Abstract
Tumor immunoevasion is an advanced phase of cancer immunosurveillance in which tumor cells acquire the ability to circumvent host immune systems and exploit protumorigenic inflammation. T-cell immunoglobulin mucin (TIM) gene family members have emerged as critical checkpoint proteins that regulate multiple immune response phases and maintain immune homeostasis. Accumulating evidence demonstrates that tumor cells exploit TIM gene family members to evade immunosurveillance, whereas TIM gene family members facilitate the prevention of inflammation-related tumor progression. Thus, a comprehensive analysis to clarify the relative contributions of TIM gene family members in tumor progression may elucidate immunosurveillance systems in cancer patients.
Keywords: antitumor immunity, immunoevasion, immunosurveillance, TIM, tumorigenic inflammation
Introduction
The interaction between tumor cells and host immune cells plays an important role in multiple stages of tumorigenesis, and recent clinical evidence suggests a potential contribution of host immune responses in modulating the clinical outcome of cancer patients.1,2 Moreover, manipulation of the endogenous immune system has emerged as an effective anticancer therapy in patients with advanced cancer.3,4
Interestingly, accumulating evidence has revealed that the tumor microenvironment has a significant impact on the functional properties of certain immunoregulatory components that regulate whether host responses promote or antagonize tumor growth. Tumor cells and tumor-infiltrating lymphocytes adopt strategies to evade antitumor processes and may enhance the metastatic potential through the activation of chronic inflammatory signals.5,6 Together, these observations underscore the complexity of host immune system regulatory pathways in the regulation of tumorigenesis.
In this article, we describe the potential impact of the T-cell immunoglobulin mucin (TIM) gene family in tumor immunosurveillance and immunoevasion and the impact of different tumor microenvironments on the therapeutic responses of TIM-targeted therapies.
Mechanisms of tumor immunosurveillance and immunoevasion
Transformation is established by overcoming multiple intrinsic and extrinsic tumor suppression mechanisms. Transformed cells are detected intrinsically using checkpoint mechanisms that survey genetic and epigenetic abnormalities such as oncogene-induced senescence, DNA damage responses or apoptotic/necrotic cell death programs.7 Extrinsic tumor surveillance systems detect transformed cells by utilizing non-transformed cells within tumor microenvironments. In particular, the innate and adaptive immune systems play a critical role in detecting and eliminating transformed cells by activating multiple sets of myeloid cells and lymphocytes.2 Interestingly, tumor-infiltrating immune cells also contribute to tumor progression by triggering tumor angiogenesis and immune suppression.8,9,10 These findings suggest that the host immune system contributes to tumor initiation and progression in a contradictory manner.
Although the mechanisms that regulate tumor immune responses require further clarification, the recent concept of ‘cancer immunoediting' might explain the differential temporal and spatial dynamism of tumor immunosurveillance and immunoevasion, as evidenced by the antitumorigenic and protumorigenic host immune responses during different phases of tumorigenesis. Classically, cancer immunoediting has been divided into three phases: elimination, equilibrium and escape. In the elimination phase, innate and adaptive lymphocytes detect the presence of transformed cells and remove them. However, tumor immunosurveillance selectively eliminates highly immunogenic transformed cells, while poorly immunogenic cells survive and enter into the equilibrium phase. The interaction between surviving tumor variants and immune cells creates a homeostasis in which low-level malignant and/or quiescent tumor cells coexist with lymphocytes. After a long-term equilibrium between tumor cells and lymphocytes, additional genetic and epigenetic alterations allow tumor cells to evade tumor immunosurveillance. During this immunoevasion phase, tumor cells not only circumvent antitumor immunity, but also promote tumorigenic activities through multiple tumor-intrinsic and extrinsic machineries.8,10,11 These immunoevasion mechanisms elicited by the tumor microenvironment include the secretion of immunosuppressive cytokines, the emergence of tumor antigen-loss variants and the subversion of antigen-specific CTL responses by immunosuppressive antigen-presenting cells (APCs).12,13,14,15,16
Moreover, the tumor microenvironment adopts multiple strategies to subvert tumor immunosurveillance by recruiting immunosuppressive myeloid cells, such as IDO+ dendritic cells (DCs), B7-1+ macrophages, angiogenic DCs and Foxp3+ regulatory T cells (Tregs), which severely compromise endogenous immunogenicity and impair therapeutic responses to immunotherapy.17,18,19 More importantly, tumor cells exploit host immunity to create intimal networks among tumor cells, stromal cells and endothelial cells and to promote pro-tumorigenic inflammation-associated carcinogenic responses such as angiogenesis and epithelial–mesenchymal transitions.20,21 Tumor immunoevasion has multiple impacts on tumorigenesis by triggering tolerogenic responses to tumor cells and by exploiting pro-tumor inflammation.
