Abstract
Aim:
To study whether calcium-modulating proteins CASQ2, FKBP12.6 and SERCA2a participate in diabetic cardiomyopathy, and whether the beneficial actions of testosterone, sildenafil or fructose diphosphate Sr (FDP-Sr) in the treatment of diabetic cardiomyopathy result from suppressing these molecules.
Methods:
Fifty male Sprague-Dawley (SD) rats were divided into five groups. Except for the normal group (non-diabetic), the other four groups were injected with streptozotocin (STZ, 60 mg/kg, ip) to induce diabetes. Four weeks after STZ injection, the four groups received sildenafil (12 mg·kg-1·d-1, ig, for 4 week), FDP-Sr (200 mg/kg, ig, for 4 week), testosterone propionate (4 mg·kg-1·d-1, sc, for 4 week), or no treatment, respectively.
Results:
In the diabetic rats, blood glucose, free fatty acids, triglycerides, total cholesterol, and low-density lipoprotein cholesterol (LDL-C) were significantly increased, while high-density lipoprotein cholesterol (HDL-C) was significantly reduced, as compared to the non-diabetic rats. Cardiac dysfunction and myocardial hypertrophy of the diabetic rats were associated with increased mRNA and protein expression of iNOS, OBRb, and PKCɛ, while expression of CASQ2, SERCA2a, and FKBP12.6 was significantly down-regulated. Sildenafil and FDP-Sr, but not testosterone, significantly attenuated the biomarker abnormalities, without changing the metabolic abnormalities.
Conclusion:
CASQ2, FKBP12.6 and SERCA2a were down-regulated in diabetic cardiomyopathy. Sildenafil and FDP-Sr, but not testosterone, attenuated the cardiac dysfunction in diabetic cardiomyopathy, without changing the metabolic abnormalities, which may results from inhibiting oxidative and inflammatory cytokines and improving calcium homeostasis.
Keywords: diabetic cardiomyopathy, CASQ2, FKBP12.6, SERCA2a, sildenafil, FDP-Sr, testosterone, calcium homeostasis
Introduction
By 2025, there will be an estimated 300 million people worldwide suffering from diabetes mellitus (DM)1. Up to 80% of morbidity and mortality in diabetic patients is due to cardiovascular disease (CVD). Initially, cardiac changes are asymptomatic. Later, left ventricular hypertrophy as well as diastolic and systolic dysfunction develops, and finally, symptomatic heart failure occurs. This unique pathological process is referred to as diabetic cardiomyopathy2, 3.
The main cause of diabetic cardiomyopathy is metabolic changes characterized by a sustained elevation of blood glucose and lipids, which promotes the formation of advance glycation end products (AGEs) over time4, 5. AGE excess stimulates glycosylation, which adversely affects cardiac contractility, likely through calcium-modulating proteins in the sarcoplasmic reticulum (SR). Sarcoplasmic reticulum calcium pump 2a (SERCA2a) is responsible for the uptake of intracellular calcium back into the SR and is essential for cardiac function. SERCA2a is very sensitive to posttranslational modification, and glycosylation of SERCA2a leads to cardiac dysfunction6, 7. The systolic function of myocardial cells relies mainly on ryanodine receptor type 2 (RyR2), FOK binding protein/calstabin 2 (FKBP12.6), SERCA2a, and troponin C, whereas diastolic function is dependent on SERCA2a and sodium calcium exchangers (NCX). Calcium homeostasis, the basis of cardiac function, relies on the release (RyR2 and FKBP12.6), uptake (SERCA2a and phospholamban, PLB), and storage (calsequestin 2, CASQ2) of Ca2+ in the SR. RyR2, FKBP12.6, SERCA2a, and PLB were found to be downregulated in failing hearts and diabetic cardiomyopathy, but these changes can be alleviated by drug intervention8, 9. Furthermore, SERCA2a overexpression is protective against STZ-induced cardiotoxicity in rats10. CASQ2 holds Ca2+ in the ER lumen and affects calcium release during the cardiac cycle. CASQ2 or RyR2 mutations may lead to a dangerous arrhythmia called catecholaminergic polymorphic ventricular tachycardia (CPVT)11. Additionally, abnormal CASQ2 expression can be caused by isoproterenol and relieved by CPU0213, an endothelin receptor antagonist11, 12. We aimed to explore if downregulation of CASQ2 disrupts calcium homeostasis in diabetic cardiomyopathy. Currently, no studies have investigated how changes in CASQ2 expression affect diabetic cardiomyopathy.
Recent evidence has suggested that low serum testosterone, as seen in male hypogonadism, is an independent risk factor for cardiovascular disease (CVD) due to increased inflammatory factors and cytokines in the myocardium13. Testosterone replacement therapy (TRT) has become an attractive intervention that may relieve the cardiac dysfunction associated with late-onset hypogonadism, a potential oxidative stress, through its antioxidant activity14, 15, 16.
