Background: Ion selectivity of voltage-gated channels is governed by selectivity filters.
Results: Alternative turret region in domain II promotes highly sodium-permeable T-type channels without major changes to gating and kinetic features.
Conclusion: T-type channels can generate variable sodium or calcium permeability by gene splicing.
Significance: Ion selectivity in T-type channels can be altered using extracellular domains outside the ion selectivity filter.
Keywords: Alternative Splicing, Calcium Channels, Heart, Patch Clamp Electrophysiology, Permeability, Sodium Channels
Abstract
T-type (Cav3) channels are categorized as calcium channels, but invertebrate ones can be highly sodium-selective channels. We illustrate that the snail LCav3 T-type channel becomes highly sodium-permeable through exon splicing of an extracellular turret and descending helix in domain II of the four-domain Cav3 channel. Highly sodium-permeable T-type channels are generated without altering the invariant ring of charged residues in the selectivity filter that governs calcium selectivity in calcium channels. The highly sodium-permeant T-type channel expresses in the brain and is the only splice isoform expressed in the snail heart. This unique splicing of turret residues offers T-type channels a capacity to serve as a pacemaking sodium current in the primitive heart and brain in lieu of Nav1-type sodium channels and to substitute for voltage-gated sodium channels lacking in many invertebrates. T-type channels would also contribute substantially to sodium leak conductances at rest in invertebrates because of their large window currents.
Introduction
Cav3 T-type channels are members of the 4×6TM (4 heterologous domains of 6 trans-membrane segments) family of voltage-gated ion channels, which includes calcium (Cav) and sodium (Nav) channels and sodium leak conductance channel (NALCN) (1–3). The four heterologous domains of 4×6TM channels are considered to have evolved from two rounds of duplication of ancestral channels with one domain, such as the voltage-gated potassium channels (4). Key elements of each domain are a voltage sensor region (segments 1–4) and a re-entrant pore loop that extends from segments 5 and 6 and contains the selectivity filter thought to govern ion selectivity. The potassium channel pore mimics the hydration shell oxygen atoms that surround potassium ions in solution using conserved selectivity filter residues consisting of backbone carbonyl oxygens (5). This configuration makes it energetically feasible for surrogate oxygen groups to displace hydrating water molecules as potassium ions permeate (5). P-(Pore-) loops are contributed by four asymmetrical domains of calcium and sodium channels to a wider and shorter selectivity filter, which allows sodium ions to pass in a semi-hydrated (6) or hydrated state (7). Side chains of glutamate residues (EEEE) in the selectivity filter project carboxyl oxygens into the pore to create a high affinity pore locus for calcium ions in Cav1 (L-type) and Cav2 (non-L-type) calcium channels (8), where Na+ ions are prohibited from passing in the presence of low concentrations of external Ca2+ ions (∼1 μm) (9). T-type channels ubiquitously have a similar negatively charged ring of residues as Cav1 and Cav2 channels in the selectivity filter, but they are notably different, with aspartate residues replacing two of the glutamate residues in the 3rd and 4th positions (EEDD). Mammalian T-type channels (Cav3.1, Cav3.2, and Cav3.3) have an ∼10-fold lower calcium selectivity over sodium than Cav1 and Cav2 channels, but they are still impermeant to sodium in the presence of low (10 μm) calcium (10, 11). Here, we show that the LCav3 T-type channel from the pond snail Lymnaea stagnalis with a standard EEDD selectivity filter becomes highly permeant to monovalent cations through splicing of a novel extracellular turret, even in the presence of physiological (millimolar) concentrations of Ca2+ ions. These sodium-permeant T-type currents serve as a major pacemaker current of primitive hearts, and are likely proxy for sodium channels when they are absent in many invertebrates. Our work confirms the capacity of snail channel currents to switch sodium and calcium permeabilities by an extracellular determinant, first postulated by Kostyuk et al. (12) more than 30 years ago.
EXPERIMENTAL PROCEDURES
Cloning and Expression of Novel LCav3 Exon 12a Splice Variant
The original cloning and expression work of LCav3 T-type calcium channel containing exons +8b, 12b, and −25c was described in Senatore and Spafford (13), and the original gene sequence was deposited as GenBankTM accession number AF484084. Subsequently, we described LCav3 T-type calcium channel splicing of exon 8b deposited as GenBankTM accession number JQ313138 and exon 25c deposited as GenBankTM accession number JQ313139. LCav3 −8b and +25C splice variants were described in Senatore and Spafford (14). Here, we describe novel exon 12a isoform (+8b, 12a, −25C), created by PCR using the original full-length clone of LCav3 in pIRES2 vector (+8b, 12b, −25c) as template. The novel splice variant (+8b, 12a, −25C) has been deposited as GenBankTM accession number JX292155. The inclusion of exons +8b (54%) and 12a (56%) and −25C (77%) is the most common configuration of these three exons in the adult brain, whereas the adult heart does not commonly have a configuration of +8b (16%) or −25C (21%) (14). The adult heart configuration of exons most commonly found is −8b (84%), 12a (99%), and +25C (79%) (14). Novel exon 12a was confirmed in multiple PCR amplifications from snail brain cDNA (Fig. 1). All expressed plasmids were confirmed by sequencing (TCAG DNA Sequencing Facility, Sick Kids Hospital, Toronto, Canada). Human Cav3.1 expressible clone was a gift from Gerald Zamponi, University of Calgary, and cloned by Aaron Beedle.
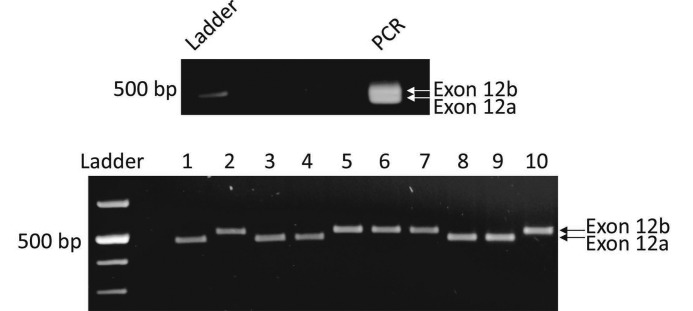
FIGURE 1.
Expression of exons 12a and 12b in L. stagnalis cDNA. Top, PCR amplification of snail embryonic cDNA with nested primers flanking the mutually exclusive exons 12a and 12b produced two DNA fragments that could be distinguished in size on an agarose gel. Bottom, plasmid DNA inserts from A were excised from the pGEM-T Easy vector with EcoRI, and electrophoresed inserts were restricted to two sizes (451 bp for exon 12a and 484 bp for exon 12b, plus additional sizes for polylinker sequence from the pGEM vector). Sequencing of multiple inserts of each size confirmed that the smaller plasmid insert encoded exon 12a and the larger clone encoded exon 12b.
Culturing of Snail Heart Cells
Heart ventricle cells were cultured from anesthetized adult snails (25–35 mm shell length) using a modified protocol (15). Cut up ventricles were placed in 0.5 mm Ca2+ Leibowitz medium containing gentamycin (400 mg/ml) and 30 mm glucose, trypsinized (0.25% (w/v) trypsin, Sigma T9201) for 10 min, and then treated with 0.1% collagenase (Sigma type II) in 0.5 mm Ca2+ Leibowitz medium for 30 min. The digested hearts were then washed three times with 3.5 mm Ca2+ Leibowitz medium and then plated on acid-etched circular glass coverslips (16) and left for 24 h to adhere at room temperature in 3.5 mm Ca2+ Leibowitz medium containing 400 mg/ml gentamycin, 30 mm glucose, and 2% fetal bovine serum, before patch clamp recording 24 h later.
Whole Cell Patch Clamp Recording
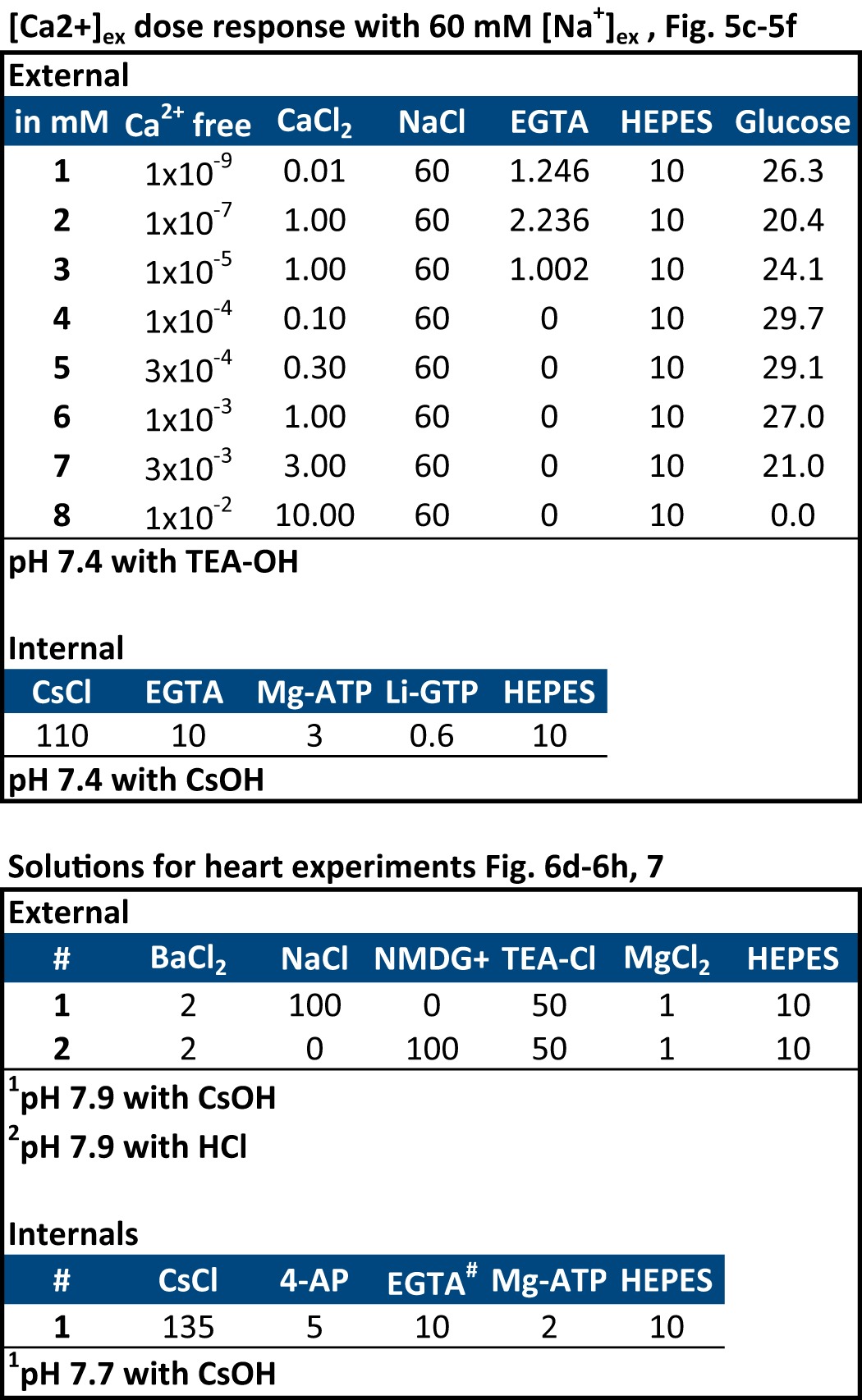
We have detailed our optimized technique for the culture and maintenance of mammalian HEK-293T cells, calcium phosphate transfection and expression of ion channels, and their recording using whole cell patch clamp in an on-line video journal (17). There are five sets of solutions for recording (Tables 1 and 2). For comparing ion permeabilities or drug block, a Valvelink8.2® gravity flow Teflon perfusion system (AutoMate Scientific, Berkeley, CA) was used to toggle between differing external solutions during electrophysiological recording. Mibefradil was diluted in 5 mm water. A stock solution of isradipine was made in DMSO (dimethyl sulfoxide). Final concentration of isradipine was in 0.01% DMSO.
TABLE 1.