The role of TIM molecules in the regulation of immune homeostasis
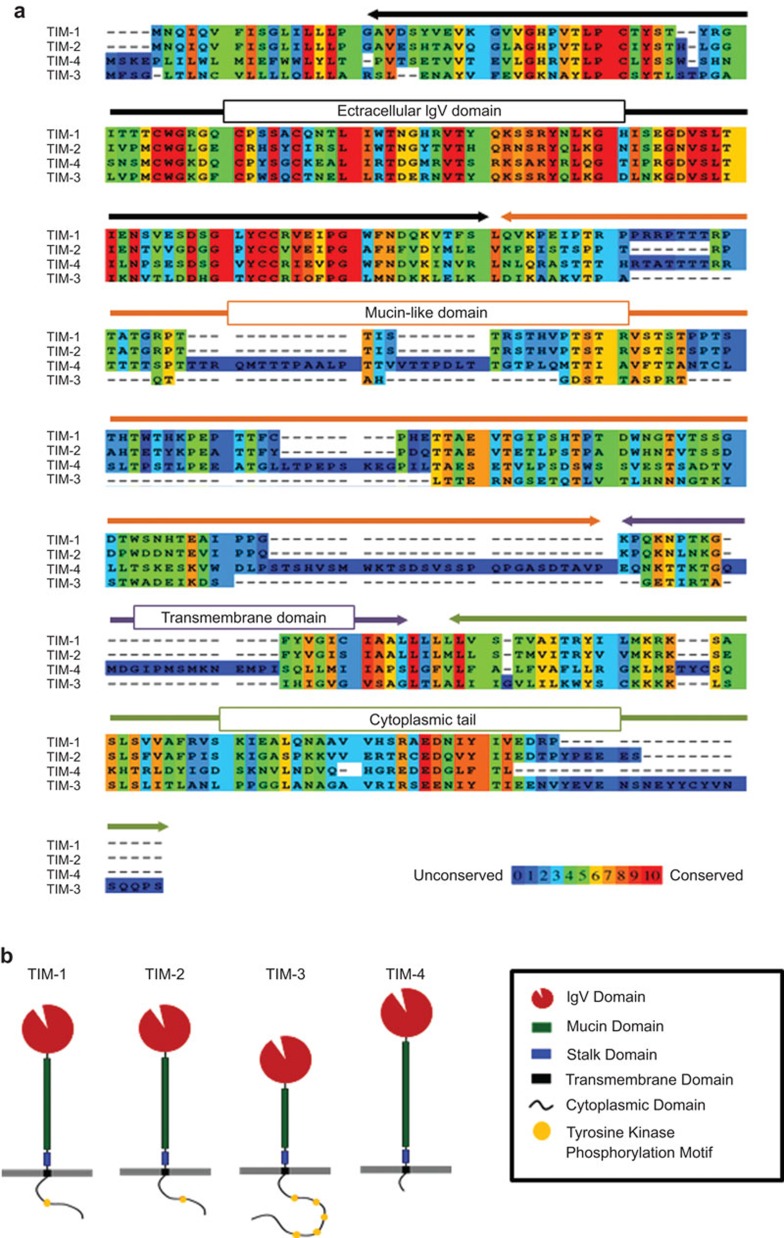
TIM proteins are type-I cell-surface glycoproteins composed of a signal peptide, an extracellular IgV domain, a mucin-like domain, a transmembrane domain, and an intracellular cytoplasmic tail. All members share a conserved sequence homology in the IgV domains, while other domains demonstrate poor homology (Figure 1). Under healthy conditions, the TIM family is largely confined to restricted sets of lymphoid and myeloid lineage cells and kidney epithelial cells. However, the induction of TIM gene family members is frequently observed on cell types such as stromal cells, endothelial cells and transformed cells in chronic viral infections and cancer, implying that TIM proteins play a critical role in the regulation of these pathological conditions.22,23
Figure 1.
