Abstract
Tuberculosis (TB) infection poses substantial challenges for obstetricians and gynecologists globally, as gynecologic involvement may cause infertility, irregular bleeding, and pelvic pain. If TB-infected women are able to conceive, obstetric complications include intrauterine growth restriction and, more rarely, congenital transmission. Appropriate screening for high-risk populations is crucial for diagnosis and treatment of latent and active TB infection, which may prevent reproductive sequelae for individual patients and, eventually, contribute to complete eradication of the disease.
Key words: Infertility, Pregnancy, Tuberculosis, Tuberculosis screening, Tuberculosis treatment
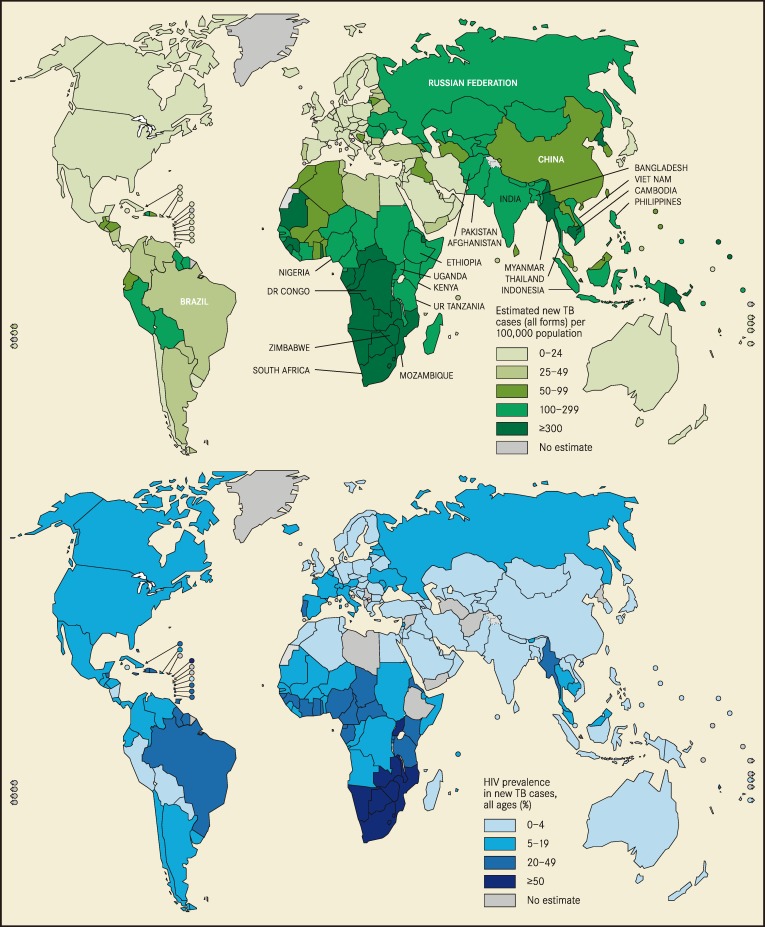
Despite global efforts to eradicate tuberculosis (TB), high rates of TB infection persist, especially where human immunodeficiency virus (HIV) remains prevalent (Figure 1). Worldwide, there were 8.7 million incident cases of TB in 2011, 13% of which occurred in the setting of HIV-1 coinfection.1 The risk of developing TB is 20- to 37-fold higher in those infected with HIV than in the HIV-uninfected population, and more than 25% of deaths among people with HIV are due to TB.2 TB infection is often affected by social factors that further disadvantage the poor and marginalized. The most potent risk factors for TB transmission include overcrowded living conditions, malnutrition, and immunosuppression. Other biologic risk factors, such as genetic polymorphisms and vitamin D deficiency, are areas of ongoing research.3
Figure 1.
