Abstract
Background
Parenteral nutrition (PN) is often used in severely injured patients when caloric goals are not achieved enterally. The purpose of this study is to determine whether early administration of parenteral nutrition is associated with an increased risk for infection following severe injury.
Methods
Retrospective cohort study of severely injured blunt trauma patients enrolled from eight trauma centers participating in the “Inflammation and the Host Response to Injury” (Glue Grant) study. We compared patients receiving PN within seven days following injury to a control group who did not receive early PN. We then focused on patients who tolerated at least some enteral nutrition during the first week and evaluated the potential influence of supplemental PN on outcomes in this “enteral tolerant” subgroup. The primary outcome was the occurrence of a nosocomial infection following the first post-injury week. Secondary outcomes included the type of infection and hospital mortality.
Results
Of the 567 patients enrolled, 95 (17%) received early PN. Early PN use was associated with a greater risk of nosocomial infection (relative risk 2.1; 95% CI 1.6-2.6, P=<0.001). In the enteral tolerant subgroup (n=249), early PN was also associated with an increase in nosocomial infections (RR=1.6; 95% CI 1.2-2.1, P=0.005) in part due to an increased risk of blood stream infection (RR=2.8; 95% CI 1.5-5.3, P=0.002). Mortality tended to be higher in patients receiving additional EN + PN versus EN alone (RR 2.3; 95% CI 1.0-5.2, P=0.06).
Conclusion
In critically ill trauma patients who are able to tolerate at least some EN, early PN administration may contribute to increased infectious morbidity and a worse clinical outcome.
Introduction
Adequate nutritional support is vital to managing the severely injured patient. Enteral nutrition is preferable and when compared to the parenteral route, results in fewer complications and seems to lead to improved patient outcomes 1-4. However, not all severely injured patients tolerate enteral feeding to achieve pre-specified caloric, protein and micronutrient goals early in the post injury period. This raises the notion of what the role of parenteral nutritional support is in the early post-injury period in patients slow or unable to tolerate adequate enteral nutrition.
Although well-studied in patients undergoing elective surgical procedures, there is little evidence supporting the use of PN in severely injured patients 5, 6. In this setting, most published studies comparing parenteral and enteral routes have concluded that EN leads to better outcomes than PN. However, the relative benefits and potential harm of PN in the context of partial EN are not known. It is assumed that any detrimental effects of starvation might be overcome by parenteral support in patients unable to tolerate full enteral support. This assumption is the basis for recommendations by several organizations that include the administration of PN when adequate enteral nutrition cannot be reliably achieved within 7 days of injury in critically ill patients7. It is unknown whether earlier institution of PN would be beneficial particularly in circumstances where enteral support may be partially successful, but usual caloric targets are not achieved. There are conflicting recommendations regarding the use of supplemental PN when nutritional support targets are not reached. Some suggest that underfeeding is associated with adverse outcomes whereas others note that there is no advantage conferred by supplemental PN and recommend that PN not be used in combination with EN8, 9.
Given that “adequate” or “sufficient” enteral nutrition remains poorly defined and that few studies address when PN should be used, we undertook this study in an attempt to determine whether the institution of PN in the initial post-injury week influenced outcome. Our initial analysis includes all patients who received PN during the first week. Acknowledging differences between patients receiving PN and those that do not, we further focused our analyses on patients who received at least a modest amount of enteral support in the first post-injury week, testing the null hypothesis that supplemental PN did not influence outcome.
Methods
Study Subject Inclusion and Data Collection
This study was performed as a secondary analysis of the prospective, large scale collaborative research program, entitled, Inflammation and the Host Response to Injury (2 U54 GM-062119-07), the details of which have been reported elsewhere10. In this program, severely injured adult patients are enrolled following blunt trauma if they have evidence of shock as manifested by either a base deficit ≥ 6 or hypotension (systolic blood pressure <90 mm Hg) within 60 minutes of arrival to the emergency department and also require blood transfusion. Exclusion criteria include isolated severe traumatic brain injury, significant pre-existing organ dysfunction and ongoing immunosuppression. All patient demographics, injury, and outcome data are collected prospectively from eight participating centers, de-identified and entered into a web based database. Institutional Review Board approval from Harborview Medical Center, University of Washington was obtained to extract information from the database. Data collection for the parent study began on September 25th, 2003. For this study, the trauma related database was accessed on May 25th, 2006 and all patients 16 years of age or older who survived 72 hours were included.
Demographic data and clinical measures of anatomic and physiologic severity of illness or injury were collected and include abbreviated injury score (AIS), injury severity score (ISS), APACHE II score, admission base deficit, and initial red blood cell transfusion requirement. Procedural data, although available for both abdominal and non-abdominal procedures, was limited in detail to those relating to laparotomy (non-therapeutic, splenectomy, nephrectomy, laparotomy NOS) and laparotomy with a bowel perforation (stomach, small intestine, colon or rectum). An “open abdomen” was defined as any time the abdominal fascia was left open as part of “damage control” laparotomy or where the diagnosis of “abdominal compartment syndrome” was recorded. The administration of all medications, including corticosteroids during the patient's hospital course was also documented.
