Background: Unique glycan structures on glycoprotein hormones are recognized by glycan-specific receptors that control hormone half-life in the blood.
Results: Clearance rates for recognized glycoproteins are determined by the level of receptor expression in the liver.
Conclusion: Expression of glycan-specific receptors is modulated at critical points in the reproductive cycle.
Significance: Glycans and glycan-specific receptors play critical roles in reproduction.
Keywords: Carbohydrate-binding Protein, Carbohydrate Function, Carbohydrate Glycoprotein, Endocrinology, Endocytosis, Hormones, Circulatory Half-life
Abstract
The rate at which glycoproteins are cleared from the circulation has a critical impact on their biologic activity in vivo. We have shown that clearance rates for glycoproteins such as luteinizing hormone (LH) that undergo regulated release into the circulation determine their potency. Two highly abundant, carbohydrate-specific, endocytic receptors, the asialoglycoprotein receptor (ASGR) and the mannose receptor (ManR) are expressed in the liver by parenchymal and sinusoidal endothelial cells, respectively. We demonstrate that the ManR mediates the clearance of glycoproteins such as LH that bear N-linked glycans terminating with β1,4-linked GalNAc-4-SO4, as well as glycoproteins bearing glycans that terminate with Man. Steady state levels of mRNA encoding the ASGR and the ManR are regulated by progesterone in pregnant mice, reaching maximal levels on day 12.5 of pregnancy. Protein expression and glycan-specific binding activity also increase in the livers of pregnant mice. In contrast, ManR mRNA, but not ASGR mRNA, decreases in male mice at the time of sexual maturation. We show that levels of ManR and ASGR expression control the clearance rate for glycoproteins bearing recognized glycans. Thus, reduced expression of the ManR at the time of sexual maturation will increase the potency of LH in vivo, whereas increased expression during pregnancy will reduce LH potency until progesterone and receptor levels fall prior to parturition.
Introduction
Our quest to understand the biologic significance of unique N-linked glycans terminating with β1,4-linked GalNAc-4-SO4, that we originally described on the glycoprotein hormones luteinizing hormone (LH)2 and thyroid-stimulating hormone (1–3), led us to the realization that these glycans are recognized by a receptor in hepatic sinusoidal endothelial cells (SEC) that determine their circulatory half-life following regulated release into the blood (4, 5). Differences in the branching pattern and terminal sugars on glycans can alter their recognition by carbohydrate-specific receptors and as a consequence their circulatory half-life. It is also possible that altered levels of carbohydrate specific receptors modulate the clearance rates for such glycoproteins at critical times such as during pregnancy or at the time of sexual maturation. However, to date, no technology has been available to define and compare clearance rates in vivo for glycoprotein species that only differ in their respective pattern of terminal glycosylation. We have overcome this limitation by expressing recombinant chimeric glycoproteins containing Gaussia luciferase in cells with different complements of glycosyltranferases (6–8). The clearance of these chimeric glycoproteins bearing different glycan structures from the blood of mice can be characterized by analyzing the decline in Gaussia luciferase activity in blood with time following injection. We have used this novel strategy to illuminate the role of carbohydrate-specific receptor expression in regulating glycoprotein hormone clearance rates from the circulation in vivo.
The asialoglycoprotein receptor (ASGR) and the mannose receptor (ManR) are carbohydrate-specific, endocytic receptors expressed by hepatic parenchymal cells and SECs, respectively (5, 9–11). The ASGR consists of two subunits, Asgr-1 and Asgr-2 (12–14), both of which must be expressed to generate an active receptor able to bind glycans terminating with β-linked Gal or GalNAc (15–17). SECs in the liver predominantly express a dimeric form of the ManR that binds glycans terminating with β1,4-linked GalNAc-4-SO4 via a ricin-type β-trefoil domain, the Cys-rich domain, located at the amino terminus (18–20). Monomeric forms of the ManR that bind glycans terminating with mannose via multiple C-type lectin domains located near the transmembrane domain predominate in macrophages (19, 21). Based on ligand binding studies with isolated cells, 5 × 105 binding sites for glycans with terminal Gal are present at the surface of hepatocytes (22), and 5.8 × 105 binding sites for glycans with terminal GalNAc-4-SO4 are present at the surface of SECs (5). The number of receptors expressed at the cell surface, their rapid rate of internalization and recycling, and the large number of parenchymal cells and SECs that constitute the liver result in carbohydrate receptor systems with an enormous capacity to remove glycoproteins bearing distinct glycans from the circulation.
Despite the capacity of these receptor systems for clearance, the number of endogenous glycoproteins identified that are cleared from the circulation in vivo by either the ASGR (23–25) or the ManR (26, 27) is relatively limited, and little is known about regulation of the expression of either receptor in relation to function. As a consequence, our understanding of the biologic role played by these receptors in vivo remains incomplete. We have previously shown that LH terminating with the sequence SO4-4-GalNAcβ1,4GlcNAcβ1,2Man is rapidly cleared from the circulation by SECs in the liver (4, 5). We demonstrated that the Cys-rich domain of the ManR binds terminal GalNAc-4-SO4 and mediates the rapid clearance of LH in vivo (5, 18, 27). Ablation of GalNAc-4-sulfotransferase-1 (GalNAc-4-ST1, CHST8) prevents the addition of SO4 to LH glycans resulting in modification with α2,6-linked sialic acid to form Siaα2,6GalNAcβ1,4GlcNAcβ1,2Man. Following GalNAc-4-ST1 ablation LH half-life is increased, testosterone and estrogen levels are increased, sexual maturation is precocious, and seminal vesicles and uteri are enlarged, indicating that LH is more potent due to its extended half-life (28).
The ManR must be dimeric to simultaneously engage two terminal GalNAc-4-SO4 moieties on separate N-linked glycans with an association constant of 2.32 × 10−7 m (19). Because circulating LH levels are well below the association constant for binding, the LH clearance rate will not be sensitive to LH concentration but should be sensitive to the amount of receptor expressed at the cell surface and the rate of receptor internalization. In the present study, we have defined the regulation of expression for both ASGR and ManR clearance systems during critical times in the reproductive life cycle in both female and male mice. We have found that receptor expression is sensitive to progesterone in female mice. In contrast, receptor levels do not change in response to progesterone in male mice. Our demonstration of marked differences in expression of both receptor message and protein levels underscores how the amount of receptor indeed regulates the rate of clearance of glycoproteins bearing recognized glycans from the blood. Thus changes in ManR expression will modulate clearance rate and as a consequence LH potency at critical times such as pregnancy and sexual maturation.
EXPERIMENTAL PROCEDURES
Materials
SO4-4-GalNAcβ1,4GlcNAcβ1,2Manaβ-bovine serum albumin (S4GGnM-BSA) and asialoorosomucoid (ASOR) were prepared as described previously (5, 22). Asgr-2−/− mice (15, 16) were obtained from Jackson ImmunoResearch Laboratories, and ManR−/− mice (26) were generously provided by Dr. M.C. Nussenzweig (Rockefeller University).
Radiolabeling
S4GGnM-BSA (25 μg) and ASOR (25 μg), respectively, in 95 μl of 20 mm Tris-HCl, 150 mm NaCl, adjusted to pH 7.4 (TBS) were incubated on ice for 15 min with 5 μCi of 125I and one IODO-BEAD® (Pierce). S4GGnM-[125I]BSA and [125I]ASOR were separated from free 125I by gel filtration using Bio-Spin® 6 Tris columns (Bio-Rad) spun for 4 min at 1,000 × g. After adding BSA (1 mg/ml), the radiolabeled proteins were stored at −20 °C. The amount of radiolabel incorporated into protein was determined by precipitation with 10% (w) trichloroacetic acid.
Preparation of Liver Membranes
Anesthetized mice were sacrificed by cervical dislocation and the livers immediately harvested, rinsed with 25 mm NaPO4, 150 mm NaCl, adjusted to pH 7.4, and weighed. Individual livers were suspended in 10 volumes (Vol:Wt) of TBS and homogenized 3 times for 15 s each on ice using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) with a PT10 probe. After sedimenting the homogenates for 5 min at 1,500 × g, the resulting supernatants were collected and sedimented for 75 min at 100,000 × g at 4 °C. The pellets were suspended in TBS-2 mm CaCl2 containing 0.5% (w/v) Triton X-100 using a 1-ml glass Dounce homogenizer and stored at −80 °C. Protein concentrations were determined using the non-interfering protein method (G-Biosciences, St. Louis, MO).
