Abstract
The ever-improving technology to generate induced pluripotent stem cells (iPSCs) has increased their potential use as novel candidates for disease modeling, drug screening, regenerative medicine and cell therapy. Indeed, iPSCs offer extensive capacity for self-renewal without the ethical concerns faced by embryonic stem cells (ESCs). With respect to potential applications in the immune system, many studies provide evidence to support that there are exclusive advantages to using iPSCs over other systems. Both hematopoietic stem cells and several types of mature immune cells have successfully been reprogrammed to iPSCs and vice versa, paving a path toward our ability to effectively model patient-specific diseases and provide potentially alternative cell sources for transfusion medicine. Despite these potential advances, some limitations regarding the use of iPSCs in the clinic still remain, including the immunogenicity of iPSCs and their derivatives, which is currently under debate in the field. In this review, we mainly focus on discussing the recent progress being made in the latest differentiation methods and clinical implications of iPSCs with respect to the immune system. Additionally, current issues regarding the clinical application of iPSCs are addressed, especially the controversy surrounding immunogenicity, along with various other perspectives.
Keywords: differentiation, hematopoietic cell, immunogenicity, immunotherapy, induced pluripotent stem cells
Introduction
Stem cells have unlimited self-renewal capacity and can differentiate into specific cell types. In light of their differentiation potential, stem cells are divided into the following categories with decreasing plasticity: totipotent, pluripotent and multipotent.1 While totipotent cells can differentiate into all cell types, including extra-embryonic tissue, pluripotent cells are more limited and include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Multipotent stem cells, like hematopoietic stem cells (HSCs), are even more limited and can only commit to several lineages within a tissue. Although ESCs, first isolated from in vitro cultures of mouse blastocytes,2 have broad applications to regenerative medicine and for tissue replacement, acquiring ESCs requires oocytes and embryo destruction, which raises ethical debates. Moreover, because immunogenicity between the ESCs and the recipients is different, cell transfusion therapy could potentially lead to immunological rejection. Therefore, to avoid these potential concerns, many scientific approaches have successfully used pluripotent stem cells instead of ESCs. In 2006, Dr Shinya Yamanaka showed that the pluripotency of iPSCs was comparable to ESCs and that the technique he used to obtain iPSCs without oocytes or embryos was easy and feasible; indeed, this novel technique was revolutionary enough to earn him the 2012 Nobel Prize in medicine. One of the most exciting applications of iPSCs is its potential use in generating hematopoietic cells and/or various specific immune cells for broad clinical application in numerous diseases. In this review, we highlight the past and present strides in iPSC generation methods and discuss the potential immunotherapeutic applications of iPSCs with its advantages and remaining limitations, including iPSC immunogenicity, as well as some perspectives and potential improvements for the future.
Advances in iPSC generation methods
Various reprogramming techniques have been used to generate iPSCs, including integrating vectors, non-integrating vectors, excisable integrating vectors and non-vector systems. For integrating vectors, Takahashi and Yamanaka converted mouse embryonic fibroblasts (MEFs) and mouse tail-tip fibroblasts to iPSCs for the first time by retroviral vector-mediated delivery of four reprogramming factors (Oct4, Sox2, Klf4 and c-Myc).3 In 2007, Yu et al.4 used lentiviruses carrying Oct4, Sox2, Nanog and Lin-28 to reprogram human fibroblasts into iPSCs. Although this method can successfully reprogram cells into iPSCs, integrating retroviral DNA into the host genome may lead to insertional mutagenesis, which can cause malignant tumor formation or leukemia. Therefore, approaches to generate iPSCs without altering the host genome are preferred for future clinical therapy purposes.
