Abstract
Endometriosis is a debilitating disease common in women of reproductive age characterized by pain and infertility. Macrophage migration inhibitory factor (MIF) is a cytokine whose expression is elevated in endometriotic tissue from women with the disease but the functional role of this factor in the pathogenesis of the disease is uncertain. To examine the role of MIF in the pathogenesis of endometriosis, we induced experimental disease in mice and examined the ability of the MIF antagonist, ISO-1, to reduce endometriotic implant size. Administration of ISO-1 resulted in a significant reduction in implant size and vascularity (as assessed by Flk1 mRNA expression) which was not associated with an alteration in the reproductive cycle. These data suggest that inhibition of MIF activity is associated with a significant reduction in endometriotic implant size and leads us to speculate that a similar approach of targeting MIF may prove useful in treating endometriosis in humans.
Keywords: endometriosis, macrophage migration inhibitory factor, uterus, ISO-1
Introduction
Endometriosis is a debilitating disease that affects as many as 10% of all women of reproductive age and as many as 40 to 50% of all women with infertility. The disease occurs in women of reproductive years (i.e. menstruating females) and is characterized by chief complaints of pelvic pain, dysmenorrhea and infertility. Endometriosis is classically defined as the presence of ectopic endometrial stromal and glandular tissue and is thought to develop via reverse menstruation of viable endometrial tissue into the peritoneal cavity. However, because almost all women of reproductive age exhibit some degree of retrograde menstruation (1, 2), it is postulated that some other factors must contribute to the development and progression of the disease. Strong evidence supports the notion that endometriosis development involves and requires cell/tissue adhesion, tissue remodeling, angiogenesis, and cell proliferation (3). Further, endometriosis shares many characteristics with autoimmune/inflammatory diseases (3, 4). As such, emphasis has been placed upon pro-inflammatory factors which elicit these biological effects as they may be strong candidates for endometriosis therapy (3, 5). Recently, macrophage migration inhibitory factor (MIF) has been proposed to play a role in the pathophysiology of endometriosis as well as other inflammatory/autoimmune diseases (6 – 8).
MIF is a potent mitogenic factor for human endothelial cells in vitro and tumor angiogenesis in vivo (9). A similar effect of MIF in patients with endometriosis was demonstrated by Yang and colleagues (10) who first identified MIF as a potent endothelial cell growth-promoting factor released by endometriotic cells in vitro. Subsequent studies have demonstrated a positive correlation between MIF levels and endometriosis. Specifically, MIF has been shown to be markedly expressed in active and early/stage I endometriotic implants (11) as well as over-expressed in eutopic endometrium from women with the disease (12). MIF levels are also elevated in the peritoneal fluid (13) and peripheral blood (14) of women with endometriosis and MIF secretion is enhanced in peritoneal macrophages from women with the disease (15).
In vitro, MIF has been shown to stimulate prostaglandin E2, cyclooxygenase-2 (16), vascular endothelial growth factor, interleukin-8 and monocyte chemotactic protein-1 (17), all of which have been associated with a proliferative and angiogenic phenotype conducive to endometriotic establishment and/or growth. As such, there is ample evidence to suggest a strong association between elevated MIF expression/levels and endometriosis in vivo as well as in vitro evidence which indicates that MIF can induce factors which are believed to be essential for endometriosis to develop. However, to date there is no information which suggests that MIF functionally contributes to endometriosis growth/viability in vivo. As such, the current studied was designed to determine if MIF plays a functional role in the development/progression of endometriosis using a mouse model for endometriosis. To address this issue, we induced experimental endometriosis in intact mice and then treated them with the MIF antagonist ISO-1 using a similar approach to that described in the literature for treatment of other inflammatory diseases (18-20).
Materials and Methods
Mouse model of endometriosis
Endometriosis was experimentally induced using a modification of the EGFP mouse model described by Nowak and colleagues (21). Our model consisted of female C57BL/6-Tg(ACTB-EGFP)1Osb/J mice which ubiquitously express green fluorescent protein (EGFP; Jackson Laboratories, Bar Harbor, ME) which were used as recipients and immature C57BL/6 wild-type female mice (Jackson Labs) which served as uterine tissue donors. All mice were housed within environmentally controlled conditions under the supervision of a licensed veterinarian. All animal procedures for these experiments were approved by the University of Kansas Medical Center Institutional Animal and Use Committee. Mice were maintained on a 14 L : 10 D photo period and provided water and mice chow ad libitum.
