Foxp3+ regulatory T cells (Tregs) are unique in their immunosuppressive abilities and contribution to immune regulation. However, the homeostatic processes and survival programs that maintain the Treg population remain unclear. Here, we highlight the recent study by Pierson et al.,1 which dissected the regulatory mechanisms of Treg homeostasis and survival. By utilizing transgenic models, the authors provide evidence to support the notion that peripheral Tregs are able to alter their proliferative and apoptotic rates to rapidly restore a numerical deficit through both interleukin 2 and costimulation-dependent pathways.
Tregs are characterized by their capacity to modulate immune responses and can typically be identified by their specific expression of Foxp3, the transcription factor that endows T cells with their regulatory functions.2,3 Tregs are critical and indispensable for maintaining peripheral tolerance, and there is an active balance between regulatory and effective immune responses under the steady state. Indeed, deterioration of the balance between regulatory and effective immune responses can lead to the induction of several types of diseases. For instance, effective immune responses are often hindered by an excessive number of Tregs in the tumor microenvironment,4 whereas decreases in the number and functionality of Tregs are observed in many autoimmune diseases and inflammatory conditions.5,6,7
Given that Tregs are postulated to be valuable targets for immune therapies against tumor and autoimmune diseases, there is an urgent need to understand both the cellular and molecular mechanisms contributing to Treg homeostasis. Unlike effector T cells, Tregs are considered a group of anergic and quiescent cells, as supported by in vitro studies.8 However, Tregs undergo homeostatic expansion and vigorous proliferation, particularly in a lymphopenic host,9 suggesting that these cells are active under such circumstances and that an intact immune system may help to maintain Treg homeostasis and vice versa.
Programmed cell death (apoptosis) is the predominant underlying mechanism for maintaining T cell homeostasis.10,11 The immune response against foreign antigens begins robustly with effector T-cell activation and proliferation, and the population of activated T cells is then restrained and controlled through apoptosis to circumvent an excessive immune response. Apoptosis itself is a delicate process that can be initiated via either an extrinsic or intrinsic signaling cascade.12 The Bcl-2 protein family, including Bcl-2, Bcl-XL, Mcl-1, Blf-1 and A1, is composed of anti-apoptotic proteins that are able to suppress the activation of the apoptotic regulators Bax and Bak. Conversely, the anti-apoptotic Bcl-2 protein family can be antagonized by pro-apoptotic BH3-only proteins, including Bim, Bik, Puma and Bad.13 In T cells, the dependence on apoptotic-related proteins varies during different developmental stages.14,15,16,17
The regulation of Treg homeostasis and survival has been dissected by a study recently reported in Nature Immunology by Pierson et al. These researchers utilized a transgenic model to study the cellular and molecular mechanisms that contribute to Treg population homeostasis.1 5-bromo-2'-deoxyuridine (BrdU) was administrated to identify the proliferative profile of Tregs in vivo and to assess whether the Treg population is stable and quiescent. It was found that Tregs actually proliferate more rapidly than conventional T cells under a static condition, indicating that the Treg population is dynamic. By using transgenic female mice heterozygous for Thy1.1 and DTR (diphtheria toxin receptor) in the Foxp3 locus of the X chromosome (Foxp3DTR/Thy1.1), the authors demonstrated that Thy1.1+ Tregs (which constitute 50% of the Treg population due to X chromosome inactivation) proliferate vigorously and restore the Treg population shortly after DTR+ Tregs are removed by DT administration. Signals provided by IL-2 are essential for peripheral Treg maintenance, and IL-2 appears to be critical for the niche-filling process because IL-2 acts as a mediator along with costimulatory signals to stimulate the proliferation and to reduce the apoptosis of Tregs.