External and internal recording solutions (part 1)
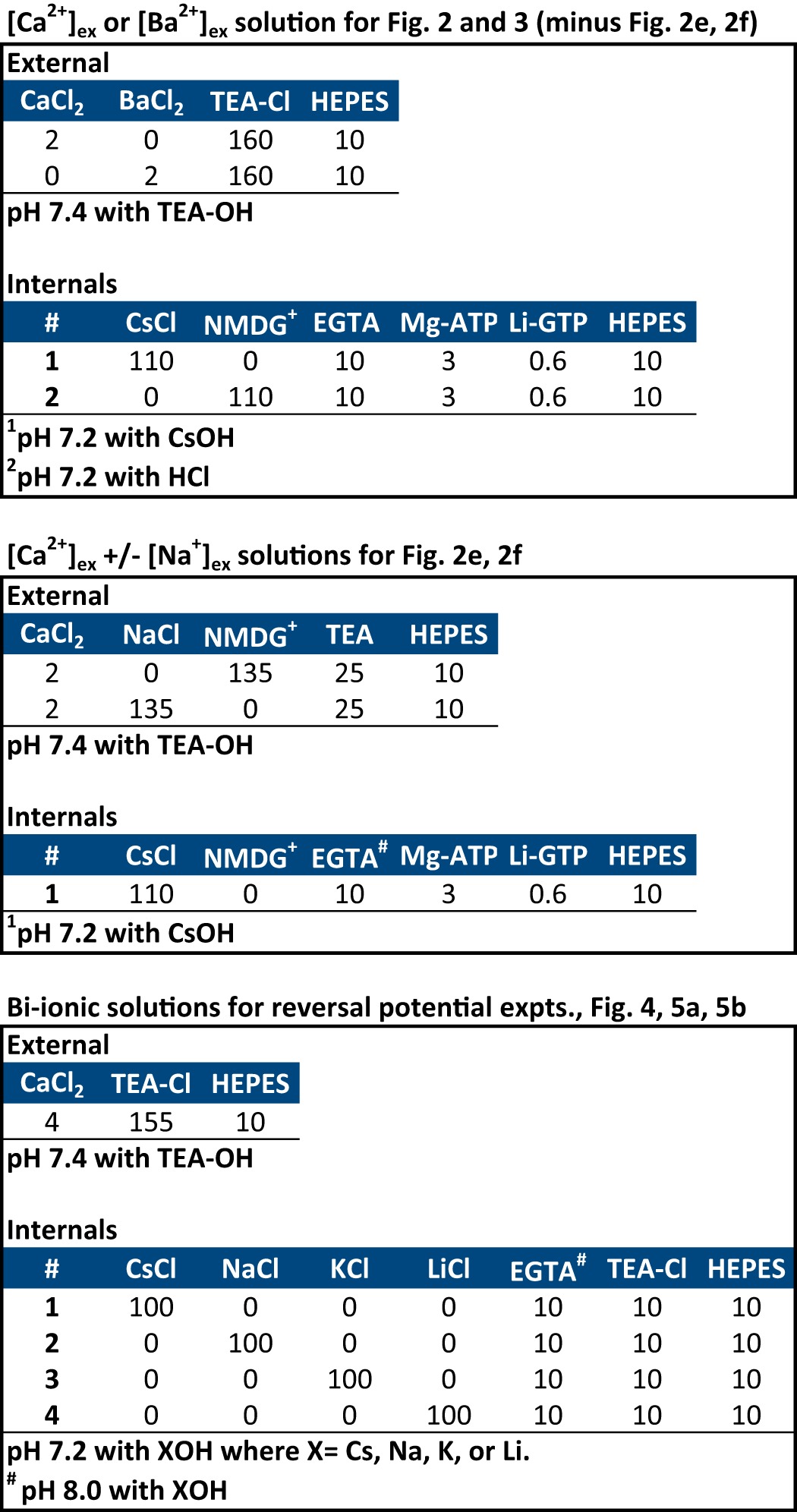
TABLE 2.
External and internal recording solutions (part 2)
Whole cell patch clamp recordings were carried out with an AxoPatch 200B amplifier, combined with a Digidata® 1440A data acquisition system and pCLAMP 10 software. Patch pipettes for recording had pipette resistances of 2–5 MΩ (HEK-293T cells) or 5–10 MΩ (heart cells) and with typical access resistance maintained after breakthrough between 4 and 6 MΩ (HEK-293T cells) or 10 and 14 MΩ (heart cells). Only recordings with minimal leak (<10% of peak) and small current sizes (<500 pA) in HEK-293T cells were used due to loss of voltage clamp above 500 pA. Series resistance was compensated to 70% (prediction and correction, 10-μs time lag). Off-line leak subtraction was carried out using the Clampfit 10.1 software (Molecular Devices). Protocols for measuring the voltage sensitivity and kinetics and curve fitting data are described in Refs. 13, 14. The relative permeability of PCa/Px, where x is monovalent ion (Li+, Na+, K+, and Cs+), was calculated by the following bi-ionic Equation 1 (18),
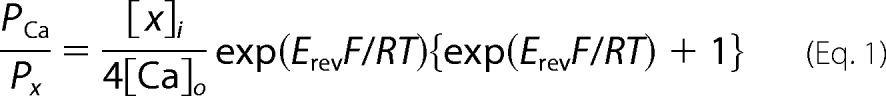 |
Measurement of mRNA Expression of Exon 12a and Exon 12b Splice Variants Using qPCR
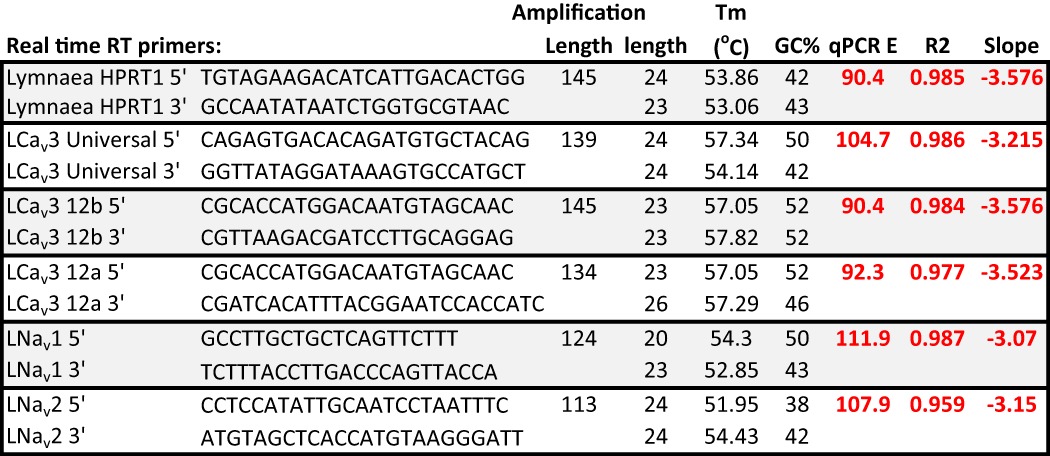
qPCR4 was carried out as described previously (14, 19, 20). RNA was extracted from entire juvenile and adult snails, where sexually immature juveniles have shell lengths of 1.0 to 1.5 cm, and reproduction-capable adults have shell lengths of 2.0 to 2.5 cm (21). qPCR primer sets (Table 3) were designed to selectively amplify universal and specific exon 12a and exon 12b splice variants of Lymnaea Cav3. The specificity of PCR primer pairs was initially assessed by comparing the size of PCR products amplified from a pooled cDNA library with those amplified from cloned cDNAs. PCR primer efficiency for each primer set was then determined by generating relative standard curves using the 1:5 serial dilutions of pooled cDNA (1:5, 1:25, 1:125, and 1:625) as template for real time RT-PCR amplification. For each dilution, triplicate reactions were carried out in rigid 96-well PCR plates (Bio-Rad), with each well containing 0.5 μl of serially diluted cDNA, 5 μl of SsoFastTM EvaGreen® Supermix (Bio-Rad), 0.5 μl of each 10 μm primer from a set, and 3 μl of water. PCR amplification, fluorescence reading, and melt curve analyses were done using a Bio-Rad C1000TM Thermal Cycler equipped with a CFX96TM Real-Time System and run by CFX Manager software (Bio-Rad). All cycle threshold values used for analysis were determined relative to the average cycle threshold value of the control gene, HPRT1 (hypoxanthine phosphoribosyltransferase 1).
TABLE 3.
Primers used for qPCR of control gene HPRT1, LCav3, LNav1 and LNav2
LCav3 primer sets include universal sequence primers, exon 12a isoform-specific primers, and exon 12b isoform-specific primers. PCR efficiency (E), goodness of fit R2 values, and slopes for the standard curves used to characterize qPCR primer pairs are shown in red.
RESULTS
Novel Spliced Turret Alters Monovalent Ion Permeability of Snail T-type Channels
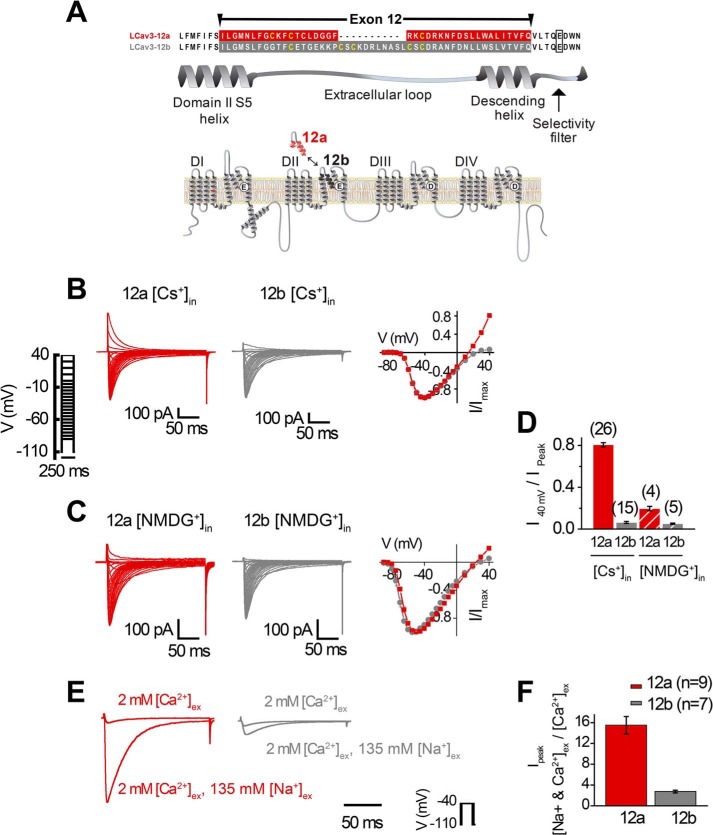
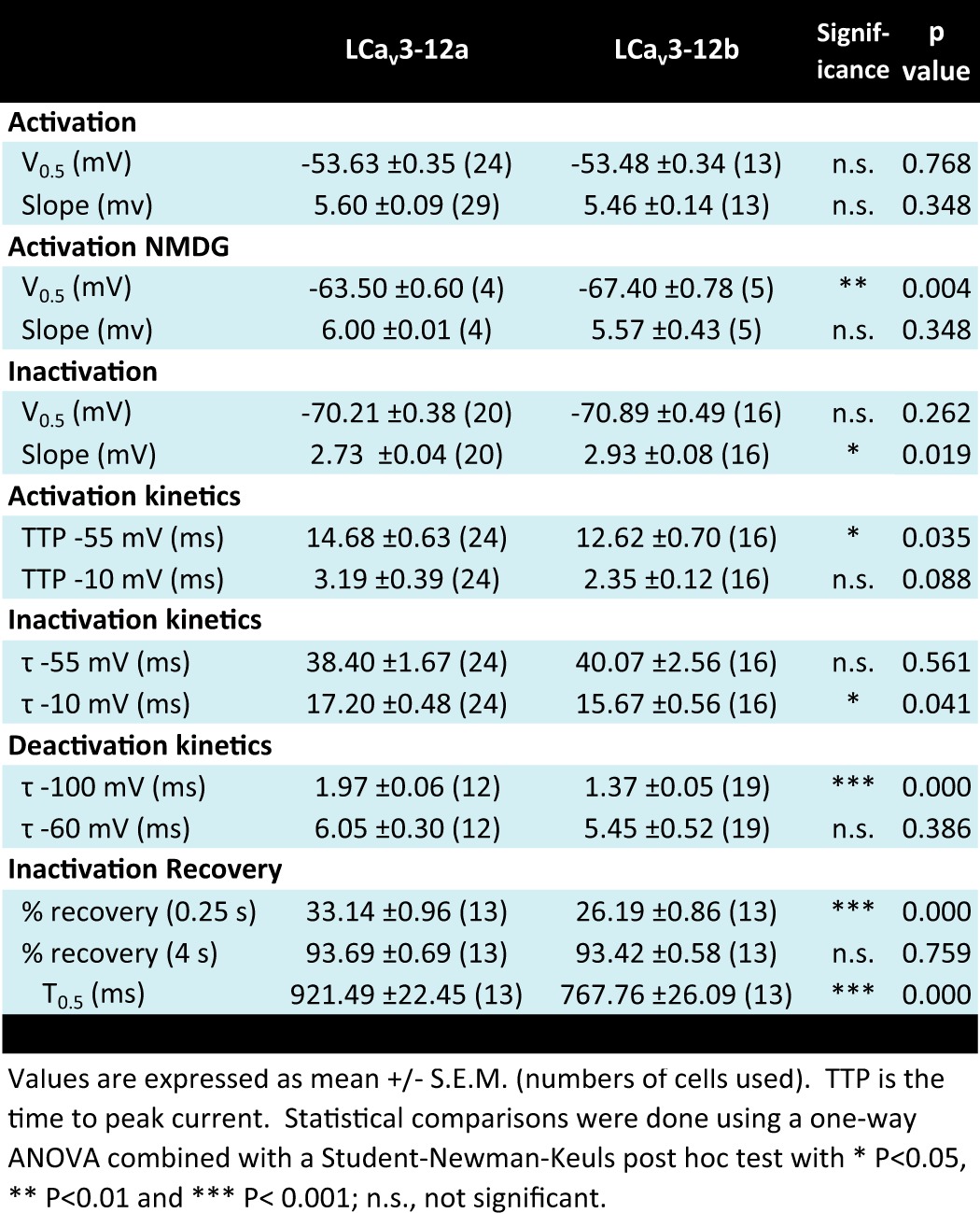
T-type channels diversified into three vertebrate genes but are rooted as a single T-type channel gene in extant relatives of animals spanning tissue and nervous system evolution (Trichoplax and cnidarians) and identified as the LCav3 gene in the pond snail, L. stagnalis (13, 14). Expressible snail LCav3 channel resembles mammalian T-type channels in their rapid channel kinetics, and low threshold of activation for generating rhythmic pacemaker currents to threshold for eliciting action potential spikes (13). Snail channels also resemble particular mammalian T-type channels in their conserved and developmentally regulated exon splicing, such as with optional exons 8b and 25c, which confers membrane expression changes and changes to kinetic properties, respectively (14). We have identified unique exon splicing for exon 12 in the invertebrate T-type channel that spans from the S5 membrane helix of domain II, the extracellular loop, dubbed the “turret,” to the descending pore helix that projects into the pore toward the selectivity filter (Fig. 2A). Remarkably, the 3′-splice sites for exons 12a and 12b occur five amino acids upstream but do not include the invariant selectivity filter (Fig. 2A), whose residues define the channel's characteristic ion selectivity (22). Exon 12a is shorter (39 aa long) with a characteristic cysteine structure, CxxC … C, whereas exon 12b has a penta-cysteine structure C … CxC … CxC and is longer (50 aa long) (Fig. 2A). Snail LCav3-12a and LCav3-12b were recorded with 2 mm Ca2+ as the external charge carrier in transfected HEK-293T cells, using whole cell patch clamp as described previously (13, 14), with the cell culturing methods we describe in an on-line video publication (JoVE) (17). A notable difference for LCav3-12a compared with LCav3-12b in the ensemble of voltage-gated calcium currents was a large outward current with voltage steps above 0 mV (Fig. 2, B and D). We suspected that the large outward current with LCav3-12a was carried by the outflow of 110 mm Cs+ from the intracellular patch pipette, a constituent normally present in the patch pipette to block K+ currents. Replacement