Binding Assays
Assays were performed in 100 μl of TBS and 2 mm CaCl2 containing 0.5% (w/v) Triton X-100, 150 μg of bovine IgG, 2 × 104 cpm of S4GGnM-[125I]BSA or [125I]ASOR and 100–300 μg of liver membrane protein. Following incubation for 30 min at 25 °C, 90 μl of ice cold 20% (w/v) PEG 8000 in TBS, 2 mm CaCl2 was added, and the samples were vortexed for 2 s. The samples were then incubated for 30 min at 4 °C to precipitate receptor-ligand complexes. Receptor-ligand complexes were collected by filtration using MultiScreen®-FC 96 well plates (Millipore, Bedford, MA) previously treated with 5 mg/ml BSA in TBS and 2 mm CaCl2 to prevent nonspecific binding. Filters were rinsed 3 times with ice cold 10% (w/v) PEG 8000 in TBS and 2 mm CaCl2, and the amount of 125I-labeled ligand captured determined using a γ-counter. Incubation in the presence of 100 μm GalNAc-4-SO4 or GalNAc blocked binding of S4GGnM-[125I]BSA and [125I]ASOR, respectively. In addition, incubation with membrane preparations from ManR−/− or Asgr-2−/− mice did not exhibit binding of S4GGnM-[125I]BSA and [125I]ASOR, respectively.
Western Blot
Proteins (10 μg) were separated on NuPAGE® Bis-Tris 4–12% gels without reduction and electrophoretically transferred to Immobilon-P® (Millipore). Membranes were then incubated in 1% casein in TBS plus 0.005% Tween 20 Surfactant-Amps Detergent (Pierce) (TBS-T) for 2 h at 25 °C and washed three times with TBS-T. After overnight incubation at 4 °C with either rabbit anti-rat ASGR (1:20,000) or rabbit anti-rat ManR (1:10,000) in TBS-T, the membranes were washed three times with TBS-T and incubated with stabilized goat anti-rabbit-IgG horseradish peroxidase-conjugated secondary antibody (1:300,000) for 2 h at 4 °C, washed with TBS-T, and exposed with SuperSignal® West Femto maximum sensitivity substrate (Thermo Scientific, Rockford, IL) for up to 5 min. The specificity of the rabbit anti-rat ASGR and anti-rat ManR antibodies was established using pre-immune serum from the same rabbits and by examining membrane preparations from Asgr−/− and ManR−/− mice. Upon blotting samples with pre-immune serum, none of the bands used to quantitate either the ASGR or the ManR with immune serum in either pregnant or non-pregnant mice were present. In addition, the bands that reacted with the anti-rat ASGR were retained by GalNAc-bearing affinity columns (24).
Progesterone Implantation
Pellets (Innovative Research of America, Sarasota, FL) designed to release 2.5 mg of progesterone over 21 days were implanted subcutaneously in the dorsal side of the neck using a 10-gauge trochar. After 72 h, the mice were sacrificed, and the livers were harvested for liver membrane preparation as described above.
Analysis of mRNA Expression by Real-time Quantitative PCR
Tissues were homogenized in TRIzol (Invitrogen) according to the manufacturer's instructions. RNA purity and concentration were determined with Nanodrop 2000c (NanoDrop Products, Wilminton, DE). One microgram of total RNA was reverse-transcribed using Omniscript Reverse Transcriptase (Qiagen, Valencia, CA) in a volume of 20 μl using the protocol supplied by the manufacturer. For real-time PCR, each reaction contained 9 μl of cDNA diluted 1:25 and 10 μl of Taqman Universal.
Fast Master Mix (Applied Biosystems), 1 μl of primer mix, and primers (see Table 1) were used to perform real-time PCR in triplicate using a StepOnePlus (Applied Biosystems). Melting curve analysis was performed to confirm the absence of nonspecific product amplification for each primer set.
TABLE 1.
Primers used for PCR
| Gene/Construct primer | Primer sequences (5′-3′) | Accession no. |
|---|---|---|
| Hprt1 | NM 013556 | |
| Probe | CTTGCTGGTGAAAAGGACCTCTCGAA | |
| Forward | AACAAAGTCTGGCCTGTATCC | |
| Reverse | CCCCAAAATGGTTAAGGTTGC | |
| MR1 (Mrc1) | NM 008625 | |
| Probe | ACGAAATCCCTGCTACTGAACCTCC | |
| Forward | ATGGATGTTGATGGCTACTGG | |
| Reverse | TTCTGACTCTGGACACTTGC | |
| Asgr-1 | NM 009714 | |
| Probe | TGACTGAAGCTCTGAAAGGACCTGC | |
| Forward | GAGTCGAAGCTGGAAAAACAG | |
| Reverse | CCTTCATACTCCACCCAGTTG | |
| Asgr-2 | NM 007493 | |
| Probe | ATTGCCCCGAAATGCAGCCAC | |
| Forward | ACGTGAAGCAGTTAGTGTCTG | |
| Reverse | CCTTCATACTCCACCCAGTTG | |
| GLucPSG23 | ||
| Gau3′-PSG23-5′-AS-B | ACGTGTTGTGGTGACTGGATGGTGACACTGTCACCACCGGCCCCCTTGATC | |
| Gaussia-3′-S-r | GATCAAGGGGGCCGGTGGTGArC | |
| PSG23-3′-MYC-5′-S-B | TCCCAGTCAGCCTGGCCGTGATGAATGAGTCTAGAGGGCCCGAACAAAAACTCATCTC | |
| LMYC-AS-r | GTCACCAGTGGAACCTGGAACrC | |
| Gaussia-3′-AS-r | GTCACCACCGGCCCCCTTGATrC | |
| LMYC-S-r | GAGGGCCCGAACAAAAACTCATCTrC | |
| mFut9 | ||
| mFUT9-BamHI-S | CGAGCTCGGATCCATGACATCAACATCCAAAGG | |
| mfut9-EcoRI-as | TGCAGAATTCCATTAATTCCAAAACCATTTCTCTAAATTACC | |
For quantification, ΔΔCT method was used to determine the relative expression between groups (29). The threshold cycle (CT) was determined for genes of interest and the housekeeping gene Hprt1. The data were normalized by subtracting the CT for each gene of interest from the CT for Hprt1 to determine the ΔCt. After this loading control correction, the relative expression levels of the genes of interest in an experimental sample was compared with that of calibrator sample by subtracting their ΔCT values. In our experiments, we used a cDNA pool of all the samples as the calibrator. By using the same calibrator, the relative expression for every sample was calculated using the following formula: relative fold change = 2−x, where x is the difference between the ΔCT of the experimental sample and the calibrator sample.
In Vivo Clearance Studies Using Gaussia Luciferase PSG-23 Chimeric Proteins
A chimeric glycoprotein, GLuc-PSG23, consisting of Gaussia luciferase (GLuc) followed by pregnancy-specific glycoprotein-23 (PSG23) (NM_064657.2), and the epitope tag Myc-His, was prepared by amplifying reverse transcribed RNA obtained from day 15 mouse placenta using the primers in Table 1, Gau3′PSG235′-AS-B and the riboprimer Gaussia-3′-S-r versus PSG233′MYC5′-S-B and the riboprimer LMYC-AS-r using Klentaq long and accurate DNA polymerase. The Myc-His epitope tag was added to pCMV-GLuc obtained from New England Biolabs and amplified using riboprimers Gaussia-3′-AS-r and LMYC-S-r. PSG23 devoid of the leader sequence was ligated into pCMV-GLuc-Myc-His following digestion with ribonuclease (6).
mFut9 (NM_010243) was prepared by amplifying reverse-transcribed RNA obtained from day 15 mouse placenta by PCR amplification with Klentaq long and accurate DNA polymerase using primers mFut9-BamHI-S and mFut9-EcoRI-AS. Following digestion with BamHI and EcoRI, the cDNA was ligated into the expression vector pcDNA3.1 (Invitrogen).
pCMV-GLuc-PSG23 was expressed in CHO, CHO/T3, and CHO-Lec8 cells grown in serum-free ultra CHO medium (Lonza 12-724Q) as described previously (6). Products were analyzed by SDS-PAGE. The efficiency of N-glycan modification was monitored using a combination of glycosidase digestion and lectin binding. For example, GLuc-PSG23 expressed in CHO cells did not bind to RCA-I prior to digestion with neuraminidase. Following neuraminidase digestion GLuc-PSG23 was quantitatively bound by RCA-I, and binding could be abolished by digestion with diplococcal β-galactosidase. In contrast, co-expression of GLuc-PSG23 with mFut9 in CHO cells prevented sialic acid addition and allowed GLuc-PSG23:Galβ1,4[Fucα1,3]GlcNAc to be bound by RCA-I. Digestion with diplococcal β-galactosidase was not effective at reducing binding to RCA-I, indicating the terminal sequence on the vast majority, if not all, of N-linked glycans consisted of the LewisX epitope Galβ1,4[Fucα1,3]GlcNAc. Similarly, GLuc-PSG23 expressed in CHO/T3 cells was not modified with sialic acid and was bound by Wisteria floribunda agglutinin, a lectin specific for terminal β1,4-linked GalNAc (30), indicating that the GLuc-PSG23 glycans were modified with GalNAcβ1,4GlcNAc (GLuc-PSG23:GalNAcβ1,4GlcNAc). Binding to WFA was abolished when GLuc-PSG23 was co-expressed with GalNAc-4-ST1 in CHO/T3 reflecting modification of all the terminal GalNAc with SO4 to generate the terminal sequence SO4-4-GalNAcβ1,4GlcNAc (Gluc-PSG23:SO4-4-GalNAcβ1,4GlcNAc). The absence of sialic acid on the N-glycans of GLuc-PSG23 expressed in CHO/T3 in the presence or absence of GalNAc-4-ST1 indicated that no β1,4-linked Gal is present on the N-linked glycan termini because the sialyltransferase expressed in CHO cells will add α2,3-linked sialic acid to terminal Gal but not to terminal GalNAc (31).
