Abstract
Aim:
To investigate the effect of genipin on apoptosis in human leukemia K562 cells in vitro and elucidate the underlying mechanisms.
Methods:
The effect of genipin on K562 cell viability was measured using trypan blue dye exclusion and cell counting. Morphological changes were detected using phase-contrast microscopy. Apoptosis was analyzed using DNA ladder, propidium iodide (PI)-labeled flow cytometry (FCM) and Hoechst 33258 staining. The influence of genipin on cell cycle distribution was determined using PI staining. Caspase 3 activity was analyzed to detect apoptosis at different time points. Protein levels of phospho-c-Jun, phosphor-c-Jun N-terminal kinase (p-JNK), phosphor-p38, Fas-L, p63, and Bax and the release of cytochrome c were detected using Western blot analysis.
Results:
Genipin reduced the viability of K562 cells with an IC50 value of approximately 250 μmol/L. Genipin 200–400 μmol/L induced formation of typical apoptotic bodies and DNA fragmentation. Additionally, genipin 400 μmol/L significantly increased the caspase 3 activity from 8–24 h and arrested the cells in the G2/M phase. After stimulation with genipin 500 μmol/L, the levels of p-JNK, p-c-Jun, Fas-L, Bax, and cytochrome c were remarkably upregulated, but there were no obvious changes of p-p38. Genipin 200–500 μmol/L significantly upregulated the Fas-L expression and downregulated p63 expression. Dicoumarol 100 μmol/L, a JNK1/2 inhibitor, markedly suppressed the formation of apoptotic bodies and JNK activation induced by genipin 400 μmol/L.
Conclusion:
These results suggest that genipin inhibits the proliferation of K562 cells and induces apoptosis through the activation of JNK and induction of the Fas ligand.
Keywords: genipin, apoptosis, K562 cells, p-JNK, cell cycle, Fas-L, Bax, cytochrome c, p63, dicoumarol
Introduction
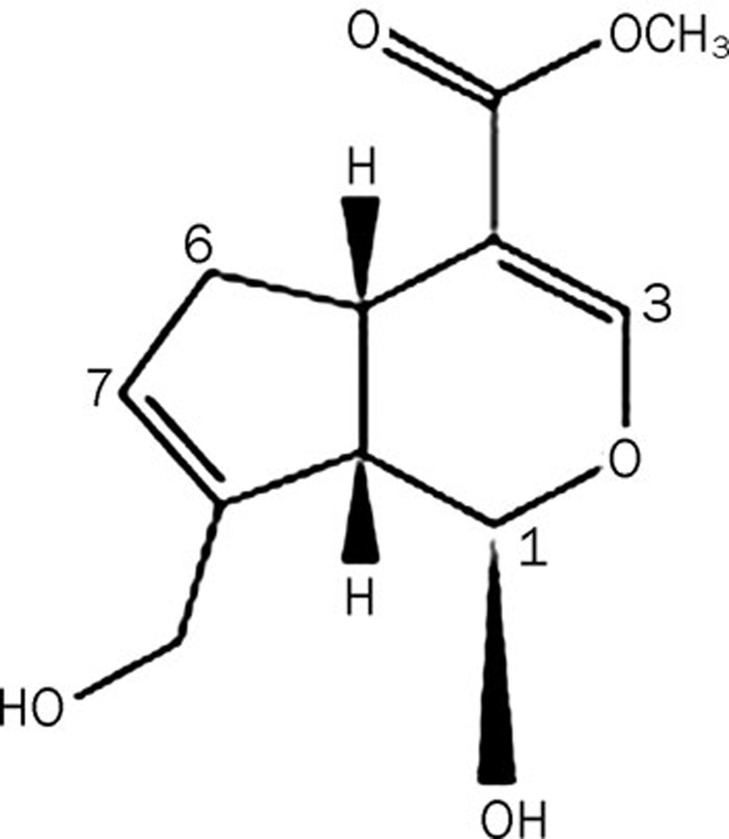
Genipin (Figure 1), an iridoid compound, is an aglycone derived from geniposide. It is the major active ingredient of Gardenia jasminoides Ellis fruit, which has long been used in traditional Chinese medicine1, 2. Genipin has a molecular weight of 226 and a white crystalline structure. It is soluble in ethanol and ethyl acetate and slightly soluble in water. It also has a low cytotoxicity2. Pharmacokinetic studies suggested that geniposide is hydrolyzed into genipin by β-D-glucosidases in the intestine and liver3. It is genipin, not geniposide, which functions as the main bioactive compound and exhibits the pharmacological activities of the gardenia4. Genipin is used to prepare blue colorants in the food industry and as a crosslinking reagent for biological tissue fixation5. Additionally, genipin has also been shown to possess various pharmacological actions such as anti-inflammatory2, 6, antiangiogenic7, antithrombotic8, anti-diabetic9, and antioxidative10 properties, the inhibition of NO production2 and the protection of neurotrophic activities11. Genipin inhibits endothelial exocytosis in human umbilical vein endothelial cells (HUVECs), suppresses vascular and endothelial cell inflammatory activation, and may target acute inflammatory events12. Notably, genipin has been shown to induce dose-dependent apoptosis in FaO rat hepatoma cells, human hepatocarcinoma Hep3B cells13 and PC3 human prostate cancer cells14. However, whether genipin shows a potential effect on human leukemia K562 cells and the cellular signaling pathway involved in this potential effect have not been well elucidated.
Figure 1.
Chemical structure of genipin.
Stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK1/2), a member of the mitogen-activated protein kinase (MAPK) family, is highly activated in response to a variety of stress signals including chemotherapy drugs, tumor necrosis factor, hyperosmotic stress and ultraviolet irradiation. Its activation is most frequently associated with the induction of apoptosis15, 16, 17. Activated JNK specifically catalyzes the phosphorylation of c-Jun on its N-terminal transactivation domain at Ser63 and Ser73. Phosphorylation of c-Jun, a major target of JNK, is a signal of JNK activation18. The death domain-containing receptor Fas and its ligand Fas-L have mainly been studied with respect to their ability to induce apoptosis. JNK/Fas mediation of the apoptotic pathway has been reported in previous studies of genipin19. In the present study, we investigated the effect of genipin on apoptosis in human leukemia K562 cells and elucidated the molecular mechanism of cell apoptosis. The results showed that genipin induced apoptosis in K562 cells via blockage of cell cycle progression at the G2/M phase and the subsequent progression into apoptosis through a multi-signaling pathway.
