Abstract
Aim:
20(S)-Ginsenoside Rh2 (Rh2) has shown potent inhibition on P-glycoprotein (P-gp), while most HIV protease inhibitors are both substrates and inhibitors of P-gp and CYP3A4. The aim of this study was to investigate the potential pharmacokinetic interactions between Rh2 and the HIV protease inhibitor ritonavir.
Methods:
The effects of Rh2 on the cellular accumulation and transepithelial transport of ritonavir were studied in Caco-2 and MDCK-MDR1 cells. Male rats were administered Rh2 (25 or 60 mg/kg, po) or Rh2 (5 mg/kg, iv), followed by ritonavir (25 mg/kg, po). The P-gp inhibitors verapamil (20 mg/kg, po) or GF120918 (5 mg/kg, po) were used as positive controls. The concentrations of ritonavir in plasma, bile, urine, feces and tissue homogenates were analyzed using LC-MS.
Results:
Rh2 (10 μmol/L) significantly increased the accumulation and inhibited the efflux of ritonavir in Caco-2 and MDCK-MDR1 cells, as verapamil did. But Rh2 did not significantly alter ritonavir accumulation or transport in MDCK-WT cells. Intravenous Rh2 significantly increased the plasma exposure of ritonavir while reducing its excretion in the bile, and oral verapamil or GF120918 also increased plasma exposure of ritonavir but without changing its excretion in the bile. Interestingly, oral Rh2 at both doses did not significantly change the plasma profile of ritonavir. Moreover, oral Rh2 (25 mg/kg) significantly elevated the ritonavir concentration in the hepatic portal vein, and markedly increased its urinary excretion and tissue distribution, which might counteract the elevated absorption of ritonavir.
Conclusion:
Rh2 inhibits the efflux of ritonavir through P-gp in vitro. The effects of Rh2 on ritonavir exposure in vivo depend on the administration route of Rh2: intravenous, but not oral, administration of Rh2 significantly increased the plasma exposure of ritonavir.
Keywords: pharmacokinetics, drug interactions, 20(S)-ginsenoside Rh2, HIV protease inhibitors, ritonavir, P-glycoprotein, absorption, tissue distribution, excretion
Introduction
HIV protease inhibitors (PIs) are commonly used as part of highly active anti-retroviral therapy (HAART) for HIV infection to control virus replication and disease progression1. Despite the clinic significance of PIs, accumulating clinical evidence indicates that the incorporation of HIV PIs into HAART is associated with various adverse effects, such as metabolic syndrome characterized by dyslipidemia, insulin resistance, and lipodystrophy2,3.
20(S)-Ginsenoside Rh2 (Rh2), a component isolated from red ginseng4, is one of the most studied ginsenosides due to its various pharmacological activities, such as anti-cancer effects, anti-hyperglycemic effects, and anti-obesity effects5,6,7,8. Rh2 has been approved as a complementary food by the China State Food and Drug Administration since 2006, and it can be speculated that Rh2 is popular among HIV-infected patients, especially in the individuals with AIDS and cancer.
Most PIs are both substrates and inhibitors of P-glycoprotein (P-gp)9,10 and CYP3A411,12. Recently, it was found that Rh2 is a potent noncompetitive inhibitor of P-gp13,14,15, indicating that there may be a potential pharmacokinetic interaction between Rh2 and the P-gp substrate of PIs. Ritonavir (RTV), one of the most commonly used HIV PIs, has been demonstrated to be a substrate and inhibitor of P-gp both in vitro and in vivo9,10,16,17. However, few studies have focused on the interactions between ginsenosides and antiretrovirals18,19. To our knowledge, the pharmacokinetic interactions between HIV PIs and Rh2 remain unknown. Thus, the goal of this study was to evaluate the potential pharmacokinetic interactions between a typical PI agent — RTV — and Rh2 using both in vitro and in vivo models.
Materials and methods
Chemicals and reagents
Rh2 (purity >98%) was purchased from Jilin University (Changchun, China). RTV was kindly donated by Dr Hui-ping ZHOU (Virginia Commonwealth University, Richmond, VA, USA). Verapamil (Ver) and digitoxin were purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). GF120918 was purchased from J&K Scientific Ltd (Shanghai, China). Acetonitrile was purchased from Merck Co (Darmstadt, Germany). Deionized water was prepared using a Milli-Q system (Millipore, Milford, MA, USA). Ethylacetate and all other reagents and solvents were purchased from commercial sources and were of analytical grade.
Cell culture
Caco-2 cells were purchased from the American Type Culture Collection (Rockville, MD, USA). Wild-type MDCK cells (MDCK-WT) and MDCK cells transfected with the human MDR1 gene (MDCK-MDR1) were obtained from Zhejiang University (Hangzhou, China). All of the cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 110 mg/L pyruvic acid sodium, 3.7 mg/L HEPES, 62.5 mg/L penicillin, and 100 mg/L streptomycin (Invitrogen) and incubated at 37 °C in an atmosphere of 5% carbon dioxide and 90% relative humidity. The cell culture medium was refreshed every 2 d until the cells reached confluence. All of the cells were used between passages 30 and 45 and tested negative for mycoplasma infection.
Cellular accumulation study
The accumulation studies were performed with confluent epithelial monolayers of Caco-2, MDCK-WT, and MDCK-MDR1 cells. The cells were pre-treated with Hanks' balanced salt solution (HBSS, pH 7.4) containing Rh2 or 0.1% dimethyl sulfoxide (DMSO, control) at 37 °C for 0.5 h, followed by the addition of RTV for specified time. After the retention period had passed, the drugs were withdrawn, and the cells were rinsed with ice-cold HBSS. Ver was used as a positive control P-gp inhibitor. The cells were lysed by three freeze-thaw cycles, and the protein concentrations were measured using a Bradford protein assay kit (BioRad, Hercules, CA, USA) according to the manufacturer's instructions. The RTV concentrations were determined by liquid chromatography-mass spectrometry (LC-MS) and were expressed as ng/mg protein. All experiments were conducted in triplicate. All of the drugs (RTV, Rh2, and Ver) were prepared by dissolution in DMSO and dilution with HBSS. The final concentration of DMSO was no more than 0.2% (v/v).
Transepithelial transport studies
For the transport experiments, cells at a density of 1.5×105 cells/insert were seeded on a permeable polycarbonate insert (Millicell cell culture inserts, 0.4 μm pore size, 12 mm diameter, Millipore, USA) in 24-well culture plates and were used for the experiment at 18–21 d (Caco-2 cells) or 5–6 d (MDCK-WT and MDCK-MDR1 cells) after seeding. TEER measurements (Millicell-ERS Epithelial Voltohmmeter, Millipore Co, Milford, MA, USA) were used to evaluate the integrity of the cell monolayers. The monolayers used in the transport studies had TEER values exceeding 500 Ω·cm2 for the Caco-2 and MDCK-MDR1 cells and 180 Ω·cm2 for the MDCK-WT cells.