of 110 mm internal Cs+ with equimolar impermeant monovalent cation N-methyl-d-glucamine (NMDG+) eliminated most of the outward current for LCav3 isoforms, leaving a residual current, possibly carried by NMDG+, but also the 0.6 mm Li+ in the compound Li-GTP and other monovalent contaminants contained in the intracellular solution (Fig. 2, C and D). Calcium channels have a high affinity for Ca2+ ions, and monovalent ions are normally excluded in the presence of physiological levels of extracellular calcium, [Ca2+]ex (10). Thus, it was surprising to observe that LCav3-12a generated >15-fold greater peak inward currents in the presence of 2 mm Ca2+ with physiological levels of [Na+]ex at 135 mm, whereas LCav3-12b was significantly less permeable with only an ∼2.5-fold increase (Fig. 2, E and F). With such a dramatic change in the pore's ion permeability for LCav3-12a, it is surprising that both snail LCav3-12a and -12b isoforms have completely overlapping biophysical properties, such as voltage sensitivities and kinetics of activation and inactivation (Fig. 3, A and B), as well as the size of the currents in divalent cations (Ba2+ currents being ∼1.3-fold larger than Ca2+ currents) (Fig. 3C) and similar sensitivity to block by Ni2+ ions (Fig. 3D). Outside of permeability changes, there is a notably faster recovery rate from inactivation (Fig. 3E) and slower deactivation rate for the LCav3-12a isoform (Fig. 3F). Yet, taking it all together (see Table 4), it is surprising how an extracellular turret, constituting ∼1% of the channel's length, has remarkably little effect on the T-type channel while dramatically altering monovalent cation permeability.
FIGURE 2.
Splicing of extracellular turret isoform (exon 12a) in the pore loop of domain II in snail LCav3 channels generates monovalent ion-permeant T-type channels. A, aligned sequences illustrate mutually exclusive exons (12a and 12b) that correspond to a portion of the domain II S5 helix, the turret, and the descending helix that descends into the pore vestibule. B, dramatic difference in outward Cs+ currents between LCav3-12a and -12b in 2 mm [Ca2+]ex and 0.6 mm [Li+-GTP] is mostly eliminated by replacement of 110 mm internal Cs+ with equimolar impermeant NMDG+ (C). Sample traces (left) and current voltage curves (right) are shown in B and C. D, ratio of maximum outward currents (at +40 mV) to peak inward current for B and C. n values are shown in parentheses. E, sample currents; F, bar graph illustrating the 15-fold peak inward current size with addition of 135 mm [Na+]ex to 2 mm [Ca2+]ex with LCav3-12a, compared with the ∼2.5-fold change with LCav3-12b. Statistics for Fig. 2, E and F, are shown in Table 5.
FIGURE 3.
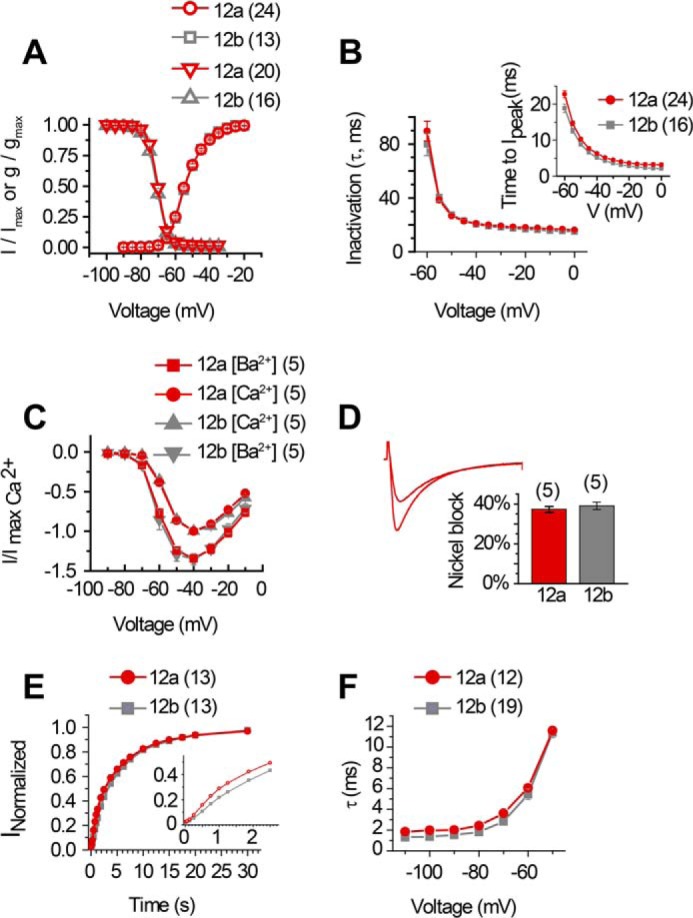
Differing extracellular turrets in domain II P-loop (exons 12a and 12b) do not alter major features outside of monovalent ion permeability. There were no major differences in most biophysical properties between LCav3-12a and LCav3-12b in 2 mm [Ca2+]ex, including the following: A, current-voltage relationship for activation and steady-state inactivation; B, inactivation kinetics and time to peak (inset); C, current size differences in 2 mm [Ba2+]ex versus [Ca2+]ex, and D, blockade by 300 μm Ni2+. A sample of the blocking effect for nickel for LCav3-12a is shown in the inset of D. A sample of a similar block of nickel for LCav3-12b is shown in Fig. 3, A and B, of Ref. 13. There are measurable differences in faster recovery rate from inactivation (E) and slower deactivation kinetics for LCav3-12a (F). n values are shown in parentheses. The differences in the rate of recovery is observed at the earliest time point, when the time scale range is limited from 0 to 2 s (E, inset). Statistics for Fig. 3 are shown in Table 4.
TABLE 4.
Comparison of the biophysical parameters for snail LCav3 channel currents recorded harboring either exon 12A or exon 12B in HEK-293T cells
Snail T-type Channel with Exon 12a Is More Permeant to Sodium Ions than Calcium Ions
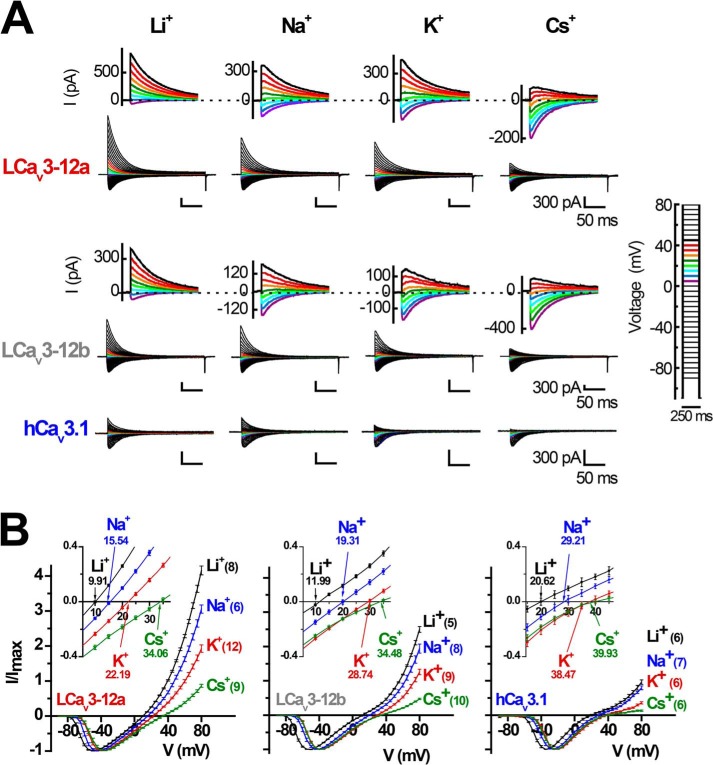
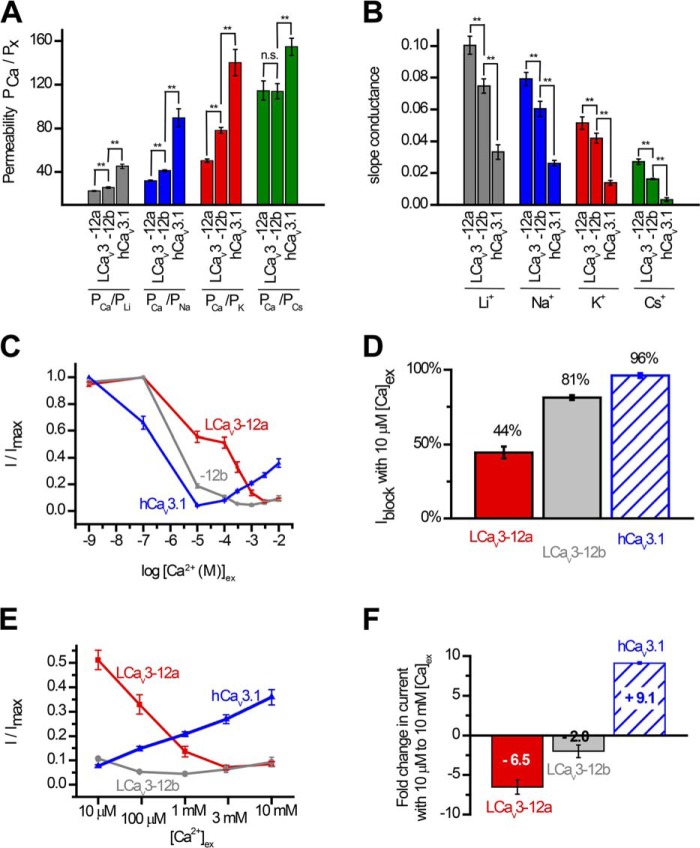
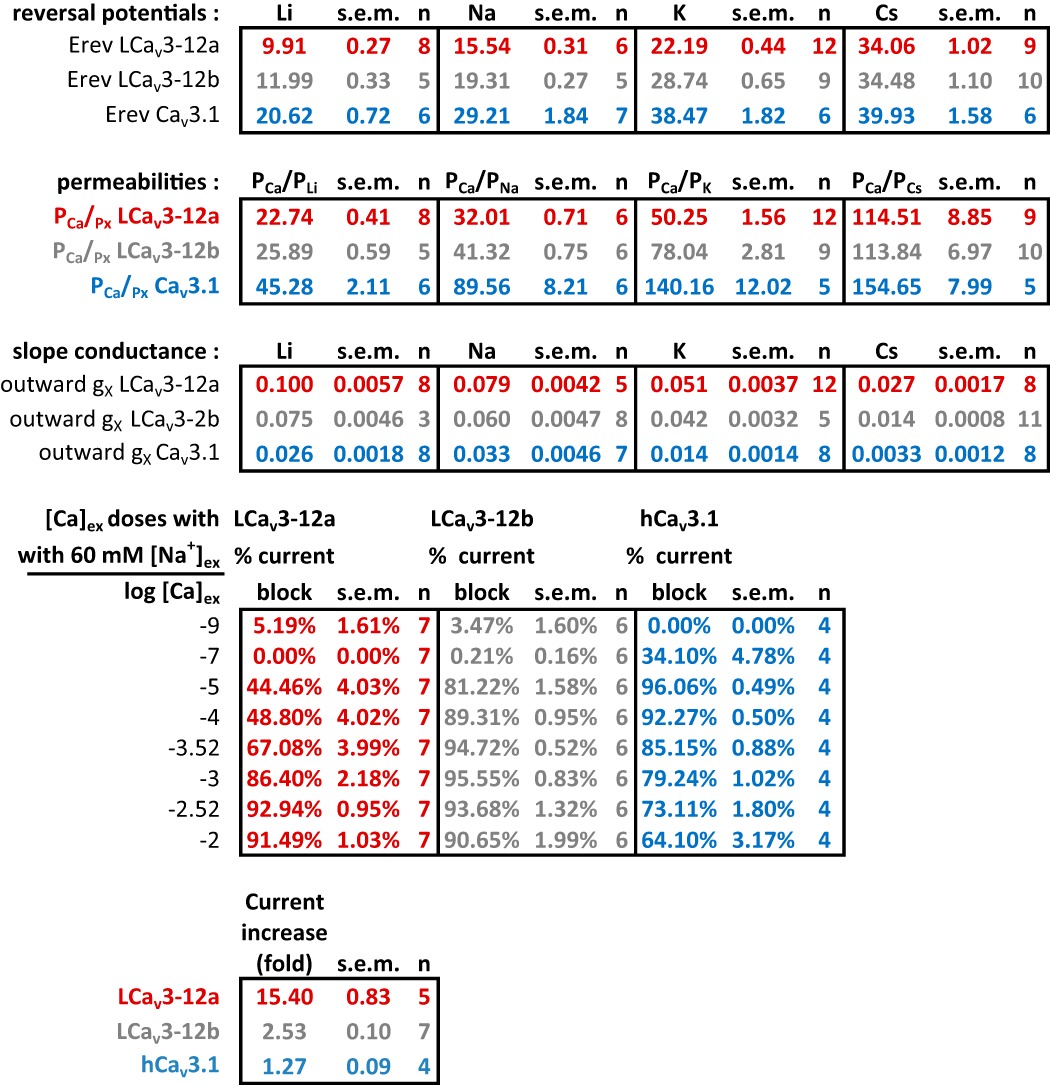
To quantify ion permeability differences, we measured peak sizes of T-type channel currents generated in bi-ionic conditions with intracellular solutions containing 100 mm of differing monovalent ions (Li+, Na+, K+, or Cs+) in the presence of external calcium ions (4 mm [Ca2+]ex). We compared the more sodium-permeant snail LCav3-12a and snail LCav3-12b isoforms to the most sodium-impermeant human T-type channel, Cav3.1. Sizes of outward currents were elicited from a −110-mV holding potential in voltage steps from −90 to +80 mV in 5-mV increments (Fig. 4A). The relative permeability of monovalent ions followed the ion selectivity model by Eisenman et al. (23), where Li+ > Na+ > K+ > Cs+, and is consistent with the decreasing permeability with increasing crystal radii of monovalent ions from Li+ < Na+ < K+ <Cs+ (Ao) = 0.60, 0.95, 1.33, and 1.69, respectively (Fig. 4, A and B). The contribution of each monovalent ion to a change in reversal potential reflects its relative permeability to the reference external calcium ion. Calculating the relative Li+, Na+, K+, and Cs+ permeability in the bi-ionic Nernst equation reveals PCa/Px with approximate averages of 23, 32, 49, and 118 for LCav3-12a channels, 26, 41, 78, and 114 for LCav3-12b channels, and 45, 