Background: Simultaneous fluctuation of the inner membrane potential in filamentous mitochondria suggests electrical and functional connection.
Results: Transient contraction of mitochondrial matrix induces depolarization through the inner membrane fusion dynamin OPA1.
Conclusion: Transient mitochondrial contraction is a previously unrecognized cellular mechanism that induces depolarization.
Significance: Transient morphological contraction of mitochondria represents a new mechanism regulating mitochondrial activity.
Keywords: Bioenergetics, Electron Transfer, Membrane, Membrane Fusion, Mitochondria, Drp1, OPA1, Dynamin, Mitochondrial Fission, Proton Leak
Abstract
Dynamin-related membrane remodeling proteins regulate mitochondrial morphology by mediating fission and fusion. Although mitochondrial morphology is considered an important factor in maintaining mitochondrial function, a direct mechanistic link between mitochondrial morphology and function has not been defined. We report here a previously unrecognized cellular process of transient contraction of the mitochondrial matrix. Importantly, we found that this transient morphological contraction of mitochondria is accompanied by a reversible loss or decrease of inner membrane potential. Fission deficiency greatly amplified this phenomenon, which functionally exhibited an increase of inner membrane proton leak. We found that electron transport activity is necessary for the morphological contraction of mitochondria. Furthermore, we discovered that silencing the inner membrane-associated dynamin optic atrophy 1 (OPA1) in fission deficiency prevented mitochondrial depolarization and decreased proton leak without blocking mitochondrial contraction, indicating that OPA1 is a factor in coupling matrix contraction to mitochondrial depolarization. Our findings show that transient matrix contraction is a novel cellular mechanism regulating mitochondrial activity through the function of the inner membrane dynamin OPA1.
Introduction
Mitochondrial morphology in cells varies greatly from small spheres to filamentous tubules and reticular networks. This morphological diversity of mitochondria is largely determined by fission and fusion, which are mediated through the dynamin-related membrane-remodeling proteins. Dynamin-like/related protein 1 (DLP1/Drp1) mediates mitochondrial fission, whereas optic atrophy 1 (OPA1) and mitofusin mediate fusion of the inner and outer membranes, respectively (1–4). Mitochondrial dysfunction is often associated with disruption of mitochondrial fission or fusion (5), suggesting a close relationship between mitochondrial morphology and function (6). However, the molecular mechanisms by which mitochondrial function is regulated by morphology or vice versa are not fully understood.
It has been recognized that long filamentous mitochondria are electrically connected and function as signal- and power-transmitting cables, facilitating the delivery of energy and metabolites from one place to another within cells (7, 8). The electrical continuum of filamentous mitochondria has been illustrated by simultaneous fluctuation of membrane potential (ΔΨm) in connected mitochondria using the mitochondrial potentiometric probe, tetramethylrhodamine ethyl ester (TMRE)2 or tetramethylrhodamine methyl ester. These ΔΨm fluctuations have been shown in many different cell types, including neurons (9), astrocytes (10, 11), cardiomyocytes (12–14), and COS-7 cells (15). Many of these ΔΨm fluctuations were ascribed to reactive oxygen species (ROS) generated from photoactivation of TMRE/tetramethylrhodamine methyl ester (10, 14–16). In contrast, several studies indicated that there is spontaneous ΔΨm fluctuation independent of ROS, Ca2+, or mitochondrial permeability transition (9, 17, 18). A recent report describes spontaneous pH flashes in the mitochondrial matrix that mirror transient depolarization of mitochondria (19). This study demonstrated that sudden matrix alkalization (pH flash) is linked with ΔΨm loss, reflecting a compensatory increase in proton pumping immediately following the spontaneous loss of ΔΨm (19). These spontaneous pH flashes and thus ΔΨm fluctuation were shown to be independent of ROS, Ca2+, and mitochondrial permeability transition (19). We have shown that inhibiting mitochondrial fission by expression of a dominant-negative fission mutant, DLP1-K38A, induces a spontaneous large scale oscillation of TMRE with random frequencies and intervals (20). Unlike the TMRE photooxidation-induced ΔΨm fluctuation, the fission inhibition-induced TMRE oscillation is insensitive to ROS scavenger and mitochondrial permeability transition inhibitor (20); hence, it resembles the spontaneous ΔΨm fluctuation associated with pH flash (19). Furthermore, as a functional consequence of the frequent large scale depolarization, cells expressing DLP1-K38A exhibited increased proton leak in respiration measurements (20). In this study, through morphological examination of TMRE flickering, we discovered a unique cellular process in which transient contraction along the mitochondrial tubule mediates inner membrane depolarization. We found that the inner membrane dynamin OPA1 is necessary for mitochondrial contraction-induced depolarization, whereas electron transport activity is required for the transient contraction. Our results demonstrate that mitochondrial contraction-depolarization coupling is a previously unidentified cellular process regulating mitochondrial activity. These findings provide a new mechanistic link between mitochondrial morphology and function as well as insight for fine temporal and spatial regulation of mitochondrial activity through a unique morphological modulation.
EXPERIMENTAL PROCEDURES
Cell Culture
DLP1-KO MEFs along with the wild-type MEFs were obtained from Dr. Hiromi Sesaki (The Johns Hopkins University). MEFs and H9c2 (ATCC CRL-1446) were maintained in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Mitochondrial matrix-targeted GFP and DLP1-K38A were expressed in cells through adenoviral infection. Small hairpin RNAs (Santa Cruz Biotechnology) were used for gene silencing through lentiviral transduction. Stable cells expressing OPA1 shRNA were isolated by puromycin selection.
Fluorescence Microscopy
For TMRE imaging, cells were loaded with 20 nm TMRE for 20 min in Krebs-Ringer bicarbonate/HEPES buffer (KRBH: 135 mm NaCl, 3.6 mm KCl, 5 mm NaHCO3, 0.5 mm NaH2PO4, 0.5 mm MgCl2, 1.5 mm CaCl2, and 10 mm HEPES, pH 7.4, and 0.1% bovine serum albumin) plus 5 mm glucose at 37 °C. After rinsing to wash out TMRE, cell fluorescence was imaged in KRBH plus 5 mm glucose using the digital microscope iMIC (TILL Photonics) equipped with the Dichrotome dual emission extension and the Live Acquisition imaging software (TILL Photonics). For nutrient starvation, DLP1-KO MEFs were incubated in BSA-free KRBH for 4–5 h. After TMRE (20 nm) loading and rinsing in BSA-free KRBH, TMRE imaging was performed in BSA-free KRBH supplemented with pyruvate or pyruvate/oligomycin. In membrane potential assessments, TMRE imaging was done in the presence of 20 nm TMRE in the imaging medium. Excitation/emission wavelengths for GFP and TMRE were 478/517 and 555/613 nm, respectively. In dual imaging, the emission beams were split through 560 DCXR. Images were quantified or post-processed using Offline Analysis (TILL Photonics), ImageJ (Wayne Rasband, National Institutes of Health), and Photoshop (Adobe).
