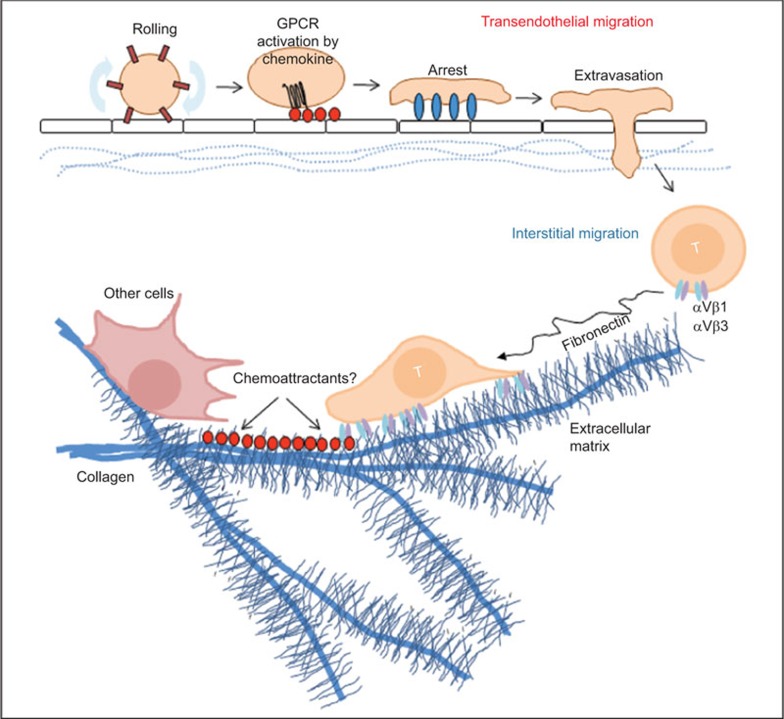
T cells are one of the most migratory cells in the body. The development and function of T cells depend on their interaction with other cells, which is, in turn, dependent on optimal cell migration. T cells migrate largely in three different ways: rapid distribution via blood vessels through hemodynamic movement, transendothelial migration and interstitial movement. Transendothelial migration involves sequential activation of selectins, chemokine receptors and integrin molecules expressed by migrating T cells, leading to extravasation from blood vessel into local tissue environments. Interstitial migration involves T-cell migration within a local tissue environment. While the critical roles of integrins for transendothelial migration are well established, integrins appear to be largely dispensable for interstitial migration in three-dimensional extracellular matrix (ECM).1,2,3 A leading theory is an actin-myosin-based and non-adhesive ‘crawling and squeezing amoeboid motility'. Pertussis toxin-sensitive signaling from G protein-coupled receptors, activated mainly by chemoattractants regulates interstitial migration. It is thought that interstitial migration involves both hapto- and chemo-taxis (or –kinesis), but the exact migration mechanisms specific for cell types, tissues and conditions remain unclear. Because of the roles of integrins in cell adhesion to extracellular matrix proteins such as collagens, fibronectin and vitronectin, searches for integrins that can guide cell migration in specialized tissues and conditions continued. Recently, Overstreet et al.4 reported that αV-containing integrins play important roles in interstitial T-cell migration in inflamed tissues.4
The integrin chain (Itg) αV can pair with Itg β1, β3, β5, β6 and β8 to make functional heterodimers on cell membranes. Activated T cells highly express Itg β1 and β3, whereas the expression of the other αV-pairing integrins has not been established. This suggests that αVβ1 and αVβ3 would play important roles in migration of effector T cells. Itg αV is absent on naive CD4+ T cells, but is upregulated in lymph node-emigrating effector T cells. Overstreet et al. reported that T-cell motility is increased in inflamed tissues. They examined T-cell migration in dermis inflamed with complete Freund's adjuvant. Collagen fibers became condensed into thicker bundles with more dense presence of fibronectin in the inflamed dermis, and T cells migrated around the network formed by these ECM fibers. This indicates that ECM fibers would guide T-cell migration in inflamed tissues (Figure 1). This localized migration on ECM may increase the likelihood of T-cell interaction with other cell types such as antigen-presenting dendritic cells or phagocytes. ECM proteins typically contain the amino acid sequence arginine–glycine–aspartic acid (RGD), which is a major binding motif for interaction with integrins. Blocking of integrins with a RGD peptide suppressed T-cell motility, indicating positive roles of integrins. Th1 cells highly expressed αV, β1 and β3 along with α2, α4, αL and β2 integrins in inflamed skin or influenza virus-infected lung. The RGD blocking experiment, however, could not specifically identify the integrins that mediate the process. Thus, Overstreet et al. also employed blocking antibodies to Itg β1 and β3 and found that these antibodies were effective in suppressing T cell motility. Because Itg β1 can pair with Itg α1–α7 and αv, and Itg β3 can pair with αIIb and αV, they also employed antibodies to Itg α chains. Blocking Itg α1, α2 and α4 did not change the T-cell motility in inflamed skin, but blocking Itg αV was effective. This indicates positive roles of αVβ1 and αVβ3, but does not rule out the potential function of other β1 integrins and αIIbβ3.
