Abstract
Aim:
To compare the vasorelaxing effects of hydrogen sulfide (H2S) on isolated aortic and pulmonary artery rings and to determine their action mechanisms.
Methods:
H2S-induced vasorelaxation of isolated rat aortic versus pulmonary artery rings under 95% O2 and 5% CO2 was analyzed. The expression of cystathinonine gamma-lyase (CSE), cystathionine beta synthase (CBS), 3-mercaptopyruvate sulfurtransferase (3MST), SUR2B and Kir6.1 was examined.
Results:
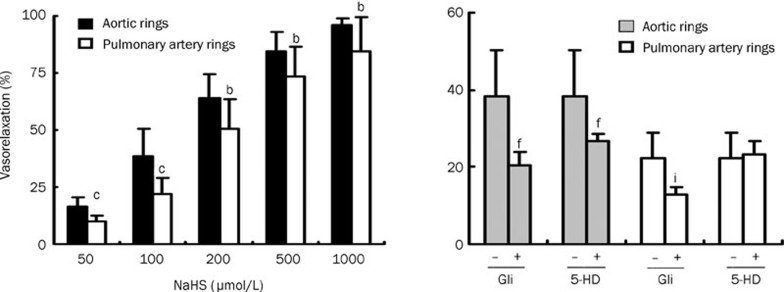
NaHS caused vasorelaxation of rat aortic and pulmonary artery rings in a dose-dependent manner. NaHS dilated aortic rings to a greater extent (16.4%, 38.4%, 64.1%, 84.3%, and 95.9% at concentrations of 50, 100, 200, 500, and 1000 μmol/L, respectively) than pulmonary artery rings (10.1%, 22.2%, 50.6%, 73.6%, and 84.6% at concentrations of 50, 100, 200, 500 and 1000 μmol/L, respectively). The EC50 of the vasorelaxant effect for aortic rings was 152.17 μmol/L, whereas the EC50 for pulmonary artery rings was 233.65 μmol/L. The vasorelaxing effect of H2S was markedly blocked b y cellular and mitochondrial membrane KATP channel blockers in aortic rings (P<0.01). In contrast, only the cellular membrane KATP channel blocker inhibited H2S-induced vasorelaxation in pulmonary artery rings. SUR2B mRNA and protein expression was higher in aortic rings than in pulmonary artery rings. Cystathinonine gamma-lyase (CSE) but not cystathionine beta synthase (CBS) expression in aortic rings was higher than in pulmonary artery rings. 3-Mercapto pyruvate sulfurtransferase (3MST) mRNA was lower in aortic rings than in pulmonary artery rings.
Conclusion:
The vasorelaxing effect of H2S on isolated aortic rings was more pronounced than the effect on pulmonary artery rings at specific concentrations, which might be associated with increased expression of the KATP channel subunit SUR2B.
Keywords: hydrogen sulfide; aortic rings; pulmonary rings; vasorelaxation; cystathinonine gamma-lyase; cystathionine beta synthase; 3-mercapto pyruvate sulfurtransferase; glibenclamide, 5-hydroxydecanoate
Introduction
The regulation of systemic and pulmonary circulation is a very important issue in cardiovascular research. Systemic circulation differs from pulmonary circulation in several important aspects. The same pathological stimuli may elicit different responses from either systemic or pulmonary circulation. For example, under hypoxia (20–60 mmHg pO2), pulmonary arteries contract while systemic arteries relax1. Vasoactive substances such as endothelin (ET) and angiotensin II (Ang II) play very important roles in the cardiovascular system but induce different vascular responses in pulmonary and systemic circulation2, 3, 4. The above-mentioned studies have revealed many differences in the responses of systemic and pulmonary circulation to pathophysiological stimuli.
In their carefully performed study, Olson et al examined vertebrate vessels and found that H2S produced temporally and quantitatively identical responses even though the responses varied from constriction (lamprey dorsal; IDA) to dilation (rat aorta; rA) to multiphasic (rat and bovine pulmonary arteries; rpA and bPA, respectively)5. They discovered that the concentration of vasoactive H2S in the vessel was governed by a balance between endogenous H2S production and its oxidation by available O25. In our study, we tried to analyze the difference between rat aorta and pulmonary artery at the vasorelaxant stage and further explored the role of KATP channels in the regulation of the vasorelaxant effect by hydrogen sulfide.
