Abstract
Luciferase transfected cell lines are used extensively for cancer models, revealing valuable biological information about disease mechanisms. However, these genetically encoded reporters, while useful for monitoring tumor response in cancer models, can impact cell metabolism. Indeed firefly luciferase and fatty acyl-CoA synthetases differ by a single amino acid, raising the possibility that luciferase activity might alter metabolism and introduce experimental artifacts. Therefore knowledge of the metabolic response to luciferase transfection is of significant importance, especially given the thousands of research studies using luciferase as an in vivo bioluminescence imaging (BLI) reporter. Untargeted metabolomics experiments were performed to examine three different types of lymphoblastic leukemia cell lines (Ramos, Raji and SUP T1) commonly used in cancer research, each were analyzed with and without vector transduction. The Raji model was also tested under perturbed starvation conditions to examine potential luciferase-mediated stress responses. The results showed that no significant metabolic differences were observed between parental and luciferase transduced cells for each cell line, and that luciferase overexpression does not alter cell metabolism under basal or perturbed conditions.
Keywords: Luciferase, metabolomics, bioluminescence imaging, reporter gene
Introduction
The clinical relevance of cancer models depends in large part on their similarity to primary tumors, and whether treatment responses can be discerned from other sources of variation [1]. Tumor cell lines expressing reporter genes are widely employed as cancer models, and their use has revealed valuable biological information about disease mechanisms and treatment responses. Bioluminescence imaging (BLI) for example is used to monitor the abundance, localization, and function of tumor cells over time in vivo, most often via engineered expression of insect luciferase enzymes. However, these types of genetic manipulations or variations in culture conditions can alter cell physiology and may affect cell metabolism.
Luciferases are oxidative enzymes best known for their light-producing, ATP- and oxygen-dependent metabolism of luciferin substrates [2]. Firefly luciferases (FLuc) and fatty acyl-CoA synthetases (FACS) are structurally and functionally related [3, 4]; both enzyme activities can be localized to peroxisomes [5, 6], and both can catalyze the synthesis of dinucleoside polyphosphates and acyl-CoA derivatives [7, 8]. Indeed, a single amino acid change converts FLuc to a FACS [9]. Numerous small molecule luciferase inhibitors have been identified [10] and luciferase activity can be modulated by xenobiotics and endogenous conditions in vivo [11–14]. These findings and the increasing use of BLI-optimized FLuc raise the possibility that highly expressed, long-lived luciferases might alter cellular metabolism and introduce experimental artifacts.
Recent studies have examined luciferase bioluminescence effects on tumor models and come to contradictory conclusions. We [15] and others [16] have observed that under certain circumstances luciferase-expression is associated with altered cell growth in vivo. Furthermore, Brutkiewicz and colleagues [12] noted that tumor growth retardation occurred after serial imaging of ovarian tumor cells expressing high levels of luciferase, an affect the authors attributed to the bioluminescence reaction itself. In contrast, Tiffen et al. [17] examined the growth characteristics of breast tumor and melanoma clones expressing different levels of luciferase activity, and found that neither luciferase expression nor biophotonic activity caused detectable cytotoxicity. Similarly conflicting results were obtained on examination of luciferase bioluminescence in the presence of photosensitizing agents. Luciferase activity was reported to be sufficient to drive photodynamic cytotoxicity in NIH 3T3 cells [18], but this finding was not generalizable to other cells [19].
Increasingly, researchers are using unbiased profiling methods to assess experimental cell models. Untargeted metabolomics permits comprehensive analyses of all the small molecules within a tissue or cell population, which cumulatively reflect the activity of all cellular processes [20]. Recent advances in liquid chromatography/mass spectrometry (LC/MS)-based metabolomics have enabled more complete recovery and identification of metabolites [20–24], making it an ideal technology for identifying metabolic perturbations caused by genetic or environmental stresses.
In the present study, we use untargeted metabolomics to examine the impacts of genetic manipulations on lymphoma lines used in BLI. We show that even seemingly minor nutrient stress induces significant metabolic responses in the lines tested. In contrast, the same lines were metabolically stable in sub-confluent cultures, even after luciferase vector transduction and antibiotic selection. The implications of these findings for BLI models, and the general utility of this approach for unbiased characterization of experimental cell manipulations are discussed.