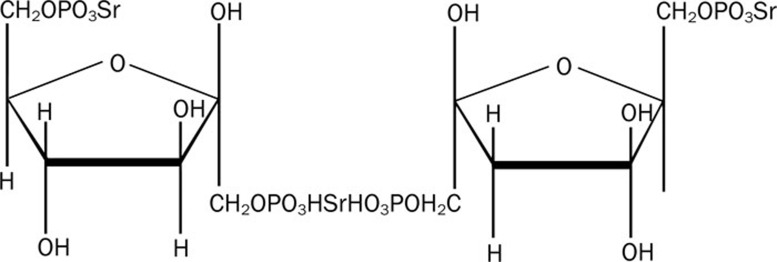
Sildenafil, a phosphodiesterase type 5 (PDE5) selective inhibitor, has been shown to improve heart failure by correcting vascular dysfunction through increasing vascular cyclic guanosine monophosphate (cGMP), endogenous NO, and free radical-scavenging activity17, 18. However, the efficacy of sildenafil in attenuating diabetic cardiomyopathy has not been shown. FDP-Sr, a derivative of FDP that contains three extra adenosine triphosphates (ATPs) in the moiety, has been shown to be effective in attenuating diabetic cardiomyopathy19, 20 (Figure 1).
Figure 1.
The chemical structure of FDP-Sr.
We hypothesized that diabetic cardiomyopathy was a unique disorder characterized by cardiac dysfunction associated with low serum testosterone and downregulated calcium-handling proteins at the SR, including CASQ2, FKBP12.6, and SERCA2a. In this study, the effects of FDP-Sr, sildenafil, and testosterone on diabetic cardiomyopathy were tested in terms of relieving oxidative stress and normalizing calcium homeostasis in the myocardium.
Materials and methods
Animals
Adult male Sprague-Dawley (SD) rats weighing 200–220 g were used. They were housed in a controlled environment and allowed free access to water and food. Animal handling and experimental procedures were in accordance with the regulations of the Jiangsu Provincial Government and the Principles of Laboratory Animal Care published by the US National Institutes of Health (NIH Publication No 85-23, revised 1996).
Experimental protocol
Fifty SD male rats were randomly divided into five groups. Except for the non-diabetic group, rats were injected with STZ (60 mg/kg, intraperitoneal) once. On days 7, 14, 21, and 28 after STZ administration, blood glucose was measured. Blood glucose consistently greater than 16.7 mmol/L was considered to be indicative of diabetes. During weeks 5 to 8 after STZ injection, animals were treated with sildenafil (12 mg/kg, ig), FDP-Sr (200 mg/kg, ig), or testosterone propionate (4 mg/kg, subcutaneous). Rats in the non-diabetic or STZ untreated groups were given an equal amount of citric acid buffer.
Hemodynamic changes
On day 57, the rats were anesthetized with urethane (1.5 g/kg, intraperitoneal). A catheter (PE 50, ID 0.58 mm, OD 0.965 mm, Becton Dickinson, San Jose, CA, USA) was inserted into the left ventricular (LV) chamber through the right carotid artery to measure LV systolic blood pressure (LVSP), LV end diastolic blood pressure (LVEDP), maximum rising rate of LV pressure (LV+dp/dtmax), and minimum declining rate of LV pressure (LV-dp/dtmin), as described previously21.
LV weight index
After hemodynamic measurements, the rats' hearts were excised and dissected into the LV free wall plus septum (LV) and right ventricle (RV). The LV weight index was assessed as the weight of the LV divided by body weight (LVW/BW).
Cell culture
Cardiomyocytes isolated from neonatal rats (1–3 d old) were cultured for 72 h in DMEM medium containing 20% fetal bovine serum and 0.1 mmol/L bromodeoxyuridine. The adherent cell density was 0.5×106/mL. Then the cardiomyocytes were incubated for 24 h with testosterone (10-5, 10-6, or 10-7mol/L), FDP-Sr (10-5, 10-6, or 10-7mol/L), or sildenafil (10-5, 10-6, or 10-7mol/L). The control cells were treated with DMSO. CASQ2 mRNA and protein expression were measured to investigate if changes in CASQ2 could be induced by testosterone, FDP-Sr, or sildenafil in the absence of high-glucose medium.
RT-PCR
Total RNA was extracted using Trizol reagent (Biouniquer Technology Company, Nanjing, China) according to the manufacturer's instructions. Five milligrams of RNA was used to synthesize cDNA using SUPERSCRIPT II RNase H-Reverse Transcriptase (Biouniquer Technology Company, Nanjing, China) according to the manufacturer's protocol. This cDNA was used as a template for the PCR reactions22. PCR primers specific for iNOS, OBRb, PKCɛ, CASQ2, FKBP12.6, SERCA2a, and GAPDH were designed as indicated in previous reports12, 21.