As an attempt to reprogram cells into iPSCs without requiring genome integration, the Hochedlinger group utilized non-integrating adenoviruses transiently expressing Oct4, Sox2, Klf4 and c-Myc to generate iPSCs from fibroblast and liver cells. Intriguingly, no tumor formation was observed in any chimera progeny up to 20 weeks of age.5 Another team led by Shinya Yamanaka confirmed that adenoviral transgenes did not integrate into the iPSC host genome, and they used plasmids to reset somatic cells into the pluripotent state.6 Similarly, Yu et al.7 created human iPSCs (hiPSCs) by first using non-integrating orip/EBNA1-based episomal vectors, which stably replicated themselves in a stable extra-chromosomal form in mammalian cells, and then subcloning by drug selection to separate the hiPSCs. Although these non-integrating vectors can also successfully generate iPSCs, their main drawback is the relatively low reprogramming efficiency compared to the integrating vectors.
Later, other investigators moved away from genetically based techniques and focused instead on directly using the necessary proteins. As the first demonstration that iPSCs could be generated by the protein-transduction method, the four reprogramming factors were fused with a poly-arginine protein transduction domain, and the fusion protein was delivered to reprogram OG2/Oct4-GFP reporter MEF cells.8 The conspicuous advantage of this reprogramming method is that it effectively excludes any risk posed by manipulating the genome of the targeted cell. Subsequently, another protein-based reprogramming approach adopted a certain group of embryonic stem cell-derived extract proteins, rather than DNA or RNA, to fully reprogram adult fibroblasts.9 Recently, Hou et al. transformed mouse somatic cells to the pluripotent state using only small-molecule compounds, providing another convenient pathway to generate iPSCs without genetic intervention.10 Encouragingly, Israeli scientists11 uncovered the crucial molecular hurdle in the reprogramming process—Mbd3, a core member of the Mbd3/nucleosome remodeling and deacetylation repressor. By applying OSKM transduction plus Mbd3 depletion, they synchronically reprogrammed mouse/human somatic cells with efficiencies near 100% within 7 days, a giant leap forward in reprogramming.
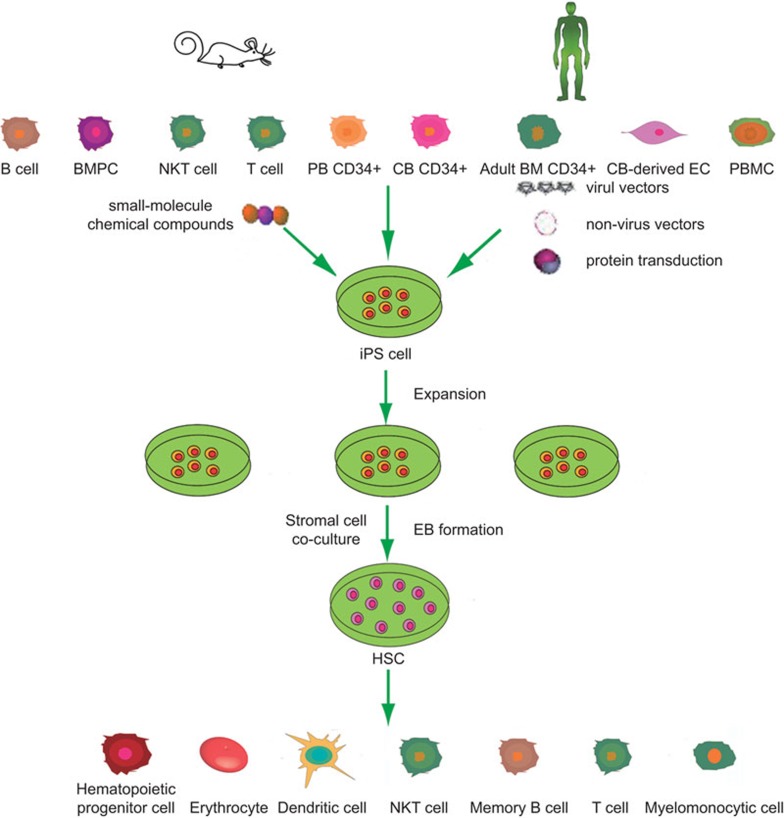
While the above-mentioned studies were achieved completely in vitro, whether iPSCs could be induced directly in vivo remained unclear. Recently, Abad et al.12 achieved this difficult task. In this striking study, the authors generated reprogrammable mice carrying a doxycycline-inducible cassette encoding the four reprogramming factors and demonstrated that the reprogrammed iPSCs acquired totipotent characteristics that were passed on by the zygote and its immediate daughter cells, which in vitro-generated ESCs and iPSCs could not accomplish. In summary, various cell types have already been reprogrammed to iPSCs, and, accordingly, iPSCs have been guided to differentiate into many different cell types. The remainder of this review focuses on the induction and redifferentiation of iPSCs to cells in the immune system, specifically to HSCs and fully differentiated immune cells (Figure 1).