Experimental Procedures
Cell culture and biological reagents
Ramos (CRL-1596), Raji (CCL-86) and SUP-T1 (CRL-1942) cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Unless otherwise noted, cell culture conditions are as described [22], and all plasticware, buffers, and media components were from single manufacturing lots. Fetal bovine serum (FBS) was purchased from GE Healthcare Bio-Sciences Corp. (PAA #A15-204, Piscataway, NJ, USA); Sigma Aldrich (#F4135, St. Louis, MO, USA); and Thermo Fisher (Hyclone #SH30070.03, Waltham, MA, USA). According to the manufacturer, PAA FBS used in these studies consisted of ~77% FBS supplemented with a bulk additive (Hypep 1510, consisting of 31.5 g/L Bovine Serum Albumin (BSA) 30.25 g/L Soy Peptone, 3.8 g/L Sodium Chloride, and 1g/L glucose. Soy peptones are used to enhance protein production in bioreactors.
LucSh, is a codon-optimized Photinus pyralis luciferase (FLuc) fused to the Streptoalloteichus hindustanus bleomycin resistance protein ble domain [25] in place of the carboxy-terminal 13 amino acids of FLuc (Invivogen technical service). LucSh was sub-cloned from the pMOD-LucSh vector (InvivoGen; San Diego, CA, USA) into the retroviral transfer vector pMSCV (Clontech; Mountain View, CA, USA). Amphotrophic pseudotyped retrovirus was prepared by co-transfection of 293T cells (ATCC) with LucSh or empty retroviral transfer vector plus pCL10A1, a retroviral packaging vector (Imgenex, San Diego). Viral supernatants were used to transduce Raji, Ramos, and Sup-T1 cells in the presence of 8 μg/ml polybrene. Transduced cells were grown in either 2 mg/ml puromycin or 200 mg/ml Zeocin™ to select drug resistant cell pools. LucSh expression was confirmed using the Bright Glo™ luciferase assay (Promega, Madison, WI, USA), and by flow cytometry using a goat anti-luciferase polyclonal antibody (#G745A, Promega, 1:200 dilution) and an Alexa-fluor 488 conjugated donkey anti-goat IgG detection antibody (#A11055, Invitrogen, Carlsbad, CA, USA).
Lymphoma metabolomic profiling was performed as described [22] with the following modifications. Sixteen to 24 hours prior to isolation and extraction, cell viability was assessed, and cultures with greater than 93% viability were re-suspended to a final dilution of approximately 500,000 cells per mL in fresh growth medium containing antibiotics as indicated. One hour prior to isolation, cell viability was again measured, and cultures with greater than 93% viability were adjusted to 1 × 106 cells per mL in fresh medium containing FBS and antibiotics as indicated, then incubated at 37 °C in a 5 % CO2 incubator until isolation. Twenty-five million cells were processed per experimental replicate, split into five equal aliquots, then analyzed by LC/MS in 5 technical replicates.
Serum withdrawal stress
Sub-confluent cultures of Raji cells transduced with pMSCV-empty vector (puromycin selection; one passage post-transduction) or pMSCV-LucSh (FLuc-Sh ble fusion gene; passage 2 post-transduction) and were maintained in growth medium supplemented with antibiotics as described. Aliquots of 25 million cells were removed and centrifuged at 400 × g at room temperature for 2 to 4 minutes. Twenty-four mL of each supernatant was immediately removed by aspiration, and cell pellets were re-suspended by adding 24 mL of either RPMI 1640 (starvation medium) or growth medium consisting of RPMI 1640 supplemented with 10% fetal bovine serum (FBS), and incubated for 4 hours at 37 °C in a 5 % CO2 incubator. Adherent cells were dislodged from flasks by gentle pipetting to enable cell counting and cell viability assessment using the Viacount™ assay as described [22]. Cells were processed and frozen for extraction and metabolite profiling as described above.
Sample preparation for LC/MS and untargeted metabolomic analyses
Cell extractions and analysis were performed as described [22]. For normalization, the cells were counted using the Guava Viacount® assay Millipore, Billerica, MA, USA) and placed into 5 mL aliquots of 10 million cells per replicate before extraction. This ensured uniform cell numbers in each replicate.