On the day of experiment, the cell monolayers were pre-treated with HBSS containing Rh2 (1, 5, and 10 μmol/L) or 1‰ DMSO (control) at 37 °C for 0.5 h. Then, 2 μmol/L RTV was added to either the apical (AP) or basolateral (BL) side to evaluate transport in the absorptive and secretory directions, respectively. The cell monolayers were incubated for another 1 h. Ver was used as the positive control. At the end-point, samples were taken from the receiving chamber for analysis by LC-MS. All experiments were conducted in triplicate.
Animals
Healthy male Wistar rats (180–220 g) were obtained from the Experimental Animal Breeding Center of Yangzhou University (Yangzhou, China) and housed under environmentally controlled conditions with a 12-h light-dark cycle. The animals were acclimated to the environment for 5 d before the experiment. Prior to each experiment, the rats were fasted overnight (12 h) with free access to water. The animals were randomly assigned to different experimental groups. The animal experiments were carried out in accordance with the Guidelines for Animal Experimentation of China Pharmaceutical University (Nanjing, China), and the protocol was approved by the Animal Ethics Committee of this University.
Pharmacokinetic studies in rats
To evaluate the effect of Rh2 on the pharmacokinetics of RTV, the rats were divided into seven groups. Two groups of rats were intragastrically administered a single dose of Rh2 at 25 mg/kg or 60 mg/kg suspended in 0.5% CMC-Na. One group of rats was injected with a single dose of Rh2 (5 mg/kg) via the caudal vein. Two groups received the typical inhibitors of P-gp, Ver (20 mg/kg, po) and GF120918 (5 mg/kg, po). The two blank control groups received either the intragastric vehicle (0.5% CMC-Na) or the intravenous vehicle (20% ethanol+30% propylene glycol+50% dextrose, 5%). Thirty minutes (the two intravenous groups) or one hour (all other groups) later, RTV (25 mg/kg) in 0.5% CMC-Na was orally administered to the rats. Blood samples (0.3 mL) were collected at 0, 0.25, 0.5, 1, 2, 3, 4, 6, 9, 12, 18, and 24 h in heparinized tubes. Plasma was prepared by centrifugation at 5000×g for 10 min and stored at -20 °C until analysis.
Collection of rat bile, urine, feces, and hepatic portal vein plasma
The rats were randomly classified into four groups, ie, the vehicle control (0.5% CMC-Na, po) group, the Rh2 (25 mg/kg, po) group, the Rh2 (5 mg/kg, iv) group, and the GF120918 (5 mg/kg, po) group. For the collection of rat bile, cannulas were surgically inserted into the bile duct after anaesthetization with diethyl ether. Thirty minutes (the iv group) or one hour (all other groups) later, RTV (25 mg/kg) in 0.5% CMC-Na was administered orally to the rats. Bile samples were collected -2 to 0 h before drug administration and 0–2, 2–4, 4–6, 6–8, and 8–12 h after drug administration. A solution of 100 mmol/L taurocholic acid salt in 0.9% saline-0.5% KCl was infused at 0.5 mL/h into the duodenum. Samples were stored at -20 °C until analysis.
For the collection of urine and feces, the four groups of rats (mentioned above) were housed in individual metabolic cages. Urine was collected and the volume was measured between -12 and 0 h before drug administration and during the periods of 0–6, 6–12, 12–24, 24–36, and 36–48 h after drug administration. Feces were collected for the periods -12 to 0 h before drug administration and 0–12, 12–24, 24–36, 36–48, and 48–72 h after drug administration. The samples were weighed and mechanically homogenized in water (1:5, w/w) for 5 min and stored at -20 °C until analysis.
For the collection of rat hepatic portal vein plasma, heparinized cannulas were surgically inserted into the vicinity of the junction of the gastroduodenal vein after anaesthetization. Thirty minutes (the iv group) or one hour (all other groups) later, RTV (25 mg/kg) in 0.5% CMC-Na was administered orally to the rats. Blood samples were collected at 0, 0.5, 1, 2, 4, 6, and 8 h in heparinized tubes. Plasma was obtained by centrifugation at 5000×g for 10 min and stored at -20 °C until analysis.
Rat tissue distribution
The rats were intragastrically administered a single dose of Rh2 (25 mg/kg) or vehicle (0.5% CMC-Na) one hour before the oral administration of RTV (25 mg/kg, po). At 3 and 6 h after RTV administration, the male Wistar rats (four at each time point) were sacrificed immediately after blood sampling. The primary tissues (heart, liver, spleen, lungs, kidneys, brain, stomach, small intestine, fat, and testes) were harvested, weighed, and homogenized with 0.8% NaCl saline containing 0.01 mol/L Tris-HCl, 0.001 mol/L Na2EDTA, and 0.01 mol/L saccharose (pH 7.4). The supernatants of the tissue homogenates obtained after centrifugation were stored at -80 °C until analysis.
LC-MS analysis
Samples of the cell lysates, rat plasma, urine, feces, and bile spiked with 10 μL of internal standard solution (10 μmol/L digitoxin) were extracted with ethyl acetate. After centrifugation, the upper organic phase was evaporated to dryness (Thermo Savant SPD 2010 SpeedVac System, Thermo Electron Corporation, USA). The residue was reconstituted in 100 μL of acetonitrile, followed by another centrifugation. Five microliters of supernatant was injected into the LC-MS system and separated with an Inertsil ODS-3 column (150 mm×2.1 mm id, 5 μm, GL Science Inc, Japan) fitted with a C18 guard column (4.6 mm×12.5 mm, 5 μm, Agilent, USA) at 40 °C. A Shimadzu 2010A LC-MS system (Shimadzu, Kyoto, Japan) with an ESI source was used to perform the analysis. Data acquisition was performed with LCMSsolution Version 2.04 (Shimadzu Corp, Japan). The mobile phase consisted of solvent A (0.002% ammonium chloride in water) and solvent B (acetonitrile) using the following gradient: 40%–60% B (linear, 0.03 min), 60%–80% B (B Curve Value=-3, 4.97 min), 80%–88% B (linear, 0.03 min), 88% B (1.97 min), 88%–40% B (linear, 0.5 min), and 40% B (4.5 min) for equilibration. The flow rate was set at 0.2 mL/min. In SIM mode, the negative ions of RTV, Rh2, and digitoxin (internal standard) were monitored at m/z 755.15 ([M+Cl]−), 657.3 ([M+Cl]−), and 799.30 ([M+Cl]−), respectively.
Data analysis
The apparent permeability (Papp) of RTV across the cell monolayers used for transport studies was calculated with the following formula: Papp=(1/C0)(1/S)(ΔQ/Δt), where C0 is the concentration of the test drug in the donor chamber, S is the surface area of the monolayer, and ΔQ/Δt is the permeability rate (nmol/s).
The efflux ratio (ER), which is the ratio of BL-to-AP transport to AP-to-BL transport, was calculated using the following equation: ER=Papp (BL-AP)/Papp (AP-BL), where Papp (BL-AP) and Papp (AP-BL) represent the apparent permeability of the test compound in the BL-to-AP direction and the AP-to-BL direction of the cellular monolayer, respectively.