Western blotting
For quantitative analysis of myocardial phosphorylated PKCɛ (pPKCɛ), CASQ2, FKBP12.6, and SERCA2a protein levels, 100 mg of heart tissue was homogenized in 1 mL of extraction buffer and centrifuged at 10 000×g for 10 min as previously described21. After determining protein concentration, the supernatants were stored at -20 °C. An aliquot was heated to 100 °C for 10 min and size fractionated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The extracted protein was transferred to a nitrocellulose membrane and blocked with nonfat milk (5% w/v) followed by incubation with primary antibody for another 1–2 h at 37 °C. After three washes, the blot was incubated with horseradish peroxidase-conjugated goat secondary antibody IgG (Affinity Bioreagents; 1:500) for an additional 1 h at 37 °C22. Antigen was detected with a DAB kit. The density of the bands was analyzed using Labworks imaging acquisition and analysis software (GDS8000, Syngene, UK).
Statistical analysis
SPSS 11.5 (SPSS company, Chicago, USA) was used to analyze the results. Data were presented as the mean±SD. For statistical evaluation, one-way analysis of variance was used followed by Dunnett's test. The Student Newman Keuls test was performed when the variance was equal, and the Games-Howell test was performed when the variance was not equal. A P-value of less than 0.05 was considered statistically significant.
Results
Serum glucose, lipids, and androgens
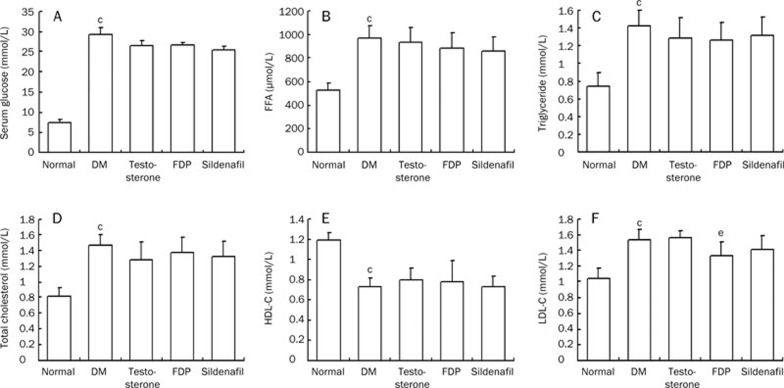
In the diabetic group, blood glucose, free fatty acids, triglycerides, total cholesterol, and LDL-C were significantly increased, and HDL-C was significantly decreased, indicating that the STZ-injected rats had marked metabolic changes (P<0.01). Single treatment with testosterone, Sildenafil, and FDP-Sr had no effects on the metabolic changes (Figure 2). Serum androgens (nmol/L) were sharply reduced in the STZ-injected rats (4.6±1.9, P<0.01) compared to the non-diabetic group (15.6±4.1). Serum androgens were significantly elevated in the testosterone group compared to the diabetic untreated group (12.9±2.1, P<0.01). There was no difference compared to the non-diabetic rats. The FDP-Sr group had a moderate increase in serum androgen compared to the diabetic untreated group (10.6±2.6, P<0.01). Sildenafil did not raise serum androgen (5.8±2.1).
Figure 2.
Changes in blood sugar and lipid were found in STZ-injected rats. The metabolic changes in DM rats were significant and testosterone propionate, sildenafil, and FDP-Sr had no effects on the altered sugar and lipid metabolism. (A) Serum glucose; (B) Free fatty acid (FFA); (C) Triglyceride; (D) Total cholesterol; (E) HDL-C; (F) LDL-C. Mean±SD. n=10. cP<0.01 vs normal. eP<0.05 vs DM.
Cardiac performance
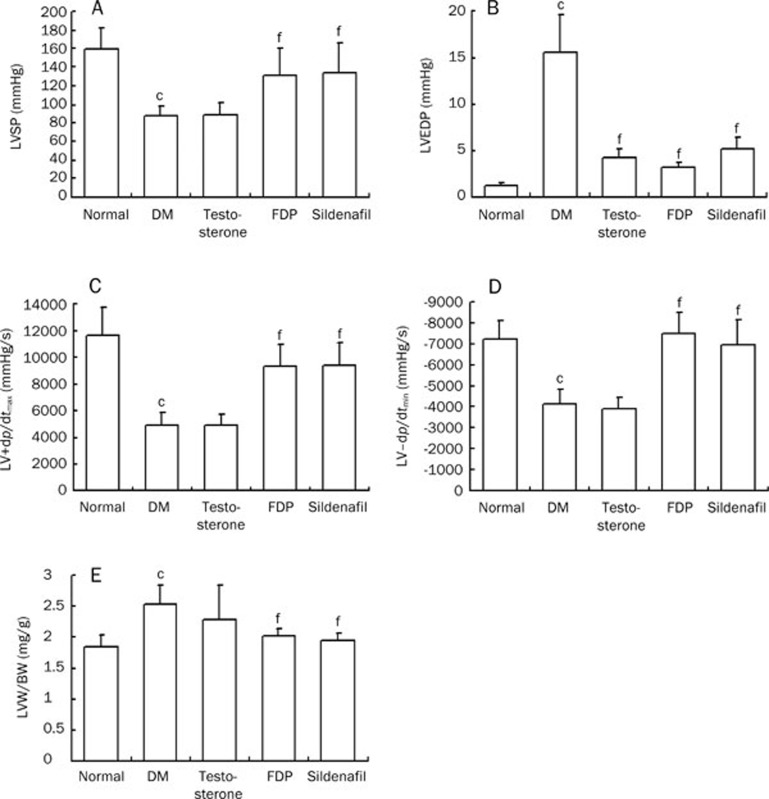
In the diabetic group, LVSP and LV plus dp/dtmax decreased significantly, while LVEDP and LV-dp/dtmin significantly increased compared to the non-diabetic group (P<0.01). Sildenafil and FDP-Sr led to a significant recovery in cardiac dysfunction (P<0.01). Additionally, the diabetic untreated group had a significantly increased LV mass index compared to the normal group (P<0.01). This increase was blunted by FDP-Sr and sildenafil. In contrast, testosterone had no effect on the hemodynamic or LV mass index abnormalities (Figure 3).