Patient Care Protocols
Patients at each institution received care based upon standard operating procedures developed to minimize treatment variability between centers. These guidelines covered the following aspects of critical care: sedation in the ICU, mechanical ventilation, diagnosis of ventilator associated pneumonia, trauma resuscitation, venous thromboembolism, nutritional support, transfusion threshold, and strict glycemic control11-16. The remainder of the care was determined by the individual practitioners and local intensive care unit protocols. Preference was given to enteral support and PN was reserved for cases where enteral support was deemed inappropriate or inadequate, according to the treating physician.
Definition of Nutritional Support Groups
Daily enteral and parenteral caloric totals were available for post-injury days 2-28. Enteral calories were administered through a gastric or small bowel feeding tube. Patients who received ≥1000 enteral calories on any day during the first week after injury were considered “enteral tolerant” (EN group). This was an arbitrary selection and corresponds to an estimate of 10 – 30 kcal/kg/day. The specific enteral formula used was at the discretion of the treating physician.
Parenteral calories consisted of all nutritional support provided as intravenously administered protein, carbohydrate and lipid. In the database, propofol (1.1 kcal/ml) is included in the parenteral calorie total but not distinguished from PN calories. We used a threshold value of 750 parenterally administered calories per day as indicating that the patient did, indeed receive PN. This threshold was chosen given that it would be unlikely that this amount of calories would be given as propofol and 5% dextrose. For example, the maximum daily dose of propofol is approximately 3mg/kg/hour × 70 kg × 24 hours × 1.1 kcal/ml of 1% solution which would contribute 554 kcal.
Study Outcomes
The primary outcome was the occurrence of any nosocomial infection that occurred after the 7th post-injury day. This time point was chosen in order to exclude early infections that would unlikely be influenced by the mode of nutritional support during the initial 7 days after injury. Further analysis of the type of infection counted each site separately based on standard CDC criteria 17. Ventilator associated pneumonia was diagnosed using quantitative culture of bronchoalveolar lavage fluid in the majority of cases.
Secondary outcomes included mortality, non-infectious complications, duration of mechanical ventilation, intensive care unit and hospital length of stay. Non-infectious complications of interest were determined prior to data abstraction and were limited to acute respiratory distress syndrome (ARDS) and venous thromboembolism (combined pulmonary embolism and deep venous thrombosis) occurring after the 7th post-injury day. We also examined nosocomial infections occurring before 7 days, given that we would expect PN support to have little if any effect on these early complications.
Statistical Analysis
All statistical analyses were performed using STATA 9.2 (College Station, TX.) and SPSS 11.0 (Chicago, Ill). Infectious complications were treated as dichotomous outcomes, regardless of the number of nosocomial infections experienced by an individual patient. Statistical comparisons between patients receiving and not receiving early PN were conducted using Pearson's chi-square and the Students t-test for categorical and continuous data, respectively. Non parametric continuous data were analyzed using the Wilcoxin rank sum test. Actual p-values are reported as well as the relative risk (RR) or odds ratio (OR) with associated 95% confidence intervals. Adjustment for covariates was performed using logistic and linear regression where appropriate. The initial covariates included age, gender, injury severity score (ISS), APACHE II, red blood cell transfusion during the first 12 hours, admission base deficit, steroid administration, abdominal abbreviated injury score (AIS), laparotomy with and without hollow viscus injury, , and the presence of an open abdomen. ISS, admission base deficit, and red blood cell transfusion volume were analyzed as both continuous and dichotomous variables. In addition, baseline comorbid conditions such as diabetes, liver disease and home steroid use were also included in analyses. The final model incorporated age, gender, injury severity score (<25 vs. ≥25), APACHE II score, massive transfusion (> 6 units in the first 12 hours post-injury), steroid administration, abdominal AIS (as a continuous variable), and any laparotomy. The inclusion of hollow viscus injuries as a separate covariate from laparotomy, did not alter the results. Our final analysis included a variable indicating which of the 8 consortium hospitals the patient was admitted.
Results
In the initial analysis of 567 patients, those who received early PN (95/567, 17%) had more severe injuries, had a greater severity of shock and received more red blood cell transfusions in the first 12 hours than patients who did not receive early PN (Table 1). Abdominal injuries were more severe (higher abdominal AIS score, a greater percentage with hollow viscus injuries and treatment with laparotomy and/or an open abdomen) in those receiving early PN. Patients received PN for a median of 4 days (25th – 75th percentile = 2 – 9 days). A substantial number (63/95) of patients receiving early PN received little or no enteral support during the first post-injury week. Of the entire 567 patients, those receiving early PN were more likely to develop a nosocomial infection than those who did not receive early PN (relative risk (RR) = 2.1; 95% CI = 1.6 – 2.6, p < 0.001). Adjusting for the variables indicated in the methods by logistic regression, the association between early PN and nosocomial infection remained (Table 2). In addition to including patients receiving the vast majority of their nutritional support parenterally, this analysis also included less seriously injured patients who were tolerating an oral diet within 3 – 4 days and therefore did not receive any enteral or parenteral support. As these factors would likely bias the risk associated with PN in an unfavorable way, we subsequently focused on the group of patients who received a modest amount of EN during the first week (≥ 1000 kcal for at least 1 day during the initial post-injury week; referred to as “enteral tolerant”), a proportion of whom received additional parenteral calories
Table 1. Baseline Characteristics of Study Cohort (n=567).