Estimated new tuberculosis (TB) cases and human immunodeficiency syndrome (HIV) prevalence in new TB cases. Reproduced with permission from World Health Organization.31
TB is one of the leading nonobstetric causes of maternal mortality in the developing world.4 Approximately one-sixth of maternal deaths in referral hospitals in Southern Africa are attributable to TB.5 Furthermore, TB poses infectious risks to family members, including newborns, in the puerperium. Like many other infectious conditions, TB can be easily screened for during pregnancy, and many women seek primary care only when pregnant. As stated above, TB of the gynecologic tract is a major cause of infertility, pelvic pain, and irregular vaginal bleeding. As immigration rates continue to rise in the United States, obstetrician-gynecologists in urban centers will continue to see patients with gynecologic manifestations of TB.
Pathogenesis
Mycobacterium tuberculosis is an aerobic, acid-fast, nonmotile, non-encapsulated bacillus. It thrives in tissues with high oxygen saturation, which explains its predilection for infecting the lungs. The microorganism is most commonly transmitted from person to person via respiratory tract droplets from those with active pulmonary disease. M tuberculosis replicates slowly, allowing it to persist in tissues for months before causing clinically significant symptoms. The classic histopathologic marker of TB infection is the granuloma with central caseating necrosis, which is the cornerstone of the immune response to M tuberculosis at the tissue level. Granulomas are dynamic collections of macrophages that play a crucial role in the host immune response. However, immunocompromised hosts are unable to form effective granulomas in response to M tuberculosis infection.3
The Centers for Disease Control and Prevention (CDC) define latent TB infection (LTBI) as “the presence of Mycobacterium tuberculosis bacteria in the body as evidenced by a significant reaction to a Mantoux tuberculin skin test or positive interferon gamma release assay,” without active disease symptoms.6 Notably, those with LTBI are not infectious. With immune compromise, the primary infection may be reactivated and become active disease. Such conditions include HIV coinfection, diabetes mellitus, corticosteroid use, end-stage renal disease, and use of tumor necrosis factor-α inhibitors. Approximately 10% of women with LTBI will eventually develop reactivation TB.7
TB/HIV Coinfection
Women living with HIV are at increased risk of TB infection, regardless of their CD4 count.8 However, those with severe immunodeficiency (CD4 < 200) may have more extrapulmonary manifestations, including acute sepsis.9 Immune reconstitution inflammatory syndrome (IRIS), which occurs after initiation of antiretroviral therapy, can acutely unmask active TB as early as 7 days after initiation of treatment.9 This syndrome is thought to be a paradoxical immunologic reaction against tubercular antigens, leading to an inflammatory life-threatening response.9 Coinfection with HIV may also pose a diagnostic challenge for the clinician, as there may be no overt classic clinical symptoms of TB.
TB and the Obstetrician-Gynecologist
Although the predominant site of TB infection is the lung, hematogenous spread may lead to manifestations in other sites, such as the lymph nodes, meninges, peritoneum, skin, bones, and genitourinary tract. Hematogenous dissemination of M tuberculosis from caseous lymph node foci to various organs can lead to miliary deposits (known as miliary TB), which account for 1% to 2% of all TB cases.10 Mother-to-child transmission can occur via either the placenta and amniotic fluid (congenital TB) or respiratory droplets (neonatal TB).
Gynecologic Complications
Approximately 1% of women with M tuberculosis infection have gynecologic involvement, which may result in salpingitis, endometritis, cervicitis, or peritonitis. However, the prevalence of gynecologic TB is much higher in developing countries. One study from India estimated the prevalence of gynecologic TB increased from 13% in 1976 to 30% in 1997 in developing countries.11 Additionally, there remains a large burden of undiagnosed TB among women in impoverished areas who may not reach the health care system. Several studies have examined the links between genital TB and pelvic pain, infertility, menstrual irregularities, and postmenopausal bleeding.12,13 In one study, 75.6% of women with genital TB were found to be infertile.13 In developing countries, infertility is a common presentation of TB in women of reproductive age. Duration of treatment for genital TB in women remains controversial, as there are insufficient data regarding risk of relapse and failure in this population to guide clinical management. In the setting of known infertility and genital TB, some experts advocate aggressive treatment for up to 1 year before advising patients to attempt spontaneous pregnancy or assisted reproductive technologies.