To induce endometriosis, 22 - 24 day old C57BL/6 female mice (N=13) were injected s.c. with pregnant mare serum gonadotropin (PMSG; 2 IU; Sigma Chemical Company, St. Louis, MO) to stimulate an estrogenic response within the uterus. Uteri were then harvested from these donors 48 h after PMSG injection. Uterine tissue (stroma and epithelium intact) was separated from myometrium and dissected into 8 to 10 fragments (1 mm3). Each donor uterus was used for every 2 recipients. Uterine fragments were suspended in 0.2 mL of sterile saline and then injected into 2 to 4 month old female (EGFP) recipients as described below.
Recipient mice (N = 32 total) were anesthetized with ketamine/xylazine. The area over the right rib cage was prepared for surgery and a small incision (approximately 3 mm) was made exposing the peritoneal cavity. Tissue fragments were injected into the peritoneal cavity through the incision and the incision was then closed with wound clips. Carprofen was given for post-operative pain at the conclusion of the surgery and 24 h later. For the first experiment, a group of sham-operated animals (in which only sterile saline and no tissue fragments were transferred) served as controls. After induction of endometriosis, mice were sacrificed at either one week or one month after the initial transplantation of endometrial fragments. In this initial experiment, we sought to validate the experimental model and assess endometriotic implant as well as eutopic endometrial Mif mRNA and protein levels. Uterine tissue was obtained from the sham group as served as a reference control for Mif mRNA and protein expression.
Based upon the pattern of MIF expression, we next sought to determine if inhibition of MIF activity impacted the growth of endometriotic implant tissue. To do so, the remaining 20 females with experimentally-induced endometriosis were equally divided between the control and treatment groups (N=10/group). Mif mRNA expression was analyzed in one endometriotic implant/mouse from each of the 6 mice, while protein was assessed by pooling remaining implants from 5/6 mice which harbored more than one implant.
Treatment administration and assessment of endometriotic implant size
One week after induction of endometriosis, mice were randomly assigned to receive either the MIF antagonist, (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1; 35 mg/kg BW) or vehicle (5% DMSO in saline). Mice were injected i.p. twice daily (12 hours apart) for 3 consecutive days. Mice were then sacrificed one week after the last injection. At the time of sacrifice, the number of endometriotic implants present was counted and an average implant size (in mm3) calculated. Implants were then separated from underlying peritoneum with the aid of a dissecting microscope. Implants were snap-frozen and stored at −80 C until analyzed for mRNA or protein. Reproductive cyclicity/estrous cycle was determined as previously described (22) beginning 4 days prior to treatment injection and continuing up until the time of sacrifice (for a total period of 14 days).
Assessment of Mif activity
To verify that ISO-1 inhibited Mif activity, dopachrome tautomerase assays were conducted as described by Dagia and colleagues (19). Briefly, peritoneal fluid was obtained by injecting 2 ml of sterile saline prior to sacrificing the mice. The peritoneal cavity was massaged. After mice were sacrificed, peritoneal fluid was aspirated with a 1 cc syringe. Protein concentrations were assessed for all peritoneal fluid samples in one assay. Protein concentration was normalized and equated for all samples by adjusting the volume to 180 μl which was added to 100 μl of assay buffer (50 mM potassium phosphate, pH 6.0; 1 mM EDTA) and the entire volume added to wells of 96-well plates. Samples were analyzed for enzymatic activity by adding dopachrome methyl ester (20 μl per well). Absorbance was read at 475 nm using a microwell plate spectrophotometer (Molecular Devices; Sunnyvale, CA) and compared with that of the controls (i.e., endometriotic implant homogenates from vehicle-treated animals). Values were expressed as arbitrary units (fold change from controls). Dopachrome methyl ester was prepared as described by Al-Abed and colleagues (18) by adding equal volumes of 4 mM solution of L-3,4-dihydroxyphenylalanine methyl ester and 8 mM sodium periodate.