| Early EN only (n = 472) | Early EN + PN (n = 95) | p-value | |
|---|---|---|---|
| Age | 40 (26 – 52) | 43 (26 – 53) | 0.35 |
| Male (%) | 297 (63) | 69 (73) | 0.07 |
| Admission base deficit (mmol/L) | 7.8 (5 – 10) | 9.5 (7 – 13) | <0.001 |
| Total 12 hour PRBC transfusion (ml) | 1500 (700 – 2800) | 2300 (1250 – 4900) | <0.001 |
| Abdominal AIS Score ≥3 (%) | 189 (40) | 52 (55) | 0.008 |
| ISS | 27 (20 – 38) | 34 (27 – 43) | <0.001 |
| APACHE II | 28 (23 – 32) | 33 (28 – 36) | <0.001 |
| Number Requiring Laparotomy (%) | 191 (40) | 56 (59) | 0.001 |
| Number with Hollow Viscus Injuries Including | 18 (4) | 5 (5) | 0.51 |
| Stomach, Small Intestine, Colon, and Rectum) (%) | |||
| Open Abdomen (%) | 64 (14) | 25 (26) | 0.002 |
Continuous data are presented as median with interquartile range in parentheses and categorical data are presented as number with percentage in parentheses.
Table 2. Infectious and Noninfectious Outcomes in Study Cohort (n=567).
| Complications (%) | No PN (n = 472) | PN (n = 95) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Late Infectious | ||||
| Nosocomial Infection (excluding SSI) | 128 (27) | 53 (56) | 2.1 (1.3 - 3.5) | 0.003 |
| Pneumonia | 68 (14) | 31 (33) | 1.7 (1.0 - 3.0) | 0.06 |
| Blood Stream Infection (BSI) | 38 (8) | 25 (26) | 2.9 (1.6 – 5.4) | 0.001 |
| Catheter Related BSI | 8 (2) | 4 (4) | 3.8 (0.9 – 15.6) | 0.06 |
| Urinary Tract Infection | 39 (8) | 21 (22) | 2.5 (1.3 – 4.9) | 0.006 |
| Surgical Site Infection (SSI) | 47 (10) | 17 (18) | 1.5 (0.8 – 2.9) | 0.24 |
| Non-Infectious | ||||
| Late ARDS (onset after 7 days) | 7 (1) | 8 (8) | 3.4 (1.0 – 11.0) | 0.04 |
| Late Thromboembolic (onset after 7 days) | 29 (6) | 9 (9) | 1.4 (0.6 – 3.2) | 0.48 |
| Death | 38 (8) | 22 (23) | 1.5 (0.8 – 3.0) | 0.24 |
Data are presented as number of patients in each group with percentage in parentheses. Associated p-values are based upon aOR.
Influence of PN on Outcomes in “Enteral Tolerant” Patients
A total of 249 (44% of the original 567 patients) were “enteral tolerant” during the first week. Of these, 217 (87%) received only EN during the initial week and 32 (13%) received additional PN. Demographic and clinical information for these 2 groups are shown in Table 3. The median day of PN initiation was 3 days post-injury (IQR: 2 – 5 days).
Table 3. Baseline Characteristics-“Enteral Tolerant” Subgroup.
| Early EN only (n = 217) | Early EN + PN (n = 32) | p-value | |
|---|---|---|---|
| Age | 41 (27 – 54) | 43 (25 – 53) | 0.74 |
| Male (%) | 133 (61) | 24 (75) | 0.13 |
| Admission base deficit (mmol/L) | 8.4 (6 – 11) | 9.1 (8 – 12) | 0.08 |
| Total 12 hour PRBC transfusion (ml) | 2100 (1100 – 3200) | 2250 (1200 – 3325) | 0.70 |
| Abdominal AIS Score ≥3 (%) | 79 (36) | 14 (44) | 0.42 |
| ISS | 34 (24 – 41) | 33 (28 – 44) | 0.24 |
| APACHE II | 30 ( 26 – 34) | 30 ( 26 – 34) | 0.91 |
| Number Requiring Laparotomy (%) | 83 (38) | 14 (44) | 0.55 |
| Number with Hollow Viscus Injuries Including | 8 (4) | 1 (3) | 0.87 |
| Stomach, Small Intestine, Colon, and Rectum) (%) | |||
| Open Abdomen (%) | 36 (17) | 5 (16) | 0.89 |
Continuous data are presented as median with interquartile range in parentheses and categorical data are presented as number with percentage in parentheses.