Mice were anesthetized with 87 mg/kg ketamine HCl and 13.4 mg/kg xylazine HCl and placed on a 37 °C warm plate. The tail was warmed with 37 °C water for 1–2 min and washed with a 70% alcohol pad. Two hundred and fifty microliters of tissue culture medium containing GLucPSG23 was injected into the lateral tail vein in under 5 s. Immediately following injection, 10–15 μl of blood was drawn retro-orbitally with a heparinized capillary tube as the first time point. Subsequent time points were taken at the times indicated. Blood was immediately transferred from heparinized capillary tubes to non-heparinized tubes, allowed to clot, and serum-harvested. For each time point, 1 μl of serum was diluted with 49 μl of PBS and assayed using the Renilla luciferase assay system (Promega). Samples were analyzed on a PerkinElmer Victor 2 luminometer injecting 100 μl of substrate, shaking for 1 s, followed by a 2-s delay and measuring the output over 10 s. Data were analyzed using Prism (version 6.0).
RESULTS
Steady State Levels of ManR and ASGR mRNA Rise during Pregnancy in Response to Progesterone
Steady state levels of ManR and ASGR (Asgr-1 and Asgr-2) mRNA in the liver were determined by qPCR during the course of pregnancy (Fig. 1). Compared with control non-pregnant mice, mRNA levels increased in pregnant mice by 3–5-fold for ManR, Asgr-1, and Asgr-2 by day 3.5 of pregnancy where identification of a vaginal plug was set as day 0.5. Levels were maximal on day 12.5 and then began to decline. The increase in mRNA levels was 7-fold for ManR, 14-fold for Asgr-1, and 30-fold for Asgr-2 by day 12.5 of gestation when compared with virgin mice.
FIGURE 1.
ManR and ASGR mRNA levels increase during pregnancy. Mice were monitored for the presence of vaginal plugs and sacrificed on days 0, 3.5, 6.5, 9.5, 12.5, 15.5, and 18.5 of pregnancy and day 2 following delivery (PN2). Livers were collected, and RNA was prepared for qPCR analysis of mRNA levels. A, ManR; B, Asgr-1 of the ASGR; C, Asgr-2 of the ASGR. Error bars, S.E. with n = 4.
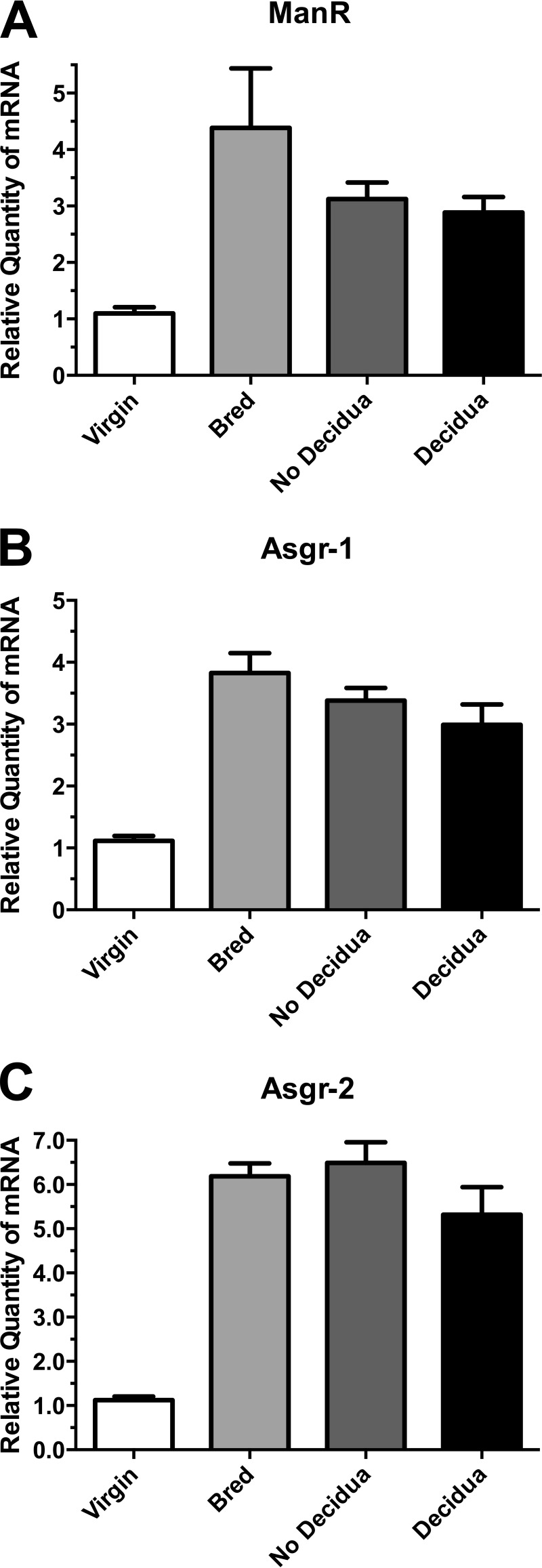
Because implantation of fertilized eggs occurs on day 3.5, we examined pseudopregnant mice to determine whether implantation and/or decidua formation is required for the increase in mRNA levels. Female mice were bred with vasectomized male mice. The hormonal changes associated with fertilization and implantation occur in the absence of fertilized embryos in pseudopregnant mice. Furthermore, injection of oil into the uterus on day 3.5 induces decidua formation in roughly half the pseudopregnant mice. As illustrated in Fig. 2, mRNA levels for ManR, Asgr-1, and Asgr-2 increase in pseudopregnant mice bred with sterile males as compared with virgin mice. Induction of decidua by injection of oil does not have an impact on the increase in message levels. Thus, neither implantation or decidua formation is required to induce the increase in message levels at day 3.5.
FIGURE 2.
ManR and ASGR mRNA levels increase in pseudopregnant mice. Virgin female mice were mated with vasectomized male mice. The presence of a vaginal plug was utilized to assign day 1 of pseudopregnancy. On day 4 of pseudopregnancy, oil was injected in the uterine horn of a subset of the bred mice. On day 9 of the pseudopregnancy, the mice were sacrificed, and their livers were collected for analysis of mRNA levels by qPCR. A, ManR; B, Asgr-1; C, Asgr-2. The results are shown for: virgin mice (Virgin), pseudopregnant mice that were not further manipulated (Bred), pseudopregnant mice treated with oil that did not develop decidua (No Decidua), and pseudopregnant mice treated with oil that developed decidua (Decidua). In each case, the level of message differed significantly (p < 0.05) from that found in virgin mice using the unpaired t test. Error bars, S.E. with n = 4.
Progesterone levels increase rapidly beginning on day 3 following copulation with male mice and peak around day 12.5. We therefore examined the impact of exogenous progesterone administration on mRNA levels for the ManR and ASGR in female and male mice. The steady state levels of ManR, Asgr-1, and Asgr-2 mRNA increased in response to exogenous progesterone (Fig. 3) in female mice. In contrast, administration of exogenous estrogen did not have any impact on the mRNA levels for either receptor (data not shown). The mRNA levels for both the ManR and the ASGR therefore rise and fall in response to progesterone levels in the pregnant mouse. In contrast, male mice do not display a statistically significant change in ManR, Asgr-1, or Asgr-2 message in response to progesterone (Fig. 3). The ability to respond to progesterone is characteristic of female but not male mice.
FIGURE 3.