Because the Treg apoptotic rate initially declined but eventually reversed, and proliferative rate was inversely correlated with the apoptotic rate during the niche-filling process, the authors further clarified the role of apoptosis in Treg homeostasis. Specifically, knockout of Bak and Bax in Tregs resulted in the peripheral accumulation of Tregs in the host, indicating that the integral intrinsic apoptosis pathway is required for peripheral Treg homeostasis. The interactions between anti-apoptotic proteins, pro-apoptotic proteins and apoptosis regulators determine the fate of cells. Among the anti-apoptotic proteins, the role of Bcl-2 and Bcl-XL in Treg homeostasis was ruled out via hematopoietic reconstitution and specific knockout, respectively.
In addition, to understand the importance of Mcl-1 during T-cell development, the authors utilized a fate-mapping strategy, as follows. Human CD4 (hCD4) was introduced adjacently to the Mcl-1 locus flanking with loxP sequence (Mcl1fl). Mice heterozygous of this allele (Mcl1fl/+) were crossed with mice bearing a CD127-promoter-driven Cre recombinase (CD127CreMcl1CD4/+), resulting in expression of hCD4 serving as a reporter for Mcl-1 expression during the development of T cells. Higher levels of Mcl-1 expression were identified in DP (CD4+CD8+ double-positive) T cells and in CD4+Foxp3+ T cells but not in CD8+ or CD4+Foxp3− T cells. The host with the specific ablation of Mcl-1 in Tregs exhibited reduced immune homeostasis, as implicated by decreases in the Treg population and the development of a scurfy phenotype. Furthermore, the essential role of Mcl-1 inTreg survival was assessed by hematopoietic chimeras. Mcl-1 or Bcl-2 was ablated via tamoxifen-inducible Cre in 50% of the bone marrow cells transferred; only the Treg population lacking a functional Mcl-1 declined soon after tamoxifen administration, strongly suggesting that Mcl-1 is the dominant anti-apoptotic factor involved in maintaining Treg homeostasis.
Pro-apoptotic BH3-only proteins are able to antagonize the anti-apoptotic effects of the anti-apoptotic Bcl-2 family proteins.13 An elevated percentage of CD4+Foxp3+ T cells was identified in mice with Tregs that had been specifically Bim-ablated, although the increase was not as obvious as that in mice with Bax and Bak double-knockout Tregs. As IL-2 is crucial for maintaining Tregs, particularly in the periphery, the researchers first crossed CD127CreMcl1CD4/+ with FoxP3DTR/+ to substantiate the correlation between IL-2 and Mcl-1 expression and found elevated Mcl-1 expression during the homeostatic expansion of Tregs. Serum IL-2 and IL-2-secreting CD4+ T cells increased during this process; thus, IL-2 plus an anti-IL-2 antibody complex, which usually causes the rapid expansion of Tregs, were introduced into CD127CreMcl1CD4/+ mice. Mcl-1 expression (as reflected by human CD4 expression) was specifically increased in the Treg population receiving IL-2 plus the anti-IL-2 antibody complex.
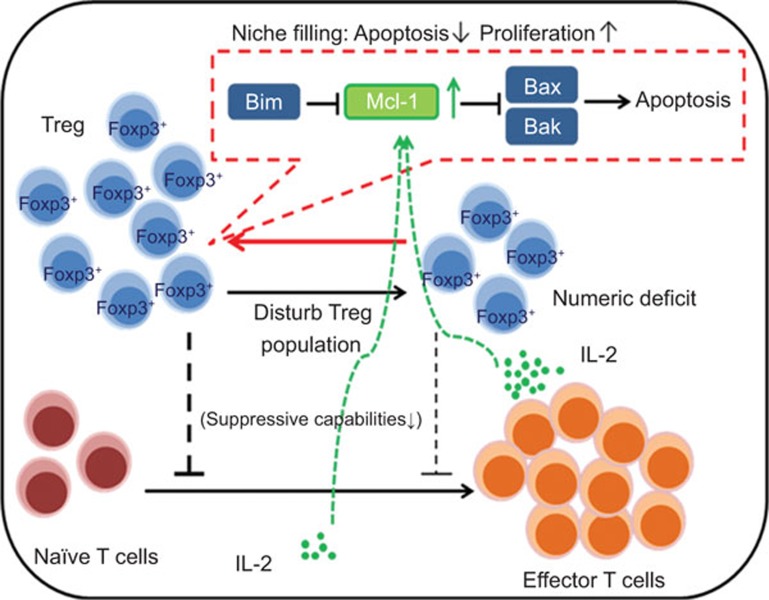
In summary, Tregs proliferate when their homeostatic population is disturbed through an IL-2 and costimulatory signal-dependent mechanism, which in turn downregulates apoptosis and facilitates the restoration of the Treg population. In addition, the Treg population is constrained through the Bax and Bak-mediated intrinsic apoptosis pathway. The molecular mechanisms involved in the process include the signals provided by IL-2, which elevates anti-apoptotic Mcl-1 expression during expansion, whereas Bim is the major antagonist of Mcl-1 in Tregs (Figure 1).