The pharmacokinetic parameters of RTV were obtained by noncompartmental analysis using WinNonLin Phoenix version 6.3 (Pharsight Corp, Mountain View, CA, USA). The area under the plasma concentration-time curve (AUC) was calculated using the trapezoidal method. The terminal half-life (t1/2) was calculated as ln2/k, where k, the elimination rate constant, was determined from the slope of the terminal regression line. Absolute bioavailability (F) was determined by the equation (AUCpo×Doseiv)/(AUCiv×Dosepo).
In the excretion studies, the cumulative excretion rate was calculated by the following equation: (cumulative excretion/total amount)×100%.
Statistical analysis
The data are presented as the mean±SEM. Comparisons between groups were performed using Student's t-test. For multiple comparisons, a one-way analysis of variance followed by Dunnett's test was applied. A probability value of <0.05 was considered significant.
Results
Rh2 increased the accumulation and inhibited the efflux of RTV in Caco-2 cells
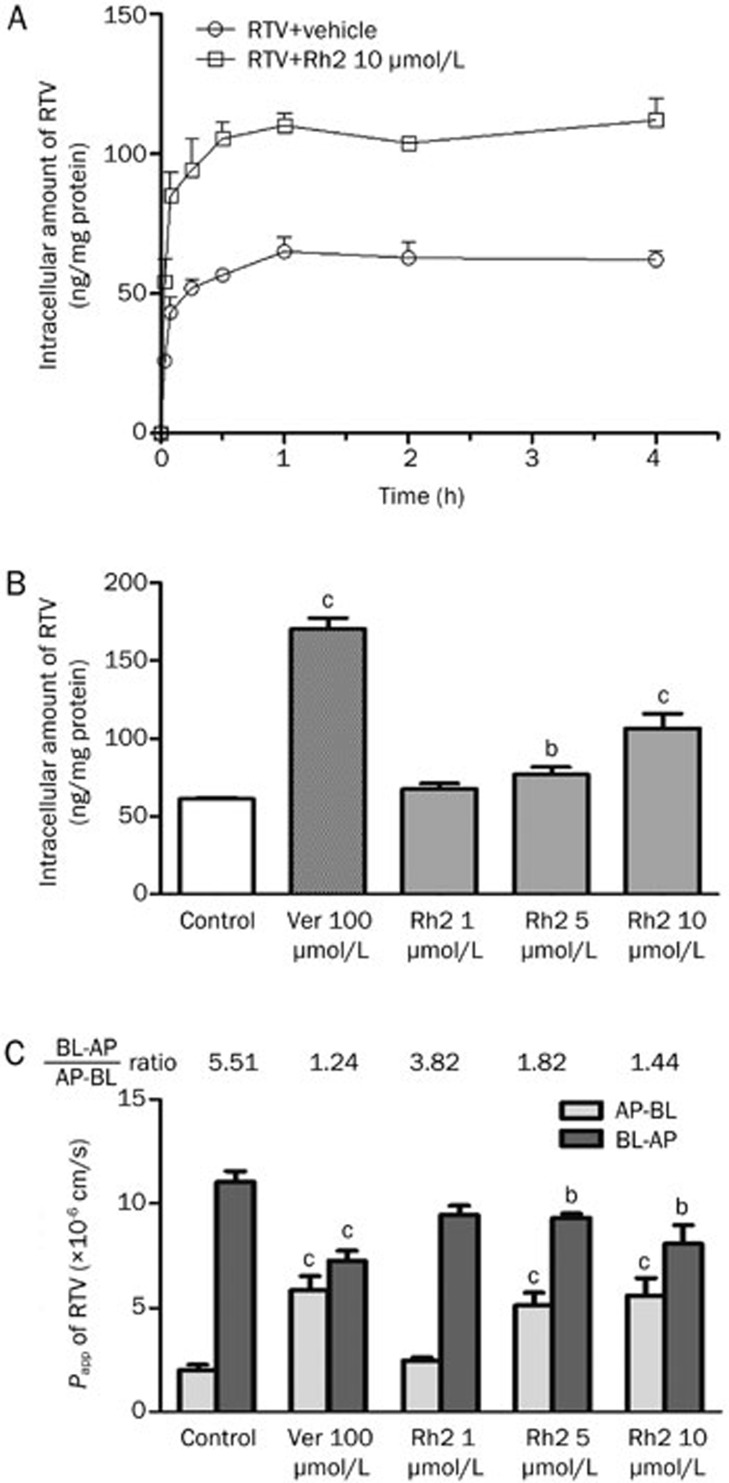
As shown in Figure 1A, RTV rapidly accumulated in Caco-2 cells. The amount of accumulated RTV at 1 h was similar to that at 4 h. Therefore, we used 1 h as the time point for the following accumulation and transport studies in Caco-2 cells.
Figure 1.
Effects of Rh2 on the accumulation and transport of ritonavir (RTV) in Caco-2 cells. (A) Intracellular accumulation of RTV in Caco-2 cells treated with or without Rh2 (10 μmol/L) for 5, 15, 30, 60, 120, or 240 min. (B) Intracellular accumulation of RTV in Caco-2 cells treated with verapamil (Ver) or Rh2. (C) The transportation of RTV across Caco-2 monolayers cells. The cells were preincubated with Rh2 (1, 5, and 10 μmol/L) or Ver (100 μmol/L) for 30 min, respectively, followed by a further incubation in the presence of 2 μmol/L RTV for 60 min. The bars represent the mean±SEM of three independent experiments. bP<0.05, cP<0.01 vs control. AP, apical; BL, basolateral; Papp, apparent permeability.
Rh2 increased the cellular accumulation of RTV in Caco-2 cells in a concentration-dependent manner, with increases of 1.10-, 1.25-, and 1.74-fold in the presence of 1, 5 (P<0.05), and 10 μmol/L (P<0.01) Rh2, respectively (Figure 1B). The classic P-gp inhibitor Ver (100 μmol/L) exhibited a potent inhibitory effect on P-gp, resulting in a significant 2.78-fold increase in the intracellular accumulation of RTV (P<0.01).
In addition, the effect of Rh2 on RTV transport across Caco-2 monolayers was examined. As shown in Figure 1C, the Papp values of RTV in the BL-to-AP direction were markedly higher than those in the AP-to-BL direction, with an efflux ratio (BL-to-AP/AP-to-BL) of approximately 5.51. In the presence of 1, 5, and 10 μmol/L Rh2, the efflux ratio of RTV gradually decreased from 3.82 to 1.82 and finally 1.44. Ver (100 μmol/L) decreased the efflux ratio of RTV to 1.24.
