Abstract
Hepatitis C imposes a significant burden on global healthcare. Chronic infection is associated with progressive inflammation of the liver which typically manifests in cirrhosis, organ failure and cancer. By virtue of elaborate evasion strategies, hepatitis C virus (HCV) succeeds as a persistent human virus. It has an extraordinary capacity to subvert the immune response enabling it to establish chronic infections and associated liver disease. Chemokines are low molecular weight chemotactic peptides that mediate the recruitment of inflammatory cells into tissues and back into the lymphatics and peripheral blood. Thus, they are central to the temporal and spatial distribution of effector and regulatory immune cells. The interactions between chemokines and their cognate receptors help shape the immune response and therefore, have a major influence on the outcome of infection. However, chemokines represent a target for modulation by viruses including the HCV. HCV is known to modulate chemokine expression in vitro and may therefore enable its survival by subverting the immune response in vivo through altered leukocyte chemotaxis resulting in impaired viral clearance and the establishment of chronic low-grade inflammation. In this review, the roles of chemokines in acute and chronic HCV infection are described with a particular emphasis placed on chemokine modulation as a means of immune subversion. We provide an in depth discussion of the part played by chemokines in mediating hepatic fibrosis while addressing the potential applications for these chemoattractants in prognostic medicine.
Keywords: chemokine, chemokine receptor, hepatic fibrosis, hepatitis C virus, immune evasion
Hepatitis C virus (HCV): the non-A, non-B causative agent of viral hepatitis
Over 170 million people globally are infected with the HCV,1,2 the majority of whom will establish chronicity with long term complications such as cirrhosis, liver failure and hepatocellular carcinoma (HCC).3,4,5 HCV is a hepatotrophic, non-cytopathic, enveloped particle of 40–70 nm in diameter.6,7 It belongs to the Hepacivirus genus within the Flaviviridae family and has a host range limited to just chimpanzees and humans.6,8 The HCV genome comprises a positive sense ssRNA molecule which measures 9.6 Kb in length and contains 3′ and 5′ untranslated regions flanking a single open reading frame. The open reading frame encodes a 3000 residue polyprotein which is cleaved by host and viral proteases into four structural and seven non-structural components.9 The virus is categorized into seven known genotypes which can be delineated further into several distinct subtypes.10 Individual HCV genotypes and subtypes may exhibit extensive sequence divergence contributing to variable pathogenicity and treatment susceptibility.11 Treatment of chronic HCV infection currently involves 24–48 weeks of combinational therapy consisting of pegylated interferon-α (IFN-α) and the guanosine analogue ribavirin.12 Sustained virological response (SVR) rates with antiviral therapy are highly variable and success with treatment depends on both host and viral factors. Furthermore, the lack of an efficacious vaccine and the meagre success rates offered by existing regimens means that current medical treatment remains limited.13
Microanatomy and immunology of the liver
Aforementioned, HCV is typically hepatotrophic. Therefore, it is worth briefly discussing the unique structure and immune properties of the liver. The hepatic vascular bed is dually supplied by the hepatic arteries and portal vein which drain towards a network of specialized capillaries termed hepatic sinusoids.14 The sinusoids are lined with fenestrated endothelial cells (ECs) and luminal Kupffer cells (KCs), and run parallel to one another through liver parenchyma allowing perfusion of the tissue with an oxygenated, nutritive and antigen rich blood supply.14,15 On return to the systemic circulation, blood passes to the central veins and from there to the hepatic veins before exiting via the extrahepatic inferior vena cava.16 Between sinusoidal endothelium and liver parenchyma lies the space of Disse which contains fat storing hepatic stellate cells (HSC) and liver resident dendritic cells (DCs).17 Immune surveillance and tolerance within the liver is mediated by a system of innate defences which features sentinel cells, natural killer (NK) cells and natural killer T (NKT) cells. Additionally, the liver is populated by all typical classes of effector and memory T and B cells as well as CD25+FoxP3+ regulatory T cells (Tregs).19 For a more detailed account of the immunological properties of the liver, several comprehensive reviews have been previously published.17,18,20,21,22,23,24,25,26,27,28
Determinants of acute and chronic HCV infection
Acute infections are typically asymptomatic, therefore studies on immunity to HCV infection have been for the large part limited to chronically infected patients or immune chimpanzees.13 The outcome of acute infection is generally configured within the initial 6 months and ultimately depends on the magnitude, breadth and specificity of the adaptive immune response.13 Acute resolving infections are characterized by early expansion of polyclonal CD4+ and CD8+ T-cell populations which are sustained through to clearance.8 By contrast, chronic infections are associated with transient delayed responses that are weak and target a narrow range of MHC class I and II restricted epitopes29 which may explain the apparent deficiency or absence of antigen-specific cytotoxic T lymphocytes (CTLs). The adaptive response in chronic infection is generally short-lived and regardless of any initial vigour in helper cell proliferation, CD4+ effector functions are significantly reduced 30,31 and CD8+ T cells appear functionally exhausted.32 Unlike acute infections, chronic infections are characterized by poor memory induction and increased programmed death-1 expression on exhausted CD8+ T cells in both the liver and peripheral blood.31,33 Blunt and short-lived responses in chronically infected individuals may be explained in part by defects in antigen presentation. Such impairment might give rise to insufficient T-cell priming, late expansion of HCV-specific clones and subsequent delay in IFN-γ production.34 Defective myeloid dendritic cell (mDC) allostimulatory capacity has been reported in some patient studies and is associated with decreased IL-12 production and a reciprocal increase in IL-10.34 This is further supported by the observations that HCV viral proteins have ability to modulate monocyte and DC secretion profiles in cell culture.35 Although the frequency of plasma cells is increased during chronic HCV infection, the majority of these are nonspecific and in non-resolving infections, neutralizing antibodies fail to clear the virus.13 Despite these impairments, antibodies and CTLs exert selective pressure and contribute to the emergence of viral escape mutants.36 Why exactly persistent infections are established is not yet clear and the reasons for such extensive variation in patient responses against infection remain obscure. Current hypotheses aimed at explaining these phenomena include; the potential role of Tregs within the liver, defects in secretory mechanisms, susceptibility conferred by particular HLA types and chemokine modulation.29,37,38,39
Chemokine signalling and leukocyte migration
Chemokines are a family of small (8–12 kDa), chemotactic glycoproteins which regulate cell trafficking.40 These proteins are commonly referred to as homeostatic or inflammatory depending on the context in which they are expressed.41 Through ligation of their cognate receptors, they initiate signalling pathways culminating in the polarization, migration and extravasation of leukocytes from blood into tissues.42 They are classified into four groups; CXC (α), CC (β), XC (γ) and CX3C (δ) chemokines based on an N-terminal tetra cysteine motif. CXC chemokines display a generic amino acid (X) between their first two cysteine residues while the first two cysteine residues of a CC chemokine lie adjacently.43,44 XC chemokines lack the typical first and third cysteine residues whereas the only known CX3C chemokine, Fractalkine displays a sequence of 3 amino acids between its first two cysteines.45 Chemokine receptors belong to the rhodopsin-like GPCR superfamily. They are structured into seven transmembrane helices, an extracellular ligand binding domain and a cytoplasmic carboxy terminus rich in serine and threonine residues.43,46,47 Phosphorylation of the chemokine receptor upon activation promotes dissociation of its heterotrimeric G protein, transduction of intracellular signals and the subsequent binding of β-arrestins.46 β-arrestins induce receptor desensitization and internalisation by sterically preventing the G protein from re-associating with the GPCR.42,44 This feedback mechanism permits regulation of chemotaxis and cell migration at the point of the receptor or at the level of its downstream effectors.42,44 The extravasation of leukocytes from peripheral blood into underlying tissues during inflammation is a process which is heavily reliant on chemokine signalling for its initiation.48,49 Chemokines released by vascular endothelium, through various chemokine receptors, initiate a signalling cascade downstream of the GPCR. This cascade leads to activation of Rap-1-GTP which triggers integrin clustering and talin-mediated stabilization.42,50 This results in enhanced affinity of integrins for adhesion receptors such as ICAM-1 and subsequent arrest of the leukocyte upon the vessel wall.48 The cell then uses amoeboid ‘crawling' motion to navigate along the apical side of the endothelium towards chemokine gradients.49 The link between heterotrimeric G protein activation and that of the small GTPases is not completely understood. However, activation of the small GTPases Rho, Rac and Cdc42 is mediated by guanine exchange factors downstream from the heterotrimeric G protein and results in the polymerisation of G-actin into F-actin, lamellipod formation and a migratory phenotype.42,44
The role of chemokines in HCV infection
Type 1 helper T cell (Th1)-associated CXC chemokine receptor 3 (CXCR3)- and CC-chemokine receptor 5 (CCR5)-binding chemokines are detected in the peripheral blood approximately 2–8 weeks after initial infection with HCV.51 However, antigen-specific intrahepatic T cells are not observed until 8–12 weeks post infection which suggests that a late priming of specific CD8+ T cells is responsible for delayed liver infiltration rather than a defect in chemokine synthesis.52 While chemokine production does not appear to dictate the outcome of acute infection, it represents a target in HCV evasion with implications for effector cell recruitment and viral elimination. Inflammatory CXC and CC chemokines are upregulated in the liver and peripheral blood during chronic HCV infection.53 Moreover, their distribution within the liver directs migration to specific anatomical sites54 (Figure 1). Th1-polarized clones which dominate the HCV-infected liver are characterized by CXCR3, CCR5 and CCR7 expression.55 Their secretion profile includes an array of pro-inflammatory (IL-1B, IL-6, tumour-necrosis factor (TNF)-α) and Th1-type (IFN-γ, IL-12, IL-18) cytokines which dictate the local chemokine response.56 Th1-type CXCR3 and CCR5 ligand synthesis may be induced by IFN-γ or TNF-α in JAK/STAT- or NF-κB-mediated pathways respectively.57 These ligands may also be induced by IL-12 and IL-18 production and may depend on IL-6 amplifier activity for efficient gene expression.58,59,60,61,62,63 Moreover, IFN-γ, TNF-α and IL-1β are capable of stimulating CXCL2 and CCL2 expression in vitro.64 Th2 cell-derived cytokines (IL-10, IL-4, IL-5 and IL-13), on the other hand, downregulate Th1 responses and promote humoral immunity.65 Stimulation of DCs with IL-10 in particular has been shown to downregulate IL-12, IFN-α and CCR7 expression while upregulating CCR5 mRNA.56,66,67 T cells polarized towards a Th2 response typically express CCR3, CCR4 and CCR8 and the classical Th2-type chemokines include CCL17 and CCL22.68,69 HCV may interfere with DC trafficking by modulating chemokine expression and thus prevent efficient antigen presentation. Natterman et al.70 reported that CCL5 secretion induced by E2 crosslinking of CD81, sequesters immature dendritic cells (iDCs) expressing CCR5 within the liver and in doing so obstructs their use of CCR7 to enter the lymphatics. Moreover, expression of lymphoid CCL21 which binds CCR7 is increased in the chronically infected liver suggesting its role in the development of follicles during fibrosis.71 Table 1 summarizes the roles of major chemokines implicated in the immunopathogenesis of HCV infection.
