Background: Sec13 is a core component in both the protein trafficking complex and the nuclear pore complex (NPC).
Results: The role of Sec13 in retina development has been investigated.
Conclusion: The NPC function of Sec13 is essential for retina development.
Significance: This is the first genetic evidence to differentiate the contributions of the two functions of Sec13 during organogenesis.
Keywords: Cell Biology, Endoplasmic Reticulum (ER), Molecular Genetics, p53, Zebrafish, Sec13, Sec31, Nuclear Pore Complexes, Retina Development
Abstract
Sec13 is a dual function protein, being a core component of both the COPII coat, which mediates protein trafficking from the endoplasmic reticulum to the Golgi apparatus, and the nuclear pore complex (NPC), which facilitates nucleo-cytoplasmic traffic. Here, we present a genetic model to differentiate the roles of these two functions of Sec13 in vivo. We report that sec13sq198 mutant embryos develop small eyes that exhibit disrupted retinal lamination and that the mutant retina contains an excessive number of apoptotic cells. Surprisingly, we found that loss of COPII function by oligonucleotide-mediated gene knockdown of sec31a and sec31b or brefeldin A treatment did not disrupt retinal lamination, although it did result in digestive organ defects similar to those seen in sec13sq198, suggesting that the digestive organ defects observed in sec13sq198 are due to loss of COPII function, whereas the retinal lamination defects are due to loss of the NPC function. We showed that the retinal cells of sec13sq198 failed to form proper nuclear pores, leading to a nuclear accumulation of total mRNA and abnormal activation of the p53-dependent apoptosis pathway, causing the retinal defect in sec13sq198. Furthermore, we found that a mutant lacking Nup107, a key NPC-specific component, phenocopied the retinal lamination phenotype as observed in sec13sq198. Our results demonstrate a requirement for the nuclear pore function of Sec13 in development of the retina and provide the first genetic evidence to differentiate the contributions of the NPC and the COPII functions of Sec13 during organogenesis.
Introduction
COPII vesicles are responsible for mediating protein trafficking from the ER2 to the Golgi apparatus, whereas NPCs facilitate nucleo-cytoplasmic traffic. Despite their distinct functions within the cell, structural studies have revealed that these two protein complexes share a common ancestral coatomer element that leads to the formation of a similar lattice-like structure (1–3). Sec13 is a member of the WD-repeat protein family and functions as a core component of both the COPII complex in the cytoplasm (4, 5) and the NPCs across the nuclear envelope. Moreover, Sec13 shuttles between the nucleus and the cytoplasm (6). This fact suggests that Sec13 occupies a unique position as a link between these two evolutionarily related fundamental processes.
Sec13 interacts with Sec31 to form the outer cage of the COPII vesicle coat required for vesicular protein transport from the ER to the Golgi apparatus. Studies of zebrafish genetic mutant sec13sq198 (7) and of the morpholino-mediated gene knockdown approach (8, 9) have shown that the COPII function of Sec13 is essential for the organogenesis of digestive organs and craniofacial cartilage. Sec23 and Sec24 are two inner coat components essential for the formation of the COPII vesicle. Previous reports have shown that mutants or morphants with compromised COPII function, due to a loss of function of Sec23 or Sec24, exhibit diverse developmental defects, including a deformed craniofacial skeleton structure, small eyes, and a defective digestive system (10–13).
NPCs are large macromolecular assemblies comprising about 30 different proteins known collectively as the nucleoporins (Nups) (14, 15). In the NPCs, Sec13 stably interacts with Nup145C (yeast) or Nup96 (vertebrates) and, together with other proteins, forms a lattice (16, 17). The main role of the nuclear pore is to provide a passage to facilitate the nuclear and cytoplasmic transport of mRNA and proteins. Different NPC components have been found to have critical roles in diverse developmental processes, including oogenesis (18), gastrulation (19, 20), neurogenesis (21), and the formation of digestive organs, pharyngeal cartilage, and eyes (22–24). Specifically, the study of a zebrafish flotte lotte (flo) mutant that carries a mutation in the elys gene showed that, in addition to displaying hypoplastic digestive organs, the flo mutant also exhibited a malformed retina due to the failure of proliferating precursors to differentiate neurons from their stem cell niche (22–24). By studying the zebrafish nup107 mutant, Zheng et al. (25) showed that Nup107 is essential for the development of most organs, including pharyngeal cartilage, intestines, and eyes.
Considering that COPII and the NPC are critical components of fundamental cellular processes, it is striking that loss of function of different COPII or NPC components leads to diverse and distinct phenotypes in vertebrates. Because Sec13 plays an important role in the function of both of these complexes, it is uniquely placed to increase our understanding of these complexes and their relationship with each other. A possible approach is to identify which elements of the Sec13 mutant phenotype are due to the loss of the COPII function and which elements are due to the loss of the NPC function or even to determine whether these are distinct phenotypes. To address this question, we used a zebrafish sec13sq198 mutant caused by a point mutation altering the normal splicing of the sec13 transcripts, which results in the addition of 8 bp from the seventh intron leading to a frameshift (7). The aberrant sec13sq198 transcript encodes the mutant protein Sec13sq198, which lacks the C-terminal 85 amino acids of normal Sec13 (7).
Zebrafish retinogenesis is a precisely regulated process involving a number of intrinsic and extrinsic factors and finely tuned regulatory networks (26, 27). As in other vertebrates, a mature zebrafish retina includes one class of glial cells, the Müller glia, six classes of neurons comprising ganglion, amacrine, bipolar, and horizontal cells, and cone and rod photoreceptors. These different cell types are organized into three distinguishable layers demarcated by two plexiform layers as follows: the innermost ganglion cell layer (GCL) is formed by ganglion cells; the inner nuclear layer (INL) is formed by amacrine, bipolar, and horizontal cells, and the outer nuclear layer (ONL) is formed by cone and rod photoreceptors (26).
Here, we report that, in addition to hypoplastic digestive organs, the sec13sq198 mutant also displayed a small eye phenotype, with the overall structure of the mutant eye, especially its retinal INL-ONL lamination, being affected. Recently, Schmidt et al. (28) also reported a small eye phenotype exhibited by Sec13 knockdown morphants. However, they did not explore the possible contribution of the NPC function of Sec13 to this phenotype (28). In this report, we show the following: 1) the retina lamination phenotype in sec13sq198 is not entirely due to the compromised COPII function, and 2) disruption of the NPCs in the sec13sq198 retina is the primary cause for developmental defects of the retina. Furthermore, this conclusion is supported by the fact that a zebrafish mutant lacking Nup107 phenocopies the sec13sq198 retina lamination phenotype. Taken together, our data clearly demonstrate an important role for the NPC function of Sec13 in the process of retinal development.
EXPERIMENTAL PROCEDURES
Zebrafish Strains
All animal procedures were performed in full accordance with the requirements of the Regulation for the Use of Experimental Animals in Zhejiang Province (Ethics Code Permit ZJU2011-1-11-009Y). The fish lines used included the wild-type AB line (WT), sec13sq198 (7, 29), and nup107tsu068Gt (25).
Hematoxylin and Eosin (H&E) Staining
WT, sec13sq198, nup107tsu068Gt, and different morphants (including standard control MO morphant, sec31a ATG MO, and sec31b splice MO double morphant) were fixed, embedded, and cryosectioned as described previously (7, 30). After being washed briefly with distilled water, the cryosections were stained with filtered 0.1% hematoxylin solution for 2 min at room temperature (RT). Following a brief wash in distilled water, the sections were treated with 1% acid alcohol for 30 s, washed with water for 4 min, and then counterstained in 0.5% eosin for about 30 s at RT. After being washed with water for 3 min, the sections were gradually dehydrated in 95 and 100% ethanol and then in xylene three times for 15 min each. Finally, the sections were mounted with balsam, and images were taken under a Nikon ECLIPSE 80i microscope. The Sec23b MO- and Sec24C MO-injected embryos were fixed at 120 or 144 hpf, respectively, and embedded in plastic resin, sectioned, and stained with toluidine blue as described previously (31).
Whole-mount in Situ Hybridization (WISH)
WISH was performed as described previously (7). Gene-specific primers were designed based on available information, and RT-PCR products were cloned into pCS2+ vectors. RNA probes rod opsin, blue opsin, red opsin, brn3b, vsx1, otx2, and rx1 were labeled with digoxygenin (Roche Diagnostics) and used at a concentration of 0.5 μg/ml.
BrdU Incorporation Analysis, PH3 Antibody Staining, and Apoptosis Assay
For the BrdU incorporation assay, embryos at 36 and 48 hpf were injected with 1 nl of 10 mm BrdU solution. Four hours after injection, embryos were harvested for the immunostaining of BrdU-positive cells using an anti-BrdU antibody (AbD Serotec, OBT0030) in a 1:200 dilution as described previously (7). PH3 immunostaining used the polyclonal antibody against PH3 (Santa Cruz Biotechnology, SC-8656-R) in a 1:200 dilution. An apoptosis assay was performed using the in situ cell death detection kit, tetramethylrhodamine red (Roche Diagnostics, 12 156 792 910).
mRNA Rescue and Morpholino Mimicking of the sec13sq198 Phenotype
Full-length sec13 cDNA was cloned into the pCS2+ vector. The sec13 mRNA was synthesized using the mMessage mMachine SP6 kit (Ambion, AM1340). Two hundred pg of sec13 mRNA was injected into the one-cell stage embryos, and the embryos were subjected to bright field microscopy and WISH. The sec13 and sec31a morpholinos (Gene Tools, Philomath, OR), designed to target their respective translation start sites, were used as described previously (7). Two sec31b splicing morpholinos were designed to target the intron 3-exon 4 junction (sec31b-MO1, 5′-GATCAGATTACTCT GAGATGAAGGA-3′) and exon4-intron 4 junction (sec31b-MO2, 5′-GACTACAGAAACGAGCTGTACCTGT-3′), respectively. One nanoliter of Sec13-ATG MO (1 μm), Sec31a-ATG MO (0.75 μm), sec31b-MO1 or sec31b-MO2 (1 μm) was injected into the one-cell stage embryos. A human β-globin morpholino (5′-CCTCTTACCTCAGTTACAATTT-3′) was used in parallel as the standard negative control morpholino.
Fluorescence Microscopy
The zebrafish embryos were fixed, embedded, and cryosectioned. Primary antibodies against NPCs (Abcam, ab24609), serotonin (Sigma, S5545), collagen II (Lifespan Biosciences, LS-C41831), and PDI (Sigma, P7496) were used in a 1:100 dilution and Zpr-1 (Zebrafish International Resource Center) and Sec13 in a 1:300 dilution. Secondary antibodies conjugated with Alexa Fluor 488, 549, and 647 were used in a 1:400 dilution. DAPI was used to label the nuclei. Images were taken under a Leica TCS SP5 confocal microscope and an Olympus FLUOVIEW FV1000 microscope.
Transmission Electron Microscopy (TEM)
The zebrafish embryos were fixed in 2% glutaraldehyde and 1% paraformaldehyde in PBS for 2 h followed by post-fixation in 1% osmium tetroxide for 1 h at RT. Fixed embryos were gradually dehydrated in ethanol and embedded individually into resin blocks, and 50-nm cross-sections were obtained with an Ultracut E Microtome (Leica) and collected on copper grids. This was followed by staining with 5% uranyl acetate and 2% lead citrate. Images were acquired using a Philips CM-10 TEM or a Hitachi H-7650 TEM.