Figure 3.
FDP-Sr and sildenafil improved hemodynamic parameters and heart weight index in STZ injected rats. (A) LVSP; (B) LVEDP; (C) LV+dp/dtmax; (D) LV-dp/dtmin; (E) the LV weight index. Mean±SD. n=10. bP<0.05, cP<0.01 vs normal; fP<0.01 vs DM.
Oxidative stress
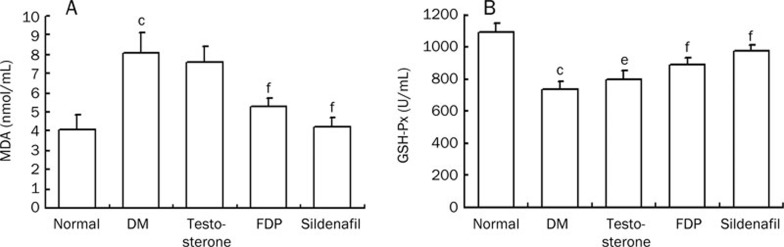
Plasma malondialdehyde (MDA), a measure of oxidants, was significantly elevated, and GSH-Px, an antioxidant marker, was significantly decreased in the diabetic untreated group compared to the non-diabetic group (P<0.01, respectively). Therefore, the diabetic rats had significant oxidative stress. Sildenafil and FDP-Sr suppressed MDA and raised GSH-PX levels compared to the diabetic untreated group (P<0.01, Figure 4). Thus, the antioxidant activity of sildenafil and FDP-Sr ameliorated the pathological changes in diabetic cardiomyopathy. Testosterone did not significantly block these pathological changes (Figure 4).
Figure 4.
Changes of MDA and GSH-PX in serum were improved by FDP-Sr or sildenafil in diabetic rats. (A) MDA; (B) GSH-Px. Mean±SD. n=10. cP<0.01 vs normal; eP<0.05, fP<0.01 vs DM.
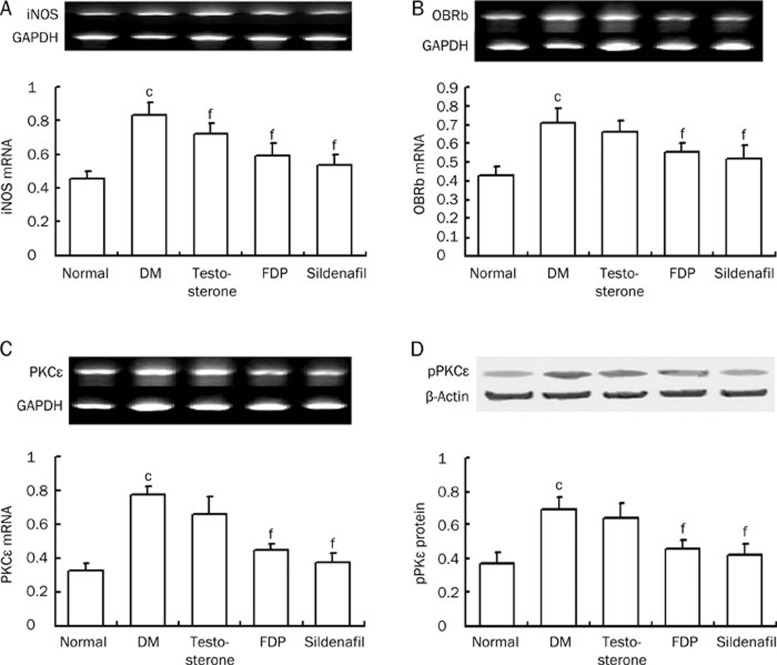
Cardiac iNOS, OBRb, and PKCɛ
In the diabetic rats the mRNA expression of iNOS and OBRb, a leptin receptor, was significantly increased (P<0.01). Additionally, PKCɛ mRNA expression was significantly increased (P<0.01). Sildenafil and FDP-Sr ameliorated the abnormal expression of iNOS, OBRb, and PKCɛ mRNA (P<0.01). In contrast, testosterone did not affect any of these biomarkers except iNOS mRNA (Figure 5).
Figure 5.
FDP-Sr and sildenafil inhibited abnormal mRNA expression of iNOS, OBRb, PKCɛ, and protein exprsssion of pPKCɛ. (A) iNOS mRNA; (B) OBRb mRNA; (C) PKCɛ mRNA; (D) pPKCɛ protein. Mean±SD. n=10. cP<0.01 vs normal; eP<0.05, fP<0.01 vs DM.