Obstetric Complications
During pregnancy, congenital TB is rare and usually associated with HIV infection. However, one study reported vertical transmission of TB in approximately 16% of infected mothers, and HIV-1 coinfection did not seem to contribute significantly to vertical TB transmission. 14 Compared with uninfected women, rates of intrauterine growth restriction and low birth weight among infected mothers were dramatically higher (66% and 49%, respectively). Some hypotheses for this association include placental infection and insufficiency, and maternal malnutrition, anemia, and cachexia. In Sudan, where its incidence is 275 per 100,000 pregnant women, maternal TB was associated with anemia, low birth weight, and preterm birth.12
Maternal HIV/TB coinfection increases vertical transmission of HIV. Gupta and colleagues found that 30% of HIV/TB coinfected mothers transmitted HIV to their newborns, compared with only 12% of mothers uninfected with TB.15 After controlling for maternal CD4 count, viral load, antiretroviral use, and breastfeeding duration, maternal TB was associated with a 2.51-fold increased odds ratio of mother-to-child transmission of HIV.
Screening and Diagnosis
The CDC and World Health Organization (WHO) recommend that all adults and adolescents living with HIV, including pregnant women, undergo screening for TB. The tuberculin skin test (TST), which is based on a purified protein derivative of M tuberculosis, was the first test for infection. A positive reading indicates cell-mediated hypersensitivity to tubercular antigens, but does not differentiate between LTBI and active disease. The TST requires a clinical visit to determine the result 48 to 72 hours after the intradermal test is administered.
Interferon-γ release assays (IGRAs) are alternative screening tests that have been proven to be more specific than TSTs, especially in populations that have been immunized against TB with Bacillus Calmette-Guérin vaccine.16,17 IGRAs measure interferon-γ (IFN-γ), which is released from T cells in response to specific antigens presented by M tuberculosis. However, there are discordant data regarding the accuracy of TSTs and IGRAs in detecting LTBI in HIV-infected people.18,19 Positive IGRA results during pregnancy (IFN-γ > 8) have been shown to persist into the postpartum period,20 indicating that pregnancy does not have an anergic effect on the pathogenesis and manifestations of TB infection. In immunosuppressed women (CD4 count < 250), a positive IGRA result was associated not only with increased overall maternal mortality, but also with increased maternal and infant active TB and their attendant mortality. 21 The CDC now states that IGRAs can be used in all situations in which the TST is recommended.22 However, the cost of IGRAs and the laboratory requirements for their interpretation remain prohibitive in resource-poor settings.
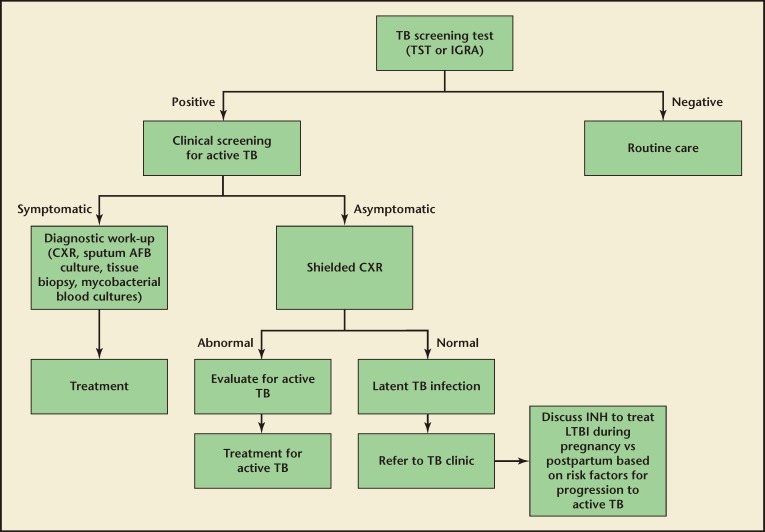
Among those who have a positive screening test result, chest radiographs are performed to identify cases of active pulmonary infection. Additionally, sputum samples are obtained and examined for the presence of acid-fast bacilli if there is a suspicion of active pulmonary disease. If extrapulmonary TB is suspected, a tissue biopsy is useful for diagnosis. Blood cultures for mycobacteria may be drawn in cases where there is a high suspicion of disseminated disease. Drug susceptibility tests are subsequently performed on any specimens with M tuberculosis to guide clinical treatment. An algorithm for TB screening in pregnancy is shown in Figure 2.