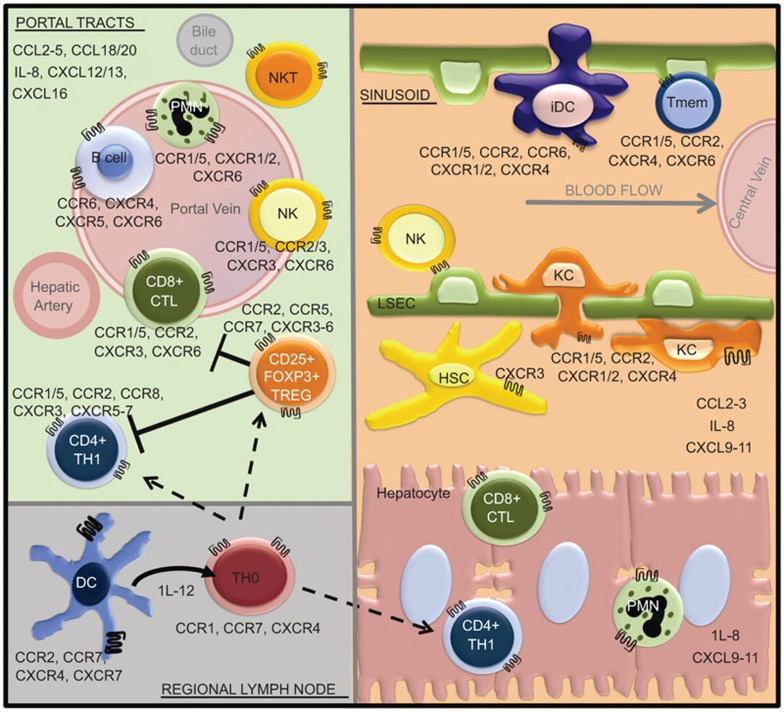
Figure 1.
Chemokine expression profile compartmentalizes the immune response to HCV in the chronically infected liver. Blood enters the liver lobule from the hepatic artery and portal vein, and flows via the sinusoids towards the central vein. Extravasation of leukocytes out of the blood and physical trapping of specific effector subsets is mediated by chemokine secretion profiles in discrete anatomical compartments. Potent cytotoxic reactions ensue within the PORTAL TRACTS retained by upregulated expression of CCR5 associated chemokines (CCL3, CCL4 and CCL5) on portal endothelium, while a nonspecific CCR5+/CXCR3+ Th1 subset dominates the helper cell population in the SINUSOIDS and hepatic parenchyma attracted by an upregulated expression of CXCL9–11. Upon maturation, dendritic cell and T-cell subtypes change surface expression of chemokine receptor CCR, facilitating their migration between secondary lymphatic tissues (REGIONAL LYMPH NODES) and the liver. CCR, CC-chemokine receptor; CTL, cytotoxic T lymphocyte; CXCR, CXC chemokine receptor; HCV, hepatitis C virus; HSC, hepatic stellate cell; iDC, immature dendritic cell; KC, Kupffer cell; LSEC, liver sinusoidal endothelial cell; NK, natural killer; PMN, polymorphonuclear neutrophil; Tmem, memory T cell; Th1, type 1 helper T cell; Treg, regulatory T cell.
Table 1. Chemokine and chemokine receptors in the immunopathogenesis of HCV infection.
| Ligand | Synonym | Receptor | Source | Target | Classification | Role in HCV pathogenesis |
|---|---|---|---|---|---|---|
| CC chemokines | ||||||
| CCL2 | MCP-1 | CCR2 | KC, HSC | Monocyte, KC, memory T cell, CTL, Treg, pDC | Inflammatory | Innate and adaptive immunity, Th1 inflammation, fibrosis |
| CCL3 | MIP-1α | CCR1, CCR5 | Portal endothelium, LSEC, KC, HSC, lymphocyte | Monocyte, NK, NKT, neutrophil, Th1, CTL, Treg, naive T cell, immature DC | Inflammatory | Initial transmigration, portal tract fibrosis, innate and adaptive immunity, Th1 inflammation |
| CCL4 | MIP-1β | CCR1, CCR5 | Portal endothelium, LSEC, KC, HSC, hepatocyte | Monocyte, NK, NKT, neutrophil, Th1, Treg, immature DC | Inflammatory | Initial transmigration, portal tract fibrosis, innate and adaptive immunity, Th1 inflammation |
| CCL5 | RANTES | CCR5 | Portal endothelium, biliary epithelium, hepatocyte, KC, HSC | Monocyte, memory T cell, NK, Th1, immature DC | Inflammatory | Initial transmigration, portal tract fibrosis, innate and adaptive immunity, Th1 inflammation |
| CCL7 | MCP-3 | CCR2, CCR3 | Portal endothelium, LSEC, monocyte | Monocyte, memory T cell, naive T cell, Th1, Treg, NK, DC | Homeostatic | Th2 response, Th1 inflammation, T cell and Monocyte Chemotaxis |
| CCL13 | MCP-4 | CCR2, CCR3, CCR5 | Widely expressed | KC, NK, memory T cell, Th1, Th2 | Homeostatic | Adaptive immunity, follicle formation, T-cell migration |
| CCL14 | HCC-1 | CCR1 | Widely expressed | KC, NK, memory T cell, Th1, Th2 | Homeostatic | Adaptive immunity, follicle formation, T-cell migration |
| CCL15 | MIP-5 | CCR1, CCR3 | Widely expressed | KC, NK, memory T cell, Th1, Th2, neutrophil | Homeostatic | Adaptive immunity, follicle formation, T-cell migration |
| CCL16 | LEC | CCR1, CCR2, CCR5, CCR8 | Hepatocyte, biliary epithelium | KC, NK, memory T cell, Th1, Th2 | Homeostatic/inflammatory | Adaptive immunity, follicle formation, T-cell migration |
| CCL18 | DC-CK1 | Unknown | Monocyte | Naive T cell | Homeostatic/inflammatory | Adaptive immunity, necro-inflammatory tissue damage, fibrosis |
| CCL19 | ELC | CCR7 | Vascular endothelium, lymphatics, LSEC | Naïve B and T cell, mature mDC | Homeostatic/inflammation | DC homing to lymphoid tissue, follicle formation |
| CCL20 | MIP-3α | CCR6 | Monocyte, DC | Memory B and T cell, immature mDC, Th17 | Homeostatic/inflammatory | Necro-inflammatory damage, fibrosis, HCC |
| CCL21 | SLC | CCR7 | Vascular endothelium, lymphatics, LSEC, HSC, CD8+ CTL, fibroblast | Naive B and T cells, Th1, Th2, Treg, mature mDC | Homeostatic | Homing of DC and T cells to lymphoid tissues, follicle formation, fibrosis |
| CXC chemokines | ||||||
| CXCL8 | IL-8 | CXCR1, CXCR2 | LSEC, KC, HSC, hepatocyte, neutrophil, biliary epithelium, portal endothelium | KC, neutrophil | Inflammatory | Innate immunity, fibrosis, angiogenesis, vasodilation, cirrhosis, HCC |
| CXCL9 | MIG | CXCR3 | Macrophage, Th1 cell, portal endothelium, LSEC, hepatocyte | Memory T cell, Th1, Th2, CTL, Treg, NKT, HSC | Inflammatory | Adaptive immunity, Th1 cell compartmentalisation, antifibrotic |
| CXCL10 | IP-10 | CXCR3 | Hepatocyte, LSEC | Memory T cell, Th1, Th2, CTL, Treg, NKT, HSC | Inflammatory | Recruitment and retention of intrahepatic Th1 cells, lobular inflammation, HCC |
| CXCL11 | I-TAC | CXCR3, CXCR7 | Hepatocyte, LSEC | Memory T cell, Th1, Th2, CTL, Treg, NKT, HSC | Inflammatory | Recruitment and retention of intrahepatic Th1 cells |
| CXCL12 | SDF-1 | CXCR4, CXCR7 | Widely expressed | Monocyte, KC, stromal cell, naive and memory B and T cells, NKT | Homeostatic | Portal fibrosis, angiogenesis, follicle formation, HCC, metastasis |
| CXCL13 | BLC | CXCR5 | Follicular DC, macrophages and lymphocytes | B lymphocyte, CD4+ T cell, CTL | Homeostatic/inflammatory | Follicle development |
| CXCL16 | SRPSOX | CXCR6 | Biliary epithelial cell, portal endothelium, hepatocyte | Memory T cell, CD4+ T cell, CTL, NK, NKT, B cell, neutrophil | Inflammatory | T-cell and NK migration, retention, integrin-mediated adhesion of liver infiltrating lymphocytes |
Abbreviations: BLC, B lymphocyte chemoattractant; CCR, CC-chemokine receptor; CTL, cytotoxic T lymphocyte; CXCR, CXC chemokine receptor; DC, dendritic cell; DC-CK1, dendritic cell chemokine-1; ELC, EBI1 ligand chemokine; HCC, hemofiltrate CC-chemokine; HCV, hepatitis C virus; HSC, hepatic stellate cell; I-TAC, interferon-inducible T-cell alpha chemoattractant; IP-10, interferon gamma-induced protein 10; KC, Kupffer cell; LEC, liver-expressed chemokine; LSEC, liver sinusoidal endothelial cell; MCP, monocyte chemotactic protein; mDC, myeloid dendritic cell; MIG, monokine induced by gamma interferon; NK, natural killer; NKT, natural killer T; pDC, plasmacytoid DC; RANTES, regulated and normal T cell expressed and secreted; SDF-1, stromal cell-derived factor-1; SLC, secondary lymphoid chemokine; SRPSOX, scavenger receptor for phosphatidylserine and oxidized low density lipoprotein; Th1, type 1 helper T cell; Treg, regulatory T cell.