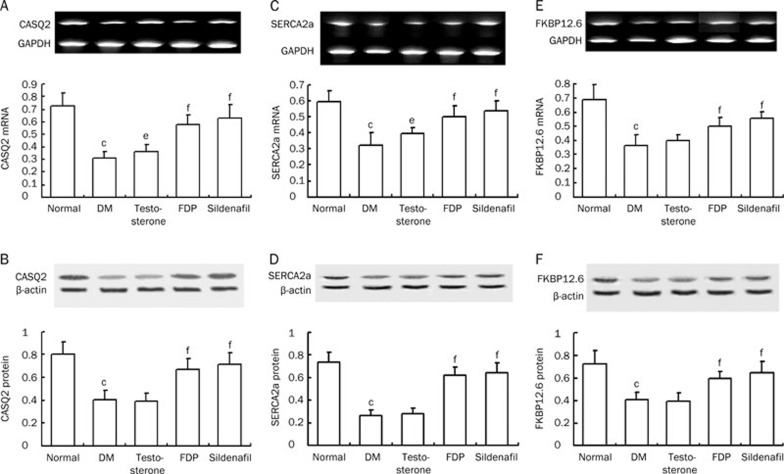
Calcium homeostasis
Calcium homeostasis in the myocardium is maintained by the calcium-handling proteins, which control intracellular calcium release, uptake, and storage in the SR and are manipulated by bioactive molecules, including FKBP12.6, the main regulator of RyR2, as well as SERCA2a and CASQ2. In the diabetic rats, mRNA and protein expression of CASQ2, SERCA2a, and FKBP12.6 were reduced (P<0.01). This indicates that aspects of calcium homeostasis, such as calcium release, uptake during diastole, and calcium-storing capacity in the SR are impaired in diabetic cardiomyopathy. The biomarker abnormalities may lead to heart failure and severe arrhythmias, which are likely to appear in diabetic cardiomyopathy. Sildenafil and FDP-Sr attenuated the abnormal expression of these molecules (P<0.01). Testosterone ameliorated CASQ2 and SERCA2a mRNA expression but had no effects on their protein expression. Testosterone had no effects on both mRNA and protein expression of FKBP12.6 (Figure 6).
Figure 6.
FDP-Sr and sildenafil improved abnormal mRNA and protein expressions of the calcium handing system. (A) CASQ2 mRNA. (B) CASQ2 protein. (C) SERCA2a mRNA. (D) SERCA2a protein. (E) FKBP12.6 mRNA. (F) FKBP12.6 protein. Mean±SD. n=10. bP<0.05, cP<0.01 vs normal; eP<0.05, fP<0.01 vs DM.
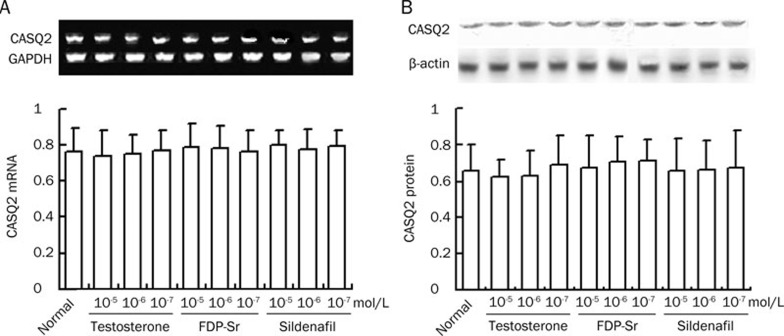
CASQ2 mRNA and protein expression were measured in cardiomyocytes grown in normal medium with normal glucose as well as sildenafil, FDP-Sr, and testosterone. No difference in CASQ2 expression was found in these cells compared to the cardiomyocytes in the absence of drug interventions (Figure 7). Therefore, we believe that sildenafil and FDP-Sr are effective in improving abnormal calcium homeostasis and cardiac dysfunction by suppressing the pathological processes due to hyperglycemia in the diabetic rats.
Figure 7.
The mRNA and protein expression of CASQ2 in the absence of diabetes mellitus with the three compounds in cardiomyocytes. (A) CASQ2 mRNA. (B) CASQ2 protein. Mean±SD. n=10.
Discussion
In diabetic cardiomyopathy, low serum testosterone and changes in glucose and lipid metabolism are the main factors responsible for disease progression. The cardiac dysfunction and hypertrophy in diabetic rats responded significantly to sildenafil and FDP-Sr but not testosterone. However, sildenafil and FDP-Sr did not improve hyperglycemia or hypercholesterolemia. The metabolic changes were not linked directly to cardiac dysfunction. Intermediate events, such as ROS and inflammatory factors, are critical in the pathogenesis of diabetic cardiomyopathy.