Materials and methods
Materials
The human leukemia cell line K562 was kindly provided by Professor Sheng-fu LI (Laboratory of Transplant Engineering and Immunology, West China Hospital, Sichuan University, Chengdu, China). Genipin (98%, Figure 1) was purchased from Haikang Biotechnology Co, Ltd (Chengdu, China) and dissolved in distilled water (13.1 mg/mL, pH 7.4). The solution of genipin was filtered with Millipore filtration and stored at -20 °C. Triton X-100, Tris base, SDS, acrylamide, bisacrylamide, ammonium persulfate and TEMED were from Amresco Co (Solon, OH, USA). RPMI-1640 medium was from Gibco Co (Langley, USA). Fetal bovine serum (FBS) was from HyClone Co (Beijing, China). Antibodies against p63, β-actin, Bax and cytochrome c were from Cell Signaling Technology Co (Boston, USA). Phosphorylated antibodies (anti-phosphor-JNK, anti-phosphor-c-Jun, and anti-phosphor-p38) were purchased from Cell Signaling Technology Co (Boston, USA). The antibody against Fas-L was from Millipore Co (Billerica, USA). The 0.25% Trypsin/EDTA solution, penicillin and streptomycin were from Beijing Solarbio Science & Technology Co, Ltd (Beijing, China). Peroxidase-conjugated AffiniPure goat anti-rabbit and goat anti-mouse immunoglobulin were from ZSGB-BIO Co, Ltd (China). Dicoumarol (a JNK inhibitor) was purchased from NICPBP (Beijing, China). PVDF paper and the enhanced chemiluminescence (ECL) Western blot detection system were purchased from Millipore Co (Billerica, USA). Trypan blue was from Sigma Co (USA). The Apoptosis DNA Ladder Detection Kit and the Caspase 3 Activity Assay Kit were from the Beyotime Institute of Biotechnology Co (Nanjing, China). The Apoptotic Body/Nuclear DNA Staining Kit was from Bio Basic Inc (Toronto, Canada). All stock solutions were stored at 4 or -20 °C. All other chemicals were of analytical grade.
Cell culture
K562 cells were cultured in RPMI-1640 medium with 10% (v/v) heat-inactivated FBS, 100 U/mL penicillin and 100 μg/mL streptomycin in humidified atmosphere at 37 °C with 5% CO2. Cells were seeded at a density of 5×104 in 100-mm Petri dishes and incubated for 6 h before drug treatment. Various concentrations of genipin were added to each well. Experiments were performed in triplicate.
Cell viability assays
Cell viability was determined by direct cell counting. K562 cells were cultured in 24-well plates at a density of 2×104 cells/well for 6 h and then incubated with various concentrations of genipin (100, 200, 300, 400, and 500 μmol/L) for 24 h. The cells were then stained with trypan blue dye and counted with a hemocytometer. The result was expressed as a percentage relative to the control, and the IC50 values were calculated.
Morphological observation of cells
After being cultured at 37 °C for 6 h in 100-mm Petri dishes, K562 cells were treated with various concentrations of genipin (0, 200, 300, and 400 μmol/L) for 24 h. K562 cells were observed with a phase-contrast microscope (Leica DMIRB, Germany).
Analysis of DNA fragmentation
After incubation with the designated concentrations of genipin (200, 300, 400, and 500 μmol/L) for 24 h, 1×106 K562 cells were collected. Genomic DNA was extracted from untreated and genipin-treated cells using the Apoptosis Ladder Detection Kit. The DNA was then electrophoresed in a 1.0% agarose gel and visualized by ethidium bromide (EB) staining. The gel was photographed under ultraviolet light.
Caspase 3 activity assay
Caspase 3 activity in cytosolic extracts was determined using a spectrophotometric assay as described by Song et al20. Briefly, supernatants from cell lysates treated with 400 μmol/L genipin for different amounts of time were incubated at 37 °C with Ac-DEVE-MCA, a fluorogenic substrate of caspase 3. Cleavage of the substrate was monitored at 405 nm. Data were normalized for the protein content of each supernatant and expressed as the relative value compared to the untreated group (time 0 h).
Flow cytometric analysis of the cell cycle and apoptosis
To further measure the apoptosis induction activity of genipin, K562 cells were incubated for 24 h in 6-well plates with genipin. Cells were then harvested, washed twice with ice-cold phosphate-buffered saline (PBS) and fixed with 70% ethanol at 4 °C. Before the samples were analyzed the DNA was stained on ice with 200 μL of cold (4 °C) propidium iodine (PI) for 10 min in the dark. DNA-bound PI fluorescence was measured using a 15 mW air-cooled argon ion laser at 488 nm as the excitation source and an EPICS ELITE ESP flow cytometer (Beckman Coulter, USA). Analysis of the cell cycle was performed with Coulter Elite 4.5 Multicycle software. Ten thousand events were recorded for each sample21.
Electrophoresis and immunoblotting
K562 cells were treated for 24 h with different concentrations of genipin (200, 300, 400, and 500 μmol/L). The cells were then harvested and washed with PBS. Whole cell lysates were obtained using cell lysis solution [250 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 7.4), 0.1% SDS, 1% Triton X-100, 1 mmol/L EDTA, 50 mmol/L NaF, and 1 mmol/L PMSF], followed by centrifugation (12 000×g, 30 min). Protein samples were heated at 95 °C for 5 min, normalized to a total of 100 μg per lane, resolved by SDS-PAGE, transferred onto PVDF membranes (Millipore) by electroblotting, and probed with antibodies against phosphor-JNK, phosphor-c-Jun, phosphor-p38, p63, Bax, Fas-L, and cytochrome c. Primary antibodies were detected with goat anti-rabbit antibody conjugated to horseradish peroxidase using enhanced chemiluminescence with the ImmobilonTM Western Chemiluminescent HRP Western blotting detection system (Millipore). Photographs were taken with the ChemiDoc XRS automatic photomicrograph system (Bio-RAD, USA) and analyzed using Quantity One software.
Analysis of cytochrome c release
K562 cells were collected by centrifugation at 300×g for 5 min at 4 °C and lysed with cell lysis solution for the mitochondrial cytochrome c release assay. Samples were then centrifuged at 12 000×g at 4 °C for 30 min. Supernatants containing the cytosolic proteins were recovered and analyzed using Western blotting.