CCR2 and associated ligands mediate effector cell recruitment in the HCV-infected liver
CCR2 is expressed on monocytes, macrophages, DCs and T cells. Ligands for CCR2 include CCL2, CCL7, CCL8 and CCL13, all of which are expressed in the hepatic environment.70,72 mRNA transcripts for CCL2 and CCR2 are significantly increased in HCV-infected livers.73,74 Moreover, an increase in serum CCL2 correlates with progressive liver inflammation in infected persons compared to healthy individuals.75 IFN-inducible CCL2 is released by KCs in the early phase of infection and its secretion promotes the first wave of infiltrating monocytes including CCR2+ plasmacytoid DCs.76,77 Activated plasmacytoid DCs release type I IFN, TNF-α, CCL4 and CXCL10, and stimulate CCL2 secretion by other IFN-inducible cell types.76 This effectively triggers a cascade which amplifies leukocyte recruitment; CCL2 recruits CCL3 secreting monocytes which in turn attract IFN-γ producing NK cells. Subsequent secretion of IFN-γ potentiates macrophage activation and secretion of CCL3 and CXCL9 which mediate recruitment of CD4+ effector T cells.78,79,80 In addition, CCR2 expressing CD8+ T cells are enriched in the inflamed liver during persistent infection with HCV.81 Thus, CCR2 is not only required for innate cell recruitment and antigen presentation, but must also play some part in the extravasation of CTLs.
Pro-inflammatory IL-8 and neutrophil chemotaxis in chronic HCV infection
Neutrophils provide first-line defence against invading viral pathogens. They generate reactive oxygen species and secrete inflammatory cytokines including IL-8, IL-10 and IL-12.82 IL-8 secretion results in an increased recruitment of neutrophils to the liver which in turn increases hepatic levels of IL-8 and exacerbates the necro-inflammatory process.82 In addition, CXCR1-positive CTLs have been detected in the HCV-infected liver suggesting that IL-8 may also mediate CD8+ T cell recruitment to sites of hepatic inflammation.54 IL-8 production is mainly induced by TNF-α, but may also be regulated via the RIG1/IRF3 pathway.83,84 Moreover, the induction of IL-8 may be amplified via ligation of the Toll-like receptors TLR2, TLR3 and TLR7 which recognize components of the HCV polyprotein, dsRNA and host anti-viral compounds respectively.85 In fact, activation of signalling networks downstream of TLR2 culminates in an NF-κB-dependent increase in TNF-α and direct transactivation of the IL-8 promoter resulting in increased expression of this pro-inflammatory chemokine.86 Neutrophil chemotaxis may be enhanced further by an increase in vascular permeability mediated by TNF-α, thus contributing to increased levels of IL-8 within the liver.75 Furthermore, the release of reactive oxygen species from granulocytes and macrophages is known to modulate the transcriptional activity of NF-κB, thereby affecting IL-8 expression.82 Hepatic IL-8 is detected at low maintenance levels during acute HCV infection, although marked increases in serum and hepatic levels have been observed in HCV-infected patients with progressive inflammation and cirrhosis as compared to healthy controls.75,84 This increase in hepatic and peripheral IL-8 correlates positively with an increase in TNF-α and advancing fibrosis as indicated by histological activity index and serum levels of alanine aminotransferase.75 The most pronounced increase in IL-8 is observed in patients with a higher degree of neutrophil infiltration, cirrhosis and impaired liver function.75 HCV Core, NS4A, NS4B and NS5B are capable of inducing IL-8 mRNA and protein expression in vitro via transactivation of the IL-8 promoter.87,88 In addition to this, HCV Core and NS3A proteins have been shown to trigger inflammation through the TLR2/NF-κB pathway.35,89 This mechanism may represent an evasion strategy whereby HCV inhibits IFN production and exacerbates chronic hepatic inflammation. In contrast, type I IFNs and the NS3/4A protease complex have been shown to downregulate IL-8 expression.90 Thus, the virus has evolved a number of elaborate pathways for modulating host responses via manipulation of the IL-8 promoter. HCV as a quasispecies is likely to generate antigenic variants of structural proteins including NS5A. Different isolates of the virus may therefore exhibit different potencies for transactivation of the IL-8 promoter resulting in varying responses to antiviral therapy.84,91
Th1-type CXCR3-associated chemokines and liver-infiltrating lymphocytes
CXCR3 acts as a receptor for CXCL9 (monocyte induced by IFN-γ), CXCL10 (IFN-γ inducible protein-10) and CXCL11 (IFN-inducible T-cell α chemoattractant), all of which are highly expressed in the peripheral blood and liver during chronic HCV infection.92,93,94 Upregulated surface expression of CXCR3 on liver infiltrating CD4+ effector lymphocytes and intrahepatic memory T cells has been reported in the livers of HCV-infected individuals.95,96,97 This upregulation is associated with an increase in migration towards CXCL9 and CXCL10 released by sinusoidal ECs and hepatocytes.97,98 Moreover, the importance of the CXCR3-binding chemokines in the adhesion and transmigration of effector lymphocytes across sinusoidal endothelium has been demonstrated in in vitro adhesion assays by two independent groups.96,99 HCV replication may induce CXCR3-associated chemokines during the early phase of infection by a dsRNA or viral peptide mediated synthesis of type I interferon. It is presumed that this response is further enhanced by secretion of the Th1-type cytokines IFN-γ and TNF-α within the local inflammatory environment.83 Gradients of CXCR3-associated chemokines are important for the migration and retention of T cells from peripheral blood into the chronically infected liver. However, it is proposed that CXCR3 ligands do not mediate the initial extravasation of lymphocytes into parenchyma, but rather they are involved in preferential compartmentalisation of a CXCR3+/CCR5+ Th1 cell subtype.100 This hypothesis was drawn from the observation that CXCL9 and CXCL11 were differentially expressed in the liver and peripheral blood, while CXCL10 levels were found to be as high in the liver as in the sera of HCV-infected patients and may therefore neutralize any initial gradient.83 Instead, the role of initial extravasation has been attributed to CCR5 and its associated ligands.100 Persistent HCV infection is associated with a CXCR3+high/CCR5+high T-cell infiltrate and a nonspecific Th1 response resulting in impaired CD8+ cytotoxicity and progressive necro-inflammatory damage. Larubia and colleagues101 believe that this Th1 phenotype is likely to dominate the helper cell population within hepatic parenchyma, possibly due to an increased proliferative capacity or susceptibility of other CD4+ lymphocytes to cell death. HCV replication significantly increases CXCL11 in infected hepatocytes and sinusoidal endothelial cells, and it has been proposed that an upregulation of CXCL11 in the sinusoids might benefit the virus by selectively recruiting CXCR3+/CCR5+ effector cells to this compartment resulting in decreased delivery of antigen-specific lymphocytes to the portal tracts.52 This may promote chronic localized inflammation within the portal tracts where a potentially nonspecific subset predominates. High serum levels of CCL3 and CXCL10 have been correlated with a normal frequency of CXCR3/CCR5 expressing CD8+ T cells in the peripheral blood.101 Thus, it is possible these cells are strategically retained in the liver or that CXCR3 and CCR5 are downregulated in response to overstimulation from a high circulatory ligand concentration.70,102
CCR1/CCR5 and associated Th1-type chemokines in portal tract inflammation
CCR1 and CCR5 are expressed on activated Th1/Tc1 cells, memory T cells, NK cells, and antigen presenting cells.103,104 The ligands for CCR1 and CCR5 include CCL3 (macrophage inflammatory protein-1α MIP-1α), CCL5 (regulated upon activation, normal T-cell expressed and secreted; RANTES) and CCL8.54,81 In addition, CCR1 has affinity for the monocyte chemoattractant CCL7 and the homeostatic chemokines CCL13–16, whereas CCR5 alone binds to CCL4.70 CCL3, CCL4 (MIP-1β) and CCL5 are implicated in portal tract inflammation and may be induced by proinflammatory Th1-type cytokines and type I IFNs.105 CCR5-associated chemokines direct recruitment of effector T cells to the portal tracts, whereas CXCR3 ligands by comparison appear to attract infiltrates of macrophages and lymphocytes into liver parenchyma.101 Co-expression of CCR5 and CXCR3 on activated T lymphocytes is thought to contribute to the recruitment and retention of hepatic infiltrates and involve selective compartmentalisation of nonspecific CCR5+/CXCR3+ T cells within the portal tracts.52 Evidently, CCL3, CCL4 and CCL5 expression is upregulated by portal endothelium during chronic HCV infection.54,97,102 In agreement with this finding, hepatic inflammation and fibrosis in persistent HCV infection is concentrated within the portal tracts.106 Furthermore, the interaction of CCL3 with CCR5 has been shown to mediate recruitment of effector Th1/Tc1 cells to the portal tracts in a murine model of host versus graft disease.72 The CCR1 receptor is also known to play a role in regulating hepatic inflammation in mice.107,108 Differential expression of CCR5 and CCR1 has been demonstrated in peripheral blood and intrahepatic T cells from HCV-infected persons. Expression of both CCR5 and CCR1 was found to be elevated on intrahepatic CD4+ T cells from individuals with HCV infection, whereas all subsets of peripheral blood derived T cells showed a significant reduction in the respective receptors when compared to healthy subjects.100 In vitro chemotaxis studies on blood and liver derived lymphocytes from chronic HCV-infected patients revealed a reduced migration of peripheral blood T cells to CCL3, CCL4 and CCL5 in comparison to those obtained from the infected liver.100 Interestingly, it was confirmed that CD8+ effector cells from blood and liver displayed the greatest difference in receptor levels in the infected cohort.100 A plausible explanation for a decrease of these receptors in the peripheral blood relative to the infected liver may be a marked increase in intrahepatic migration due to increased chemokine synthesis in the liver.101 A higher concentration of the CCR5-associated chemokines in the portal tracts may sequester these cells within the liver and in the context of nonspecific CTLs contribute to disease progression. At first, a prominent CD8+ response is observed which correlates with transient upregulation of CCR5 expression.109 However, during chronic infection, virus-specific CD8+ T cells exhibit reduced expression of CCR1 and CCR5 which are normally associated with a robust Th1/Tc1 response.100,102 The pathology of chronic HCV infection often involves gradual progression of fibrosis leading to cirrhotic liver disease. It has been suggested that hepatic inflammation is kept at low levels over the course of infection ensuring host cell viability and maintenance of virus numbers.110 It is hypothesized that the virus achieves this by reducing migration of antigen-specific CTLs into the liver via impairment of CCR5 and CCR1 receptor expression.100 Soo and colleagues111 were first to demonstrate the ability of full-length HCV cDNA to induce CCL5 mRNA and protein levels in a number of cell lines. The HCV genome has since been reported to modulate transactivation of the CCL5 promoter via IRFs and NF-κB in a HCV culture system and in various cell lines.112 Additionally, the various regulatory effects of HCV proteins on CCL5 expression in vitro have been studied in a number of experimental models. The interaction of HCV E2 with CD81 leads to an increase in CCL5 expression and