In this study, cardiac dysfunction in rats likely correlates with low serum testosterone; thus, we treated the mice with exogenous testosterone, which is a common way to correct this deficiency23. FDP-Sr moderately raises serum testosterone, likely through improving pathological changes in the testes. In contrast, sildenafil plays no role in correcting low serum testosterone. As a result, sildenafil and FDP-Sr, rather than testosterone supplement, dramatically attenuate cardiac dysfunction and oxidative stress. Exogenous testosterone did not alleviate diabetic cardiomyopathy, likely because it lacks significant antioxidant activity and cannot overcome the pathological changes induced by hyperglycemia.
Increased oxidative stress in vivo has been shown to contribute to the pathogenesis of diabetic cardiomyopathy24, 25. MDA, a lipid oxidation end product, affects the mitochondrial respiratory chain complex and the activities of key enzymes in the mitochondria. Excess reactive oxygen species (ROS) come from mitochondria and result from the activities of NADPH oxidase and induced NOS (iNOS) activity, which have been implicated in diabetic insults26. Increased ROS disturb many of the normal functions of the ER, induce cellular damage, and lead to apoptosis27. Activated iNOS can be combined with NO to form toxic peroxynitrite (ONOO· –), which impairs the subcellular apparatus of the myocardium. Upregulation of iNOS is associated with xanthine oxidase activation and upregulation of NADPH oxidase, p22phox, p40phox, and p47phox, leading to more cardiac dysfunction in diabetic cardiomyopathy28. These changes in calcium homeostasis and oxidative stress are consistent with our previous findings8, 9. In addition, oxidants and inflammatory cytokines, such as ROS, iNOS, endothelin-1, and leptin, damage DNA, leading to myocardial cell death.
Diabetes is often accompanied by obesity, abnormal lipid metabolism, and leptin upregulation29, 30. Leptin is a peptide hormone secreted by fat cells that stimulates the satiety center in the hypothalamus. However, in pathological conditions, prolonged leptin upregulation eventually leads to leptin resistance and increased expression of the leptin receptor OBRb. OBRb serves as an inflammatory factor that mediates myocardial injury22.
Disorder of calcium-modulating proteins, such as FKBP12.6 and SERCA2a, has been shown to play a role in diabetic or isoproterenol-induced cardiomyopathy, which causes oxidative stress and inflammation8, 12, 21. Downregulation of FKBP12.6 and SERCA2a leads to more intracellular Ca2+ at the end of diastole, known as calcium leak, which we found in L-thyroxine-induced cardiomyopathy30. Thus, downregulation of FKBP12.6 is a marker of calcium leak, which causes cardiac dysfunction and severe arrhythmias. Endothelin receptor antagonists and CPU86017, a berberine derivative, relieve these conditions23, 30.
Mutation of CASQ2 may cause an autosomal recessive form of the life-threatening arrhythmia CPVT. Additionally, mutations of RyR2 can lead to an autosomal dominant form of CPVT25. Normally, CASQ2 and RyR2 form tetramers. When SR calcium is low, RyR2 places the tetramer in an inhibitory state. When SR calcium increases, the CASQ2-RyR2 complex is weakened. If CASQ2 is mutated, the tetramer is even more unstable, and RyR2 sensitivity to calcium increases. As a result, SR calcium leak was more likely31, 32. We found that downregulation of CASQ2 is an important event in diabetic cardiomyopathy. Also, downregulation of FKBP12.6 and SERCA2a are likely involved. For example, an increase in the cytosol:SR calcium ratio disrupts SERCA2a function. Therefore, concentrations of calcium in the cytoplasm increase, and excessive calcium flows through sodium calcium exchanger (NCX), leading to increased Na+ influx into myocardial cells, delayed repolarization, and arrhythmias33. Interestingly, in addition to mutation, CASQ2 can be altered by posttranscriptional modifications due to either isoproterenol or hyperglycemia, which also lead to cardiac failure and tachyarrhythmias. CASQ2, FKBP12.6, and SERCA2a are sensitive markers of oxidative stress in the myocardium. Isoproterenol-induced downregulation of FKBP12.6, SERCA2a, and CASQ2 has been associated with increased ROS34, 35. ROS produced by hyperglycemia and AGEs are considered to be mediated by the same mechanism as the downregulation of FKBP12.6, SERCA2a, and CASQ2 in diabetic cardiomyopathy. Therefore, sildenafil and FDP-Sr were effective in blunting the abnormalities in calcium-handling proteins and proinflammatory cytokines, such as leptin, iNOS, and endothelin-1, through their antioxidant activities. As demonstrated in this study, changes in CASQ2, FKBP12.6, and SERCA2a are not affected by exogenous testosterone even though the low serum androgen levels were corrected. This is likely due to the mild antioxidant activity of testosterone.
Myocardial diacylglycerol (DAG) activates protein kinase C (PKC) signaling pathways. This process has been found in the vascular tissue of diabetic animals and leads to increased blood vessel susceptibility to hyperglycemia. The PKC/NFκB/c-fos pathway is activated in neonatal rat cardiomyocytes in high-glucose medium36. Activated PKCɛ has been found in isoproterenol-induced cardiomyopathy and in the vasculature of STZ-injected rats37, 38. PKCɛ overexpression may participate in downstream events, including K+ channel and calcium-handling protein abnormalities, that are involved in diabetic or isoproterenol-induced cardiomyopathy9, 12, 35. In this study, PKCɛ mRNA expression was elevated, and pPKCɛ was increased in association with cardiac dysfunction. FDP-Sr and sildenafil decreased PKCɛ mRNA and pPKCɛ protein significantly, likely through their antioxidant activity .