Rh2 increased the accumulation and inhibited the efflux of RTV in MDCK-MDR1 cells
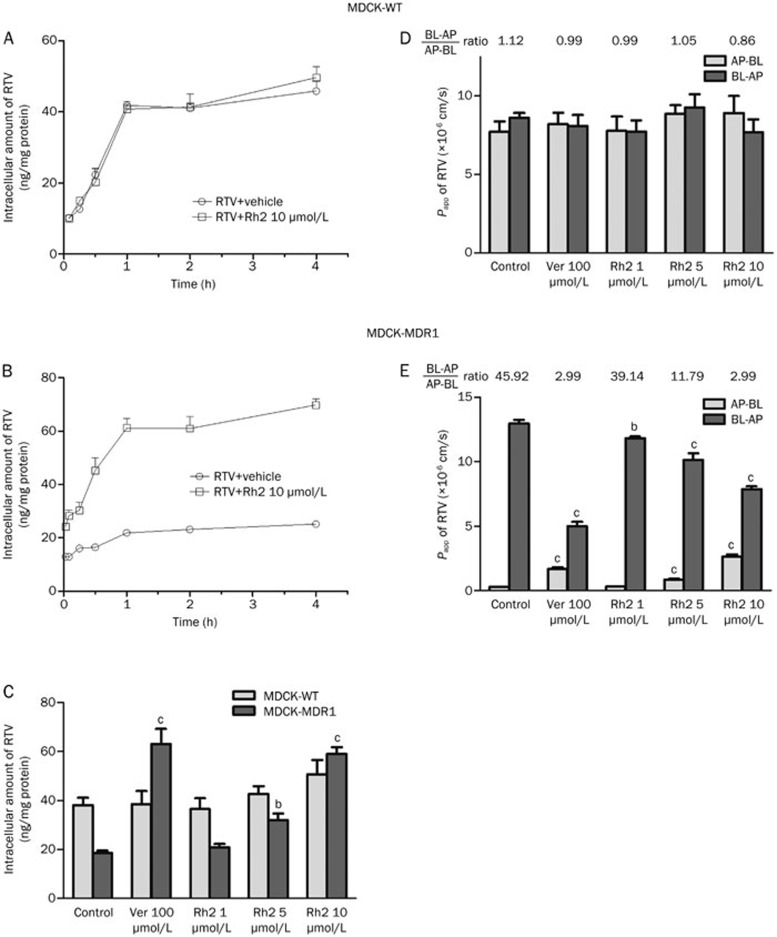
RTV rapidly accumulated in MDCK-WT (Figure 2A) and MDCK-MDR1 (Figure 2B) cells. As in Caco-2 cells, in the MDCK-WT and MDCK-MDR1 cells, there was not a significant difference between the levels of RTV accumulation at 1 h and 4 h. Therefore, 1 h was chosen as the time point for the following accumulation and transport studies in both MDCK-WT and MDCK-MDR1 cells. The accumulation of RTV was significantly lower in MDR1-transfected MDCK-MDR1 cells than that in the wild-type cells (Figure 2B).
Figure 2.
Effects of Rh2 on the accumulation and transport of ritonavir (RTV) in MDCK-WT and MDCK-MDR1 cells. (A) Intracellular accumulation of RTV in MDCK-WT cells treated with or without Rh2 (10 μmol/L) for 5, 15, 30, 60, 120, or 240 min. (B) Intracellular accumulation of RTV in MDCK-MDR1 cells treated with or without Rh2 (10 μmol/L) for 5, 15, 30, 60, 120, or 240 min. (C) The accumulation of RTV in MDCK-WT and MDCK-MDR1 cells in the presence of Rh2 or verapamil (Ver) for 60 min. (D) The transportation of RTV across MDCK-WT monolayer cells. (E) The transportation of RTV across MDCK-MDR1 monolayer cells. The cells were preincubated with Rh2 (1, 5, and 10 μmol/L), or Ver (100 μmol/L) for 30 min, respectively, followed by a further incubation in the presence of 2 μmol/L RTV for 60 min. The bars represent the mean±SEM of three independent experiments. bP<0.05, cP<0.01 vs control. AP, apical; BL, basolateral; Papp, apparent permeability.
The Rh2 increased the intracellular accumulation of RTV in MDCK-MDR1 cells in a concentration-dependent manner, with increases of 1.12-, 1.72-, and 3.17-fold in the presence of 1, 5 (P<0.05), and 10 μmol/L (P<0.01) Rh2, respectively (Figure 2C). The established P-gp inhibitor Ver (100 μmol/L) exhibited a potent inhibitory effect on P-gp, resulting in a significant 3.40-fold increase in the intracellular accumulation of RTV (P<0.01). However, neither Rh2 nor Ver exhibited any significant effect on the accumulation of RTV in MDCK-WT cells (Figure 2C).
The effect of Rh2 on RTV transport across MDCK-MDR1 monolayers was examined. In MDCK-MDR1 cells, the Papp value of RTV in the BL-to-AP direction was markedly higher than that in the AP-to-BL direction, with an efflux ratio (BL-to-AP/AP-to-BL) of approximately 45.92 (Figure 2E). In the presence of 1, 5, and 10 μmol/L Rh2, the efflux ratio of RTV decreased from 39.14 to 11.79 and finally 2.99. Ver (100 μmol/L) decreased the efflux ratio of RTV to approximately 2.99. However, in MDCK-WT cells, the permeability of RTV was comparable in both the absorptive (AP-BL) and secretory (BL-AP) directions, with efflux ratios (BL-to-AP/AP-to-BL) of approximately 0.86–1.12 (Figure 2D). The presence of Ver and Rh2 did not have any significant effect on RTV transport.
Differential effects of intragastrically and intravenously administered Rh2 on the pharmacokinetics of RTV
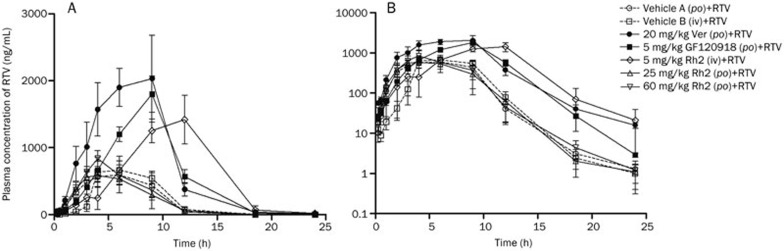
The intragastric administration of Rh2 did not have any significant effect on the pharmacokinetic profile or the parameters of RTV (Figure 3 and Table 1). The increased dose of Rh2 had little effect on the pharmacokinetics of RTV. However, the pre-administration of Ver (20 mg/kg, po) or GF120918 (5 mg/kg, po) significantly increased the AUC of RTV by 3.60 and 2.71-fold, respectively. The maximum plasma concentration (Cmax) increased by 3.62- and 2.49-fold in the Ver- and GF120918-pretreated groups, respectively, relative to the vehicle control-treated group.
Figure 3.
Plasma concentration-time curve of ritonavir (RTV) in male Wistar rats. The rats were intragastrically administered a single dose of Rh2 (25 or 60 mg/kg), verapamil (Ver) (20 mg/kg), GF120918 (5 mg/kg), or intragastric vehicle (vehicle A, 0.5% CMC-Na) 1 h before receiving RTV (25 mg/kg, po) or were intravenously injected with a single dose of Rh2 (5 mg/kg) or intravenous vehicle (vehicle B, 20% ethanol+30% propylene glycol+50% dextrose, 5%) 30 min before receiving RTV (25 mg/kg, po). The data are expressed as the mean±SEM (n=5). A, normal scale; B, semi-log scale.