Figure 2.
Algorithm for TB screening in pregnancy. This algorithm excludes patients with prior positive TB screening. AFB, acid-fast bacilli; CXR, chest x-ray (radiograph); IGRA, interferon-g release assay; INH, isonicotinylhydrazine; TB, tuberculosis; TST, tuberculin skin test.
Given the global distribution of TB and the poor infrastructure in many developing areas, symptom screening is an effective clinical alternative to TSTs and IGRAs. One study showed that the negative predictive value for screening using fever, cough, night sweats, or weight loss was 99.7% for detecting active TB among HIV-infected pregnant women.23 However, clinical screening does not identify those with LTBI, nor is it validated for detecting gynecologic involvement.
Treatment in Pregnant Women Without HIV Infection
Latent TB
The CDC recommends isoniazid (isonicotinylhydrazine [INH]) treatment either daily or twice weekly with supplemental pyridoxine for 9 months in pregnant women with LTBI (Table 1).24 However, treatment can be deferred to the postpartum period in those at low risk for progression to active TB disease, due to the potential increased risk of hepatotoxicity with INH during pregnancy.25
Table 1.
Recommended for LTBI Treatment in Pregnant Women
| CDC | WHO | |
|---|---|---|
| Regimen | INH 300 mg/d × 6-9 mo + pyridoxine 25 × mg/d 6–9 mo OR | INH 300 mg/d × 6 moa + pyridoxine 10–25 mg/d × 6 mo |
| INH 900 mg 2 × wk × 9 mo + pyridoxine 25 × mg/d 9 mo | ||
| HIV-positive | Immediate treatmentb | Immediate treatment |
| HIV-negative | May defer treatment until 2–3 mo postpartum as long as no other risk factors for progression to active TB are present | No guidelines |
aConsider treatment for 36 mo if in a country with a high TB burden.
bFor HIV-positive women who are on antiretroviral drugs during pregnancy strictly to prevent vertical transmission, may be deferred to postpartum period. CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; INH, isonicotinylhydrazine; LTBI, latent tuberculosis infection; TB, tuberculosis; WHO, World Health Organization.
Active TB
For pregnant women with active infection, the recommended treatment regimen includes INH, rifampin, and ethambutol for 9 months. The antimicrobial agents that are contraindicated in pregnancy are streptomycin (due to fetal ototoxicity), kanamycin, amikacin, capreomycin, and fluoroquinolones (Table 2). Notably, the CDC does not recommend pyrazinamide in pregnancy, as there are limited data on such use. Other international organizations, such as the WHO and the International Union Against Tuberculosis and Lung Diseases, do include pyrazinamide in treatment guidelines in pregnancy.8 The CDC recommends that these women have surveillance hepatic panels every month of treatment, due to potentially increased rates of isoniazidinduced hepatotoxicity.8 Although rifampin is not a known teratogen in humans, the CDC recommends a single prophylactic dose of vitamin K to minimize the potential increased risk of hemorrhagic problems in neonates of women undergoing anti-TB treatment with rifampin (Table 3).8
Table 2.
Use of Antituberculosis Drugs in Pregnancy, Lactation, and in the Newborn Baby
| Drug | Pregnancy | Lactation | Newborn |
|---|---|---|---|
| Rifamycins | |||
| Rifampicin | Safe | 0.05% of adult dose can be detected | Safe 10–20 mg/kg/d |
| Rifabutin | Congenital defects in animal studies | — | Use not established |
| Rifapentine | Rarely causes bleeding in mother and newborn if administered in last few weeks of pregnancy | — | Use not established |
| Isoniazid | Safe; supplement with pyri-doxine to avoid peripheral neuropathy | 0.75%–2.3% of adult dose can be detected | Safe 5–10 mg/kg/d |
| Pyrazinamide | Limited data but recom-mended | — | Safe 20–30 mg/kg/d |
| Ethionamide | Safe in humans; cleft palate, skull and spine defects | In minute amounts | Retrobulbar neuritis; not recommended |
| Aminoglycosides | Ototoxicity in fetus; renal damage | 0.05%–0.5% of adult dose can be detected; not well absorbed orally | Use with caution |
| Quinolones | Bone development abnor-malities in animals | — | Use with caution |
Reprinted with permission from Pillay T et al.5
Table 3.