Quantitative Real Time-PCR (qPCR)
For qPCR, the embryos were collected for total RNA extraction, and a reverse transcription was performed using a reverse transcriptase kit (Invitrogen, 28025-021) according to the manufacturer's protocol. The amount of transcribed cDNA was normalized based on elongation factor 1a (elf1a). Prepared cDNA and gene-specific primers (0.5 μl of each) were added to SsoFast EvaGreen Supermix (Bio-Rad, 172-5201AP). The CFX96 real time system (Bio-Rad) was used to obtain the threshold cycle (Ct) value. The relative expression of each gene was determined after being normalized to elf1a. The primer sequences are listed in Table 1.
TABLE 1.
Primers used in the qPCR
| Gene | Sequence |
|---|---|
| p53 forward | TggAgAggAggTCggCAAAATCAA |
| p53 reverse | gACTgCgggAACCTgAgCCTAAAT |
| D113p53 forward | ATATCCTggCgAACATTTggAggg |
| D113p53 reverse | CCTCCTggTCTTgTAATgTCAC |
| mdm2 forward | CTCgCAgTgAgggCAgTgAAg |
| mdm2 reverse | TCTAggCACgTAgCgggAAgg |
| cyclin-G1 forward | gCCCTTTACAgTCCAgCCCAAATC |
| cyclin-G1 reverse | CTgTgCCTCAAgCCTCTCgATgTA |
| p21 forward | gAAgCgCAAACAgACCAACAT |
| p21 reverse | gCAgCTCAATTACgATAAAgA |
| apaf1 forward | CCgggCTgggTgACTgTATTTg |
| apafl reverse | gTgCTgggTggCCgCTgACT |
| baxa forward | CAgAgTggCCCgTgAgAT |
| baxa reverse | gggggTgCCAAAATAACTg |
| bcl2 forward | gATAgCCCgggTCACTCgTTCAgA |
| bcl2 reverse | CCAgTggCCCgTTCAggTAgTCAg |
| caspase8 forward | AgCggCCTCTTggATACTgTCTA |
| caspase8 reverse | gCCCAAAACTgTgCCCTTCT |
| fadd forward | gCCCggCTggTggTCTgg |
| fadd reverse | CACTCgggCgTTCTCCTTCTTgAT |
| gadd45aa forward | TggCgCTTCAgATTCACTTCACT |
| gadd45aa reverse | AgCggTTCACTTTTCCCAgAg |
| puma forward | CACTgCCCCACATCCCCTCACAT |
| puma reverse | gTACggCCACCCTCTCCACAgC |
| elf1a forward | CTTCTCAggCTgACTgTgC |
| elf1a reverse | CCgCTAgCATTACCCTCC |
Protein Analysis
Protein extraction and Western blot were performed as described previously (7). A mouse monoclonal antibody against zebrafish p53 and rabbit monoclonal antibody against GAPDH (Epitomics, 2251-1) were used as described previously (32). The Western blot bands were visualized by SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, 34096) and quantified with Adobe Photoshop CS5. The ratio was obtained by dividing the value for p53 or Sec13 by the value for GAPDH before being compared.
mRNA Export Assay
WT, sec13sq198, sup107tsu068Gt, different morphants (including standard control MO morphant and sec31a ATG MO and sec31b splice MO double morphant), and ethanol-treated and brefeldin A-treated embryos were fixed, embedded, cryosectioned, and stored at −80 °C. When used, the sections were washed with 1× cold PBS and refixed with 4% paraformaldehyde for 20 min on ice. Following permeabilization in PBST (with 0.5% Triton X-100) for 10 min on ice, the sections were washed in 2× SSC for 5 min, prehybridized for 1 h at 37 °C, hybridized with Cy3-oligo(dT)50 (Invitrogen) at a concentration of 1000 pg/μl overnight at 37 °C, and then washed with 2× SSC at 37 °C three times for 5 min each. Finally, the stained sections were mounted with DAPI. The images were taken under an Olympus Fluoview FV1000 microscope.
Brefeldin A (BFA) Treatment
Five mg/ml BFA (Sigma, B7651) in 100% ethanol was diluted in egg water to make a 1.5 μg/ml final concentration. Embryos at 22 hpf were incubated in BFA solution for about 62 h. The embryos were then fixed for RNA in situ hybridization, protein immunostaining, or H&E staining at 84 hpf.
RESULTS
Retinal Lamination Is Disrupted in the sec13sq198 Mutant
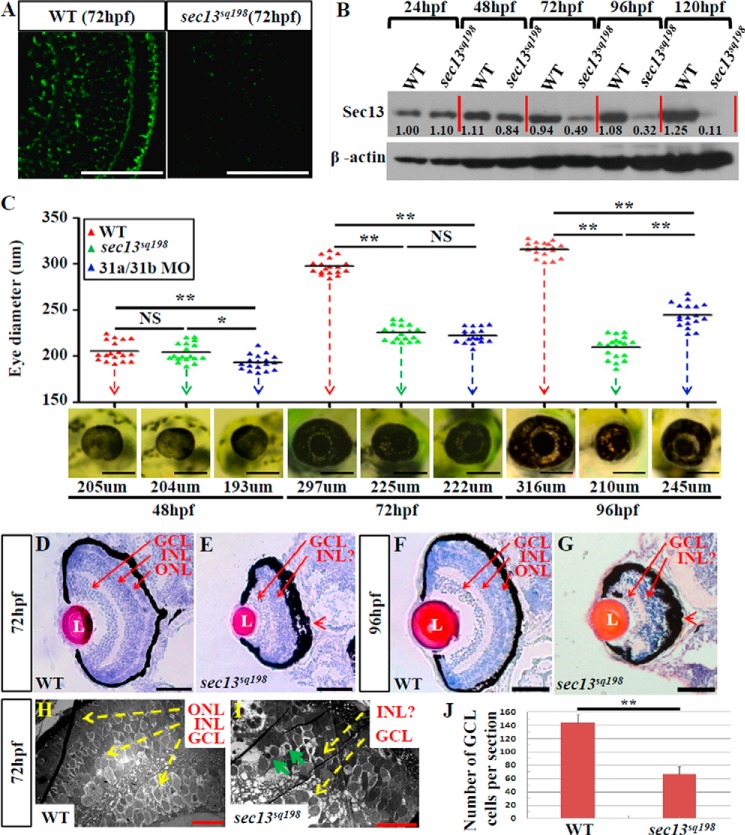
Immunostaining showed that Sec13 was highly expressed in the WT retinal cells but was only barely detectable in the sec13sq198 mutant retinal cells (Fig. 1A), suggesting that Sec13 is likely to play a role in retinogenesis. In addition to exhibiting dysplastic digestive organs as reported previously (7), we found that sec13sq198 mutant embryos also displayed a small eye phenotype from 72 h post-fertilization (hpf) onward, despite the size of the eyes appearing normal at 48 hpf (Fig. 1C and data not shown). Both H&E staining and TEM analysis showed that the WT embryos developed a typical three-layered retina at 72 and 96 hpf (Fig. 1, D, F, and H). In contrast, the INL and ONL in mutant embryos could not be defined, and the ONL appeared lost or greatly diminished (Fig. 1, E, G, and I). In addition, the retinal pigment epithelium (RPE) in mutant embryos was significantly expanded (Fig. 1, E and G). All of the eye phenotypes exhibited by the sec13sq198 mutants were identical to those displayed by the sec13 morphant reported recently (28).
FIGURE 1.
Retinal lamination is disrupted in the sec13sq198 mutant. A, immunostaining of Sec13 protein in the WT and sec13sq198 mutant retina at 72 hpf. B, Sec13 is a maternal deposited protein. Western blot analysis of Sec13 protein in WT and sec13sq198 mutant from 24 to 120 hpf is shown. Value for Sec13 protein in WT at 24 hpf is set as 1. C, measurements of eye diameter in WT, sec13sq198 mutant, and sec31a/sec31b double morphant at 48, 72, and 96 hpf (n = 18). D–G, H&E staining analysis of WT (D and F) and mutant (E and G) retina at 72 and 96 hpf. WT embryos at 72 and 96 hpf developed normally laminated retinas consisting of a differentiated GCL, an INL, and an ONL (indicated by the red arrows). However, retinal lamination in sec13sq198 mutant embryos was disrupted (indicated by the red arrows), and retinal pigment epithelium was clearly expanded in width (shown by the red arrowheads). H and I, TEM analysis of the WT and sec13sq198 mutant retina at 72 hpf showed that compared with well developed WT retina (H), the sec13sq198 mutant embryos developed disrupted and disorganized retina containing many apoptotic cells (shown by the green arrows) (I). J, average number of GCL cells per section/per embryo in each genotype is shown (n = 3, four sections from each embryo were used for counting the GCL cells). L, lens. Scale bar, 50 μm (A and D–G), 150 μm (C), and 20 μm (H and I). NS, no significance. *, p < 0.05; **, p < 0.01.
The specification and differentiation of the retinal cell are sequential events and are completed by 72 hpf in zebrafish (26, 28, 33). WISH using probes for otx2 and rx1, the two key genes for early eye development (34, 35), revealed no drastic differences between the WT and mutant embryos at 36 hpf (Fig. 2A), suggesting that the initiation of eye development is not affected by sec13sq198, likely because Sec13 is a maternal deposited protein (Fig. 1B). WISH using the ganglion cell marker brn3b, which encodes a member of the POU domain family of transcription factors, showed that a specification of ganglion cells did occur, although the GCL area and number of cells was reduced at 72 hpf (Figs. 1J and 2, B and C). Immunostaining with serotonin (to mark the amacrine cells) and WISH with a vsx1 RNA probe (to mark the bipolar cells) showed that these two classes of cells were also successfully differentiated (Fig. 2, D–G). In the ONL, rod opsin expression was barely detectable, and the expression of red and blue opsin was sharply reduced (Fig. 2, H–M) (27, 36). The small eye phenotype and deficiency in the rod opsin signal in the ONL were largely rescued by sec13 mRNA injection and were mimicked in the sec13 morphant (Fig. 2N). Taken together, our results demonstrate that, although retinal cell specification and differentiation are not disrupted in sec13sq198 mutants, retinal development and the layering of photoreceptors were severely affected. This observation is consistent with that previously observed in the sec13 morphant (28).
FIGURE 2.