Table 1. The effect of 20(S)-ginsenoside Rh2 and standard P-gp inhibitors on the pharmacokinetic parameters of ritonavir (RTV).
| Regimen | Parameter (mean±SEM) |
||||
|---|---|---|---|---|---|
| T1/2 (h) | Tmax (h) | Cmax (ng/mL) | AUC0–24 (h·μg/mL) | Cl/F (mL·h−1·kg−1) | |
| Vehicle A (po)+RTV | 1.9±0.3 | 5.3±1.0 | 728.4±109.8 | 4.8±1.0 | 7062±1495.5 |
| Vehicle B (iv)+RTV | 1.6±0.3 | 6.3±0.8 | 938.4±142.9 | 4.8±0.5 | 5462.8±602.3 |
| 20 mg/kg Ver (po)+RTV | 2.7±0.3 | 6.4±1.0 | 2634.7±536b | 17.3±3.1c | 1661.4±257.8b |
| 5 mg/kg GF120918 (po)+RTV | 1.6±0.1 | 8.4±0.5b | 1812.0±290.9b | 13.0±1.6c | 2072.5±242.6b |
| 5 mg/kg Rh2 (iv)+RTV | 2.6±0.2b | 9.8±0.7c | 1724.5±380b | 14.9±3.9b | 2332.2±479c |
| 25 mg/kg Rh2 (po)+RTV | 2.3±0.5 | 4.3±0.9 | 782.6±117.7 | 4.5±1.5 | 8008.9±2042.6 |
| 60 mg/kg Rh2 (po)+RTV | 2.0±0.2 | 3.8±0.2 | 884.1±99.8 | 5.2±0.6 | 5198.0±609.2 |
The rats were intragastrically administered a single dose of Rh2 (25 or 60 mg/kg), Ver (20 mg/kg), GF120918 (5 mg/kg), or intragastric vehicle (vehicle A, 0.5% CMC-Na) 1 h before receiving RTV (25 mg/kg, po) or intravenously injected with a single dose of Rh2 (5 mg/kg) and intravenous vehicle (vehicle B, 20% ethanol+30% propylene glycol+50% dextrose, 5%) 30 min before receiving RTV (25 mg/kg, po). The data are expressed as the mean±SEM (n=5). bP<0.05, cP<0.01 vs Vehicle A control. T1/2, terminal half-life; Tmax, time to maximum plasma concentration; Cmax, the maximum plasma concentration; AUC, area under the concentration-time curve; Cl/F, apparent clearance.
The intravenous injection of Rh2 distinctly altered the pharmacokinetic profile and the RTV parameters. The pharmacokinetic profile of RTV was characterized by a delayed absorption phase and Tmax and an elevated Cmax (1.84-fold) and AUC (3.10-fold) relative to the intravenous vehicle control. The terminal t1/2 of RTV was increased after the intravenous injection of Rh2 (1.40-fold). However, there were no significant differences in the Tmax, Cmax, or AUC between the two groups treated with the intragastric and intravenous vehicles, indicating that the intravenous vehicle did not significantly affect the pharmacokinetic profile of RTV.
Differential effects of intragastrically and intravenously administered Rh2 on the concentration of RTV in the hepatic portal vein
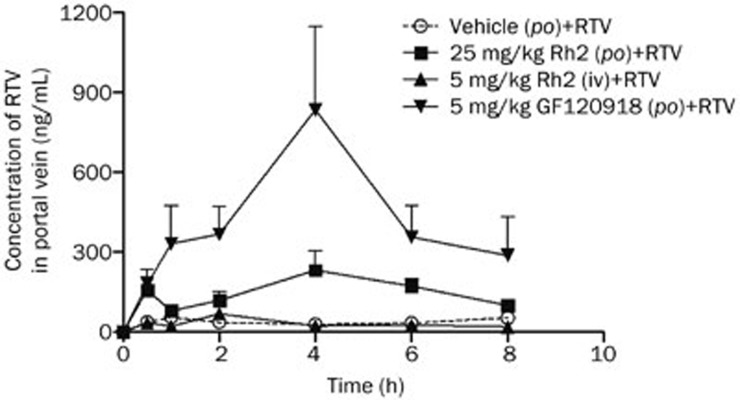
The hepatic portal vein is a blood vessel that conducts blood from the gastrointestinal tract and spleen to the liver. This vein contains the drugs and metabolites absorbed from the gastrointestinal tract before reaching the systemic circulation. Quantitative measurement of the RTV concentration in the hepatic portal vein revealed that the intragastric administration of Rh2 or the established P-gp inhibitor GF120918 obviously increased the absorption of RTV, with increases from 301.4±77.9 h·ng/mL (AUC0–8 h) to 1234.8±277.3 h·ng/mL (P<0.05) and 3565.2±683.0 h·ng/mL (P<0.01), equivalent to 4.1- and 11.8-fold increases, respectively. The intravenous administration of Rh2 did not significantly affect the RTV profile in the hepatic portal vein, and the RTV profile under these conditions was similar to the profile associated with the blank vehicle (Figure 4).
Figure 4.
Concentration-time curves of ritonavir (RTV) in the hepatic portal vein. The rats were intragastrically administered a single dose of 20(S)-ginsenoside Rh2 (Rh2) (25 mg/kg), GF120918 (5 mg/kg), or intragastric vehicle (0.5% CMC-Na) 1 h before the oral administration of RTV (25 mg/kg, po) or were intravenously injected with a single dose of Rh2 (5 mg/kg) 30 min before receiving RTV (25 mg/kg, po). Each value represents the mean±SEM (n=5).