The endogenous gaseous signaling molecule hydrogen sulfide (H2S) functions as a physiological regulator6, 7, 8, 9, 10, 11, 12. Recent studies have shown that cystathinonine gamma-lyase (CSE), cystathionine beta synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3MST) are three H2S generating enzymes13, 14. It is shown that H2S relaxes blood vessels and lowers blood pressure by opening ATP-sensitive K+ channels in vascular smooth muscle cells15. To examine and compare the pathways used for the endogenous production of H2S in aortic and pulmonary arteries, we tested the expression of the above-mentioned enzymes. It has been demonstrated that H2S acts as an endogenous KATP channel opener to regulate vascular tone and that Kir6.1 and SUR2B are the main KATP channel subunits expressed in the vascular smooth muscle cells. So we tested the protein and mRNA expression of SUR2B and Kir6.1 in aortic and pulmonary arteries to investigate the possible mechanisms responsible for the regulation of vasorelaxation by H2S. H2S deficiency has been observed in animal models of systemic and pulmonary hypertension16, 17, 18. It also plays important roles in the pathogenesis of cardiovascular diseases16, 17, 18, 19, 20, 21, 22, 23, 24. The main mechanism for the cardiovascular actions of H2S was considered to be the activation of KATP channels, because H2S increased whole-cell KATP channel currents in rat aortic vascular smooth muscle cells15. However, whether H2S exerts different vasorelaxing effects on aortic and pulmonary artery rings is unknown. If it does, the potential mechanisms behind these effects are not understood. Therefore, this study was designed to observe the vasorelaxing effect of H2S on isolated aortic and pulmonary artery rings of rats in vitro and to identify the possible mechanisms.
Materials and methods
Reagents
Glibenclamide (Gli), 5-hydroxydecanoate (5-HD), and nicardipine were purchased from American Sigma Aldrich Company. NaHS was dissolved in deionized water and freshly prepared solution was used.
Animal preparation
The animal experimental procedures conformed to the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH) in the United States and was approved by the Animal Research Committee of Peking University. Adult male Wistar-Kyoto (WKY) rats weighing 220–250 g were purchased from Vital River (Beijing, China). Rats were housed in cages and fed a standard laboratory diet and fresh water. The cages were kept in a room with controlled temperature (24 °C±1 °C), relative humidity (65%–70%), and a 12-h light/dark cycle.
Preparation for aortic and pulmonary artery rings
Male Wistar rats (n=10) were anesthetized with urethane (1 g/kg body weight) intraperitoneally. The thoracic cavity was opened quickly, and the thoracic aorta and pulmonary artery were rapidly dissected and cleaned from fat and connective tissues. The artery was separated as carefully as possible to maintain the vascular activity. Rings 2–3 mm in length were cut and placed in 0 °C Krebs solution and immersed in 20 mL of organ baths containing pre-warmed Krebs' bicarbonate buffer filled with 95% O2–5% CO2 at 37 °C. The composition of the Krebs solution was as follows (mmol/L): NaCl: 120, KCl: 5.5, CaCl2: 2.5, MgCl2·6H2O: 1.2, NaH2PO4: 1.2, NaHCO3: 20, EDTA-Na2: 0.03, glucose: 10, and pH: 7.2–7.4. Organ baths were filled with oxygenated (95% O2–5% CO2) Krebs solution.
Changes in tension were recorded using force transducers connected to a PowerLab (BL Newcentrary, TaiMeng, Chengdu, China). First, the aortic rings were stretched passively to a tension of 1 g, while the pulmonary rings were stretched at 0.6 g. The rings were equilibrated for 1 h before starting the experiment. The endothelia of the rings were kept functionally unbroken, as confirmed by their relaxation after acetylcholine treatment (1 μmol/L). The rings were contracted with norepinephrine (NE, 1 μmol/L). When the vasoconstriction curves of the rings reached the plateau phase of maximum tension, H2S at 50–1000 μmol/L was given and the changes in tension were recorded. In another experiment, aortic and pulmonary artery rings were incubated with two kinds of KATP channel blockers for 30 min (at a concentration of 1×10−6mol/L) before the physiological dose of 100 μmol/L NaHS was given to observe whether the vasorelaxing effect of H2S could be blocked. The relaxation ratio was calculated by the relaxation degree and preshrinking degree and expressed as a percentage (%). The relaxation degree and shrinking degree, in grams, were recorded by electrophysiolograph.
Measurement of CSE, CBS, SUR2B and Kir6.1 expression in aortic and pulmonary artery rings by Western blotting
Aortic and pulmonary artery rings from Wistar rats (n=10) were homogenized and lysed. Equal amounts of proteins were boiled and separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes according to the experimental protocol. The primary antibody dilutions were 1:1000 for CSE, 1:4000 for CBS, 1:500 for SUR2B, 1:200 for Kir6.1, and 1:4000 for GAPDH antibodies. Secondary antibody (Santa Cruz) was used at a 1:10000 dilution. The immunoreactions were visualized by electrochemiluminescence (ECL) and exposed to X-ray film (Kodak Scientific Imaging film).
