elevated intra-abdominal pressure (>25 cm H20) associated with at least one of the following: oliguria (<30 mL urine per hour), diminished cardiac output (<2.5 L/min/m2), elevated plateau (static) pressures (>45 cm H20) or PaO2/FiO2 ratio of less than 200.
Upper Gastrointestinal Bleeding
Requires evidence of overt bleeding and clinical signs (defined below) occurring more than 48 hours after trauma. Overt bleeding is characterized by hematemesis (gross blood or coffee grounds), hematochezia, or melena. Clinical signs include increase in heart rate >20 bpm, decrease in blood pressure >20 mm Hg, decrease in hemoglobin >2 g/dL (or hematocrit >6 points), and blood transfusion. Ideally, the origin of upper gastrointestinal bleeding should be confirmed endoscopically, but rarely this is not feasible or safe.
Acknowledgments
Supported by a Large-Scale Collaborative Project Award (U54-GM62119) from The National Institute of General Medical Sciences, National Institutes of Health.
Additional participating investigators in the Large Scale Collaborative Research Agreement entitled, “Inflammation and the Host Response to Injury” include
Henry V. Baker, PhD, Timothy R. Billiar, MD, Bernard H. Brownstein, PhD, Steven E. Calvano, PhD, Irshad H. Chaudry, PhD, J. Perren Cobb, MD, Chuck Cooper, MS, Ronald W. Davis, PhD, Adrian Fay, PhD, Robert J. Feezor MD, Richard L. Gamelli, MD, Nicole S. Gibran, MD, Doug Hayden, MS, David N. Herndon, MD, John Lee Hunt, MD, Krzysztof Laudanski, MD, MA, James A. Lederer, PhD, Stephen F. Lowry, MD, John A. Mannick, MD, Carol L. Miller-Graziano, PhD, Michael Mindrinos, PhD, Lyle L. Moldawer, PhD, Grant E. O’Keefe, MD, MPH, Laurence G. Rahme, PhD, Daniel G. Remick, Jr, MD, David Schoenfeld, PhD, Robert L. Sheridan, MD, Geoffrey M. Silver, MD, Richard D. Smith, PhD, Gregory Stephanopoulos, PhD, Ronald G. Tompkins, MD, ScD, Mehmet Toner, PhD, H. Shaw Warren, MD, Steven E. Wolf, MD, Wenzhong Xiao, PhD, Martin Yarmush, MD, PhD, Vernon R. Young, PhD, ScD.
References
- 1.Maier RV, Bankey PE, McKinley BA, et al. Inflammation and the host response to injury, a Large-Scale Collaborative Project: patient-oriented research core—standard operating procedures for clinical care: foreward. J Trauma. 2005;59:762–763. [PubMed] [Google Scholar]
- 2.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 3.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. quiz 279–280. [DOI] [PubMed] [Google Scholar]
- 4.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- 5.Heyland DK, Cook DJ, Marshall J, et al. The clinical utility of invasive diagnostic techniques in the setting of ventilator-associated pneumonia. Canadian Critical Care Trials Group. Chest. 1999;115:1076–1084. doi: 10.1378/chest.115.4.1076. [DOI] [PubMed] [Google Scholar]
- 6.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury Large Scale Collaborative Research Program Investigators. Inflammation and the host response to injury, a Large-Scale Collaborative Project: patient-oriented research core–standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 7.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51:1–29. [PubMed] [Google Scholar]
- 8.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28:140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 10.Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56B:408–416. [PubMed] [Google Scholar]
- 11.Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132:435–439. doi: 10.1001/archsurg.1997.01430280109019. [DOI] [PubMed] [Google Scholar]
- 12.Pell AC, Hughes D, Keating J, Christie J, Busuttil A, Sutherland GR. Brief report: fulminating fat embolism syndrome caused by paradoxical embolism through a patent foramen ovale. N Engl J Med. 1993;329:926–929. doi: 10.1056/NEJM199309233291305. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs I, Nadkarni V, Bahr J, et al. International Liaison Committee on Resuscitation; American Heart Association; European Resuscitation Council; Australian Resuscitation Council; New Zealand Resuscitation Council; Heart and Stroke Foundation of Canada; InterAmerican Heart Foundation; Resuscitation Councils of Southern Africa; ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 15.The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]