Conclusion
In this study, CASQ2 was found to be abnormally expressed in diabetic cardiomyopathy. CASQ2 is actively involved in the pathogenesis of diabetic cardiomyopathy and is closely related to oxidative stress and inflammation. Furthermore, other calcium-handling proteins, such as SERCA2a and FKBP12.6, were abnormally expressed. Oxidants, iNOS, OBRb, and pPKCɛ serve as markers of the metabolic changes responsible for inducing diabetic cardiomyopathy. Sildenafil and FDP-Sr are beneficial for treating diabetic cardiomyopathy through their antioxidant and anti-inflammatory properties. However, FDP-Sr and sildenafil did not affect blood glucose or lipid metabolism, leaving the abnormal metabolic changes intact. Testosterone did not improve cardiac dysfunction or calcium homeostasis in the myocardium.
Author contribution
Yu-si CHENG conducted the project, processed the data, and wrote the manuscript. Qi ZHANG provided the sildenafil. Hui JI assisted with the project and discussion of the manuscript. De-zai DAI and Yin DAI designed the hypothesis and project and revised the manuscript.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No 30873112).
References
- Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:715–24. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–57. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Asghar O, Al-Sunni A, Khavandi K, Khavandi A, Withers S, Greenstein A, et al. Diabetic cardiomyopathy. Clin Sci (Lond) 2009;116:741–60. doi: 10.1042/CS20080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani M. Diabetic cardiomyopathy: how much does it depend on AGE. Br J Pharmacol. 2008;154:725–26. doi: 10.1038/bjp.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Zhong M, Shang Y, Lin H, Deng J, Jiang H, et al. Differential regulation of collagen types I and III expression in cardiac fibroblasts by AGEs through TRB3/MAPK signaling pathway. Cell Mol Life Sci. 2008;65:2924–32. doi: 10.1007/s00018-008-8255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107:539–57. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, et al. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes. 2004;53:463–73. doi: 10.2337/diabetes.53.2.463. [DOI] [PubMed] [Google Scholar]
- Qi MY, Xia HJ, Dai DZ, Dai Y. A novel endothelin receptor antagonist CPU0213 improves diabetic cardiac insufficiency attributed to up-regulation of the expression of FKBP12.6, SERCA2a, and PLB in rats. J Cardiovasc Pharmacol. 2006;47:729–35. doi: 10.1097/01.fjc.0000211765.52012.aa. [DOI] [PubMed] [Google Scholar]
- Qi MY, Liu HR, Dai DZ, Li N, Dai Y. Total triterpene acids, active ingredients from Fructus Corni, attenuate diabetic cardiomyopathy by normalizing ET pathway and expression of FKBP12.6 and SERCA2a in streptozotocin-rats. J Pharm Pharmacol. 2008;60:1687–94. doi: 10.1211/jpp/60.12.0016. [DOI] [PubMed] [Google Scholar]
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- Györke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2009;6:123–9. doi: 10.1016/j.hrthm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Li N, Jia N, Dai DZ, Hu C, Dai Y. Role of endothelin in the effects of isoprenaline on potassium currents and calsequestrin 2 expression in the heart. Clin Exp Pharmacol Physiol. 2010;37:557–63. doi: 10.1111/j.1440-1681.2009.05349.x. [DOI] [PubMed] [Google Scholar]
- Maggio M, Basaria S. Welcoming low testosterone as a cardiovascular risk factor. Int J Impot Res. 2009;21:261–4. doi: 10.1038/ijir.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease. Trends Endocrinol Metab. 2010;21:496–503. doi: 10.1016/j.tem.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Francomano D, Bruzziches R, Natali M, Aversa A, Spera G. Cardiovascular effect of testosterone replacement therapy in aging male. Acta Biomed. 2010;81:101–6. [PubMed] [Google Scholar]
- Mancini A, Leone E, Festa R, Grande G, Silvestrini A, de Marinis L, et al. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J Androl. 2008;29:622–9. doi: 10.2164/jandrol.107.004838. [DOI] [PubMed] [Google Scholar]
- Montani D, Chaumais MC, Savale L, Natali D, Price LC, Jaïs X, et al. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther. 2009;26:813–25. doi: 10.1007/s12325-009-0064-z. [DOI] [PubMed] [Google Scholar]
- Guazzi M. Sildenafil and phosphodiesterase-5 inhibitors for heart failure. Curr Heart Fail Rep. 2008;5:110–4. doi: 10.1007/s11897-008-0018-9. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu HR, Ying HJ, Dai DZ, Tang XY, Dai Y. Strontium fructose 1,6-diphosphate early diabetic testopathy by suppressing abnormal testicular matrix metalloproteinase system in streptozocin-treated rats. J Pharm Pharmacol. 2009;61:229–36. doi: 10.1211/jpp/61.02.0013. [DOI] [PubMed] [Google Scholar]