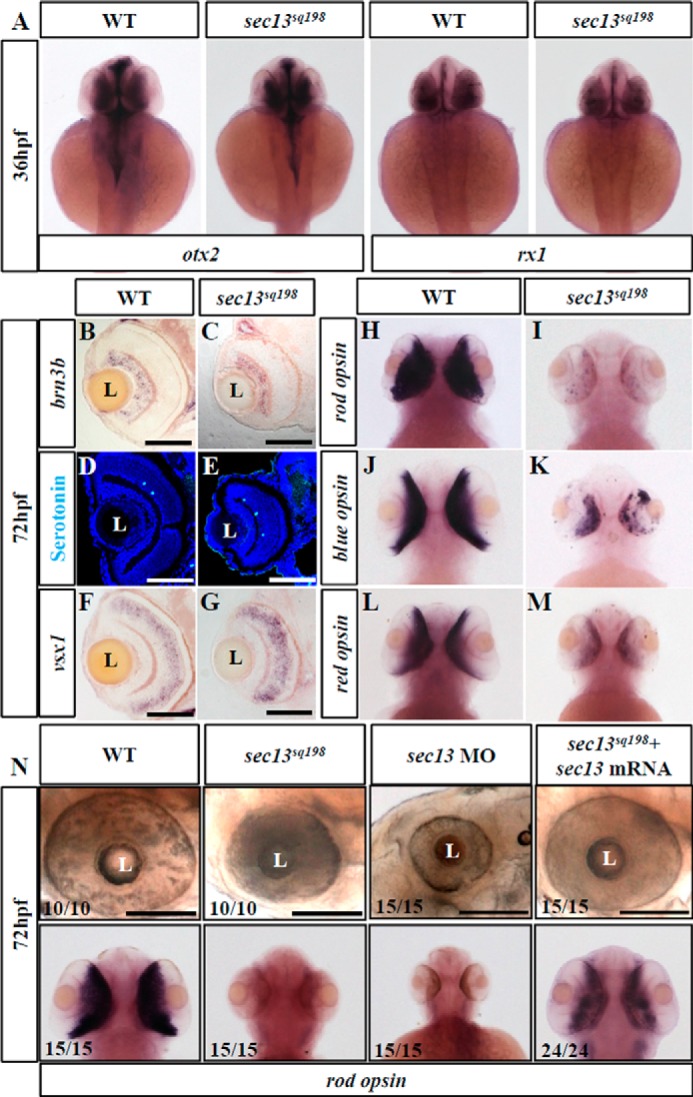
Differentiation of the retinal cells is not affected in the sec13sq198 mutant. A, WISH analysis of early eye markers otx2 and rx1 in the WT and sec13sq198 mutant embryos at 36 hpf. B–M, in situ hybridization analysis of different retinal cell types in the WT and sec13sq198 mutant at 72 hpf. Examination of each cell type in the WT (B, D, F, H, J, and L) and sec13sq198 mutant (C, E, G, I, K, and M) retina are shown. Ganglion cells were detected with the following: brn3b probe (B and C); amacrine cells with an anti-serotonin antibody (D and E); bipolar cells with a vsx1 probe (F and G); rod photoreceptors with a rod opsin probe (H and I); blue cones with a blue opsin probe (J and K); and red cones with a red opsin probe (L and M). N, bright field images (top panel) of the retina of the WT, sec13sq198 mutant, sec13 morphant, and sec13sq198 mutant embryos injected with sec13 mRNA at 72 hpf and WISH analysis (lower panel) of rod opsin expression in the retina of the WT, sec13sq198 mutant, sec13 morphant, and sec13sq198 mutant embryos injected with sec13 mRNA at 72 hpf. L, lens. Scale bar, 50 μm (B–G) and 150 μm (N).
COPII Dysfunction Is Not the Primary Cause of Retinal Lamination Lesions in the sec13sq198 Mutant
In a previous report, Schmidt et al. (28) examined the relationship between opsin secretion and ONL/RPE development, and they concluded that the ONL/RPE lesion resulted from a failure to secrete opsin and collagen in a cell autonomous manner. Sec13 interacts with Sec31 to form the outer coat of the COPII complex, which is critical for protein trafficking from the ER to the Golgi apparatus (37). We found that Sec13sq198 mutant protein is a short lived protein in vivo (data not shown), and we have previously reported that the Sec13sq198 mutant protein lost its ability to form a complex with Sec31 in the cultured human cells, thus disrupting COPII function and leading to cell cycle arrest and activated apoptosis, which resulted in hypoplasia of the digestive organs in the sec13sq198 mutant (7). Here, we asked whether the eye defect in the sec13sq198 mutant is also due to COPII dysfunction. TEM analysis reveals that the ER structure is disrupted in sec13sq198 mutant retina cells (Fig. 3), albeit to a lesser extent compared with the digestive organs (7). We have also previously shown that knockdown of Sec31a produces even more severe dysplastic digestive organ phenotypes (7). In most vertebrates, including zebrafish, Sec31a has a lone paralog Sec31b. To determine whether the retinal phenotype in the sec13sq198 mutant was caused by a disruption to the COPII function, we knocked down both Sec31a and Sec31b with their respective specific morpholinos (Fig. 4A) (7). We assessed the sec31a/sec31b double morphant phenotypes. Like the sec31a morphant (7), both the sec31b morphant and the sec31a/sec31b double morphant displayed small liver, intestine, and pancreas phenotypes (Fig. 4, B–D), demonstrating that COPII plays an important role in the organogenesis of the digestive organs. In terms of eye development, the results showed that the sec31b morphant exhibited a more profound eye phenotype than the sec31a morphant (data not shown). Simultaneous knockdown of Sec31a and Sec31b caused an even more severe eye phenotype (Fig. 1C) in addition to a curved body (data not shown). However, the results from H&E staining, WISH using brn3b and vsx1 probes, and immunostaining of Zpr-1 (green/red double cones marker) showed that the co-knockdown of Sec31a and Sec31b did not totally destroy retinal lamination (Fig. 4, E and F) and cell differentiation (Fig. 4, G–L), although it triggered a mild level of cell apoptosis (0.78 apoptotic cells in the standard control MO morphant and 17 in a Sec31a/Sec31b double morphant) (Fig. 4M). In fact, previous reports have shown that mutations in sec24d, which encodes an inner coat component of the COPII complex, did not cause obvious defects in retinal development in either medaka fish (38) or zebrafish (13), although these mutants were defective in its craniofacial skeleton development. Similarly, the loss-of-function of Sec23a, Sec23b, and Sec24c did not eliminate retinal lamination (Fig. 4, N–R) (13, 39).
FIGURE 3.
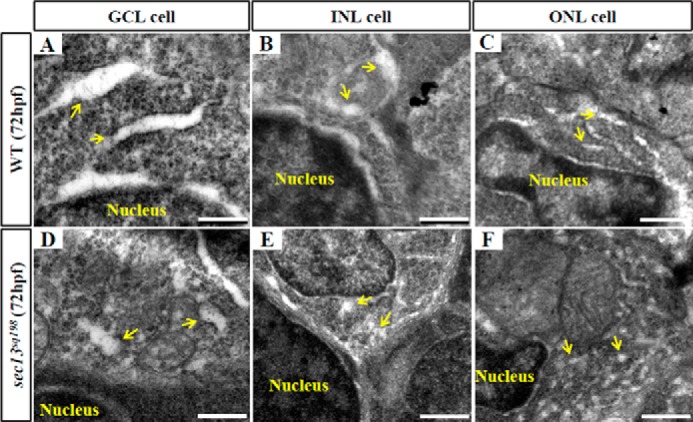
ER lumen in sec13sq198 mutant retinal cells. A–F, TEM analysis of the ER structure in a GCL cell (A and D), INL cell (B and E), and ONL cell (C and F) in WT (A–C) and sec13sq198 mutants (D–F) at 72 hpf. ER lumens are indicated with yellow arrows. Scale bar, 0.5 μm.
FIGURE 4.
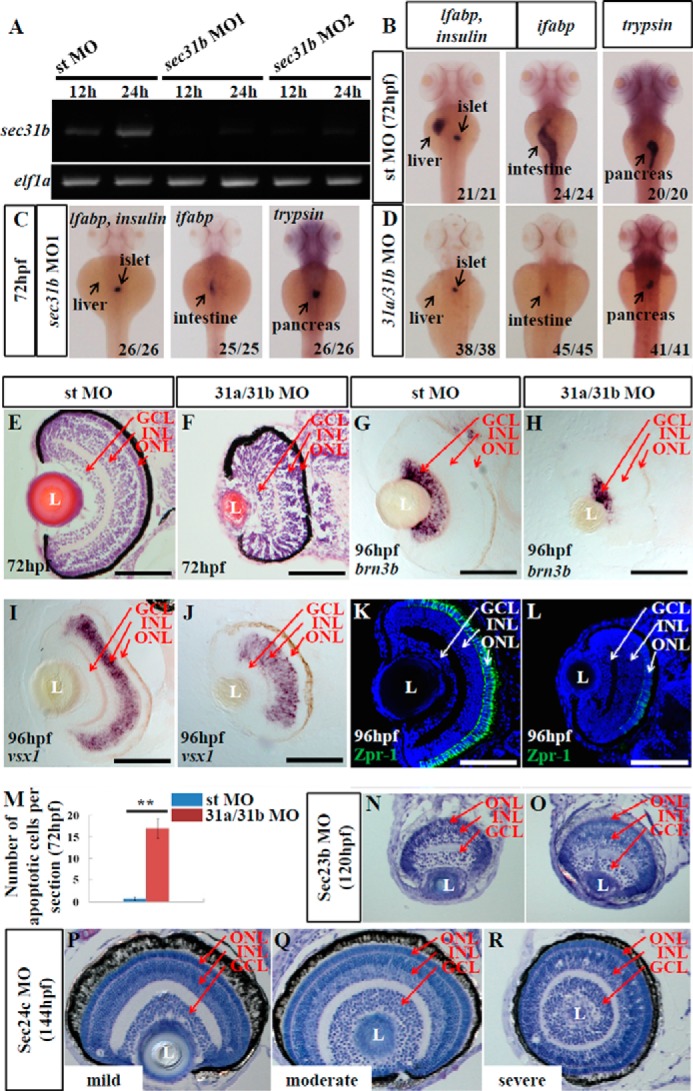
Loss-of-function of COPII does not disrupt retinal lamination. A, expression of sec31b in the embryos injected with the standard control morpholino (st MO) or two sec31b splicing morpholinos (sec31b MO1 and sec31b MO2) at 12 and 24 hpf were determined by qPCR with the expression of elf1a as the normalization control. B–D, WISH analysis of digestive organs in the control morphant (st MO), sec31b morphant, and sec31a/sec31b double morphant at 72 hpf. In each case, the number of total embryos examined (as denominator) and the number of embryos exhibiting the displayed phenotype (as numerator) are shown on the bottom right. E and F, H&E staining of the sections of eyes in the control (E) and sec31a/sec31b double (F) morphant. G–L, ganglion cells were detected with a brn3b probe (G and H), bipolar cells with a vsx1 probe (I and J), and green/red double cones with an anti-Zpr-1 antibody (K and L). M, comparison of the number of apoptotic cells in the control and sec31a/sec31b double morphant at 72 hpf (n = 3, four sections from each embryo were used for counting the apoptotic cells). N–R, histological analysis of the retinal structure in Sec23b morphant at 120 hpf and in Sec24c morphant at 144 hpf. L, lens. Scale bar, 50 μm (E–L).