Quantification of TMRE Flickering and Mitochondrial Contraction
TMRE flickering was quantified in a stack of time-sequence images (10-s intervals) acquired with a ×10 objective, typically containing 100–300 cells. The “Stack-T function” of ImageJ was used to generate a stack of “ΔF,” which is the fluorescence difference between the two images in succession. Therefore, TMRE losses in flickering are shown as black holes in the ΔF image stack. The black holes were extracted from ΔF by thresholding and noise removal. A stack of three-dimensional surface plots was then generated from the ΔF image stack. Peaks in the surface plot represent the TMRE loss and were counted as flickering events. TMRE flickering was presented as the number of events per 100 frames per 100 cells. For quantifying mitochondrial contraction, DLP1-KO MEFs expressing matrix-targeted GFP were treated with or without 1 μm antimycin A for 2 min or 2 μm carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) for 5 min and immediately fixed in 1% glutaraldehyde in phosphate-buffered saline for 15 min. Mitochondrial images were viewed at ×600 magnification (Olympus IX71), and the number of cells containing nodulated mitochondrial tubules was counted to assess mitochondrial contraction.
Whole Cell Respiration
Oxygen consumption was measured using a Clark-type O2 electrode in a sealed chamber (Mitocell 200 system, Strathkelvin Instruments). Decreases of the O2 concentration in the chamber were measured as whole cell oxygen consumption. Respiration was measured in glucose-free DMEM supplemented with 5 mm pyruvate, 2 mm glutamine, and 5 mm HEPES. Oligomycin (1 μm) was added to measure oxygen consumption in the absence of the ATP synthase activity. Oxygen consumption rate (OCR) in the presence of oligomycin represents the leak rate. Maximal respiration was obtained by adding FCCP (2 μm). OCR in the presence of antimycin A (1 μm) was subtracted to obtain mitochondrial oxygen consumption. To assess the extent of proton leak, the leak ratio was calculated as the proportion of leak rate to maximum rate (OCROlm/OCRFCCP).
RESULTS
Identification of Transient Contraction of Mitochondrial Matrix
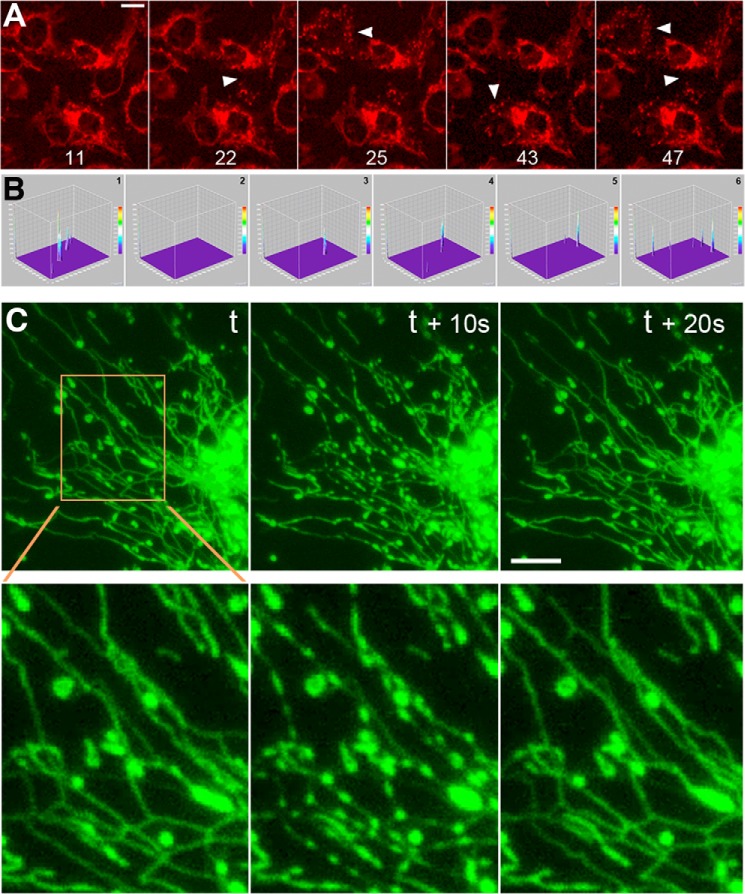
We observed that interconnected mitochondria resulting from fission inhibition by the dominant-negative DLP1-K38A in hepatocytes showed repeated loss and recovery of TMRE fluorescence (TMRE flickering) (20). Because this event was not readily observed in normal hepatocytes, it is likely that fission inhibition by DLP1-K38A greatly amplifies TMRE flickering (20). We speculated that the mutant DLP1-K38A protein co-assembles with endogenous DLP1 to form a functionally defective DLP1 ring structure that may cause membrane leakage through incomplete membrane constriction during fission. However, we observed robust TMRE flickering in DLP1 knock-out mouse embryonic fibroblasts (DLP1-KO MEFs) (Fig. 1A and supplemental Movie 1), indicating that the act of fission is not necessary for this large scale depolarization. TMRE flickering was quantified from time-sequence images by generating a stack of three-dimensional surface plots (Fig. 1B). Peaks in the surface plot represented TMRE loss and were counted as flickering events. Typically, DLP1-KO MEFs showed 50–80 events/100 frames/100 cells. Wild-type MEFs showed very little discernable TMRE flickering (supplemental Movie 1).
FIGURE 1.
TMRE flickering and transient synchronous nodulation of mitochondrial tubules in DLP1-KO MEFs. A, time-sequence images of DLP1-KO cells show loss and recovery of TMRE fluorescence. Cells with TMRE loss are indicated by arrowheads. Time-lapse images were acquired at 10-s intervals. Frame numbers are given at the bottom. Scale bar, 20 μm. B, three-dimensional surface plots of a fluorescence image time-sequence for quantifying TMRE flickering in DLP1-KO MEFs. Six sequential frames of the TMRE imaging field (1344 × 1024 pixels, x and y axes) containing 221 cells are shown. Typically, 100 sequential frames were processed using the “Stacks-T-functions” of ImageJ. Images were inverted to display the loss of TMRE fluorescence as upward peaks. Peaks in the z axis represent losses/decreases of TMRE fluorescence compared with those in the preceding frame. The peaks in each frame were counted and presented as events/100 frames/100 cells. C, matrix-targeted GFP shows transient contraction of mitochondrial tubules. Synchronous nodulation appeared and disappeared within 20 s. t, time. Scale bar, 10 μm.