Measurement of CSE, SUR2B and Kir6.1 expression in aortic and pulmonary rings by immunohistochemistry
After dewaxing by dimethylbenzene, sections of aortic and pulmonary artery rings were placed in distilled water and treated with 3% H2O2 for 12 min. The slides were washed with PBS three times for 5 min each. The antigens were then exposed for 15 min. The slides were rinsed again, and the samples were blocked for 30 min with goat serum working fluid. Polyclonal antibodies to CSE (1:150), SUR2B (1:50), and Kir6.1 (1:50) were added and incubated at 4 °C overnight. On the following day, slides were rinsed three times for 5 min in PBS and then incubated with biotinylated anti-mouse, goat, or rabbit IgG at 37 °C for 60 min. Slides were rinsed again in PBS three times, and horseradish peroxidase streptavidin was added for 30 min at 37 °C, followed by three 5 min-washes with PBS. DAB was added for color development, and the sections were counter-stained with hematoxylin. The sections were dehydrated through a graded ethanol series, treated with dimethylbenzene, and then mounted on slides. The presence of brown granules in aortic and pulmonary smooth muscle cells and endothelial cells was defined as positive signal. For negative controls, sections were processed as described above except that the primary incubation was performed with nonimmune goat serum instead of primary antibodies.
Measurement of SUR2B, Kir6.1, and 3MST mRNA expression in aortic and pulmonary artery rings using quantitative real-time polymerase chain reaction (PCR)
RNA from aortic and pulmonary artery rings of rats (n=7) was extracted using Trizol reagent (GibcoBRL) and reverse transcribed using an oligo d(T)18 primer and M-MLV reverse transcriptase. Primers and TaqMan probes used for the quantification of cDNAs in samples were designed using the Primer Express 3.0 software (Applied Biosystems, Foster City, CA, USA). Primers and probes were synthesized by the SBS Company, Limited (Beijing, China). Quantitative real-time PCR was carried out using an ABI PRISM 7300 instrument (Applied Biosystems). The sequences of the primers and probes are shown in Table 1. The PCR condition for SUR2B, Kir6.1 and 3MST were as follows: pre-denaturation at 94 °C for 5 min, then 94 °C for 30 s, 59.5 °C for 30 s, and 70 °C for 1 min for 40 cycles. The PCR condition for β-actin was 95 °C for 5 min, 95 °C for 15 s, and 60 °C for 1 min for 40 cycles. The amount of β-actin cDNA in the sample was used to calibrate the amount of sample needed for quantification.
Table 1. The sequence of the primers and probes of SUR2B, Kir6.1, 3MST and β-actin.
| Forward primer | SUR2B-F: 5′-ACCCGCGAGTACAACCTTCTT-3′ | |
| SUR2B | Reverse primer | SUR2B-R: 5′- TCTCATCGCTCAAGAGAAACTCAT-3′ |
| Probe | SUR2B-P: 5′-AGCCATCATCAGCGTTCAGAAGCT-3′ | |
| Forward primer | Kir6.1-F: 5′-ACCCGCGAGTACAACCTTCTT-3′ | |
| Kir6.1 | Reverse primer | Kir6.1-R: 5′-TATCGTCATCCATGGCGAACT-3′ |
| Probe | Kir6.1-P: 5′-AGGTCATTCACTTCTGCGTTTCTCTTCTCCAT-3′ | |
| Forward primer | 3MST-F: 5′-CGGCGCTTCCAGGTAGTG-3′ | |
| 3MST | Reverse primer | 3MST-R: 5′-CTGGTCAGGAATTCAGTGAATGG-3′ |
| Probe | 3MST-P: 5′-CGCAGCTGGCCGTTTCCA-3′ | |
| Forward primer | β-Actin-F: 5′-ACCCGCGAGTACAACCTTCTT-3′ | |
| β-Actin | Reverse primer | β-Actin-R: 5′-TATCGTCATCCATGGCGAACT-3′ |
| Probe | β-Actin-P: 5′-CCTCCGTCGCCGGTCCACAC-3′ |
Statistical analysis
The data were analyzed by Excel and SPSS 13.0 statistical software, and all values were expressed as mean±standard deviation. The relaxation reaction at different concentrations of NaHS on aortic and pulmonary artery rings was analyzed by an independent sample t-test. The relaxation reaction to treatment of aortic and pulmonary artery rings with physiological concentrations of NaHS (the WKY+NaHS, Gli+NaHS, and 5-HD+NaHS groups) was analyzed by one-way ANOVA. LSD analysis was used for comparing data between the two groups. The expression of SUR2B, Kir6.1, CSE, CBS, and 3MST in aortic and pulmonary arteries was compared using the paired-sample t analysis. A level of P<0.05 was set as statistically significant.
Results
The maximum diastolic effect of aortic and pulmonary artery rings to different concentrations of NaHS in rats
NaHS caused vasorelaxation in rat thoracic aortic and pulmonary artery rings pre-contracted with 1 μmol/L NE in vitro in a dose-dependent manner. H2S at concentrations of 50–1000 μmol/L dilated aortic rings more noticeably than pulmonary artery rings (P<0.05, Figure 1). The EC50 of the vasorelaxant effect on aortic rings was 152.17 μmol/L, while the effect on pulmonary artery rings had an EC50 of 233.65 μmol/L.
Figure 1.