90, 140, and 155 for human Cav3.1 channels (Fig. 5A). LCav3 channels with the exon 12a turret are significantly more permeable to monovalent ions than LCav3 channels bearing the exon 12b turret. Both snail channel splice variants are much more sodium-permeant than human Cav3.1, which is considered the most sodium-impermeant of the three mammalian T-type channels, Cav3.1, Cav3.2, and Cav3.3 (10, 11). L-type channels are much more selective for calcium ions, and their monovalent ion permeability is barely detectable. L-type calcium channels are 10–60-fold more selective for calcium ions than T-type channels with relative Li+, Na+, K+, and Cs+ permeability ratios reported as PCa/Px of 424, 1170, 3000, and 4200 (9, 18). Another way to gauge the relative permeabilities is in the linear slope conductances between voltage steps from +70 to +80 mV, where the outward monovalent ion currents possess steeper conductance slopes in current voltage relations, reflecting the much greater observed outward monovalent current through snail LCav3-12a compared with snail LCav3-12b channels or human Cav3.1 (Fig. 5B). Monovalent ions such as sodium are competing with calcium ions for pore-binding sites (18), so we measured the changing peak amplitudes of the inward-permeating sodium current in 60 mm Na+ex with 10-fold increment doses of extracellular calcium, in a range from 10−9 to 10−2 m. As calcium levels rose, there was an increased blocking of the Na+ current by calcium ions (Fig. 5C), reflecting a decrement of the sodium current with increasing external calcium. A striking difference for channels with the exon 12a turret is a weak calcium blocking effect (44.5 ± 4.0%) compared with almost a complete block for channels with exon 12b turret (81.2 ± 1.6%) or human Cav3.1 channels (96.1 ± 1.6%) at 10 μm [Ca2+]ex (Fig. 5D). Above 10 μm [Ca2+]ex, Ca2+ out-competes sodium in the pore and the observed in current rises through Cav3.1 channels as calcium reaches physiological (millimolar) levels (Fig. 5E). Current sizes through Cav3.1 rise 9.1-fold from 10 μm to 10 mm external calcium, in the presence of a constant 60 mm [Na+]ex reflecting the much greater permeability of the human Cav3.1 channel for calcium ions. The lack of calcium permeability of snail LCav3 channels is reflected in the 6.5- and 2.0-fold monotonic decline (LCav3-12a and LCav3-12b, respectively) in current size when calcium levels are rising between 10 μm and 10 mm external calcium (Fig. 5, E and F). The switch from a calcium block of Na+ current to permeation and rise of calcium currents generates a characteristic U-shaped dose-response curve over the range of calcium concentrations for mammalian T-type channels (Fig. 5C) (10). The consequence of the extracellular turret from exon 12a is to alter T-type channel ion selectivity, shifting a preference to passage of sodium ions over calcium ions especially at physiological concentrations and reducing the effectiveness of calcium ions to prevent sodium ions from permeating the channel pore. A summary of the monovalent and calcium permeability results are shown in Table 5.
FIGURE 4.
Monovalent ion (Li+, Na+, K+, and Cs+) permeability through snail LCav3 T-type channels is especially dramatic with exon 12a compared with exon 12b extracellular turrets or human Cav3. 1 channels. Inward calcium currents in 4 mm [Ca2+]ex and outward monovalent T-type channel currents were generated with intracellular solutions containing 100 mm of Li+, Na+, K+, or Cs+ and elicited from a −110-mV holding potential in voltage steps from −90 to +80 mV in 5-mV increments. A, representative currents; B, their current-voltage relationships. n values are shown in parentheses. A or B, inset, close-up of currents crossing the reversal potential, with reversal potentials labeled in B. Statistics for Fig. 4 are shown in Table 5.
FIGURE 5.
Snail LCav3 channels with exon 12a are highly permeable to sodium ion and weakly permeant to calcium ions, compared with LCav3 channels with exon 12b or human Cav3. 1 channels. A, relative permeabilities of calcium to monovalent cations (PCa/Px) generated using reversal potentials (shown in Fig. 3B) inputted into a bi-ionic Nernst equation (see under “Experimental Procedures”). B, slope conductance of outward currents (shown in Fig. 3B) measured as the linear fit of currents generated from steps between +70 and +80 mV. C, increasing block of maximal ionic current (I/Imax) in 60 mm Na+ external with increasing [Ca2+]ex from 10−9 to 10−5 m reflects the competition between sodium and calcium ions to permeate the pore. D, bar graph illustrates the weak block of LCav3-12a channel current (44%) compared with LCav3-12b (81%) and human Cav3.1 (96%) channels at 10 μm [Ca2+]ex. E, X-scale shown for calcium dose-response curves (C) limited to rise in [Ca2+]ex from 10 μm to 10 mm with corresponding bar graph (F), reflecting the dramatic fold decrease (×6.5 and ×2.0) in calcium permeability with snail LCav3-12a and -12b channels, respectively, compared with the dramatic fold increase (×9.1) in calcium current through the physiological range for external calcium ions. Statistical comparisons in A and B were done using a one-way analysis of variance combined with a Student-Newman-Keuls post hoc test with the following: *, p < 0.05; **, p < 0.01, and ***, p < 0.001; n.s., not significant. Statistics for Fig. 5 are shown in Table 5.
TABLE 5.