secretion by CD8+ T cells as reported by Nattermann and co-workers.70 By comparison, HCV Core protein has been shown to mediate inhibition of the CCL5 promoter in two cell lines.92 However, Ruggieri et al.113 found that when coexpressed with either E1/E2 or NS2/NS3 complexes, the inhibitory effect of HCV Core on the CCL5 promoter could be reversed. Conversely, Soo et al.111 found that when stimulated by E1/E2, Core mediated activation of a RANTES promoter construct could be suppressed in a cancer cell line. The regulatory activity of HCV Core has been further clarified by Boisvert and co-workers81 and it now appears as though this structural component either induces or inhibits CCL5 expression depending on cellular context and endogenous transcription factors. The CCR5/CCL5 interaction appears to skew adaptive immunity in the HCV-infected liver towards a Th1 phenotype. In comparison, effectors with a Th2 profile are predominantly CCR4+.100 A potent Th1 response is necessary to support HCV-specific cytotoxicity which is central to the control of viral replication. However, a nonspecific inflammatory load and an exhausted CD8+ response characterized by excessive inflammation often ensues.38,114 It may be possible that localized expression of CCR5 ligands within the liver influences whether an acute infection is spontaneously eliminated or progresses to chronicity. Likewise, CCR5/CCL5 interactions could be a predisposing factor for the success of antiviral treatment. The manipulation of CCR5 and CCL5 expression and receptor–ligand interactions by HCV is likely to favour subversion of antiviral immunity and chronicity culminating in persistent inflammation and hepatic tissue damage. However, the pathological significance of CCR5 in HCV infection presently lacks definition.103
The CXCR6–CXCL16 axis in the recruitment and retention of distinct lymphocyte subsets
CXCL16 is a potent chemoattractant for infiltrating CD4+ and CD8+ effector T cells in addition to NK cells and NKT cells with which the liver is enriched.115,116,117,118 CXCL16 is highly expressed in epithelial cells of the biliary ducts and on portal endothelium of chronic HCV-infected patients.117 Lymphocyte subsets recruited by CXCL16 display an increased surface expression of CXCR6 in patients with persistent hepatitis C and are characteristically Th1-polarized.119,120 However, the CXCR6+ infiltrate likely contributes to excessive tissue damage in chronic infection. In addition, intrahepatic CXCL16 expression has been linked to an influx of CXCR6 and IL-8 expressing neutrophils.121 Inflammatory Th17 cells and Tregs expressing CXCR6 are thought to be recruited to the liver by CXCL16 during chronic HCV infection.72 It is possible that chronic infection requires a balanced recruitment of effector and regulatory subtypes such that low-grade inflammation and suppression of the antiviral response is established.32 Furthermore, a unique population of intrahepatic HCV-specific CXCR6+ CTLs secreting both IL-17 and IFN-γ has recently been described, the significance of which is currently under investigation.72,122 The interaction of CXCL16 with CXCR6 on infiltrating lymphocytes has been shown to promote β1-integrin-mediated adhesion which may function in the retention of an inflammatory load within the portal tracts.117 Thus, the CXCR6–CXCL16 axis may be important for regulatory and effector lymphocyte recruitment, but could have potential implications for the retention of these cells resulting in prolonged and inappropriate inflammatory responses.
Chemokines in HCV-related hepatic and non-hepatic disease
Necro-inflammatory damage secondary to chronic HCV infection accumulates over several decades and is believed to initiate fibrosis through the direct activation of HSCs.123 Activated HSCs are characterized by a loss of retinoid and an increased responsiveness to mitogenic and chemotactic stimuli resulting in their enhanced proliferation and migration into regions of hepatic injury.124 HSCs play a dominant role in liver fibrosis through their recruitment of an inflammatory load, their secretion of profibrotic and pro-inflammatory cytokines, and their increased deposition of extracellular matrix proteins.109,113 Consequently, the liver undergoes a remodelling process characterized by expansion of HSCs, epithelial cell and hepatocyte proliferation, the generation of fibrotic septa and the formation of lymphoid-like follicles.53,110 Cirrhosis represents the most advanced stages of liver fibrosis, at which point, significant changes to the tissue architecture begin to compromise crucial immunological and metabolic functions which often manifests in liver failure and/or HCC.111 Histological analyses of cirrhotic livers reveal a replacement of normal parenchyma with thickened interlobular fibrous septa and the formation of regenerative hepatocyte nodules.112 As the disease progresses, portal blood flow becomes severely impeded and hepatocyte numbers become diminished.125 A mechanistic link between neovascularisation and hepatic fibrosis has been firmly established.126,127,128,129 These processes appear to act in concert and may be co-dependently linked through chronic inflammation.130,131 HCC relies heavily on angiogenesis to meet its metabolic needs for tumour growth and metastasis 132,133,134 and local tissue hypoxia may act as a trigger for synthesis of angiogenic factors through a HIF-1α-dependent pathway. Increased intrahepatic vascular resistance associated with cirrhosis may also stimulate compensatory vascular remodeling which further exacerbates the condition.134 In fact, blood vessel density and VEGF expression correlate directly with Child–Pugh score and the proliferative index, size and grade of HCC tumours.135,136,137,138,139,140 A number of chemokines and their receptors are upregulated in the diseased liver and peripheral blood in the progression of liver fibrosis to cirrhosis and in the pathogenesis of malignant HCC. These molecules regulate inflammation, fibroproliferation, angiogenesis, carcinogenesis, metastasis, and anti-tumour immunity.59,133,141,142,143,144 CCL2 is a major HSC-produced chemokine and a critical factor in the induction of liver fibrosis.145,146 Expression of this chemokine is significantly increased in HSCs and promotes infiltration of the portal tracts with activated macrophages and T lymphocytes.147,148 CCL2 also exerts a direct chemotactic effect on HSCs, ensuring a continuous release of angiogenic and fibrotic factors.149 Interestingly, Farci and co-workers146 found that sustained high levels of this chemokine were predictive of rapid progression to cirrhosis, while high levels of CCL4 were typical of slow progressors. Presence of the −2518 CCL2G allele in HCV-infected patients has also been reported as a predisposition to advanced stage disease.119 CCL2 is strongly angiogenic as demonstrated by its ability to upregulate VEGF expression and stimulate EC chemotaxis.150,151 When compared to non-tumourous neighbouring tissue or healthy specimen, HCC tumours expressed significantly higher levels of CCL2.152 Moreover, CCL2 treatment enhances the migration and invasion of Huh7 hepatoma cells in vitro.153 Macrophage- and biliary epithelial cell-produced CCL3 is also upregulated in the fibrotic liver and expression of CCR1 and CCR5 has been detected on effector T cells within lymphoid-like follicles.53 CCL3 appears to be an important mediator of injury-induced liver fibrosis as demonstrated by the reduction in immune cell infiltration and attenuation of fibrosis in CCL3-deficient mice.154 Furthermore, CCL3 is capable of stimulating HSC proliferation and migration.154 The Th2-type cytokines IL-4 and IL-13 induce both CCL2 and CCL3. Thus, it is possible that a system is established whereby constant Th2 cell recruitment and CCL2/CCL3 production is maintained.120 CCR1 and CCL3 expression is significantly higher in HCC than in fibrosis, cirrhosis or non-tumourous surrounding tissue.155,156 Moreover, the CCR1–CCL3 axis appears to play an integral role in promoting angiogenesis and tumour burden.143,155,157 Both mRNA and protein content of CCL5 are significantly increased in patients with high histological activity index and serum alanine aminotransferase, and its expression correlates positively with disease severity.60 Stimulation of activated HSCs with either TNF-α or IL-1β potently induces CCL5 production through an NF-κβ-dependent mechanism.158 Autocrine CCL5 is then thought to induce downstream phosphorylation of ERK and focal adhesion kinase in HSCs with ensuing increases in HSC proliferation and migration.158 Upregulation of homeostatic CCL19, CCL21 and CXCL13 at sites of chronic inflammation promotes follicle formation and has been implicated in the recruitment of CCR7+ and CXCR5+ lymphocytes to B- and T-cell zones.59 CCL20 functions as a chemoattractant for CCR6+ lymphocytes and DCs.121,122 Serum levels of CCL20 are significantly elevated in chronic hepatitis and continue to increase with advancing fibrosis and cirrhotic disease.122 Expression of this chemokine is also markedly increased in hepatoma cells and its levels correlate directly with VEGF expression and tumour size.159,160 Accordingly, a role for this chemokine in regulating cirrhosis- and HCC-associated angiogenesis is strongly implied. Elevated levels of CCR6 mRNA have also been detected on Th17 cells and Tregs within the inflammatory tumour microenvironment.133,159,161 As such, the CCR6–CCL20 axis is suggested to be an important factor in carcinogenesis, tumour cell trafficking, proliferation and metastasis. CXC chemokines play a mechanistic role in mediating fibrosis and neovascularisation.162,163,164 Differentiation is made between those that promote the formation of immature blood vessels and those that act by inhibiting that same process. Proangiogenic CXC chemokines contain a conserved Glu–Leu–Arg (ELR) motif in their amino terminal and as such are referred to as ELR+.129 They mediate their angiogenic activity through CXCR2 and CXCR1 on vascular ECs, while the IFN-inducible CXC chemokines CXCL9-11, and CXCL4 which lack the ELR motif are potently angiostatic and signal through the CXCR3B splice variant.165 CXCR2 is the main functional receptor for mediating angiogenic and proliferative effects of ELR+ chemokines like CXCL2, CXCL5 and IL-8.129,165 CXCR2 is also considered to be a crucial determinant of liver regeneration and hepatic cell death in response to chronic inflammatory damage.166 Upregulation of CXCR2 mRNA in HCC tissue and its correlation with intrahepatic metastasis also suggests a role for this receptor in the proliferation of tumour cells.167 CXCR3+ effector cells are another important determinant of HCV-related fibrosis. These cells proved indispensable in a mouse model of fibrogenesis when deletion of CXCR3 resulted in a reduction of intrahepatic IFN-γ and increased liver fibrosis.117 Furthermore, CXCR3+ effector cells are typically Th1 polarized and when outweighed by a Th2 response, fibrosis tends to be more aggressive.120 Zeremski et al.79 found that while CXCL9 and CXCL10 were associated with severity of fibrosis as indicated by histological activity index and serum alanine aminotransferase, CXCL11 levels were not.79 CXCR3 ligands were also associated with features of advanced cirrhosis such as portal hypertension.126 However, CXCL9 and CXCL10 appear to have opposing effects in the context of liver fibrosis. Although increased in advanced fibrosis, CXCL9 induced an inhibitory effect on expression of TGF-β and collagen in an in vitro model, suggesting an antifibrotic role for this chemokine in vivo.117 In contrast, binding of CXCR3 on HSCs by CXCL10 stimulates activation of the MAPK pathway resulting in a proliferative response.127 Moreover, induction of