The frequencies of infectious and non-infectious complications for patients who were “enteral tolerant” are presented in Table 4. The risk of nosocomial infection was higher in the group receiving early EN+PN (RR =1.6, 95% CI = 1.2 – 2.1, p = 0.005). After adjustment, early EN+PN remained associated with an increased risk for nosocomial infection when compared to patients receiving EN alone. Considering the association between EN+PN use and specific infections, the largest increase was observed in the risk of blood stream infections (RR = 2.8, 95% CI = 1.5 – 5.3, p = 0.002). The patients in this cohort were enrolled from 8 member hospitals. While there were differences between these institutions in numbers of patients enrolled (range 1 – 133) and the frequency of early PN use (0 – 23%), there was no difference between institutions in the frequency of nosocomial infections. Adjusting for the hospital of admission did not affect the association between early EN+PN and subsequent nosocomial infections.
Table 4. Infectious and Noninfectious Outcomes in “Enteral Tolerant” Group.
| Complications (%) | Early EN only (n = 217) | Early EN + PN (n = 32) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Late Infectious | ||||
| Nosocomial Infection (excluding SSI) | 92 (42) | 22 (69) | 2.5 (1.1-5.9) | 0.03 |
| Pneumonia | 55 (25) | 12 (38) | 1.4 (0.6-3.3) | 0.39 |
| Blood Stream Infection (BSI) | 24 (11) | 10 (31) | 3.4 (1.4-8.3) | 0.007 |
| Catheter Related BSI | 2 (1) | 2 (6) | 9.8 (0.9-112.9) | 0.07 |
| Urinary Tract Infection | 24 (11) | 5 (16) | 1.5 (0.5-4.6) | 0.48 |
| Surgical Site Infection (SSI) | 31 (14) | 6 (19) | 1.3 (0.5-3.4) | 0.65 |
| Non-Infectious | ||||
| Late ARDS (onset after 7 days) | 4 (2) | 3 (9) | 5.4 (1.1-27.4) | 0.04 |
| Late Thromboembolic (onset after 7 days) | 15 (7) | 4 (13) | 1.7 (0.5-5.7) | 0.37 |
| Death | 18 (8) | 6 (19) | 2.7 (0.8-9.3) | 0.10 |
Data are presented as number of patients in each group with percentage in parentheses. Associated p-values are based upon aOR.
Enteral tolerant patients who received additional PN had an approximate 2-fold increased risk of death before (RR = 2.3; 95% CI = 1.0-5.2, p = 0.06) and after adjustment (aOR = 2.7; 95% CI= 0.8-8.8, p = 0.10). Other adverse outcomes were more common in patients receiving EN+PN. Late ARDS (defined as ARDS whose onset was after the 7th post-injury day) was also more common in those receiving EN+PN (RR = 5.1, 95% CI = 1.2-21.7, p = 0.016), whereas early ARDS was not. This difference persisted after adjustment for potential confounding variables (Table 4). Duration of mechanical ventilation (14 versus 19 days, p = 0.007), and ICU length of stay (18 versus 23 days, p = 0.008) were longer in patients receiving early EN+PN. There was no significant difference in hospital length of stay (29 versus. 