In examining DLP1-KO MEFs, we found that TMRE flickering occurs in a compartmentalized manner, as not all mitochondria within a cell lose TMRE at the same time. This observation suggests that mitochondria in DLP1-KO cells are not a single continuous network but are composed of several units. It is possible that there may be an alternative DLP1-independent fission process that separates and maintains these compartments. This potential DLP1-independent fission may not be tightly regulated and thus may result in leaky fission, possibly responsible for TMRE flickering in DLP1-KO MEFs. Although time-lapse imaging found occasional fission events in DLP1-KO cells, which appeared to be mediated by forceful stretching of the mitochondrial tubule along the cytoskeleton, this type of fission was extremely rare and not accountable for TMRE flickering. Instead, time-lapse imaging of mitochondrial matrix-targeted green fluorescent protein (GFP) in DLP1-KO MEFs revealed transient contraction of the mitochondrial matrix occurring within one or a group of tubules in a synchronous manner (Fig. 1C). The mitochondrial matrix contracted at multiple places in a tubule simultaneously, forming a beads-on-a-string morphology. This morphological change was transient as the synchronous nodulation rapidly disappeared in 10–20 s (Fig. 1C). The transient nature of mitochondrial contraction is particularly appreciable in animation of image sequences acquired at 10-s intervals (supplemental Movie 2).
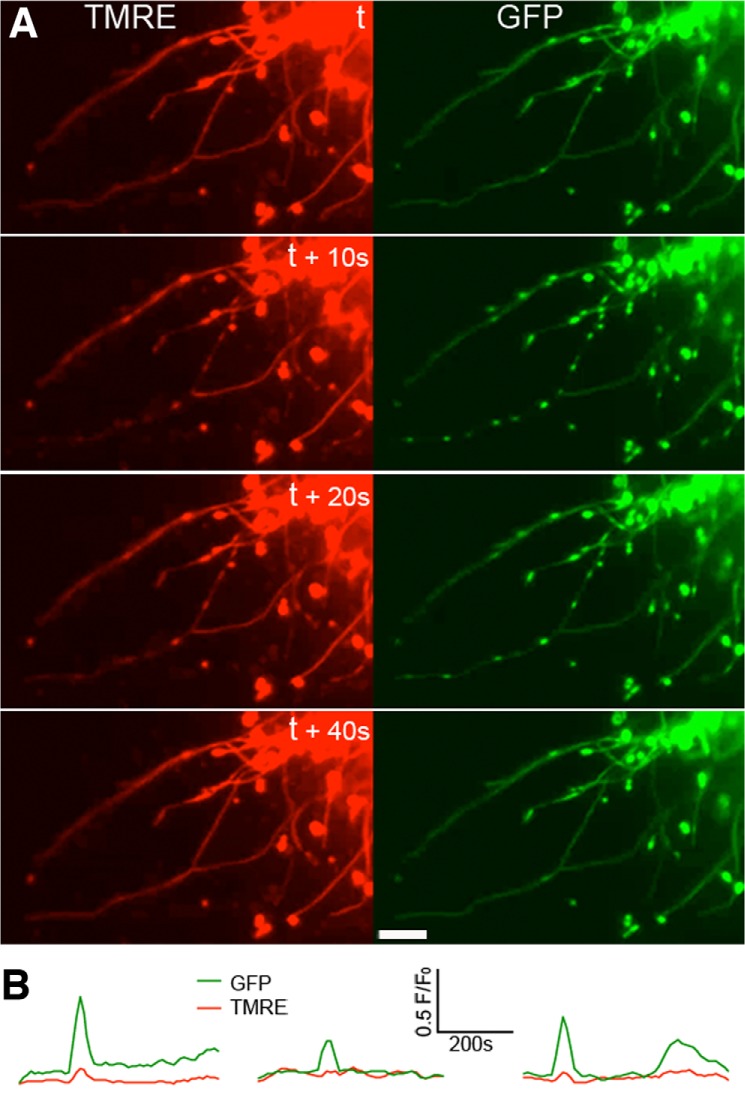
Transient Matrix Contraction Is Coupled to TMRE Loss
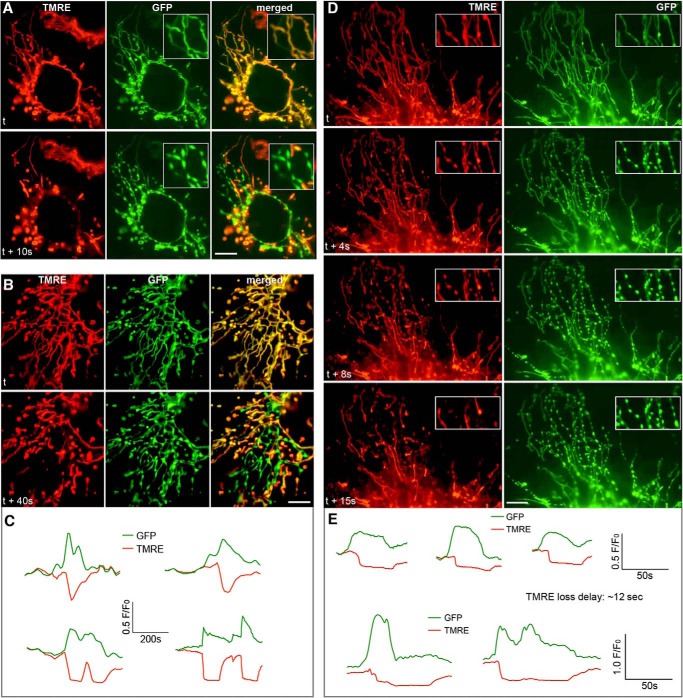
In general, we were able to detect transient mitochondrial contraction once or twice at most in a 100-frame (1,000 s) imaging. However, similar to TMRE flickering in which some cells are active and others are quiescent in a given imaging period, the occurrence of the matrix contraction was unpredictable. Because the frequency and randomness of this event were similar to those of TMRE flickering, we examined whether TMRE flickering was associated with the synchronous contractions of the mitochondrial matrix. Remarkably, dual imaging of TMRE and the mitochondrial matrix revealed that loss of TMRE faithfully coincided with matrix contractions, and both recovered afterward (Fig. 2A and supplemental Movie 3). This phenomenon was consistently reproducible over numerous imaging experiments. TMRE and GFP fluorescence at the contracted nodules showed that increased GFP intensity due to contraction was accompanied by depolarization (Fig. 2C). In net-like mitochondria, matrices were contracted toward the tubule branching points (Fig. 2B and supplemental Movie 3), indicating variation in the morphological contraction associated with depolarization. In many cases, the disappearance of contracted nodules preceded the recovery of the TMRE fluorescence. The delay in repolarization after the morphological restoration varied greatly up to a minute or longer. This delayed TMRE recovery suggests that morphological contraction is not the consequence of depolarization. These time-lapse sequences were acquired at 10-s intervals, potentially missing information about the order of occurrence between matrix contraction and TMRE loss. In the images acquired at 1-s intervals, the loss of TMRE and matrix contraction still occurred with exact concomitance in many cases (supplemental Movie 4). On several occasions, however, we observed a delay in TMRE loss after matrix contraction (Fig. 2D and supplemental Movie 4). Mitochondria were fully contracted for several seconds before TMRE fluorescence disappeared. The onset of the contraction preceded depolarization by an average of 12 s, ranging from 9 to 15 s (Fig. 2E), although the significance of the duration of these delays is currently unclear. These observations indicate that matrix contraction is the preceding event that leads to depolarization.