- Xu M, Dai DZ, Zhang Q, Cheng YS, Dai Y. Upregulated NADPH oxidase contributes to diabetic testicular complication and is relieved by strontium fructose 1,6-diphosphate. Exp Clin Endocrinol Diabetes. 2010;118:459–65. doi: 10.1055/s-0030-1248325. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Dai DZ, Dai Y. Stress-induced cardiac insufficiency relating to abnormal leptin and FKBP12.6 is ameliorated by CPU0213, an endothelin receptor antagonist, which is not affected by the CYP3A suppressing effect of erythromycin. J Pharm Pharmacol. 2009;61:569–76. doi: 10.1211/jpp/61.05.0004. [DOI] [PubMed] [Google Scholar]
- Na T, Dai DZ, Tang XY, Dai Y. Upregulation of leptin pathway correlates with abnormal expression of SERCA2a, phospholamban and the endothelin pathway in heart failure and reversal by CPU86017. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:39–49. doi: 10.1007/s00210-007-0134-1. [DOI] [PubMed] [Google Scholar]
- Casey RW, Barkin J. Testosterone replacement therapy for the primary care physician. Can J Urol. 2008;15:71–7. [PubMed] [Google Scholar]
- Bilginoglu A, Seymen A, Tuncay E, Zeydanli E, Aydemir-Koksoy A, Turan B. Antioxidants but not doxycycline treatments restore depressed beta-adrenergic responses of the heart in diabetic rats. Cardiovasc Toxicol. 2009;9:21–9. doi: 10.1007/s12012-009-9032-8. [DOI] [PubMed] [Google Scholar]
- Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem. 2004;261:187–91. doi: 10.1023/b:mcbi.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- Violi F, Basili S, Nigro C, Pignatelli P. Role of NADPH oxidase in atherosclerosis. Future Cardiol. 2009;5:83–92. doi: 10.2217/14796678.5.1.83. [DOI] [PubMed] [Google Scholar]
- Münzel T. Endothelial dysfunction: pathophysiology, diagnosis and prognosis. Dtsch Med Wochenschr. 2008;133:2465–70. doi: 10.1055/s-0028-1100941. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Mukhopadhyay B, Patel V, Haskó G, et al. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:2330–41. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iciek R, Wender-Ozegowska E, Seremak-Mrozikiewicz A, Drews K, Brazert J, Pietryga M. Leptin gene, leptin gene receptor polymorphisms and body weight in pregnant women with type 1 diabetes mellitus. Ginekol Pol. 2008;79:592–601. [PubMed] [Google Scholar]
- Zhang Y, Huang ZJ, Dai DZ, Feng Y, Na T, Tang XY, et al. Downregulated FKBP12.6 expression and upregulated endothelin signaling contribute to elevated diastolic calcium and arrhythmogenesis in rat cardiomyopathy produced by L-thyroxin. Int J Cardiol. 2008;130:463–71. doi: 10.1016/j.ijcard.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Liu N, Rizzi N, Boveri L, Priori SG. Ryanodine receptor and calsequestrin in arrhythmogenesis: what we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol. 2009;46:149–59. doi: 10.1016/j.yjmcc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Györke S, Hagen BM, Terentyev D, Lederer WJ. Chain-reaction Ca2+ signaling in the heart. J Clin Invest. 2007;117:1758–62. doi: 10.1172/JCI32496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, et al. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117:1814–23. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Jia N, Dai DZ, Hu C, Dai Y. Role of endothelin in the effects of isoprenaline on potassium currents and calsequestrin 2 expression in the heart. Clin Exp Pharmacol Physiol. 2010;37:557–63. doi: 10.1111/j.1440-1681.2009.05349.x. [DOI] [PubMed] [Google Scholar]
- Li N, Jia N, Dai DZ, Dai Y. Endothelin receptor antagonist CPU0213 and vitamin E reverse downregulation of FKBP12.6 and SERCA2a: a role of hyperphosphorylation of PKCepsilon. Eur J Pharmacol. 2008;591:211–8. doi: 10.1016/j.ejphar.2008.06.080. [DOI] [PubMed] [Google Scholar]
- Min W, Bin ZW, Quan ZB, Hui ZJ, Sheng FG. The signal transduction pathway of PKC/NF-kappaB/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovasc Diabetol. 2009;8:8. doi: 10.1186/1475-2840-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Dai DZ, Dai Y. Isoproterenol disperses distribution of NADPH oxidase, MMP-9, and pPKCepsilon in the heart, which are mitigated by endothelin receptor antagonist CPU0213. Acta Pharmacol Sin. 2009;30:1099–106. doi: 10.1038/aps.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YF, Dai DZ, Dai Y. NaHS ameliorates diabetic vascular injury by correcting depressed connexin 43 and 40 in the vasculature in streptozotocin-injected rats. J Pharm Pharmacol. 2010;62:615–21. doi: 10.1211/jpp.62.05.0009. [DOI] [PubMed] [Google Scholar]