34 days, p = 0.23). We also compared nosocomial infections occurring in the initial 7 post injury days between patients receiving early EN+PN and those receiving only enteral support. The risk was similar in patients receiving early EN+PN and those receiving only enteral support (5/32 and 50/217, p = 0.49). We would expect that early EN+PN should, in fact, have minimal if any effect on the risk for these early infections. This similar incidence of early infection supports the notion that the overall baseline risk for nosocomial infection did not differ between patients who did and did not receive early supplemental PN.
Nutritional Support, Caloric Intake and Serum Albumin Levels
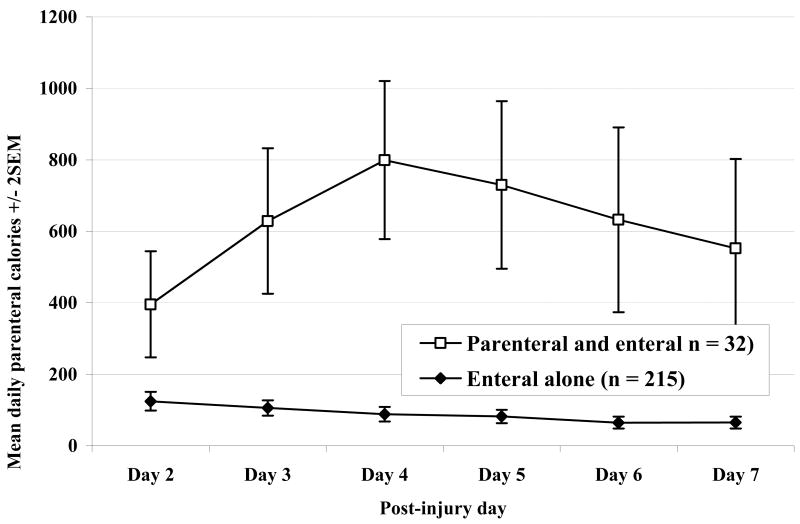
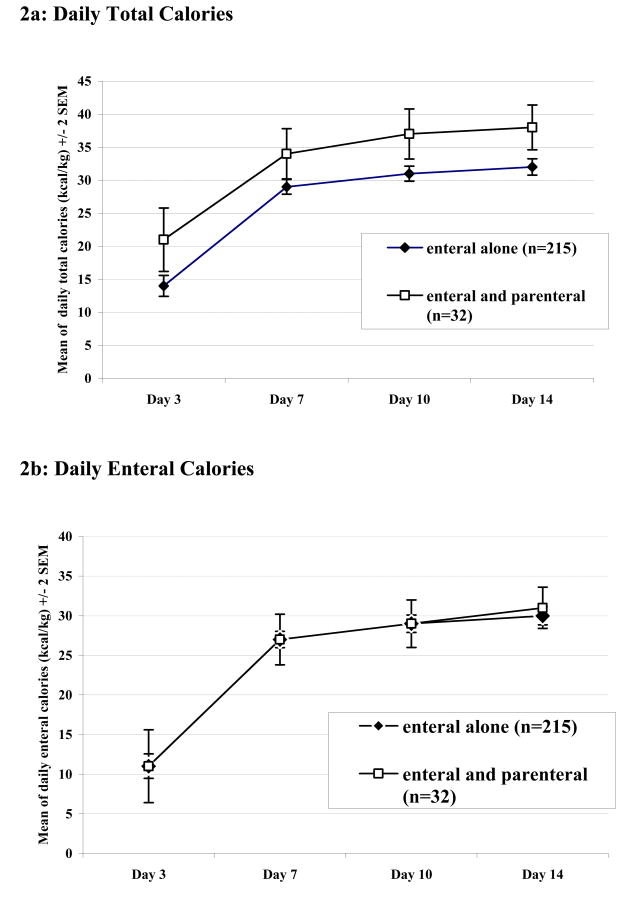
The mean daily parenteral calories received over the first 7 days in the enteral tolerant group are shown in Figure 1. The groups differed substantially in regard to the amount of parenteral support received over this period indicating that the 2 groups did, in fact, experience distinctly different nutritional support. This difference in parenteral calories translated into a difference in total caloric intake over the first 2 weeks, whereas the enteral intake was similar between the 2 groups. When adjusted for predicted or adjusted body weight (ideal + [actual-ideal/2]), the patients given EN+PN received more calories per day over the first 2 weeks than did patients who did not receive PN support. These data are summarized in Figures 2a and 2b, which show average maximum caloric intake by days 3, 7, 10 and 14 after injury. In summary, the parenteral support seemed only to add to the caloric intake rather than replace relatively less enteral support.
Figure 1. Daily Parenteral Calories during Initial Post-Injury Week.
Figure 2. Daily Total and Enteral Caloric Intake Adjusted for Body Weight.
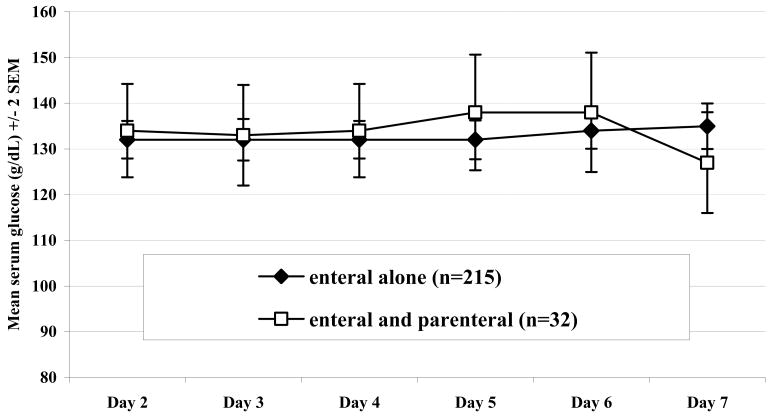
There was no evidence that patients receiving EN+PN had higher blood glucose concentrations nor were they more likely to be “hyperglycemic” when using different serum glucose concentrations to define hyperglycemia (110 g/dL or 130 g/dL, for example). The maximum serum glucose concentration measured during the first week was similar in patients who did and did not receive early PN (165 ± 28 g/dL versus 164 ± 34 g/dL, p-value = 0.5). Figure 3 shows the maximum daily glucose concentrations for enteral tolerant patients during day's 2 – 7 post injury, indicating no effect of PN on serum glucose concentration in this subgroup.
Figure 3. Maximum Daily Serum Glucose Concentrations on Post-Injury Days 2 – 7.