Recommendations for Active Tuberculosis Treatment in Pregnant Women
| CDC | WHO | |
|---|---|---|
| Regimen (regardless of HIV status) | INH, RIF or rifabutin, and EMB × 2 mo Followed by: INH and RIF or rifabutin × 4–7 moa Pyridoxine supplementation Pyridoxine supplementation | INH, RIF, pyrazinamide, and EMB × 2 mo Followed by: INH and RIF × 4 moa Pyridoxine supplementation |
| Dosing frequency | Daily | Daily; 3× wk allowed with DOTS |
| Monitoring | Monthly hepatic panels | — |
| Newborn assessment | — | After active TB ruled out, new-born should receive INH × 6 mo, followed by BCG vaccine |
| Other recommendations | — | Vitamin K administration to newborn if mother taking RIF to decrease risk of neonatal hemorrhage |
aVaries based on drug resistance and anatomic location of TB infection (central nervous system, bone, joint involvement may require longer treatment). BCG, Bacillus Calmette-Guérin; CDC, Centers for Disease Control and Prevention; DOTS, directly observed treatment strategy; EMB, ethambutol; HIV, human immunodeficiency virus; INH, isonicotinylhydrazine; TB, tuberculosis; RIF, rifampin; WHO, World Health Organization.
Treatment in Pregnant Women With HIV Infection
Latent TB
Among HIV-infected pregnant women, a positive IGRA result has been associated with a 4.5-fold increased risk of active TB.21 According to the WHO, those who have an unknown or positive TST or IGRA result should receive at least 6 months of isoniazid preventive therapy.2 The CDC also recommends universal treatment for LTBI among HIV-infected adults.8 Additionally, the guidelines state that treatment is indicated for HIV-positive adults who either have had close contact with individuals with active pulmonary TB or have a history of inadequately treated TB, regardless of screening test results.8 Pregnant women with HIV and LTBI should undergo treatment whenever possible. However, due to the potential of INH/antiretroviral drug interactions, the CDC does allow for deferment of LTBI treatment in pregnant women who are already on antiretroviral drugs strictly for preventing vertical transmission and plan to stop antiretroviral drugs after delivery.8
Active TB
Treatment guidelines for active TB in HIV-positive pregnant women are no different than those for other adults. The CDC recommends a 6- to 12-month regimen of isoniazid, rifampin or rifabutin, pyrazinamide, and ethambutol, depending on the organs involved. For pregnant women infected with HIV, risks and benefits of anti-TB regimens must be balanced with antiretroviral regimens. Rifampin induces cytochrome P450 enzymes, which metabolize nonnucleoside reductase inhibitors (NNRTIs). Efavirenz is the preferred NNRTI with concomitant rifampin-based TB regimens, because it has been shown to maintain its potency in the presence of rifampicin.26 However, animal and retrospective studies have produced some concerns that efavirenz is associated with neural tube defects.27,28 Despite these findings, a meta-analysis of 11 prospective cohorts and 5 retrospective reviews showed no increased risk of birth defects among women exposed to efavirenz in the first trimester compared with other antiretroviral drugs.29 If HIV and active TB are diagnosed concurrently, the CDC recommends TB treatment before initiating antiretroviral drugs, given the risk of IRIS. However, because there are no clear guidelines for this clinical situation in pregnant women, management should be conducted in collaboration with infectious disease specialists.