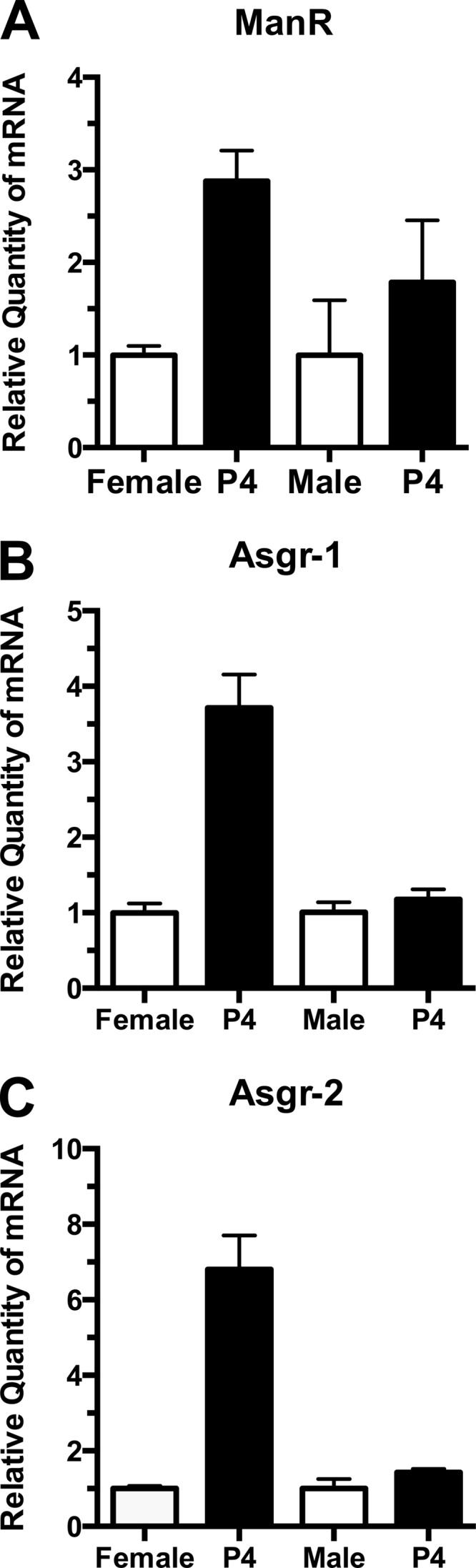
ManR and ASGR mRNA levels increase in response to exogenous progesterone. Female and male mice were given a single intraperitoneal dose of 100 μg of progesterone in oil. Six days later, the mice were sacrificed, and the levels of message in the liver were determined by qPCR for A (ManR), B (Asgr-1), and C (Asgr-2). The steady state mRNA levels for female mice treated with progesterone (P4) increased 3-fold (p = 0.005) for the ManR, 4-fold (p = 0.004) for Asgr-1, and 7-fold (p = 0.003) for Asgr-2. Changes in message levels for male mice treated with progesterone were not significant. Significance was determined by unpaired t test analysis. Error bars are S.E. with n = 6.
ManR and ASGR Protein and Binding Activity Increase in Response to Progesterone during Pregnancy
The increase in mRNA encoding the ManR and the two subunits of the ASGR is remarkable because both receptors are already abundantly expressed at the plasma membranes of SECs and parenchymal cells of non-pregnant mice and are highly efficient endocytic receptors. Western blot analysis with ManR- and ASGR-specific antibodies was used to compare levels of protein expression in membranes prepared from livers of mice that had or had not been treated with progesterone. In the case of ManR, a protein band corresponding to the intact receptor was observed migrating with a molecular mass of 180 kDa. We also observed two proteolytic fragments migrating with apparent molecular masses equal to species of 60 and 50 kDa (Fig. 4A). All three species increase 2–3-fold following progesterone treatment.
FIGURE 4.
Progesterone increases expression of the ManR and ASGR in the liver of female mice. Progesterone pellets (2.5 mg/pellet) were implanted subcutaneously in female mice. After 72 h, the mice were sacrificed, and livers were collected. Following homogenization, membranes were prepared by sedimentation on a sucrose cushion. Ten μg of total protein was loaded onto each lane for analysis by SDS-PAGE. Following electrophoresis, the proteins were electrophoretically transferred to PVDF membranes, and the amount of ManR (A) and ASGR (B) was determined by Western blot analysis using antibodies specific for the ManR and ASGR, respectively. The intensity of each band was determined using ImageJ software. The bar graphs show the mean for three treated or three control mice with error bars = S.E. Lanes 1–3 and white bars indicate control mice. Lanes 4–6 and solid black bars indicate progesterone-treated mice.
Expression of Asgr-1 (42 kDa) and Asgr-2/3 (50 and 60 kDa, respectively) of the ASGR also increase in response to progesterone (Fig. 4B). In the case of Asgr-1, a band migrating roughly 2 kDa faster than the form of Asgr-1 seen in the absence of progesterone treatment is detected. The faster moving form of Asgr-1 accounts for much of the increase in Asgr-1 seen by Western blot. The basis for the difference in migration remains to be determined. Asgr-1 increases 2-fold, whereas Asgr-2 increases 4-fold following progesterone treatment, consistent with the greater increase in mRNA seen for Asgr-2 than Asgr-1 (Figs. 1 and 2).
The GalNAc-4-SO4-specific form of the ManR predominates in SECs of the liver (19). We therefore examined liver membranes from mice on day 0 (P0), day 10 (P10), and day 15 (P15) of pregnancy for binding of S4GGnM-BSA. Binding activity per μg of membrane protein increased 2–3 fold in membranes from P10 and P15 mice as compared with P0 (Fig. 5A), indicating that the dimeric form of the ManR that binds glycoprotein hormones such as LH bearing terminal GalNAc-4-SO4 increases during pregnancy. ASGR binding activity was determined using the same membranes and ASOR. Binding activity increased 3-fold by P10 and 10-fold by P15 of pregnancy as compared with day 0 (Fig. 5B). Thus, both protein expression and binding activity of the ManR and the ASGR increase during pregnancy and in response to exogenous progesterone. Because the size of the liver increases in pregnancy (32, 33), the increase in the total mass of both the ManR and the ASGR and their capacity to capture glycoproteins bearing recognized glycans is enhanced to an even greater extent than indicated by their increase in activity per μg of membrane protein.
FIGURE 5.
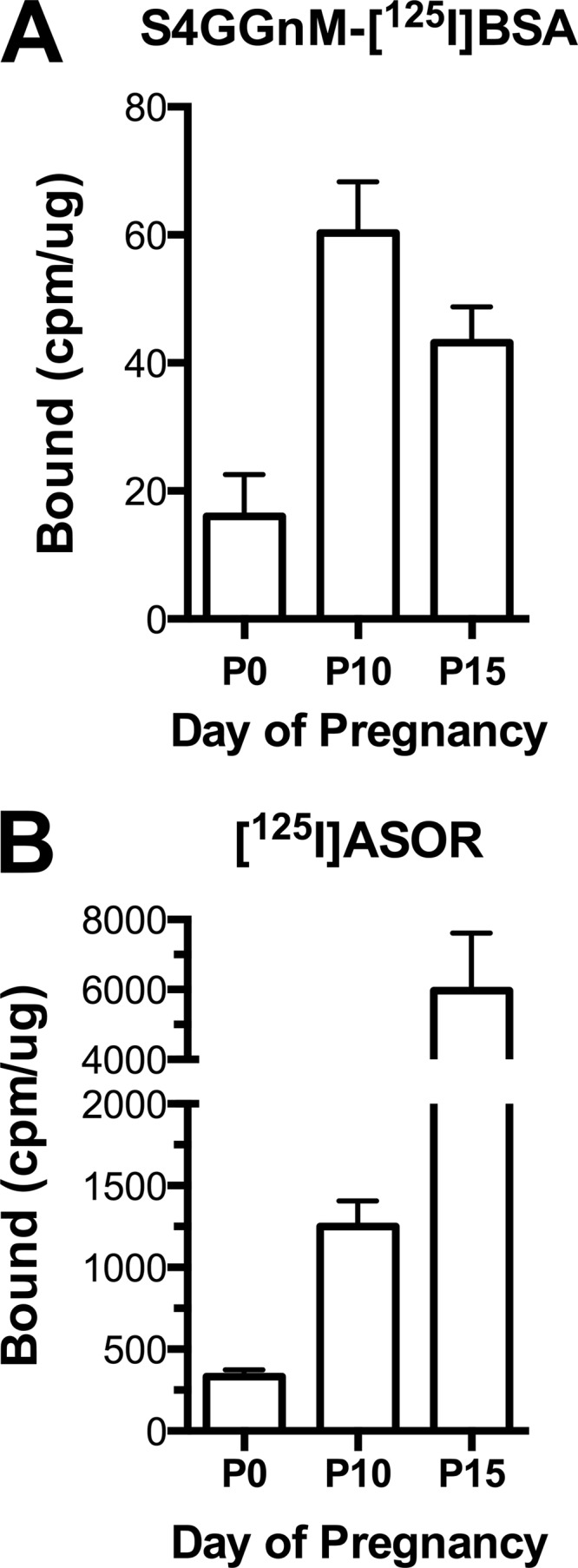
Binding activity for ligands containing terminal β1,4-linked GalNAc-4-SO4 and β1,4-linked Gal, respectively, increases during pregnancy. Livers were collected from female mice sacrificed on days 0, 10, and 15 of pregnancy. The amount of S4GGnM-[125I]BSA and [125I]ASOR that could be bound per μg of protein was determined. A, S4GGnM-[125I]BSA; B, [125I]ASOR. Values are presented as the mean for n = 6 with error bars = S.E.