mRNA assessment by qRT-PCR
Real-time quantitative RT-PCR (qRT-PCR) was performed as previously described (23). cDNA was synthesized from 2 μg total RNA isolated with Trizol reagent using Maloney murine leukemia virus reverse transcriptase and random hexamer primer. The resulting cDNAs were diluted 1:10 in sterile water, and 1 μl aliquots was used in the quantitative real-time PCR reactions. Primers for Mif and Flk1 mRNA were designed with Primer Express 2.0 and qPCR reactions were carried out on an Applied Biosystems HT7900 Sequence Detector. A no-template reaction was included during each experiment to control for DNA contamination in the reagents. Amplification of 18S rRNAs was used to normalize the level of mRNA expression. A standard curve was run in each assay, with an arbitrary value assigned to the highest standard and corresponding values to the subsequent dilutions. Each cDNA sample was run in triplicate and the relative abundance of each target divided by the relative abundance of 18S in order to normalize for the starting quantity of cDNA.
Western Analysis
Total protein was extracted from frozen uteri or implant samples using RIPA buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1% SDS) containing a protease/phosphatase inhibitor cocktail (0.1 mg/ml PMSF, 30 μl/ml aprotinin, 5 μg/ml leupeptin, 1 mM sodium orthovanadate; Sigma). Protein concentration in each sample was determined using the DC Protein Assay (Bio-Rad Laboratories, Richmond, CA). The same amount of protein (50 μg) was subjected to 4-12% Bis-Tris (Invitrogen, Carlsbad, CA, USA) gel electrophoresis and electroblotted onto PVDF membranes (Invitrogen). Rabbit anti-Mif (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA) and goat anti-rabbit secondary antibody (1:5000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) were used. Stripping and reprobing for β-actin (Santa Cruz) was conducted to normalize Mif protein expression levels. Immunodetection was carried out using an enhanced chemiluminescence (ECL) kit (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
All data were analyzed using GraphPad Instat 3 (GraphPad Software, Inc., La Jolla, CA). ANOVA was used for comparison across treatment regimes. When an F test indicated statistical significance, post-hoc analysis was made using the Tukey HSD procedure. Significance was set at p < 0.05 for all comparisons.
Results
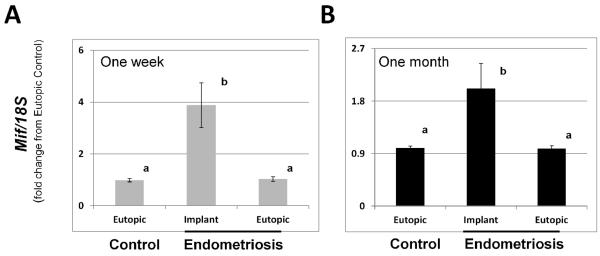
To begin to assess the potential functional role of Mif in endometriotic implant growth in vivo, we first validated our mouse model for the disease. Using this model, we found that all mice in each group (6/6) contained endometriotic implants (range 1 – 4 implants per mouse). Analysis of endometriotic implant Mif mRNA expression revealed that endometriotic implant tissue expressed significantly greater (P<0.05; N=4) levels of Mif mRNA compared to Mif mRNA levels in corresponding eutopic uterine tissue from mice with endometriosis or from sham-control mice at both 1 week (Fig. 1A) and 1 month (Fig. 1B) post-induction.
Figure 1.
Macrophage migration inhibitory factor mRNA expression in eutopic and ectopic endometrium from mice with surgically induced endometriosis at 1 week (A) and 1 month (B) post induction. Endometriosis was induced as described in Materials and Methods and eutopic uterine tissue was obtained from control/sham mice as well as mice with endometriosis in addition to implant tissue obtained from mice with endometriosis. Macrophage migration inhibitory factor (Mif) transcript levels were quantitated as described in Materials and Methods by qRT-PCR and normalized to 18S rRNA levels. Data are displayed as the mean ± SD and are representative of 6 independent samples per time point per treatment (N=6). Data was analyzed by one-way ANOVA for comparison among groups and expressed as fold-change from eutopic control values. Different letters indicate statistical significance as determined by one-way ANOVA (P<0.05).