Hoechst 33258 staining
Nuclear fragmentation of K562 cells treated with 400 μmol/L genipin was visualized by Hoechst 33258 staining following the use of the Apoptotic body/Nuclear DNA Staining Kit (Canada). Briefly, K562 cells were cultured in 6-well plates for 6 h and then co-incubated for 1 h with 100 μmol/L dicoumarol, an inhibitor of JNK activation22, before treatment with 400 μmol/L genipin. After treatment for 24 h, the cells were washed with PBS, fixed in 10% formaldehyde solution for 5 min at room temperature and resuspended in 50 μL of PBS before deposition on cover slips. The adhered cells were incubated with Hoechst 33258 for 20 min at room temperature. Cover slips were rinsed with PBS and imaged by fluorescence microscopy (Nikon Eclipse ET2000-E, Japan). Three replicate wells were analyzed for each treatment by the quantitative and qualitative examination of three random fields in each well. Cell viability was calculated from the number of viable cells excluding apoptotic nuclei vs the total number of nuclei in each well.
Statistical analysis
Data were presented as the mean±SD and were representative of three independent experiments. Statistical differences were evaluated using the Student's t-test.
Results
Effect of genipin on cell viability
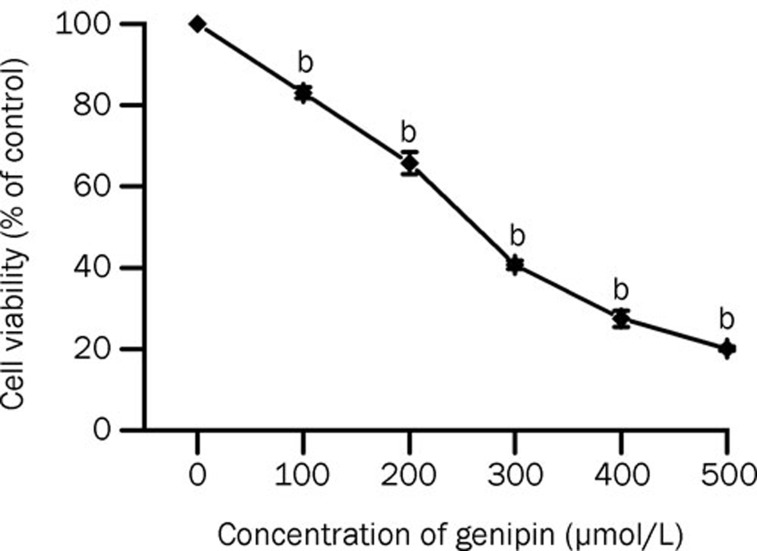
The effect of genipin on cell viability was evaluated by the trypan blue dye exclusion method; cells were counted with a hemocytometer. A significant decrease in viable cells and total cell number was detected after treatment with genipin for 24 h. Cell viability decreased to 22.75% of the control after treatment with genipin 500 μmol/L (Figure 2). The IC50 of genipin in K562 cells was approximately 250 μmol/L. Genipin showed dose-dependent cytotoxic and antiproliferative effects on K562 cells.
Figure 2.
Effect of genipin on cell viability in K562 cells. Cells were treated in vitro for 24 h with various concentrations of genipin (0, 100, 200, 300, 400, and 500 μmol/L). Cell viability was determined by cell counting. Data are the mean±SD of three independent experiments. bP<0.05 vs control.
Morphological changes in genipin-treated K562 cells
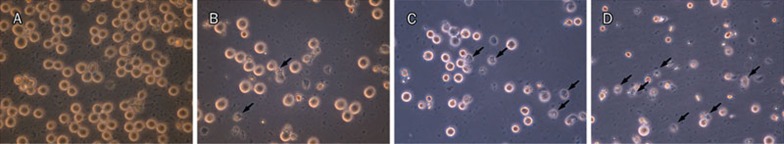
To better clarify the changes in cell morphology induced by genipin, K562 cells were exposed to the indicated concentrations of genipin for 24 h and then observed under a microscope. As shown in Figure 3, characteristic morphological changes were observed in K562 cells. Control K562 cells had normal features with round and homogeneous nuclei (Figure 3A). Significant morphological changes were observed in the cells after treatment with genipin. Cells exhibited the characteristic features of apoptosis such as cell shrinkage, membrane blebbing, and the appearance of apoptotic bodies (Figure 3B-3D, arrows). Genipin was found to inhibit the growth of K562 cells and increase the number of apoptotic cells in a dose-dependent manner. The exposure of K562 cells to 400 μmol/L genipin resulted in the most obvious apoptotic phenomena.
Figure 3.
Morphological changes in K562 cells when treated for 24 h with different concentrations of genipin. The cells were observed under a microscope (phase-contrast microscopic view). (A) Control; (B) K562 cells treated with 200 μmol/L genipin; (C) K562 cells treated with 300 μmol/L genipin; (D) K562 cells treated with 400 μmol/L genipin. Apoptotic cells are shown with arrows, 400× magnification.
DNA fragmentation
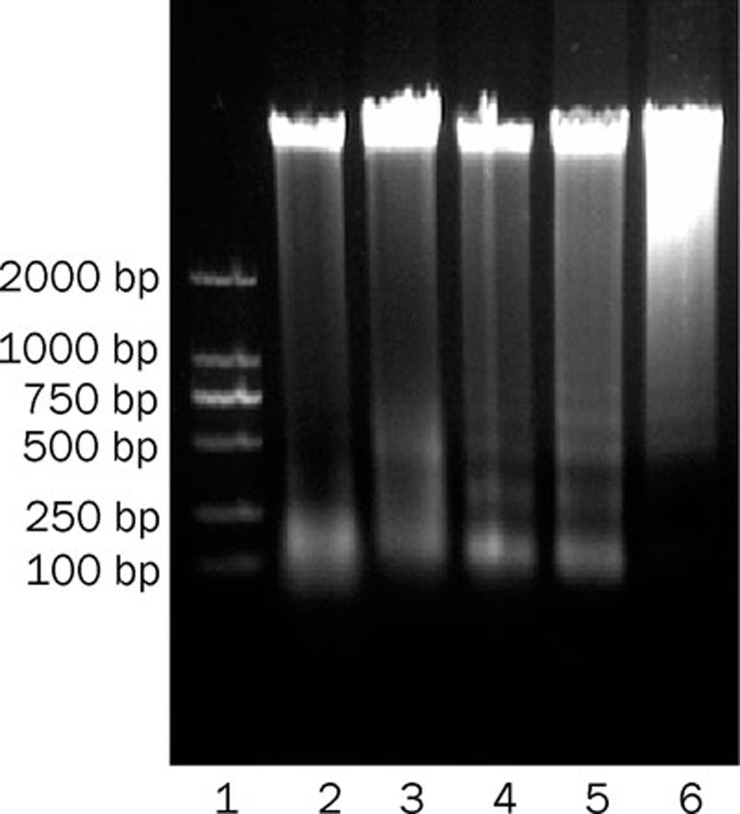
An important feature of apoptosis is the fragmentation of genomic DNA into integer multiples of 180-200 bp, which results in a characteristic ladder upon agarose gel electrophoresis. Experiments showed that the DNA of K562 cells presented typical ladder-like pattern after treatment with genipin at the indicated concentration (Figure 4). Genipin induced apoptosis in K562 cells.
