Nitric oxide (NO) is a short-lived free radical produced endogenously in biological tissues by nitric oxide synthases (NOSs)1, 2. Three NOS isoforms, namely NOS1 or neuronal NOS (nNOS), NOS2 or inducible NOS (iNOS), and NOS3 or endothelial NOS (eNOS) are present in most cell types, including cardiac myocytes and vascular endothelial cells. Vascular relaxation to mediators such as acetylcholine or increased blood flow depends on NO produced by the eNOS. The discovery of NO as the endothelium-derived relaxing factor (EDRF) and its crucial function as a signaling molecule in cardiovascular system was awarded the Nobel Prize in Physiology or Medicine in 19981.
Under normal circumstances the main function of eNOS is to produce NO. It catalyzes the conversion of L-arginine (L-Arg) to L-citrulline and NO via electron transfer from the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) through a flavin containing reductase domain to oxygen bound at the heme of an oxygenase domain containing tetrahydrobiopterin (BH4) and L-Arg binding sites (Figure 1). The eNOS-derived NO activates the guanylate cyclase/cGMP/protein kinase G (PKG) pathway and modulates protein properties and function through nitrosylation of tyrosine and thiol-groups of cysteine in proteins, which are usually effective protection mechanisms against oxidative stress.
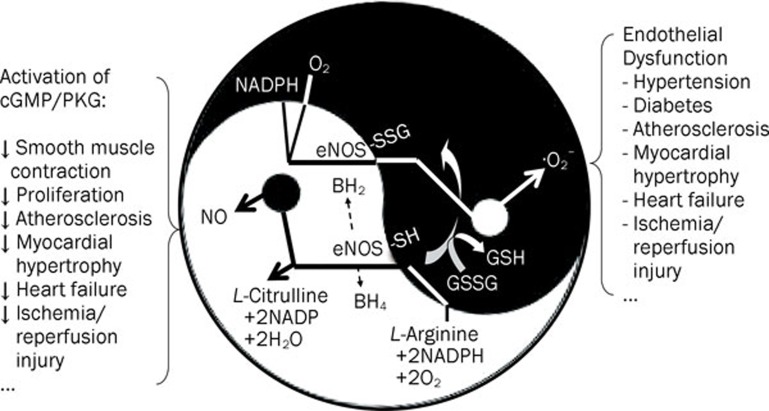
Figure 1.
Schematic representation of S-glutathionylation of eNOS as a molecular switch of eNOS function from generation of NO to production of superoxide anion. The endothelial NO synthase (eNOS) uses L-arginine to generate NO. NO activates the guanylate cyclase/cyclic GMP (cGMP)/protein kinase G (PKG) pathway and modulates protein properties and function through nitrosylation of tyrosine and thiol-groups of cysteine in proteins, which are effective protection mechanisms against oxidative stress. Under pathological conditions NO production and bioavailability could be attenuated due to decreased supply of L-arginine or oxidation of the cofactor tetrahydrobiopterin (BH4) of eNOS, leading to eNOS uncoupling and superoxide anion (·O2−) formation. Under oxidative stress, eNOS is S-glutathionylated through reversible thiol-disulfide exchange with oxidized glutathione (GSSG) or reaction of oxidant-induced protein thiolyl radicals with reduced glutathione (GSH). S-glutathionylation of eNOS in endothelial cells switch off NO synthesis and turns on ·O2− generation, which is a pivotal mechanism for many diseases associated with epithelial dysfunction.
Under pathophysiological conditions such as hypertension, diabetes, septic shock and atherosclerosis, oxidative stress alters many functions of the endothelium and leads to endothelial dysfunction when the endothelium fails to serve its normal physiologic and protective mechanisms. A common feature of endothelial dysfunction is the reduced bioavailability of NO and increased production of superoxide (·O2−) and other reactive oxygen species (ROS) in the vasculature3. Multiple mechanisms may underlie the impaired NO availability4. These include a reduction in the expression level of eNOS mRNA or protein, changes in subcellular compartmentalization of eNOS activity, and compromised availability of the substrates and/or enzymatic cofactors for eNOS5, 6. Depletion of the substrate L-arginine, accumulation of methylarginines, and oxidation of the cofactor BH4 of eNOS can uncouple the electron transfer reactions and revert eNOS to function as an NADPH oxidase, thus producing ·O2− instead of NO (Figure 1). The rapid reaction of NO with ·O2− can form the most potent oxidant peroxynitrite anion (OONO−) and causes cellular injury associated with many pathophysiologic conditions, such as hypertension, atherosclerosis, diabetes, myocardial hypertrophy, heart failure, and ischemia/reperfusion injury7. The precise molecular mechanisms underlying the “switch” of the eNOS function from NO synthesis to ·O2− production under oxidative stress conditions, however, are still not fully understood.