Results and Discussion
Metabolomic comparisons of three lymphoma lines grown under optimized conditions
These studies sought to determine whether expression of a BLI-optimized FLuc reporter gene affected lymphoma metabolism in a manner that might alter tumor treatment responses. Experiments were performed in vitro using pools of transduced cells to minimize clone- specific artifacts. To isolate the effects of transductant selection and vector expression from environmental effects, the metabolism of parental and vector-transduced cells were first examined in nutrient-replete, sub-confluent cultures. Previously optimized conditions were used, including careful control of cell culture reagents and plasticware; cell density and media freshness; metabolite extraction and data analysis; and strict adherence to cell viability criteria at the time of sample collection [22].
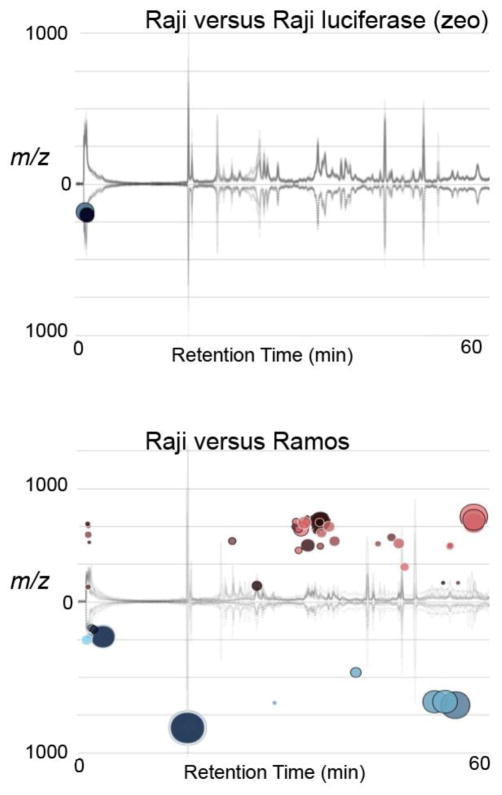
Raji, Ramos, and SupT1 lymphoblastic cells were grown under identical conditions, harvested, and stored at −80°C before extraction. Samples were analyzed by LC/MS and the data were exported into XCMS Online software for pair-wise analysis [23]. The data was filtered for noise, statistical significance, and the magnitude of dysregulation (fold change from comparator ≥2). Cloud plots [20], were generated to display dysregulated features after inter-line metabolomic comparisons (Figures 1 and Table 1). Data sets for all analyses are available to view on XCMS Online under the Public Shares tab. The analysis shows total ion chromatogram plots, cloud plots, multidimensional scaling plots and principal component analysis plots. Metabolite profiles observed were consistent within each line (see below) and, as expected, significant metabolomic differences were seen (46–272 dysregulated features) between parental lines.
Figure 1.
Cloud plots [6] of dysregulated features of Raji parental versus Raji luciferase (zeo), and Raji parental compared to Ramos parental cells. Cloud plots, which display the dysregulated (differentially regulated) features, provide a bubble which represents a metabolic feature. Features upregulated can be seen the upper portion of the plot, while features downregulated can be seen in the lower portion of the plot. The larger and darker the bubbles correspond to larger fold change and smaller the p-values, respectively. The data was filtered for noise, p-value ≤0.001 and fold change ≥2.
Table 1.
Pairwise comparisons of lymphoma cells with luciferase analyzed by XCMS Online.
| Cell comparison | Total number of features | Number of dysregulated features |
|---|---|---|
| Raji parental vs. Raji luciferase (zeo) | 4657 | 2 (NS) |
| Raji parental vs. Raji luciferase (puro) | 5862 | 1 (NS) |
| Raji Empty vector vs. Raji luciferase (puro) | 4106 | 1 (NS) |
|
| ||
| SUP-T1 parental vs. SUP-T1 luciferase (zeo) | 4726 | 0 (NS) |
| SUP-T1 parental vs. SUP-T1 luciferase (puro) | 4701 | 1 (NS) |
|
| ||
| Ramos parental vs. Ramos luciferase (zeo) | 7791 | 6 (NS) |
| Ramos parental vs. Ramos luciferase (puro) | 5547 | 6 (NS) |
|
| ||
| Raji parental vs. Raji Empty vector | 4736 | 0 (NS) |
|
| ||
| Raji parental vs. Ramos parental | 9732 | 73 |
| Raji parental vs. SUP-T1 parental | 9332 | 46 |
| SUP-T1 parental vs. Ramos parental | 7154 | 118 |
NS = Not statistically significant where the number of dysregulated features is less than 0.01%.