The structure and main domains of TIM gene family members. The sequences (a) and schematic structures (b) are shown for each TIM gene family member. TIM molecules are type-I cell-surface glycoproteins that comprise an extracellular IgV domain, a mucin-like domain, a transmembrane domain and an intracellular cytoplasmic tail. All TIM molecules have a conserved sequence homology in the IgV domains, while other domains show little similarity. The sequence data were generated using the PRALINE multiple sequence alignment function. TIM, T-cell immunoglobulin mucin.
Moreover, the TIM gene family has multiple roles in the regulation of immune activation and tolerance, which may have a large impact on the clinical consequences of sterile inflammation, antimicrobial defense and antitumor immune responses.24,25 For example, continuous blockade of TIM-3 causes autoimmune nephritis in immune competent animals, and TIM-3 triggers antimicrobial immunity by interacting with galectin-926 and impedes innate antitumor responses.27,28 Moreover, TIM-1 promotes pro-inflammatory responses that may be associated with aggressive graft-versus-host disease.29 Thus, these findings raise the possibility that TIM family members serve as critical checkpoints to regulate immune homeostasis and inflammation. More importantly, accumulating evidence has unveiled the critical roles of TIM family members in the regulation of antitumor immunosurveillance. Thus, we will provide an overview of TIM-mediated regulation of tumor immunity and perspectives on the potential of TIM family members for tumor immunosurveillance and immunoevasion.
The role of TIM molecules in the immune regulation of tumors
TIM-1
TIM-1 is mainly expressed on T cells and kidney epithelial cells.24,25 Several lines of evidence suggest a dual role for TIM-1 in the regulation of T cell-mediated immunity. TIM-1 may act as a costimulatory molecule for T-cell activation 30,31 or transduce a negative signal that leads to the inhibition of T-cell effector function.32 Several studies have reported multiple roles for TIM-1 in creating immunostimulatory or immunosuppressive environments. For example, treatment with anti-TIM-1 antibodies in the effector phase impedes the development of inflammation, while treatment during the priming phase results in a break in immune tolerance.31,32,33 In addition, TIM-1 on the surface of macrophages acts as a phosphatidylserine receptor that phagocytoses apoptotic cells.34 Thus, it is highly likely that TIM-1 play a role in the efficient clearance of apoptotic cells and the maintenance of tumor microenvironment homeostasis.