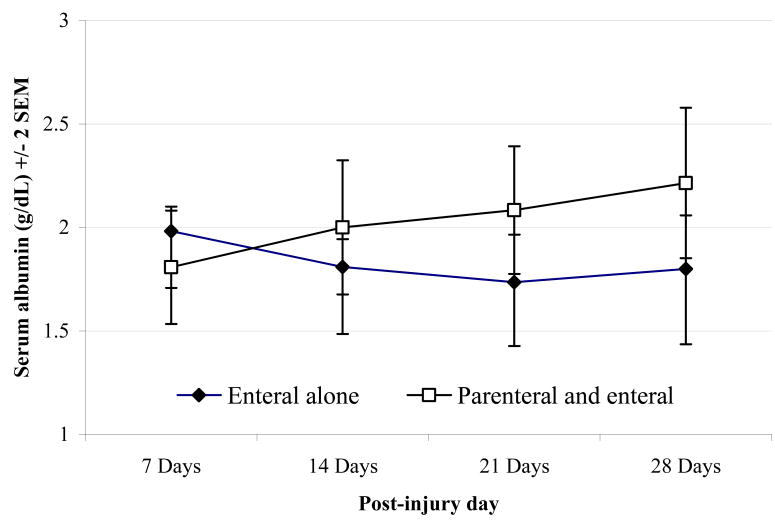
There was no difference in the maximum serum albumin between groups during the first week, with serum albumin in both groups generally below normal (2.0 versus 1.8 g/dL, p = 0.11). However, during the subsequent days and weeks, the maximal albumin was, indeed higher in patients receiving EN+PN support as shown in Figure 4. Although these data do suggest a beneficial biochemical effect of PN that is manifest by serum albumin concentrations, this was not accompanied by an improved clinical outcome.
Figure 4. Weekly Maximum Serum Albumin Concentration.
Discussion
In this study we found that early PN administration, when administered along with a modest quantity of enteral nutrition, was associated with an increased risk of nosocomial infection and an increased mortality in severely injured trauma patients. The analysis involving the entire cohort of 567 patients is likely biased against patients receiving early PN given that those patients (n = 63) who received PN exclusively during the first 7 days were more severely injured than the remainder of the cohort. We therefore focused our analyses on those patients who tolerated a modest amount of enteral nutrition, of whom, 32 of 249 (13%) still received early PN. The results of this analysis were not markedly different from the analysis of the entire cohort with regard to the increased risk of nosocomial infection associated with receiving PN within 7 days of injury.
The beneficial and adverse effects of PN on infectious morbidity have been explored previously. In the majority of cases, PN is compared to enteral feeding regimens. The common finding is generally an increase in infectious morbidity and mortality3, 18, 19. These observations are often attributed to the protective effect of enteral nutrition on gut mucosal function and innate immunity20. Our data do not refute such interpretations, but given that the average daily amount of enteral calories (in the 249 enteral tolerant patients) was similar in patients who did and those who did not receive early PN, the data suggest that nosocomial infections may, in part, be due to the administration of PN.
Our results are similar to a recent observational study in trauma patients documenting a significant decrease in the use of TPN at a single institution over a six year period. The authors noted that the administration of TPN was associated with increased infections, ARDS and multi-organ dysfunction syndrome21. Interestingly, TPN use was associated with the greatest risk for bacteremia whereas the risk of pneumonia and urinary tract infections were only minimally or not at all associated with TPN. Although these findings are similar to our own, we feel that this should be interpreted with caution as their analysis did not attempt to account for the relationship between the timing of PN initiation, EN administration, and outcome.
One explanation for our observations is that the PN itself is harmful. Studies in elective surgical, acute burn, trauma, and critically ill patients have failed to demonstrate a beneficial effect of PN on patient outcome 18, 21-24. Given that the majority of these studies were performed prior to the widespread use of protocols for tight glycemic control, it is possible and has been posited, that poorly controlled hyperglycemia contributes to the increased morbidity seen with PN use. In our study, patients were managed using a protocol for tight glycemic control when even mild hyperglycemia was present (>110 mg/dl). We saw no evidence that patients receiving PN had poorer glycemic control than those receiving only EN. In addition, there was no relationship between hyperglycemia and infectious morbidity. Although it is possible that unmeasured hyperglycemia contributed to infectious morbidity in this cohort, we feel that this is unlikely to have resulted in significant bias, either for or against PN, given that the sampling protocol was applied to both groups.
Other explanations for the association between early PN and nosocomial infection include differences in caloric quantity and the composition of administered PN. Patients given EN+PN received more calories than those receiving EN alone. When corrected for body weight (using adjusted body weight in patients with a BMI > 30), a greater number of those given EN+PN received over 35 kcal/kg at some point during the first week (47% versus 23%). It is not certain, however, exactly how an increased number of calories might lead to an increased infection rate, beyond the possibility of an excess of specific caloric sources. However, the lack of differences in glucose concentrations and hyperglycemia risk suggests that excess glucose calories are not causative. It is possible that excess lipid calories contributed to infectious morbidity. The use of intravenous fat emulsion has been observed to suppress both neutrophil and lymphocyte function in animal and human studies 25-28. In addition, intravenous fat emulsions have been shown to affect pulmonary function in patients with lung injury and have been associated with mortality 27, 28. This is consistent with our finding that patients receiving early EN+PN had a greater risk of later developing ARDS. It is possible therefore, that it is the lipid administration which is responsible for detrimental effects of parenteral support.