Figure 1.
Known reprogramming of hematopoietic cells and immune cells to iPSCs and redifferentiation of iPSCs to cells of hematopoietic and immune system. BM, bone marrow; BMPC, bone-marrow progenitor cell; CB, cord blood; EC, endothelial cell; iPSC, induced pluripotent stem cell; NKT, natural killer T; PB, peripheral blood; PBMC, peripheral blood mononuclear cell.
iPSCs can redifferentiate into to HSCs
To date, it remains difficult to expand HSC or hematopoietic progenitor cell (HPC) populations in vitro, limiting their wide use in bone marrow transplantation. However, combining iPSC technology with hematopoietic differentiation protocols has the potential to produce a multitude of histocompatible HSCs for clinical use. To this end, with the availability of hiPSCs, the next step of obtaining HSCs/HPCs from hiPSCs can now be achieved. Since various hematopoietic-derived cell types have been successfully induced from human ESCs (hESCs), including red blood cells,13,14,15,16 monocytes,17 dendritic cells (DCs),18 megakaryocytes19 and lymphocytes,20 it is anticipated that hiPSCs can also differentiate into hematopoietic cells using similar methods previously applied to hESCs.
Practically speaking, several reports already demonstrate that hiPSCs can commit to hematopoietic lineages, imitating the hESCs differentiation process. Park et al.21 first validated this using hematopoietic colony-forming assays. They identified apparent myeloid and erythroid colony formation from embryoid bodies (EBs) differentiated from hiPSC lines. Later, human CD34+ cell-derived iPSCs were induced to differentiate into CD34+CD45+ cells in feeder- and serum-free conditions following a modified method to EB formation, demonstrating that purified CD34+CD45+ cells could be derived from these hiPSCs.22 Another study reported that a step-wise generation of osteoclasts from iPSCs yielded a monocyte–macrophage lineage population using defined factors in the absence of serum and feeder cells through an EB formation intermediate.23 Aside from using EB formation to guide iPSCs into the hematopoietic lineage, mouse OP9 stromal cells can also be harnessed to direct the differentiation of hiPSCs through coculture. Choi et al.24 used this system to generate hematopoietic cells from seven hiPSC lines, finally obtaining CD34+CD43+ hematopoietic progenitors. Using the same coculture system, they also yielded myelomonocytic cells from pluripotent stem cell-derived lin−CD34+CD43+CD45+ progenitors via a protocol similar used to the one used to derive these cells from hESCs.25 The Douay research group reported for the first time that differentiation from initial hiPSCs into enucleated, fetal hemoglobin-containing red blood cells could occur in its entirety through a process involving an EB formation intermediary and additives, including essential cytokines, which provided the final push toward differentiation into mature red blood cells.26 Undoubtedly, this technical achievement will open new avenues for transfusion medicine. Collectively, these studies offer the proof-of-principle evidence to support that hiPSCs reprogrammed from different cellular origins can redifferentiate into hematopoietic cells via EB formation or coculture with mouse OP9 stromal cell lines.