Metabolomic effect of growth medium serum additives
Lymphoma metabolism was next examined in media prepared with different lots of FBS. Specifically, Ramos cells were grown for 2 days in manufacturing grade media containing FBS and soy peptone, washed, and split into three separate cultures using either fresh, identical media, or media supplemented with one of two different lots of FBS. After 16 hours of culture, between 8 and 20 metabolite differences were seen among these cultures, indicating that both media freshness and FBS lots have significant effects on Ramos cell metabolism.
Metabolomic comparisons of vector-transduced lymphoma lines grown in different antibiotic selection media
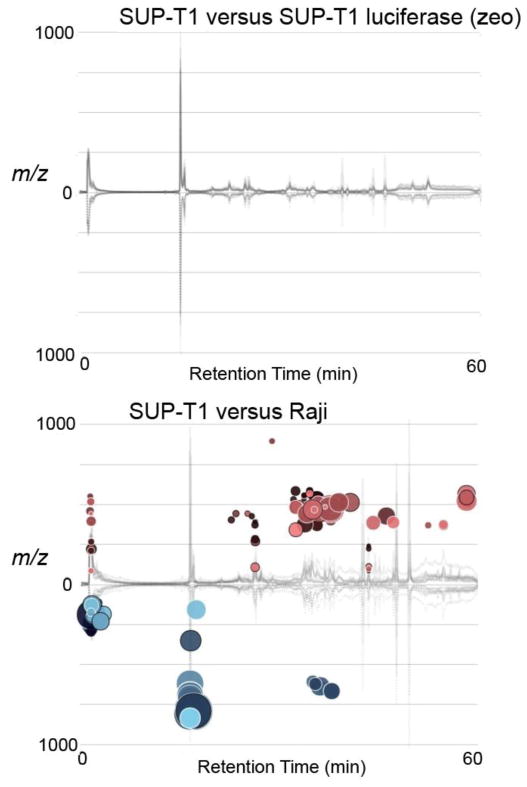
Since genetic modification of cells typically requires antibiotic selection, we asked whether vector-driven resistance to a protein synthesis inhibitor (puromycin) or a DNA damaging agent (Zeocin™) alters lymphoma metabolism. Specifically, cells were transduced with vectors encoding a puromycin N-acetyl transferase antibiotic resistance gene alone or in combination with LucSh, firefly luciferase fused to Sh ble; a bleomycin-binding protein that confers additional resistance to Zeocin™. LucSh transduced cells had comparable luciferase protein expression (Figure S1) and in vitro bioluminescence (data not shown) under either antibiotic selection regime. Figures 1 and 2 compare Raji, Ramos and Sup-T1 parental metabolism to those of derivative lines expressing LucSh under Zeocin™ selection (LucSh-zeo). Table 1 shows the number of features observed for each experiment, and the number of features significantly dysregulated when p≤0.001. No significant differences were seen between parental and LucSh-zeo cell lines. For example, parental Ramos cells compared to Ramos LucSh-zeo cells revealed 7791 total aligned features, of which only six were dysregulated. At p≤0.001, these six were most likely to occur by chance or were attributable to noise. Zeocin™ detoxification can be incomplete in resistant cells, leading to ongoing DNA damage [26]. Our results show that these cells are metabolically insensitive to Zeocin™ exposure, suggesting that either DNA damage is minimal or cells metabolically adapt to the effects of this antibiotic. It is also worth noting that Zeocin™ fragments are too large to be detected using these methods, and are not expected to appear among dysregulated metabolites.
Figure 2.
Cloud plots of dysregulated features p<0.001, fold change >2, intensity >10,000 of SUP-T1 parental cells compared to SUP-T1 luciferase (zeo), and SUP-T1 parental compared to Raji parental.
We also examined the effect of LucSh expression with puromycin selection on Raji cells. Again, only six features were significantly different between parental Raji cells and their LucSh-transduced derivative line, which would most likely have occurred due to chance (the total number of aligned features was 5862 and p≤0.001) whereas only one metabolite was different between empty vector and Raji cells. Notably, that single difference was identified as N-acetyl puromycin, a product of the detoxifying antibiotic resistance enzyme puromycin N-acetyl-transferase; this metabolite was identified in the METLIN database, and confirmed by accurate mass measurement and tandem MS. The absence of puromycin itself in the empty vector samples demonstrates the efficiency of cell washing procedures, and suggests that the metabolites being measured are intracellular products of puromycin metabolism.