Global Efforts to Eradicate TB
Directly observed treatment strategy (DOTS) has historically been the cornerstone of TB surveillance and treatment. Because of its evidence-based success, DOTS has been applied globally to TB, HIV, and other infectious diseases. The five components of the strategy are (1) political commitment, (2) early case detection through qualityassured diagnosis, (3) standardized treatment with supervision and patient support, (4) drug supply and management system, and (5) monitoring and evaluation.30 Because of the extraordinary burden of TB among HIV-infected individuals, multinational organizations have focused specifically on that population. In 2011, the WHO published the “Three I’s” for TB/HIV: intensified casefinding of TB, isoniazid preventive therapy, and infection control for TB.2 Due to increasing findings of drug-resistant strains of M tuberculosis, the WHO endorsed the Xpert® MTB/RIF (Foundation for Innovative New Diagnostics [Geneva, Switzerland]/Cepheid [Sunnyvale, CA]) test in 2010 as an effective, rapid point-of-care screen for resistant strains during a single clinical encounter.31
Despite the publication of various international guidelines for TB detection and treatment, the implementation of such guidelines in resource-limited settings remains hampered by deficiencies in health system infrastructure. For example, South Africa has experienced difficulty in integrating vertical programs for TB, HIV, and prevention of mother-to-child transmission at the different health system levels.32 Clinical screening for active TB symptoms in HIV-positive pregnant women has led to improvements in case detection and takes advantage of the unique TB, HIV, and prevention of mother-to-child transmission interaction between pregnant women and the health care system.33 As immigration rates continue to rise, obstetrician-gynecologists have the opportunity to intervene in the TB infection cycle both locally and globally by creating appropriate screening and treatment programs for at-risk women.
Main Points.
Gynecologic tuberculosis (TB) is a substantial cause of infertility among women in resource-poor settings.
TB and HIV act synergistically, exacerbating poor obstetric and gynecologic outcomes.
Interferon-g release assays are recommended by the Centers for Disease Control and Prevention for TB screening and have better specificity than tuberculin skin tests.
Pregnancy is an ideal window during which to screen and treat women with latent TB infection who have risk factors for progression to active TB.
References
- 1.World Health Organization, authors. Global Tuberculosis Report 2012. Geneva, Switzerland: World Health Organization; 2012. [Accessed November 12, 2013]. [Google Scholar]
- 2.World Health Organization, authors. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Department of HIV/AIDS and Stop TB Department. Geneva, Switzerland: World Health Organization; 2011. [Accessed November 12, 2013]. [Google Scholar]
- 3.Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 4.Grange J, Adhikari M, Ahmed Y, et al. Tuberculosis in association with HIV/AIDS emerges as a major nonobstetric cause of maternal mortality in Sub-Saharan Africa. Int J Gynaecol Obstet. 2010;108:181–183. doi: 10.1016/j.ijgo.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Pillay T, Khan M, Moodley J, et al. Perinatal tuberculosis and HIV-1: considerations for resource-limited settings. Lancet Infect Dis. 2004;4:155–165. doi: 10.1016/S1473-3099(04)00939-9. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Web site, authors. Tuberculosis: menu of suggested provisions for state tuberculosis prevention and control laws. [Accessed November 12, 2013].
- 7.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Web site, authors. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. [Accessed November 12, 2013].