Figure 4.
K562 cells were cultured for 24 h in the presence or absence of the indicated concentration of genipin. DNA was isolated and visualized on a 1.0% agarose gel stained with ethidium bromide. Lane 1, DL2000 Marker; lane 2, control; lane 3, 200 μmol/L; lane 4, 300 μmol/L; lane 5, 400 μmol/L; lane 6, 500 μmol/L.
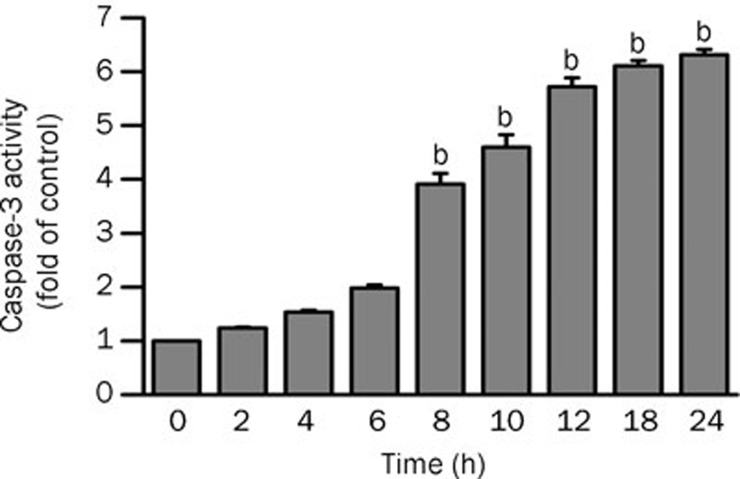
Involvement of caspase 3 in genipin-induced apoptosis
We examined whether apoptosis is induced in genipin-treated K562 cells and when the cells become apoptotic. We measured the activity of caspase 3, the critical mediator of apoptosis, in cytosolic extracts using Ac-DEVD-MCA as its fluorogenic substrate. Genipin significantly activated caspase 3 after 6 h of treatment; the trend increased slightly near 24 h (Figure 5). Caspase 3 activity increased within 24 h in a time-dependent manner in K562 cells treated with 400 μmol/L genipin.
Figure 5.
Activation of caspase 3 in K562 cells treated with 400 μmol/L genipin. Enzymatic activity is represented as ΔA405 nm per min per mg protein. Data are mean±SD of three independent experiments. bP<0.05 vs control.
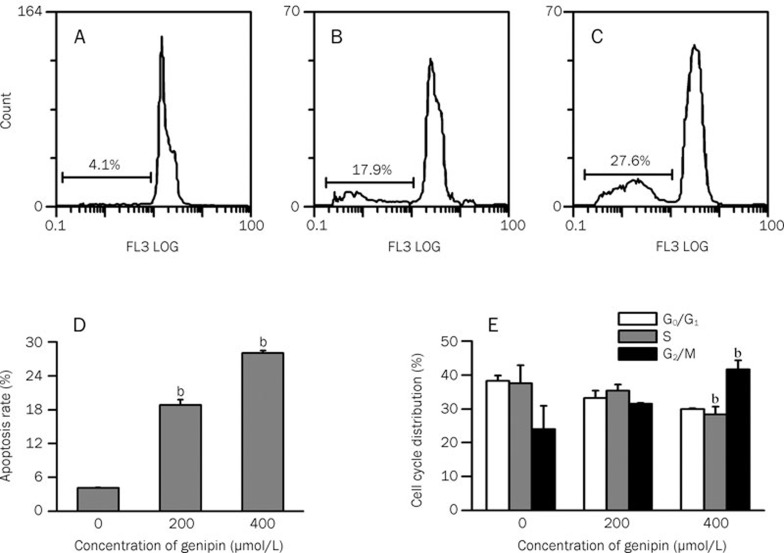
Effect of genipin on apoptosis and the induction of G2/M cell cycle arrest in K562 cells
Apoptosis and cell cycle distribution were examined at the indicated concentration of genipin. The flow cytometry results shown in Figures 6A–6C demonstrated that the population of apoptotic cells (propidium iodide-positive cells) gradually increased to 17.9% and 27.6% (4- and 7-fold) when the cells were treated with 200 and 400 μmol/L genipin, respectively; the increase in the control group was 4.1%. A dramatic hypodiploid (sub-G1) population was seen with 400 μmol/L genipin and is shown in Figure 6D. The results suggested that genipin could induce the apoptosis of K562 cells in a dose-dependent manner.
Figure 6.
Effect of genipin on the sub-G1 population and cell cycle distribution in K562 cells. K562 cells were incubated for 24 h in the absence (A) or presence of (B) 200 μmol/L and (C) 400 μmol/L genipin. Cells were then collected, stained with propidium iodide and analyzed by flow cytometry. The percentage of apoptotic cells is shown in each chart, and the apoptosis rate (D) and cell cycle distribution (E) are shown in the histogram. The histogram was obtained using Origin Version 8.0 software. Data are the mean±SD of three independent experiments. bP<0.05 vs control.
Cell cycle distribution was analyzed by comparing the percentage of cells in the G0/G1, S, and G2/M populations between the control group and cells treated with genipin for 24 h (Figure 6E and Table 1) without taking into consideration the apoptotic cell (sub G0/G1) population. In the control cells, the G2/M population represented 24.1% of the total number of viable cells. After 24 h of treatment with 200 μmol/L and 400 μmol/L genipin, the G2/M population increased to 31.5% and 41.7% (near 1.5- and 2-fold) of the total viable cells, respectively. This change was accompanied by a decrease in the G1 and S populations (Table 1). Moreover, it occurred in a dose-dependent manner. Treatment with genipin significantly altered the cell cycle profile and increased the number of apoptotic cells, demonstrating that the decrease in cell viability is due to an induction in cell apoptosis and is related to cell cycle arrest. These findings support the conclusions that the induction of cell death is due to apoptosis and that genipin arrests K562 cells at the G2/M phase.