FIGURE 2.
Concomitance of mitochondrial contraction and TMRE loss in DLP1-KO MEFs. A, dual-color imaging shows that matrix contraction (GFP) in the filamentous mitochondrial tubules coincides with TMRE loss. Scale bar, 10 μm. B, matrix contraction occurs toward branch points in mitochondrial networks and coincides with depolarization. Scale bar, 10 μm. C, analyses of fluorescence intensity at the contracted nodules. Increases in GFP signals are accompanied by decreases in TMRE fluorescence. D, images from 1-s interval time sequences. TMRE is retained in the contracted nodules for ∼10 s before its disappearance. Scale bar, 10 μm. E, fluorescence intensity analyses at the contracted nodules show that increases of GFP signals precede the loss of TMRE.
Mitochondrial Contraction-Depolarization Coupling Is a Normal Cellular Process
We next examined whether the observed coupling of mitochondrial contraction to TMRE loss is a common cellular phenomenon occurring in normal cells. Unlike in wild-type MEFs, the occasional small scale TMRE flickering was observed in a normal culture of the cardiac myoblast cell line H9c2, suggesting that the tendency for TMRE flickering is cell type-dependent. Dual imaging of TMRE and matrix GFP in these cells showed that the loss or decrease of TMRE fluorescence was consistently associated with matrix contraction (Fig. 3 and supplemental Movie 5). Even a small single mitochondrion displayed a contracted morphology as it lost the membrane potential (supplemental Movie 5). On many occasions, TMRE fluorescence dimmed without a complete loss and was rapidly recovered. Matrix contraction still coincided with TMRE dimming (Fig. 3B and supplemental Movie 5), suggesting that inner membrane depolarization, whether large or small, is generally associated with mitochondrial contraction. These observations indicate that transient mitochondrial contraction is a normal cellular process coupled to mitochondrial depolarization.
FIGURE 3.
Mitochondrial contraction-depolarization coupling in H9c2 cells. A–A″, dual imaging of TMRE and GFP in mitochondria of normal H9c2 cells shows transient contraction accompanied by depolarization. Scale bar, 10 μm. B–B‴, partial mitochondrial depolarization indicated by TMRE dimming in normal H9c2 cells coincides with mitochondrial contraction. Scale bar, 10 μm. C, inhibition of mitochondrial fission in H9c2 cells by expression of DLP1-K38A significantly increased TMRE flickering. D, merged images from TMRE-GFP dual imaging in the DLP1-K38A-expressing H9c2 cell show prominent mitochondrial contractions associated with TMRE loss (arrows). Scale bar, 10 μm.
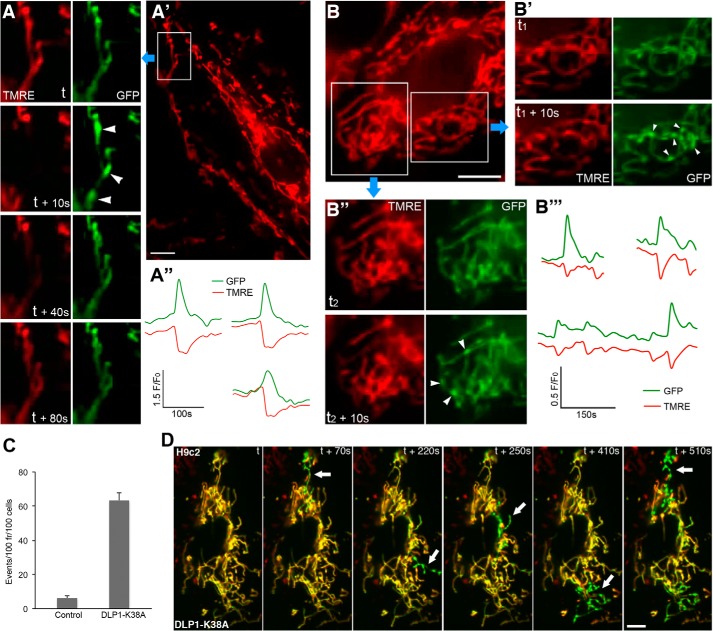
We also expressed DLP1-K38A in H9c2 cells to examine TMRE flickering and matrix contraction. DLP1-K38A expression significantly increased TMRE flickering by ∼10-fold (Fig. 3C), indicating the amplification of TMRE flickering by fission deficiency. Dual imaging demonstrated that elongated mitochondrial tubules formed by DLP1-K38A expression in H9c2 cells show a more pronounced matrix contraction that is coupled to depolarization, similar to that seen in DLP1-KO MEFs (Fig. 3D and supplemental Movie 6). In this particular cell, merged images from dual color imaging show that four compartments of connected mitochondria, generated by DLP1-K38A expression, were alternately contracted and depolarized. These observations indicate that contraction-depolarization coupling is a normal cellular process, which is amplified by fission deficiency that increases mitochondrial interconnection.