The maximum relaxation response of aortic and pulmonary artery rings to different concentrations of NaHS in rats, and the effect of a KATP channel blocker on the vasorelaxing effect of hydrogen sulfide on aortic and pulmonary artery rings (n=10). bP<0.05, cP<0.01 compared to aortic rings. fP<0.01 compared to aortic rings without giving KATP channel blocker. iP<0.01 compared to pulmonary artery rings without giving KATP channel blocker. Gli: glibenclamide, 5-HD: 5-hydroxydecanoate.
The effects of a cytomembrane KATP channel blocker and mitochondrial membrane KATP channel blocker on the vasorelaxing effect of H2S on aortic and pulmonary artery rings
The vasorelaxing effect of H2S was markedly blocked by cytomembrane and mitochondrial membrane KATP channel blockers (Gli and 5-HD) in aortic rings (P<0.01, Figure 1). In contrast, the H2S-induced vasorelaxing effect on pulmonary artery rings could only be blocked by the cytomembrane KATP channel blocker (P<0.01, Figure 1) but not by the mitochondrial membrane KATP channel blocker (P>0.05, Figure 1).
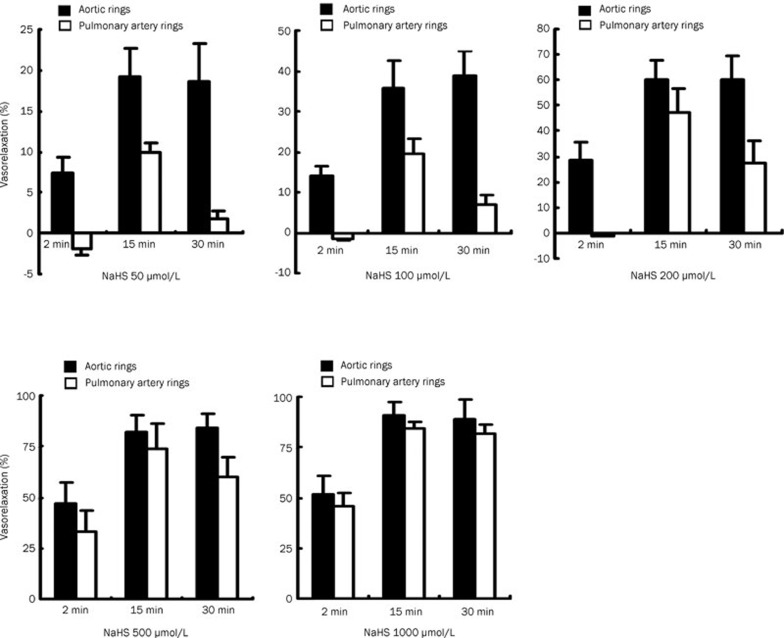
The different vasoactive response of aortic and pulmonary artery rings to different concentrations of NaHS in rats
Within 30 min, all concentrations of hydrogen sulfide (50–1000 μmol/L) gradually relaxed the rat aorta over time until it reached its maximum level of vasorelaxation. However, in rat pulmonary arteries, NaHS (concentrations of 50, 100 and 200 μmol/L) produced a constriction followed by a relaxation, and then followed by a reduced relaxation. This effect was not obvious at higher concentrations (500 and 1000 μmol/L) in rat pulmonary arteries (Figure 2).
Figure 2.
The different vasoactive response of aortic and pulmonary artery rings to different concentrations of NaHS in rats at the different time points.
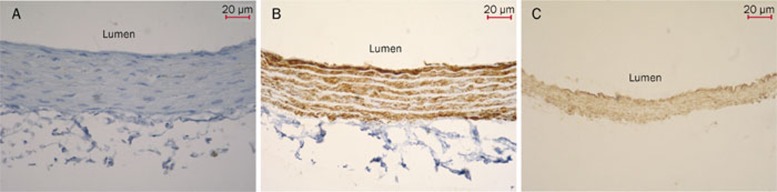
Immunohistochemical analysis of CSE expression in aortic and pulmonary artery rings
CSE protein in aortic and pulmonary artery rings of rats in the control group was strongly expressed in the inner membrane and tunica media vasorum. The presence of brown granules in aortic and pulmonary artery smooth muscle cells and endothelial cells was defined as a positive signal (Figure 3).
Figure 3.
Immunohistochemical analysis of CSE expression in aortic and pulmonary artery rings (DAB×200). (A) The aortic ring negative control was processed without CSE primary antibody. The thickness of the inner elastic layer was uniform and the structure of smooth muscle cells was normal. (B) The aortic ring was processed with CSE antibody. CSE protein was strongly expressed in the inner membrane and tunica media vasorum. The brown granules in aortic smooth muscle cells and endothelial cells were defined as positive signals. (C) The pulmonary artery ring was processed with CSE antibody. The brown granules were observed in pulmonary artery smooth muscle cells and endothelial cells. CSE, cystathinonine gamma-lyase.