Table 1. Effect of genipin on cell cycle distribution in K562 cells. The cells in G0/G1, S and G2/M phase was shown in the form. Data are expressed as mean±SD of 3 independent experiments.
| Cell cycle distribution | Concentration of genipin (24 h) | ||
|---|---|---|---|
| Control | 200 μmol/L | 400 μmol/L | |
| % G0/G1 | 38.3±1.6 | 33.2±2.2 | 30±0.2 |
| % S | 37.6±5.3 | 35.4±1.8 | 28.4±2.4 |
| % G2/M | 24.0±6.8 | 31.5±0.3 | 41.7±2.6 |
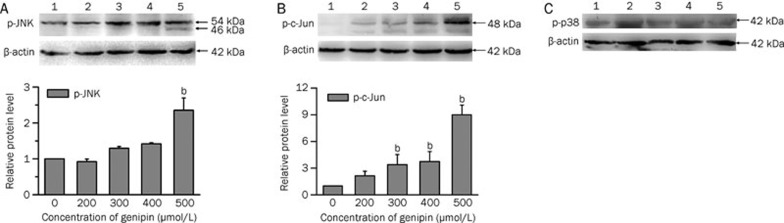
Activation of JNK and phosphor-c-Jun expression in genipin-treated K562 cells
To elucidate the signaling pathways involved in the genipin-induced apoptosis of K562 cells, we evaluated the expression of phosphor-JNK and phosphor-c-Jun, a target of JNK, by using Western blotting. JNK has three isoforms (JNK1, JNK2, and JNK3) with splicing variants that are either p46 or p54 in size23. Obvious p-JNK and p-c-Jun bands were detected. The results showed that the protein levels of p-JNK and p-c-Jun were increased in a dose-dependent manner; they were dramatically increased after treatment with 500 μmol/L genipin in comparison to the control (Figure 7A, 7B). The protein level of p-p38, another stress kinase and member of MAPK family, did not change significantly in the K562 cells exposed to genipin (Figure 7C).
Figure 7.
Effect of genipin on phospho-JNK (A), phospho-c-Jun (B) and phospho-p38 (C) activation in K562 cells. K562 cells were treated for 24 h with the indicated concentrations of genipin. The cells were harvested and lysed. Total protein (100 μg per lane) was loaded on the SDS gel, electrophoresed, transferred to PVDF membranes, and probed with antibodies against p-JNK, p-c-Jun and p-p38. Lane 1, control; lane 2, 200 μmol/L; lane 3, 300 μmol/L; lane 4, 400 μmol/L; lane 5, 500 μmol/L. Histograms indicated the changes in p-JNK and p-c-Jun expression as compared to the control. Relative protein levels (fold of the control) were obtained using Quantity One software. Histograms were obtained using Origin Vision 8.0 software. Data are the mean±SD of three independent experiments. bP<0.05 vs control.
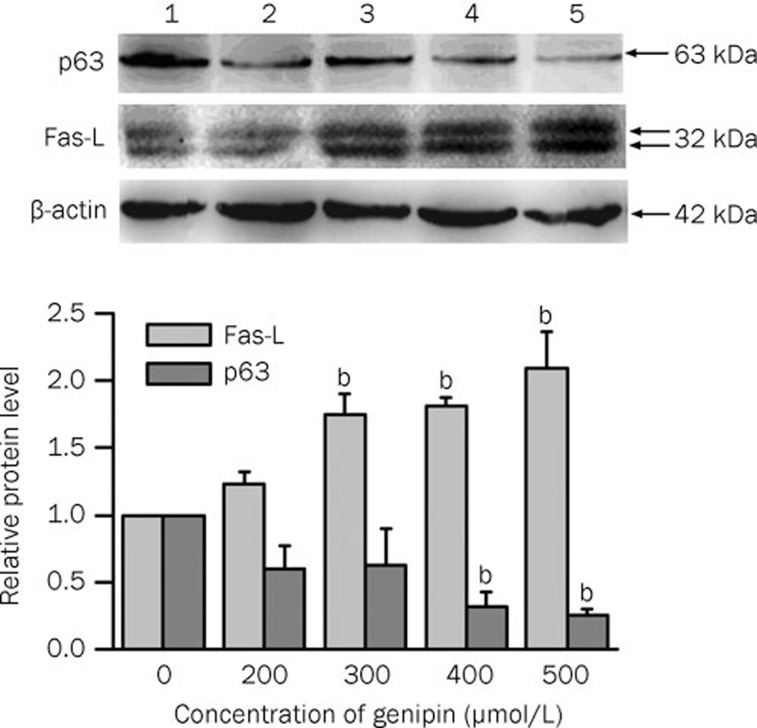
Fas-L and p63 expression in genipin-treated K562 cells
To further confirm that apoptosis was induced by genipin through the death-receptor pathway, Fas-L and p63, two signaling proteins in the apoptotic pathways, were detected by Western blot analysis. The expression of death-receptor Fas-L was induced by genipin in a concentration-dependent manner (Figure 8). Because K562 cells lack functional p5324, the expression of p63 in genipin-treated K562 cells was investigated. Genipin downregulated the expression of p63 (Figure 8). p63 in general and one of its specific isoforms, ΔNp63α, are overexpressed in a variety of squamous cell cancers. However, their expression is downregulated in apoptotic cells25. These results demonstrated that the genipin-induced apoptosis is accompanied by the up-regulation of Fas-L and the downregulation of p63 in K562 cells.
Figure 8.
Effect of genipin on Fas-L and p63 expression in K562 cells. K562 cells were treated for 24 h with the indicated concentration of genipin. The Western blot was analyzed using antibodies against Fas-L and p63. Lane 1, control; lane 2, 200 μmol/L; lane 3, 300 μmol/L; lane 4, 400 μmol/L; lane 5, 500 μmol/L. The histogram indicated the changes in Fas-L and p63 expression as compared to the control. The mean densities were obtained using Quantity One software. The histogram was obtained using Origin Vision 8.0 software. Data are the mean±SD of three independent experiments. bP<0.05 vs control.