the MEK/ERK pathway by CXCL10 may reflect a key event in the development of HCC. CXCL12 is expressed in bone marrow stroma, lymphoid-like follicles and bile duct epithelial cells.128 Hepatic expression of this chemokine is induced in response to pro-inflammatory stimuli and increases with progression of HCV-induced cirrhosis.58,168,169 Furthermore, CXCR4 expression is increased on cells within the foci of cirrhotic livers.128 Strieter and colleagues129 also demonstrated an essential role for CXCL12 in VEGF-driven angiogenesis within the murine liver. In review of these findings, it was suggested that CXCL12 mediates formation of lymphoid-like structures in the cirrhotic liver and its redistribution along fibrotic septa promotes the development of immature blood vessels within these structures.128 A range of pathological features have been attributed to the CXCR4–CXCL12 axis in the development and progression of intrahepatic tumours. Expression of CXCL12 and its corresponding receptor has been detected at significantly higher levels in HCC tissue when compared to non-tumourous tissue or tissue from cirrhotic livers.170 Moreover, CXCL12 levels correlate with tumour cell proliferation and distant lymphoid metastases.171 The ability to recruit endothelial precursors into tumour sites suggests that the CXCR4–CXCL12 axis is also a key mediator of neovascularisation during the progression of HCC and therefore, must play an intricate role in supporting outgrowth and metastasis of malignant CXCR4+ tumours.172,173 Furthermore, CXCL12 appears to be involved in regulating the secretion of MMP-2 and -9 which promote detachment from the basement membrane and metastasis.174 Overstimulation of CXCR4 by its ligand may also stimulate hyperproliferation and induce abnormal differentiation patterns in these cells, thus having a direct tumorigenic effect.143
Chronic infection with HCV can also give rise to a spectrum of extrahepatic diseases (HCV-EHDs) including lymphoproliferative disorders, metabolic syndrome, thyroid dysfunction and cancer.175,176 Moreover, extrahepatic disease is believed to complicate prognosis and further increase the risk of developing HCC and type II diabetes (T2D).60 HCV-EHDs are characterized by sustained Th1 responses, the potencies of which correlate with disease severity.175,176 A signature Th1 cytokine profile has been implicated in the induction of HCV-related autoimmune disease and the observed ability of HCV to infect islet cells and thyrocytes in vitro has raised the hypothesis that Th1 responses in the infected liver are analogous to those mediating autoimmune and systemic HCV-EHDs.177 Interestingly, CXCL9-11 appear to play a significant role in the development of these diseases.56 Similar to HCV-induced inflammation of the liver, HCV-EHDs may be brought on by excessive production of the Th1-type cytokines IFN-γ and TNF-α in target tissues.56 A consequential increase in CXCR3 ligand expression might further perpetuate the immune response by augmenting selective recruitment of CXCR3+ Th1 cells to peripheral sites of inflammation.176 Additionally, the expression of CXCL13 may be modulated by HCV and has been associated with lymphocyte trafficking in lymphoproliferative disorders and B cell neoplasms.56,178
Chemokines as biomarkers in the outcome of treatment
Chemokines have been acknowledged for their potential application as biomarkers in determining response to IFN/ribavirin therapy.81,94,98,112,179,180,181,182,183,184 Depending on prediction accuracies, serum chemokines may be capable of indicating success or failure in response to treatment, thus offering a rapid and non-invasive alternative to liver biopsy. In a study investigating the relationship between serum levels of CXCR3-associated chemokines and response to antiviral therapy, a significant increase in all three chemokines was observed prior to treatment.98 The same study found that CXCL9 and CXCL10 were decreased in those for whom treatment had been successful, whereas CXCL11 levels did not decline during treatment or in the initial 6 months after. In contrast, plasma levels of these molecules remained elevated in the group of non-responders. Low baseline levels of CXCL10 were associated with an early response to therapy while higher pre-treatment levels indicated a failure to achieve SVR. Furthermore, higher CXCL10 mRNA and protein in the peripheral blood of infected individuals were predictive of advanced fibrosis and non-response in two separate investigations.94,180 Levels of CXCL10 were also higher in African Americans who are generally less responsive to treatment than Caucasians. However, no race-specific differences were associated with CXCL9 and CXCL11 in either the infected or uninfected cohort.98 Elevated levels of IL-8 in serum are strongly associated with resistance to standard antiviral therapy.84,183 It is thus likely that IL-8 expression is induced by HCV NS5A and at least partially inhibits IFN activity in vivo. In a study by Yamauchi et al.,185 all patients with chronic infection had similar pre-treatment serum levels of CCL20. However, CCL20 levels were reduced within two weeks of therapy in successful responders, but remained relatively unchanged in the group of non-responders.185 Higher levels of CCL20 following standard combinational therapy may therefore predict failure in achieving SVR. While early induction of CCL5 may be predictive of viral clearance, a decrease in CCL3 and CCL4 following treatment correlated well with SVR suggesting a reduction in the migration and retention of CCR5+ inflammatory cells.101 The CCR5δ32 polymorphism was associated with an increased CCL5 gene expression in the peripheral blood, and mild portal inflammation probably due to reduced migration of CCR5+ T cells into the liver.102 CCL5 was also found to be useful as a marker for distinguishing disease stage in the fibrotic liver.112,186 ‘Cytokinomics' involves the use of high throughput experimental techniques and computational methods to gain a genome-wide insight of the cytokine family and to investigate the functional relationships of these molecules in a complex biological system.187 Using this approach, a wealth of information can be yielded on the hierarchical structure of chemokine signalling in both health and disease. Interactomic studies make it possible to evaluate the regulation of ligand and receptor expression, determine pleiotropy, synergy, antagonism and functional redundancy within an interaction network and explore correlations with disease.187 Ultimately, these tools will grant an important understanding of the roles played by chemokines in the pathogenesis of diseases such as chronic HCV infection, liver fibrosis and HCC. Moreover, a comprehensive global vision of protein networks in the context of disease will allow for more effective drug developments and the validation of useful clinical markers. In a recent study, Capone and colleagues applied an interatomic study to characterize progression of chronic HCV infection, cirrhosis and HCC.58 By evaluating a panel of cytokines, chemokines and growth factors in patient serum, they identified a profile of pro-inflammatory cytokines that were upregulated in HCC and correlated with β-NGF, thus gaining a greater understanding of the link between cancer and inflammation. Constantini et al.60 also adopted a systems approach to discriminate between isolated cases of chronic hepatitis C infection and HCV infection with associated hepatic disease or T2D. They found that CXCL1 and CXCL9 were consistently upregulated in chronic HCV infection and associated liver disease with or without T2D as a disease modifier. However, this observation appeared to be specific to inflammatory liver disease as it was absent in serum from patients with T2D alone. In the quest for prognostic markers and indicators of treatment outcome, it seems that chemokines may be ideal candidates. Evaluating serum chemokines that reflect HCV pathology or predict whether or not a patient responds to IFN therapy would have major clinical benefits and impact upon treatment decisions. However, to fully assess the feasibility of these biomarkers in the assessment of HCV-related disease, wider studies are required. In particular, it is of fundamental importance that variable responses across different ethnic groups and viral genotypes are investigated.
Conclusions
Chemokines mediate control of HCV replication and the inflammatory response within the hepatic environment. They play an essential role in coordinating extravasation, and are required for both spontaneous clearance in acute infection and elimination with immunotherapy. However, they represent a target for immune evasion. During chronic infection, a sustained expression of chemokines drives persistent low-grade inflammation in the absence of a robust Th1 response, the net effect of which is collateral tissue damage and failure to eliminate the virus. HCV antagonizes the chemokine response to perturb infiltration of antigen-specific effector clones, thus allowing for uninterrupted generation of escape mutants and dissemination of virus particles. In addition, continuous infiltration of the liver by a nonspecific inflammatory load is a key hallmark of persistent HCV infection (Figure 2). In this review, the roles of the chemokine receptors CCR1, CCR2, CCR5, CXCR1, CXCR3 and CXCR6 in the pathogenesis of HCV have been discussed. The interactions between these receptors and their respective ligands are illustrated in Supplementary Figure 1. Overexpression of CCL3, CCL4 and CCL5 on portal endothelium appears to selectively recruit and retain a continuous wave of nonspecific CCR1+/CCR5+ inflammatory and cytotoxic effectors within this compartment. The CCR2-associated chemokines CCL2 and CCL8 are also upregulated in the portal tracts and an influx of CCR2+ mononuclear cells has been linked to hepatic fibrosis. Moreover, manipulation of the CXCR6-CXCL16 axis may favour persistence of the virus through the recruitment of Tregs and suppression of rigorous CD8+ T cell responses within the portal tracts. In addition, preferential migration of HCV-specific CTLs to CXCR3-associated chemokines in the hepatic parenchyma is thought to impair delivery of an efficient antiviral response to the portal and periportal regions. Based on experimental findings, it is postulated that HCV modulates expression and localisation of these chemokines in vivo and in doing so escapes the immune response. Chemokines have been suggested as potential non-invasive biomarkers for determining disease outcome and evaluating responses to pegylated IFN/ribavirin treatment. This is not surprising given the strength of correlation between serum levels of specific chemokines and resistance to antiviral therapy. CXCL10 in particular represents a putative prognostic marker in the treatment of chronic HCV as baseline levels can predict whether or not SVR is achieved. In addition, elevated serum levels of IL-8 and CCL20 following treatment may indicate resistance to antiviral therapy. Levels of CXCL12, CCL2, CCL3 and CCL5 may also help in gauging inflammatory activity and liver fibrosis. Early CCL5 responses in the peripheral blood may on the other hand translate to successful outcome and less severe inflammation within the liver. Furthermore, from analysis of polymorphisms in the genes encoding CCL2 and CCL5, it appears as though targeting chemokines therapeutically may be an approach to consider in the future treatment of chronic HCV infection. Further investigations of chemokines in the context of hepatitis C infection will undoubtedly provide a clearer definition of their role in HCV immunopathogenesis and thus allow the development of novel therapeutic interventions and predictive clinical markers.