Differential effects of intragastrically and intravenously administered Rh2 on the excretion of RTV in bile, feces, and urine
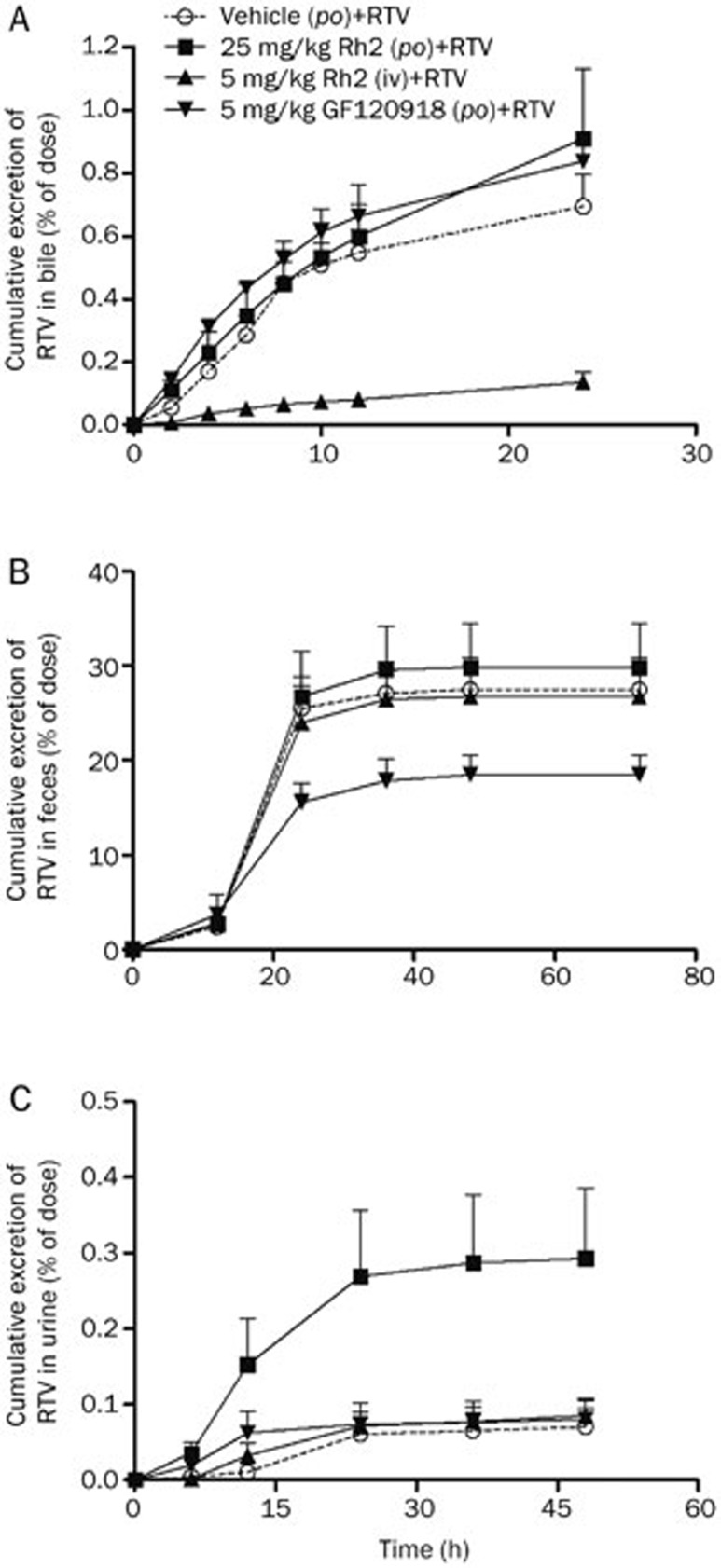
The level of RTV in the bile was profiled, and its kinetics in the presence of vehicle were compared with those in the presence of Rh2. It was shown that RTV was extensively metabolized in the liver, and the metabolite peaks were much larger, which agrees with the results of a previous study20 (data not shown). The intravenous administration of Rh2 significantly decreased the cumulative excretion of RTV in bile from 0.69%±0.11% to 0.14%±0.03% (P<0.01), whereas the intragastric administration of Rh2 and GF120918 did not significantly affect the excretion of RTV, which is similar to the results obtained following the administration of the blank vehicle (Figure 5A).
Figure 5.
Effects of Rh2 and GF120918 on the cumulative excretion of ritonavir (RTV) in the bile, feces and urine. The rats were intragastrically administered a single dose of Rh2 (25 mg/kg), GF120918 (5 mg/kg), or intragastric vehicle (0.5% CMC-Na) 1 h before the administration of RTV (25 mg/kg, po) or intravenously injected with a single dose of Rh2 (5 mg/kg) 30 min before the administration of RTV (25 mg/kg, po). (A) Cumulative excretion of RTV in the bile; (B) Cumulative excretion of RTV in the feces; (C) Cumulative excretion of RTV in the urine. Each value represents the mean±SEM (n=5).
The measurement of the RTV level in feces indicated that 27.6%±3.3% of the dose was recovered in the feces in the original unchanged form of RTV after the intragastric administration of RTV. The oral administration of GF120918 significantly reduced the cumulative excretion of unchanged RTV in the feces to 18.5%±2.3% (P<0.05). The intragastric and intravenous administration of Rh2 did not significantly affect the excretion of RTV, similar to the administration of the blank vehicle. These results indicate that GF120918 greatly enhanced the gastrointestinal absorption of RTV by inhibiting P-gp.
The RTV concentration in the urine was also profiled and compared. The intragastric administration of Rh2 significantly promoted the cumulative excretion of RTV in the urine from 0.07%±0.02% to 0.29%±0.09% (P<0.05), whereas the administration of Rh2 (iv) and GF120918 (po) did not significantly affect the excretion of RTV, similar to the administration of the blank vehicle (Figure 5C).
Plasma pharmacokinetics of intragastrically and intravenously administered Rh2 in male Wistar rats
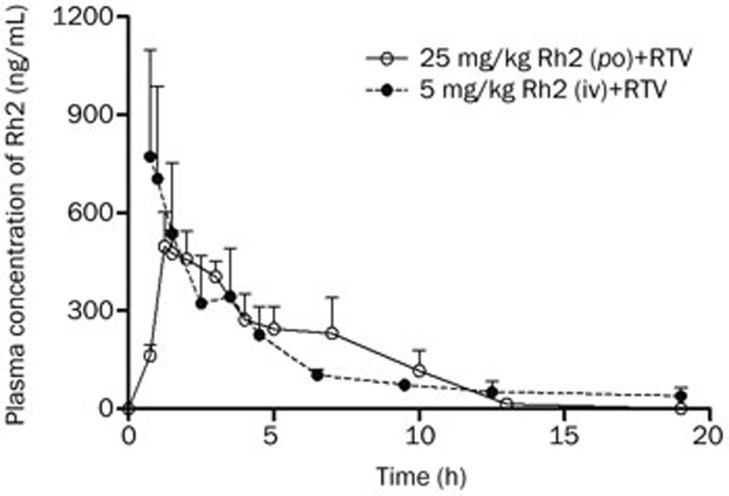
The concentration-time curve of Rh2 was also profiled in the same experiment used to evaluate the effect of Rh2 on the pharmacokinetics of RTV. Up to 19 h, the calculated AUC0–19 h of orally administered Rh2 of 4.6±0.6 h·μg/mL was statistically equivalent to the value associated with intravenous administration (5.9±1.6 h·μg/mL). The absolute bioavailability was 15.4% (Figure 6).
Figure 6.
Plasma concentration-time curves of intragastrically and intravenously administered Rh2 in rats. The rats were intragastrically administered a single dose of Rh2 (25 mg/kg) 1 h before receiving ritonavir (RTV) (25 mg/kg, po) or intravenously injected with a single dose of Rh2 (5 mg/kg) 30 min before receiving RTV (25 mg/kg, po). The data are expressed as the mean±SEM (n=5).