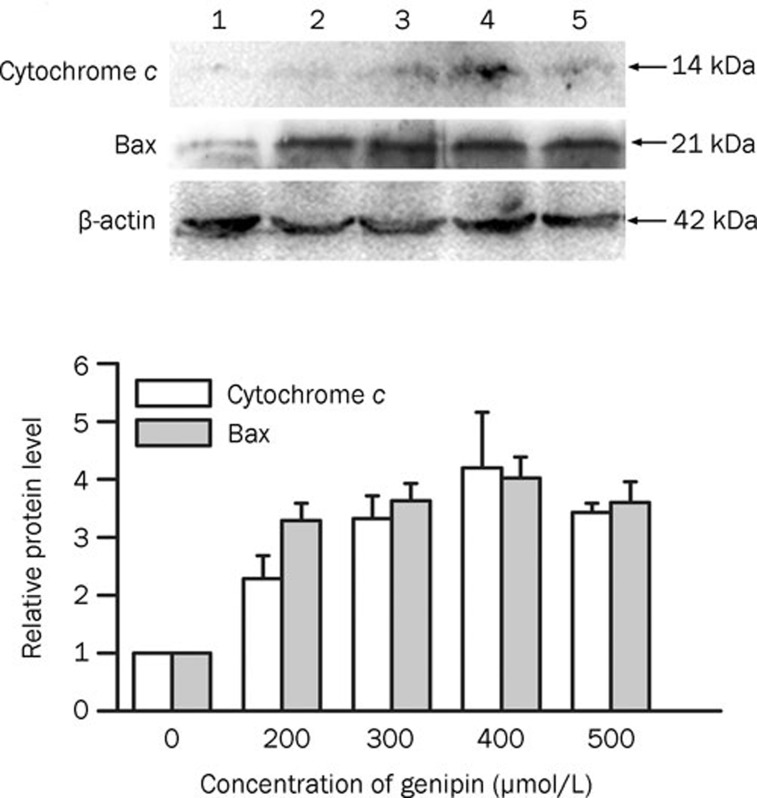
Increase in Bax expression and cytochrome c release after genipin treatment
To clarify whether the mitochondria-dependent pathway is involved in genipin-induced apoptosis, the expression of Bax and the efflux of cytochrome c into the cytosol were examined in genipin-treated K562 cells using Western blotting. As shown in Figure 9, accumulation of the Bax protein was observed and cytochrome c content increased significantly in the cytosol of genipin-treated K562 cells. These results showed that genipin-induced apoptosis resulted in cytochrome c release from the mitochondria into the cytosol in K562 cells.
Figure 9.
Genipin induced Bax expression and cytochrome c release into the cytosol of K562 cells. K562 cells were treated for 24 h with the indicated concentrations of genipin. The Western blot was analyzed using antibodies against Bax and cytochrome c. Lane 1, control; lane 2, 200 μmol/L; lane 3, 300 μmol/L; lane 4, 400 μmol/L; lane 5, 500 μmol/L. The histogram indicated the changes in cytochrome c release into the cytosol and Bax expression as compared to the control. The mean densities were obtained using Quantity One software. The histogram was obtained using Origin Vision 8.0 software. Three independent experiments were conducted and revealed similar patterns of changes. Data are the mean±SD of three independent experiments. bP<0.05 vs control.
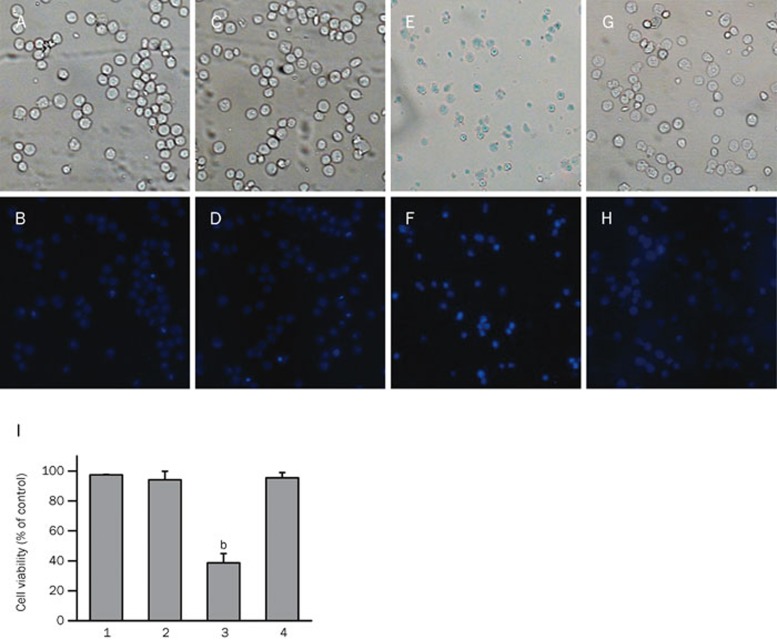
Activation of JNK in genipin-induced apoptosis
As shown in Figure 7, the activation of JNK was involved in genipin-induced apoptosis in K562 cells. To clarify the role of JNK activation, we tested the effect of dicoumarol, a JNK inhibitor22, on genipin-induced apoptosis and JNK activation. Nuclear fragmentation in K562 cells treated for 24 h with 400 μmol/L genipin was determined by Hoechst 33258 staining (Figures 10E and 10F). Apoptotic bodies containing nuclear fragments were found only in genipin-treated cells; the nuclear envelope appeared lytic and the cytoplasm had shrunk (Figures 10E and 10F). In contrast, cells cultured with dicoumarol only (Figures 10C and 10D) or with genipin and dicoumarol (Figures 10G and 10H) showed normal cell nuclei morphology as in the control (Figures 10A and 10B). These results showed that dicoumarol markedly suppressed the formation of apoptotic bodies and inhibited genipin-induced JNK activation. Additionally, the results of cell viability analyses in Figure 10I revealed that genipin-induced apoptosis was effectively inhibited by the addition of a JNK inhibitor. Taken together, these results indicated that the JNK signaling pathway mediated the genipin-induced apoptosis in K562 cells.
Figure 10.
Effect of a JNK inhibitor on genipin-induced apoptosis. K562 cells were treated for 24 h with vehicle or 400 μmol/L genipin in the absence or presence of the JNK1/2 inhibitor dicoumarol (100 μmol/L). Morphological changes in K562 cells treated with 400 for 24 h were observed by Hoechst 33258 staining and fluorescence microscopy. Fragmented or condensed nuclei indicative of apoptosis could be observed in the genipin-treated groups (E, F). However, the dicoumarol-treated cells (C, D) and the dicoumarol and genipin-treated cells (G, H) showed normal cell nuclei morphology as observed in untreated cells (A, B). (400×magnification). Cell viability of each group is shown in the histogram (I), which was obtained using Origin Version 8.0 software. 1, control; 2, dicoumarol-treated cells; 3, genipin-treated cells; 4, dicoumarol and genipin-treated cells. Three independent experiments were conducted and revealed similar patterns of changes. Mean±SD. n=3. bP<0.05 vs control.
Discussion
In recent years, genipin has been found to display potent anticancer effects. Genipin has been reported to induce apoptosis in FaO rat hepatoma cells, human hepatocarcinoma Hep3B cells and PC3 human prostate cancer cells13, 14. However, the exact mechanism of genipin-induced apoptosis has not yet to be determined. The present study demonstrates that genipin, a plant-derived iridoid, induces apoptosis in human K562 leukemia cells in a dose-dependent manner involving the activation of JNK/Fas-L signaling and G2/M arrest.