Potential clinical applications of hiPSC-derived hematopoietic cells
On the clinical side, iPSCs have the potential to treat monogenetic diseases either by correcting somatic cell defects through reprogramming or by gene-targeting techniques. In a humanized knock-in mouse model of sickle-cell anemia, iPSCs were transfected with the human βA wild-type globin gene by homologous recombination. Then, HPCs derived from these corrected iPSCs were transplanted to irradiated male hβs/hβs mice, restoring levels of all hematological indexes of sickle cell anemia back to normal.27 Raya et al.28 successfully derived patient-specific pluripotent cells from genetically corrected fibroblasts and keratinocytes, which were phenotypically disease-free; these corrected iPSCs were then successfully differentiated into hematopoietic erythroid and myeloid lineage progenitors that continued to maintain the disease-free phenotype. These studies are significant, as they establish new pathways for implementing iPSC therapy, especially the disease-modified, patient-specific iPSCs. They further indicate that iPSCs may become a platform for personalized medicine by allowing a patient's own autologous cells to be used as a source of therapeutic tissue, including HSCs.
In another interesting potential application of this technology, we can use iPSCs to recapitulate disease conditions to better understand the underlying mechanisms and pathogenesis of specific diseases. As an example, Ye et al.22 harvested peripheral blood CD34+ cells from patients with myeloproliferative disorders and reprogrammed them into iPSC lines, which were then redifferentiated into hematopoietic lineages. The resulting HPCs exhibited disease-specific gene expression patterns and abnormal erythropoiesis, resembling the primary CD34+ cells from the patient. This study corroborates that iPSCs can also be used as a powerful tool to model blood diseases characteristic of acquired somatic mutations.
Redifferentiating iPSCs into terminally differentiated immune cells for immunotherapy and the corresponding implications in clinical medicine
iPSCs can be derived from immune cells as equally as they can redifferentiate into specific immune cell types for modeling diseases or clinical immunotherapy (Table 1). Lei et al.29 reported for the first time that iPSCs could differentiate into a functional T lymphocyte lineage via coculture with OP9 stromal cells by referring to previous studies showing that ESCs30 and HSCs31 could commit to T lymphocytes. This study also demonstrated that iPSC-derived T cells could be successfully utilized for adoptive immunotherapy in a mouse system, laying the foundation for ultimately generating and applying disease-specific iPSC-derived T cells in clinical medicine. Nevertheless, B cell- or T cell-originated iPSC lines for cell replacement therapy should be adopted with discretion, considering that such iPSC-derived adaptive immune cells may result in a limited antigen-recognition repertoire.32 Confirming this presumption, one report showed that iPSCs originating from different cell types manifested different transcriptional and epigenetic patterns, as well as disparate differentiation potentials, although these differences were weakened by continued passaging of iPSCs.33
Table 1. Approaches to develop different immune cells from different iPSCs.
| Immune cell | Differentiation method | Reference |
|---|---|---|
| T cell | Coculture with DL1-expressing OP9 stromal cells, adding Flt-3 ligand and IL-7 | 29 |
| NKT cell | Coculture on OP9/DLL-1 in the presence of various cytokine combinations (Flt3L, IL-7, IL-15, IL-2) on different times | 36 |
| DC | Suspending in α-MEM supplemented with 20% FCS and seeding onto OP9 cell layers (steps 1 and 2) and later in RPMI-1640 medium supplemented with 10% FCS in addition to GM-CSF, 2-ME, IL-4, TNF-α and anti-CD40 mAb (step 3) | 37 |
Abbreviations: DC, dendritic cell; DL1, delta-like 1 ligand; FCS, fetal calf serum; GM-CSF, granulocyte-macrophage colony-stimulating factor; iPSC, induced pluripotent stem cell; mAb, monoclonal antibody; MEM, minimum essential medium; NKT, natural killer T cell.