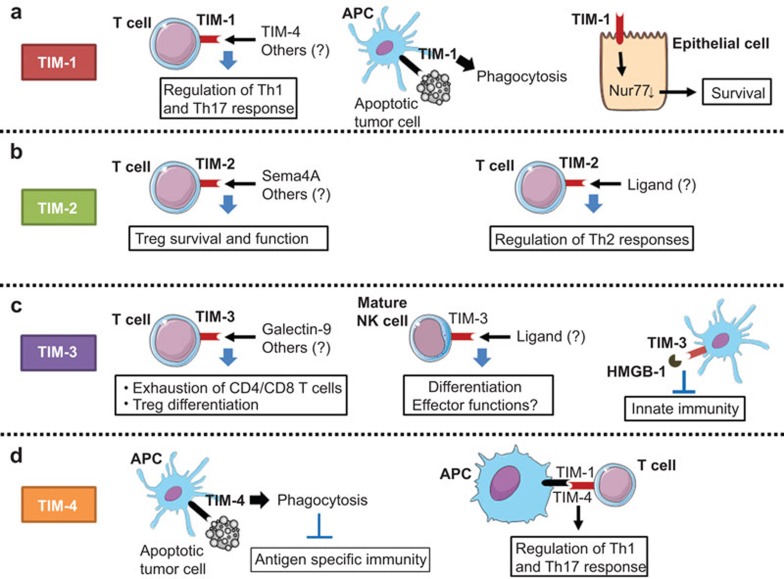
The molecular mechanism of TIM-1-mediated immune regulation has been recently demonstrated. In this study, TIM-1 recycles from the cell surface to endosomes using clathrin-dependent endocytosis pathways, and it suppresses the expression of the orphan nuclear receptor NUR77 through lysosomal degradation pathways, thus protecting kidney epithelial cells from NUR77-mediated apoptotic death signals.35 NUR77 also serves as a lineage-specific factor that promotes monocyte differentiation from BM precursors.36 Additionally, NUR77 inhibits pro-inflammatory activities by myeloid cells in murine atherosclerosis models.37 Thus, NUR77 may contribute to the creation of tolerogenic microenvironments by generating immunosuppressive myeloid cell lineages. It is tempting to speculate that TIM-1 generates tolerogenic myeloid cells that promote pro-inflammatory responses by interfering with NUR77-dependent immunosuppressive programs (Figure 2).
Figure 2.
The role of the TIM gene family in antitumor immune responses. TIM molecules regulate multiple immune pathways. (a) TIM-1 expression on T cells regulates the differentiation of T helper subsets,32,33 whereas TIM-1 expressed on APCs serves as a phagocytosis receptor that facilitates the removal of apoptotic cells.34 TIM-1 is also expressed on kidney epithelial cells and promotes cell survival through the degradation of nuclear factor NUR77.35 (b) TIM-2 promotes the differentiation of Th2 cells and regulates T-cell survival and activation upon interaction with Sema4A on myeloid cells.38,40 (c) TIM-3 on DC suppresses innate immune signals mediated by nucleic acids or DAMPs,27,28 whereas TIM-3 expressing T cells display exhausted phenotypes and trigger apoptosis by interacting with galectin-9.46 TIM-3 also regulates NK cell differentiation and function.55 (d) TIM-4 expression is largely restricted to APCs, and it serves as a phosphatidylserine receptor that regulates the engulfment of apoptotic cells; it interacts with TIM-1 on T cells to regulate the differentiation of Th1 and Th17 cells.34,57,59 APC, antigen-presenting cell; DAMP, damage-associated molecular pattern; DC, dendritic cell; NK, natural killer; Sema4A, Semaphorin 4A; TIM, T-cell immunoglobulin mucin.
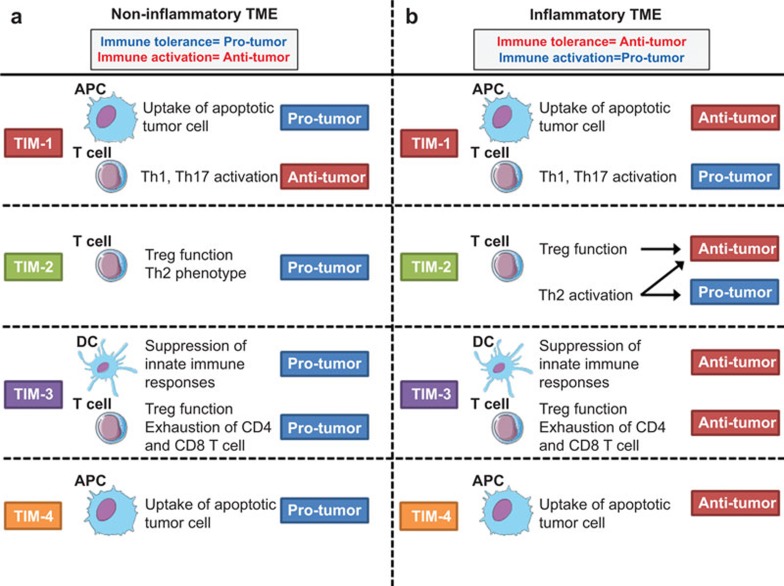
TIM-1 has multiple functions in the regulation of tolerance and immunity depending on the cell type and microenvironment; it remains unclear whether the functional multiplicity of TIM-1 could affect the direction and quality of antitumor immunity and tumor-associated inflammation (Figure 3).