Receptor Levels Determine Clearance Rates
The rate at which a glycoprotein is removed from the circulation by receptors such as the ManR and the ASGR will be determined by the combined effect of multiple parameters in vivo, including the affinity for the glycan, the rate of receptor internalization, the on rate for binding, and the amount of receptor. The change in ManR and ASGR expression levels during pregnancy provided us with an opportunity to determine whether increased levels of receptor expression would result in more rapid clearance from the blood. In the glycobiology field, detailed studies of clearance have been hampered by the inability to generate glycoproteins that differ only in the terminal modification of their N-linked glycans and whose levels could be quantified in plasma with sufficient sensitivity to allow sampling at multiple time points in mice. We thus have developed a novel strategy that overcomes these obstacles and indeed does not even require purification of glycoproteins bearing different glycan modifications prior to examining their clearance rate.
Our approach to quantitation of clearance parameters is based on using our recently described generation of chimeric fusion proteins consisting of Gaussia luciferase followed by a glycoprotein of interest, that allowed us to characterize protein-specific forms of glycosylation in vivo by expression in CHO cells (6, 7). PSG23 is a member of the CEA antigen family that is expressed during pregnancy in the mouse (34). PSG23 bears seven N-linked glycans distributed among three Ig variable-like domains. Expression of PSG23 in CHO cells with different repertoires of glycosyltransferases makes it possible to generate GLuc-PSG23 bearing N-linked glycans with different structures. High levels of Gaussia luciferase activity (expressed as light units) obtained in CHO serum-free culture medium following transfection means that injection of 300 μl of this medium in under 5 s into the tail vein yields sufficient activity for multiple analyses using 25 μl of blood per time point. We followed the clearance of various injected GLuc-PSG23 species bearing different N-linked glycan structures from the blood by monitoring the decline in Gaussia luciferase activity with time. The same amount of luciferase activity was injected for each of the studies performed. Examples of clearance curves for GLuc-PSG23 bearing glycans with different termini are shown in Fig. 6 for wild type (WT), ManR-deficient (ManR−/−) (26), and ASGR-deficient (ASGR−/−) (15) mice.
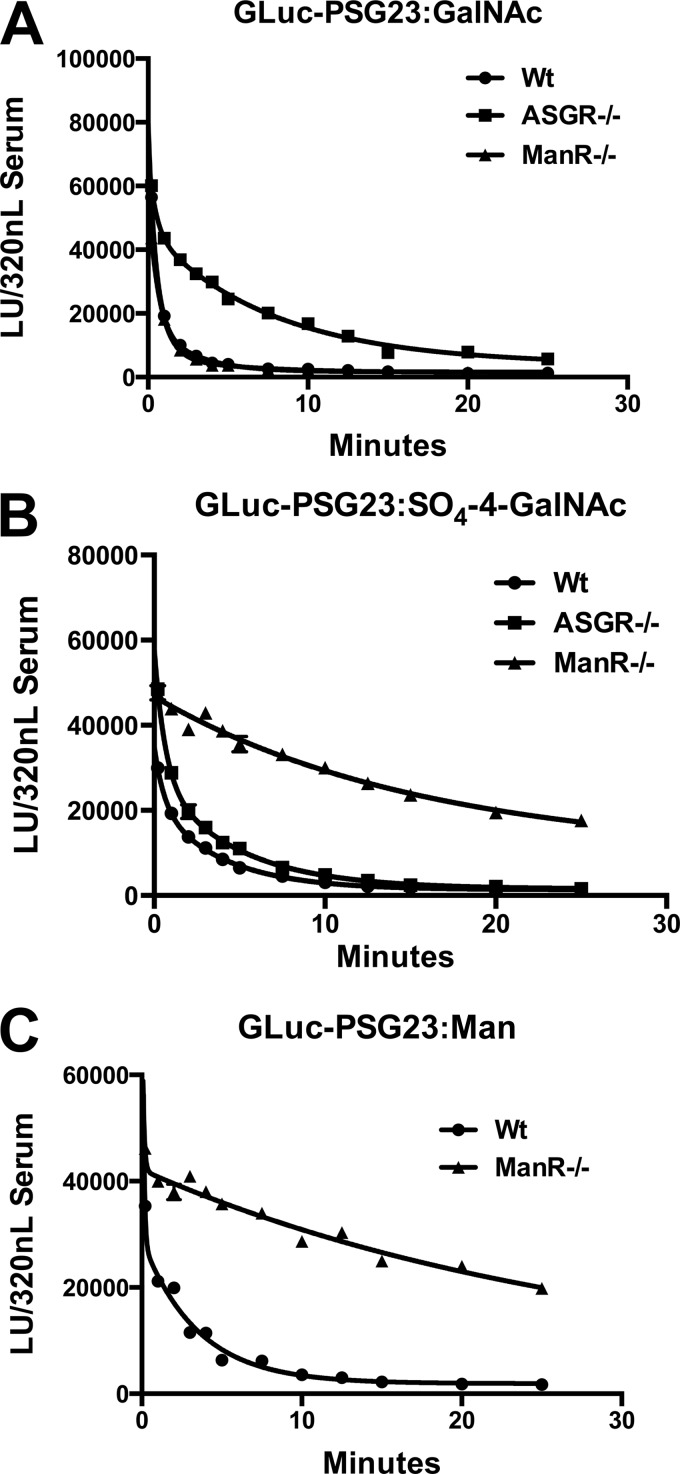
FIGURE 6.
The ManR accounts for clearance of glycoproteins bearing N-linked glycans terminating with either β1,4-linked GalAc-4-SO4 or α-linked Man. GLuc-PSG23 was expressed in CHO/T3 cells, CHO/T3 cells co-transfected with GalNAc-4-ST1, or CHO/Lec1 cells to generate N-linked glycans terminating with GalNAcβ1,4GlcNAcβ1,2Man (GLuc-PSG23:GalNAc) (A), SO4-4-GalNAcβ1,4GlcNAcβ1,2Man (GLuc-PSG23:SO4-4-GalNAc) (B), and Man (GLuc-PSG23:Man) (C), respectively. WT, ASGR-deficient (ASGR−/−), and ManR-deficient (ManR−/−) male mice were injected with the GLuc-PSG23 constructs indicated, and samples were collected immediately, then at 1-min intervals for 5 min, 2.5-min intervals from 7.5 to 15 min, and 5-min intervals from 15 to 25 min. The amount of GLuc activity present in the equivalent of 320 nl of plasma was determined for each time point after dilution of plasma aliquots. Individual time points were analyzed in triplicate, and error bars = S.E. but are not visible because they are smaller than the symbols used. LU, light units.
GLuc-PSG23 expressed in CHO/T3 cells bears N-glycans terminating with β1,4-linked GalNAc. This protein species designated GLuc-PSG23:GalNAc has a glycan structure that is efficiently bound by the ASGR. GLuc-PSG23:GalNAc is rapidly cleared from the blood by WT and ManR−/− mice (Fig. 6A). The clearance curve is biphasic with 79–90% of the GLuc-PSG23:GalNAc cleared with a t½ of 0.3–0.5 min. Clearance of GLuc-PSG23:GalNAc in ASGR−/− mice is also biphasic, but only 35% is cleared with a t½ of 0.3 min. whereas 65% is cleared with a t½ of 5.4 min. The ASGR accounts for the rapid clearance of the majority of GLuc-PSG23:GalNAc (Fig. 6A).
Co-expression of GLuc-PSG23 and GalNAc-4-ST1 in CHO/T3 cells yields a species denoted as GLuc-PSG23:SO4-4-GalNAc, which has the same GLuc-PSG23 protein structure as GLuc-PSG23:GalNAc above but now bears N-glycans terminating with β1,4-linked GalNAc-4-SO4. N-Linked glycans terminating with GalNAc-4-SO4 are bound by the Cys-rich domain of the ManR (18). GLuc-PSG23:SO4-4-GalNAc is rapidly cleared from the blood of WT and ASGR−/− mice (Fig. 6B). The clearance curves are biphasic with 40 and 54% cleared with half-life of 0.4 min, whereas the remaining 60 and 46% are cleared with half-lives of 2.7 and 3.3 min in WT and ASGR−/− mice. In contrast, 89% of GLuc-PSG23:SO4-4-GalNAc is cleared with a half-life of 12.9 min in ManR−/− mice. The clearance curves demonstrate both that the ManR accounts for the clearance of glycoproteins bearing N-glycans with terminal β1,4-linked GalNAc-4-SO4 and that little if any terminal GalNAc is present on GLuc-PSG23:SO4-4-GalNAc.