Monovalent ion and calcium permeability parameters for snail LCav3–12a or LCav3–12ba channel or human Cav3.1 channel currents expressed in HEK-293T cells
Sodium-permeant T-type Channel Is the Only Major Sodium Current in the Snail Heart
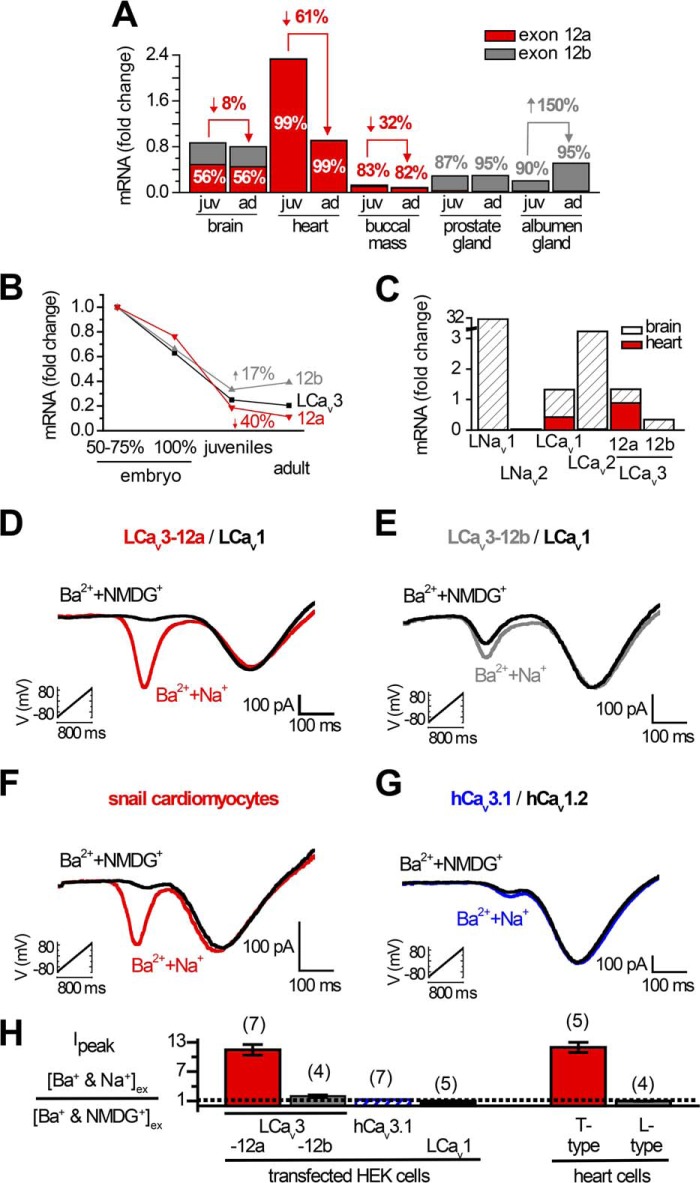
We evaluated mRNA density of the differing extracellular turrets (exon 12a and 12b) in snail tissues by qPCR to determine where we could expect to find sodium-permeable T-type channels. The highest density of T-type channels was detected in the snail heart, where the expression pattern of LCav3 is exclusively the sodium-permeant LCav3-12a isoform and where the exon 12b isoform is not detectable (Fig. 6A). Whole animal Cav3 transcript levels steeply decline during embryonic development, and LCav3-12a continues to fall from juvenile to adults (Fig. 6B), which likely is largely attributable to the sharp decline of LCav3 expression specifically in the heart (Fig. 6A). The sodium-impermeant isoform LCav3-12b is of equal abundance as LCav3-12a in the brain but is almost exclusive in secretory reproductive tissue (prostate and albumen gland), where LCav3 expression rises from juvenile to adult animals (Fig. 6A). We examined the relative density of mRNA for all relevant voltage-gated cation channels comparing the heart and brain (Fig. 6C). Snail hearts only express two cation channel genes, the L-type channel LCav1 (24) and the highly sodium permeant T-type channel LCav3-12a (Fig. 6C). Brain-enriched genes such as the classical sodium channel serving to generate the action potential upstroke (LNav1 (15)) is completely lacking in the snail heart. Also, the snail heart does not express the brain-enriched synaptic non-L-type calcium channel LCav2 (25). Note the line break in the y axis scale in Fig. 6C to accommodate the scale of the Nav1 sodium channel expression in the snail brain (∼30-fold higher than control HPRT1 gene) that is dramatically above the range of expression of the other LCav1, LCav2, and LCav3 calcium channel genes (up to ∼3-fold higher than control HPRT1 gene). The dramatic absence of expression of LCav3-12b and the highly brain-enriched LNav1 and LCav2 in the snail heart is evidence for the limited repertoire of four domain cation genes to LCav3-12a and LCav1 and a specialized signaling output in the snail heart that is highly different from the snail brain. We co-expressed and analyzed LCav1 (plus its required accessory β and α2δ subunits) and LCav3-12a channels in HEK-293T cells, which is a configuration expected to emulate the major cation channels in snail cardiomyocytes. Voltage ramps generated from −100 to +80 mV separate low voltage-activated (LVA) T-type and high voltage-activated (HVA) L-type currents in 2 mm [Ba2+]ex and impermeant [NMDG+]ex at 100 mm (black curve, Fig. 6D). The high sodium permeation through LCav3-12a channels is revealed after replacing the 100 mm NMDG+ with equimolar Na+ (red curve, Fig. 6D). In the same recording, the HVA L-type current is unchanged when external NMDG+ is replaced with Na+, reflecting the channel's barium-selective currents and a lack of sodium permeability for L-type channels (Fig. 6D). A voltage ramp recording of adult snail cardiomyocytes (Fig. 6F) generates a remarkably similar profile of the sodium-permeant LVA T-type current and sodium-impermeant HVA L-type current. The ∼11-fold increase in size of the combined sodium and barium LVA current, upon addition of Na+ ions, is identical in cells transfected with LCav3-12a channels and the endogenous LVA T-type current in cardiomyocytes (Fig. 6H). On the contrary, the ∼2-fold current increase in HEK cells transfected with LCav3-12b (Fig. 6, E and H) or human Cav3.1 (Fig. 6, G and H) is not reflected in the T-type currents of cardiomyocytes, corroborating the qPCR data that LCav3-12a is the exclusive T-type isoform in the snail heart. The similarity between cardiomyocytes and transfected LCav3-12a/LCav1 channels is also revealed in the makeup of current ensembles initiated from differing holding potentials (Fig. 7A). We used 2 mm Ba2+ and 100 mm Na+ external solution to separate the kinetics of the slow barium L-type currents from the fast T-type currents mostly carried by sodium ions. A holding potential of −60 mV will inactivate T-type sodium currents, leaving a residual L-type current (gray color) and a mostly pure T-type sodium current (red color) from the difference current generated from −110 and −60 mV holding potentials (Fig. 7A). We examined drug responses of currents in cardiomyocytes elicited by voltage ramp (Fig. 7, B and C). Pharmacologically, we could separate the L-type from the T-type current using 1 μm isradipine, but both the L-type and T-type currents were similarly blocked by nickel and mibefradil (Fig. 7, B and C). The sum of the electrophysiological data for cardiomyocytes (current kinetics, drug block, and sodium permeability) (Figs. 6 and 7) is consistent with the mRNA expression profiles in the heart, which indicate an exclusive expression of sodium-permeant LCav3-12a and sodium-impermeant LCav1 channels.
FIGURE 6.
Snails express two cardiac cation currents, the highly sodium-permeant T-type channel LCav3 and the calcium-selective L-type channel LCav1. A–C, quantitative RT-PCR standardized to Lymnaea HPRT1 control gene. A, relative mRNA expression levels of exon 12a and 12b in juvenile (juv) and adult (ad) snails, reflecting the almost exclusive expression of exon 12a in the snail heart and exon 12b in prostate and albumen glands. B, dramatic decline in LCav3 channel expression in whole animals can mostly be attributed to the decline of LCav3-12a expression in the developing heart. C, snail hearts lack mRNA expression of any sodium channel gene (LNav1 and LNav2). Snail hearts also lack any expression of the LCav2 synaptic non-L-type channel or the Cav3 T-type channel gene with exon 12b. Note the break in the y axis scale, illustrating that the range of expression of the sodium channel gene of LNav1 in the snail brain is ∼30-fold higher than the control HPRT1 gene, compared with the low levels of expression of the other calcium channel genes (which vary up to ∼3-fold higher than the control HPRT1 gene). D–G, ramp protocols (−110 to +100 mV in 1 s) carried out in the presence of external solutions as follows: 2 mm Ba2+ and 100 mm Na+ ions or 2 mm Ba2+ and 100 mm NMDG+ reveal a covert sodium-permeant, T-type current superimposed on the Ba2+ current in cardiomyocytes in the presence of a sodium-impermeant, L-type current (F). The sodium-permeant T-type currents in cardiomyocytes are indistinguishable from mammalian HEK-293T cells transfected with LCav3 with exon 12a with co-expression of LCav1 and accessory subunits (α2δ and β2a) (D). E, LCav3 with exon 12b; G, human Cav3.1 has some Na+ permeability but is not as Na+-permeant as the LCav3-12a variant expressed in cardiomyocytes. H, bar graph ± S.E., including sample data in D–G.
FIGURE 7.
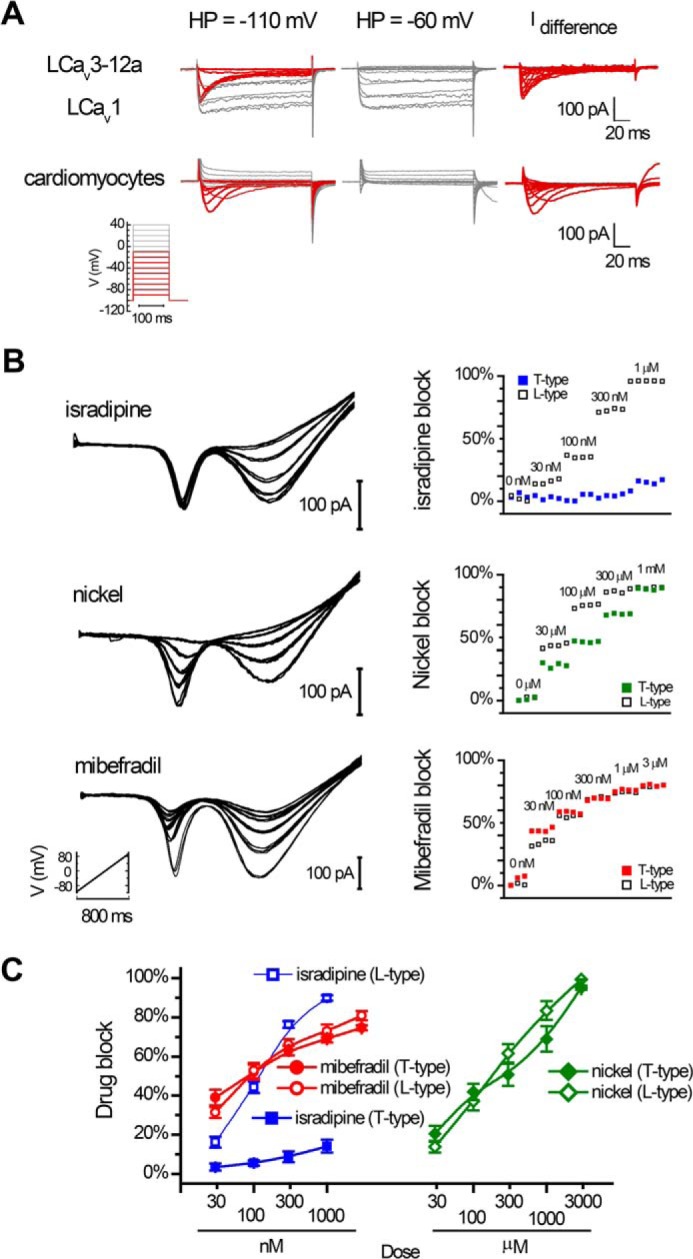
Separation of snail cardiac LCav3-12a (T-type) and LCav1 (L-type) currents using holding potentials and drugs (isradipine, mibefradil, and nickel) in whole cell patch clamp recording. A, fast T-type currents (red color) isolated by difference current in 2 mm [Ba2+]ex and 100 mm [Na+]ex with a holding potential (HP) of −110 mV versus −60 mV in whole cell recording of native LCav3/LCav1 channel currents in snail cardiomyocytes. B, ramp protocols (−110 to +100 mV in 800 ms) carried out in the presence of external solutions 2 mm [Ba2+]ex and 100 mm [Na+]ex and differing doses of drug perfused on cardiomyocytes. Isradipine at 1 μm is a specific blocker of L-type currents, whereas mibefradil and nickel are nonspecific blockers of L-type and T-type currents. C, dose-response curves ± S.E. for isradipine (n = 6), mibefradil (n = 8), and nickel (n = 8); sample data are shown in B.