Figure 3.
The dual role of TIM family members in sterile or inflammatory tumor microenvironments. TIM members may serve as dual regulators of antitumor immune responses depending on the quality of the tumor microenvironment. (a) In sterile, non-inflammatory TMEs, TIM-1 and TIM-4 on APCs suppress antigen-specific immune responses by facilitating tolerogenic phagocytosis. Moreover, TIM-2 may create tolerogenic environments by activating Treg populations, whereas TIM-3 negatively regulates DAMP-mediated innate immune signals and compromises tumor-specific CTL responses. (b) In contrast, immunoregulatory activities mediated by TIM-3 and TIM-4 may have a beneficial role in preventing protumorigenic inflammation, while TIM-1 and TIM-2 have dual roles in tumorigenesis by regulating T helper cell differentiation. DAMP, damage-associated molecular pattern; TIM, T-cell immunoglobulin mucin; Treg, regulatory T cell.
TIM-2
TIM-2 is preferentially expressed in differentiated Th2 cells.24,25 TIM-2 blockade results in T cell hyperproliferation and the production of Th2 cytokines such as IL-4 and IL-10, thus demonstrating a critical role for TIM-2 in the regulation of Th2-mediated immunity.38,39
TIM-2 has also been identified as a potential ligand for the semaphorin family member Semaphorin 4A (Sema4A).40 Sema4A is expressed on antigen presenting cells such as dendritic cells and activated B cells, and it plays an important role in supporting the effector function and survival of Tregs.41 Because Tregs are a major population that inhibit intratumoral effector responses and tumor infiltration of Treg may be associated with poor prognosis in cancer patients,42 the interaction between TIM-2 and Sema4A may act as a barrier against efficient antitumor immunity by activating Treg-dependent mechanisms (Figure 2). The relevance of TIM-2-mediated regulation in Th2 and/or Treg function remains largely unknown. However, it is plausible that TIM-2 may contribute to an immunosuppressive environment that favors tumor cell survival at early stages of immunosurveillance; TIM-2 might additionally have a dual role in inflammation-driven tumorigenesis by regulating protumorigenic Th2 and anti-inflammatory Treg responses 43,44 (Figure 3).
TIM-3
TIM-3 negatively regulates Th1 cell responses by binding with galectin-9, and under normal conditions, TIM-3-mediated immune regulation is fine-tuned through negative regulation by HLA-B-associated transcript-3.45,46 Consistent with its immunoregulatory actions, several studies have revealed that the interaction between TIM-3 and galectin-9 causes exhaustion and apoptosis of antigen-specific CTLs in chronic viral infection and cancer.47,48,49 It has been demonstrated that the TIM-3/galectin-9 pathway is responsible for repressing intratumoral immune responses in patients with hepatitis B virus-associated hepatocellular carcinomas.50 Moreover, the exhaustion phenotype characterized by the coexpression of TIM-3 and programmed death 1 (PD-1) is frequently detected on CD8+ T cells in tumor-bearing hosts and correlates with impaired antitumor immune responses in murine acute myelogenous leukemia models and in melanoma patients.51,52 TIM-3 expression has also been identified on Foxp3+ Tregs, although the functional relevance of this population compared to other subsets should be explored in future studies.53
In addition, recent studies have revealed that TIM-3 regulates innate immune responses. The upregulation of TIM-3 on DC in the tumor microenvironment negatively regulates innate responses to nucleic acids released from apoptotic tumor cells through its interaction with HMGB1. The interaction between TIM-3 and HMGB1 interferes with the endocytosis of nucleic acids into DC endosomes, which blocks innate immune signaling pathways upstream of PRR-sensing systems.27,28,54 Moreover, TIM-3 expression has been demonstrated on a specific subset of natural killer cells, which might regulate cytokine profiles and cytotoxic activities by distinct mechanisms55 (Figure 2).