Similarly, endometriotic implant expression of Mif protein was also significantly higher compared to eutopic uterine tissue from both endometriosis and sham-control mice at both time points (Fig. 2).
Figure 2.
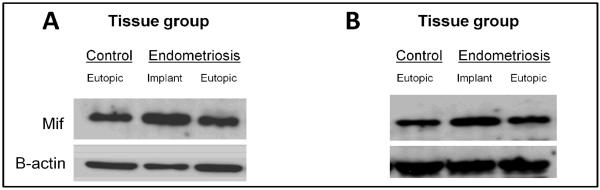
Macrophage migration inhibitory factor protein expression in eutopic and ectopic endometrium from mice with surgically induced endometriosis at 1 week (A) and 1 month (B) post-induction. Endometriosis was induced as described in Materials and Methods and eutopic uterine tissue was obtained from control/sham mice as well as mice with endometriosis in addition to implant tissue obtained from mice with endometriosis. Macrophage migration inhibitory factor (Mif) protein levels were quantitated as described in Materials and Methods by Western blot analysis and normalized to β-actin levels. Data are representative of 5 independent samples per time point per treatment (N=5).
These observations validated the use of our murine model in that Mif expression at both the transcript and protein level is elevated in endometriotic tissue; an event which also occurs in the human disease. To determine if Mif protein is functionally associated with endometriotic implant growth in vivo, we treated mice with experimentally-induced endometriosis with the Mif antagonist, ISO-1(24 – 26). Administration of ISO-1 was associated with a significant reduction in average implant size (Fig. 3) as well as vascularity of the implant (data not shown).
Figure 3.
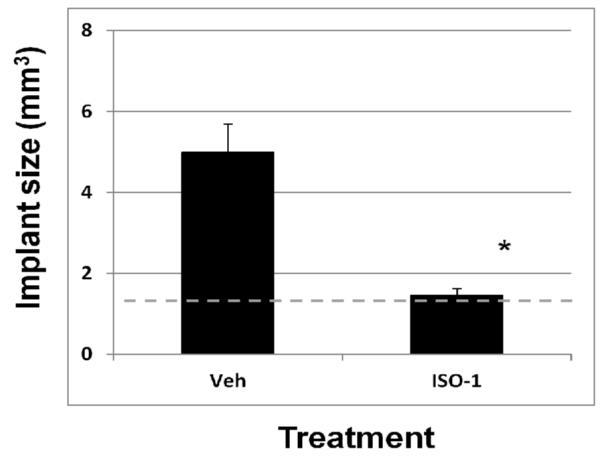
The MIF inhibitor, ISO-1 reduces endometriotic implant mass. Endometriosis was induced and mice were subsequently treated as described in Materials and Methods. Average endometriotic implant size (mm3) was calculated between treatment groups. Data are displayed as the mean ± SD and are representative of 10 mice per treatment group (N=10). Data was analyzed by unpaired t-tests. Significance was set at p < 0.05 for all comparisons. Asterisks (*) indicates statistical significantly different means. Broken line indicates size of implants at start of experiment (1 mm3).
There was no significant difference in neither the number of mice which contained implants or the range of implants which developed in mice of either treatment group (data not shown).
To gain a more quantitative index of implant vascularity, we assessed mRNA levels of VEGF receptor 2 (Flk1) which has been shown to be elevated in human implant tissue and correlates with vascularity. Compared to vehicle-treated mice, Flk1 expression in endometriotic implants from mice treated with ISO-1 was significantly lower (Fig. 4).
Figure 4.