Contraction-Depolarization Coupling of Mitochondria Requires Electron Transport Chain Activity
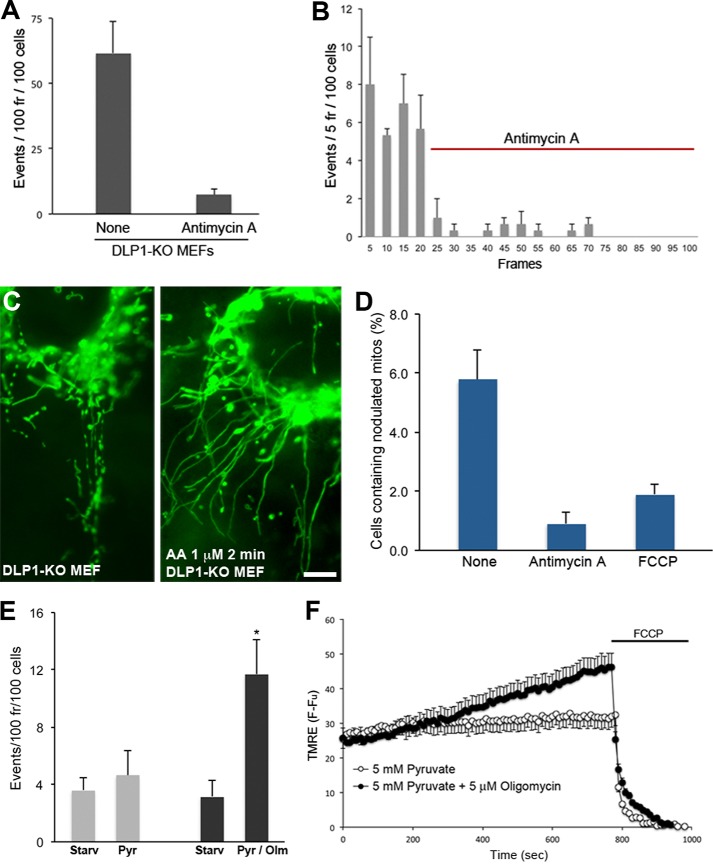
The abrupt loss of TMRE suggests that TMRE flickering may involve sudden openings of a channel or pore, most likely the permeability transition (PT) pore. However, we observed continued TMRE flickering in the presence of the PT pore inhibitor cyclosporin A (20). The TMRE fluctuation associated with pH flashes has also been shown to be insensitive to cyclosporin A (19). In addition, similar to our observation, inhibition of fission increased the pH flash caused by TMRE loss, suggesting that the TMRE flickering we observed is the same phenomenon as the one associated with pH flash. To gain insight as to what causes mitochondrial contraction and TMRE flickering, we treated DLP1-KO cells with agents that perturb different cellular processes. Among those, we found that blocking mitochondrial electron transport with antimycin A greatly decreased TMRE flickering in DLP1-KO MEFs. Quantification of TMRE flickering showed that the antimycin A treatment resulted in an ∼8-fold decrease in TMRE flickering (Fig. 4A). Additionally, quantification in every five frames revealed that antimycin A minimized TMRE flickering immediately after the addition (Fig. 4B and supplemental Movie 7), suggesting that active electron transport is necessary for TMRE flickering. Furthermore, the immediate abolition of TMRE loss by the antimycin A treatment suggests that TMRE flickering is not due to the photooxidation of TMRE. Because TMRE loss is tightly coupled with morphological contraction, we further tested the effect of antimycin A on matrix contraction. By counting cells containing nodulated mitochondrial tubules in fixed DLP1-KO cells (Fig. 4C), we found that the antimycin A treatment substantially decreased mitochondrial contraction (Fig. 4D). Because of the transient nature of the matrix contraction, the number of untreated DLP1-KO cells containing contracted tubules was small in a fixed snapshot, consisting of about 6%. In contrast, however, contracted mitochondria were found much more rarely in antimycin A-treated cells (∼0.9%), indicating that antimycin A inhibited mitochondrial contraction. In addition, we also found a significant decrease of mitochondrial nodulation in the presence of the uncoupler FCCP (Fig. 4D). These results indicate that electron transport chain activity and membrane potential are important factors for transient matrix contraction and depolarization.
FIGURE 4.
TMRE flickering requires electron transport chain activity and membrane potential. A, antimycin A treatment diminished TMRE flickering in DLP1-KO MEFs. B, TMRE flickering quantified for every five frames. Antimycin A was added in frame 21. Error bars are S.E. C, examples of fixed snapshots showing mitochondrial morphologies in DLP1-KO MEFs, either with or without antimycin A for 2 min. Nodulated mitochondrial tubules in untreated DLP1-KO MEFs and un-nodulated mitochondria in antimycin A-treated DLP1-KO cells are shown. Scale bar, 10 μm. D, counting cells containing nodulated mitochondria shows that a 2-min treatment with antimycin A and a 5-min treatment with FCCP significantly decreased mitochondrial contraction. Error bars are S.E. E, nutrient starvation (Starv) of DLP1-KO MEFs abolished TMRE flickering. Addition of pyruvate (Pyr) alone did not increase TMRE flickering. Pyruvate/oligomycin (Olm) induced a substantial increase of TMRE flickering. Error bars are S.E. F, membrane potential assessments by TMRE. TMRE fluorescence in completely uncoupled mitochondria after the FCCP treatment was subtracted (F − Fu). Pyruvate/oligomycin increased membrane potential, whereas pyruvate alone had no significant effect. Error bars are S.E.
To further elaborate the role of the electron transport activity and membrane potential in TMRE flickering, DLP1-KO cells were incubated in nutrient-free conditions for 4–5 h. We found that nutrient starvation greatly decreased TMRE fluorescence and flickering, suggesting that diminished electron transport caused reduced membrane potential and TMRE flickering. We then added pyruvate to increase the electron transport activity. However, adding pyruvate did not increase TMRE flickering significantly (Fig. 4E). In contrast, we observed a substantial increase in TMRE flickering by addition of pyruvate along with oligomycin to nutrient-starved DLP1-KO cells (Fig. 4E and supplemental Movie 8). Assessments of membrane potential under these conditions showed that the combination of pyruvate and oligomycin increased membrane potential, whereas no significant change was found with pyruvate alone (Fig. 4F). These observations suggest that a buildup of membrane potential through electron transport activity is necessary for TMRE flickering. These results suggest that matrix contraction-depolarization coupling is a physiological process governed by the energetic states of mitochondria (see under “Discussion”).