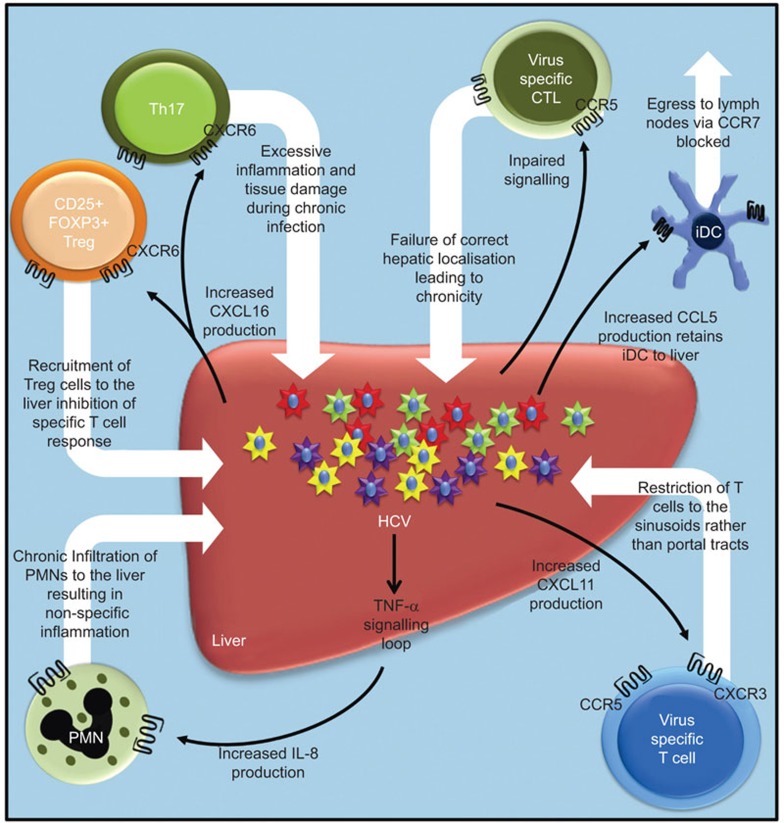
Figure 2.
HCV subverts the immune response and establishes chronic infection within the liver through modulation of hepatic chemokine networks. HCV modulates inflammation and cytotoxicity within the liver via manipulation of the CCL5 and IL-8 promoters. Increased CCL5 secretion sequesters iDCs expressing CCR5 within the liver and in doing so obstructs their use of CCR7 to enter the lymphatics, thus impairing antigen presentation and the induction of efficient CD4+ T-cell help. Intrahepatic IL-8 may be directly induced by HCV viral peptides and RNA, and cytokine signalling loops resulting in chronic infiltration of neutrophils to the liver and extensive necro-inflammatory damage. CXCR3-associated chemokines (CXCL9–11) may be induced by a HCV RNA or peptide mediated synthesis of type I interferon. An increase of CCR5- and CXCR3-associated chemokines within the liver with simultaneous downregulation of these receptors on peripheral T cells may be responsible for trapping these cell types in the liver. Gradients of CXCR3-associated chemokines restrict the recruitment of virus-specific CCR5+/CXCR3+ T cells to hepatic parenchyma while a nonspecific inflammatory load is retained within the portal tracts by CCR5 ligands. Increased expression of CXCL16 by biliary epithelial cells and portal endothelium may direct recruitment of CXCR6+ Th17 cells and Tregs within the liver, thus contributing to the suppression of antigen-specific cytotoxicity and maintenance of persistent low grade inflammation. CCR, CC-chemokine receptor; CTL, cytotoxic T lymphocyte; CXCR, CXC chemokine receptor; HCV, hepatitis C virus; iDC, immature dendritic cell; PMN, polymorphonuclear neutrophil; Th, helper T cell; TNF-α, tumour-necrosis factor α Treg, regulatory T cell.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology website.
Supplementary Information
References
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973–978. doi: 10.1038/nature04083. [DOI] [PubMed] [Google Scholar]
- Pickett BE, Striker R, Lefkowitz EJ. Evidence for separation of HCV subtype 1a into two distinct clades. J Viral Hepat. 2011;18:608–618. doi: 10.1111/j.1365-2893.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527–535. doi: 10.1002/hep.21321. [DOI] [PubMed] [Google Scholar]
- Nielsen SU, Bassendine MF, Martin C, Lowther D, Purcell PJ, King BJ, et al. Characterization of hepatitis C RNA-containing particles from human liver by density and size. J Gen Virol. 2008;89 Pt 10:2507–2517. doi: 10.1099/vir.0.2008/000083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari FV. Unscrambling hepatitis C virus–host interactions. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5–19. doi: 10.1002/hep.20032. [DOI] [PubMed] [Google Scholar]
- Nakano T, Lau GM, Sugiyama M, Mizokami M. An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver Int. 2012;32:339–345. doi: 10.1111/j.1478-3231.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Callendret B, Walker C. A siege of hepatitis: immune boost for viral hepatitis. Nat Med. 2011;17:252–253. doi: 10.1038/nm0311-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20:3–7. doi: 10.1034/j.1600-0676.2000.020001003.x. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Baird AW, O'Farrelly C. Microanatomy of the liver immune system. Seminars in immunopathology. 2009;31:333–343. doi: 10.1007/s00281-009-0173-4. [DOI] [PubMed] [Google Scholar]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43 2 Suppl 1:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmunity. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Billiar TR, Machida K, Crispe IN, Seki E. Toll-like receptor signaling in liver diseases. Gastroenterol Res Pract. 2010;2010:971270. doi: 10.1155/2010/971270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Li Z, Diehl AM. Innate immunity in the liver. Curr Opin Gastroenterol. 2003;19:565–571. doi: 10.1097/00001574-200311000-00009. [DOI] [PubMed] [Google Scholar]
- Bottcher JP, Knolle PA, Stabenow D. Mechanisms balancing tolerance and immunity in the liver. Dig Dis. 2011;29:384–390. doi: 10.1159/000329801. [DOI] [PubMed] [Google Scholar]
- Petrovic D, Stamataki Z, Dempsey E, Golden-Mason L, Freeley M, Doherty D, et al. Hepatitis C virus targets the T cell secretory machinery as a mechanism of immune evasion. Hepatology. 2011;53:1846–1853. doi: 10.1002/hep.24327. [DOI] [PubMed] [Google Scholar]
- Kanto T, Hayashi N. Immunopathogenesis of hepatitis C virus infection: multifaceted strategies subverting innate and adaptive immunity. Intern Med. 2006;45:183–191. doi: 10.2169/internalmedicine.45.1530. [DOI] [PubMed] [Google Scholar]
- Petrovic D, Dempsey E, Doherty DG, Kelleher D, Long A. Hepatitis C virus–T-cell responses and viral escape mutations. Eur J Immunol. 2012;42:17–26. doi: 10.1002/eji.201141593. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2012;61:1226–1234. doi: 10.1136/gutjnl-2011-300620. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, et al. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Fisicaro P, Tola D, Orlandini A, Sacchelli L, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–139. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- Agrati C, Nisii C, Oliva A, D'Offizi G, Montesano C, Pucillo LP, et al. Lymphocyte distribution and intrahepatic compartmentalization during HCV infection: a main role for MHC-unrestricted T cells. Arch Immunol Ther Exp. 2002;50:307–316. [PubMed] [Google Scholar]
- Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond) 2007;112:141–155. doi: 10.1042/CS20060171. [DOI] [PubMed] [Google Scholar]
- Tripathy AS, Shankarkumar U, Chadha MS, Ghosh K, Arankalle VA. Association of HLA alleles with hepatitis C infection in Maharashtra, western India. Indian J Med Res. 2009;130:550–555. [PubMed] [Google Scholar]
- Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res. 2006;34:211–227. doi: 10.1385/IR:34:3:211. [DOI] [PubMed] [Google Scholar]
- Choi WT, An J. Biology and clinical relevance of chemokines and chemokine receptors CXCR4 and CCR5 in human diseases. Exp Biol Med (Maywood) 2011;236:637–647. doi: 10.1258/ebm.2011.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- Eberlein J, Nguyen TT, Victorino F, Golden-Mason L, Rosen HR, Homann D. Comprehensive assessment of chemokine expression profiles by flow cytometry. J Clin Invest. 2010;120:907–923. doi: 10.1172/JCI40645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Petri B, Bixel MG. Molecular events during leukocyte diapedesis. FEBS J. 2006;273:4399–4407. doi: 10.1111/j.1742-4658.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- Thimme R, Binder M, Bartenschlager R. Failure of innate and adaptive immune responses in controlling hepatitis C virus infection. FEMS Microbiol Rev. 2012;36:663–683. doi: 10.1111/j.1574-6976.2011.00319.x. [DOI] [PubMed] [Google Scholar]
- Kang W, Shin EC. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med J. 2011;52:871–878. doi: 10.3349/ymj.2011.52.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald O, Weiss ID, Galun E, Peled A. Chemokines in hepatitis C virus infection: pathogenesis, prognosis and therapeutics. Cytokine. 2007;39:50–62. doi: 10.1016/j.cyto.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology. 2009;49:676–688. doi: 10.1002/hep.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- Fallahi P, Ferri C, Ferrari SM, Corrado A, Sansonno D, Antonelli A. Cytokines and HCV-related disorders. Clin Dev Immunol. 2012;2012:468107. doi: 10.1155/2012/468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MS, Chen X, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappaB-dependent/NO-independent chemokine gene expression in MIN6 beta cells. J Surg Res. 2003;110:295–303. doi: 10.1016/s0022-4804(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, Scala S, et al. Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw. 2010;21:99–104. doi: 10.1684/ecn.2010.0192. [DOI] [PubMed] [Google Scholar]
- Airoldi I, Ribatti D. Regulation of angiostatic chemokines driven by IL-12 and IL-27 in human tumors. J Leukoc Biol. 2011;90:875–882. doi: 10.1189/jlb.0511237. [DOI] [PubMed] [Google Scholar]
- Costantini S, Capone F, Guerriero E, Marfella R, Sorice A, Maio P, et al. Cytokinome profile of patients with type 2 diabetes and/or chronic hepatitis C infection. PLoS ONE. 2012;7:e39486. doi: 10.1371/journal.pone.0039486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma G, Pena R, Blanco J, Rosell A, Borras FE, Este JA, et al. Treatment of monocytes with interleukin (IL)-12 plus IL-18 stimulates survival, differentiation and the production of CXC chemokine ligands (CXCL)8, CXCL9 and CXCL10. Clin Exp Immunol. 2006;145:535–544. doi: 10.1111/j.1365-2249.2006.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MA, Mansfield PJ, Pakozdi A, Campbell PL, Ahmed S, Martinez RJ, et al. Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways. Arthritis Rheum. 2007;56:1787–1797. doi: 10.1002/art.22705. [DOI] [PubMed] [Google Scholar]
- Murakami M, Hirano T. The pathological and physiological roles of IL-6 amplifier activation. Int J Biol Sci. 2012;8:1267–1280. doi: 10.7150/ijbs.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen ML, Ronn SG, Bruun C, Larsen CM, Eizirik DL, Mandrup-Poulsen T, et al. IL-1beta-induced chemokine and Fas expression are inhibited by suppressor of cytokine signalling-3 in insulin-producing cells. Diabetologia. 2009;52:281–288. doi: 10.1007/s00125-008-1199-1. [DOI] [PubMed] [Google Scholar]
- Farci P, Wollenberg K, Diaz G, Engle RE, Lai ME, Klenerman P, et al. Profibrogenic chemokines and viral evolution predict rapid progression of hepatitis C to cirrhosis. Proc Natl Acad Sci USA. 2012;109:14562–14567. doi: 10.1073/pnas.1210592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Lau AH, de Creus A, Lu L, Thomson AW. Liver tolerance mediated by antigen presenting cells: fact or fiction. Gut. 2003;52:1075–1078. doi: 10.1136/gut.52.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Henderson NC, Bone-Larson CL, Lukacs NW, Hogaboam CM, Kunkel SL. Chemokines in the pathogenesis of liver disease: so many players with poorly defined roles. Clin Sci (Lond) 2003;104:47–63. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Nattermann J, Zimmermann H, Iwan A, von Lilienfeld-Toal M, Leifeld L, Nischalke HD, et al. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–1076. doi: 10.1016/s0016-5085(03)01194-6. [DOI] [PubMed] [Google Scholar]