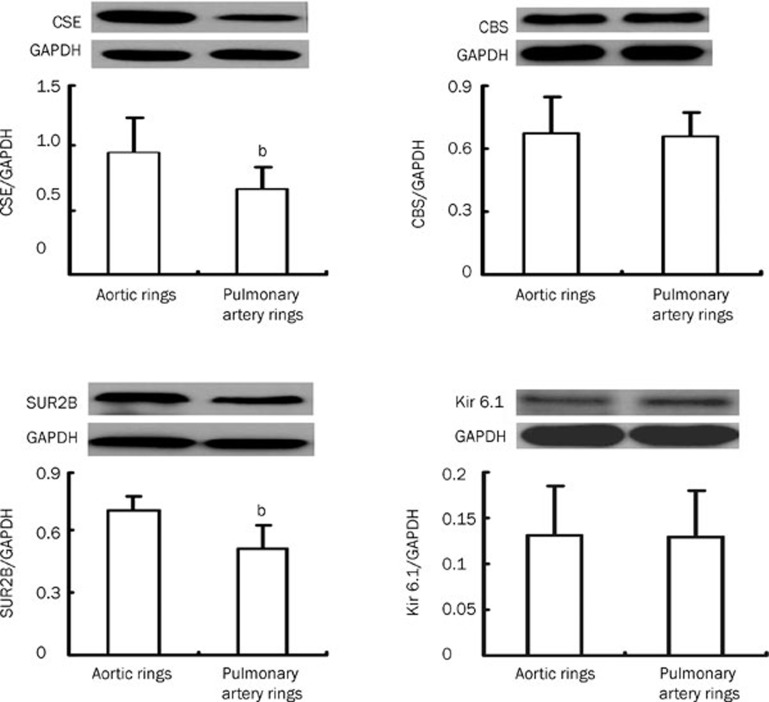
CSE and CBS expressions in aortic and pulmonary artery rings by Western blotting
Compared to that in pulmonary artery rings, the expression of CSE protein in aortic rings was notably enhanced (P<0.05, Figure 4). However, there was no difference in CBS protein expression between aortic and pulmonary artery rings (P>0.05, Figure 4).
Figure 4.
CSE, CBS, SUR2B and Kir6.1 expression in aortic and pulmonary artery rings as detected by Western blotting (n=10, mean±SD). bP<0.05 compared to aortic rings. CSE, cystathinonine gamma-lyase; CBS, cystathionine beta synthase; SUR2B, a KATP channel subunit; Kir6.1, a KATP channel subunit.
Expression of SUR2B, Kir6.1 and 3MST by real-time PCR
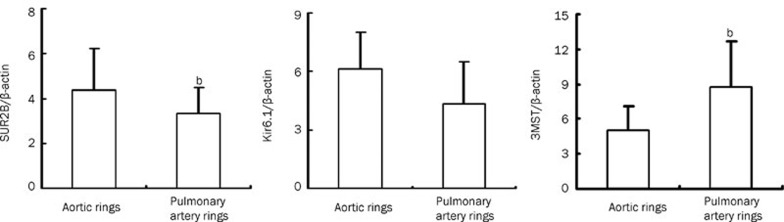
As measured by semi-quantitative real-time PCR, SUR2B mRNA was higher in aortic rings than in pulmonary artery rings (P<0.05, Figure 5). However, Kir6.1 mRNA expression did not differ between aortic rings and pulmonary artery rings (P>0.05, Figure 5). 3MST mRNA was lower in aortic rings than in pulmonary artery rings (P<0.05, Figure 5).
Figure 5.
Expression of SUR2B, Kir6.1 and 3MST by real-time PCR (n=7, mean±SD). bP<0.05 compared to aortic rings. SUR2B, a KATP channel subunit. Kir6.1, a KATP channel subunit. 3MPST, 3-mercaptopyruvate sulfurtransferase.
Immunohistochemical analysis of SUR2B and Kir6.1 expression in aortic and pulmonary artery rings
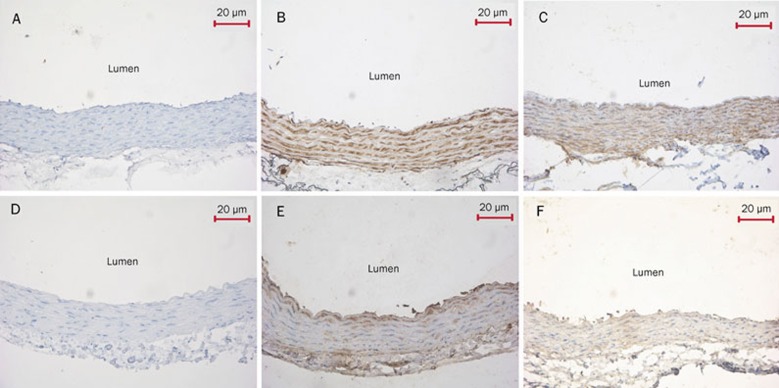
The SUR2B and Kir6.1 proteins in aortic and pulmonary artery rings from WKY rats were mainly expressed in the medial layer of the vessel. The brown granules in both aortic and pulmonary artery smooth muscle cells and endothelial cells viewed were defined as positive signals (Figure 6).