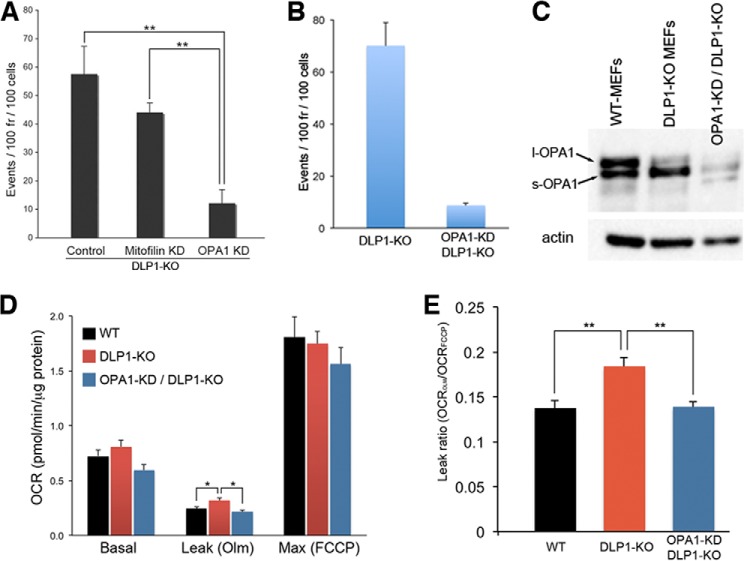
Silencing OPA1 in Fission Deficiency Diminishes TMRE Flickering and Normalizes Inner Membrane Proton Leak
Considering the active role of electron transport in the transient contraction of mitochondria, it is likely that this morphological change is mediated by a factor inside mitochondria. We speculated that a membrane remodeling protein in the inner membrane may induce the physical contraction of mitochondria in response to their functional state (see under “Discussion”). Therefore, we tested OPA1 and mitofilin, which regulate inner membrane organization, for their role in TMRE flickering. OPA1 is a dynamin-related inner membrane-remodeling protein, and mitofilin is a component of the inner membrane-scaffolding complex (21–23). Remarkably, we found that OPA1 knockdown in DLP1-KO cells markedly decreased TMRE flickering, whereas silencing mitofilin had no significant effect (Fig. 5A), suggesting an involvement of OPA1 in fission deficiency-induced TMRE flickering. Quantification with a stable clone expressing OPA1-shRNA in DLP1-KO MEFs showed that OPA1 silencing significantly decreased TMRE flickering in DLP1-KO cells (Fig. 5B and supplemental Movie 9). OPA1 undergoes protein processing that cleaves the long form of OPA1 (l-OPA1) into a short soluble form (s-OPA1). Interestingly, we detected a decrease of l-OPA1 and an increase of s-OPA1 in DLP1-KO cells (Fig. 5C). OPA1 has been shown to sense changes in membrane potential, as depolarization induces OPA1 cleavage (24, 25). It is possible that repeated depolarization may have led to the accumulation of s-OPA1 in DLP1-KO MEFs.
FIGURE 5.
OPA1 silencing decreases TMRE flickering and proton leak in DLP1-KO MEFs. A, quantification of TMRE flickering at 72 h post-transduction shows that down-regulation of OPA1 (OPA1 KD) substantially decreased TMRE flickering, whereas mitofilin knockdown had no significant effect. Error bars are S.E. **, p < 0.01. B, marked 8-fold decrease in TMRE flickering in a stable clone expressing OPA1 shRNA in DLP1-KO MEFs (OPA1-KD/DLP1-KO). n = 4. Error bars are S.E. C, DLP1-KO MEFs show increased processing of l-OPA1 to s-OPA1. D, cellular oxygen consumption analyses. Leak respiration in the presence of oligomycin (Olm) was higher in DLP1-KO MEFs. Knockdown of OPA1 in DLP1-KO cells normalized the leak respiration. E, DLP1-KO cells showed a substantial increase in the leak ratio compared with wild-type MEFs (WT) due to frequent large scale depolarization during TMRE flickering. Silencing OPA1 in DLP1-KO cells normalized leak ratio. OCR, oxygen consumption rate. n = 6. Error bars are S.E. **, p < 0.01.
We have shown that the large scale TMRE flickering in fission inhibition by DLP1-K38A is functionally reflected as an increased proton leak in respiration measurements (20). Similarly, DLP1-KO MEFs showing frequent large scale depolarization had increased inner membrane proton leak, as the leak respiration in the presence of oligomycin was significantly higher compared with that of wild-type cells (Fig. 5D). The calculated leak ratio (leak rate/maximum rate) was substantially higher in DLP1-KO MEFs (Fig. 5E). Importantly, we found that the diminished TMRE flickering resulting from OPA1 silencing in DLP1-KO cells restored proton leak to a level similar to that of wild-type MEFs (Fig. 5, D and E), demonstrating that OPA1 silencing normalized respiration coupling efficiency in DLP1-KO cells. These results suggest that OPA1-mediated transient depolarization is a novel mechanism of proton leak and regulates oxidative phosphorylation coupling.
OPA1 Couples Matrix Contraction to Membrane Leak
OPA1 is primarily known as an inner membrane fusion protein (26), and its down-regulation causes mitochondrial fragmentation (27–29). However, long tubular mitochondria were maintained with OPA1 silencing in DLP1-KO MEFs due to the absence of fission. Because OPA1 knockdown greatly reduced TMRE flickering, we tested whether OPA1 function is necessary for the synchronous matrix contraction that leads to TMRE loss. However, we found that the transient synchronous contraction of mitochondrial tubules still occurred with OPA1 silencing. Remarkably, dual imaging revealed that TMRE was still retained during transient matrix contractions (Fig. 6A and supplemental Movie 10). Fluorescence intensity analyses showed no decrease in TMRE upon the contraction-induced GFP increase (Fig. 6B). These observations suggest that the coupling between matrix contraction and depolarization is largely decreased by OPA1 silencing. These data indicate that, while OPA1 does not participate in matrix contraction, it is the factor that couples matrix contraction to depolarization. These observations also support the notion that matrix contraction is an upstream event leading to depolarization. Furthermore, because mitochondrial matrices are already in a fused state regardless of OPA1 silencing in DLP1-KO cells, our results suggest that OPA1-mediated depolarization does not involve the inner membrane fusion activity of OPA1. We identified the inner membrane dynamin OPA1 as a factor that mediates inner membrane leak during transient matrix contraction. Our findings suggest that the mitochondrial contraction-depolarization coupling facilitated by OPA1 is a novel cellular process regulating mitochondrial energetic states.
FIGURE 6.
OPA1 silencing prevents mitochondrial depolarization without inhibiting mitochondrial contraction. A, silencing OPA1 in DLP1-KO cells prevents TMRE loss during mitochondrial contraction. Scale bar, 5 μm. B, fluorescence intensity analyses show no TMRE loss with increased GFP signal during mitochondrial contraction in OPA1-KD/DLP1-KO cells.