Apoptosis is characterized by specific morphological changes such as condensation of chromatin, nuclear fragmentation, blebbing of the plasma membrane, and the presence of apoptotic bodies26. We observed the typical characteristics of apoptosis in genipin-treated K562 cells using phase-contrast microscopy (Figure 3) and fluorescence microscopy (Figure 10). DNA fragmentation, a hallmark of apoptosis, was demonstrated by fragments ranging in size from 180 to 200 bp (Figure 4). Inhibition of cell viability (Figure 2) and the formation of a sub-G1 peak of apoptosis (Figure 6) were observed in a dose-dependent manner. These results demonstrated that the death of K562 cells induced by genipin was due to apoptosis. Caspase 3, one of the crucial mediators of apoptosis, is an executioner caspase that can be activated by a mitochondrial pathway or a death receptor pathway27. Activation of caspase 3 was observed in genipin-treated cells and increased dramatically after 6 h of incubation (Figure 5).
Perturbation of the progression of the cell cycle is a signal that can trigger apoptotic cell death28. Several studies have reported that various cytotoxic drugs can induce G2/M phase arrest. As shown in Figure 6, genipin induced the dose-dependent accumulation of cells in the G2/M phase of the cell cycle with subsequent accumulation in the sub-G1 phase suggesting the sequential events of cell cycle arrest followed by apoptosis. Innocente et al29 reported that p53 arrested the cells at the G2/M phase. However, we determined by Western blot that K562 cells were deficient in functional p53 (data not shown), which was consistent with the results of Lubbert et al24. Mutation or dysfunction of p53 is found in more than half of all human cancers. However, p63, a transcription factor homologous to p53, was found to be gradually reduced in a dose-dependent manner in our study (Figure 8). p63 has a critical physiological function. There is evidence that p63 in general and its specific ΔNp63α isoform are overexpressed in a variety of squamous cell cancers and in other types of cancers and could act as oncogenes25, 30, 31. Stravopodis et al32 reported the dose-dependent attenuation of p63 expression in RT4 cells at the mRNA level in doxorubicin-induced apoptosis. This result suggested that p63 might regulate apoptosis in genipin-treated K562 cells.
The prolonged and persistent activation of JNK and c-Jun induce apoptotic cell death. Previous studies have shown upregulation of JNK activation in FaO rat hepatoma cells and human hepatocarcinoma Hep3B cells with genipin-induced apoptosis13. It has also been reported that JNK plays an important role in IQDMA-mediated G2/M arrest and apoptosis of K562 cancer cells33. Although JNK and p38 MAPKs are collectively termed stress-activated protein kinases and are activated by a variety of stress-related stimuli and chemotherapy drugs15, 16, there was no obvious change in p-p38 (Figure 7C) in our studies. Kim et al13 reported that the phosphorylation of JNK was clearly detectable in genipin-treated FaO cells, whereas MEK1/2 and p38 MAPKs were not activated after genipin treatment. Here, genipin dose-dependently upregulated the phosphorylation levels of JNK in K562 cells (Figure 7A). The effect of JNK was supported by dose-dependent phosphorylation of c-Jun, a substrate of JNK (Figure 7B). Furthermore, the involvement of JNK activation was confirmed by the use of dicoumarol (a JNK inhibitor)22 (Figure 10). These results suggest that the genipin-induced apoptosis in K562 cells occurs through the JNK activation pathway.
The Fas/Fas-L pathway is an important cellular pathway regulating the induction of apoptosis in a variety of cell types. JNK has been found to contribute to death receptor apoptotic signaling via c-Jun/AP-1, which leads to the transcriptional activation of Fas-L19. Peng et al34 suggested that JNK/Fas plays a pivotal role in (Ac)5GP-induced apoptosis. In the present study, the expression of Fas-L in genipin-treated K562 cells was found to increase dose-dependently (Figure 8), suggesting that genipin triggered Fas-L signaling following JNK activation and induced the death receptor apoptotic pathway in K562 cells. Mundt et al35 suggested that the ΔNp63α isoform of p63 negatively regulated genes encoding Fas. Thus, p63 downregulation may regulate Fas/Fas-L activation. These results indicate that the activation of Fas-L might be attributed to both JNK activation and p63 downregulation in genipin-treated K562 cells; Fas-L is then able to activate caspases to induce apoptosis.
Bax is a member of Bcl-2 family and also plays a crucial role in apoptosis as a proapoptotic protein. Accumulation of the Bax protein was observed (Figure 9) and cytochrome c content was significantly increased in the cytosol of genipin-treated K562 cells suggesting that genipin-induced apoptosis also occurs through the mitochondrial apoptotic pathway. It has been reported that genipin induces apoptotic cell death in PC3 cells via JNK-dependent activation of the mitochondrial pathway14. Several experiments also revealed that activation of the JNK pathway can also cause cytochrome c release from the mitochondria to the cytosol and that apoptotic stimuli fail to release cytochrome c in JNK null cells36. Additionally, the ΔNp63α isoform repressed apoptosis-related genes of the mitochondrial apoptotic pathway35.
In conclusion, a signaling pathway associated with genipin-induced apoptosis in K562 cells was preliminarily examined in the present study. Treatment of K562 cells with genipin resulted in G2/M phase cell cycle arrest and cell apoptosis in a dose-dependent manner. Additionally, the activation of JNK was shown to play a crucial role in genipin-induced apoptosis in K562 cells. Downregulation of p63 may result in both Fas-L activation and cytochrome c release, which are related to the death receptor pathway and mitochondrial apoptotic pathway, respectively. These changes lead to caspase 3 activation and the ultimate induction of apoptosis in K562 cells. Further studies will be performed on the specific apoptotic signaling pathways in genipin-induced apoptosis in K562 cells. Taken together, our research provided important insights into the anticancer activity of genipin.
Author contribution
Qian FENG, Hou-li CAO, and Lin-fang DU designed the research; Qian FENG, Hou-li CAO, Wei XU, Xiao-rong LI, and Yan-qin REN performed the research; Qian FENG, Hou-li CAO, and Lin-fang DU contributed new analytical reagents and analyzed the data; Qian FENG and Lin-fang DU wrote the paper.
Acknowledgments
This study was supported by the grants from the Key Technologies R&D Program of Sichuan Province (2008SG0025), the Program for New Century Excellent Talents in University (NCET-04-0861) and Sichuan University Research Grant 985. We thank Dr Yong-sheng LIU for kindly providing the electron microscope, Yong-qiu MAO for providing the flow cytometer and assistance, and Sheng-fu LI for providing K562 cells.