Figure 1.
A proposed regulatory mechanism of Treg homeostasis and survival. In the steady state, the interactions between pro-apoptotic Bim, anti-apoptotic Mcl-1 and apoptotic regulators Bax and Bak actively maintain the homeostatic population of Tregs. When the homeostatic population of Tregs is disturbed, signals provided by IL-2 and costimulatory signals upregulate anti-apoptotic Mcl-1 expression, which in turn inhibits the Bak and Bax-mediated intrinsic apoptosis pathway and subsequently allows Tregs to proliferate during the niche-filling process. Treg, regulatory T cell.
In the steady state, Tregs are a stable but dynamic population due to their high turnover rate. However, Tregs tend to lose their suppressive function under inflammatory/disease conditions. For example, the Treg/TH17 ratio is decreased in multiple sclerosis patients, and the ratios are negatively correlated with the disease severity.18 The importance of cytokines was also implied in the study by Pierson et al, whereby the neutralization of IL-2 using antibodies could not completely antagonize the Treg homeostatic expansion.1 These results suggest that cytokines other than IL-2 may synchronize with the costimulatory signals to sustain Treg homeostatic expansion and the Treg population. Indeed, TGF-β, IL-35 and IL-4 have also been proven to be related to Treg homeostasis.19,20,21 It is therefore important to consider all the factors related to Treg homeostasis, including cytokines and costimulatory signals, and also take the molecular balance between pro- and anti-apoptotic responses into consideration for the design of effective Treg-based therapeutic strategies.
Acknowledgments
The authors declare that they have no conflicts of interest and acknowledge the financial support of grants from the National Health Research Institutes, National Science Council (101-2320-B-182-027-MY3), and Chang Gung Memorial Hospital (CMRPD3B0052 and BMRP440). CRS and WCY contributed equally to this work.
References
- Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–3018. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- Shi Y, Radvanyi LG, Sharma A, Shaw P, Green DR, Miller RG, et al. CD28-mediated signaling in vivo prevents activation-induced apoptosis in the thymus and alters peripheral lymphocyte homeostasis. J Immunol. 1995;155:1829–1837. [PubMed] [Google Scholar]
- Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991;351:150–153. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DC, Strasser A. BH3-only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Bouillet P, O'Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9:514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- Hernandez JB, Newton RH, Walsh CM. Life and death in the thymus—cell death signaling during T cell development. Curr Opin Cell Biol. 2010;22:865–871. doi: 10.1016/j.ceb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 2007;19:516–521. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh-Esfahani SH, Gharagozloo M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. J Neuroimmunol. 2013;262:106–112. doi: 10.1016/j.jneuroim.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey NJ, Chapoval SP, Smith EP, Skupsky J, Scott DW, Keegan AD. STAT6 controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation. J Immunol. 2013;191:1517–1528. doi: 10.4049/jimmunol.1300486. [DOI] [PMC free article] [PubMed] [Google Scholar]