DISCUSSION
In this study, we discovered a previously unrecognized cellular phenomenon in which transient matrix contraction is coupled to mitochondrial depolarization. We found that fission deficiency greatly increases this phenomenon. We speculate that the mitochondrial interconnection resulting from fission deficiency is one of the factors regulating the extent of TMRE flickering. Studies indicate that transient opening of the PT pore occurs in normal conditions (16, 30). Our observations support this finding, as small transient inner membrane leak/depolarization was seen in normal cells, although it is unlikely that the conventional cyclosporin A-sensitive pore is involved in this phenomenon (19, 20). In normal cells, these small leaks occur in discrete mitochondria as isolated events. Therefore, at any given time, the population of depolarized mitochondria is small and negligible and thus does not affect the overall cellular mitochondrial function. However, when mitochondria become interconnected due to fission deficiency, the same small leak depolarizes all connected mitochondria simultaneously. Therefore, mitochondrial interconnection can serve as an amplifying factor for depolarization, as evidenced by increased proton leak in respiration measurements. In the same manner, matrix contractions are more readily observable in the long connected mitochondria in fission-deficient cells. The matrix contractions in long mitochondrial tubules were manifested as multiple “nodules.” Consistent with simultaneous depolarization, we found that all connected mitochondria contract synchronously. Alternatively, other factors that are increased in DLP1-KO cells could play a role in this phenomenon. Endoplasmic reticulum tubules have been shown to mediate constriction of mitochondrial tubules, marking future fission sites (31). It is possible that endoplasmic reticulum mitochondria contacts may be increased in DLP1-KO cells, which, in the absence of DLP1-mediated fission, may predispose constricted mitochondria to depolarization. Of note, the sustained presence of nodules was observed in some mitochondrial tubules; however, only transient contraction is associated with TMRE loss.
Mechanistically, our data indicate that the inner membrane fusion dynamin OPA1 plays a role in mitochondrial contraction-depolarization coupling. A recent report showed that spontaneous mitochondrial depolarization induces matrix alkalinization (pH flash), which represents a compensatory increase of proton pumping (19). This work by Santo-Domingo et al. (19) indicated that OPA1-mediated inner membrane fusion is necessary for depolarization-induced pH flash because pH flash was absent in OPA1-KO MEFs and was increased upon re-introduction of OPA1, which restored inner membrane fusion. Similarly, our experiments using OPA1 silencing in DLP1-KO MEFs demonstrated that TMRE flickering requires OPA1 function. However, our experimental results provide novel information that the OPA1 function necessary for depolarization does not involve inner membrane fusion. Unlike the fragmented, small discrete mitochondria seen in OPA1-KO MEFs, the mitochondrial matrices in the long mitochondria of DLP1-KO cells are already fused together, and OPA1 silencing in DLP1-KO MEFs maintains this pre-existing matrix continuity during contraction. Our results demonstrated that OPA1 mediates depolarization even in the absence of inner membrane fusion in DLP1-KO MEFs, and OPA1 silencing inhibited it. Therefore, it is unlikely that fusion between inner membranes by OPA1 is involved in depolarization/membrane leak. It has been shown that OPA1 has the capacity to tubulate the membrane (32). Furthermore, the presence of OPA1 in only one of the fusing partners is sufficient for fusion (33). Matrix bulging during mitochondrial contraction would generate close contacts between the inner and outer membranes. It is possible that inner membrane tubulation and protrusion by OPA1 at these contacts may transiently fuse the inner membrane to the outer membrane, opening a fusion pore and allowing content leakage. Our findings suggest that OPA1 may have an additional role of regulating mitochondrial bioenergetics through a function other than inner membrane fusion.
It is currently unclear what induces matrix contraction. Considering that electron transport is necessary for the matrix contraction that accompanies depolarization, it is likely that TMRE flickering, whether it is small (in normal cells) or large (in fission-deficient cells), is closely linked to the mitochondrial electron transport chain activity. Concerning this, the original observations by Hackenbrock (34) may provide insight to the matrix contraction we observed. With isolated mitochondria in vitro, Hackenbrock (34) demonstrated that there is a reversible ultrastructural transition between “orthodox” and “condensed” conformations, which represent resting and rapidly respiring mitochondria, respectively. Condensed mitochondria display expanded cristae and condensed matrix area. Recent studies suggest that this structural change observed in isolated mitochondria also holds true for mitochondria in cells (35, 36), raising a possibility that matrix contraction may be a reflection of the dynamic transition of mitochondrial internal structure. In this regard, it is of interest to investigate whether the transient contraction of mitochondria is associated with dynamic assembly of respiratory chain supercomplexes or respirasome (37, 38). Interestingly, a recent report showed that OPA1 activity is linked to the regulation of crista structure and respiratory complex assembly (39), suggesting a potential connection between matrix contraction, OPA1, and depolarization.
Our results indicate that the membrane potential generated by electron transport is necessary for the TMRE flickering associated with matrix contraction, suggesting that contraction-depolarization coupling has a physiological role. Transient opening of the PT pore normally occurs in individual mitochondria (16, 30). Because mitochondrial hyperpolarization causes ROS overproduction and Ca2+ overload, the transient PT would be necessary to alleviate these harmful effects of hyperpolarization. TMRE fluorescence intensity varies among cells in a population as well as mitochondria within a cell, suggesting that individual cells and mitochondria have different membrane potentials. Furthermore, our observations indicate that there is no correlation between TMRE flickering and the intensity of TMRE fluorescence, as mitochondria with low TMRE fluorescence can undergo robust flickering, whereas those with higher TMRE fluorescence can be quiescent. We speculate that individual mitochondria not only are in different energetic states but also have their own set ceilings for the maximum tolerable membrane potential. It is possible that a buildup of membrane potential above a certain limit set by individual mitochondria may signal internally to evoke transient depolarization through matrix contraction. Transient mitochondrial contraction-depolarization occurs locally in normal cells, as we observed in H9c2 cells. Therefore, this transient contraction-depolarization could be a protective mechanism for energetically active mitochondria to prevent hyperpolarization. In this context, this newly identified phenomenon can be considered a novel form of transient PT. While more factors are likely involved in this process, the mitochondrial contraction-depolarization coupling reported in this study will provide new insight for the mechanisms regulating morphology-function correlation in mitochondria.
Supplementary Material
Acknowledgments
We thank Hiromi Sesaki for providing WT and DLP1-KO MEFs. We also thank Dawn O'Brien for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK078618 and DK061991 (to Y. Y.).

This article contains supplemental Movies 1–10.
- TMRE
- tetramethylrhodamine ethyl ester
- ROS
- reactive oxygen species
- MEF
- mouse embryonic fibroblast
- FCCP
- carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- OCR
- oxygen consumption rate
- l-OPA1
- long OPA1
- s-OPA1
- short OPA1
- PT
- permeability transition.