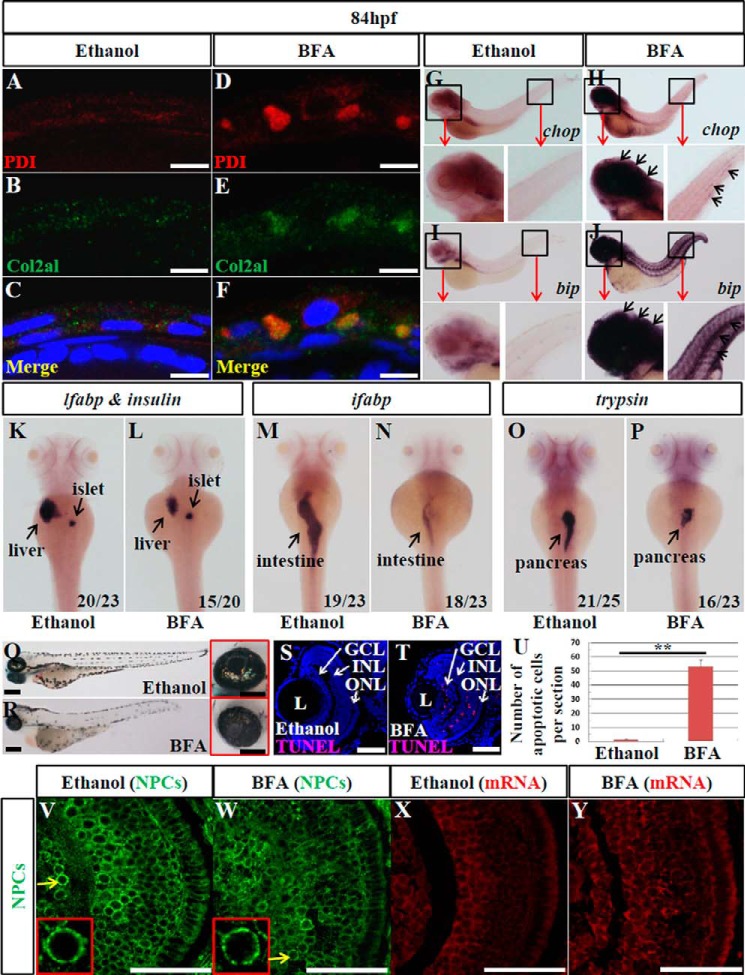
As an alternative approach to reduce COPII function, we utilized the small molecule BFA (40, 41) and examined its effect on retinal development. BFA is a lactone-type antibiotic drug that inhibits the activation of Arf1 to stop vesiculation at the Golgi, causing Golgi resorption into the ER and effectively preventing all anterograde transport (42, 43). Differentiation of the ganglion cells initiates at about 32 hpf (28), so we treated the embryos at 22 hpf and carried out an analysis at 84 hpf. The efficacy of BFA in blocking COPII function was confirmed by the accumulation of collagen II in the ER, the dilatation of ER lumen in epidermal cells (Fig. 5, A–F), and by strong up-regulation of chop and bip (two ER stress markers) in the brain, eye, and tail, compared with the ethanol-treated control embryos (Fig. 5, G–J). BFA treatment caused overall developmental abnormalities, including shortened body length, heart edema, moderately reduced eye size, and defective digestive organs (Fig. 5, K–R), as observed in the sec31a/sec31b double morphant. Moreover, BFA treatment triggered apoptosis, mainly in the GCL and INL (on average, 1.5 apoptotic cells in ethanol-treated retina and 53 in BFA-treated retina per section) (Fig. 5, S–U). However, unlike the sec13sq198 mutant, H&E staining, brn3b and vsx1 RNA WISH, and Zpr-1 protein immunostaining all showed that retinal lamination was clearly visible, although Zpr-1 expression was greatly reduced in embryos treated with BFA (Fig. 6, A–O). Combined, these data suggest that disruption of the COPII function is not the primary cause of the retinal lamination lesions observed in the sec13sq198 mutant.
FIGURE 5.
BFA treatment destroys the COPII function and impedes digestive organ development. A–F, double immunostaining of PDI (ER marker) and collagen II (Col2a1, secretory protein) in ethanol-treated (A–C) and BFA-treated (D–F) embryos at 84 hpf. A and D, immunostaining of PDI (shown in red). B and E, immunostaining of Col2a1 (in green). C and F, merge of PDI and Col2a1 staining. G–J, WISH analysis of chop and bip in ethanol-treated (G and I) and BFA-treated (H and J) embryos at 84 hpf. K–P, WISH analysis using lfabp (for the liver) plus insulin (for the endocrine pancreas) (K and L), ifabp (for the intestine) (M and N), and trypsin (for the exocrine pancreas) (O and P) probes for the analysis of digestive organs in ethanol-treated (K, M, and O) and BFA-treated (L, N, and P) embryos at 84 hpf. In each case, the number of total embryos examined (as denominator) and the number of embryos exhibiting the displayed phenotype (as numerator) are shown on the bottom right. Q and R, bright field images of ethanol-treated (Q) and BFA-treated (R) embryos at 84 hpf. Higher magnification of the eye image is shown on the right correspondingly. S–U, representative image of TUNEL analysis of apoptosis in ethanol-treated (S) and BFA-treated (T) retina at 84 hpf. U, average number of apoptotic cells in these samples were shown (n = 3, four sections from each embryo were used for counting the apoptotic cells). V and W, immunostaining of nuclear pores in ethanol-treated (V) and BFA-treated (W) embryos at 84 hpf. X and Y, mRNA export assay in ethanol-treated (X) and BFA-treated (Y) retinas at 84 hpf. L, lens. Scale bar, 10 μm (A–F), 300 μm (Q and R), 150 μm (inset in Q and R), and 50 μm (S, T, and V–Y). **, p < 0.01.
FIGURE 6.
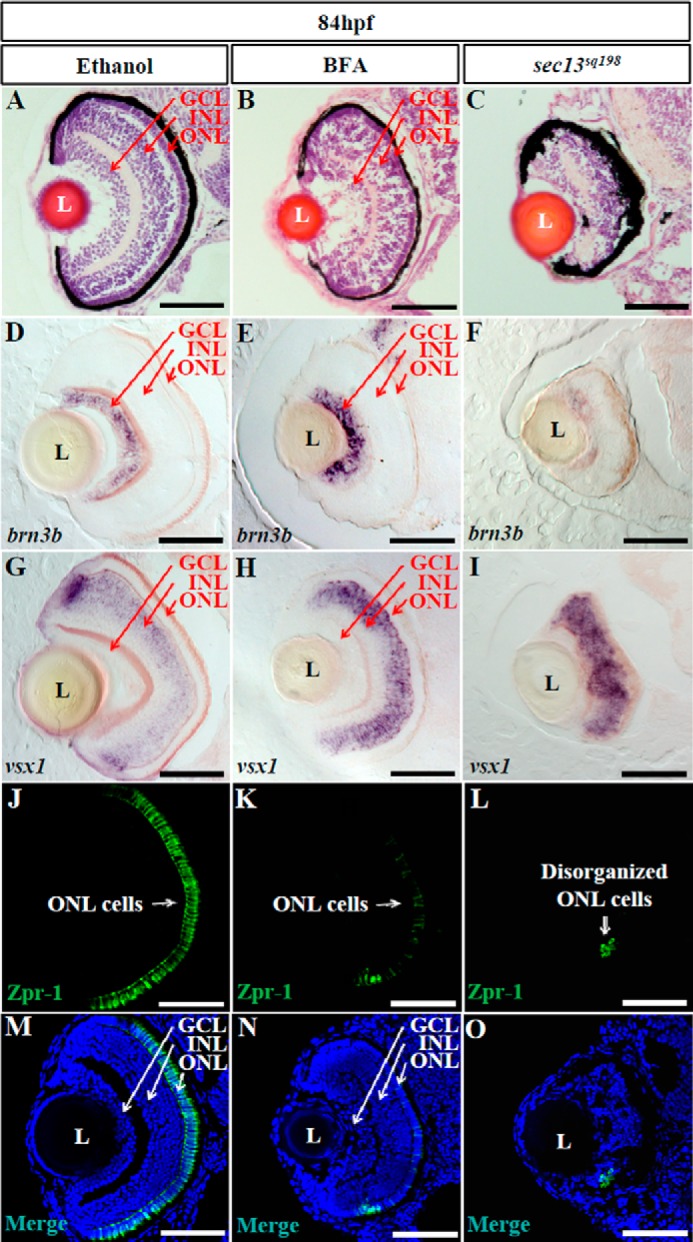
BFA treatment does not disrupt retinal lamination. A–C, H&E staining analysis of the retina in ethanol-treated (A), BFA-treated (B), and sec13sq198 mutant (C) embryos at 84 hpf. D–O, ganglion cells were detected with a brn3b probe (D–F), bipolar cells with a vsx1 probe (G–I), and green/red double cones with an anti-Zpr-1 antibody (J–O). L, lens. Scale bar, 50 μm.
Formation of the Nuclear Pores Is Impaired in the sec13sq198 Mutant Retina
Sec13 also serves as a key component in NPCs. Because COPII dysfunction did not fully phenocopy the retinal phenotype in the sec13sq198 mutant, we explored whether the retinal lesion was a result of the combination of the dysfunctions of Sec13's COPII and NPC functions in the sec13sq198 mutant. To assess the formation of nuclear pores in the retinal cells of the mutant, we performed immunostaining using a mouse Mab414 antibody that recognizes Nup153, Nup214, and Nup358 (44), and we compared the nuclear pores in the WT, sec31a/sec31b double morphant, and the sec13sq198 mutant. At 48 hpf, both the WT and sec31a/sec31b double morphant retinal cells showed well formed nuclear pores characterized by a regular ring of these Nups surrounding the nucleus (Fig. 7, A and D). In contrast, sec13sq198 mutant retinal cells had an irregular sporadic distribution of Nups (Fig. 7G). At 72 and 96 hpf, normal nuclear pores were present in both the WT and sec31a/sec31b double morphant retinal cells (Fig. 7, B, C, E, and F). In contrast, only a faint signal was detected in the sec13sq198 mutant at 72 hpf, and the signal was almost undetectable at 96 hpf, suggesting an eventual total disruption of the nuclear pores in the sec13sq198 mutant (Fig. 7, H and I). In fact, Western blot of the NPCs using the Mab414 antibody revealed that the levels of proteins in the NPCs were obviously down-regulated in the sec13sq198 mutant retina (data not shown). Similarly, nuclear pores were also malformed in the mutant digestive organs (Fig. 9, A–F), suggesting that dysfunction in the NPCs probably also contributed to the dysplastic digestive organs in sec13sq198. We further examined the nuclear pores with TEM. Nuclear pores were clearly visible in all of the retinal cells in the GCL, INL, and ONL in the WT and sec31a/sec31b double morphant embryos at 72 hpf (Fig. 7, J, K, and M, and data not shown). However, the number of identifiable nuclear pores in the sec13sq198 mutant retinal cells in all three layers was markedly reduced (Fig. 7, L and M, and data not shown).
FIGURE 7.