Intragastric administration of Rh2 increased the tissue/plasma ratio of RTV
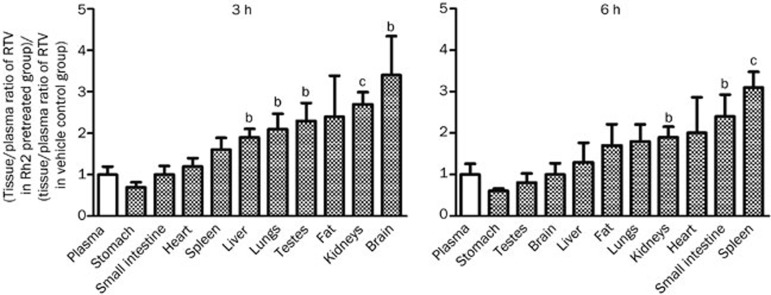
Compared with the vehicle control, pre-treatment with orally administered Rh2 had little effect on the plasma concentration of RTV but enhanced the movement of RTV from plasma into most tissues such as the liver, lungs, testes, fat, kidneys, and brain at 3 h and the lungs, kidneys, heart, small intestine, and spleen at 6 h, resulting in an elevated tissue/plasma ratio of RTV relative to that in the vehicle control group (Figure 7).
Figure 7.
Effect of the intragastric administration of 20(S)-ginsenoside Rh2 (Rh2) on the tissue/plasma distribution ratios of ritonavir (RTV). The rats were intragastrically administered a single dose of Rh2 (25 mg/kg) or vehicle (0.5% CMC-Na) 1 h before the oral administration of RTV (25 mg/kg, po). The longitudinal axis represents the tissue/plasma ratio of RTV in the Rh2-pretreated group compared with the vehicle control group. Each value represents the mean±SEM (n=4). bP<0.05, cP<0.01.
Discussion
MDCK-WT cells transfected with the human MDR1 gene, referred to as MDCK-MDR1 cells, expressed higher levels of P-gp following transfection. Compared with the effect in MDCK-WT cells, Ver significantly enhanced the accumulation and efflux inhibition of RTV in MDCK-MDR1 cells, confirming that RTV is a substrate of P-gp9,17. In vitro experiments using MDCK-MDR1 and MDCK-WT cells indicated that Rh2 enhanced the accumulation of RTV in MDCK-MDR1 cells and exhibited a concentration-dependent effect on the inhibition of RTV efflux across MDCK-MDR1 monolayers in the same manner as the standard P-gp inhibitor Ver. However, the accumulation and efflux of RTV in MDCK-WT cells were only marginally affected. The differential effect of Rh2 on the accumulation and efflux of RTV in MDCK-MDR1 and MDCK-WT cells confirmed the inhibitory effect of Rh2 on P-gp13. Further experiments using the human intestinal epithelial cancer cell line Caco-2 revealed that Ver enhanced the accumulation and efflux inhibition of RTV, indicating that RTV is an effective substrate of intestinal P-gp. Rh2 enhanced the accumulation of RTV and had a concentration-dependent effect on the inhibition of RTV efflux across Caco-2 monolayers in the same manner as the standard P-gp inhibitor Ver. These data strongly suggest a potential pharmacokinetic interaction between RTV and Rh2 following oral administration.
The in vivo experiments demonstrated that the co-administration of oral Ver distinctly increased the bioavailability of RTV. Because Ver is an inhibitor of P-gp and CYP3A21, both of which may contribute to the increased bioavailability of RTV9,11, another P-gp inhibitor, GF120918, which is not an inhibitor of CYP3A, was used in combination with RTV22. The co-administration with GF120918 significantly enhanced the RTV bioavailability, increased the level of RTV by 11.8-fold (AUC) in the hepatic portal vein and decreased the amount of RTV in feces, suggesting that the effect of GF120918 on P-gp inhibits the efflux of RTV in gastrointestinal cells and thus enhances the absorption of RTV. The above results confirm the effectiveness of P-gp inhibition on the enhancement of the gastrointestinal absorption of RTV.
Interestingly, despite the distinct inhibition by Rh2 of the P-gp-related accumulation and efflux of RTV in vitro, the intragastric administration of Rh2 at either 25 or 60 mg/kg had little effect on the bioavailability of RTV in rats. However, the intravenous injection of Rh2 at an amount equivalent to that administered intragastrically in terms of bioavailability (Figure 6) significantly increased the AUC and Cmax of RTV. RTV was administered 30 min after the intravenous injection of Rh2 and 60 min after the intragastric administration of Rh2 to give approximately the same plasma concentrations of Rh2. In contrast to our group's previous findings of increased exposure for P-gp substrates, digoxin, fexofenadine, and etoposide by concomitant intragastric administration of Rh2, RTV exposure failed to increase after the intragastric administration of Rh213. However, RTV exposure was increased after the concomitant intravenous administration of Rh2. The gradually ascending concentration of RTV and the obviously delayed Tmax (Figure 3) suggest that intravenous Rh2 had little effect on the early stage of RTV absorption in the gastrointestinal tract but instead primarily affected the profile of RTV at later stages. This hypothesis was also supported by the experimental data indicating that intravenous Rh2 had little effect on the level of RTV in the hepatic portal vein compared with the vehicle control.
To further explore the mechanism underlying the differential effects of the intragastric and intravenous administration of Rh2 on the bioavailability of RTV, the levels of RTV excreted in the bile, the urine, and feces were examined (Figure 5). Compared with the vehicle control, the intravenous administration of Rh2 obviously decreased the cumulative excretion of RTV in the bile, indicating that intravenous Rh2 inhibited transporters involved in the efflux of RTV from the liver into the bile. In contrast, the intravenous administration of Rh2 had little effect on RTV excretion in urine and feces. These results suggest that the effect of intravenous Rh2 on the bioavailability of RTV is mainly dependent on the inhibition of a transporter (eg, P-gp) involved in the efflux of RTV from the liver to the bile. Considering that RTV can be extensively metabolized in male rats and that the original form of RTV was only a very small fraction of the total amount of RTV and its metabolites in the bile according to a previous study20, the total amount of RTV and its metabolites in the bile accounted for a fairly high fraction of the original dose in the rats receiving Rh2 intravenously. Thus, the decreased cumulative excretion of RTV and its metabolites in the bile most likely contributed to the elevated bioavailability of the RTV when co-administered with intravenous Rh2, although the biliary excretion of the original form of RTV accounted for less than 1% of the RTV recovery.
The effect of the intragastric administration of Rh2 on the bioavailability of RTV is difficult to elucidate. Although the co-administration of oral Rh2 did not increase the bioavailability of RTV, there was a distinct elevation in the RTV level in the hepatic portal vein, suggesting the enhancement of the absorption of RTV in the gastrointestinal tract and the enhancement of the effect of Rh2 on P-gp. One possible reason for the marginally affected bioavailability may be the increased elimination of RTV. Therefore, the effects of Rh2 on the distribution, metabolism, and excretion of RTV were studied. The data indicate that Rh2 had little effect on the metabolism of RTV (Supplementary Figure 1), whereas the intragastric administration of Rh2 increased urinary excretion and the distribution of RTV in tissues, such as the liver, lungs, and kidneys. Although the urinary excretion of RTV accounted for less than 1% of the RTV recovery, there were also many metabolites of RTV in the urine, which account for much more of the administered dose than the original form of RTV (data not shown). Compared with the vehicle control, the intragastric administration of Rh2 did not significantly affect the cumulative excretion of RTV in the bile, whereas it increased the absorption of RTV in the gastrointestinal tract, indicating that, in fact, the intragastric administration of Rh2 inhibited the excretion of RTV into the bile as intravenous administration did. The elevated absorption of RTV may have efficiently compensated for the increased urinary excretion and distribution in tissues following the intragastric administration of Rh2.