Figure 1.
Integrins regulate interstitial migration of effector T cells. Circulating memory and effector T cells in the blood circulation enter into tissues through the endothelial cell layer. Adhesion molecules and chemokines cooperatively induce the endothelial cell migration process. Constitutively expressed tissue-specific or inflammation-induced chemokines and adhesion molecules regulate the migration. Integrins play critical roles in transendothelial cell migration by mediating the rolling and firm adhesion process. After transendothelial migration, T cells still need to migrate to specialized microenvironments for their effector functions. This migration is called interstitial migration and involves crawling and squeezing movement on ECM fibers (e.g., collagen and fibronectin), which undergo dramatic alteration to support T-cell migration in inflamed tissues. In inflamed skin, integrins, particularly αV-containing integrins (e.g., αVβ1 and αVβ3), are required for normal interstitial migration of effector T cells. Chemoattractants are likely to be involved in regulating the migration of effector T cells on ECM fibers. Considerable diversity is expected in terms of chemokines and integrins that regulate T-cell migration in different tissues and conditions. ECM, extracellular matrix.
Overstreet et al. utilized multiphoton microscopy to examine T-cell migration in the inflamed skin. Multiphoton microscopy is an ideal tool to determine 3D T-cell migration in tissues. The advantages include deep tissue imaging up to ∼500 µm in live unfixed tissues. In soft tissues like, brain tissues, even deeper imaging up to ∼1 mm is possible. Multiphoton microscopy involves relatively faster resonance scanners which can readily acquire images of T cells migrating at speeds up to 30 µm/min. However, it is not the best instrument to image faster moving cells on blood vessels, which can be better imaged by other methods such as charge-coupled device camera-based spinning disk microscopy. Multiphoton microscopy is particularly useful in multichannel intravital imaging of cell–cell interaction in tissues of live animals. T cells are chemically labeled with membrane dyes or genetically labeled with fluorescent proteins, which are excited by one to two infrared lasers. Sophisticated software such as Imaris and Volocity are used for 3D image analysis.
Migration is important for T cells to undergo activation, differentiation and survival because it allows T cells to colocalize with antigen presenting cells. Moreover, migration is required to interact with other target cells that T cells would activate or kill. Interactions mediated by LFA-1-ICAM-1, α4β1-VCAM and αEβ7-E-cadherin pairs are well established.5,6,7,8 In the work of Overstreet et al., blocking Itg αV during an antigen-specific immune response decreased the formation of IFN-γ-producing T cells and clearance of pathogens (Leishmania major), indicating the importance of Itg αV for generating or maintaining effector T cells.