Alternatively Spliced Turrets for Generating Sodium-permeable T-type Channels Are Widespread in Protostome Invertebrates
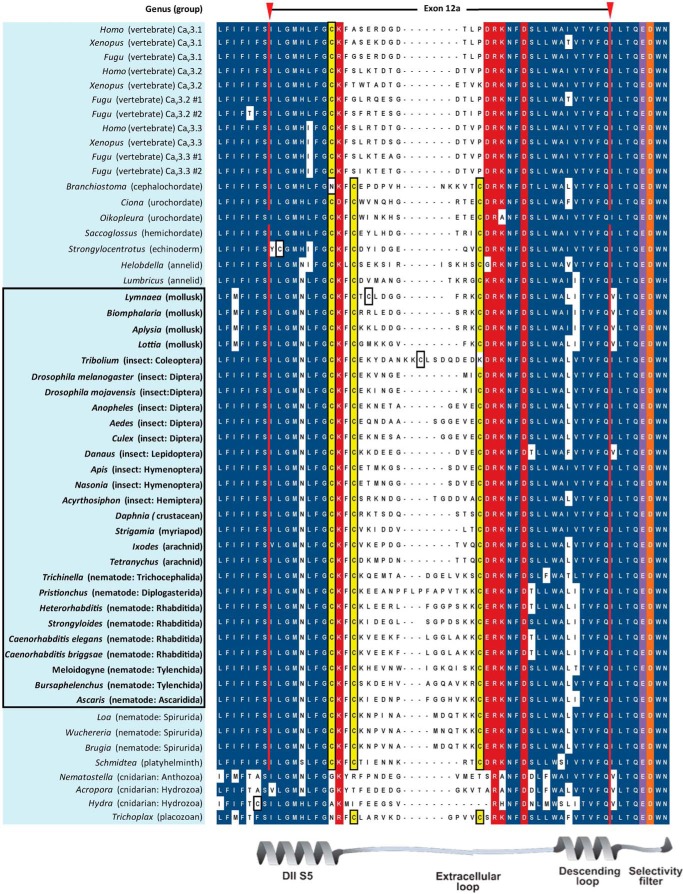
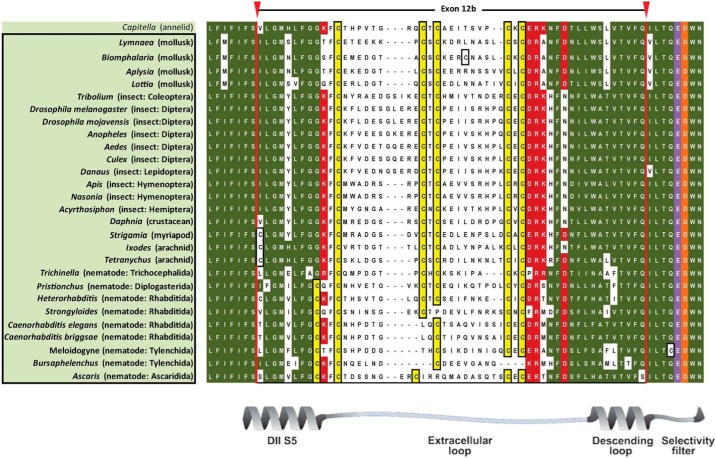
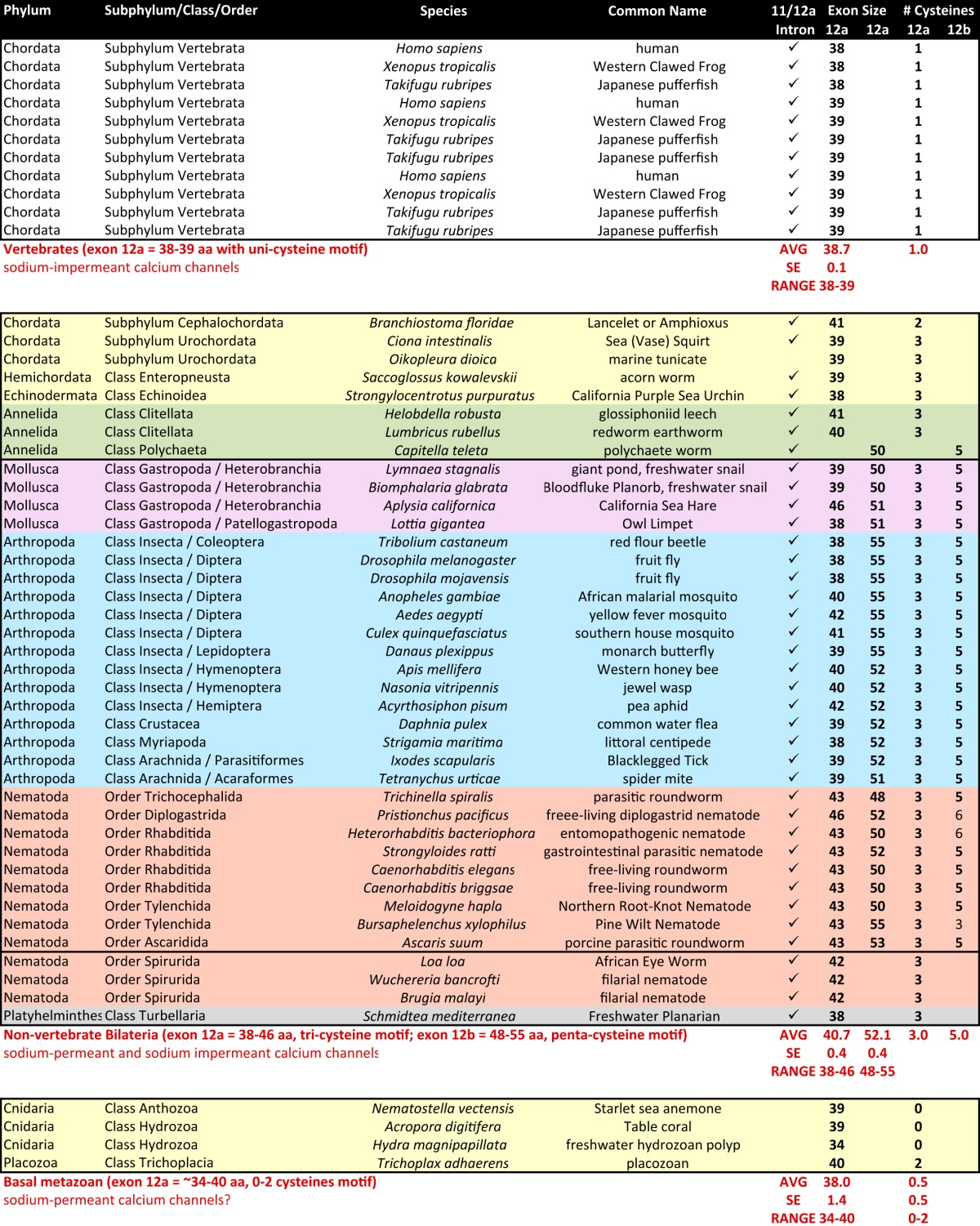
Analyses of Cav3 genomic sequences reveal that alternative splicing of exon 12 that we discovered in the snail L. stagnalis is widely present but limited to protostomes (i.e. non-echinoderm invertebrates) (Figs. 8 and 9 and Table 6). Exon 12a is characteristically short (38–46 aa long, average = 40.7 aa) with a nearly invariant tri-cysteine structure, CXXC … C (Fig. 8). Exon 12b has a penta-cysteine structure C … CXC … CXC (most protostomes) or CXXC … C … CXC (some nematodes) and is always longer (range, 48–55 aa long; average = 52.1 aa) than exon 12a (Fig. 9). The three vertebrate T-type channels, Cav3.1, Cav3.2, and Cav3.3, have an exon 12 with only one conserved cysteine (Fig. 8) compared with three and five cysteines in invertebrate exons 12a (Fig. 8) and 12b (Fig. 9), respectively. Paradoxically, the vertebrate exon 12 resembles the shorter exon 12a of invertebrates in size (38–39 aa long, average = 38.7 aa) which in snail LCav3 we have shown imparts the properties of extreme sodium ion permeability for exon 12a-containing T-type channels.
FIGURE 8.
Aligned amino acid sequences of exon 12A in metazoan Cav3 channel homologs. Exon 12a codes for novel extracellular turret residues from the middle of domain II, segment five through the descending pore helix to five amino acids upstream of selectivity filter glutamate (purple) residue, and invariant aspartate residue after it, conserved in all calcium channels (orange residue). Exon 12a is flanked by conserved charged residues (red), surrounding a set of variable amino acids, outside of very conserved cysteine (yellow) residues. Exon 12a is ∼40 aa with a tri-cysteine motif (CXXC … C). Vertebrate T-type channels resemble the size of exon 12A in length (∼39 aa) but are missing the most downstream two cysteines of invertebrate exon 12a. Animals bearing T-type channels below the protostome invertebrates (placozoan and cnidarians) have zero or two cysteines in exon 12a and lack an exon 12b. Most protostome invertebrates possess an alternative exon 12b (see Fig. 9) turret in addition to exon 12a, and these are highlighted and outlined.
FIGURE 9.
Aligned amino acid sequences of exon 12B found in protostome Cav3 channel homologs. Exons 12B code for novel extracellular turret residues from the middle of domain II, segment five through the descending pore helix to five amino acids upstream of selectivity filter glutamate (purple) residue, and invariant aspartate residue after it, conserved in all calcium channels (orange residue). 12b exons are flanked by conserved charged residues (red), surrounding a set of variable amino acids, outside of very conserved cysteine (yellow) residues. Exon 12b is ∼52 aa with a penta-cysteine motif (C … CXC … CXC) or more rarely (CXXC … C . . CXC), in some nematodes. Most protostome invertebrates possess a shorter alternative exon 12a turret (see Fig. 8) in addition to longer exon 12b, and these are highlighted and outlined.
TABLE 6.
Features of exons 11 and 12 of Cav3 channel homologs in sequenced metazoan genomes
DISCUSSION
Unique Splicing of Exon 12 in Invertebrate T-type Channels
We have functionally characterized the first nonvertebrate T-type calcium channel (13), which reveals a high conservation of quintessential features of vertebrate Cav3.1 and Cav3.2 T-type channels, including a similar low voltage range of activity with a peak current at −40 to −35 mV, with similar rapid activation and inactivation kinetics and slow deactivation kinetics. Also conserved is developmentally regulated splicing where T-type channels that lack exon 25c are highly down-regulated from embryo to adult animals, leading to a battery of biophysical changes among LCav3, Cav3.1, and Cav3.2 (14). We also found that the presence of optional exon 8b in the I-II linker specifically down-regulates the level of protein expression of snail T-type channels in a manner consistent with Cav3.1 (14). Here, we describe unique exon splicing that generates highly sodium-permeant T-type channels utilizing an alternative exon 12a, which spans the extracellular turret of domain II upstream of the highly conserved EEDD selectivity filter of T-type channels.
We show that invertebrate LCav3 T-type channels will generate large outward currents in the presence of 100 mm intracellular monovalent ions accompanying the large driving force at high voltage steps much more so than human Cav3.1, while also generating an inward calcium current in the presence of 4 mm extracellular calcium ions at voltage steps below the reversal potential (Figs. 2 and 4). Permeability of monovalent ions decreases with increasing ion diameter size where Li+ > Na+ = K+ > Cs+ > NMDG+. All T-type channels pass sodium ions when external calcium concentration is at exquisitely low levels (<10 μm external calcium ions). However, the sodium current through human Cav3.1 has less physiological relevance because it is almost completely blocked (96%) by low (10 μm) external calcium. With rising external calcium concentrations to physiological levels, nearly all of the current through human Cav3.1 is carried by calcium (Fig. 5). The snail T-type channel isoforms, especially those harboring exon 12a, are not calcium-selective like human Cav3.1. Calcium ions are not effective at blocking the sodium current at 10 μm external calcium, with snail T-type channels with exon 12a resisting the calcium block (44%), much more than isoforms with exon 12b (81%) or human Cav3.1 (96%) (Fig. 5). Increasing external Ca2+ above 10 μm to physiological (millimolar) levels does not generate the larger calcium currents for LCav3 as is reflected in the “U” shape calcium dose-response curve of human Cav3.1 (10). Rather, the total current size dramatically falls monotonically (6.5-fold) for snail channels harboring exon 12a reflecting their reduced affinity for Ca2+ in the pore that allows for greater permeability of Na+ ions (Fig. 5).
The extreme sodium permeability of LCav3-12a is evident in voltage ramp-generated currents with amplitudes that are ∼11-fold greater when external Na+ replaces impermeant cation NMDG+ in the presence of barium. The sodium permeability is not evident in the co-expressed snail L-type (LCav1) channel in the same voltage ramp and a much reduced (∼2-fold instead of ∼11-fold) sodium permeability for LCav3 channels harboring exon 12b (Fig. 6). Snail heart cells only express T-type channels with exon 12a and express the same sodium-permeable T-type current as the in vitro expressed gene, resembling its fast kinetics, low voltage of activation, nickel (millimolar) and mibefradil (millimolar) sensitivity, and isradipine (micromolar) insensitivity (Figs. 6 and 7).
Unique Functions for Highly Sodium-permeant (Exon 12a) and Less Sodium-permeant (Exon 12b) T-type Channels
The distinct tissue expression patterns of LCav3 exons 12a (heart and muscle) and 12b (secretory glands) suggest specialized roles of different permeable T-type channels in different cell types. T-type channel transcript levels steeply decline during embryonic development through adulthood, reflected by a sharp decline in LCav3-12a expression in juvenile to adult heart (Fig. 6). A down-regulation of LCav3-12a may relate to allometric scaling during development, where more prominent LCav3 currents are found in smaller animals (non-adults) coinciding with faster heart rhythms (26). Expression of LCav3-12a isoform indicates a significantly slower deactivation rate and faster recovery rate from inactivation when compared with LCav3-12b (Fig. 3). This is consistent with LCav3-12a and adaptations in the snail heart, where a slower deactivation would serve to prolong the repolarization phase of the cardiac action potential, and a faster inactivation recovery prevents rundown between heart beats due to cumulative inactivation. Snails have only one (nonspecialized) sodium channel gene (Nav1), compared with 10 in vertebrates, and this gene is only expressed in the snail brain and is not expressed in the snail heart (Fig. 6) (15). Thus, LCav3-12a is the only voltage-gated sodium channel generating pacemaker currents in ventricular cells of the primitive two-chambered snail heart, which lacks the appearance of specialized vertebrate sodium channels, like Nav1.5 and other neuronal Nav channels for fast conduction of heart rhythms.
The more calcium-selective isoform LCav3-12b is of equal abundance to LCav3-12a in the brain, and it is the almost exclusive transcript associated with reproductive tissue of the hermaphroditic snail (prostate = male organ and albumen gland = female organ) (Fig. 6). The rise in transcripts of LCav3-12b from juvenile to adults (Fig. 6) coincides with sexual maturation where the albumen gland takes up a role secreting factors that facilitate egg mass formation (27). Our observations of a much faster deactivation rate and a slower recovery rate from inactivation of LCav3-12b channels promotes the typical pulsatile, burst firing of vertebrate T-type channels, where the rise in intracellular calcium may contribute to pacemaking and possibly also to excitation-secretion coupling (14).
Snail LCav3 (like its vertebrate counterparts) also has prominent “window” currents, where an estimated 1–2% of available channels are open at rest (13). LCav3-12a variants are highly abundant in the brain and likely contribute to reported persistent sodium currents (28, 29) serving as a significant sodium leak conductance (30). Even though highly sodium-permeant T-type channels are likely widespread in invertebrates, it would be hard to discern them with voltage-gated sodium channel currents if you were not looking for them. Drugs are not selective in most invertebrates, like snails that are insensitive to classical sodium blockers such as tetradotoxin (15), and neither are snail T-type channels easily discernible by Ni2 block (13). Some species, including nematodes like Caenorhabditis elegans, lack a sodium channel (Nav) gene in their genomes (31), which means that their sodium-permeant T-type channels with exon 12a likely serve as a critical source for generating sodium-dependent spikes in these organisms. Most invertebrates do contain gene homologs of hyperpolarization-activated cyclic nucleotide-gated channels, which presumably contribute to sodium currents in invertebrate pacemaker rhythms (32). A hyperpolarization-activated cyclic nucleotide-gated channel homolog is also notably absent in C. elegans (31, 32), which limits the possibilities of sodium current generating spikes in these animals from the conventional palette of ion channel genes. We suggest that T-type sodium channels have a role to play in lieu of sodium channels in C. elegans.