- 9.Müller M, Wandel S, Colebunders R, et al. IeDEA Southern and Central Africa, authors. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–430. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Sumana G, Mittal S. Genital tuberculosis: a leading cause for infertility in women seeking assisted conception in North India. Arch Gynecol Obstet. 2008;278:325–327. doi: 10.1007/s00404-008-0590-y. [DOI] [PubMed] [Google Scholar]
- 12.Ali AA, Abdallah TM. Clinical presentation and epidemiology of female genital tuberculosis in eastern Sudan. Int J Gynaecol Obstet. 2012;118:236–238. doi: 10.1016/j.ijgo.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Namavar Jahromi B, Parsanezhad ME, Ghane-Shirazi R. Female genital tuberculosis and infertility. Int J Gynaecol Obstet. 2001;75:269–272. doi: 10.1016/s0020-7292(01)00494-5. [DOI] [PubMed] [Google Scholar]
- 14.Pillay T, Sturm AW, Khan M, et al. Vertical transmission of Mycobacterium tuberculosis in KwaZulu Natal: impact of HIV-1 co-infection. Int J Tuberc Lung Dis. 2004;8:59–69. [PubMed] [Google Scholar]
- 15.Gupta A, Bhosale R, Kinikar A, et al. Six Week Extended-Dose Nevirapine (SWEN) India Study Team, authors. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011;203:358–363. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–354. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 17.Wolf T, Goestsch U, Oremek G, et al. Tuberculosis skin test, but not interferon-γ-releasing assays is affected by BCG vaccination in HIV patients. J Infect. 2013;66:376–380. doi: 10.1016/j.jinf.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Talati N, Seybold U, Humphrey B, et al. Poor concordance between interferon-γ release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis. 2009;9:15. doi: 10.1186/1471-2334-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos JM, Robledano C, Masiá M, et al. Contribution of Interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: a comparison of QuantiFERON-TB Gold In Tube, T-SPOT.TB and tuberculin skin test. BMC Infect. 2012;12:169. doi: 10.1186/1471-2334-12-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonnalagadda SR, Brown E, Lohman-Payne B, et al. Consistency of Mycobacterium tuberculosis-specific interferon-gamma responses in HIV-1 infected women during pregnancy and postpartum. Infect Dis Obstet Gynecol. 2012;2012:950650. doi: 10.1155/2012/950650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonnalaggada S, Lohman Payne B, Brown E, et al. Latent tuberculosis detection by interferon γ release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J Infect Dis. 2010;202:1826–1835. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection — United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 23.Gupta A, Chandrasekhar A, Gupte N, et al. Byramjee Jeejeebhoy Medical College-Johns Hopkins University Study Group, authors. Symptom screening among HIV-infected pregnant women is acceptable and has high negative predictive value for active tuberculosis. Clin Infect Dis. 2011;53:1015–1018. doi: 10.1093/cid/cir605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control Web site, authors. TB elimination: tuberculosis in pregnancy. [Accessed November 12, 2013].
- 25.Boggess KA, Myers ER, Hamilton CD. Antepartum or postpartum isoniazid treatment for latent tuberculosis infection. Obstet Gynecol. 2000;96(5 Pt 1):757–762. doi: 10.1016/s0029-7844(00)01039-5. [DOI] [PubMed] [Google Scholar]
- 26.Patel A, Patel K, Patel J, et al. Safety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral-naïve patients in India who are coinfected with tuberculosis and HIV-1. J Acquir Immune Defic Syndr. 2004;37:1166–1169. doi: 10.1097/01.qai.0000135956.96166.f0. [DOI] [PubMed] [Google Scholar]
- 27.Fundarò C, Genovese O, Rendeli C, et al. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002;16:299–300. doi: 10.1097/00002030-200201250-00025. [DOI] [PubMed] [Google Scholar]
- 28.De Santis M, Carducci B, De Santis L, et al. Periconceptional exposure to efavirenz and neural tube defects. Arch Intern Med. 2002;162:355. doi: 10.1001/archinte.162.3.355. [DOI] [PubMed] [Google Scholar]
- 29.Ford N, Mofenson L, Kranzer K, et al. Safety of efavirenz in first-trimester of pregnancy: a systematic review and meta-analysis of outcomes from observational cohorts. AIDS. 2010;24:1461–1470. doi: 10.1097/QAD.0b013e32833a2a14. [DOI] [PubMed] [Google Scholar]
- 30.Stop TB Partnership, authors. The Global Plan to Stop TB 2011–2015: Transforming the Fight Towards Elimination of Tuberculosis. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 31.Global Tuberculosis Control 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 32.Uwimana J, Jackson D, Hausler H, Zarowsky C. Health system barriers to implementation of collaborative TB and HIV activities including prevention of mother to child transmission in South Africa. Trop Med Int Health. 2012;17:658–665. doi: 10.1111/j.1365-3156.2012.02956.x. [DOI] [PubMed] [Google Scholar]
- 33.DeLuca A, Chaisson RE, Martinson NA. Intensified case finding for tuberculosis in prevention of mother-to-child transmission programs: a simple and potentially vital addition for maternal and child health. J Acquir Immune Defic Syndr. 2009;50:196–199. doi: 10.1097/QAI.0b013e3181900201. [DOI] [PMC free article] [PubMed] [Google Scholar]