Figure 6.
Immunohistochemical analysis of SUR2B and Kir6.1 expression in aortic and pulmonary artery rings (DAB×200). (A) The aortic ring negative control was processed without SUR2B primary antibody. This control had normal smooth muscle cell and endothelial cell structure without brown granules. (B) The aortic ring was treated with SUR2B antibody. SUR2B protein was strongly expressed in the inner membrane and tunica media vasorum. The presence of the brown granules in aortic smooth muscle cells and endothelial cells was defined as positive signals. (C) The pulmonary artery ring was processed with SUR2B antibody. Brown granules were observed in pulmonary artery smooth muscle cells and endothelial cells. (D) The aortic ring negative control was treated without Kir6.1 primary antibody. The structure of smooth muscle cells and endothelial cells was normal and had no brown granules. (E) The aortic ring was treated with Kir6.1 antibody. Brown granules were strongly expressed in the medial layer of aortic ring. (F) The pulmonary artery ring was processed with Kir6.1 antibody. The brown granules were observed in pulmonary artery smooth muscle cells and endothelial cells. SUR2B, a KATP channel subunit. Kir6.1, a KATP channel subunit.
SUR2B and Kir6.1 expression in aortic and pulmonary artery rings as shown by Western blotting
Compared to the pulmonary artery rings, the expression of SUR2B protein increased in the aortic rings of the Wistar rats (P<0.05, Figure 4), but there was no difference in Kir6.1 protein expression between aortic and pulmonary artery rings (P>0.05, Figure 4).
Discussion
In the present study we found that NaHS resulted in the vasorelaxation of rat thoracic aortic rings in a dose-dependent manner, which was more pronounced than the vasorelaxation that occurred in pulmonary artery rings. In aortic rings, both cellular and mitochondrial membrane KATP channel blockers markedly inhibited H2S-induced vasorelaxation. In contrast, H2S-induced vasorelaxation in pulmonary artery rings could only be blocked by a cellular, but not mitochondrial, membrane KATP channel blocker. The expression of SUR2B protein and mRNA in aortic rings increased compared to pulmonary artery rings.
H2S, a novel gaseous signaling molecule, has been considered to play an important role in the regulation of cardiovascular functions6, 7, 8, 9, 10. Endogenous cardiovascular H2S is mainly produced by CSE8, 9, 10. R ecent evidence from CSE knockout mice suggests that loss of CSE gene expression results in a decrease in H2S production and a subsequent rise in blood pressure16. Furthermore, Shibuya et al and Olson et al showed that CSE, CBS, and 3MST were the three important H2S generating enzymes13, 14. In our study, we found that the mRNA expression of 3MST in rat aorta was lower than that in the pulmonary artery, whereas the CSE protein was higher in the rat aorta than in the pulmonary artery. However, there was no difference in CBS protein expression in the rat aorta and pulmonary artery.
H2S plays an important role in the regulation of systemic and pulmonary circulation25, 26, 27, 28, 29, 30. Olson et al showed that in rat pulmonary arteries, NaHS produced a transit constriction followed by relaxation for 20 to 30 min, which was then followed by a second constriction5. In a later study, this same group carefully examined the effects of Na2S on the conductance and resistance responses of the cow and sea lion pulmonary arteries and showed that the sea lion arteries had vasodilating characteristics31. We analyzed the vascular response to H2S for 30 min. We found that in rat pulmonary arteries, NaHS at concentrations of 50, 100, and 200 μmol/L produced a transit constriction followed by a relaxation for about 20 min, which was then followed by a reduced relaxation. However, this did not occur in the rat aorta.
In this study, we found that NaHS caused vasorelaxation of rat thoracic aortic and pulmonary artery rings pre-contracted with 1 μmol/L NE in vitro in a dose-dependent manner. The mechanism for the vasoconstrictive response to norepinephine is the action of NE on the vascular alpha adrenaline receptors, resulting in the vasoconstrictive response. A previous study showed that in sheep in vivo, the vasoconstrictor response to alpha-adrenergic stimulation was less in the pulmonary circulation compared to the systemic circulation of the fetus32. This same study also indicated that alpha-adrenergic receptor density was less pronounced in fetal intrapulmonary vascular smooth muscle than that in fetal aortic VSM32. The vasorelaxing effect of H2S on aortic and pulmonary rings is dependent on this initial pre-contraction. As far as we know, the differences in the vasorelaxing effects of H2S between the aortic and pulmonary rings involve the following mechanisms: the mechanical properties of the blood vessels, the targeting ion channel KATP expressions and density where H2S acts on. Thus, in our study, we attempted to examine if there were any KATP expression-mediated mechanisms in which H2S acts on the different arteries. H2S at concentrations of 50–1000 μmol/L dilated aortic rings more significantly than pulmonary artery rings. This result indicates that H2S at the same dose induces a stronger vasorelaxing effect in aortic rings compared to pulmonary artery rings.