Recently, Chen et al reported that S-glutathionylation of eNOS may be a unique mechanism for the redox regulation of eNOS8. It has been demonstrated previously that cysteine residues are critical for the maintenance of normal eNOS function9. Protein S-glutathionylation has been known as a specific post-translational modification of protein cysteine residues by adding the tripeptide glutathione through reversible thiol-disulfide exchange with oxidized glutathione (GSSG) or reaction of oxidant-induced protein thiyl radicals with reduced glutathione (GSH). Under oxidative stress, therefore, protein S-glutathionylation can serve to prevent irreversible oxidation of protein thiols10. S-glutathionylation has now emerged as a potential mechanism for dynamic, post-translational regulation of a variety of regulatory, structural, and metabolic proteins. Increasing lines of evidence point to the important role of S-glutathionylation in the regulation of signaling and metabolic pathways in intact cellular systems. Indeed, Chen et al found that GSSG induced dose-dependent S-glutathionylation of human eNOS that was reversed by reducing agents β-mercaptoethanol or dithiothreitol8. S-glutathionylation of eNOS reversibly decreases NOS activity with a concomitant increase in ·O2− generation primarily from the reductase domain. Two highly conserved cysteine residuals are identified as sites of S-glutathionylation and found to be critical for redox-regulation of eNOS function. They further demonstrated that S-glutathionylation of eNOS in endothelial cells turned off NO synthesis and turned on ·O2− generation (Figure 1). This conversion of eNOS function by S-glutathionylation is closely associated with impaired endothelium-dependent vasodilatation. In hypertensive vessels, S-glutathionylation of eNOS is increased with impaired endothelium-dependent vasodilatation. Thio-specific reducing agents that reverse the S-glutathionylation of eNOS are able to restore the endothelium-dependent vasodilatation of the hypertensive vessels. Thus, S-glutathionylation of eNOS may represent a novel and pivotal molecular switch providing redox regulation of cellular signaling and endothelial function. Control of this molecular switch will perhaps provide new therapeutic targets and specific strategy to correct endothelial dysfunction under pathophysiological conditions. Importantly, it may also shed new mechanistic light on the action of some antihypertensive herbal extracts that elicit potent antioxidant properties and relax blood vessels in an endothelium-dependent and NO-mediated manner. It can be speculated that herbs with varying degrees of redox potential may exert different, or even opposite, vascular effects by flipping the switch to preferentially cause the formation of NO or ·O2 11, thus lending scientific support for the Yin-Yang temperaments emphasized in the theory of traditional Chinese medicine.
References
- Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19:235–51. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone. Recent Prog Horm Res. 1998;53:43–59. [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Wang Y, Marsden PA. Nitric oxide synthases: gene structure and regulation. Adv Pharmacol. 1995;34:71–90. doi: 10.1016/s1054-3589(08)61081-9. [DOI] [PubMed] [Google Scholar]
- Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–4. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic Biol Med. 2009;47:1108–19. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–8. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PF, Tsai AL, Wu KK. Cysteine 184 of endothelial nitric oxide synthase is involved in heme coordination and catalytic activity. J Biol Chem. 1994;269:25062–6. [PubMed] [Google Scholar]
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–88. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achike FI, Kwan CY. Nitric oxide, human diseases and the herbal products that affect the nitric oxide signalling pathway. Clin Exp Pharmacol Physiol. 2003;30:605–15. doi: 10.1046/j.1440-1681.2003.03885.x. [DOI] [PubMed] [Google Scholar]