Expression of GLuc-PSG23 in CHO/Lec1 cells yields a species designated GLuc-PSG23:Man, which bears oligomannose type N-glycans due to the presence of an inactive form of the glycosyltransferase GnT1 (35). Glycans with this structure are bound by the CRD domains of the ManR. Seventy-nine percent of GLuc-PSG23:Man is cleared with a half-life of < 0.05 min in WT mice (Fig. 6C). In contrast, GLuc-PSG23:Man is cleared with a half-life of 19.8 min in ManR−/− mice. The ManR therefore accounts for clearance of glycoproteins with N-glycans bearing either β1,4-linked GalNAc-4-SO4 or Man in vivo.
The experiments shown in Fig. 6 verify that GLuc-PSG23 can be used to quantitate the clearance of glycoproteins bearing N-glycans with differing termini in vivo. Our goal was to establish that differences in levels of glycan-specific receptors can indeed alter their rates of clearance in vivo. The binding studies shown in Fig. 5 indicate that in wild-type mice, based on the increase in [125I]ASOR bound per μg of liver membrane protein at P15 compared with P0 and the increase in liver weight, there is a 10–15 fold increase in the binding capacity of the ASGR in P15 livers. Likewise, there is a 2–3-fold increase in ManR binding capacity. Because blood volume increases as much as 50% during pregnancy, we compared the clearance of a form of GLuc-PSG23 that is not recognized by either the ASGR or the ManR to determine whether the increased volume would reduce the rate of clearance. GLuc-PSG23 expressed in CHO cells bears N-linked glycans that terminate with Siaα2,3Gal. This species designated GLuc-PSG23:Siaα2,3Gal is cleared slowly from the blood of non-pregnant mice and P15 of pregnancy (Fig. 7A). Even though identical light units of GLuc-PSG23:Siaα2,3Gal were injected, the initial values for light units/320 nl of serum were 35% lower in pregnant mice than non-pregnant mice, reflecting the greater blood volume in pregnant animals. When the values obtained for P15 mice were multiplied by 1.5, there was excellent correspondence between the pregnant and non-pregnant mice (Fig. 7A). Therefore, all of the values for the clearance studies in P15 mice were multiplied by 1.5 to allow direct comparison of the clearance rates to non-pregnant animals.
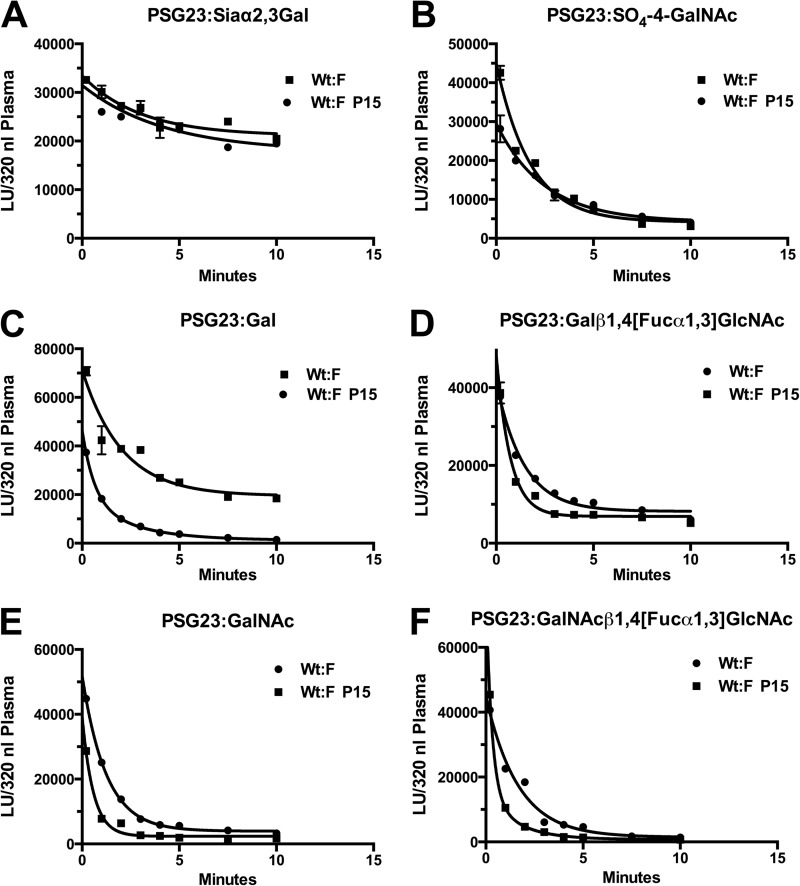
FIGURE 7.
Increased expression of the ASGR and ManR during pregnancy increases the rate of GLuc-PSG23 clearance from the blood. Gluc-PSG23 was expressed in CHO cells (A), CHO/T3 cells co-transfected with GalNAc-4-ST1 (B), CHO cells and digested with neuraminidase prior injection (C), CHO cells co-transfected with α1,3-fucosyltransferase-9 (D), CHO/T3 cells (E), and CHOT3 cells co-transfected with α1,3-fucosyltransferase-9 to generate N-glycans with the termini shown (F). The GLuc-PSG23 products were injected into WT female mice on day 15 of pregnancy. Blood was collected immediately and at the times shown. The amount of GLuc activity present in the equivalent of 320 nl of plasma was determined for each data point following dilution of aliquots. The light units (LU) of activity in samples from pregnant animals were corrected for the 50% great blood volume seen during pregnancy. Individual time points were analyzed in triplicate, and error bars = S.E. but are not visible because they are smaller than the symbols used.
As demonstrated above (Fig. 6), the ManR mediates clearance of glycoproteins such as LH that bear glycans terminating with SO4-4-GalNAc. Clearance of GLuc-PSG23:SO4-4-GalNAc is biphasic in non-pregnant and P15 pregnant mice with 45 and 42%, respectively, of the GLuc-PSG23:SO4-4-GalNAc being cleared during the fast phase. The half-life is 0.08 min for the P15 mice and 0.41 for the non-pregnant mice (Fig. 7B). Clearance is very rapid in both instances; however, it is significantly more rapid in P15 mice with the higher level of ManR expression.
The ASGR increases to an even greater extend during pregnancy than the ManR. In addition, the ASGR recognizes a number of glycans with different termini. We therefore examined the clearance of GLuc-PSG23 bearing four different N-linked glycan structures each of which is recognized by the ASGR (Fig. 7, C–F). In each instance, GLuc-PSG23 was cleared from the blood significantly faster, 2-fold or more, on day 15 of pregnancy than in non-pregnant mice. The greater blood volume in the pregnant mouse would be predicted to reduce rather than enhance the rate of clearance, making the difference in rate even more significant. In the case of GLuc-PSG23:Gal, the second phase of clearance (Fig. 7C) in non-pregnant mice is sufficiently slow that the curve at 10 min gives the appearance of a plateau even though the GLuc-PSG23 levels continue to decline after 10 min in longer studies (data not shown). Thus, the increase in ASGR in the liver of pregnant mice significantly increases the rate of clearance of glycoproteins bearing glycans that are recognized by this receptor.
ManR Levels Decline at the Time of Sexual Maturation in Male Mice
Increasing or decreasing ManR expression in hepatic SECs will alter the rate at which hormones such as LH are cleared from the blood. Because male mice undergo sexual maturation between postnatal days 15 and 35, we compared mRNA expression for ManR, Asgr-1, and Asgr-2 over this time (Fig. 8). ManR message levels decrease by roughly 50% between postnatal days 15 and 35. In contrast, differences in Asgr-1 and Asgr-2 message levels are not statistically significant. The change in message level is specific for ManR, consistent with its role in regulating the half-life of LH in vivo.