TIM-3 serves as a negative regulator of both innate and adaptive immunity in the tumor microenvironment. We hypothesize that in an early phase of cancer immunoediting, TIM-3 could reduce antitumor immunosurveillance through coordinated and distinct suppressive actions on innate and adaptive antitumor immune responses. However, TIM-3 might dampen protumorigenic inflammation by negatively regulating inflammation in the tumor microenvironment. Moreover, TIM-3-mediated suppression of innate immune signals in inflammation-associated tumor microenvironments may modulate the tumorigenic activities of myeloid cells and attenuate tumor progression, metastasis and resistance to anticancer therapies56 (Figure 3). Although further studies are needed to clarify the role of TIM-3 during immunoediting, our hypothesis further highlights the complex features of immunoregulatory molecules in the regulation of cancer immunosurveillance and immune subversion.
TIM-4
TIM-4 is another member of the TIM family that mainly functions as a phosphatidylserine receptor to enhance the engulfment of apoptotic cells.34,57 In contrast to TIM-3, which is expressed on multiple immune and non-immune cells, TIM-4 expression is restricted to APCs such as DCs and macrophages.58 Recent analysis of TIM-4-deficient mice has shown that TIM-4 dampens inflammatory responses and maintains immune tolerance, implying that TIM-4 may have a potential role in the regulation of immune responses in the tumor microenvironment59,60 (Figure 2).
The role of TIM-4 in tumor immunosurveillance, and in particular the regulation of antitumor immune responses and inflammation-associated carcinogenesis, remains obscure. It is tempting to speculate that TIM-4 plays an important role in controlling tumor-specific responses by regulating the processing and presentation of antigens from phagocytosed apoptotic tumor cells. Consistent with this hypothesis, phagocytic systems for apoptotic cells such as MFG-E8/integrin-αvβ3 and Gas-6/TAM (Mer/Axn/tyro-3) create an immunosuppressive milieu that contributes to the maintenance of immune homeostasis and impaired tumor immunosurveillance.61,62
Consistent with this hypothesis, we recently found that vaccination with irradiated B16 melanoma cells expressing Flt3 ligand, combined with antibody blockade of TIM-4, elicited potent antitumor responses against B16-OVA melanoma tumors by activating the antitumor effector responses of intratumoral CD8+ T cells.63
Thus, TIM-4 suppresses antigen-specific responses by repressing the presentation of immunogenic tumor-associated antigens and by establishing tolerized tumor environments. In contrast, tumor-associated APCs may utilize TIM-4 to counteract tumorigenic inflammation through apoptotic cell phagocytosis and the induction of immunosuppressive lymphocytes. Consistent with this idea, the presence of dying tumors due to impaired phagocytic systems provides potential sources for sterile inflammation and immune responses, which should create favorable circumstances for inflammation-driven tumorigenesis.21,64 Thus, depending on the inflammatory milieu within the tumor microenvironment, it is critical to evaluate whether TIM-4-mediated immune regulation is beneficial or detrimental (Figure 3).