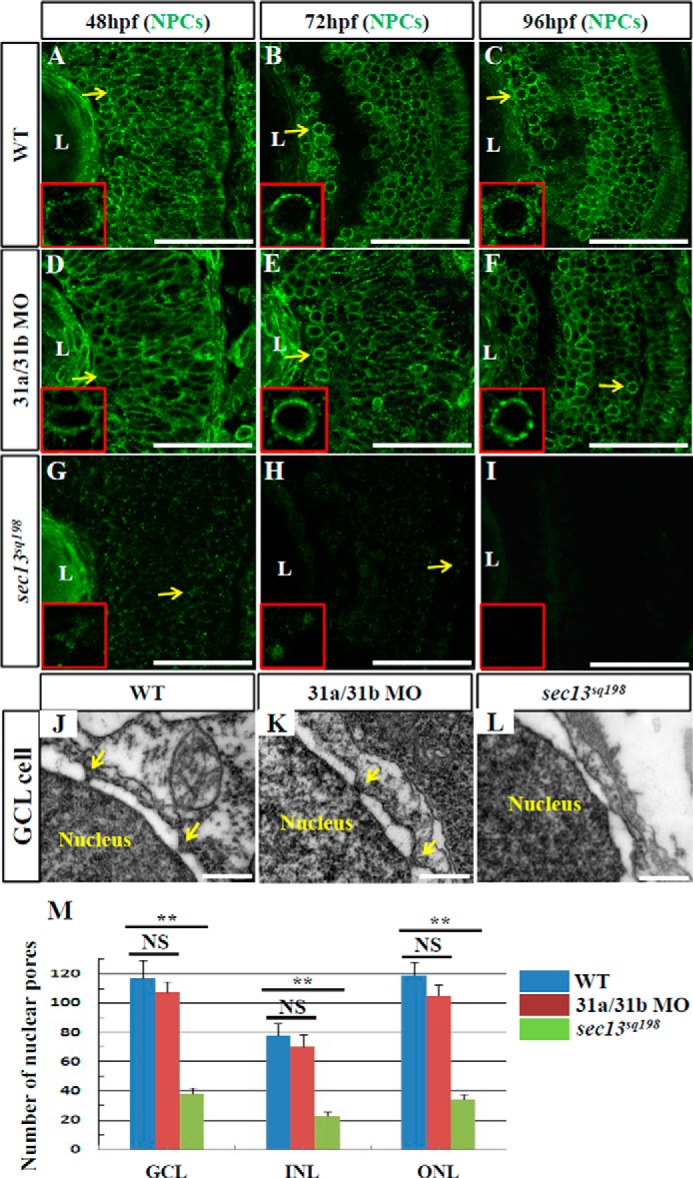
Formation of nuclear pores is impaired in the sec13sq198 mutant retina. A–I, immunostaining of nuclear pores in WT (A–C), sec131a/sec31b double morphant (D–F), and sec13sq198 mutant (G–I) retina with Mab414 antibody. The nuclear pores in both WT (A–C) and the sec31a/sec31b double morphant (D–F) retina were well formed (shown by the yellow arrows and insets), whereas formation of the nuclear pores in the sec13sq198 mutant retina gradually failed (G–I) (shown by the yellow arrows and insets). J–L, TEM analysis of the nuclear pores in the GCL cells in WT (J), sec31a/sec31b double morphant (K), and sec13sq198 mutant (L) retina at 72 hpf. Note that both the WT (J) and the sec31a/sec31b double morphant (K) retinal cells displayed characteristic nuclear pores (indicated by the yellow arrows). In contrast, the mutant retina was defective in the formation of nuclear pores in cells from all three layers. M, comparison of the number of nuclear pores in the GCL, INL, and ONL in the WT, sec31a/sec31b double morphants, and sec13sq198 mutant retina at 72 hpf. Three ultrathin sections from two embryos for each genotype were used to count the number of nuclear pores in the GCL, INL, and ONL, respectively. For each ultrathin section, six cells in each retinal layer were selected for counting, and the total number of nuclear pores from 18 cells in each retinal layer is shown here. L, lens. Scale bar, 50 μm (A–I) and 1 μm (J–L). NS, no significance. **, p < 0.01.
FIGURE 9.
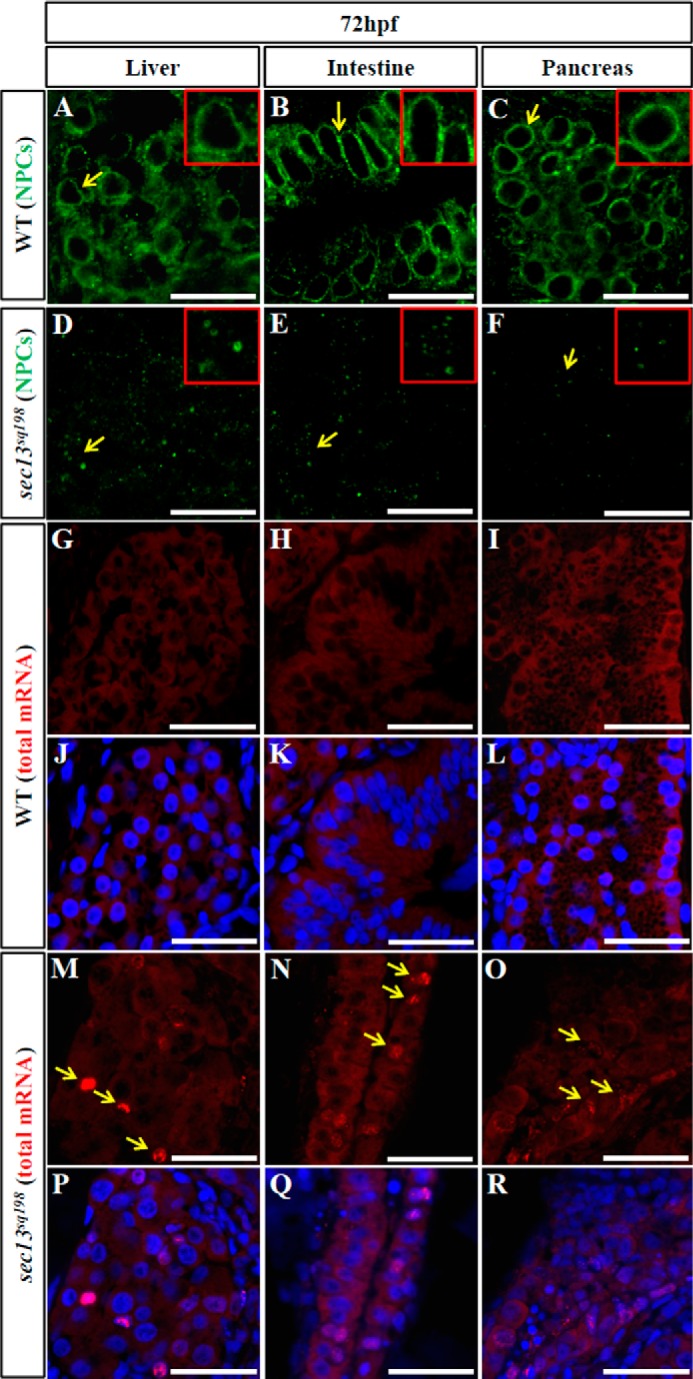
sec13sq198 mutant digestive organs are also defective in the formation of nuclear pores and mRNA export. A–F, immunostaining of nuclear pores with a mouse monoclonal Mab414 antibody in the WT (A–C) and sec13sq198 mutant (D–F) liver, intestine, and pancreatic cells at 72 hpf. G–R, mRNA export assay in WT (G–L) and sec13sq198 mutant (M–R) liver, intestine, and pancreatic cells at 72 hpf. The nuclei were stained with DAPI. Scale bar, 15 μm (A–F) and 25 μm (G–R).
Total mRNA Accumulates in the Retinal Cell Nucleus in the sec13sq198 Mutant
Nuclear pores serve as essential channels for the nucleo-cytoplasmic transport of RNA and proteins. Because the formation of nuclear pores was impaired in the sec13sq198 mutant retinal cells, we wondered whether the export of total polyadenylated mRNA was blocked. We performed fluorescent in situ hybridization using Cy3-labeled oligo(dT)50 as a molecular probe to determine the cellular distribution of total mRNA. We found that the mRNA was exported out of the nucleus and evenly distributed throughout the cytoplasm in both the WT and sec31a/sec31b double morphant retinal cells at 48 hpf (Fig. 8, A–F). In contrast, a fraction of the sec13sq198 mutant retinal cells began to accumulate mRNA in their nuclei at 48 hpf (Fig. 8, G–I). By 72 hpf, the nuclear accumulation of mRNA became more prominent in sec13sq198 mutant retinal cells compared with WT and sec31a/sec31b double morphant embryos (Fig. 8, J–R). Accumulated mRNAs formed particle-like clusters (Fig. 8, P–R). However, BFA treatment neither led to disruption of formation of the NPCs (Fig. 5, V and W) nor to the accumulation of total RNA of the nuclei in the retina (Fig. 5, X and Y). These data demonstrate that the NPC function was disrupted in the retinal cells in the sec13sq198 mutant. Interestingly, we noticed that although the sec13sq198 mutant's digestive organs exhibited defective nuclear pores, a much smaller number of cells in these organs accumulated total mRNA in the nuclei (Fig. 9, G–R).
FIGURE 8.
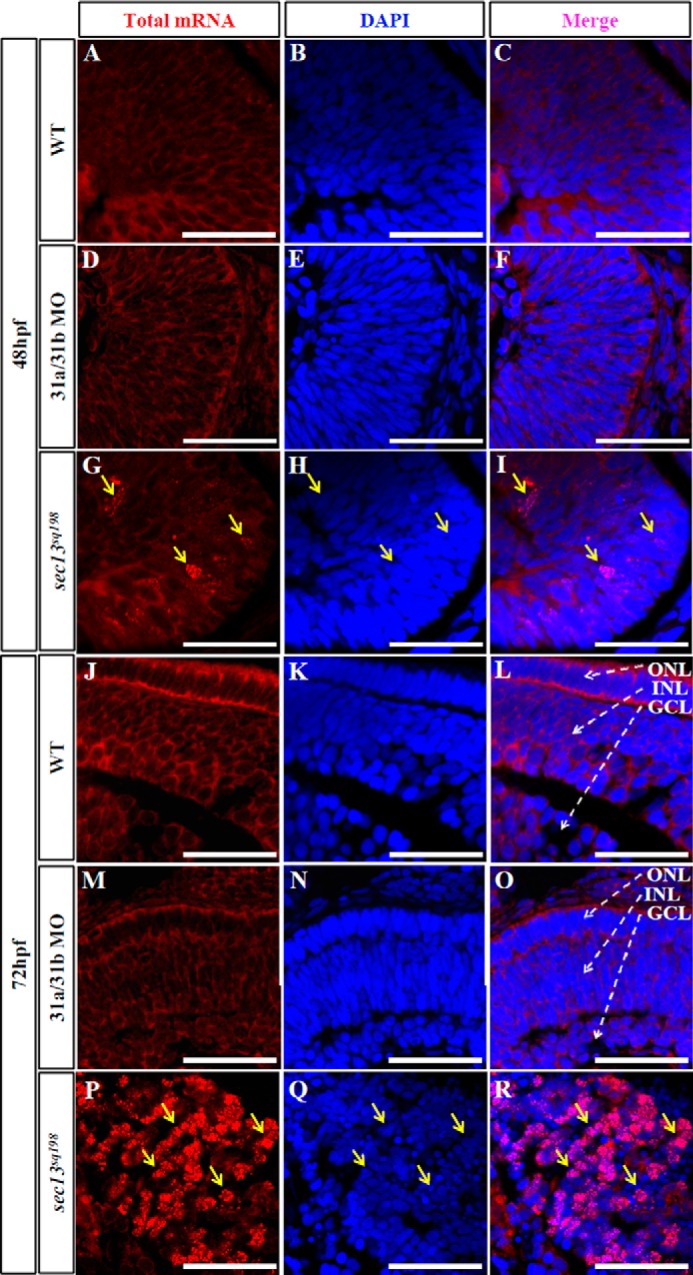
Total polyadenylated mRNAs accumulate in retinal cell nuclei in the sec13sq198 mutant. A–R, mRNA export assay as marked by Cy3-labeled oligo(dT)50 in WT (A–C and J–L), sec31a/sec31b double morphant (D–F and M–O), and sec13sq198 mutant (G–I and P–R) retinas at 48 and 72 hpf. In both WT (A–C and J–L) and sec31a/sec31b double morphant (D–F and M–O) retinal cells, mRNA was evenly distributed throughout the cytoplasm. However, in the sec13sq198 mutant retinal cells (G–I and P–R), export was blocked, and mRNA accumulated in the nuclei (shown by the yellow arrows). Scale bar, 50 μm.