H2S acts as a regulator of cardiovascular function33, 34. The opening of smooth KATP channels by H2S has been suggested to be one of the mechanisms responsible for H2S-induced vasorelaxation in vascular smooth muscle both in vitro and in vivo24. H2S can open KATP channels in the cell membrane of aortic vascular smooth muscle, causing cytomembrane hyperpolarization. The KATP channel is very important in the cardiovascular system35, 36, 37, 38, 39, 40, 41 and H2S acts as an endogenous KATP channel opener. KATP channels are recognized for their cardioprotective role in ischemia35. Evidence suggests that Kir6.1 and SUR2B are the main KATP channel subunits expressed in the vascular smooth muscle42, 43, 44.
Therefore, we investigated the possible mechanisms responsible for the differences in vasorelaxation between aortic and pulmonary artery rings induced by H2S by targeting KATP channels using cell and mitochondrial membrane KATP channel blockers. The results showed that cellular (Gli) and mitochondrial (5-HD) membrane KATP channel blockers could block H2S-induced vasorelaxation in aortic rings. In contrast, in pulmonary artery rings, only the cell membrane KATP channel blocker effectively blocked H2S-induced vasorelaxation. In aortic rings, vasorelaxation by NaHS was 38.4% at a concentration of 100 μmol/L, which was reduced to 20.4% and 26.9% when aortic rings were pre-treated with cell and mitochondrial membrane KATP channel blockers, respectively. In pulmonary artery rings, the percent of vasorelaxation was 22.2% following 100 μmol/L NaHS, which was reduced to 12.8% when pre-treated with the cell membrane KATP channel blocker. However, pre-treatment with the mitochondrial membrane KATP channel blocker did not alter pulmonary artery ring vasorelaxation. We presume that H2S likely induces more obvious vasorelaxation in aortic rings because H2S opens the KATP channels more widely in aortic rings than in pulmonary artery rings.
Next, we further examined whether there was any differences in KATP channel expression between aortic and pulmonary artery rings. The results showed that protein expression of the KATP channel subunit SUR2B was higher in aortic than pulmonary artery rings. Furthermore, the mRNA expression of SUR2B was higher in aortic rings than in pulmonary artery rings. These findings suggested that the relatively higher density of KATP channels in aortic rings was partly responsible for the pronounced vasorelaxation observed in isolated aortic rings compared to those observed in pulmonary artery rings at specific concentrations. The identification of more profound mechanisms involved in the H2S-induced vasorelaxation of aortic and pulmonary artery rings requires further investigation.
Author contribution
Jun-bao DU and Hong-fang JIN designed the research; Yan SUN and Hong-fang JIN performed the research and contributed new analytical reagents and tools; Yan SUN, Hong-fang JIN, Chao-shu TANG, and Jun-bao DU analyzed data; and Yan SUN, Hong-fang JIN, and Jun-bao DU wrote the paper.
Acknowledgments
This work was supported by the Foundation of the Ministry of Education, People's Republic of China (20070001702 and 20070001770); the National Natural Science Foundation of China 81070212, 30821001, and 30801251); the Major Basic Research Development Program of the People's Republic of China (2011CB503904); and the Beijing Natural Science Foundation (7082095).
We thank Shu-xu DU and Wei LU for their technical assistance.