Therapeutic potential of targeting TIM molecules against tumors
Accumulating evidence has demonstrated the therapeutic potential of targeting TIM-3 to activate the antitumor immune responses of T cells infiltrating human tumors. For example, combined blockade of TIM-3 and PD-1 reversed the exhaustion phenotype and enhanced tumor antigen-specific CTL activities.65 Moreover, recent studies unveiled the therapeutic potential of targeting TIM-3 and TIM-4 in preclinical murine tumor models. For example, treatment with anti-TIM-3 monoclonal antibody (mAb) alone or in combination with immunotherapy or chemotherapy augmented antitumor responses against established subcutaneous tumor models.51,53,66 In addition, treatment with anti-TIM-4 mAb augmented antitumor responses against B16 melanoma models.63 More importantly, combined treatment with anti-TIM-3 and anti-TIM-4 mAb further maximized the efficacy of cancer vaccines by increasing the numbers and effector functions of tumor-infiltrating natural killer and CD8+ T cells compared to either mAb alone.63 Taken together, this experimental evidence has supported the therapeutic potential of targeting TIM-3 and TIM-4 to stimulate antitumor immune responses and improve the clinical responses of conventional anticancer regimens. Interestingly, a recent study suggested that an siRNA that disrupted the interaction between TIM-4 on DCs and TIM-1 on T cells by targeting the FG–CC′ loop enhanced the therapeutic efficacy of DC vaccines against gastric cancer; this demonstrated that the FG–CC′ loop in TIM-1/4 may be a suitable target to develop novel immunotherapeutics.67
In contrast, anticancer strategies to suppress TIM-3 and TIM-4 activities might be detrimental in controlling tumorigenesis during sterile inflammation; further sterile inflammation caused by the blockade of TIM-3 and/or TIM-4 might accelerate protumorigenic inflammation. In the condition above, pharmacological inhibition of TIM-1 and TIM-2 in T cells might be useful for attenuating tumor progression by targeting protumorigenic inflammation and suppressing tumorigenic microenvironments.
Finally, we speculate that the therapeutic potential of TIM inhibitors that has been demonstrated in animal tumor models does not necessarily translate into clinical therapies for treating human cancer patients because exogenous implantation of established tumor cell lines does not reflect the natural course of immunosurveillance and immunoevasion in tumor microenvironments. Therefore, it is critical to evaluate the clinical implications of immune regulatory drugs such as TIM inhibitors by utilizing genetically modified animal models to evaluate and compare the pro- and anti-tumorigenic actions of host immunity on the temporal and spatial processes of tumorigenesis.68,69
Conclusions
We describe the potential role of the TIM gene family in the regulation of anti- and pro-tumorigenic responses during cancer immunosurveillance and evasion. The role of TIM family members in the temporal dynamics of immunosurveillance should be broadly applicable to other molecules that have immune modulatory and antitumor activities. For example, therapeutic strategies for targeting immunosuppressive factors (CTLA-4, PD-1, LAG3, IDO, etc.) or boosting immunostimulatory pathways (CD28, CD137, OX40, etc.) may be promising therapeutic strategies to stimulate antitumor immunity and trigger tumor regression during the early phase of tumor immunosurveillance. In addition, immunomodulation may be useful for treating advanced stage cancer patients in whom tumor-mediated immune regulation does not result in immunoevasion and pro-tumor inflammation. In contrast, targeting immunoregulatory pathways such as TIM, CTLA-4 and PD-1 might be detrimental for controlling tumorigenic activities during the advanced stages of carcinogenesis; restoring innate and antigen-specific immune responses might further amplify protumorigenic inflammation and promote tumor progression.
Recent advances to develop immunoregulatory drugs targeting immune checkpoints such as anti-CTLA-4 mAb and anti-PD-1 mAb elicited potent antitumor responses in patients with advanced solid cancers.70,71 Moreover, accumulating evidence has revealed that multiple subsets of immunoregulatory populations, including Treg, MDSC, B7-1 and B7-4-expressing macrophages, contribute to immune suppression and are suitable therapeutic targets in combination with immune checkpoint molecules such as TIM family members.17 However, it remains largely unknown whether particular immune cell subtypes exist that elicit antitumor responses and promote tumorigenic inflammation by these immune modulatory drugs. Thus, a deep understanding of the spatial and temporal regulatory mechanisms of immunosurveillance and immunoevasion during tumorigenesis should help to correctly regulate the homeostatic and inflammatory balance between host immunity and tumorigenicity in clinical settings.