The questions of when and whether PN should be given to severely injured patients are difficult to answer and our data address only one aspect of this issue. Several studies have demonstrated critically ill patients frequently receive enteral nutrition in quantities insufficient to meet nutritional goals yet the addition of parenteral calories seems to come at a significant cost8, 29-31. While available guidelines recommend PN when caloric goals are not met during the first week, it has not been shown that this approach is beneficial. Given the potential consequences of malnutrition in trauma victims, the risk to benefit ratio will eventually favor PN over prolonged starvation 32-35. However, our data suggest that the time point where the benefits of PN outweigh its complications does not occur in the first post-injury week. We, however, note that this conclusion applies only to patients who are able to tolerate some enteral calories and not necessarily to patients unable to take any enteral support during the first week.
As this is not a randomized trial, this study is limited by the possibility that patients receiving EN+PN differed systematically, in unmeasured, but important ways from those receiving only EN. It is possible that we failed to account for factors leading to an increased risk for nosocomial infections, particularly bloodstream infections that also lead to, or were associated with the need for parenteral nutritional support in the initial post injury week. Despite this observed association, we cannot determine if the nutrient composition or simply the provision of additional parenteral calories was contributory.
Clearly there are additional limitations of our study, such as our choice of various thresholds and definitions. First, our selection of 7 days to define both early PN and subsequent late infections could be considered arbitrary. However, 7 days seems a reasonable threshold by which to define early PN given existing literature and nutritional support recommendations. Second, we characterized patients as enteral tolerant if they received ≥ 1000 kcal/day at any point within the first week. Of these patients, 190 never received PN, 32 received early PN and 27 ultimately required delayed (after 7 days) PN. This indicates that most of these patients were successfully managed with EN alone. Importantly, delaying PN until after 7 days did not appear to be detrimental, given that those receiving late PN did not have a higher mortality than those who were managed solely with EN (8% versus 7%).
Finally, the interpretation of our data would change little if we altered the threshold to define enteral tolerance from 1000 kcal/day on any day in the first post-injury week. For example, lowering the threshold to 500 kcal/day would increase the number of “enterally tolerant” patients from 249 to 295, of whom 43 (15%) received early EN+PN. Early EN+PN remained associated with an increased risk of nosocomial infection even with this lower threshold of enteral intake in the first week (RR = 2.5, 95% CI = 1.4 – 4.5). We do not take this to indicate that PN is not beneficial under any circumstances in the first post-injury week, but rather that benefits of enteral support may be achieved at lower caloric intake than previously considered necessary.
Given that a number of patients received PN for relatively few days it could be questioned whether early PN could have any effects, whether beneficial or detrimental. However, despite a short duration of EN+PN, patients did, in fact, demonstrate an improvement in serum albumin concentrations beginning by 14 days post injury. While this does not confirm that parenterally delivered calories caused or led to infections, it does indicate that the supplemental parenteral support achieved higher caloric intake and did seem to influence biochemical nutritional parameters.
Conclusion
Our data suggest that parenteral nutritional support, when commenced during the first post-injury week along with a moderate amount of enteral calories, does not provide measurable clinical benefit and is associated with an increase in the risk of nosocomial, particularly blood stream, infections.
Acknowledgments
The investigators acknowledge the contribution of the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award # 2-U54-GM062119 from the National Institute of General Medical Sciences
Supported by the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award # 2-U54-GM062119 from the National Institute of General Medical Sciences
Disclaimer: The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This Manuscript was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gramlich L, Kichian K, Pinilla J, et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20(10):843–8. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216(2):172–83. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29(7):916–22. doi: 10.1097/00005373-198907000-00003. discussion 922-3. [DOI] [PubMed] [Google Scholar]
- 4.Peterson VM, Moore EE, Jones TN, et al. Total enteral nutrition versus total parenteral nutrition after major torso injury: attenuation of hepatic protein reprioritization. Surgery. 1988;104(2):199–207. [PubMed] [Google Scholar]
- 5.Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med. 1991;325(8):525–32. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 6.Heyland DK, Montalvo M, MacDonald S, et al. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg. 2001;44(2):102–11. [PubMed] [Google Scholar]
- 7.Jacobs DG, Jacobs DO, Kudsk KA, et al. Practice management guidelines for nutritional support of the trauma patient. J Trauma. 2004;57(3):660–78. doi: 10.1097/01.ta.0000135348.48525.a0. discussion 679. [DOI] [PubMed] [Google Scholar]