These results demonstrate that engineered lymphoma lines can be inured to BLI reporter gene expression and are metabolically indistinguishable from their parental lines grown under identical conditions. This finding increases confidence that cultured BLI reporter cells behave similarly to parental cells during tumor engraftment in vivo, but does not address potential luciferase impacts on treatment responses.
Luciferase expression does not alter stressed cell metabolism
Raji cells transduced with an empty puromycin resistance vector or the same vector expressing LucSh, were grown for 4 hours in fresh medium or under serum starvation stress, then extracted for RPLC/MS and HILIC/MS metabolite profiling. Previous experiments had shown that serum withdrawal caused increased lactate dehydrogenase release from Raji cells, after 120 minutes. Cells were therefore starved for 4 hours, tested for viability in the Viacount® assay, and extracted for metabolite profiling. By 4 hours, the starved cells were noticeably more adherent than cells growing in complete medium, and dye permeability was increased by about 10 % in both empty vector and luciferase lines (data not shown). Meta-analysis of HILIC/MS and RPLC/MS data showed that Raji cell starvation responses were pronounced, but largely unaffected by luciferase expression when compared to those of empty vector controls (p≤0.001, Table 2). HILIC results, for example, show that starvation leads to the dysregulation of 33 and 21 metabolites in LucSh- and empty vector-transduced cells, respectively when compared to fed cells. In contrast, no significant differences were seen when comparing these cell lines within a given nutrient condition.
Table 2.
Pairwise comparisons of Raji cells under nutritional stress analyzed by XCMS Online.
| Cell comparison | Total features HILIC | Dysregulated features HILIC | Total features RPLC | Dysregulated features RPLC |
|---|---|---|---|---|
| Raji empty vector starved vs. Raji luc-puro starved | 1047 | 0 (NS) | 4305 | 0 (NS) |
| Raji empty vector fed vs. Raji luc-puro fed | 1183 | 0 (NS) | 4592 | 0 (NS) |
| Raji luc-puro starved vs. Raji luc-puro fed | 1277 | 37 | 4867 | 27 |
| Raji empty vector starved vs. Raji empty vector fed | 1147 | 21 | 4819 | 9 |
NS = Not statistically significant
Conclusion
Luciferase-expressing cell lines have been used in thousands of cancer studies, including BLI studies where luciferase is highly overexpressed, highlighting the question of whether this manipulation creates metabolic perturbations. Untargeted metabolomics was used to examine three different types of lymphoblastic leukemia cell lines, Ramos, Raji and SUP-T1 to determine if they underwent significant metabolic change in response to luciferase overexpression. While metabolic differences were seen between parental Ramos, Raji and SUP-T1 cancer cell lines, no significant differences were observed when comparing the respective parental and luciferase-expressing cells, revealing that luciferase did not significantly perturb cellular metabolism. Furthermore, neither an empty expression vector nor the LucSh construct used here affected lymphoma metabolism under basal or perturbed conditions, suggesting luciferase reporter gene expression itself is unlikely to alter cellular metabolism or inject ambiguity into interpretations of treatment responses.
It is possible that luciferase effects on cellular metabolism remain undetected here because they take place during or immediately after vector transduction and antibiotic selection, and that our results instead reflect a steady-state adaptation of lymphoma cells to our luciferase expression system. The remarkable metabolic reproducibility of parental and transduced lymphoma cell lines argues against this, and further highlights the utility of our approach for examining additional aspects of luciferase biology and cellular stress responses. For example, treatment with luciferase inhibitor drugs or inducible genetic knockdown of luciferase in cells could be pursued to determine whether differences in luciferase activity alters cell metabolism over a different time course than that examined in these studies. It also remains possible that other enzymatic reporters might alter cellular metabolism, such as wild-type luciferases localized to peroxisomes [27] [28], but overall our metabolomic analysis of Raji, SUP-T1 and Ramos cancer cell lines has revealed that this BLI-optimized luciferase construct does not confer a significant metabolic signature to the cells under basal and perturbed conditions.
Supplementary Material
Flow cytometric analysis of Luciferase expression
Acknowledgments
The authors thank Cathy Zhang and Max Hallin (Pfizer Oncology Research) for insightful discussions. These studies were fully funded by Pfizer.