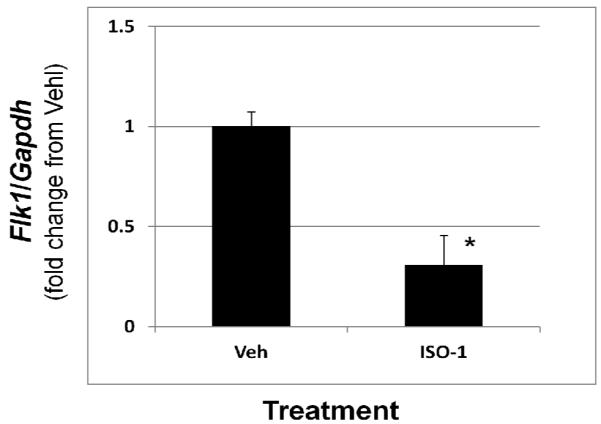
ISO-1 induced endometriotic implant regression is associated with reduced expression of the VEGF receptor, Flk1. Endometriosis was induced and mice were subsequently treated as described in Materials and Methods. Endometriotic implants were harvested after measurement and prepared for RNA isolation. Flk1 transcript levels were quantitated as described in Materials and Methods by qRT-PCR and normalized to 18S rRNA levels. Data are displayed as the mean ± SD and are representative of 6 independent samples per time point per treatment (N=6). Data was analyzed by unpaired t-tests. Significance was set at p < 0.05 for all comparisons. Asterisks (*) indicates statistical significantly different means.
Reduction in both implant size and Flk1 expression was associated with a significant reduction in Mif activity (Fig. 5) suggesting that this cytokine may directly impact endometriotic implant viability and/or growth.
Figure 5.
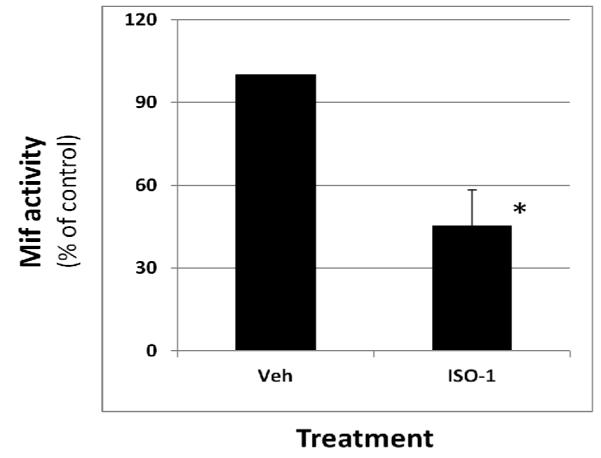
ISO-1 induced regression of endometriotic implant mass is associated with reduced peritoneal fluid MIF activity. Peritoneal fluid was obtained from mice treated with vehicle or ISO-1and MIF activity was determined as described under Materials and Methods. Data are displayed as the mean ± SD and are representative of 9 independent samples per time point per treatment (N=9). Data was analyzed by unpaired t-tests. Significance was set at p < 0.05 for all comparisons. Asterisks (*) indicates statistical significantly different means. One sample was lost during the aspiration process.
Lastly, we found that the reduction in implant mass was not associated with a disruption of reproductive cyclicity, as all mice continued to exhibit 4 to 5 day estrous cycles during the period of treatment administration (data not shown). Taken together, these results can be interpreted to suggest that the elevated levels of Mif produced by endometriotic tissue stimulate a signaling system which is conducive to endometriotic implant growth and/or maintenance of viability.
Discussion
Endometriosis is considered an inflammatory disease and shares many characteristics with autoimmune diseases (27, 28). MIF is central to the inflammatory cascade, and in accord, has been prescribed a role in the pathogenesis of autoimmune diseases (29, 30). Thus, it is not surprising that elevated MIF levels have been associated with the presence of endometriosis (10 – 16). Although current data strongly suggests an association between MIF expression and endometriosis, there is little functional data which provide insight into the potential role of this cytokine in the pathogenesis of the disease.
MIF has been shown to stimulate cell proliferation, inhibit apoptosis and stimulate tissue remodeling and angiogenesis (9, 10, 31, 32). In light of the fact that these are all necessary events which are conducive to the establishment and growth of endometriotic tissue, it is plausible that MIF may elicit similar affects on endometriotic tissue. MIF production my endometriotic implant tissue was first described in 2000 by Yang and co-workers (10). Since this initial description, MIF has been shown to be over expressed in early stage endometriotic implants (11) and eutopic endometrium of women with endometriosis (12) as well as elevated in the peritoneal fluid of women with the disease (13). In vitro evidence suggests that MIF is capable of modulating endometriotic implant stromal cell PGE2 and COX2 production (16) as well as inducing an angiogenic phenotype and triggering the production of the angiogenic factors VEGF, IL-8 and MCP-1 (17).