- Oo YH, Shetty S, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis. 2010;28:31–44. doi: 10.1159/000282062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselah T, Bieche I, Laurendeau I, Paradis V, Vidaud D, Degott C, et al. Liver gene expression signature of mild fibrosis in patients with chronic hepatitis C. Gastroenterology. 2005;129:2064–2075. doi: 10.1053/j.gastro.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Zhdanov KV, Gusev DA, Chirskii VS, Sysoev KA, Iakubovskaia LA, Shakhmanov DM, et al. Chronic HCV-infection and expression of mRNA of CC-chemokines and their receptors Zh Mikrobiol Epidemiol Immunobiol 2008473–78.Russian. [PubMed]
- Neuman MG, Benhamou JP, Marcellin P, Valla D, Malkiewicz IM, Katz GG, et al. Cytokine–chemokine and apoptotic signatures in patients with hepatitis C. Transl Res. 2007;149:126–136. doi: 10.1016/j.trsl.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Decalf J, Fernandes S, Longman R, Ahloulay M, Audat F, Lefrerre F, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–2437. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Nomiyama H, Yoneyama H, Uwatoku R. Kupffer cell-mediated recruitment of dendritic cells to the liver crucial for a host defense. Dev Immunol. 2002;9:143–149. doi: 10.1080/1044667031000137610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydtmann M. Macrophages in hepatitis B and hepatitis C virus infections. J Virol. 2009;83:2796–2802. doi: 10.1128/JVI.00996-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Mather TP, Hokeness KL. Cytokine and chemokine networks: pathways to antiviral defense. Curr Top Microbiol Immunol. 2006;303:29–46. doi: 10.1007/978-3-540-33397-5_2. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Henderson NC, Bone-Larson CL, Lukacs NW, Hogaboam CM, Kunkel SL. Chemokines in the pathogenesis of liver disease: so many players with poorly defined roles. Clin Sci. 2003;104:47–63. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- Wisniewska-Ligier M, Wozniakowska-Gesicka T, Glowacka E, Lewkowicz P, Banasik M, Tchorzewski H. Involvement of innate immunity in the pathogenesis of chronic hepatitis C in children. Scand J Immunol. 2006;64:425–432. doi: 10.1111/j.1365-3083.2006.01800.x. [DOI] [PubMed] [Google Scholar]
- Helbig KJ, Ruszkiewicz A, Lanford RE, Berzsenyi MD, Harley HA, McColl SR, et al. Differential expression of the CXCR3 ligands in chronic hepatitis C virus (HCV) infection and their modulation by HCV in vitro. J Virol. 2009;83:836–846. doi: 10.1128/JVI.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak SJ, Khabar KS, Rezeiq M, Gretch DR. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner J, Austin M, Green J, Imaizumi T, Casola A, Brasier A, et al. Regulation of CXCL-8 (interleukin-8) induction by double-stranded RNA signaling pathways during hepatitis C virus infection. J Virol. 2007;81:309–318. doi: 10.1128/JVI.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y, Kato N, Yoshida H, Wang Y, Tanaka M, Goto T, et al. Hepatitis C virus core protein and hepatitis activity are associated through transactivation of interleukin-8. J Infect Dis. 2005;192:266–275. doi: 10.1086/430924. [DOI] [PubMed] [Google Scholar]
- Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–540. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Kadoya H, Nagano-Fujii M, Deng L, Nakazono N, Hotta H. Nonstructural proteins 4A and 4B of hepatitis C virus transactivate the interleukin 8 promoter. Microbiol Immunol. 2005;49:265–273. doi: 10.1111/j.1348-0421.2005.tb03728.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Chang S, Dolganiuc A. Altered innate immunity in chronic hepatitis C infection: cause or effect. Hepatology. 2007;46:1279–1290. doi: 10.1002/hep.21938. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M, Kaukinen P, Melen K, Julkunen I. Hepatitis C virus proteins interfere with the activation of chemokine gene promoters and downregulate chemokine gene expression. J Gen Virol. 2008;89 Pt 2:432–443. doi: 10.1099/vir.0.83316-0. [DOI] [PubMed] [Google Scholar]
- Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436 7053:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- Apolinario A, Majano PL, Lorente R, Nunez O, Clemente G, Garcia-Monzon C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27–37. doi: 10.1111/j.1365-2893.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004;39:1220–1229. doi: 10.1002/hep.20167. [DOI] [PubMed] [Google Scholar]
- Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise MW, Lukens JR, Nguyen AP, Lassen MG, Waggoner SN, Hahn YS. Fas ligand is responsible for CXCR3 chemokine induction in CD4+ T cell-dependent liver damage. J Immunol. 2006;176:6235–6244. doi: 10.4049/jimmunol.176.10.6235. [DOI] [PubMed] [Google Scholar]
- Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol. 2005;167:887–899. doi: 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrubia JR, Benito-Martinez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149–7159. doi: 10.3748/wjg.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175–1182. doi: 10.1182/blood-2005-01-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Leifeld L, Nischalke HD, Rockstroh JK, Hess L, Sauerbruch T, et al. Reduced CC chemokine receptor (CCR) 1 and CCR5 surface expression on peripheral blood T lymphocytes from patients with chronic hepatitis C infection. J Infect Dis. 2002;185:1803–1807. doi: 10.1086/340829. [DOI] [PubMed] [Google Scholar]
- Larrubia JR, Calvino M, Benito S, Sanz-de-Villalobos E, Perna C, Perez-Hornedo J, et al. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632–641. doi: 10.1016/j.jhep.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Ahlenstiel G, Woitas RP, Rockstroh J, Spengler U. CC-chemokine receptor 5 (CCR5) in hepatitis C—at the crossroads of the antiviral immune response. J Antimicrob Chemother. 2004;53:895–898. doi: 10.1093/jac/dkh239. [DOI] [PubMed] [Google Scholar]
- Coenen M, Nattermann J. The role of CCR5 in HCV infection. Eur J Med Res. 2010;15:97–101. doi: 10.1186/2047-783X-15-3-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Ljunggren HG, La Cava A, van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11:658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman MG, Schmilovitz-Weiss H, Hilzenrat N, Bourliere M, Marcellin P, Trepo C, et al. Markers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis C. Int J Hepatol. 2012;2012:231210. doi: 10.1155/2012/231210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AJ, Marinos G, Ffrench RA, Lloyd AR. Immunopathogenesis of hepatitis C virus infection. Immunol Cell Biol. 2001;79:515–536. doi: 10.1046/j.1440-1711.2001.01036.x. [DOI] [PubMed] [Google Scholar]
- Choi SW, Hildebrandt GC, Olkiewicz KM, Hanauer DA, Chaudhary MN, Silva IA, et al. CCR1/CCL5 (RANTES) receptor–ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood. 2007;110:3447–3455. doi: 10.1182/blood-2007-05-087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, de Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Timm J, Spangenberg HC, Wischniowski N, Nazarova N, Kersting N, et al. Virological and immunological determinants of intrahepatic virus-specific CD8+ T-cell failure in chronic hepatitis C virus infection. Hepatology. 2008;47:1824–1836. doi: 10.1002/hep.22242. [DOI] [PubMed] [Google Scholar]
- Barnaba V. Hepatitis C virus infection: a “liaison a trois” amongst the virus, the host, and chronic low-level inflammation for human survival. J Hepatol. 2010;53:752–761. doi: 10.1016/j.jhep.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Soo HM, Garzino-Demo A, Hong W, Tan YH, Tan YJ, Goh PY, et al. Expression of a full-length hepatitis C virus cDNA up-regulates the expression of CC chemokines MCP-1 and RANTES. Virology. 2002;303:253–277. doi: 10.1006/viro.2002.1617. [DOI] [PubMed] [Google Scholar]
- Katsounas A, Trippler M, Wang B, Polis M, Lempicki RA, Kottilil S, et al. CCL5 mRNA is a marker for early fibrosis in chronic hepatitis C and is regulated by interferon-alpha therapy and toll-like receptor 3 signalling. J Viral Hepat. 2012;19:128–137. doi: 10.1111/j.1365-2893.2011.01503.x. [DOI] [PubMed] [Google Scholar]
- Ruggieri A, Franco M, Gatto I, Kumar A, Rapicetta M. Modulation of RANTES expression by HCV core protein in liver derived cell lines. BMC Gastroenterol. 2007;7:21. doi: 10.1186/1471-230X-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leuk Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Iannacone M. Effector CD8 T cell trafficking within the liver. Mol Immunol. 2013;55:94–99. doi: 10.1016/j.molimm.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, et al. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107:595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaida MM, Gunther F, Wagner C, Friess H, Giese NA, Schmidt J, et al. Expression of the CXCR6 on polymorphonuclear neutrophils in pancreatic carcinoma and in acute, localized bacterial infections. Clin Exp Immunol. 2008;154:216–223. doi: 10.1111/j.1365-2249.2008.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northfield JW, Kasprowicz V, Lucas M, Kersting N, Bengsch B, Kim A, et al. CD161 expression on hepatitis C virus-specific CD8+ T cells suggests a distinct pathway of T cell differentiation. Hepatology. 2008;47:396–406. doi: 10.1002/hep.22040. [DOI] [PubMed] [Google Scholar]
- Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–231. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43:419–428. doi: 10.1007/s00535-008-2180-y. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon S, Heindryckx F, Geerts A, van Steenkiste C, Colle I, van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int. 2011;31:146–162. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Kotsiou S, Koukourakis MI, Sivridis E. Angiogenic factor expression in hepatic cirrhosis. Mediators Inflamm. 2007;2007:67187. doi: 10.1155/2007/67187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension. J Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Burdick MD, Keane MP. CXC chemokines in angiogenesis relevant to chronic fibroproliferation. Curr Drug Targets Inflamm Allergy. 2005;4:23–26. doi: 10.2174/1568010053622902. [DOI] [PubMed] [Google Scholar]
- Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R. Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol. 2007;6:208–213. [PubMed] [Google Scholar]
- Lemos QT, Andrade ZA. Angiogenesis and experimental hepatic fibrosis. Mem Inst Oswaldo Cruz. 2010;105:611–614. doi: 10.1590/s0074-02762010000500002. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. 2010;16:347–352. doi: 10.3350/kjhep.2010.16.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu M, Osada S, Amaoka N, Goshima S, Kondo H, Kato H, et al. Expression of vascular endothelial growth factor in hepatocellular carcinoma and the surrounding liver and correlation with MRI findings. AJR Am J Roentgenol. 2005;184:832–841. doi: 10.2214/ajr.184.3.01840832. [DOI] [PubMed] [Google Scholar]
- Yu DC, Chen J, Ding YT. Hypoxic and highly angiogenic non-tumor tissues surrounding hepatocellular carcinoma: the ‘niche' of endothelial progenitor cells. Int J Mol Sci. 2010;11:2901–2909. doi: 10.3390/ijms11082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozova S, Elpek GO. Hypoxia-inducible factor-1alpha expression in experimental cirrhosis: correlation with vascular endothelial growth factor expression and angiogenesis. Apmis. 2007;115:795–801. doi: 10.1111/j.1600-0463.2007.apm_610.x. [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Mendelev N, Melamed M, Ramaswamy G. Vascular density and VEGF expression in hepatic lesions. J Gastrointestin Liver Dis. 2007;16:373–377. [PubMed] [Google Scholar]
- Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- Mathonnet M, Descottes B, Valleix D, Labrousse F, Denizot Y. VEGF in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol. 2006;12:830–831. doi: 10.3748/wjg.v12.i5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sweringen HL, Sakai N, Quillin RC, Bailey J, Schuster R, Blanchard J, et al. Roles of hepatocyte and myeloid CXC chemokine receptor-2 in liver recovery and regeneration after ischemia/reperfusion in mice. Hepatology. 2013;57:331–338. doi: 10.1002/hep.26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16:1832–1836. doi: 10.3748/wjg.v16.i15.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G765–G771. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- Farci P, Wollenberg K, Diaz G, Engle RE, Lai ME, Klenerman P, et al. Profibrogenic chemokines and viral evolution predict rapid progression of hepatitis C to cirrhosis. Proc Natl Acad Sci USA. 2012;109:14562–14567. doi: 10.1073/pnas.1210592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215–225. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- Wasmuth HE, Weiskirchen R. Pathogenesis of liver fibrosis: modulation of stellate cells by chemokines. Z Gastroenterol. 2010;48:38–45. doi: 10.1055/s-0028-1109933. [DOI] [PubMed] [Google Scholar]
- Bataller R, Lemon SM. Fueling fibrosis in chronic hepatitis C. Proc Natl Acad Sci USA. 2012;109:14293–14294. doi: 10.1073/pnas.1212048109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- Arefieva TI, Kukhtina NB, Antonova OA, Krasnikova TL. MCP-1-stimulated chemotaxis of monocytic and endothelial cells is dependent on activation of different signaling cascades. Cytokine. 2005;31:439–446. doi: 10.1016/j.cyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Yu DC, Chen J, Sun XT, Zhuang LY, Jiang CP, Ding YT. Mechanism of endothelial progenitor cell recruitment into neo-vessels in adjacent non-tumor tissues in hepatocellular carcinoma. BMC Cancer. 2010;10:435. doi: 10.1186/1471-2407-10-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagouassat M, Suffee N, Hlawaty H, Haddad O, Charni F, Laguillier C, et al. Monocyte chemoattractant protein-1 (MCP-1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. Int J Cancer. 2010;126:1095–1108. doi: 10.1002/ijc.24800. [DOI] [PubMed] [Google Scholar]
- Heinrichs D, Berres ML, Nellen A, Fischer P, Scholten D, Trautwein C, et al. The chemokine CCL3 promotes experimental liver fibrosis in mice. PLoS ONE. 2013;8:e66106. doi: 10.1371/journal.pone.0066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Nakamoto Y, Nemoto-Sasaki Y, Fujii C, Wang H, Hashii M, et al. Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin-1 in human hepatomas. Am J Pathol. 2003;162:1249–1258. doi: 10.1016/S0002-9440(10)63921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS ONE. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lu P, Fujii C, Nakamoto Y, Gao JL, Kaneko S, et al. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer. 2006;118:1869–1876. doi: 10.1002/ijc.21596. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–G958. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Soliman HH, Nagy H, Kotb N, Alm El-Din MA. The role of chemokine CC ligand 20 in patients with liver cirrhosis and hepatocellular carcinoma. Int J Biol Markers. 2012;27:e125–e131. doi: 10.5301/JBM.2012.9097. [DOI] [PubMed] [Google Scholar]
- Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, et al. Selective recruitment of regulatory T cell through CCR6–CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Friedman SL. Fibrosis in the liver: acute protection and chronic disease. Prog Mol Biol Transl Sci. 2010;97:151–200. doi: 10.1016/B978-0-12-385233-5.00006-4. [DOI] [PubMed] [Google Scholar]
- Kanzler I, Tuchscheerer N, Steffens G, Simsekyilmaz S, Konschalla S, Kroh A, et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res Cardiol. 2013;108:310. doi: 10.1007/s00395-012-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10:359–370. doi: 10.1038/sj.mn.7800200. [DOI] [PubMed] [Google Scholar]
- Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost. 2007;97:755–762. [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang L, Xu J, Zhang X, Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res. 2011;166:241–246. doi: 10.1016/j.jss.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- Hong F, Tuyama A, Lee TF, Loke J, Agarwal R, Cheng X, et al. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055–2067. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–533. [PubMed] [Google Scholar]
- Schimanski CC, Bahre R, Gockel I, Muller A, Frerichs K, Horner V, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. doi: 10.1038/sj.bjc.6603251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I, Jin D, Rafii S. The SDF-1–CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)–CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies. Clin Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007;39:197–205. doi: 10.1016/j.biocel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Ferri C, Fallahi P, Ferrari SM, Sebastiani M, Ferrari D, et al. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. Am J Gastroenterol. 2008;103:2488–2494. doi: 10.1111/j.1572-0241.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Ferri C, Ferrari SM, Colaci M, Fallahi P. Immunopathogenesis of HCV-related endocrine manifestations in chronic hepatitis and mixed cryoglobulinemia. Autoimmun Rev. 2008;8:18–23. doi: 10.1016/j.autrev.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Antonelli A, Ferri C, Ferrari SM, Colaci M, Sansonno D, Fallahi P. Endocrine manifestations of hepatitis C virus infection. Nat Clin Pract Endocrinol Metab. 2009;5:26–34. doi: 10.1038/ncpendmet1027. [DOI] [PubMed] [Google Scholar]
- Sansonno D, Tucci FA, Troiani L, Lauletta G, Montrone M, Conteduca V, et al. Increased serum levels of the chemokine CXCL13 and up-regulation of its gene expression are distinctive features of HCV-related cryoglobulinemia and correlate with active cutaneous vasculitis. Blood. 2008;112:1620–1627. doi: 10.1182/blood-2008-02-137455. [DOI] [PubMed] [Google Scholar]
- Berenguer J, Fernandez-Rodriguez A, Jimenez-Sousa MA, Cosin J, Zarate P, Micheloud D, et al. High plasma CXCL10 levels are associated with HCV-genotype 1, and higher insulin resistance, fibrosis, and HIV viral load in HIV/HCV coinfected patients. Cytokine. 2012;57:25–29. doi: 10.1016/j.cyto.2011.10.020. [DOI] [PubMed] [Google Scholar]
- Berres ML, Trautwein C, Schmeding M, Eurich D, Tacke F, Bahra M, et al. Serum chemokine CXC ligand 10 (CXCL10) predicts fibrosis progression after liver transplantation for hepatitis C infection. Hepatology. 2011;53:596–603. doi: 10.1002/hep.24098. [DOI] [PubMed] [Google Scholar]
- Diago M, Castellano G, Garcia-Samaniego J, Perez C, Fernandez I, Romero M, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55:374–379. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florholmen J, Kristiansen MG, Steigen SE, Sorbye SW, Paulssen EJ, Kvamme JM, et al. A rapid chemokine response of macrophage inflammatory protein (MIP)-1alpha, MIP-1beta and the regulated on activation, normal T expressed and secreted chemokine is associated with a sustained virological response in the treatment of chronic hepatitis C. Clin Microbiol Infect. 2011;17:204–209. doi: 10.1111/j.1469-0691.2010.03206.x. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM. Mechanisms of antiviral treatment efficacy and failure in chronic hepatitis C. Antiviral Res. 2003;59:1–11. doi: 10.1016/s0166-3542(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Akbar SM, Horiike N, Michitaka K, Onji M. Increased serum levels of macrophage inflammatory protein-3alpha in chronic viral hepatitis: prognostic importance of macrophage inflammatory protein-3alpha during interferon therapy in chronic hepatitis C. J Viral Hepat. 2002;9:213–220. doi: 10.1046/j.1365-2893.2002.00354.x. [DOI] [PubMed] [Google Scholar]
- Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C–C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415–422. doi: 10.1038/labinvest.3780046. [DOI] [PubMed] [Google Scholar]
- Costantini S, Castello G, Colonna G. Human cytokinome: a new challenge for systems biology. Bioinformation. 2010;5:166–167. doi: 10.6026/97320630005166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.