List of abbreviations
- ACN
Acetonitrile
- BLI
bioluminescence imaging
- dPBS
Dulbecco’s phosphate buffered saline
- ESI
electrospray ionization
- EV
empty vector
- FA
formic acid
- FACS
Fatty Acyl CoA synthetase
- FLuc
Photinus pyralis luciferase
- HILIC
hydrophilic interaction liquid chromatography
- HPLC
high performance liquid chromatography
- IPA
isopropanol
- LC/MS
Liquid Chromatography/Mass Spectrometry
- MeOH
methanol
- MS
Mass Spectrometry
- NH4Ac
Ammonium acetate
- NH4OH
ammonium hydroxide
- Q-TOF/MS
Quadrupole Time-Of-Flight Mass Spectrometer
- RPLC
reversed-phase liquid chromatography
Footnotes
Competing interests: The authors declare no competing interests.
Author’s contributions: PJO’B conceived the study; PJO’B, TF, CJ, BH and GS participated in the design of the study; PJO’B and TF carried out the cell culture studies; CJ and LH extracted the cells and carried out LC-QTOFMS; CJ carried out the data analysis; CJ and PJO’B wrote the manuscript. All authors read and approved the final manuscript.
Availability of supporting data
The metabolomics data sets are available on XCMS Online under the public shares tab (https://xcmsonline.scripps.edu/)
References
- 1.van Staveren WC, Solis DY, Hebrant A, Detours V, Dumont JE, Maenhaut C. Human cancer cell lines: Experimental models for cancer cells in situ? For cancer stem cells? Biochim Biophys Acta. 2009;1795:92–103. doi: 10.1016/j.bbcan.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Greer LF, 3rd, Szalay AA. Imaging of light emission from the expression of luciferases in living cells and organisms: a review. Luminescence. 2002;17:43–74. doi: 10.1002/bio.676. [DOI] [PubMed] [Google Scholar]
- 3.McElroy WD, DeLuca M, Travis J. Molecular uniformity in biological catalyses. The enzymes concerned with firefly luciferin, amino acid, and fatty acid utilization are compared. Science. 1967;157:150–160. doi: 10.1126/science.157.3785.150. [DOI] [PubMed] [Google Scholar]
- 4.Gulick AM. Conformational dynamics in the Acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem Biol. 2009;4:811–827. doi: 10.1021/cb900156h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller GA, Gould S, Deluca M, Subramani S. Firefly luciferase is targeted to peroxisomes in mammalian cells. Proc Natl Acad Sci U S A. 1987;84:3264–3268. doi: 10.1073/pnas.84.10.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins PA, Ellis JM. Peroxisomal acyl-CoA synthetases. Biochim Biophys Acta. 2012;1822:1411–1420. doi: 10.1016/j.bbadis.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guranowski A, Sillero MAG, Sillero A. Firefly luciferase synthesizes P1, P 4-bis (5′-adenosyl) tetraphosphate (Ap4A) and other dinucleoside polyphosphates. FEBS lett. 1990;271:215–218. doi: 10.1016/0014-5793(90)80409-c. [DOI] [PubMed] [Google Scholar]
- 8.Oba Y, Ojika M, Inouye S. Firefly luciferase is a bifunctional enzyme: ATP-dependent monooxygenase and a long chain fatty acyl-CoA synthetase. FEBS lett. 2003;540:251–254. doi: 10.1016/s0014-5793(03)00272-2. [DOI] [PubMed] [Google Scholar]
- 9.Oba Y, Iida K, Inouye S. Functional conversion of fatty acyl-CoA synthetase to firefly luciferase by site-directed mutagenesis: a key substitution responsible for luminescence activity. FEBS lett. 2009;583:2004–2008. doi: 10.1016/j.febslet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Thorne N, Shen M, Lea WA, Simeonov A, Lovell S, Auld DS, Inglese J. Firefly luciferase in chemical biology: a compendium of inhibitors, mechanistic evaluation of chemotypes, and suggested use as a reporter. Chem Bol. 2012;19:1060–1072. doi: 10.1016/j.chembiol.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyaerts M, Remory I, Caveliers V, Breckpot K, Bos TJ, Poelaert J, Bossuyt A, Lahoutte T. Inhibition of firefly luciferase by general anesthetics. effect on in vitro and in vivo bioluminescence imaging. PLoS One. 2012;7:e30061. doi: 10.1371/journal.pone.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brutkiewicz S, Mendonca M, Stantz K, Comerford K, Bigsby R, Hutchins G, Goebl M, Harrington M. The expression level of luciferase within tumour cells can alter tumour growth upon in vivo bioluminescence imaging. Luminescence. 2007;22:221–228. doi: 10.1002/bio.953. [DOI] [PubMed] [Google Scholar]