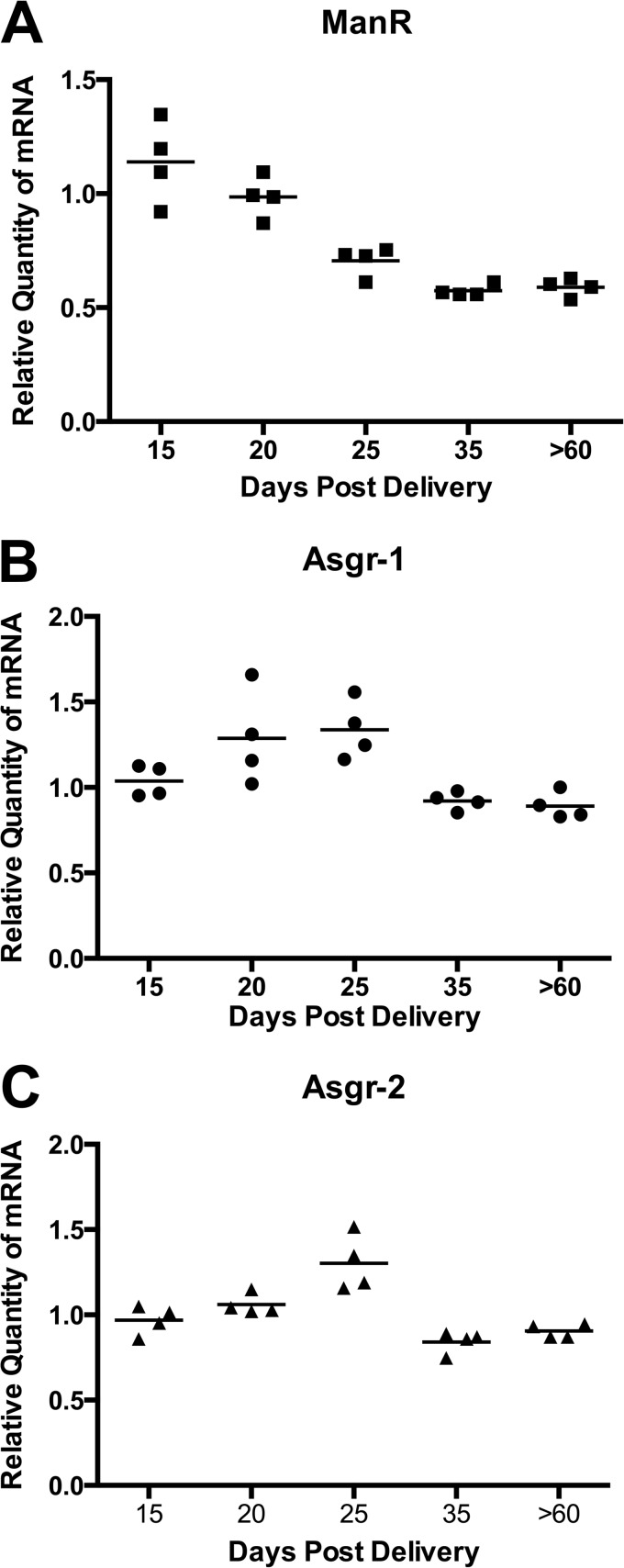
FIGURE 8.
ManR mRNA decreases in male mice at the time of sexual maturation. Livers were collected on postnatal days 15, 20, 25, 35, and >60. The steady state levels of transcripts for ManR (A), Asgr-1 (B), and Asgr-2 (C) were determined by qPCR using a cDNA pool of all the samples as the calibrator. Analyses were performed in triplicate. The difference in values for the ManR on days 25, 35, and 60 were highly significant with p = 0.0001 using an unpaired t test. The differences seen for Asgr-1 and Asgr-2 were not significant.
DISCUSSION
The studies we have presented here demonstrate that the expression of two major glycan-specific receptor systems is modulated at critical times during the reproductive cycle and that these changes in expression can alter the clearance rates for critical ligands such as LH. We have shown that progesterone modulates expression of the ASGR and the ManR during pregnancy, with steady state levels of mRNA and protein expression rising and falling in proportion to circulating levels of progesterone over the course of pregnancy. We thus establish the physiologic basis for the increase in Asgr-1 and Asgr-2 message during pregnancy noted by Collins et al. (36). Progesterone levels are critical for maintaining pregnancy in the mouse; falling progesterone levels after day 15 contribute to the initiation of parturition at the end of pregnancy. In light of the high levels of ASGR and ManR expression seen in non-pregnant mice, the increases seen during pregnancy are remarkable and indicate that both receptors have an enormous capacity to clear glycoproteins bearing recognized glycans.
The approach we developed to determine the protein specificity of glycosyltransferases (6, 7) has provided an exceptionally powerful approach to studying the clearance of glycoproteins because of the following. 1) The identical glycoproteins bearing glycans that differ in structure are examined. 2) Only the chimeric glycoprotein is detected. 3) No purification is required. 4) No radiolabeling is required. 5) Only small amounts of blood are required for analysis, allowing multiple time points to be collected. PSG23 is a member of the CEA type family of glycoproteins that are secreted by trophoblast cells of the mouse placenta during pregnancy. PSG23 has three Ig variable-like domains with a total of seven N-linked glycans. Fully glycosylated GLuc-PSG23 migrates with a molecular mass of 90 kDa when examined by SDS-PAGE and is sufficiently large to not be extensively cleared by renal filtration. By expressing GLuc-PSG23 in CHO cells, we were able to compare the clearance of the same glycoprotein bearing N-glycans differing only in their pattern of terminal glycosylation.
The use of Gaussia luciferase chimeric glycoproteins with measurement of light production as the assay overcomes many of the difficulties encountered using radiolabeled ligands for clearance studies, including damage to the ligand during radiolabeling, dissociation of non-covalently associated subunits, nonspecific labeling, high backgrounds, and an inability to choose an ideal ligand for analysis. Such issues may account for the previous conclusion using ManR−/− mice that suggested the ManR does not mediate clearance of glycoproteins such as LH bearing terminal GalNAc-4-SO4 (26). It is clear from our studies shown in Fig. 6 that the ManR does indeed mediate the rapid clearance of glycoproteins bearing terminal GalNAc-4-SO4 as well as ones bearing high mannose type glycans. Clearance of glycoproteins with high mannose glycans is consistent with the elevated levels of lysosomal enzymes reported in the ManR−/− mice (26).
Changes in the amount of ManR and ASGR expressed in the liver have the potential to alter the rate of clearance of ligands bearing recognized glycans from the blood. The circulatory half-life of endogenous LH in mice that have had their GalNAc-4-ST1 ablated increases from 7 to 10 min due to the addition of α2,6-linked sialic acid to the GalNAc in place of SO4 (28). This form of LH is more potent, resulting in increased production of estrogen in females and testosterone in males. Increased ManR expression during pregnancy should reduce the half-life and potency of LH in vivo until expression levels begin to fall prior to parturition on day 21. In contrast, the reduction in ManR expression at the time of sexual maturation in male mice would increase the potency of LH and as a consequence testosterone levels. The small size of the mice at this age, however, precluded examination of GLuc-PSG23 clearance. The circulating concentration of LH bearing two or more N-linked glycans terminating with GalNAc-4-SO4 is well below the Kd (2.5 × 10−7 m) for binding to the dimeric form of the ManR even during the preovulatory surge in LH. As a consequence, the clearance rate for LH is sensitive to the amount of ManR expressed but not to the amount of LH secreted because the ManR with its bound LH is rapidly internalized and then recycled to the SEC surface.
The increase in the expression of the ASGR during pregnancy under the direction of progesterone is even greater than for the ManR. The glycans bearing four different termini bind to the ASGR with different affinities, but in each case, the circulatory half-life is markedly reduced by the increase in ASGR expression during pregnancy. The half-life for clearance during pregnancy is sufficiently rapid that virtually all the GLuc-PSG23 is being removed from the blood in a single pass through the liver. Thus, as with the ManR, modulating the level of ASGR expression alters the rate of clearance for glycoproteins bearing recognized glycans. The marked increase in ASGR expression during pregnancy suggests that either increased capacity is required or that it is critical to reduce the concentration of certain glycoproteins released during pregnancy to zero in the blood. Even though ManR and ASGR expression respond to progesterone similarly during pregnancy, they differ in the regulation of their expression at the time of sexual maturation in the male. Unlike the ManR, the ASGR message levels do not decline as male mice become sexually mature, indicating that the ManR but not ASGR contributes to the process of sexual maturation.
Mice that have had either the ASGR or ManR or both ablated do display significant physiologic changes, particularly during pregnancy.3 Glycoproteins such as PSG23 that are undetectable in WT mice during pregnancy reach levels of 1–10 mg/ml of plasma in ASGR−/− mice, demonstrating the enormous capacity of the ASGR for clearance. Changes in estrogen and testosterone levels are seen in ManR−/− mice, consistent with its role in regulating LH clearance. Furthermore, ASGR−/− ManR−/− mice fail to initiate parturition, indicating the complex process of preparing for and initiating parturition has been severely disrupted in these mice.
Our studies demonstrate that expression of the ManR and ASGR is regulated by progesterone during pregnancy and that modulating the amount of ManR and ASGR expressed in the liver will alter the rate of clearance of glycoproteins bearing recognized glycans. Modulation of the expression of the ManR during pregnancy and sexual maturation supports the role of the ManR in regulating the potency of LH in vivo by modulating its half-life. Maintaining a constant rate of clearance that is not sensitive to ligand concentration but is sensitive to receptor level provides an elegant way to control hormone potency in vivo.