References
- Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against damage in rat primary hepatocytes. Cancer Lett. 1995;97:61–7. doi: 10.1016/0304-3835(95)03964-x. [DOI] [PubMed] [Google Scholar]
- Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, et al. Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol. 2004;495:201–8. doi: 10.1016/j.ejphar.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Akao T, Kobashi K, Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol Pharm Bull. 1994;17:1573–6. doi: 10.1248/bpb.17.1573. [DOI] [PubMed] [Google Scholar]
- Zheng HZ, Dong ZH, Yu J.Modern research and application of Chinese Traditional Medicine 4Beijing, China: Academy Press; 2000. p3166–72. [Google Scholar]
- Hou YC, Tsai SY, Lai PY, Chen YS, Chao PD. Metabolism and pharmacokinetics of genipin and geniposide in rats. Food Chem Toxicol. 2008;46:2764–9. doi: 10.1016/j.fct.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol. 2006;103:496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Park EH, Joo MH, Kim SH, Lim CJ. Antiangiogenic activity of Gardenia jasminoides. Fruit Phytother Res. 2003;17:961–2. doi: 10.1002/ptr.1259. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kondo K, Ikeda Y, Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 2001;67:807–10. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, et al. Genipin inhibits ucp2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–27. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Kano M, Suzuki S, Ohtake N, Yamamoto M, et al. Inchinkoto, a herbal medicine, and its ingredients dually exert Mrp2/MRP2-mediated choleresis and Nrf2-mediated antioxidative action in rat livers. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1450–63. doi: 10.1152/ajpgi.00302.2006. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Chiba K. Genipin exhibits neurotrophic effects through a common signaling pathway in nitric oxide synthase-expressing cells. Eur J Pharmacol. 2008;581:255–61. doi: 10.1016/j.ejphar.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wang GF, Wu SY, Rao JJ, Lü L, Xu W, Pang JX, et al. Genipin inhibits endothelial exocytosis via nitric oxide in cultured human umbilical vein endothelial cells. Acta Pharmacol Sin. 2009;30:589–96. doi: 10.1038/aps.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Kim HG, Lee SA, Lim S, Park EH, Kim SJ, et al. Genipin-induced apoptosis in hepatoma cells is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of mitochondrial pathway. Biochem Pharmacol. 2005;70:1398–407. doi: 10.1016/j.bcp.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Hong HY, Kim BC. Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem Biophys Res Commun. 2007;362:307–12. doi: 10.1016/j.bbrc.2007.07.165. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Yu JH, Wang HJ, Li XR, Tashiro S, Ikejima T. Protein tyrosine kinase, JNK, and ERK involvement in pseudolaric acid B-induced apoptosis of human breast cancer MCF-7 cells. Acta Pharmacol Sin. 2008;29:1069–76. doi: 10.1111/j.1745-7254.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- Lei XY, Yao SQ, Huang ZX, Liu LJ, Zhong M, Zhu BY, et al. Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol Sin. 2008;29:1233–9. doi: 10.1111/j.1745-7254.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. [PubMed] [Google Scholar]
- Luo J, Sun Y, Lin H, Qian Y, Li Z, Leonard SS, et al. Activation of JNK by vanadate induces a Fas-associated death domain (FADD)-dependent death of cerebellar granule progenitors in vitro. J Biol Chem. 2003;278:4542–51. doi: 10.1074/jbc.M208295200. [DOI] [PubMed] [Google Scholar]
- Song JQ, Teng X, Cai Y, Tang CS, Qi YF. Activation of Akt/GSK-3beta signaling pathway is involved in intermedin(1–53) protection against myocardial apoptosis by ischemia/reperfusion. Apoptosis. 2009;14:1299–307. doi: 10.1007/s10495-009-0398-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu Y, Zhao X, Pan X, Yin R, Huang C, et al. Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. Eur J Obstet Gynecol Reprod Biol. 2008;140:95–102. doi: 10.1016/j.ejogrb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Seanor KL, Cross JV, Nguyen SM, Yan M, Templeton DJ. Reactive quinones differentially regulate SAPK/JNK and p38/mHOG stress kinases. Antioxid Redox Signal. 2003;5:103–13. doi: 10.1089/152308603321223595. [DOI] [PubMed] [Google Scholar]
- Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinase (JNK/MAPKs) Int J Biochem Cell Biol. 2001;33:1047–63. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- Lubbert M, Miller CW, Crawford L, Koeffler HP. p53 in chronic myelogenous leukemia. Study of mechanisms of differential expression. J Exp Med. 1988;167:873–86. doi: 10.1084/jem.167.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Xia SH, Li AD, Xu X, Cai Y, Han YL, et al. Elevated expression of p63 protein in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102:580–3. doi: 10.1002/ijc.10739. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–52. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- Wang CL, Ng TB, Yuan F, Liu ZK, Liu F. Induction of apoptosis in human leukemia K562 cells by cyclic lipopeptide from Bacillus subtilis natto T-2. Peptides. 2007;28:1344–50. doi: 10.1016/j.peptides.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–52. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–21. [PubMed] [Google Scholar]
- Pruneri G, Fabris S, Dell'Orto P, Biasi MO, Valentini S, Del Curto B, et al. The transactivating isoforms of p63 are overexpressed in high-grade follicular lymphomas independent of the occurrence of p63 gene amplification. J Pathol. 2005;206:337–45. doi: 10.1002/path.1787. [DOI] [PubMed] [Google Scholar]
- Stravopodis DJ, Karkoulis PK, Konstantakou EG, Melachroinou S, Lampidonis AD, Anastasiou D, et al. Grade-dependent effects on cell cycle progression and apoptosis in response to doxorubicin in human bladder cancer cell lines. Int J Oncol. 2009;34:137–60. [PubMed] [Google Scholar]
- Yang SH, Chien CM, Lu CM, Chen YL, Chang LS, Lin SR. Involvement of c-Jun N-terminal kinase in G2/M arrest and FasL-mediated apoptosis induced by a novel indoloquinoline derivative, IQDMA, in K562 cells. Leuk Res. 2007;31:1413–20. doi: 10.1016/j.leukres.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Peng CH, Tseng TH, Huang CN, Hsu SP, Wang CJ. Apoptosis induced by penta-acetyl geniposide in C6 glioma cells is associated with JNK activation and Fas ligand induction. Toxicol Appl Pharmacol. 2005;202:172–9. doi: 10.1016/j.taap.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Mundt HM, Stremmel W, Melino G, Krammer PH, Schilling T, Müller M. Dominant negative (DeltaN) p63alpha induced drug resistance in hepatocellular carcinoma by interference with apoptosis signaling pathways. Biochem Biophys Res Commun. 2010;396:335–41. doi: 10.1016/j.bbrc.2010.04.093. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]