REFERENCES
- 1. Hoppins S., Lackner L., Nunnari J. (2007) The machines that divide and fuse mitochondria. Annu. Rev. Biochem. 76, 751–780 [DOI] [PubMed] [Google Scholar]
- 2. Palmer C. S., Osellame L. D., Stojanovski D., Ryan M. T. (2011) The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell. Signal. 23, 1534–1545 [DOI] [PubMed] [Google Scholar]
- 3. Yoon Y., Galloway C. A., Jhun B. S., Yu T. (2011) Mitochondrial dynamics in diabetes. Antioxid. Redox Signal. 14, 439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan D. C. (2006) Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 22, 79–99 [DOI] [PubMed] [Google Scholar]
- 5. Chan D. C. (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 [DOI] [PubMed] [Google Scholar]
- 6. Galloway C. A., Lee H., Yoon Y. (2012) Mitochondrial morphology–emerging role in bioenergetics. Free Radic. Biol. Med. 53, 2218–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amchenkova A. A., Bakeeva L. E., Chentsov Y. S., Skulachev V. P., Zorov D. B. (1988) Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J. Cell Biol. 107, 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skulachev V. P. (2001) Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem. Sci. 26, 23–29 [DOI] [PubMed] [Google Scholar]
- 9. Buckman J. F., Reynolds I. J. (2001) Spontaneous changes in mitochondrial membrane potential in cultured neurons. J. Neurosci. 21, 5054–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobson J., Duchen M. R. (2002) Mitochondrial oxidative stress and cell death in astrocytes–requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 115, 1175–1188 [DOI] [PubMed] [Google Scholar]
- 11. Diaz G., Falchi A. M., Gremo F., Isola R., Diana A. (2000) Homogeneous longitudinal profiles and synchronous fluctuations of mitochondrial transmembrane potential. FEBS Lett. 475, 218–224 [DOI] [PubMed] [Google Scholar]
- 12. Zorov D. B., Filburn C. R., Klotz L. O., Zweier J. L., Sollott S. J. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192, 1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duchen M. R., Leyssens A., Crompton M. (1998) Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J. Cell Biol. 142, 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aon M. A., Cortassa S., Marbán E., O'Rourke B. (2003) Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278, 44735–44744 [DOI] [PubMed] [Google Scholar]
- 15. De Giorgi F., Lartigue L., Ichas F. (2000) Electrical coupling and plasticity of the mitochondrial network. Cell Calcium 28, 365–370 [DOI] [PubMed] [Google Scholar]
- 16. Hüser J., Blatter L. A. (1999) Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem. J. 343, 311–317 [PMC free article] [PubMed] [Google Scholar]
- 17. Hattori T., Watanabe K., Uechi Y., Yoshioka H., Ohta Y. (2005) Repetitive transient depolarizations of the inner mitochondrial membrane induced by proton pumping. Biophys. J. 88, 2340–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vergun O., Votyakova T. V., Reynolds I. J. (2003) Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys. J. 85, 3358–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santo-Domingo J., Giacomello M., Poburko D., Scorrano L., Demaurex N. (2013) OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J. 32, 1927–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galloway C. A., Lee H., Nejjar S., Jhun B. S., Yu T., Hsu W., Yoon Y. (2012) Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes 61, 2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harner M., Korner C., Walther D., Mokranjac D., Kaesmacher J., Welsch U., Griffith J., Mann M., Reggiori F., Neupert W. (2011) The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 30, 4356–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoppins S., Collins S. R., Cassidy-Stone A., Hummel E., Devay R. M., Lackner L. L., Westermann B., Schuldiner M., Weissman J. S., Nunnari J. (2011) A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol. 195, 323–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von der Malsburg K., Müller J. M., Bohnert M., Oeljeklaus S., Kwiatkowska P., Becker T., Loniewska-Lwowska A., Wiese S., Rao S., Milenkovic D., Hutu D. P., Zerbes R. M., Schulze-Specking A., Meyer H. E., Martinou J. C., Rospert S., Rehling P., Meisinger C., Veenhuis M., Warscheid B., van der Klei I. J., Pfanner N., Chacinska A., van der Laan M. (2011) Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Dev. Cell 21, 694–707 [DOI] [PubMed] [Google Scholar]
- 24. Ishihara N., Fujita Y., Oka T., Mihara K. (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 25, 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song Z., Chen H., Fiket M., Alexander C., Chan D. C. (2007) OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 178, 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cipolat S., Martins de Brito O., Dal Zilio B., Scorrano L. (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc. Natl. Acad. Sci. U.S.A. 101, 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen H., Chomyn A., Chan D. C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 280, 26185–26192 [DOI] [PubMed] [Google Scholar]
- 28. Olichon A., Baricault L., Gas N., Guillou E., Valette A., Belenguer P., Lenaers G. (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 278, 7743–7746 [DOI] [PubMed] [Google Scholar]
- 29. Lee Y. J., Jeong S. Y., Karbowski M., Smith C. L., Youle R. J. (2004) Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol. Biol. Cell 15, 5001–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ichas F., Jouaville L. S., Mazat J. P. (1997) Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89, 1145–1153 [DOI] [PubMed] [Google Scholar]
- 31. Friedman J. R., Lackner L. L., West M., DiBenedetto J. R., Nunnari J., Voeltz G. K. (2011) ER tubules mark sites of mitochondrial division. Science 334, 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ban T., Heymann J. A., Song Z., Hinshaw J. E., Chan D. C. (2010) OPA1 disease alleles causing dominant optic atrophy have defects in cardiolipin-stimulated GTP hydrolysis and membrane tubulation. Hum. Mol. Genet. 19, 2113–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song Z., Ghochani M., McCaffery J. M., Frey T. G., Chan D. C. (2009) Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 20, 3525–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hackenbrock C. R. (1966) Ultrastructural bases for metabolically linked mechanical activity in mitochondria: I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol. 30, 269–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mannella C. A. (2006) Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta 1763, 542–548 [DOI] [PubMed] [Google Scholar]
- 36. Gomes L. C., Di Benedetto G., Scorrano L. (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vonck J., Schäfer E. (2009) Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochim. Biophys. Acta 1793, 117–124 [DOI] [PubMed] [Google Scholar]
- 38. Lapuente-Brun E., Moreno-Loshuertos R., Acín-Pérez R., Latorre-Pellicer A., Colás C., Balsa E., Perales-Clemente E., Quirós P. M., Calvo E., Rodríguez-Hernández M. A., Navas P., Cruz R., Carracedo Á., López-Otín C., Pérez-Martos A., Fernández-Silva P., Fernández-Vizarra E., Enríquez J. A. (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570 [DOI] [PubMed] [Google Scholar]
- 39. Cogliati S., Frezza C., Soriano M. E., Varanita T., Quintana-Cabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L. C., Perales-Clemente E., Salviati L., Fernandez-Silva P., Enriquez J. A., Scorrano L. (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.