Another lymphocyte, the natural killer T (NKT) cell, is known not only for secreting Th1 cytokines to generate antitumor activity, but also for playing multiple roles in regulating immune responses, such as those involved in antiviral immunity and transplant rejection.34,35 However, acquiring adequate NKT cell numbers from patients to induce effective immune responses is currently an obstacle for immunotherapy, which could potentially be addressed using iPSCs as a renewable source of NKT cells. Providing evidence that this method is feasible, Watarai et al.36 successfully induced functional NKT cells in vitro for the first time from iPSCs derived from either MEFs or splenic NKT cells. Importantly, these iPSC-derived NKT cells were also functional, as they produced high IFN-γ levels even without expressing mature cell surface phenotypes, fulfilling the adjuvant effect that NKT cells have on antitumor responses. Taken together, this mouse model will undoubtedly facilitate human NKT cell production from iPSCs for clinical benefit.
In terms of monocyte-lineage immune cells, DCs are the most potent antigen-presenting cells. Thus, DC-based cellular vaccination provides a powerful means for immunotherapy, especially against cancer. In addition, antigen-specific negative regulation of immune response by DCs is considered to be a promising approach to treat autoimmune diseases and in transplant medicine. At present, human DCs are normally generated from peripheral blood-derived monocytes, which cannot propagate in vitro, presenting the following issues that limit the therapeutic applicability of these DCs: the finite numbers of available monocytes in a patient's blood and the varying DC-differentiation potential among monocytes from different blood donors. Since iPSCs can overcome this limitation given their unlimited propagation capacity, iPSCs may be an ideal source for DCs that can broaden their immunotherapeutic applicability. Towards this end, Senju et al.37 reported that the iPSC-derived DCs are functional in that they effectively processed and presented antigens, stimulated T cells, and produced cytokines.
In the vaccinology field, side effects are an ever-present risk of traditional vaccine methods,38 and the functional effectiveness of vaccines depends on the ability of an individual's immune cells to handle the vaccine, which varies greatly within a population. iPSC technology presents an optional pathway to vaccination. Functionally equivalent to hESCs in many respects, hiPSCs can be genetically or chemically manipulated in vitro to become memory B cells that secrete various functional antibodies,7,21,39 which ultimately can be transfused back into the human. Since this whole process can occur outside of the human body, many of the possible adverse side effects are obviated, providing a safer way to administer vaccine protection. This iPSC-derived memory B cell-based vaccination method would be most beneficial for specific populations in which the traditional vaccination method is unsuitable, such as those with gravida, HIV and autoimmune disease.
Considering all of these potential immune-based applications of iPSCs, we are optimistic about the personalized medicine aspect of iPSC technology development.40
Immunogenicity of iPSCs
Despite the many advantages that iPSC technology can offer for immune-based therapy, there are potential concerns that must be addressed in order to ensure the safety and success of this new technology in the clinic, including the potential immunogenicity of iPSCs. Theoretically, iPSCs generated from syngeneic autologous cells can prevent or abate immune rejection, which is also the most fascinating future clinical application of iPSCs. However, Zhao et al.41 reported that teratomas generated by iPSCs derived from the retroviral integration method engendered strong immune responses. Moreover, C57BL/6 (B6)-background iPSC lines derived from non-integrated episomal and plasmid vectors consistently showed immunogenicity upon implantation into B6 mice, albeit lower than retroviral-derived iPSCs.