In addition to regulating cell proliferation and angiogenesis, MIF also induces the expression of MMP3 (33). MMP3 expression is elevated in endometriotic tissue of both humans (34) and rodents with surgically induced disease (35 – 37). Elevated levels of MMP3 protein/activity is proposed to provide the required tissue remodeling necessary for endometriotic implant establishment and growth as inhibition of MMP3 expression/activity is associated with implant regression (38, 39). Taken together, these data can be interpreted to suggest that MIF possess the ability to regulate proliferation, apoptosis, angiogenesis and cell invasion, all of which are essential for endometriotic implant growth.
While there is a wealth of information on the expression and potential function of MIF in endometriotic stromal cells (based upon in vitro studies), there is essentially no information with respect to the ability of this cytokine to modulate implant growth in vivo. Thus, the present report is to the first to suggest a functional role of MIF in the pathogenesis of endometriosis using an in vivo model. To date, the impact of MIF inhibition has been evaluated in the pathogenesis of four distinct inflammatory diseases using in vivo models. ISO-1 has been used successfully to treat sepsis (18), endotoxemia (18), colitis (19), and cancer (20). Using the same dose (35 mg/kg BW) in vivo, ISO-1 reduced LPS stimulated TNF-alpha levels by approximately 60% and increased survival rates by approximately 4-fold in mice with experimental endotoxemia and by approximately 2-fold in mice with experimentally-induced sepsis (18). Using a fluorinated analog of ISO-1 (ISO-F), Dagia and colleagues demonstrated that this compound could be given orally (at 25 mg/kg BW) to effectively suppress experimental colitis. Lastly, Meyer-Siegler and colleagues examined the ability of ISO-1 (at 20 mg/kg BW given i.p.) to prevent growth of human prostate carcinoma cells in a nude mouse model. It was revealed in this study that ISO-1 inhibited cytokine production as well as significantly reduced tumor size, tumor weight and angiogenesis compared to vehicle-treated animals. In the current study, we demonstrate that ISO-1 decreases endometriotic implant Flk1 mRNA expression, which is an indirect indicator of implant angiogenesis and show that this appears to occur independent of an impact on the mouse estrous cycle. Thus, similar to previously reported studies, this study supports the notion that anti-MIF therapy is an effective approach to treating diseases in which MIF is proposed to play a functional role in the pathology of the disease. On-going studies are currently focusing on the mechanisms which lead to this elevated Mif expression by endometriotic tissue.
In summary, we provide for the first time, evidence that Mif expression is elevated in endometriotic implant tissue from mice with experimentally-induced disease and that the MIF inhibitor, ISO-1, significantly reduces endometriotic implant mass in these mice. Further, we report that this regression occurs independently of reproductive cyclicity. The results suggest that targeting MIF may be a potential therapeutic approach for treating endometriosis that may spare effects on the reproductive cycle.
Acknowledgements
We thank Dr. Soumen Paul, Department of Pathology and Laboratory Medicine, University of Kansas Medical Center for initial assessment of qRT-PCR analysis of Flk1 expression.
Footnotes
Supported in part by NIH R03 HD064699 to WBN.
The authors have no proprietary interest or conflict and have nothing to disclose.