References
- Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, et al. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006;570:53–8. doi: 10.1113/jphysiol.2005.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GA, Webb DJ. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol Ther. 1996;72:109–48. doi: 10.1016/s0163-7258(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Sata M, Fukuda D. Crucial role of renin-angiotensin system in the pathogenesis of atherosclerosis. J Med Invest. 2010;57:12–25. doi: 10.2152/jmi.57.12. [DOI] [PubMed] [Google Scholar]
- Lipworth BJ, Dagg KD. Vasoconstrictor effects of angiotensin II on the pulmonary vascular bed. Chest. 1994;105:1360–4. doi: 10.1378/chest.105.5.1360. [DOI] [PubMed] [Google Scholar]
- Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, et al. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006;209:4011–23. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- Tang C, Li XH, Du JB. Hydrogen sulfide as a new endogenous gaseous transmitter in the cardiovascular system. Curr Vasc Pharmacol. 2006;4:17–22. doi: 10.2174/157016106775203144. [DOI] [PubMed] [Google Scholar]
- Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol Sin. 2007;28:1709–16. doi: 10.1111/j.1745-7254.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life. 2005;57:603–6. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE. What is the significance of vascular hydrogen sulphide (H2S) Br J Pharmacol. 2006;149:609–10. doi: 10.1038/sj.bjp.0706907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bełtowski J. Hydrogen sulfide as a biologically active mediator in the cardiovascular system. Postepy Hig Med Dosw. 2004;58:285–91. [PubMed] [Google Scholar]
- Łowicka E, Bełtowski J. Hydrogen sulfide (H2S)-the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–35. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–6. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, et al. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–16. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a Physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res. 2001;88:2–11. doi: 10.1161/01.res.88.1.e2. [DOI] [PubMed] [Google Scholar]
- Zhu P, Huang L, Ge X, Yan F, Wu R, Ao Q. Transdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling. Int J Exp Pathol. 2006;87:463–74. doi: 10.1111/j.1365-2613.2006.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, et al. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45:117–23. doi: 10.1016/j.cyto.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Santicioli P, Giuliani S, Maggi CA. Pharmacological investigation of hydrogen sulfide (H2S) contractile activity in rat detrusor muscle. Eur J Pharmacol. 2005;509:171–7. doi: 10.1016/j.ejphar.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–63. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313:362–8. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- Su YW, Liang C, Jin HF, Tang XY, Han W, Chai LJ, et al. Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ J. 2009;73:741–9. doi: 10.1253/circj.cj-08-0636. [DOI] [PubMed] [Google Scholar]
- Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation — a tale of three gases. Pharmacol Ther. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wang YF, Shi L, Du JB. Impact of L-arginine on hydrogen sulfide/cystathionine-γ-lyase pathway in rats with high blood flow-induced pulmonary hypertension. Biochem Biophys Res Commun. 2006;345:851–7. doi: 10.1016/j.bbrc.2006.04.162. [DOI] [PubMed] [Google Scholar]
- Yan H, Du JB, Tang CS. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–7. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P. Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. Biochim Biophys Acta. 2009;1787:864–72. doi: 10.1016/j.bbabio.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res. 2008;79:632–41. doi: 10.1093/cvr/cvn140. [DOI] [PubMed] [Google Scholar]
- Wang YF, Mainali P, Tang CS, Shi L, Zhang CY, Yan H, et al. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chin Med J. 2008;121:420–3. [PubMed] [Google Scholar]
- Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, et al. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Aterioscler Thromb Vasc Biol. 2009;29:173–9. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, et al. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul PW, Magness RR, Muntz KH, DeBeltz D, Buja LM. Alpha 1-adrenergic receptors in pulmonary and systemic vascular smooth muscle. Alterations with development and pregnancy. Circ Res. 1990;67:1193–200. doi: 10.1161/01.res.67.5.1193. [DOI] [PubMed] [Google Scholar]
- Rochette L, Vergely C. Hydrogen sulfide (H2S), an endogenous gas with odor of rotten eggs might be a cardiovascular function regulator. Ann Cardiol Angeiol (Paris) 2008;57:136–8. doi: 10.1016/j.ancard.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Du J, Hui Y, Cheung Y, Bin G, Jiang H, Chen X, et al. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels. 2004;19:75–80. doi: 10.1007/s00380-003-0743-7. [DOI] [PubMed] [Google Scholar]
- Seto SW, Ho YY, Hui HN, Au AL, Kwan YW. Contribution of glibenclamide-sensitive, ATP-dependent K+ channel activation to acetophenone analogues-mediated in vitro pulmonary artery relaxation of rat. Life Sci. 2006;78:631–9. doi: 10.1016/j.lfs.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Fan LH, Tian HY, Wang J, Huo JH, Hu Z, Ma AQ, et al. Downregulation of Kir6.1/SUR2B channels in the obese rat aorta. Nutrition. 2009;25:359–63. doi: 10.1016/j.nut.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Yang W, Yang GD, Jia XM, Wu LY, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J Physiol. 2005;569:519–31. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, et al. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol. 2006;575:627–44. doi: 10.1113/jphysiol.2006.113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang ZX, Xu YJ. Effect of mitochondrial KATP channel on voltage- gated K+ channel in 24 hour-hypoxic human pulmonary artery smooth muscle cells. Chin Med J. 2005;118:12–9. [PubMed] [Google Scholar]
- Cyrino FZ, Bottino DA, Coelho FC, Ravel D, Bouskela E. Effects of sulfonylureas on KATP channel-dependent vasodilation. J Diabetes Complications. 2003;17:6–10. doi: 10.1016/s1056-8727(02)00273-8. [DOI] [PubMed] [Google Scholar]
- Peter P, Ashcroft FM. Modeling KATP channel gating and its regulation. Prog Biophys Mol Biol. 2009;99:7–19. doi: 10.1016/j.pbiomolbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Fujita A, Kurachi Y. Molecular aspects of ATP-sensitive K+ channels in the cardiovascular system and K+ channel openers. Pharmacol Ther. 2000;85:39–53. doi: 10.1016/s0163-7258(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–43. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Tang GH, Hu DH, Wang R. Molecular basis of ATP-sensitive K+ channels in rat vascular smooth muscles. Biochem Biophys Res Commun. 2002;296:463–9. doi: 10.1016/s0006-291x(02)00892-6. [DOI] [PubMed] [Google Scholar]