- 13.Sim H, Bibee K, Wickline S, Sept D. Pharmacokinetic modeling of tumor bioluminescence implicates efflux, and not influx, as the bigger hurdle in cancer drug therapy. Cancer Res. 2011;71:686–692. doi: 10.1158/0008-5472.CAN-10-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czupryna J, Tsourkas A. Firefly luciferase and RLuc8 exhibit differential sensitivity to oxidative stress in apoptotic cells. PLoS One. 2011;6:e20073. doi: 10.1371/journal.pone.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang CC, Yan Z, Li W, Kuszpit K, Painter CL, Zhang Q, Lappin PB, Nichols T, Lira ME, Affolter T, et al. [(18)F]FLT-PET imaging does not always “light up” proliferating tumor cells. Clin Cancer Res. 2012;18:1303–1312. doi: 10.1158/1078-0432.CCR-11-1433. [DOI] [PubMed] [Google Scholar]
- 16.Milsom CC, Lee CR, Hackl C, Man S, Kerbel RS. Differential Post-Surgical Metastasis and Survival in SCID, NOD-SCID and NOD-SCID-IL-2Rgamma(null) Mice with Parental and Subline Variants of Human Breast Cancer: Implications for Host Defense Mechanisms Regulating Metastasis. PLoS One. 2013;8:e71270. doi: 10.1371/journal.pone.0071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiffen JC, Bailey CG, Ng C, Rasko JE, Holst J. Luciferase expression and bioluminescence does not affect tumor cell growth in vitro or in vivo. Mol Cancer. 2010;9:299. doi: 10.1186/1476-4598-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodossiou T, Hothersall JS, Woods EA, Okkenhaug K, Jacobson J, MacRobert AJ. Firefly luciferin-activated rose bengal: in vitro photodynamic therapy by intracellular chemiluminescence in transgenic NIH 3T3 cells. Cancer Res. 2003;63:1818–1821. [PubMed] [Google Scholar]
- 19.Schipper ML, Patel MR, Gambhir SS. Evaluation of firefly luciferase bioluminescence mediated photodynamic toxicity in cancer cells. Mol Imaging Biol. 2006;8:218–225. doi: 10.1007/s11307-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 20.Patti GJ, Tautenhahn R, Rinehart D, Cho K, Shriver LP, Manchester M, Nikolskiy I, Johnson CH, Mahieu NG, Siuzdak G. A View from Above: Cloud Plots to Visualize Global Metabolomic Data. Anal Chem. 2013;85:798–804. doi: 10.1021/ac3029745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu ZJ, Schultz AW, Wang J, Johnson CH, Yannone SM, Patti GJ, Siuzdak G. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat Prot. 2013;8:451–460. doi: 10.1038/nprot.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanisevic J, Zhu Z, Plate L, Tautenhahn R, Chen S, O’Brien PJ, Johnson CH, Marletta MA, Patti GJ, Siuzdak G. Toward ‘Omic’ Scale Metabolite Profiling: A Dual Separation – Mass Spectrometry Approach for Coverage of Lipids and Central Carbon Metabolism. Anal Chem. 2013;85:6876–6884. doi: 10.1021/ac401140h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Analytical chemistry. 2012;84:5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patti GJ, Tautenhahn R, Siuzdak G. Meta-analysis of untargeted metabolomic data from multiple profiling experiments. Nat Prot. 2012;7:508–516. doi: 10.1038/nprot.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatignol A, Durand H, Tiraby G. Bleomycin resistance conferred by a drug-binding protein. FEBS Lett. 1988;230:171–175. doi: 10.1016/0014-5793(88)80665-3. [DOI] [PubMed] [Google Scholar]
- 26.Oliva-Trastoy M, Defais M, Larminat F. Resistance to the antibiotic Zeocin by stable expression of the Sh ble gene does not fully suppress Zeocin-induced DNA cleavage in human cells. Mutagenesis. 2005;20:111–114. doi: 10.1093/mutage/gei016. [DOI] [PubMed] [Google Scholar]
- 27.Gould SG, Keller GA, Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987;105:2923–2921. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis JM, Frahm JL, Li LO. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21:212–217. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometric analysis of Luciferase expression