Evolutionary Adaptations of the Domain II Turret
A pattern of evolution of the extracellular turret in domain II of T-type channel is evident in their phylogeny (Fig. 10). The Cav3 T-type channel class first appeared in the most primitive multicellular animals, as low voltage-activated calcium-selective counterpart to the Cav1 and Cav2 calcium channels. We have isolated and characterized the singleton T-type channel, TCav3, cloned from the most primitive, extant relative to have a T-type channel, the placozoan Trichoplax, which is multicellular but lacks a tissue level organization. TCav3 generates calcium currents consistent with other basal metazoans, such as the hydrozoan jellyfish where it serves as the only calcium current generated in muscle action potentials (33). All basal metazoans have short exon 12 turrets with a small number of cysteines (0 to 2). Insertion of an intron before exon 12 in primitive protostomes, the platyzoa (acoelomates), allowed a duplication of exon 12 to generate dual turrets, one highly sodium-permeant tri-cysteine turret (exon 12a) and a more calcium-selective penta-cysteine turret (exon 12b) in ecdysozan and lophotrochozoan protostomes. Associated with protostomes is the appearance of the coelom and internal organs such as the heart, which require fast sodium-dependent spikes to coordinate the activities of their body organs. Nav1 channels in vertebrates expanded to 10 Nav1 channel genes fulfilling a universal spike generator role inside and outside the brain. Invertebrates, however, have a singleton Nav1 sodium channel gene, which is absent in many species (e.g. C. elegans) and is not abundant outside the nervous system (e.g. Lymnaea snail). Outfitting sodium-permeant pores in T-type channels is a likely adaptation in invertebrates in lieu of spikes generated by Nav1 channels.
FIGURE 10.
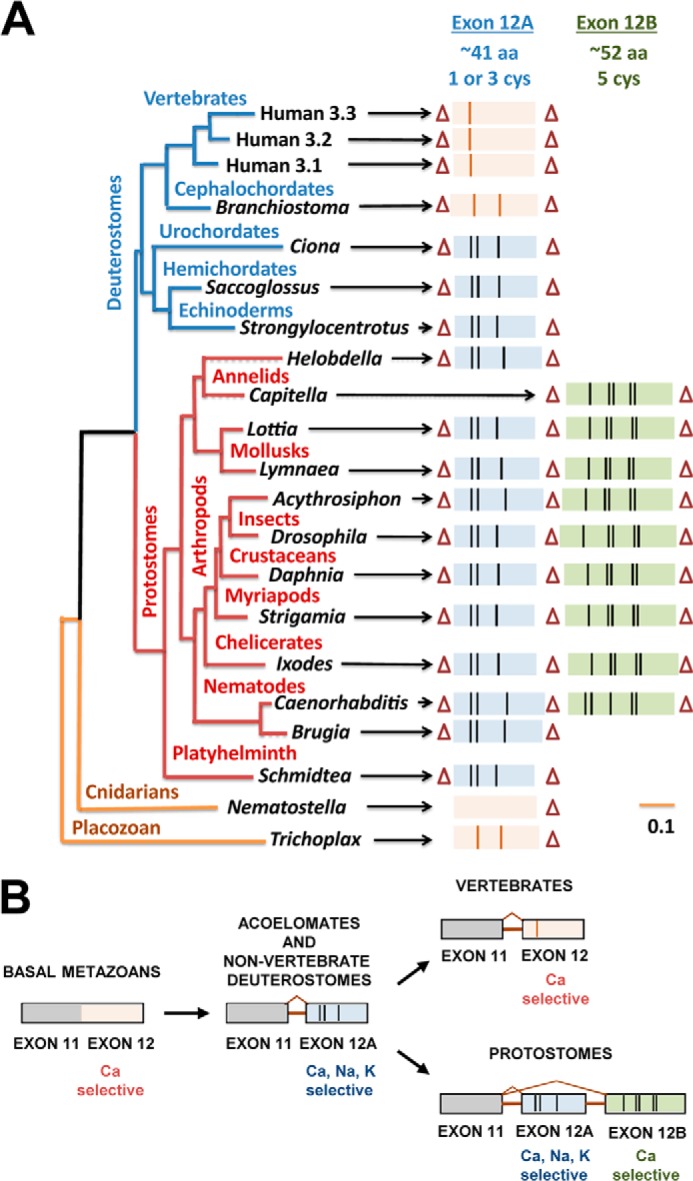
Phylogeny and proposed evolution for exon 12, which governs monovalent ion permeability in Cav3 T-type channels. A, gene tree illustrates the conservation of exon 12a (smaller, blue exons) and exon 12b (larger, green exons) with vertical black lines indicating location of cysteine residues. Introns are indicated by triangles. B, proposed evolutionary progression; an intron separating exons 11 and 12; appearance of a tri-cysteine motif characteristic of exon 12a highly sodium-permeant isoforms; an alternative exon 12b with a penta-cysteine motif characteristic of less sodium-permeant channels in protostomes. Evolution of a uni-cysteine motif in exon 12 of Cav3 channels that supports a more calcium-selective channel in vertebrates is shown.
Importance of High Field Strength Site in Selectivity Filter Governing Calcium and Sodium Permeation
Ion channels have evolved unique ion pores that are selective for the two major external cations, calcium ions and sodium ions. Flexible side chains of carboxylate oxygens from negatively charged residues are proposed to bind cations with high affinity, and permeation is facilitated by incoming ions attracted to the negatively charged extracellular surface of the pore (34). These charged residues form a “high field strength site” at the outer end of the selectivity filter in equivalent position of the selectivity filter TLESWSM in the re-entrant pore contributed by equal domains of the homomultimeric prokaryotic sodium channel, NavAb (6). Backbone carbonyl residues of the preceding two residues TLESWSM form the two innermost ion coordination sites for the sodium channel's selectivity filter (6). Replacement with three aspartates TLDDWSD in the selectivity filter transforms the sodium-selective pore into a calcium-selective one in prokaryotic channels (35, 36). Significantly, sodium and calcium ions enter, reside, and exit through the selectivity filter in a semi- or fully hydrated form through a much broader pore than a potassium-selective pore, and where strategic positioning of a limited number of residues can tip the balance in favor of whether mostly sodium or calcium ions or a mixture of both ions permeate (35). The sodium and calcium pore is a departure from the long and narrow potassium selectivity filter (TVGYG), which is a Goldilocks' “just right” fit for the dehydrated potassium ion, which underlies the potassium channels' nearly exclusive selectivity for potassium ions (5).
Identity of the single amino acid base of the equivalent field strength glutamate residue in prokaryotic sodium channels serves as the basis for categorizing metazoan sodium and calcium channels. This position is contributed by four nonequivalent residues of domains I–IV, in four domain-containing channels that contain a lysine in the domains III or II as DEKA (or DKEA) for Nav1 sodium-selective channels and lack a lysine residue in the calcium-selective Cav1 (EEEE), Cav2 (EEEE), and primordial Nav1 (DEEA) or invertebrate Nav2 channels, DEEA (37). The importance of this residue position is evident in experiments where sodium channels take on the high calcium selectivity of calcium channels after replacement of the DEKA high field strength site position with EEEE and vice versa (8, 38).
Yet it is clear that the high field strength site in the selectivity filter is not the only critical feature to govern permeation through T-type channels. All T-type channels have a unique high field strength site residue of EEDD, but when mutated to more resemble calcium-selective Cav1 and Cav2 channels (as EEED or EEDE), T-type channels become paradoxically more permeable and not less permeable to sodium ions (22). Despite the consistency in the EEDD high field strength site, T-type channels vary greatly in their sodium permeability that ranges from 20, 25, and 40% of the current for Cav3.1, Cav3.2, and Cav3.3 channels, respectively, for mammalian channels (1) and from 50 and >90% of the current through snail LCav3 channels with alternative novel extracellular S5-P turret regions in domain II. T-type channels also vary widely in their relative permeation of calcium ions as the charge carrier versus barium ions, which is expected to reflect a variable calcium affinity for the pores of T-type channels (10). This is in contrast to other calcium channels. Macroscopic barium currents are consistently ∼2-fold the size of calcium currents, regardless of whether these are invertebrate Cav1 or Cav2 channels or vertebrate Cav1 or Cav2 homologs (1).
So, how do invertebrate T-type channels become so highly sodium-permeant using alternative splicing of extracellular regions outside the selectivity filter of the pore in domain II? Snail exons 12a and 12b differ in the extracellular turret, but there are also sequence differences that may be important in the descending helix just upstream of the selectivity filter in domain II. We suggest that changes in the descending helix and the extracellular turret could contribute to the altered sodium permeation through T-type channels.
Potential Roles for Changes in the Descending Helix
The distal end of the descending helix away from the selectivity filter has three variable amino acid residues as follows: AIV, ALI, and SLV in Cav3.1 and snail LCav3-12a and LCav3-12b channels, respectively. Negatively charged residues within the selectivity filter form transient binding partners outside the selectivity filter in structural intermediates of the calcium channel pore. Changes in the descending helix may be sufficient to reorient a selectivity filter residue, altering the relative permeation of sodium and calcium ions.
Potential Similarities of the T-type Channel Turret with the Extracellular Cap Domain of K2P Channels
There are possibilities of analogy between the extracellular turret of domain II in T-type channels and the crystal structures of turret regions from two-pore potassium leak channels (K2P) channels (40, 41).
The first of two pores of K2P channels has a uniquely extended turret of 56 amino acids, which normally ranges between 5 and 20 amino acids in other potassium channels. The turret forms a highly ordered extracellular cap, reminiscent of an A-frame, extending 35 Å above the lipid membrane with a cysteine residue at its apex, which cross-links to the apex of the A-frame of a second K2P subunit, in the 2-fold symmetrical K2P channel (40, 41). The dual A-frames perpendicular to each other form a space-filled “carafe plug” that restricts ion and drug access to the channel pore from above (40, 41). K+ ion passage is limited to two funnel-shaped side portals contained in the extracellular cap domain (40, 41). Walls along the side portals are lined with negatively charged residues and variable sequences that could serve as a variable pre-filter for cations channeling to the selectivity filter below (40, 41). The extracellular cap domain approaches close to the selectivity filter where the negative C-terminal ends of the helical dipole from an extracellular helix lie just above and to the side of the selectivity filter, before sharply pivoting away from the selectivity filter at a conserved glycine residue before meeting with the descending pore helix that leads to the selectivity filter (40, 41).
The turret in domain II of T-type channels is the shortest of the four extracellular turrets preceding the pore helices that descend into the re-entrant pore and thus is likely restricted to the lower quadrant of the outer vestibule where it may likely encounter the selectivity filter (1). The constraints on size and the conserved framework of uni-, tri-, and penta-cysteine exon 12 in vertebrates and exons 12a and 12b of invertebrate T-type channels are consistent with these specialized turret variants changing the landscape in the external vestibule (analogously to K2P channels) and forming regulatory structures that make specific contacts within the pore. Differing turrets are expected to bias the selectivity filter allowing the more favored passage of monovalent over divalent cations in a pore that is expected to be similar to the wide bacterial sodium channel (e.g. NavAb (6) and NavRh (42)), which can accommodate more than one semi-hydrated ion at its more constrictive locus (the selectivity filter residues).
Possible Similarity of the Extracellular Turret Domains in K2P, KIR, and T-type Channels as Toxin Defense Shields
Eukaryotic inward-rectifying potassium channels (KIR) also possess an extended turret region, albeit a minor external appendage compared with K2P channels (43). Nonetheless, the turret of KIR channels is substantial enough to alter the external surface landscape and to contribute a unique resistance of these channels to classical invertebrate toxins (e.g. from snake, spider, and scorpion venom), which typically block voltage-gated potassium channels when applied from the outside of the channels (44–46). Resistance to external toxins is a shared property with K2P channels likely due to shielding with the elaborate extracellular cap domain of K2P channels (47, 48). Unique extracellular turret domains may also explain why there are not invertebrate pore-blocking toxins (from cone snail, spider, and snake venom) for Cav3 T-type channels compared with other (Cav1 and Cav2) calcium channels, where numbers of specific high affinity toxins have been discovered (49). The extensive extracellular turret regions in K2P (40, 41), eukaryotic KIR (43), and T-type channels are highly variable in sequence outside of key structural residues (e.g. cysteines, glycine, and prolines) or in the positioning of particular charged residues (e.g. arginine and lysine). One can imagine that some sequence variation in extracellular regions may be adaptive as counter-measures against the arsenal of specific channel toxins from the venom of invertebrate predators.