- 8.Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in canadian ICUs--opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27(1):74–83. doi: 10.1177/014860710302700174. [DOI] [PubMed] [Google Scholar]
- 9.Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24(4):502–9. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Maier R, Bankey P, McKinley B, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: Patient-Oriented Research Core-Standard Operating Procedures for Clinical Care. J Trauma. 2005;59:762–763. [PubMed] [Google Scholar]
- 11.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a Large-Scale Collaborative Project: patient-oriented research core--standard operating procedures for clinical care. II. Guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma. 2006;60(5):1106–13. doi: 10.1097/01.ta.0000220424.34835.f1. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 12.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: Patient-Oriented Research Core--standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005;59(3):764–9. [PubMed] [Google Scholar]
- 13.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care: VI. Blood glucose control in the critically ill trauma patient. J Trauma. 2007;63(3):703–8. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 14.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006;61(1):82–9. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro MB, West MA, Nathens AB, et al. V. Guidelines for sedation and analgesia during mechanical ventilation general overview. J Trauma. 2007;63(4):945–50. doi: 10.1097/TA.0b013e318142d21b. [DOI] [PubMed] [Google Scholar]
- 16.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61(2):436–9. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 17.Horan T, Gaynes R. Surveillance of nosocomial infections. In: CG M, editor. Hosptial epidemiology and infection control. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 18.Herndon DN, Barrow RE, Stein M, et al. Increased mortality with intravenous supplemental feeding in severely burned patients. J Burn Care Rehabil. 1989;10(4):309–13. doi: 10.1097/00004630-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–11. doi: 10.1097/00000658-199205000-00013. discussion 511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1545–8. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- 21.Rhee P, Hadjizacharia P, Trankiem C, et al. What happened to total parenteral nutrition? The disappearance of its use in a trauma intensive care unit. J Trauma. 2007;63(6):1215–22. doi: 10.1097/TA.0b013e31815b83e9. [DOI] [PubMed] [Google Scholar]
- 22.Bauer P, Charpentier C, Bouchet C, et al. Parenteral with enteral nutrition in the critically ill. Intensive Care Med. 2000;26(7):893–900. doi: 10.1007/s001340051278. [DOI] [PubMed] [Google Scholar]
- 23.Dhaliwal R, Jurewitsch B, Harrietha D, Heyland DK. Combination enteral and parenteral nutrition in critically ill patients: harmful or beneficial? A systematic review of the evidence. Intensive Care Med. 2004;30(8):1666–71. doi: 10.1007/s00134-004-2345-y. [DOI] [PubMed] [Google Scholar]
- 24.Hausmann D, Mosebach KO, Caspari R, Rommelsheim K. Combined enteral-parenteral nutrition versus total parenteral nutrition in brain-injured patients. A comparative study. Intensive Care Med. 1985;11(2):80–4. doi: 10.1007/BF00254779. [DOI] [PubMed] [Google Scholar]
- 25.Robin AP, Arain I, Phuangsab A, et al. Intravenous fat emulsion acutely suppresses neutrophil chemiluminescence. JPEN J Parenter Enteral Nutr. 1989;13(6):608–13. doi: 10.1177/0148607189013006608. [DOI] [PubMed] [Google Scholar]
- 26.Fischer GW, Hunter KW, Wilson SR, Mease AD. Diminished bacterial defences with intralipid. Lancet. 1980;2(8199):819–20. doi: 10.1016/s0140-6736(80)90171-3. [DOI] [PubMed] [Google Scholar]
- 27.Battistella FD, Widergren JT, Anderson JT, et al. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J Trauma. 1997;43(1):52–8. doi: 10.1097/00005373-199707000-00013. discussion 58-60. [DOI] [PubMed] [Google Scholar]
- 28.Lekka ME, Liokatis S, Nathanail C, et al. The impact of intravenous fat emulsion administration in acute lung injury. Am J Respir Crit Care Med. 2004;169(5):638–44. doi: 10.1164/rccm.200305-620OC. [DOI] [PubMed] [Google Scholar]
- 29.McClave SA, Lowen CC, Kleber MJ, et al. Are patients fed appropriately according to their caloric requirements? JPEN J Parenter Enteral Nutr. 1998;22(6):375–81. doi: 10.1177/0148607198022006375. [DOI] [PubMed] [Google Scholar]
- 30.De Jonghe B, Appere-De-Vechi C, Fournier M, et al. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered? Crit Care Med. 2001;29(1):8–12. doi: 10.1097/00003246-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Reid C. Frequency of under- and overfeeding in mechanically ventilated ICU patients: causes and possible consequences. J Hum Nutr Diet. 2006;19(1):13–22. doi: 10.1111/j.1365-277X.2006.00661.x. [DOI] [PubMed] [Google Scholar]
- 32.Rubinson L, Diette GB, Song X, et al. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med. 2004;32(2):350–7. doi: 10.1097/01.CCM.0000089641.06306.68. [DOI] [PubMed] [Google Scholar]
- 33.Bistrian BR, Blackburn GL, Scrimshaw NS, Flatt JP. Cellular immunity in semistarved states in hospitalized adults. Am J Clin Nutr. 1975;28(10):1148–55. doi: 10.1093/ajcn/28.10.1148. [DOI] [PubMed] [Google Scholar]
- 34.Haydock DA, Hill GL. Improved wound healing response in surgical patients receiving intravenous nutrition. Br J Surg. 1987;74(4):320–3. doi: 10.1002/bjs.1800740432. [DOI] [PubMed] [Google Scholar]
- 35.Larca L, Greenbaum DM. Effectiveness of intensive nutritional regimes in patients who fail to wean from mechanical ventilation. Crit Care Med. 1982;10(5):297–300. doi: 10.1097/00003246-198205000-00001. [DOI] [PubMed] [Google Scholar]