Acknowledgment
We thank Nancy L. Baenziger for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-CA21923 and R01-HD058474 (to J. U. B.).
Y. Mi, D. Fiete, and J. U. Baenziger, unpublished observations.
- LH
- luteinizing hormone
- ASGR
- asialoglycoprotein receptor
- GLuc
- Gaussia luciferase
- ManR
- mannose receptor
- ASOR
- asialoorosomucoid
- SEC
- sinusoidal endothelial cell
- WFA
- Wisteria floribunda agglutinin
- PSG23
- pregnancy-specific glycoprotein-23
- qPCR
- quantitative PCR
- P
- days of pregnancy.
REFERENCES
- 1. Green E. D., Baenziger J. U. (1988) Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J. Biol. Chem. 263, 25–35 [PubMed] [Google Scholar]
- 2. Green E. D., Baenziger J. U. (1988) Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J. Biol. Chem. 263, 36–44 [PubMed] [Google Scholar]
- 3. Green E. D., van Halbeek H., Boime I., Baenziger J. U. (1985) Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 260, 15623–15630 [PubMed] [Google Scholar]
- 4. Baenziger J. U., Kumar S., Brodbeck R. M., Smith P. L., Beranek M. C. (1992) Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc. Natl. Acad. Sci. U.S.A. 89, 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiete D., Srivastava V., Hindsgaul O., Baenziger J. U. (1991) A hepatic reticuloendothelial cell receptor specific for SO4–4GalNAc β1,4GlcNAc β1,2Man α that mediates rapid clearance of lutropin. Cell 67, 1103–1110 [DOI] [PubMed] [Google Scholar]
- 6. Fiete D., Beranek M., Baenziger J. U. (2012) Molecular basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J. Biol. Chem. 287, 29194–29203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiete D., Beranek M., Baenziger J. U. (2012) Peptide-specific transfer of N-acetylgalactosamine to O-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J. Biol. Chem. 287, 29204–29212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller E., Fiete D., Blake N. M., Beranek M., Oates E. L., Mi Y., Roseman D. S., Baenziger J. U. (2008) A necessary and sufficient determinant for protein-selective glycosylation in vivo. J. Biol. Chem. 283, 1985–1991 [DOI] [PubMed] [Google Scholar]
- 9. Ashwell G., Harford J. (1982) Carbohydrate-specific receptors of the liver. Annu. Rev. Biochem. 51, 531–554 [DOI] [PubMed] [Google Scholar]
- 10. Stockert R. J. (1995) The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol. Rev. 75, 591–609 [DOI] [PubMed] [Google Scholar]
- 11. Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. (1979) An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I: distribution of 125I-ligands among the liver cell types. J. Cell Biol. 83, 47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drickamer K., Mamon J. F., Binns G., Leung J. O. (1984) Primary structure of the rat liver asialoglycoprotein receptor: structural evidence for multiple polypeptide species. J. Biol. Chem. 259, 770–778 [PubMed] [Google Scholar]
- 13. Halberg D. F., Wager R. E., Farrell D. C., Hildreth J., 4th., Quesenberry M. S., Loeb J. A., Holland E. C., Drickamer K. (1987) Major and minor forms of the rat liver asialoglycoprotein receptor are independent galactose-binding proteins: primary structure and glycosylation heterogeneity of minor receptor forms. J. Biol. Chem. 262, 9828–9838 [PubMed] [Google Scholar]
- 14. Paietta E., Stockert R. J., Racevskis J. (1992) Alternatively spliced variants of the human hepatic asialoglycoprotein receptor, H2, differ in cellular trafficking and regulation of phosphorylation. J. Biol. Chem. 267, 11078–11084 [PubMed] [Google Scholar]
- 15. Ishibashi S., Hammer R. E., Herz J. (1994) Asialoglycoprotein receptor deficiency in mice lacking the minor receptor subunit. J. Biol. Chem. 269, 27803–27806 [PubMed] [Google Scholar]
- 16. Braun J. R., Willnow T. E., Ishibashi S., Ashwell G., Herz J. (1996) The major subunit of the asialoglycoprotein receptor is expressed on the hepatocellular surface in mice lacking the minor receptor subunit. J. Biol. Chem. 271, 21160–21166 [DOI] [PubMed] [Google Scholar]
- 17. Tozawa R., Ishibashi S., Osuga J., Yamamoto K., Yagyu H., Ohashi K., Tamura Y., Yahagi N., Iizuka Y., Okazaki H., Harada K., Gotoda T., Shimano H., Kimura S., Nagai R., Yamada N. (2001) Asialoglycoprotein receptor deficiency in mice lacking the major receptor subunit: its obligate requirement for the stable expression of oligomeric receptor. J. Biol. Chem. 276, 12624–12628 [DOI] [PubMed] [Google Scholar]
- 18. Fiete D. J., Beranek M. C., Baenziger J. U. (1998) A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. Proc. Natl. Acad. Sci. U.S.A. 95, 2089–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roseman D. S., Baenziger J. U. (2000) Molecular basis of lutropin recognition by the mannose/GalNAc-4-SO4 receptor. Proc. Natl. Acad. Sci. U.S.A. 97, 9949–9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y., Chirino A. J., Misulovin Z., Leteux C., Feizi T., Nussenzweig M. C., Bjorkman P. J. (2000) Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J. Exp. Med. 191, 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiete D., Baenziger J. U. (1997) Isolation of the SO4-4-GalNAcβ1,4GlcNAcβ1,2Manα-specific receptor from rat liver. J. Biol. Chem. 272, 14629–14637 [DOI] [PubMed] [Google Scholar]
- 22. Baenziger J. U., Fiete D. (1980) Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell 22, 611–620 [DOI] [PubMed] [Google Scholar]
- 23. Grewal P. K., Uchiyama S., Ditto D., Varki N., Le D. T., Nizet V., Marth J. D. (2008) The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat. Med. 14, 648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park E. I., Manzella S. M., Baenziger J. U. (2003) Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J. Biol. Chem. 278, 4597–4602 [DOI] [PubMed] [Google Scholar]
- 25. Steirer L. M., Park E. I., Townsend R. R., Baenziger J. U. (2009) The asialoglycoprotein receptor regulates levels of plasma glycoproteins terminating with sialic acid α2,6-galactose. J. Biol. Chem. 284, 3777–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee S. J., Evers S., Roeder D., Parlow A. F., Risteli J., Risteli L., Lee Y. C., Feizi T., Langen H., Nussenzweig M. C. (2002) Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295, 1898–1901 [DOI] [PubMed] [Google Scholar]
- 27. Fiete D., Beranek M. C., Baenziger J. U. (1997) The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAcβ or Man at independent sites. Proc. Natl. Acad. Sci. U.S.A. 94, 11256–11261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mi Y., Fiete D., Baenziger J. U. (2008) Ablation of GalNAc-4-sulfotransferase-1 enhances reproduction by altering the carbohydrate structures of luteinizing hormone in mice. J. Clin. Invest. 118, 1815–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mengeling B. J., Smith P. L., Stults N. L., Smith D. F., Baenziger J. U. (1991) A microplate assay for analysis of solution-phase glycosyltransferase reactions: determination of kinetic constants. Anal. Biochem. 199, 286–292 [DOI] [PubMed] [Google Scholar]
- 31. Smith P. L., Kaetzel D., Nilson J., Baenziger J. U. (1990) The sialylated oligosaccharides of recombinant bovine lutropin modulate hormone bioactivity. J. Biol. Chem. 265, 874–881 [PubMed] [Google Scholar]
- 32. Milona A., Owen B. M., van Mil S., Dormann D., Mataki C., Boudjelal M., Cairns W., Schoonjans K., Milligan S., Parker M., White R., Williamson C. (2010) The normal mechanisms of pregnancy-induced liver growth are not maintained in mice lacking the bile acid sensor Fxr. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dai G., Bustamante J. J., Zou Y., Myronovych A., Bao Q., Kumar S., Soares M. J. (2011) Maternal hepatic growth response to pregnancy in the mouse. Exp. Biol. Med. 236, 1322–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLellan A. S., Fischer B., Dveksler G., Hori T., Wynne F., Ball M., Okumura K., Moore T., Zimmermann W. (2005) Structure and evolution of the mouse pregnancy-specific glycoprotein (Psg) gene locus. BMC Genomics 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patnaik S. K., Stanley P. (2006) Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 416, 159–182 [DOI] [PubMed] [Google Scholar]
- 36. Collins J. C., Stockert R. J., Morell A. G. (1984) Asialoglycoprotein receptor expression in murine pregnancy and development. Hepatology 4, 80–83 [DOI] [PubMed] [Google Scholar]