Other Examples of Alternative Splicing That Generates Altered Ion Selectivity of Ion Channels
Alternative splicing that generates permeation differences in ion channels is not common. We discovered alternative exons in domain II of NALCN channels that alter the high field strength residue to generate calcium-like (EEEE) or sodium-like (EKEE) pores (of flatworms, mollusks, annelids, echinoderms, and hemichordates) (19, 50). Interestingly, some arthropods generate alternative pores in NALCN channels differently, using an alternative exon in domain III to generate calcium-like (EEEE) or sodium-like (EEKE) pores in their NALCN channels (19, 50). Finally, a completely unique approach to altering ion selectivity is the use of alternative translational start sites to generate a truncated isoform in K2P2.1 channels that are sodium-permeable, to produce offsetting depolarizing currents in a channel type that typically only serves up hyperpolarizing potassium leak currents (39).
Conclusions
T-type channels have varying sodium permeability in the presence of an extracellular veil of novel turret residues in domain II, which encode merely ∼1% of the T-type channel protein. Each splice isoform is exclusively expressed in particular tissues, where they serve highly different functions conducting variable sodium and calcium currents. It is surprising that only a few amino acids separate highly sodium- or more calcium-conducting channels, given that the ions have such different roles, where Na+ ions are relatively inert and are much more abundant serving mostly an electrogenic role, and Ca2+ ions are maintained at very low levels in cells due to cytotoxicity, and serve as an exquisitely sensitive signaling molecule. Extracellular concentrations of sodium ions are on the scale of approximately fifty times that of Ca2+ ions or K+ ions, so T-type channels that are permeant to monovalent ions are generating large currents and steeper membrane depolarizations than typical calcium-selective channels. They also generate larger currents than high voltage-activated calcium channels because of the greater driving force for T-type channels operating at their much lower voltage range.
It is remarkable that T-type channels evolved a mechanism outside the conventional selectivity filter to alter their ion selectivity. It is also intriguing to understand the physiological context (e.g. for brain, heart and glands) in which T-type channels retain similar biophysical properties but possess dramatically different ion selectivities for sodium and calcium ions.
Acknowledgments
We are grateful to Wic Wildering and Petra Hermann for their generous shipments of L. stagnalis to complete this work and Martin Rioux for assistance in generating preliminary materials for the project.
This work was supported in part by a Heart and Stroke Foundation of Canada grant-in-aid and Natural Sciences and Engineering Research Council of Canada discovery grant (to J. D. S.).
This article was selected as a Paper of the Week.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) JX292155.
- qPCR
- quantitative PCR
- aa
- amino acid
- MΩ
- megohm
- NMDG+
- N-methyl-d-glucamine
- LVA
- low voltage-activated
- HVA
- high voltage-activated.
REFERENCES
- 1. Senatore A., Guan W., Spafford J. D. (2014) Cav3 T-type channels: Regulators for its gating, membrane expression and cation selectivity. Pflugers Arch. 466, 645–660 [DOI] [PubMed] [Google Scholar]
- 2. Senatore A., Monteil A., van Minnen J., Smit A. B., Spafford J. D. (2013) NALCN ion channels have alternative selectivity filters resembling calcium channels or sodium channels. PLoS One 8, e55088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Senatore A., Zhorov B. S., Spafford J. D. (2012) CaV3 T-type calcium channels. WIREs Membr. Transp. Signal. 1, 467–491 [Google Scholar]
- 4. Strong M., Chandy K. G., Gutman G. A. (1993) Molecular evolution of voltage-sensitive ion channel genes: On the origins of electrical excitability. Mol. Biol. Evol. 10, 221–242 [DOI] [PubMed] [Google Scholar]
- 5. Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., MacKinnon R. (1998) The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280, 69–77 [DOI] [PubMed] [Google Scholar]
- 6. Payandeh J., Scheuer T., Zheng N., Catterall W. A. (2011) The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCusker E. C., Bagnéris C., Naylor C. E., Cole A. R., D'Avanzo N., Nichols C. G., Wallace B. A. (2012) Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat. Commun. 3, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinemann S. H., Terlau H., Stühmer W., Imoto K., Numa S. (1992) Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 356, 441–443 [DOI] [PubMed] [Google Scholar]
- 9. Hess P., Lansman J. B., Tsien R. W. (1986) Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J. Gen. Physiol. 88, 293–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shcheglovitov A., Kostyuk P., Shuba Y. (2007) Selectivity signatures of three isoforms of recombinant T-type Ca2+ channels. Biochim. Biophys. Acta 1768, 1406–1419 [DOI] [PubMed] [Google Scholar]
- 11. Khan N., Gray I. P., Obejero-Paz C. A., Jones S. W. (2008) Permeation and gating in CaV3.1 (α1G) T-type calcium channels effects of Ca2+, Ba2+, Mg2+, and Na+. J. Gen. Physiol. 132, 223–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kostyuk P. G., Mironov S. L., Shuba Y. M. (1983) Two ion-selecting filters in the calcium channel of the somatic membrane of mollusc neurons. J. Membr. Biol. 76, 83–93 [Google Scholar]
- 13. Senatore A., Spafford J. D. (2010) Transient and big are key features of an invertebrate T-type channel (LCav3) from the central nervous system of Lymnaea stagnalis. J. Biol. Chem. 285, 7447–7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senatore A., Spafford J. D. (2012) Gene transcription and splicing of T-type channels are evolutionarily conserved strategies for regulating channel expression and gating. PLoS One 7, e37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yeoman M. S., Brezden B. L., Benjamin P. R. (1999) LVA and HVA Ca2+ currents in ventricular muscle cells of the Lymnaea heart. J. Neurophysiol. 82, 2428–2440 [DOI] [PubMed] [Google Scholar]
- 16. Brezden B. L., Yeoman M. S., Gardner D. R., Benjamin P. R. (1999) FMRFamide-activated Ca2+ channels in Lymnaea heart cells are modulated by “SEEPLY,” a neuropeptide encoded on the same gene. J. Neurophysiol. 81, 1818–1826 [DOI] [PubMed] [Google Scholar]
- 17. Senatore A., Boone A. N., Spafford J. D. (2011) Optimized transfection strategy for expression and electrophysiological recording of recombinant voltage-gated ion channels in HEK-293T cells. J. Vis. Exp. 19, 2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hille B. (2001) Ion Channels of Excitable Membranes, 3rd Ed., pp. 441–470, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 19. Senatore A., Monteil A., van Minnen J., Smit A. B., Spafford J. D. (2013) NALCN ion channels have alternative selectivity filters resembling calcium channels or sodium channels. PLoS One 8, e55088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dawson T. F., Boone A. N., Senatore A., Piticaru J., Thiyagalingam S., Jackson D., Davison A., Spafford J. D. (2014) Gene splicing of an invertebrate β subunit (LCavβ) in the N-terminal and HOOK domains and its regulation of LCav1 and LCav2 calcium channels. PLoS One, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McComb C., Varshney N., Lukowiak K. (2005) Juvenile Lymnaea ventilate, learn and remember differently than do adult Lymnaea. J. Exp. Biol. 208, 1459–1467 [DOI] [PubMed] [Google Scholar]
- 22. Talavera K., Staes M., Janssens A., Klugbauer N., Droogmans G., Hofmann F., Nilius B. (2001) Aspartate residues of the Glu-Glu-Asp-Asp (EEDD) pore locus control selectivity and permeation of the T-type Ca2+ channel α(1G). J. Biol. Chem. 276, 45628–45635 [DOI] [PubMed] [Google Scholar]
- 23. Eisenman G., Sandblom J. P., Walker J. L., Jr. (1967) Membrane structure and ion permeation. Study of ion exchange membrane structure and function is relevant to analysis of biological ion permeation. Science 155, 965–974 [DOI] [PubMed] [Google Scholar]
- 24. Senatore A., Boone A., Lam S., Dawson T. F., Zhorov B., Spafford J. D. (2011) Mapping of dihydropyridine binding residues in a less sensitive invertebrate L-type calcium channel (LCa v 1). Channels 5, 173–187 [DOI] [PubMed] [Google Scholar]
- 25. Huang X., Senatore A., Dawson T. F., Quan Q., Spafford J. D. (2010) G-proteins modulate invertebrate synaptic calcium channel (LCa(v)2) differently from the classical voltage-dependent regulation of mammalian Ca(v)2.1 and Ca(v)2.2 channels. J. Exp. Biol. 213, 2094–2103 [DOI] [PubMed] [Google Scholar]
- 26. Ono K., Iijima T. (2005) Pathophysiological significance of T-type Ca2+ channels: properties and functional roles of T-type Ca2+ channels in cardiac pacemaking. J. Pharmacol. Sci. 99, 197–204 [DOI] [PubMed] [Google Scholar]
- 27. Nagle G. T., de Jong-Brink M., Painter S. D., Li K. W. (2001) Structure, localization and potential role of a novel molluscan trypsin inhibitor in Lymnaea. Eur. J. Biochem. 268, 1213–1221 [DOI] [PubMed] [Google Scholar]
- 28. Nikitin E. S., Vavoulis D. V., Kemenes I., Marra V., Pirger Z., Michel M., Feng J., O'Shea M., Benjamin P. R., Kemenes G. (2008) Persistent sodium current is a nonsynaptic substrate for long-term associative memory. Curr. Biol. 18, 1221–1226 [DOI] [PubMed] [Google Scholar]
- 29. Opdyke C. A., Calabrese R. L. (1994) A persistent sodium current contributes to oscillatory activity in heart interneurons of the medicinal leech. J. Comp. Physiol. A 175, 781–789 [DOI] [PubMed] [Google Scholar]
- 30. Hodgkin A. L., Katz B. (1949) The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 108, 37–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hobert O. (2013) The neuronal genome of Caenorhabditis elegans. WormBook, 2013, 1–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biel M., Wahl-Schott C., Michalakis S., Zong X. (2009) Hyperpolarization-activated cation channels: From genes to function. Physiol. Rev. 89, 847–885 [DOI] [PubMed] [Google Scholar]
- 33. Lin Y. C., Spencer A. N. (2001) Calcium currents from jellyfish striated muscle cells: Preservation of phenotype, characterisation of currents and channel localisation. J. Exp. Biol. 204, 3717–3726 [DOI] [PubMed] [Google Scholar]
- 34. Cheng R. C., Tikhonov D. B., Zhorov B. S. (2010) Structural modeling of calcium binding in the selectivity filter of the L-type calcium channel. Eur. Biophys. J. 39, 839–853 [DOI] [PubMed] [Google Scholar]
- 35. Tang L., Gamal El-Din T. M., Payandeh J., Martinez G. Q., Heard T. M., Scheuer T., Zheng N., Catterall W. A. (2014) Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature 505, 56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yue L., Navarro B., Ren D., Ramos A., Clapham D. E. (2002) The cation selectivity filter of the bacterial sodium channel, NaChBac. J. Gen. Physiol. 120, 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liebeskind B. J., Hillis D. M., Zakon H. H. (2011) Evolution of sodium channels predates the origin of nervous systems in animals. Proc. Natl. Acad. Sci. U.S.A. 108, 9154–9159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schlief T., Schönherr R., Imoto K., Heinemann S. H. (1996) Pore properties of rat brain II sodium channels mutated in the selectivity filter domain. Eur. Biophys. J. 25, 75–91 [DOI] [PubMed] [Google Scholar]
- 39. Thomas D., Plant L. D., Wilkens C. M., McCrossan Z. A., Goldstein S. A. (2008) Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brohawn S. G., del Mármol J., MacKinnon R. (2012) Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller A. N., Long S. B. (2012) Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 [DOI] [PubMed] [Google Scholar]
- 42. Zhang X., Ren W., DeCaen P., Yan C., Tao X., Tang L., Wang J., Hasegawa K., Kumasaka T., He J., Wang J., Clapham D. E., Yan N. (2012) Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486, 130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tao X., Avalos J. L., Chen J., MacKinnon R. (2009) Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 Å resolution. Science 326, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imredy J. P., Chen C., MacKinnon R. (1998) A snake toxin inhibitor of inward rectifier potassium channel ROMK1. Biochemistry 37, 14867–14874 [DOI] [PubMed] [Google Scholar]
- 45. Jin W., Lu Z. (1998) A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry 37, 13291–13299 [DOI] [PubMed] [Google Scholar]
- 46. Lu Z., MacKinnon R. (1997) Purification, characterization, and synthesis of an inward-rectifier K+ channel inhibitor from scorpion venom. Biochemistry 36, 6936–6940 [DOI] [PubMed] [Google Scholar]
- 47. Fink M., Lesage F., Duprat F., Heurteaux C., Reyes R., Fosset M., Lazdunski M. (1998) A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 17, 3297–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 15, 1004–1011 [PMC free article] [PubMed] [Google Scholar]
- 49. Doering C. J., Zamponi G. W. (2003) Molecular pharmacology of high voltage-activated calcium channels. J. Bioenerg. Biomembr. 35, 491–505 [DOI] [PubMed] [Google Scholar]
- 50. Senatore A., Spafford J. D. (2013) A uniquely adaptable pore is consistent with NALCN being an ion sensor. Channels 7, 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]