In summary, Rh2 inhibits the efflux of RTV through P-gp in vitro; however, the in vivo effect of Rh2 on RTV exposure depends on the administration route of Rh2 and involves different mechanisms. The intragastric administration of Rh2 did not significantly affect RTV pharmacokinetics in rats, whereas the intravenous administration of Rh2 increased RTV exposure. Further experiments in humans will be required to confirm this interaction.
Author contribution
Ji-ye AA, Jian SHI, Wei-bin ZHA, Guang-ji WANG, and Chang-xiao LIU designed the study; Jian SHI, Bei CAO, Xiao-lan WU, Lin-sheng LIU, Wen-jing XIAO, Rong-rong GU, Run-bin SUN, Xiao-yi YU, Tian ZHENG, Meng-jie LI, Xin-wen WANG, Jun ZHOU, Yong MAO, Chun GE, Ting MA, and Wen-juan XIA conducted the experiments; Jian SHI, Bei CAO, Ji-ye AA, Wei-bin ZHA, and Guang-ji WANG performed the data analysis; and Jian SHI, Ji-ye AA, Bei CAO, and Guang-ji WANG contributed to the writing of the manuscript.
Acknowledgments
This study was supported by the National Basic Research Program of China 973 Program 2011CB505303, the National Natural Science Foundation of China (81072692), the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (JKGP201108-AJY), and the Project for Jiangsu Province Key Lab of Drug Metabolism and Pharmacokinetics (BM2012012).
We sincerely thank Associate Professor Fang ZHOU and Dr Jing-wei ZHANG (Key Lab of Drug Metabolism and Pharmacokinetics, China Pharmaceutical University, Nanjing, China) for their assistance and advice on this article.
Footnotes
Supplementary information is available at Acta Pharmacologica Sinica website.
Supplementary Information
Inhibition of ritonavir metabolism by ketoconazole, troleandomycin or 20(S)-ginsenoside Rh2 (Rh2) in rat liver microsomes.
References
- Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–92. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–3. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- Martinez E, Domingo P, Galindo MJ, Milinkovic A, Arroyo JA, Baldovi F, et al. Risk of metabolic abnormalities in patients infected with HIV receiving antiretroviral therapy that contains lopinavir-ritonavir. Clin Infect Dis. 2004;38:1017–23. doi: 10.1086/382531. [DOI] [PubMed] [Google Scholar]
- Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. Chemical studies of crude drugs (1). Constituents of Ginseng radix rubra. Yakugaku Zasshi. 1983;103:612–22. [PubMed] [Google Scholar]
- Lai DM, Tu YK, Liu IM, Chen PF, Cheng JT. Mediation of beta-endorphin by ginsenoside Rh2 to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2006;72:9–13. doi: 10.1055/s-2005-916177. [DOI] [PubMed] [Google Scholar]
- Wu N, Wu GC, Hu R, Li M, Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol Sin. 2011;32:345–53. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei XF, Wang BX, Tashiro S, Li TJ, Ma JS, Ikejima T. Apoptotic effects of ginsenoside Rh2 on human malignant melanoma A375-S2 cells. Acta Pharmacol Sin. 2002;23:315–22. [PubMed] [Google Scholar]
- Hwang JT, Kim SH, Lee MS, Yang HJ, Kim MJ, Kim HS, et al. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun. 2007;364:1002–8. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, et al. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol. 1999;57:1147–52. doi: 10.1016/s0006-2952(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–31. [PubMed] [Google Scholar]
- Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–4. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou F, Wu X, Gu Y, Ai H, Zheng Y, et al. 20(S)-Ginsenoside Rh2 noncompetitively inhibits P-glycoprotein in vitro and in vivo: a case for herb-drug interactions. Drug Metab Dispos. 2010;38:2179–87. doi: 10.1124/dmd.110.034793. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhou F, Wu X, Zhang X, Chen Y, Zha BS, et al. Cellular pharmacokinetic mechanisms of adriamycin resistance and its modulation by 20(S)-ginsenoside Rh2 in MCF-7/Adr cells. Br J Pharmacol. 2012;165:120–34. doi: 10.1111/j.1476-5381.2011.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Gao S, Wang J, Yin T, Teng Y, Wu B, et al. Enhancement of oral bioavailability of 20(S)-ginsenoside Rh2 through improved understanding of its absorption and efflux mechanisms. Drug Metab Dispos. 2011;39:1866–72. doi: 10.1124/dmd.111.040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsenz J, Steffen H, Alex R. Active apical secretory efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 cell monolayers. Pharm Res. 1998;15:423–8. doi: 10.1023/a:1011924314899. [DOI] [PubMed] [Google Scholar]
- Meaden ER, Hoggard PG, Newton P, Tjia JF, Aldam D, Cornforth D, et al. P-glycoprotein and MRP1 expression and reduced ritonavir and saquinavir accumulation in HIV-infected individuals. J Antimicrob Chemother. 2002;50:583–8. doi: 10.1093/jac/dkf161. [DOI] [PubMed] [Google Scholar]
- Lee LS, Wise SD, Chan C, Parsons TL, Flexner C, Lietman PS. Possible differential induction of phase 2 enzyme and antioxidant pathways by American ginseng, Panax quinquefolius. J Clin Pharmacol. 2008;48:599–609. doi: 10.1177/0091270008314252. [DOI] [PubMed] [Google Scholar]
- Andrade AS, Hendrix C, Parsons TL, Caballero B, Yuan CS, Flexner CW, et al. Pharmacokinetic and metabolic effects of American ginseng (Panax quinquefolius) in healthy volunteers receiving the HIV protease inhibitor indinavir. BMC Complement Altern Med. 2008;8:50. doi: 10.1186/1472-6882-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denissen JF, Grabowski BA, Johnson MK, Buko AM, Kempf DJ, Thomas SB, et al. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug Metab Dispos. 1997;25:489–501. [PubMed] [Google Scholar]
- Molimard M, Diquet B, Benedetti MS. Comparison of pharmacokinetics and metabolism of desloratadine, fexofenadine, levocetirizine and mizolastine in humans. Fundam Clin Pharmacol. 2004;18:399–411. doi: 10.1111/j.1472-8206.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- Lam JL, Benet LZ. Hepatic microsome studies are insufficient to characterize in vivo hepatic metabolic clearance and metabolic drug-drug interactions: studies of digoxin metabolism in primary rat hepatocytes versus microsomes. Drug Metab Dispos. 2004;32:1311–6. doi: 10.1124/dmd.32.11.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of ritonavir metabolism by ketoconazole, troleandomycin or 20(S)-ginsenoside Rh2 (Rh2) in rat liver microsomes.