References
- 1.Halme J, Hammond MG, Hulka JF. Retrograde menstruation in healthy women and in patients with endometriosis. Raj SG, Talbert LM. Obstet Gyneco. 1984;64:151–4. [PubMed] [Google Scholar]
- 2.Liu DTY, Hitchcock A. Endometriosis: Its association with retrograde menstruation, dysmenorrhea, and tubal pathology. Br J Obstet Gynaecol. 1986;93:859–86. doi: 10.1111/j.1471-0528.1986.tb07995.x. 1986. [DOI] [PubMed] [Google Scholar]
- 3.Nothnick WB. Novel targets for the treatment of endometriosis. Expert Opin Ther Targets. 2004;5:459–71. doi: 10.1517/14728222.8.5.459. [DOI] [PubMed] [Google Scholar]
- 4.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Repro. 2002;10:2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 5.Kyama CM, Mihalyi A, Simsa P, Mwenda JM, Tomassetti C, Meuleman C, D’Hooghe TM. Non-steroidal targets in the diagnosis and treatment of endometriosis. Curr Med Chem. 2008;10:1006–17. doi: 10.2174/092986708784049595. [DOI] [PubMed] [Google Scholar]
- 6.Santos LL, Morand EF. Macrophage migration inhibitory factor: a key cytokine in RA, SLE and atherosclerosis. Clin Chim Acta. 2009;399:1–7. doi: 10.1016/j.cca.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Santos LL, Dacumos A, Yamana J, Sharma L, Morand EF. Reduced arthritis in MIF deficient mice is associated with reduced T cell activation: down-regulation of ERK MAP kinase phosphorylation. Clin Exp Immunol. 2008;152:372–80. doi: 10.1111/j.1365-2249.2008.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emonts M, Sweep FC, Grebenchtchikow N, Geurts-Moespot A, Knaup M, Chanson AL, Erard V, Renner P, Hermans PW, Hazelzet JA, Calandra T. Association between high levels of blood macrophage migration inhibitory factor, inappropriate adrenal response, and early death in patients with severe sepsis. Clin Infect Dis. 2007;44:1321–8. doi: 10.1086/514344. [DOI] [PubMed] [Google Scholar]
- 9.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Degranpré P, Kharfi A, Akoum A. Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Meta. 2000;85:4721–7. doi: 10.1210/jcem.85.12.7003. [DOI] [PubMed] [Google Scholar]
- 11.Kats R, Metz CN, Akoum A. Macrophage migration inhibitory factor is markedly expressed in active and early-stage endometriotic lesions. J Clin Endocrinol Metab. 2002;87:883–9. doi: 10.1210/jcem.87.2.8260. [DOI] [PubMed] [Google Scholar]
- 12.Akoum A, Metz CN, Al-Akoum M, Kats R. Macrophage migration inhibitory factor expression in the intrauterine endometrium of women with endometriosis varies with disease stage, infertility status, and pelvic pain. Fertil Steril. 2006;85:1379–85. doi: 10.1016/j.fertnstert.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 13.Kats R, Collette T, Metz CN, Akoum A. Marked elevation of macrophage migration inhibitory factor in the peritoneal fluid of women with endometriosis. Fertil Steril. 2002;78:69–76. doi: 10.1016/s0015-0282(02)03189-8. [DOI] [PubMed] [Google Scholar]
- 14.Morin M, Bellehumeur C, Therriault MJ, Metz C, Maheux R, Akoum A. Elevated levels of macrophage migration inhibitory factor in the peripheral blood of women with endometriosis. Fertil Steril. 2005;83:865–72. doi: 10.1016/j.fertnstert.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Akoum A, Kong J, Metz C, Beaumont MC. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil Steril. 2002;77:989–94. doi: 10.1016/s0015-0282(02)03082-0. [DOI] [PubMed] [Google Scholar]
- 16.Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150:3128–37. doi: 10.1210/en.2008-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 2010;95:403–12. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- 18.Al-Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, Ochani M, Tanovic M, Ochani K, Bacher M, Nicoletti F, Metz C, Pavlov VA, Miller EJ, Tracey KJ. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280:36541–4. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 19.Dagia NM, Kamath DV, Bhatt P, Gupte RD, Dadarkar SS, Fonseca L, Agarwal G, Chetrapal-Kunwar A, Balachandran S, Srinivasan S, Bose J, Pari K, B-Rao C, Parkale SS, Gadekar PK, Rodge AH, Mandrekar N, Vishwakarma RA, Sharma S. A fluorinated analog of ISO-1 blocks the recognition and biological function of MIF and is orally efficacious in a murine model of colitis. Eur. J. Pharmacol. 2009;607:201–12. doi: 10.1016/j.ejphar.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Siegler KL, Vera PL, Iczkowski KA, Bifulco C, Lee A, Gregersen PK, Leng L, Bucala R. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immun. 2007;8:646–52. doi: 10.1038/sj.gene.6364427. [DOI] [PubMed] [Google Scholar]