Loss-of-Function of the NPC Component Nup107 Phenocopies the sec13sq198 Retinal Phenotype
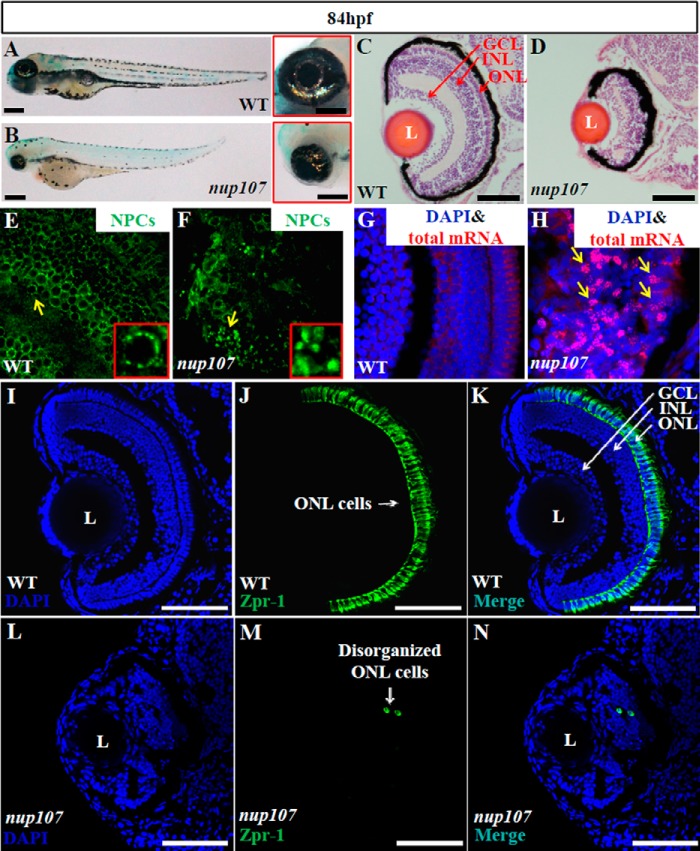
To better understand the role of NPCs in retinal development, we characterized the nup107tsu068Gt mutant, which carries a transposable element insertion in the first intron of nup107, a gene encoding another key NPC component (25). Similarly to the sec13sq198 mutant, the nup107tsu068Gt mutant developed a shortened body length, small brain, a defective pharyngeal skeleton, and small eye (Fig. 10, A and B). H&E staining showed that retinal lamination in the nup107tsu068Gt mutant embryos at 84 hpf was severely disrupted, together with a very significant loss of the ONL. This phenotype closely resembled the sec13sq198 mutant but not the embryos treated with BFA or the sec31a/sec31b double morphant (Fig. 10, C and D). Moreover, similarly to the sec13sq198 mutant, the formation of nuclear pores was impaired, and polyadenylated mRNA accumulated in the retinal cell nuclei in the nup107tsu068Gt mutant (Fig. 10, E–H). Immunostaining with Zpr-1 showed that, in contrast to the WT, the nup107tsu068Gt homozygous mutant lacked identifiable ONL, and only a few scattered cells expressed Zpr-1 (Fig. 10, I–N). This genetic evidence demonstrates that a functional nuclear pore is essential for retinal development.
FIGURE 10.
Loss-of-function of the NPC component Nup107 phenocopies the sec13sq198 retinal phenotype. A and B, WT embryo (A) and a nup107tsu068Gt mutant (nup107) embryo (B) at 84 hpf. Higher magnification of the eye image is shown on the right correspondingly. C and D, H&E staining analysis of the retina in the WT (C) and nup107tsu068Gt mutant (D) at 84 hpf. E and F, immunostaining of the NPCs in nuclear pores in WT (E) and nup107tsu068Gt mutant (F) at 84 hpf. G and H, mRNA export assay in the WT (G) and nup107tsu068Gt mutant (H) at 84 hpf. I–N, immunostaining of Zpr-1 in the WT (I–K) and nup107tsu068Gt mutant (L–N) retina at 84 hpf. L, lens. Scale bar, 300 μm (A and B), 150 μm (insets in A and B), and 50 μm (C, D, and I–N).
Activation of the p53-dependent Apoptotic Pathway Contributes to Defective Retinal Lamination in the sec13sq198 Mutant
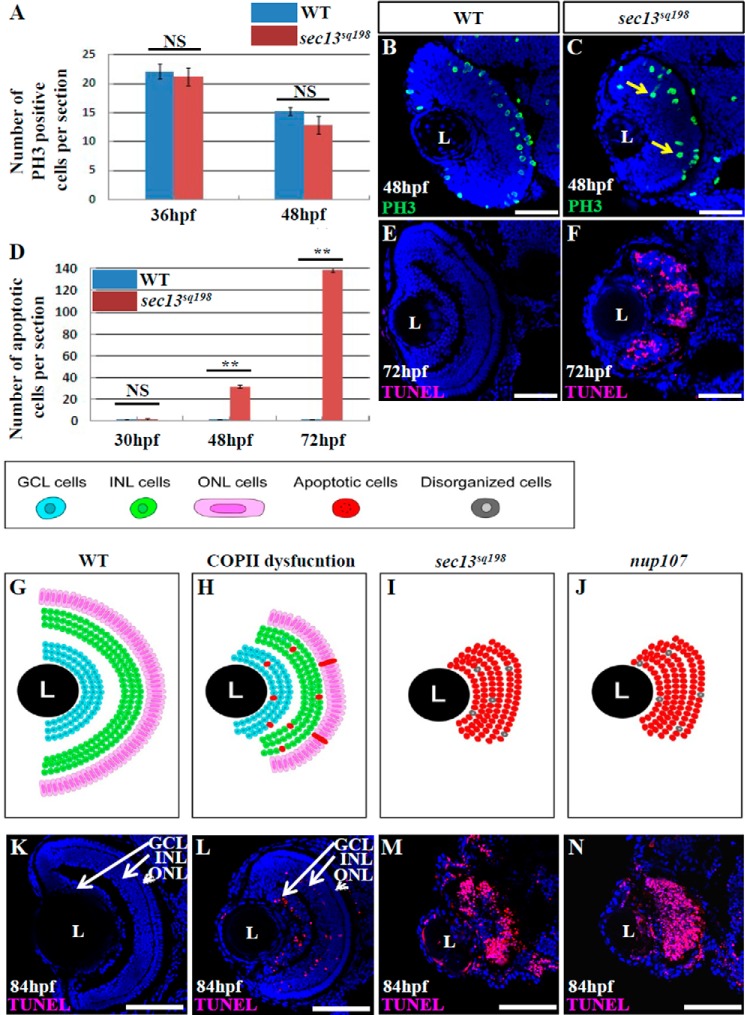
Our previous study demonstrated that the hypoplasia of digestive organs in sec13sq198 mutant embryos was caused by cell cycle arrest and activated apoptosis (7). To explore the cause of the small eye phenotype, we examined cell cycle progression through immunostaining with PH3 marking M phase cells, and the BrdU labeling assay for S phase cells. We found that PH3-positive cells were located mainly in the peripheral region of the retina at 36 hpf (Fig. 11A and data not shown) and at 48 hpf (Fig. 11, A–C) in both WT and sec13sq198 embryos, as reported previously (28). In particular, we consistently observed a small number of PH3-positive cells in the inner layers of the retina in the sec13sq198 mutant but not in the WT (Fig. 11C). No obvious difference in the distribution of BrdU-positive cells was observed between the WT and the sec13sq198 retina at 40 hpf (BrdU solution was injected at 36 hpf), with BrdU-positive cells being distributed throughout the entire retina (data not shown). At 52 hpf (BrdU solution was injected at 48 hpf), BrdU-positive cells were found in the ONL and the outer half of the INL in the WT retina. In contrast, BrdU-positive cells continued to be present in the entire retina of the sec13sq198 mutant (data not shown).
FIGURE 11.
Failure in the formation of nuclear pores triggers cell apoptosis, causing the disruption of retinal lamination in the sec13sq198 mutant. A, average number of PH3 positive cells per section/embryo in WT and sec13sq198 mutant retina is shown (n = 3, four sections from each embryo were used for counting the PH3-ositive cells). B and C, representative image of PH3 staining in the WT (B) and sec13sq198 mutant (C) retina at 48 hpf. D, average number of apoptotic cells in the WT and sec13sq198 mutant retina at 30, 48, and 72 hpf (n = 3, four sections from each embryo were used for counting the apoptotic cells). E and F, representative image of the TUNEL assays of apoptotic cells in WT (E) and sec13sq198 mutant (F) retina at 72 hpf. G–J, drawings represent the retinal structure by highlighting the cells in the GCL, INL, and ONL in the WT, BFA-treated (representing COPII-dysfunction), sec13sq198 mutant, and nup107tsu068Gt mutant (representing NPC dysfunction) at 84 hpf. K–N, drawings based on TUNEL analysis of apoptotic cells in different genotypes (entire retina). L, lens. Scale bar, 50 μm. NS, no significance. **, p < 0.01.
To assess the status of cell apoptosis in both WT and mutant retinas, we performed a TUNEL assay. We found almost no apoptotic cells in either WT or mutant retinas (on average, 0.42 apoptotic cells in WT and 0.33 apoptotic cells in mutant retina per section) at 30 hpf (Fig. 11D). However, starting from 48 hpf, some apoptotic cells appeared in the GCL and INL, but rarely in the ONL in the sec13sq198 mutant (on average, one apoptotic cell in the WT and 31.5 apoptotic cells in the mutant retina per section) (Fig. 11D). An excessive number of apoptotic cells was observed in the entire mutant retina by 72 hpf, whereas almost none were found in the WT (on average, 0.58 apoptotic cells in the WT and 139 in the mutant retina per section) (Fig. 11, D–F). We also compared the status of cell apoptosis between the WT retina (Fig. 11, G and K), a BFA-treated retina (with COPII dysfunction) (Fig. 11, H and L), a sec13sq198 mutant (with NPC dysfunction) (Fig. 11, I and M), and a nup107tsu068Gt mutant (Fig. 11, J and N) at 84 hpf. The results showed that both nup107tsu068Gt and sec13sq198 mutants exhibited extensive cell apoptosis coupled with disruption of retinal lamination (Fig. 11, I, J, M, and N).