Recently, however, several studies provided evidence to support a different point of view regarding the immunogenicity of iPSCs.42,43,44 While the above-mentioned study by Zhao et al.41 only used a single line of syngeneic ESCs, Araki et al.43 tested seven iPSC and five embryonic stem cell lines to verify that no pronounced differences in immunogenicity existed between the teratomas formed by iPSCs and ESCs. In fact, the disparities between iPSC- or ESC-derived clones perhaps deserve more attention than the differences between these two pluripotent stem cells. In a separate study, Guha and colleagues42 explored the immunogenicity of endothelial cells, hepatocytes and neuronal cells derived from ESCs and iPSCs in vitro, differing from the Araki et al.'s43 study that used grafts originating from chimeric mice. Their coculture and transplantation experiments jointly affirmed that undifferentiated, syngeneic iPSCs and their progeny—including partially differentiated EBs and tissue-specific cells—did not exhibit any immunogenicity, either in vitro or in vivo. Thus, these two studies emphasized that iPSCs and their descendants exhibit little immunogenicity, contradicting previous conclusions.41 Perhaps this seeming contradiction can be attributed to the different viral vectors and different pluripotent stem cell lines used in the respective studies. A very recent study44 using two hESC and five hiPSC strains also showed that different pluripotent cells possessed varying levels of immunogenicity. Furthermore, natural killer receptor ligand expression on hiPSCs, which are comparable to somatic cells, and enhanced HLA-E expression sharpens the prospect of using iPSCs in clinical applications, even though the compromised antigen-presentation ability of human stem cells and the upregulation of other related immune genes promoting immune privilege. Since iPSCs have been shown to manifest the capacity for highly heterogeneous hematopoietic differentiation, these studies also further support the necessity to screen large numbers of individual iPSC lines before use in downstream applications.24,45
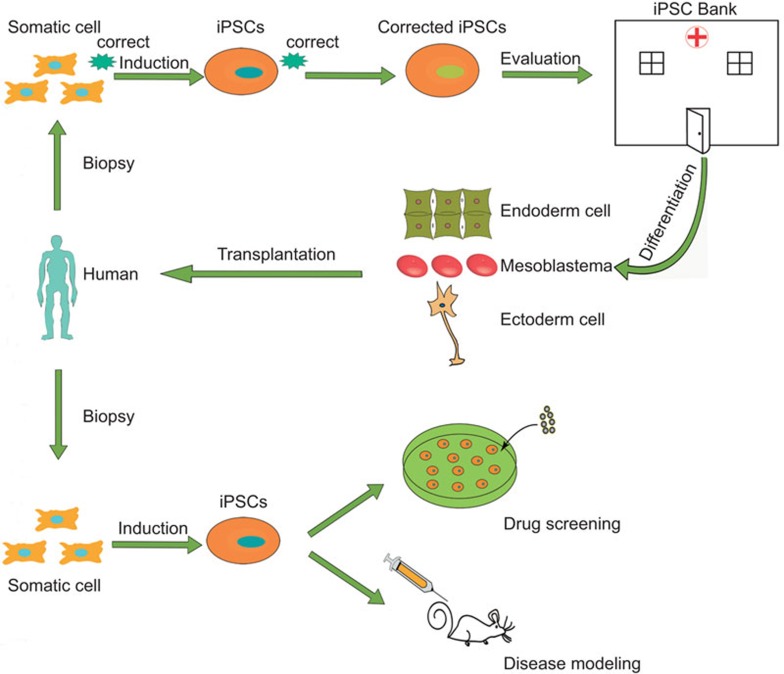
In order to completely eliminate such potential hazards when using iPSC in regenerative medicine, it is exigent and imperative for us to develop safer and more convenient approaches to producing iPSCs with less immunogenicity, such as using small-molecule compounds without artificial genetic manipulation.10 On the other hand, perhaps launching an iPSC bank containing cells from many individuals in a population for increased chances of HLA-type matching46,47 is a more practical pathway for using iPSCs in the clinic, similar to what Yamanaka has proposed48,49 and what has already been set up for ESCs.50 Such a bank may save time normally spent on inducing and evaluating iPSCs created from each specific individual. In conclusion, we should be cautiously optimistic in terms of using iPSCs in future clinical applications; moreover, we should thoroughly evaluate the immunogenicity of each specific hiPSC strain and its derivatives in order to establish an iPSC bank with iPSCs that are genuinely available for clinical use (Figure 2).
Figure 2.
The potential approach to clinical application of iPSCs. iPSC, induced pluripotent stem cell.