- 21.Nowak NM, Fischer OM, Gust TC, Fuhrmann U, Habenicht UF, Schmidt A. Intraperitoneal inflammation decreases endometriosis in a mouse model. Hum Reprod. 2008;23:2466–74. doi: 10.1093/humrep/den189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nothnick WB. Disruption of the tissue inhibitor of metalloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol Reprod. 2000;63:905–12. doi: 10.1095/biolreprod63.3.905. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Hoang E, Nothnick WB. Estrogen-induced uterine abnormalities in TIMP-1 deficient mice are associated with elevated plasmin activity and reduced expression of the novel uterine plasmin protease inhibitor serpinb7. Mol Reprod Dev. 2009;76:160–72. doi: 10.1002/mrd.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Abed Y, Vanpatten S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3:45–63. doi: 10.4155/fmc.10.281. [DOI] [PubMed] [Google Scholar]
- 25.Al-Abed Y, Dabideen D D, Aljabari B, Valster A, Messmer D, Ochani M, Tanovic M, Ochani K, Bacher M, Nicoletti F, Metz C, Pavlov VA, Miller EJ, Tracey KJ. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280:36541–36544. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 26.Lubetsky JB, Dios A, Han J, Aljabari B, Ruzsicska B, Mitchell R, Lolis E, Al-Abed Y. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem. 2002;277:24976–24982. doi: 10.1074/jbc.M203220200. [DOI] [PubMed] [Google Scholar]
- 27.Barrier BF. Immunology of endometriosis. Clin Obstet Gynecol. 2010;53:397–402. doi: 10.1097/GRF.0b013e3181db7c33. [DOI] [PubMed] [Google Scholar]
- 28.Matarese G, De Placido G, Nikas Y, Alviggi C. Pathogenesis of endometriosis: natural immunity dysfunction or autoimmune disease? 2003;9:223–8. doi: 10.1016/s1471-4914(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 29.Greven D, Leng L, Bucala R. Autoimmune diseases: MIF as a therapeutic target. Expert Opin Ther Targets. 2010;14:253–64. doi: 10.1517/14728220903551304. [DOI] [PubMed] [Google Scholar]
- 30.Stosic-Grujicic S, Stojanovic I, Nicoletti F. MIF in autoimmunity and novel therapeutic approaches. Autoimmun Rev. 2009;8:244–9. doi: 10.1016/j.autrev.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulator y role in the innate immune response. Proc Natl Acad Sci. 2002;99:345–50. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishihra J, Ishibashi T, Fukushima T, Sun B, Sato Y, Todo S. Macrophage migration inhibitory factor (MIF): its potential role in tumor growth and tumor-associated angiogenesis. Ann NY Acad Sci. 2003;995:171–82. doi: 10.1111/j.1749-6632.2003.tb03220.x. [DOI] [PubMed] [Google Scholar]
- 33.Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2002;275:444–50. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- 34.Saito T, Mizumoto H, Kuroki K, Fujii M, Mori S, Kudo R. Expression of MMP-3 and TIMP-1 in the endometriosis and influence of danazol. Nippon Sanka Fujinka Gakkai Zasshi. 1995;47:495–6. [PubMed] [Google Scholar]
- 35.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–91. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 36.Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94:2489–94. doi: 10.1210/jc.2008-2802. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelch KE, Schroder AL, Kimball PA, Sharpe-Timms KL, Wade-Davis J, Nagel SC. Aberrant gene expression profile in a mouse model of endometriosis mirrors that observed in women. Fertil Steril. 2010;93:1615–27. doi: 10.1016/j.fertnstert.2009.03.086. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mönckedieck V, Sannecke C, Husen B, Kumbartski M, Kimmig R, Tötsch M, Winterhager E, Grümmer R. model. Progestins inhibit expression of MMPs and angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol Hum Reprod. 2009;15:633–43. doi: 10.1093/molehr/gap063. [DOI] [PubMed] [Google Scholar]
- 39.Paul S, Bhattacharya P, Das Mahapatra P, Swarnakar S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J Pineal Res. 2010;49:156–68. doi: 10.1111/j.1600-079X.2010.00780.x. [DOI] [PubMed] [Google Scholar]