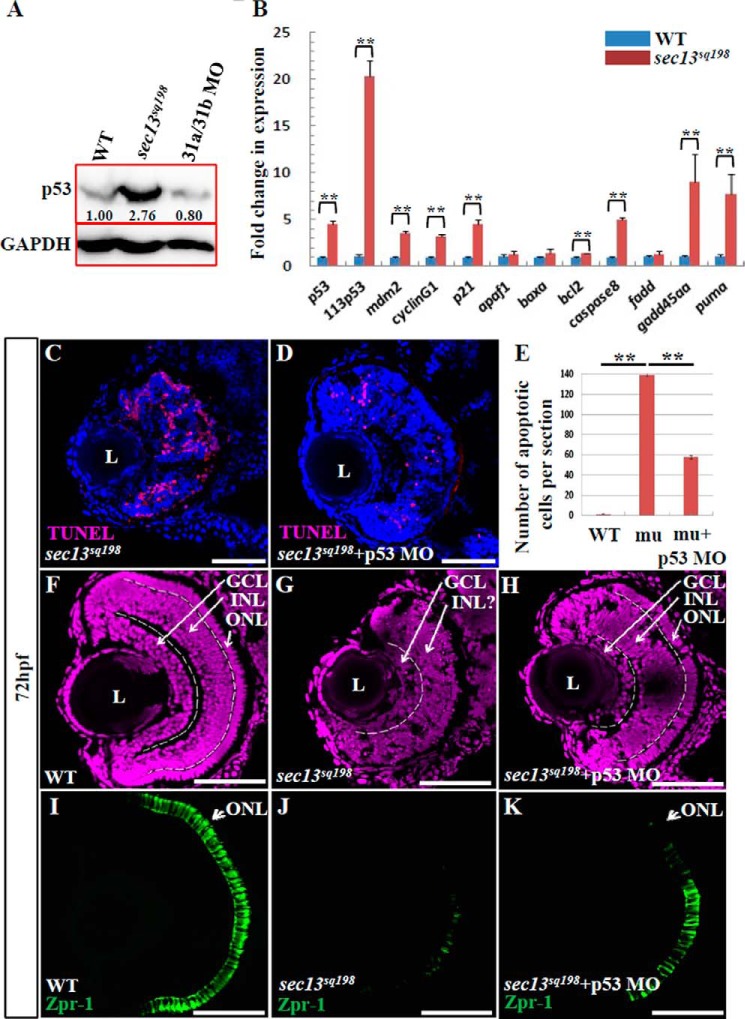
A possible outcome of the nuclear accumulation of total mRNA could be the activation of the apoptotic pathway. The tumor suppressor p53 is a well known transcription factor that functions to control the expression of genes involved in promoting cell cycle arrest and cell apoptosis (45). The activation of p53 has been found to be responsible for abnormally elevated apoptotic activity in some zebrafish mutants, including the flo mutant (46). Western blot analysis showed that the level of p53 protein was significantly up-regulated in the sec13sq198 mutant retina (Fig. 12A). We then compared the expression of p53-response genes in WT and sec13sq198 mutant retinas. The result showed that, in addition to p53, Δ113p53, p21, cyclinG1, and mdm2, genes promoting cell apoptosis, including caspase8, gadd45aa, and puma, were drastically up-regulated in the sec13sq198 mutant retina (Fig. 12B). These data suggest that significant p53-related cell death occurs in the sec13sq198 mutant retina. We therefore hypothesized that this cell death might be a major contributor to the eye phenotype. To test this, we used the p53 morpholino (47) and performed TUNEL assays on sec13sq198 mutant embryos injected with p53 morpholino. We found that a knockdown of p53 greatly reduced the number of apoptotic cells (on average, 139 apoptotic cells in the control MO-injected mutant retina and 58 apoptotic cells in the p53 MO-injected mutant retina per section) at 72 hpf (Fig. 12, C–E). Strikingly, the knockdown of p53 partially rescued the retinal development, especially the ONL, in the sec13sq198 mutant eye (Fig. 12, F–K).
FIGURE 12.
Knockdown of p53 partially restores the sec13sq198 mutant retina lesion. A, Western blot of p53 protein in the WT, sec31a/sec31b double morphant, and sec13sq198 mutant retina. Value for p53 protein in WT is set as 1. B, qPCR analysis of the expression of genes of the p53 and apoptosis pathway in the WT and sec13sq198 mutant retina. The relative expression level for each gene was expressed as the fold change in expression after normalization against elf1a. The p value was obtained by performing the two-tailed unpaired t test. C and D, representative image of the TUNEL assays of apoptotic cells in sec13sq198 mutant (C) and p53-ATG morpholino (p53 MO)-injected sec13sq198 mutant (D) retina at 72 hpf. E, average number of apoptotic cells in the WT, sec13sq198 mutant, and p53 MO-injected sec13sq198 mutant retina at 72 hpf (n = 3, four sections from each embryo were used for counting the apoptotic cells in each case). F–H, overview of the retina in WT (F), sec13sq198 mutant (G), and p53 MO-injected sec13sq198 mutant (H) embryos. I–K, immunostaining of Zpr-1 in WT (I), sec13sq198 mutant (J), and p53 MO-injected sec13sq198 mutant (K) retinas. Clearly, a knockdown of p53 partially restored the layered structure (H) (outlined with white lines) and ONL (K) in the sec13sq198 mutant retina. L, lens. Scale bar, 50 μm (C, D, and F–K). **, p < 0.01.
DISCUSSION
Sec13 serves as a key component in two functional distinct protein complexes, the COPII complex and NPCs, by interacting with distinct partners. Given the importance of these two complexes for normal cellular activities, it would be expected that the COPII and NPC functions of Sec13 are indispensable for the organogenesis of a specific organ/tissue, although the specific requirement for these two functions is likely to vary in different organs/tissues. However, no genetic model has previously been reported to distinguish these two functions of Sec13 at the level of the whole organism. Comparison of the structure of ER, the formation of NPC, and the distribution of total mRNA revealed that these subcellular organelles are defective both in the retina and digestive organs in the sec13sq198 mutant at later developmental stages, however, showing different degree of severity in different organs/tissues. Therefore, the sec13sq198 mutant serves as an ideal genetic model not only for the delineation of the molecular mechanism of the dual Sec13 function in retinogenesis but also in the organogenesis of different organs or, specifically, differentiation of different cell types.
A recent report showed that, in addition to exhibiting defective digestive organ development (9), the knockdown of Sec13 also led to a small eye phenotype (28). Schmidt et al. (28) found that the COPII function of Sec13 is essential to the secretion of opsin from the photoreceptor cells and collagen from the RPE to support the development of the ONL and RPE. However, the possible contribution of NPC function of Sec13 to the process of retinal lamination was not explored (28).
We previously reported the identification of the zebrafish sec13sq198 mutant that exhibited dysplastic digestive organs due to the dysfunction of the COPII complex (7). Here, we showed that the sec13sq198 mutant also displayed a small eye phenotype with the disruption of retinal lamination at 72 hpf as reported for the sec13 morphant (28). The relative normal initiation of eye development before 48 hpf in the sec13sq198 mutant is likely due to the maternal deposited Sec13 protein. However, by 72 hpf, the amount of Sec13 in the sec13sq198 mutant is not sufficient to support the later developmental events, thus mimicking a conditional genetic model. Surprisingly, our data showed that blocking the COPII function either by a double knockdown of Sec31a and Sec31b or by drug treatment (BFA), despite causing severe defects in the development of the digestive organs and eyes, did not abolish the retinal lamination as observed in the sec13sq198 mutant. Together with the data obtained in Sec23a, Sec23b, and Sec24c mutants/morphants, we conclude that the COPII dysfunction is not the primary cause of the disruption of retinal lamination in the sec13sq198 mutant.
Previous reports have demonstrated that NPC activity is essential for development of retinal architecture. For example, mutation of the elys gene, which encodes a component of NPCs, not only results in defective digestive organs but also leads to a disrupted retinal structure due to the failure to differentiate the proliferating precursors to neurons from their stem cell niche (22–24). We found that the sec13sq198 mutant, but not the sec31a/sec31b double morphant or the embryo treated with BFA, lacks a nuclear pore structure when examined by TEM and immunostaining of Nup153, Nup214, and Nup358. The structural deficits of the NPCs were accompanied by functional impairment, causing an abnormal accumulation of polyadenylated mRNA in mutant nuclei. The nuclear accumulation of polyadenylated mRNA is likely to ignite a cellular stress response that finally results in an up-regulation of p53-dependent apoptosis in the retinal cells resulting in the defective retinal phenotype. It is notable that the loss-of-function of Elys also activates the p53 pathway to cause apoptosis (24). This is the case for the nup107tsu068Gt mutant retina as well (25). Therefore, it appears that comprising NPC function is not compatible with cell viability and the lack of cells in these three different NPC mutants yields lamination defects. This effect is presumably mediated by the p53 pathway because p53 is activated in elys−/−, nup107tsu068Gt, and sec13sq198 mutants and because down-regulating p53 expression alleviated the sec13sq198 retinal phenotype. Thus, it can be considered that the p53 pathway is an effector of compromising NPC function. In fact, it is often seen that down-regulation of a downstream effector can fully or partially rescue the mutant phenotype in the mutant background. Reduction in cell apoptosis after p53 knockdown is likely one of the key reasons for the partial rescue of the retinal phenotype in sec13sq198. However, it is well known that p53 is a pan-transcription factor that regulates the expression of over 1000 genes. Therefore, in addition to apoptosis, we cannot rule out the possibilities that certain p53 target gene products are involved in regulating retinal lamination.
Interestingly, the loss of the Sec13 function appears to have a more profound effect on the ONL. We hypothesize that the cells in the ONL are probably more sensitive to dysfunction of both the COPII and NPC functions in the Sec13sq198 mutant, whereas other types of retinal cells can go through proper differentiation likely due to the presence of the maternal deposited Sec13 protein (7). This scenario is based on the role of Sec13 in the proper formation of nuclear pores to shuttle the mRNAs or proteins necessary for eye development between the nucleus and cytoplasm or in the formation of the COPII complex for protein trafficking. However, we cannot exclude the possibility that Sec13 and other NPC components can act alone or together to mediate specific gene expression to control eye development directly, especially given that the ONL but not the INL and GCL were severely affected in the sec13sq198 mutant, although total mRNA accumulated in the cells from all three layers. Recently, it has been reported that some NPC members participate directly in the regulation of chromatin organization and gene expression unrelated to their roles in NPCs. For instance, in Drosophila, nucleoporins Nup153 and Megator bind to continuous chromosomal regions to modulate gene expression (48). Drosophila nucleoporins Sec13, Nup98, and Nup88 were found to occupy ecdysone-induced gene loci (e.g. Sgs3 at locus 68C and gene loci 74EF and 75B) during the wandering third instar stage of larval development (49). Similarly, Nup98, Nup50, and Nup62 can directly regulate development and cell cycle genes (e.g. VEGF-related factor 2, Sema-2a, CyclinB, and Sak kinase) inside the nucleoplasm in Drosophila (50). It was also reported that the deletion of Nup210 blocked myogenesis and neurogenesis, independently of its role in nucleo-cytoplasmic transport (51). Therefore, it is possible that Sec13 teams up with an ONL-specific NPC member to control the expression of genes for the specification of the ONL cells. Future studies will be required to determine whether Sec13 and its partners in the NPCs can directly regulate the expression of genes that are important for the establishment of the eye's architecture.
Acknowledgments
We thank Dr. Jun Chen for valuable suggestions on this project and Dr. Tianhua Zhou for the generous supply of the Zpr-1 antibody.
This work was supported by “973 Program” Grant 2012CB944550 and National Natural Science Foundation of China Grant 31330050 (to J. R. P.). This work was also supported in part by National Institutes of Health Grant R01 DE018477 from NIDCR (to E. W. K.).