Perspectives of iPSCs and alternatives for the future
Since iPSCs possess similar properties to ESCs without the ethical issues, this type of pluripotent stem cell holds great promise for regenerative medicine. Additionally, patient-specific iPSCs in particular could provide an invaluable tool for remodeling genetic disease, as patient-generated iPSCs would likely retain some of the genetic imprinting of the parental somatic cells.33,51,52 However, some obstacles remain that impede our progress toward clinical use and need to be surmounted before this can become a reality. One remaining obstacle is in teratoma formation.53 iPSCs are not uniform even within a homologous clone,54,55 which could be harmful if a few aberrantly reprogrammed cells lead to immature teratoma formation after entry into patients. This effect could be exacerbated compared to hESCs, as hiPSCs exhibited higher efficiency and shorter latency than hESCs independent of the injection site in an in vivo teratoma assay.56 Thus, finding appropriate methods to direct iPSC differentiation into the desired cell type is of the utmost importance.
Another obstacle to overcome is histocompatibility. Polymorphic major histocompatibility complex class I molecules form the most formidable immunological barrier preventing successful transplantation of pluripotent stem cell-derived allografts, since these cells are targets of alloreactive CTL by either direct or indirect allorecognition.57 Intriguingly, iPSCs are killed more efficiently than MEFs, and natural killer cells also contribute to rejection in vivo.58 Consequently, it is imperative that histocompatible iPSC lines be employed in the near future, such as those derived from iPSC banks. In terms of disease modeling, effectively recapitulating disease pathogenesis by relying on cells derived from patient-specific iPSCs can be difficult, as the effects of aging and environment on the body cannot simply be simulated within in vitro conditions or in other host organisms. Moreover, many diseases may involve interactions among various cell types within the organism, which is also difficult to recapitulate in cell-autonomous conditions.
Due to various limitations associated with iPSC technology, several new alternative cells with properties similar to iPSCs are quite attractive and offer several advantages over iPSCs. A new type of human adult stem cell called the ‘multilineage-differentiating stress-enduring (Muse) cell' can differentiate into the three germ layers and do not form teratomas in immunodeficient mice, likely making them safer than iPSCs. Moreover, unlike iPSCs, they are easily obtained from human mesenchymal cells without introducing exogenous reprogramming factors.59 As another alternative, directly converting one type of somatic cell into another functional somatic cell without first passing through an intermediate undifferentiated state has proven feasible. For example, combining only three transcription factors (Ascl1, Brn2 (also called Pou3f2) and Myt1l) was sufficient to convert mouse embryonic and postnatal fibroblasts into functional neurons in vitro with high efficiency; indeed, the resultant induced neuronal cells exhibited multiple neuron-specific markers, produced action potentials and shaped functional synapses.60 Analogously, another set of three transcription factors (Ngn3, Pdx1 and Mafa) converted adult mouse pancreatic exocrine cells into cells closely resembling pancreatic β-cells, which shared similar properties to endogenous islet β-cells in terms of morphology, size, gene expression and insulin secretion.61 Thus, these new technologies may provide alternatives not only for iPSCs or ESCs, but also for regenerative medicine in general.
To sum up, iPSC technology bring a brand new hope for benefiting humans in many ways; however, the immunogenicity of iPSCs should first be taken into considerations and arouse our sufficient attentions. Next, we may need to evaluate the immunogenicity of the specific iPSC strain rather than generalizing all the iPSCs. From the optimistic point of view, in addition to the progress of generating iPSCs and mechanism study, we could also differentiate iPSCs to other immunosuppressive regulatory cells, such as regulatory DC,62 to reduce the potential immune responses elicited by iPSCs and their derivatives. Therefore, it can be wholly expected to extensively apply iPSCs in future clinical medicine.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (2011CB965202, 2013CB530502 and 2013CB944903).