In conclusion, we discuss the potential impact of TIM family members on tumor initiation and prognosis and their utility as therapeutic targets for treating patients with cancer. The development and elucidation of drugs targeting the TIM gene family should advance our understanding of the balance between beneficial antitumor immunity and harmful immune-mediated adverse inflammation and create ideal combinatorial strategies to minimize side effects and maximize clinical responses in the future.
Acknowledgments
This study was partially supported by a Grant-in-Aid for Scientific Research and Scientific Research for Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare (MJ).
The authors declare that they have no conflicts of interest.
References
- Pardoll D. Does the immune system see tumors as foreign or self. Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4:127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;9:361–371. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Harding J, Wolchok JD. Targeting immune checkpoints: releasing the restraints on anti-tumor immunity for patients with melanoma. Cancer J. 2013;18:153–159. [PMC free article] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumor environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet. Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luraishy A, Karin M, Grivennilov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35:467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Bai X, Cao Y, Wu J, Huang M, Tang D, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med. 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei F, Schiavoni G. TIM-3 as a molecular switch for tumor escape from innate immunity. Front Immunol. 2013;9:418. doi: 10.3389/fimmu.2012.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza AJ, Oriss TB, O'Malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci USA. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- Encinas JA, Janssen EM, Weiner DB, Calarota SA, Nieto D, Moll T, et al. Anti-T-cell Ig and mucin domain-containing protein 1 antibody decreases TH2 airway inflammation in a mouse model of asthma. J Allergy Clin Immunol. 2005;116:1343–1449. doi: 10.1016/j.jaci.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Xiao S, Zhu B, Jin H, Zhu C, Umetsu DT, DeKruyff RH, et al. Tim-1 stimulation of dendritic cells regulates the balance between effector and regulatory T cells. Eur J Immunol. 2011;41:1539–1549. doi: 10.1002/eji.201040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Karisola P, Peña-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–840. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Kota SK, Kuchroo VK, Humphreys BD, Strom TB. TIM family proteins promote the lysosomal degradation of the nuclear receptor NUR77. Sci Signal. 2012;5:ra90. doi: 10.1126/scisignal.2003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C-monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S, Sabatos CA, Xiao S, Illes Z, Cha EK, Sobel RA, et al. Tim-2 regulates T helper type 2 responses and autoimmunity. J Exp Med. 2005;202:437–444. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, de Souza AJ, Tosti R, Narayan P, Kane LP. Cutting edge: inhibition of T cell activation by TIM-2. J Immunol. 2006;177:4966–4970. doi: 10.4049/jimmunol.177.8.4966. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1–semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–243. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Yagita H, Yoshiyama H, Tahara H. Putting the brakes on anticancer therapies: suppression of innate immune pathways by tumor-associated myeloid cells. Trends Mol Med. 2013;19:536–545. doi: 10.1016/j.molmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Ndhlovu LC, Lopez-Vergès S, Barbour JD, Jones RB, Jha AR, Long BR, et al. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- Wong K, Valdez PA, Tan C, Yeh S, Hongo J, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci USA. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albacker LA, Karisola P, Chang YJ, Umetsu SE, Zhou M, Akbari O, et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J Immunol. 2010;185:6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–1326. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdadi M, Nagao H, Yoshiyama H, Akiba H, Yagita H, Dosaka-Akita H, et al. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol Immunother. 2013;62:629–637. doi: 10.1007/s00262-012-1371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CD, Pound JD. Cell death in the neighborhood: direct microenvironmental effects of apoptosis in normal and neoplastic tissues. J Pathol. 2011;223:177–194. doi: 10.1002/path.2792. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- Sun HW, Wu C, Tan HY, Wang QS. A new development of FG–CC′ siRNA blocking interaction of Tm-1 and Tim-4 can enhance DC vaccine against gastric cancer. Hepatogastroenterology. 2012;59:2677–2682. doi: 10.5754/hge11620. [DOI] [PubMed] [Google Scholar]
- DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, et al. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell. 2011;19:72–85. doi: 10.1016/j.ccr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]