- ER
- endoplasmic reticulum
- NPC
- nuclear pore complex
- hpf
- hours post-fertilization
- Nup
- nucleoporin
- BFA
- brefeldin A
- WISH
- whole-mount in situ hybridization
- TEM
- transmission electron microscopy
- qPCR
- quantitative real time-PCR
- GCL
- ganglion cell layer
- ONL
- outer nuclear layer
- INL
- inner nuclear layer
- PH3
- phosphorylated histone 3
- RPE
- retinal pigment epithelium
- MO
- morpholino
- PDI
- protein-disulfide isomerase.
REFERENCES
- 1. Brohawn S. G., Leksa N. C., Spear E. D., Rajashankar K. R., Schwartz T. U. (2008) Structural evidence for common ancestry of the nuclear pore complex and vesicle coats. Science 322, 1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leksa N. C., Schwartz T. U. (2010) Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges. Nucleus 1, 314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onischenko E., Weis K. (2011) Nuclear pore complex: a coat specifically tailored for the nuclear envelope. Curr. Opin. Cell Biol. 23, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antonny B., Schekman R. (2001) ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 13, 438–443 [DOI] [PubMed] [Google Scholar]
- 5. Stagg S. M., Gürkan C., Fowler D. M., LaPointe P., Foss T. R., Potter C. S., Carragher B., Balch W. E. (2006) Structure of the Sec13/31 COPII coat cage. Nature 439, 234–238 [DOI] [PubMed] [Google Scholar]
- 6. Enninga J., Levay A., Fontoura B. M. (2003) Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell. Biol. 23, 7271–7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niu X., Gao C., Jan Lo L., Luo Y., Meng C., Hong J., Hong W., Peng J. (2012) Sec13 safeguards the integrity of the endoplasmic reticulum and organogenesis of the digestive system in zebrafish. Dev. Biol. 367, 197–207 [DOI] [PubMed] [Google Scholar]
- 8. Townley A. K., Feng Y., Schmidt K., Carter D. A., Porter R., Verkade P., Stephens D. J. (2008) Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J. Cell Sci. 121, 3025–3034 [DOI] [PubMed] [Google Scholar]
- 9. Townley A. K., Schmidt K., Hodgson L., Stephens D. J. (2012) Epithelial organization and cyst lumen expansion require efficient Sec13-Sec31-driven secretion. J. Cell Sci. 125, 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang M. R., Lapierre L. A., Frotscher M., Goldenring J. R., Knapik E. W. (2006) Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat. Genet. 38, 1198–1203 [DOI] [PubMed] [Google Scholar]
- 11. Boyadjiev S. A., Fromme J. C., Ben J., Chong S. S., Nauta C., Hur D. J., Zhang G., Hamamoto S., Schekman R., Ravazzola M., Orci L., Eyaid W. (2006) Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat. Genet. 38, 1192–1197 [DOI] [PubMed] [Google Scholar]
- 12. Schwarz K., Iolascon A., Verissimo F., Trede N. S., Horsley W., Chen W., Paw B. H., Hopfner K. P., Holzmann K., Russo R., Esposito M. R., Spano D., De Falco L., Heinrich K., Joggerst B., Rojewski M. T., Perrotta S., Denecke J., Pannicke U., Delaunay J., Pepperkok R., Heimpel H. (2009) Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat. Genet. 41, 936–940 [DOI] [PubMed] [Google Scholar]
- 13. Sarmah S., Barrallo-Gimeno A., Melville D. B., Topczewski J., Solnica-Krezel L., Knapik E. W. (2010) Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS One 5, e10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beck M., Förster F., Ecke M., Plitzko J. M., Melchior F., Gerisch G., Baumeister W., Medalia O. (2004) Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science 306, 1387–1390 [DOI] [PubMed] [Google Scholar]
- 15. Alber F., Dokudovskaya S., Veenhoff L. M., Zhang W., Kipper J., Devos D., Suprapto A., Karni-Schmidt O., Williams R., Chait B. T., Rout M. P., Sali A. (2007) Determining the architectures of macromolecular assemblies. Nature 450, 683–694 [DOI] [PubMed] [Google Scholar]
- 16. Lutzmann M., Kunze R., Buerer A., Aebi U., Hurt E. (2002) Modular self-assembly of a Y-shaped multiprotein complex from seven nucleoporins. EMBO J. 21, 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagy V., Hsia K. C., Debler E. W., Kampmann M., Davenport A. M., Blobel G., Hoelz A. (2009) Structure of a trimeric nucleoporin complex reveals alternate oligomerization states. Proc. Natl. Acad. Sci. U.S.A. 106, 17693–17698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senger S., Csokmay J., Akbar T., Tanveer A., Jones T. I., Sengupta P., Lilly M. A. (2011) The nucleoporin Seh1 forms a complex with Mio and serves an essential tissue-specific function in Drosophila oogenesis. Development 138, 2133–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu X., Kasper L. H., Mantcheva R. T., Mantchev G. T., Springett M. J., van Deursen J. M. (2001) Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc. Natl. Acad. Sci. U.S.A. 98, 3191–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okita K., Kiyonari H., Nobuhisa I., Kimura N., Aizawa S., Taga T. (2004) Targeted disruption of the mouse ELYS gene results in embryonic death at peri-implantation development. Genes Cells 9, 1083–1091 [DOI] [PubMed] [Google Scholar]
- 21. Lupu F., Alves A., Anderson K., Doye V., Lacy E. (2008) Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev. Cell 14, 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Jong-Curtain T. A., Parslow A. C., Trotter A. J., Hall N. E., Verkade H., Tabone T., Christie E. L., Crowhurst M. O., Layton J. E., Shepherd I. T., Nixon S. J., Parton R. G., Zon L. I., Stainier D. Y., Lieschke G. J., Heath J. K. (2009) Abnormal nuclear pore formation triggers apoptosis in the intestinal epithelium of elys-deficient zebrafish. Gastroenterology 136, 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cerveny K. L., Cavodeassi F., Turner K. J., de Jong-Curtain T. A., Heath J. K., Wilson S. W. (2010) The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development 137, 2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davuluri G., Gong W., Yusuff S., Lorent K., Muthumani M., Dolan A. C., Pack M. (2008) Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress. PLoS Genet. 4, e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng X., Yang S., Han Y., Zhao X., Zhao L., Tian T., Tong J., Xu P., Xiong C., Meng A. (2012) Loss of zygotic NUP107 protein causes missing of pharyngeal skeleton and other tissue defects with impaired nuclear pore function in zebrafish embryos. J. Biol. Chem. 287, 38254–38264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pujic Z., Malicki J. (2004) Retinal pattern and the genetic basis of its formation in zebrafish. Semin. Cell Dev. Biol. 15, 105–114 [DOI] [PubMed] [Google Scholar]
- 27. Raymond P. A., Barthel L. K., Curran G. A. (1995) Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. J. Comp. Neurol. 359, 537–550 [DOI] [PubMed] [Google Scholar]
- 28. Schmidt K., Cavodeassi F., Feng Y., Stephens D. J. (2013) Early stages of retinal development depend on Sec13 function. Biol. Open. 2, 256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang H., Ruan H., Aw M. Y., Hussain A., Guo L., Gao C., Qian F., Leung T., Song H., Kimelman D., Wen Z., Peng J. (2008) Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development 135, 3209–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J., Ng S. M., Chang C., Zhang Z., Bourdon J. C., Lane D. P., Peng J. (2009) P53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 23, 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Granero-Moltó F., Sarmah S., O'Rear L., Spagnoli A., Abrahamson D., Saus J., Hudson B. G., Knapik E. W. (2008) Goodpasture antigen-binding protein and its spliced variant, ceramide transfer protein, have different functions in the modulation of apoptosis during zebrafish development. J. Biol. Chem. 283, 20495–20504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tao T., Shi H., Guan Y., Huang D., Chen Y., Lane D. P., Chen J., Peng J. (2013) Def defines a conserved nucleolar pathway that leads p53 to proteasome-independent degradation. Cell Res. 23, 620–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meyers J. R., Hu L., Moses A., Kaboli K., Papandrea A., Raymond P. A. (2012) Ss-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bovolenta P., Mallamaci A., Briata P., Corte G., Boncinelli E. (1997) Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J. Neurosci. 17, 4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chuang J. C., Mathers P. H., Raymond P. A. (1999) Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech. Dev. 84, 195–198 [DOI] [PubMed] [Google Scholar]
- 36. Nelson S. M., Park L., Stenkamp D. L. (2009) Retinal homeobox 1 is required for retinal neurogenesis and photoreceptor differentiation in embryonic zebrafish. Dev. Biol. 328, 24–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fath S., Mancias J. D., Bi X., Goldberg J. (2007) Structure and organization of coat proteins in the COPII cage. Cell 129, 1325–1336 [DOI] [PubMed] [Google Scholar]
- 38. Ohisa S., Inohaya K., Takano Y., Kudo A. (2010) Sec24d encoding a component of COPII is essential for vertebra formation, revealed by the analysis of the medaka mutant, vbi. Dev. Biol. 342, 85–95 [DOI] [PubMed] [Google Scholar]
- 39. Melville D. B., Knapik E. W. (2011) Traffic jams in fish bones: ER-to-Golgi protein transport during zebrafish development. Cell Adh. Migr. 5, 114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pyati U. J., Gjini E., Carbonneau S., Lee J. S., Guo F., Jette C. A., Kelsell D. P., Look A. T. (2011) P63 mediates an apoptotic response to pharmacological and disease-related ER stress in the developing epidermis. Dev. Cell 21, 492–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samali A., Fitzgerald U., Deegan S., Gupta S. (2010) Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int. J. Cell Biol. 2010, 830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson C. L., Casanova J. E. (2000) Turning on ARF: the Sec7 family of guanine-nucleotide exchange factors. Trends Cell Biol. 10, 60–67 [DOI] [PubMed] [Google Scholar]
- 43. Nebenführ A., Ritzenthaler C., Robinson D. G. (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 130, 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franz C., Askjaer P., Antonin W., Iglesias C. L., Haselmann U., Schelder M., de Marco A., Wilm M., Antony C., Mattaj I. W. (2005) Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. EMBO J. 24, 3519–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Amundson S. A., Myers T. G., Fornace A. J., Jr. (1998) Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene 17, 3287–3299 [DOI] [PubMed] [Google Scholar]
- 46. Azuma M., Toyama R., Laver E., Dawid I. B. (2006) Perturbation of rRNA synthesis in the bap28 mutation leads to apoptosis mediated by p53 in the zebrafish central nervous system. J. Biol. Chem. 281, 13309–13316 [DOI] [PubMed] [Google Scholar]
- 47. Chen J., Ruan H., Ng S. M., Gao C., Soo H. M., Wu W., Zhang Z., Wen Z., Lane D. P., Peng J. (2005) Loss of function of def selectively up-regulates Δ113p53 expression to arrest expansion growth of digestive organs in zebrafish. Genes Dev. 19, 2900–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaquerizas J. M., Suyama R., Kind J., Miura K., Luscombe N. M., Akhtar A. (2010) Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 6, e1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Capelson M., Liang Y., Schulte R., Mair W., Wagner U., Hetzer M. W. (2010) Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 140, 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalverda B., Pickersgill H., Shloma V. V., Fornerod M. (2010) Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140, 360–371 [DOI] [PubMed] [Google Scholar]
- 51. DeAngelis R. A., Markiewski M. M., Kourtzelis I., Rafail S., Syriga M., Sandor A., Maurya M. R., Gupta S., Subramaniam S., Lambris J. D. (2012) A complement-IL-4 regulatory circuit controls liver regeneration. J. Immunol. 188, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]