References
- Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Lee CS, Kwon YW, Paek JS, Lee SH, Hur J, et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood. 2010;116:386–395. doi: 10.1182/blood-2010-02-269589. [DOI] [PubMed] [Google Scholar]
- Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- Abad M, Mosteiro L, Pantoja C, Canamero M, Rayon T, Ors I, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–345. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- Chang KH, Nelson AM, Cao H, Wang L, Nakamoto B, Ware CB, et al. Definitive-like erythroid cells derived from human embryonic stem cells coexpress high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier EN, Qiu C, Velho M, Hirsch RE, Bouhassira EE. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Feng Q, Park JS, Vida L, Lee BS, Strausbauch M, et al. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KR, Cowley S, Martinez FO, Shaw M, Minger SL, James W. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol. 2008;36:1167–1175. doi: 10.1016/j.exphem.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Dravid G, Ye Z, Hammond H, Shamblott M, Gearhart J, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. . Lancet. 2004;364:163–171. doi: 10.1016/S0140-6736(04)16629-4. [DOI] [PubMed] [Google Scholar]
- Takayama N, Nishikii H, Usui J, Tsukui H, Sawaguchi A, Hiroyama T, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Kennedy M, Bozec A, Brunton F, Stenbeck G, Park IH, et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010;115:2769–2776. doi: 10.1182/blood-2009-07-234690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne H, Kobari L, Mazurier C, Tropel P, Giarratana MC, Zanella-Cleon I, et al. Red blood cells generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei F, Haque R, Weiler L, Vrana KE, Song J. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260:1–5. doi: 10.1016/j.cellimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. . Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. . Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- Ye Z, Cheng L. Potential of human induced pluripotent stem cells derived from blood and other postnatal cell types. Regen Med. 2010;5:521–530. doi: 10.2217/rme.10.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Watarai H, Fujii S, Yamada D, Rybouchkin A, Sakata S, Nagata Y, et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest. 2010;120:2610–2618. doi: 10.1172/JCI42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju S, Haruta M, Matsunaga Y, Fukushima S, Ikeda T, Takahashi K, et al. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem Cells. 2009;27:1021–1031. doi: 10.1002/stem.33. [DOI] [PubMed] [Google Scholar]
- Ales NC, Katial RK. Vaccines against biologic agents: uses and developments. Respir Care Clin N Am. 2004;10:123–146. doi: 10.1016/S1078-5337(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Li J, Gao GD, Yuan TF. Cell based vaccination using transplantation of iPSC-derived memory B cells. Vaccine. 2009;27:5728–5729. doi: 10.1016/j.vaccine.2009.07.091. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- Chen HF, Yu CY, Chen MJ, Chou SH, Chiang MS, Chou WH, et al. Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and the derivatives. Cell Transplant 2013. in press. [DOI] [PubMed]
- Kulkeaw K, Horio Y, Mizuochi C, Ogawa M, Sugiyama D. Variation in hematopoietic potential of induced pluripotent stem cell lines. Stem Cell Rev. 2010;6:381–389. doi: 10.1007/s12015-010-9150-5. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Okita K, Nagata N, Yamanaka S. Immunogenicity of induced pluripotent stem cells. Circ Res. 2011;109:720–721. doi: 10.1161/RES.0b013e318232e187. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013;12:385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC. Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS ONE. 2010;5:e8975. doi: 10.1371/journal.pone.0008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Huang S. Reprogramming cell fates: reconciling rarity with robustness. Bioessays. 2009;31:546–560. doi: 10.1002/bies.200800189. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Macia A, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saric T, Frenzel LP, Hescheler J. Immunological barriers to embryonic stem cell-derived therapies. Cells Tissues Organs. 2008;188:78–90. doi: 10.1159/000118784. [DOI] [PubMed] [Google Scholar]
- Dressel R, Nolte J, Elsner L, Novota P, Guan K, Streckfuss-Bomeke K, et al. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J. 2010;24:2164–2177. doi: 10.1096/fj.09-134957. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Guo Z, Zhang M, Wang J, Chen G, Cao X. Endothelial stroma programs hematopoietic stem cells to differentiate into regulatory dendritic cells through IL-10. Blood. 2006;108:1189–1197. doi: 10.1182/blood-2006-01-007187. [DOI] [PubMed] [Google Scholar]