What makes the environment for migrating T cells different between normal and inflamed tissues? In lymph nodes, integrins are not required for interstitial migration of leukocytes,1 whereas integrins appear to be important for their migration in inflamed tissues as shown by Overstreet et al.4 Tissues with active immune responses due to infection by pathogens, injury, or chronic tissue inflammation are different from normal tissues in tissue structure. Active immune responses increase cytokines that activate tissue cells to produce or modify ECM components such as collagen and fibronectin. Altered ECM structures would facilitate leukocyte migration by providing new paths to migrating cells (Figure 1). In addition to ECM, stromal cells can guide T-cell migration in tissues. In lymph nodes, fibroblastic reticular cells can guide T-cell migration and these cells may differ in different tissues and conditions.9
As exemplified for transendothelial cell migration, activation of integrins leads to firm adhesion of cells, which means cells get stuck to ECM or other cells, when integrins are activated. In this regard, a remaining question is how αV-containing integrins support continuous migration (or crawling) of effector T cells. The regulation of integrin activation in migrating cells in local tissues is largely unknown. Perhaps, there are intrinsic differences between cell–cell and cell–ECM interactions in affinity and activation frequency. The strength of cell–ECM interaction may be limited so that cells can crawl rather than become immobilized on ECM fibers for prolonged time periods. The identity of the factors that induce this type of integrin activation is unclear. Perhaps, chemoattractants present in local tissue environments would regulate this activation by inducing chemotaxis/kinesis and regulating integrin activation for efficient migration of effector T cells on ECM (Figure 1). If so, what are the chemoattractants that regulate the interstitial migration in inflamed tissues? The answers probably depend on the type of tissues and inflammation. Within a reactive lymph node, the CXCL9–CXCR3 axis plays a role in migration of CD8+ memory T cells and their interaction with infected macrophages.10 Similar roles are expected for CXCR3 ligands or other chemokines expressed in inflammatory tissues. ECM fragments, produced by matrix metalloproteinases and other proteases that degrade ECM, can also act as chemoattractants to guide cell migration in inflamed tissues.11
Unexpected results were observed by Overstreet et al. from their experiment to determine the motility of T cells that were genetically deleted for Itg-αV. Genetic deletion with CD4-Cre was not effective in suppressing T-cell motility, whereas the methods employing shRNA-mediated knockdown and anti-αV blocking antibody were effective in decreasing the motility and effector function of T cells. Blocking antibodies or shRNA may inadvertently block or suppress additional molecules. Itg αV expression is well established for myeloid cells and other cell types, and an Itg αV blocking antibody is likely to block αV integrins on these unintended cell types. It could be that blocking of αV integrins on phagocytes may have bigger impacts than that on T cells. It is also possible that genetic deletion may have not been really effective in decreasing Itg αV expression. Although highly speculative, compensatory expression of ECM-binding molecules in Itg αV−/− T cells could occur. Another possibility is that the Itg αVon T cells alone may not play a major role. Thus, more studies are required to fully establish the proposed non-redundant role of Itg αV in regulation of T-cell motility and effector function. Despite these shortfalls, Overstreet et al. convincingly demonstrated the importance of integrins in interstitial migration of effector T cells in inflamed tissues. While the authors demonstrated increased presence of fibronectin and restructured collagen fibers, it remains to be determined which ECM component(s) is most important for interaction with Itg αV. Vitronectin also binds Itg αV and is potentially important as well.
αV integrins have additional functions such as producing active TGF-β proteins. TGF-β proteins have latency-associated peptide which has an RGD motif. αV-integrins bind latency-associated peptide-TGF-β polypeptide chains and liberates the active TGF-β domain from the latent complex. This role of Itg αV has been documented for Itg αV-expressing dendritic cells and tissue cells such as endothelial cells, epithelial cells, and fibrobastic cells.12,13,14 αVβ3, αVβ5, αVβ6 and αVβ8 bind latent TGF-β complex and produce active TGF-β.14 Thus, expression of these integrins is required for normal production of active TGF-β1 in tissues. As demonstrated by Overstreet et al., effector T cells highly express αV integrins. It remains to be determined if effector T cells can activate TGF-β in inflamed tissues. This pathway is potentially important for limiting excessive inflammatory activity and repairing damaged tissues by T cell-activated TGF-β.
Acknowledgments
I thank B Ramsey and L Friesen at Purdue for helpful inputs. This study was supported, in part, from grants from NIH (R01AI074745, R01DK076616 and R01AI080769), National Multiple Sclerosis Society, and Michael J. Fox Foundation for Parkinson's research to CHK.
References
- Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil AM, Piel M, Sorensen CM, et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11:953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, Lambert K, et al. Inflammation-induced interstitial migration of effector CD4 T cells is dependent on integrin alpha. Nat Immunol. 2013;14:949–958. doi: 10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Schon MP, Schon M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin alphaE(CD103)-deficient mice. J Invest Dermatol. 2002;119:190–193. doi: 10.1046/j.1523-1747.2002.17973.x. [DOI] [PubMed] [Google Scholar]
- Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38:502–513. doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL. T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol. 1995;154:4379–4389. [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, et al. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–1820. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Klementowicz JE, Travis MA. TGFbeta: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]